Abstract
Background
US attributable Clostridioides difficile infection (CDI) mortality and cost data are primarily from Medicare fee-for-service populations, and little is known about Medicare Advantage Enrollees (MAEs). This study evaluated CDI incidence among MAEs from 2012 to 2019 and determined attributable mortality and costs by comparing MAEs with and without CDI occurring in 2018.
Methods
This retrospective cohort study assessed CDI incidence and associated mortality and costs for eligible MAEs ≥65 years of age using the de-identified Optum Clinformatics Data Mart database (Optum; Eden Prairie, Minnesota, USA). Outcomes included mortality, healthcare utilization, and costs, which were assessed via a propensity score–matched cohort using 2018 as the index year. Outcome analyses were stratified by infection acquisition and hospitalization status.
Results
From 2012 to 2019, overall annual CDI incidence declined from 609 to 442 per 100 000 person-years. Although the incidence of healthcare-associated CDI declined overall (2012, 53.2%; 2019, 47.2%), community-associated CDI increased (2012, 46.8%; 2019, 52.8%). The 1-year attributable mortality was 7.9% (CDI cases, 26.3%; non-CDI controls, 18.4%). At the 2-month follow-up, CDI-associated excess mean total healthcare and out-of-pocket costs were $13 476 and $396, respectively. Total excess mean healthcare costs were greater among hospitalized (healthcare-associated, $28 762; community-associated, $28 330) than nonhospitalized CDI patients ($5704 and $2320, respectively), whereas total excess mean out-of-pocket cost was highest among community-associated hospitalized CDI patients ($970).
Conclusions
CDI represents an important public health burden in the MAE population. Preventive strategies and treatments are needed to improve outcomes and reduce costs for healthcare systems and this growing population of older US adults.
Keywords: Clostridioides difficile, Medicare Advantage, incidence, mortality, costs
Clostridioides difficile infection (CDI) poses a considerable burden among the Medicare Advantage Enrollee population of US adults ≥65 years. Compared with matched controls, patients diagnosed with CDI had greater attributable mortality rates and higher excess healthcare utilization and patient out-of-pocket costs.
Clostridioides difficile is a gram-positive bacterium that forms spores and produces exotoxins primarily in the colon [1]. Symptoms of C. difficile infection (CDI) range from mild to severe diarrhea to outcomes in serious cases including pseudomembranous colitis, toxic megacolon, and death [2–4]. Risk factors for CDI include, but are not limited to, older age, hospitalization, and antibiotic use [1, 3, 5]. Patients with primary CDI can experience long-term adverse outcomes, including recurrence rates of up to 30% [1, 6–8].
The estimated CDI burden in the United States, according to the Centers for Disease Control and Prevention (CDC) Emerging Infections Program, decreased from 154.89 per 100 000 in 2011 to 143.61 per 100 000 in 2017 [9]. While historically associated with nosocomial infection, community-onset cases are increasingly contributing to CDI burden [1, 9]. In 2011, the US incidence of healthcare-associated and community-associated CDI was 92.76 and 48.16 per 100 000, respectively; in contrast, corresponding values in 2018 were 64.18 and 65.93 per 100 000, respectively [9, 10]. In 2018, the US CDI incidence in those aged 65 years and older was 431 per 100 000, accounting for 46% of the overall CDI burden, of which 61% were healthcare associated and 39% were community associated [10].
The substantial morbidity and mortality of CDI are at least partly attributed to changing virulence of the pathogen [1, 11]. Clostridioides difficile infection is also associated with prolonged hospitalization and other healthcare resource utilization, such as direct treatment costs [12]. As CDI management evolves [13], the already substantial per-patient healthcare costs and healthcare utilization associated with CDI are likely to increase [11, 12].
In the United States, attributable CDI mortality and cost data have been primarily derived from Medicare fee-for-service populations. For instance, a US study of Medicare fee-for-service enrollees (2008–2010) found that mortality rates were twice as high among patients with CDI compared with patients without CDI, and Medicare costs per patient with CDI were nearly $30 000 higher [14]. Compared with Medicare fee-for-service populations, little is known about Medicare Advantage Enrollees (MAEs; ie, those who receive Medicare benefits through Part C coverage via private insurers), who comprise an increasing proportion of Medicare beneficiaries, accounting for 39% of the Medicare population in 2020 [15]. Therefore, this study evaluated CDI incidence among MAEs from 2012 to 2019 and determined attributable mortality and costs by comparing MAEs with and without CDI occurring in 2018.
METHODS
Study Design and Data Source
This retrospective cohort study assessed CDI incidence and associated mortality and costs, stratified by acquisition and CDI hospitalization status, for eligible MAEs aged 65 years and older using the Optum Clinformatics Data Mart database (Optum; Eden Prairie, Minnesota, USA), which covers all US states. The administrative claims are verified, adjudicated, adjusted, and de-identified before inclusion in the database and comprise commercial and Medicare Advantage health plan data. In addition to medical and pharmacy claims, the database includes member eligibility, standard costs for all medical and pharmacy claims (ie, standard prices reflecting the allowed payment for all provider services), and patient out-of-pocket costs. This study was restricted to Medicare Advantage health plans that covered approximately 6.1 million elderly persons in 2019. The study was exempt from Institutional Review Board consideration because it used existing de-identified administrative claims data.
Inclusion Criteria and CDI Case Definition
For the annual incidence analyses, individuals were required to be 65 years of age or older on 1 January of a calendar year between 2012 and 2019. Individuals were followed up until the earliest of death, disenrollment, or the end of the calendar year. For the attributable mortality and cost analyses, the study period for CDI identification was 2018, with use of data from 2017 to 2019. For these analyses, patients were required to be continuously enrolled for 1 year before the CDI diagnosis date in 2018 and were followed up through the earliest of death, disenrollment, or the end of the 1-year follow-up period. Eligible controls must have met the same inclusion criteria but have had no CDI in 2017 to 2019 based on the CDI case definition.
The CDI case definition included any of the following: an inpatient claim with an International Classification of Disease, Ninth or Tenth Revision, Clinical Modification (ICD-9-CM or ICD-10-CM), CDI diagnosis code of 008.45 or A04.7x, respectively, not including admission diagnosis; a nonlaboratory outpatient claim with a CDI diagnosis plus nontopical metronidazole, oral vancomycin, or fidaxomicin therapy within ±14 days of diagnosis; or an outpatient laboratory CDI test (Current Procedural Terminology [CPT] codes 87230 [cell culture cytotoxicity assay], 87324 [immunoassay], 87493 [nucleic acid amplification]) plus nontopical metronidazole, oral vancomycin, or fidaxomicin therapy within ±14 days. To ensure a primary episode, all qualified CDI episodes, excluding the first claim during the index year, must have had no CDI claims matching any of the above criteria 60 or fewer days prior.
Each CDI episode was also classified by healthcare-associated or community-associated acquisition status according to established guidelines [6]. Healthcare-associated CDI was defined as the first CDI occurrence meeting the hospital- or other healthcare facility-onset CDI case definition with an index date more than 3 days after admission, or an inpatient, skilled nursing facility, hospice, long-term care facility, or nursing home claim more than 1 day length of stay in the 12 weeks before the CDI index date. Community-associated CDI was defined as all CDI cases other than those identified as healthcare associated (ie, cases identified from the outpatient setting or an inpatient facility ≤3 days after admission, with no inpatient stay in the previous 12 weeks).
The Charlson Comorbidity Index (CCI) score [16], based on 17 conditions, was among the baseline characteristics determined from the Optum database. Baseline individual comorbidities diagnosed 1 year or less before the index date and complications associated with CDI 2 months and 1 year post-index were identified using ICD-9-CM or ICD-10-CM diagnosis codes. Complications evaluated were primarily gastrointestinal in nature but also included blood transfusion and sepsis.
Statistical Analysis
CDI Annual Incidence (2012–2019)
The annual numbers of CDI episodes in each calendar year for 2012 to 2019 are reported overall by year and by age group. The denominator for the incidence calculations was based on the total follow-up time in person-years for the eligible study population in each calendar year, and rates are presented as episodes per 100 000 person-years. To assess the linear effect of time on incidence while accounting for individuals in more than 1 calendar year, a Poisson generalized estimating equation model with an unstructured correlation structure and calendar year as a covariate was used.
Outcomes Analysis
The first CDI diagnosis or toxin test date in 2018 was designated as the index date for CDI cases; controls were randomly assigned an index date in 2018. Patients with CDI and controls were matched 1:1 according to the propensity score (PS) obtained from a multivariable logistic regression model with 61 variables (Supplementary Figure 1), including demographics, baseline comorbidities, prior infections, and prior healthcare utilization, as well as exact matching by 5-year age groups and index date 2-week windows. Greedy nearest-neighbor matching and a caliper width of 0.1 of the standard deviation of the logit of the PS were used for matching cases and controls. Standardized differences were calculated to ensure the baseline variables included in the logistic regression were well balanced between matched CDI cases and controls, with values of 0.1 or less used as evidence of acceptable balance.
All-cause mortality was assessed 30, 60, 90, 180, and 365 days after the index date. Information on mortality was only available as year and month of death; thus, calculation of mortality risk at given intervals was determined by assuming the date of death occurred on the 15th day of the month. All-cause healthcare utilization and medical costs, which included all medical visits (ie, inpatient, emergency department, outpatient, and other visits) and outpatient pharmacy prescriptions, accrued within 2 months and 1 year of the index date were assessed. Healthcare costs and patient’s out-of-pocket expenses (eg, copay, deductibles, coinsurance) were also investigated for the same period (Supplementary Appendix). Costs were adjusted to 2019 US dollars using the medical care component of the Consumer Price Index (https://www.bls.gov/cpi/). Costs within 1 year after the index date and in patients surviving 1 year were also generated to avoid potential bias due to high mortality among cases. The attributable mortality, healthcare utilization, and cost burden of CDI were determined by calculating the difference of all-cause mortality, utilization, and costs between PS-matched cases and controls in the post-index period, with McNemar and paired t tests used to test differences. Attributable mortality and costs were also analyzed by acquisition (healthcare or community associated) and hospitalization (hospitalized or nonhospitalized) status. Hospitalized cases were those who were hospitalized on the index date or during the 60 days post-index follow-up with a diagnosis code for CDI; all other cases were considered nonhospitalized.
RESULTS
In the Optum Clinformatics Data Mart, there were 2.7 million MAEs in 2012, which increased each year, reaching 6.1 million by 2019. After applying inclusion criteria, 2.1 to 4.9 million eligible MAEs were included in the study from 2012 to 2019 (Table 1). Among MAEs with CDI by year, the mean age ranged from 76.8 to 77.6 years, nearly two-thirds were female, over one-third were from the South, and the mean CCI score ranged from 4.1 to 4.5 (Supplementary Table 1).
Table 1.
Flow of Patient Counts by Index Year and Study Population
| Index Year | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | |
| Total MAEs at risk in index year | 2 705 359 | 3 008 317 | 3 152 961 | 3 476 157 | 4 315 301 | 5 203 197 | 5 633 071 | 6 004 001 |
| ȃ ≥66 years of age | 2 260 516 | 2 509 501 | 2 678 584 | 2 976 739 | 3 600 523 | 4 347 636 | 4 754 922 | 5 092 804 |
| ȃEnrolled as of 1 January of index year | 2 145 417 | 2 362 527 | 2 447 332 | 2 836 761 | 3 391 957 | 4 110 615 | 4 520 218 | 4 856 847 |
| No. of CDI cases in index year during continuous enrollment | 11 152 | 11 864 | 11 564 | 13 321 | 16 169 | 19 328 | 19 185 | 18 308 |
Data are presented as number of patients. Abbreviations: CDI, Clostridioides difficile infection; MAE, Medicare Advantage Enrollee.
Annual CDI Incidence
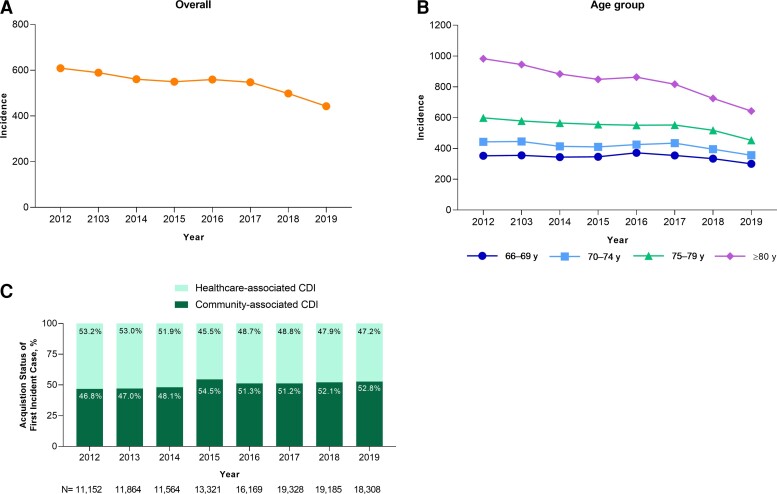
A downward trend in overall annual CDI incidence rate among MAEs was generally observed from 2012 to 2019; this trend was observed across all age groups (Figure 1A and 1B). In 2012, the CDI incidence rate was 609 per 100 000 person-years, which decreased to 442 per 100 000 person-years in 2019 (P < .001). The greatest incidence rate reductions over this period were among patients 80 years of age and older (982 and 643 per 100 000 person-years in 2012 and 2019, respectively). The proportion of patients with healthcare-associated CDI generally decreased from 53.2% in 2012 to 47.2% in 2019 (range, 45.5−53.2%), whereas community-associated CDI increased from 46.8% in 2012 to 52.8% in 2019 (range, 46.8%−54.5%) (Figure 1C).
Figure 1.
Annual incidence rate of (A) CDI overall and by (B) age and (C) proportion of healthcare-associated and community-associated cases (2012−2019). Incidence rates are presented as CDI episodes per 100 000 person-years. Abbreviations: CDI, Clostridioides difficile infection; MAE, Medicare Advantage Enrollee.
Outcome Results
In 2018, there were 15 195 CDI cases and 3 416 844 controls. Using the PS to develop CDI, 14 928 CDI cases were matched to 14 928 non-CDI controls. Patient characteristics were generally well balanced between matched groups (Table 2 and Supplementary Figure 1).
Table 2.
Patient Demographic and Baseline Clinical Characteristics in the Outcomes Cohort (Index Year = 2018)
| Before Propensity Score Matching | After Propensity Score Matching | |||||
|---|---|---|---|---|---|---|
| Characteristics | Cases (n = 15 195) |
Controls (n = 3 416 844) |
Std Diff | Case (n = 14 928) |
Control (n = 14 928) |
Std Diff |
| Age, mean (SD), years | 77.9 (6.9) | 75.9 (6.6) | .3005a | 77.9 (6.9) | 77.9 (6.9) | .0016 |
| Age, n (%) | … | … | .2971a | … | … | .0000 |
| ȃ66−69 years | 1999 (13.2) | 658 491 (19.3) | … | 1957 (13.1) | 1957 (13.1) | … |
| ȃ70−74 years | 3538 (23.3) | 1 011 620 (29.6) | … | 3466 (23.2) | 3466 (23.2) | … |
| ȃ75−79 years | 3326 (21.9) | 746 807 (21.9) | … | 3265 (21.9) | 3265 (21.9) | … |
| ȃ80−84 years | 2711 (17.8) | 487 552 (14.3) | … | 2657 (17.8) | 2657 (17.8) | … |
| ȃ≥85 years | 3621 (23.8) | 512 374 (15.0) | … | 3583 (24.0) | 3583 (24.0) | … |
| Male, n (%) | 5374 (35.4) | 1 429 668 (41.8) | −.1333a | 5289 (35.4) | 5110 (34.2) | .0252 |
| Region, n (%) | … | … | .1637a | … | … | .0239 |
| ȃNortheast | 1866 (12.3) | 442 057 (12.9) | … | 1836 (12.3) | 1803 (12.1) | … |
| ȃMidwest | 3359 (22.1) | 610 281 (17.9) | … | 3275 (21.9) | 3241 (21.7) | … |
| ȃSouth | 6565 (43.2) | 1 385 280 (40.5) | … | 6450 (43.2) | 6578 (44.1) | … |
| ȃWest | 3403 (22.4) | 977 489 (28.6) | … | 3365 (22.5) | 3306 (22.1) | … |
| ȃUnknown | 2 (0.0) | 1737 (0.1) | … | 2 (0.0) | 0 (0.0) | … |
| Type of first incident CDI case definition in 2018 | … | … | N/A | … | … | N/A |
| ȃHospital claim | 5407 (35.6) | … | … | 5215 (34.9) | … | … |
| ȃOther inpatient facility claimb | 1810 (11.9) | … | … | 1751 (11.7) | … | … |
| ȃOutpatient claim and antibiotics | 1622 (10.7) | … | … | 1609 (10.8) | … | … |
| ȃToxin test and antibiotic | 6356 (41.8) | … | … | 6353 (42.6) | … | … |
| Acquisition status of first incident case, n (%) | … | … | N/A | … | … | N/A |
| ȃHealthcare acquired | 7370 (48.5) | … | … | 7105 (47.6) | … | … |
| ȃȃHospitalized | 4021 (26.5) | … | … | 3812 (25.6) | … | … |
| ȃȃNot hospitalized | 3349 (22.0) | … | … | 3293 (22.1) | … | … |
| ȃCommunity acquired | 7825 (51.5) | … | … | 7823 (52.4) | … | … |
| ȃȃHospitalized | 2101 (13.8) | … | … | 2099 (14.1) | … | … |
| ȃȃNot hospitalized | 5724 (37.7) | … | … | 5724 (38.3) | … | … |
| Charlson Comorbidity Index, mean (SD) | 4.6 (3.2) | 1.9 (2.2) | .9862a | 4.5 (3.2) | 4.6 (3.2) | −.0192 |
| Charlson Comorbidity Index, n (%) | … | … | .9261a | … | … | .0328 |
| ȃ0 | 1471 (9.7) | 1 270 336 (37.2) | … | 1471 (9.9) | 1336 (9.0) | … |
| ȃ1 | 1538 (10.1) | 625 931 (18.3) | … | 1534 (10.3) | 1514 (10.1) | … |
| ȃ2 | 1732 (11.4) | 529 393 (15.5) | … | 1725 (11.6) | 1713 (11.5) | … |
| ȃ3+ | 10 454 (68.8) | 991 184 (29.0) | … | 10 198 (68.3) | 10 365 (69.4) | … |
Abbreviations: N/A, not applicable; Std Diff, standardized difference.
Absolute values >.1 considered significant.
Long-term care, inpatient hospice, inpatient mental health/chemical dependence, or inpatient rehabilitation facilities.
Table 3 summarizes potentially CDI-related complications during the follow-up periods for the outcomes analyses in the matched pairs. At the 2-month follow-up, abdominal and pelvic computed tomography and lower gastrointestinal endoscopy were more common in CDI cases compared with non-CDI controls (27.0% vs 8.8% and 7.8% vs 1.7%, respectively; P < .001). Sepsis of infectious origin (21.1% vs 6.8%), irritable bowel syndrome (3.3% vs 1.4%), and toxic megacolon (1.0% vs 0.1%) were also more common in CDI cases compared with the non-CDI controls (P < .001).
Table 3.
Follow-up Complications Occurring After CDI in the 2-Month and 1-Year Follow-up Periods for Medicare Advantage Enrollees in 2018
| 2-Month Follow-up Period | 1-Year Follow-up Period | |||||
|---|---|---|---|---|---|---|
| Follow-up Condition | CDI+ (n = 14 928) |
CDI– (n = 14 928) |
P a | CDI+ (n = 14 928) |
CDI– (n = 14 928) |
P a |
| Computed tomography of the abdomen or pelvis | 4030 (27.0) | 1309 (8.8) | <.001 | 6524 (43.7) | 4106 (27.5) | <.001 |
| Sepsis of infectious origin | 3149 (21.1) | 1012 (6.8) | <.001 | 4112 (27.6) | 2099 (14.1) | <.001 |
| Lower gastrointestinal endoscopy | 1168 (7.8) | 252 (1.7) | <.001 | 2611 (17.5) | 1170 (7.8) | <.001 |
| Irritable bowel syndromeb | 493 (3.3) | 205 (1.4) | <.001 | 988 (6.6) | 604 (4.0) | <.001 |
| Blood transfusion | 408 (2.7) | 204 (1.4) | <.001 | 855 (5.7) | 606 (4.1) | <.001 |
| Toxic megacolon | 156 (1.0) | 15 (.1) | <.001 | 247 (1.7) | 49 (.3) | <.001 |
| Surgical CDI complications | 62 (.4) | 19 (.1) | <.001 | 157 (1.1) | 90 (.6) | <.001 |
| Colon perforation | 91 (.6) | 26 (.2) | <.001 | 145 (1.0) | 72 (.5) | <.001 |
| Parenteral nutrition | 36 (.2) | 18 (.1) | .0143 | 90 (.6) | 56 (.4) | .005 |
Data are presented as n (%) unless otherwise indicated. Abbreviations: CDI, Clostridioides difficile infection; CDI+, CDI positive; CDI–, CDI negative.
P values derived using McNemar test.
Analysis was not restricted to patients with newly diagnosed irritable bowel syndrome.
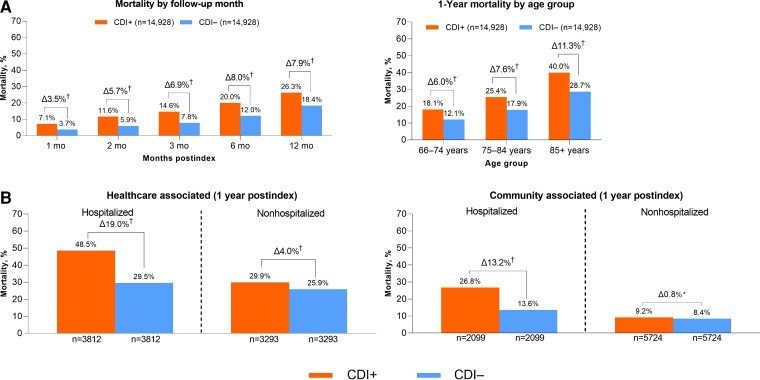
One-year mortality was 26.3% in CDI cases compared with 18.4% in non-CDI matched controls, with an attributable mortality of 7.9% (P < 0.001). The attributable mortality was highest in persons 85 years of age and older (11.3%) compared with those 75 to 84 years of age (7.5%) and 65 to 74 years of age (6.0%) (Figure 2A). The 1-year attributable mortality due to healthcare-associated CDI was higher among hospitalized patients (19.0%) compared with those not hospitalized (4.0%) and also higher in patients with community-associated CDI who were hospitalized with CDI (13.2%) compared with those who were not hospitalized (0.8%) (Figure 2B).
Figure 2.
Attributable all-cause mortality (2018 index date): (A) overall and (B) by CDI acquisition and hospitalization status. *P < .05, †P < .001. Abbreviations: CDI, Clostridioides difficile infection; CDI+, CDI positive; CDI–, CDI negative.
Supplementary Tables 2 and 3 summarize healthcare utilization findings. At the 2-month follow-up, the overall excess mean number of inpatient days was 3.5 (Supplementary Table 2) (P < .001). Hospitalized patients with healthcare-associated CDI had an excess 10.1 inpatient days, whereas patients with community-associated CDI had an excess 8.5 inpatient days. In the analysis limited to patients who survived 1 year, the overall excess mean number of inpatient days at the 1-year follow-up was also 3.5 (Supplementary Table 3).
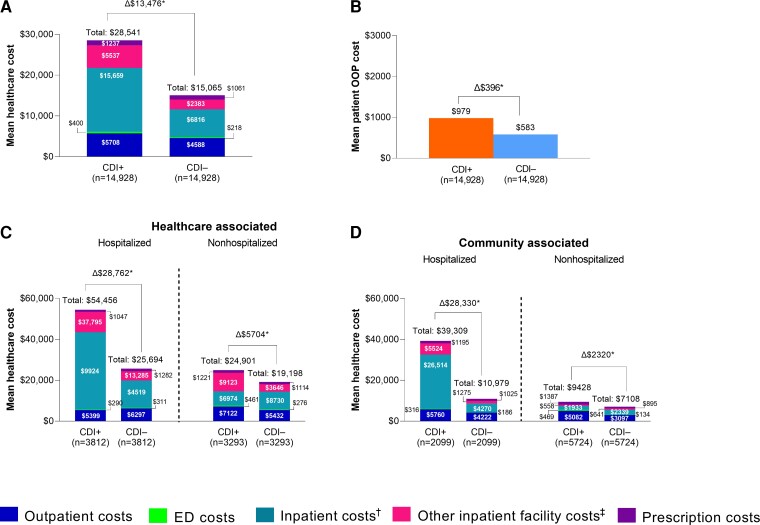
At the 2-month follow-up, mean total healthcare costs were $28 541 in the CDI cases compared with $15 065 in the non-CDI controls, a difference of $13 476 (P < .001) (Figure 3A). Total excess mean healthcare costs were greater among hospitalized patients with CDI (healthcare associated, $28 762; community associated, $28 330) than nonhospitalized patients with CDI (healthcare associated, $5704; community associated, $2320).
Figure 3.
Specific costs at the 2-month follow-up (2018 index year): (A) healthcare cost by setting, (B) total patient OOP costs, and healthcare cost by hospitalization status for (C) healthcare-associated and (D) community-associated cases. Data for the 2 months after the index date and at the 1-year follow-up are shown in Supplementary Tables 4 and 5. Data for the 1-year follow-up for those who survived 1 year are shown in Supplementary Table 6. *P < .001. †Hospital.‡Long-term care, inpatient hospice, inpatient mental health/chemical dependence, or inpatient rehabilitation facilities. Abbreviations: CDI, Clostridioides difficile infection; CDI+, CDI positive; CDI–, CDI negative; ED emergency department; OOP, out-of-pocket.
The contributions of various healthcare settings to mean costs is provided in Figure 3 and Supplementary Tables 4−6. At the 2-month follow-up, the greatest contributor to total costs was inpatient (ie, hospital) costs for both patients with CDI ($15 659) and non-CDI controls ($6816), with an excess mean inpatient cost of $8843. For hospitalized patients with CDI, the greatest cost contributor at 2 months was inpatient costs, with an excess mean cost of $24 510 for healthcare-associated and $22 244 for community-associated CDI (Figure 3C and 3D; Supplementary Table 5). For nonhospitalized patients with CDI, the greatest contributor to cost was other inpatient facility (ie, long-term care, inpatient hospice, inpatient mental health/chemical dependence, or inpatient rehabilitation facilities) costs for healthcare-associated CDI ($5477 mean excess cost) and outpatient costs (excluding emergency department) for community-associated CDI ($1984 mean excess cost). Outpatient costs (excluding emergency department) were the greatest contributor to total cost when costs were limited to patients who survived for 1 year (Supplementary Table 6).
Clostridioides difficile infection−attributable excess mean out-of-pocket cost at the 2-month follow-up was $396 in CDI cases (P < .001 vs non-CDI controls) (Figure 3B). Total excess mean out-of-pocket cost was highest among hospitalized patients with community-associated CDI ($970), followed by hospitalized patients with healthcare-associated CDI ($514), nonhospitalized patients with healthcare-associated CDI ($239), and nonhospitalized patients with community-associated CDI ($198) (Supplementary Table 4).
DISCUSSION
This retrospective cohort study of the Medicare Advantage population of older US adults assessed CDI incidence and outcomes, including mortality, healthcare utilization, and costs. We found that the overall CDI incidence rate in the MAE population of US adults 65 years of age and older gradually declined from 2012 to 2019. These findings are consistent with recent data from the CDC Emerging Infections Program showing that the burden of CDI in the general population decreased between 2011 and 2017, largely attributed to a decrease in healthcare-associated infections [9]. We also found that CDI incidence was highest among those 80 years of age and older, consistent with known CDI risk in older individuals [3].
Our study also showed that CDI represents an important public health burden in the MAE population, with 7.9% attributable mortality at 1 year after CDI diagnosis compared with a matched uninfected population. Attributable mortality was also considerably higher among hospitalized patients with CDI with healthcare-associated and community-associated infection compared with nonhospitalized patients. These findings are consistent with a study concerning an elderly (≥65 years) Medicare fee-for-service population during 2008−2010, in which CDI was associated with a significant 19% attributable mortality in a PS-adjusted analysis [14]. Notably, that publication did not report outcomes stratified by acquisition and hospitalization status. However, the reported overall 1-year mortality was higher than in our study (43% vs 26%), which may at least partly be explained by the smaller percentage of adults 85 years of age and older in our study (36% vs 24%).
We also observed that CDI was associated with a large healthcare utilization burden to the MAE population. At the 2-month follow-up, the greatest contributors to healthcare utilization were inpatient visits (overall and for hospitalized CDI cases) and inpatient facility and outpatient visits (for nonhospitalized CDI cases). In a 2010−2014 study of primary and recurrent CDI in the United States that used the IBM MarketScan database, which includes commercial and supplemental Medicare claims, a substantial increase in hospitalized days for patients with CDI was reported compared with matched patients without CDI, with more vulnerable patients incurring greater healthcare utilization [11].
In 2018, we observed significantly increased total healthcare and patient out-of-pocket costs associated with CDI among the MAE population. Healthcare costs were higher for patients with CDI compared with matched controls, with an excess mean total cost of $13 476 at the 2-month follow-up. Total excess mean healthcare costs were greater among hospitalized than nonhospitalized patients with CDI, regardless of acquisition status. Additionally, excess mean out-of-pocket cost was $396 for patients with CDI compared with matched controls and was highest in hospitalized patients with community-associated CDI.
Similar to our work, Nanwa and colleagues [17, 18] conducted 2 studies in a Canadian population using PS-matched cohorts of hospital-acquired and community-onset CDI from 2003 to 2010. Unlike our study, cost outcomes were assessed out to 3 years after CDI. Consistent with our findings, Nanwa and colleagues [17] found hospital-acquired CDI to be associated with significantly increased healthcare costs. In addition, community-onset CDI was associated with increased risk of all-cause mortality among those with CDI compared with uninfected persons, and mean costs were 1.9- to 5.1-fold higher in patients with CDI up to 1 year after diagnosis [18]. Studies of inpatient and emergency department visits indicate declines in the length of hospital stays associated with CDI since the early 2000s [19, 20]. Using claims data from 2010 to 2014, Zhang and colleagues [11] found mean CDI-attributable, nonannualized 6-month costs of $21 160 for primary CDI among individuals aged 65 years and older.
Study strengths include the derivation of the analyses from a large, representative, and contemporary US population of elderly individuals. To the best of our knowledge, this is the first study to characterize incidence and costs attributable to CDI in MAEs, including by acquisition and hospitalization status, and the first to quantify out-of-pocket costs associated with CDI using a PS-matched control population.
Our study has several limitations. As with any observational study, there is a possibility of bias due to unmeasured confounders. Additionally, the database may have included incomplete and/or inaccurate records. Other limitations include CDI identification primarily by ICD-9-CM and ICD-10-CM codes without laboratory verification, as well as lack of generalizability to fee-for-service Medicare enrollees because only 1 source of Medicare Advantage plans was used. Finally, incidence rates within calendar years were evaluated independently, so a small number of first claims may have occurred 60 days or less following an episode from the previous calendar year. This could have artificially lowered CDI cost estimates if some of the costs attributable to infection were accrued in the previous year.
This study suggests that CDI poses a considerable burden among the MAE population of US adults 65 years of age and older. Compared with matched controls, patients diagnosed with CDI had considerable attributable mortality rates and associated healthcare utilization and patient out-of-pocket costs. These results support the use of CDI-prevention strategies and treatments to improve outcomes among the growing population of older US adults.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Editorial/medical writing support was provided by Tricia Newell, PhD, at ICON (Blue Bell, PA), and was funded by Pfizer Inc. The authors also thank Pfizer colleagues Kirstin Heinrich, Birol Emir, and Deepa Malhotra for their support on the study.
Data sharing statement. Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Financial support. This work was supported by Pfizer Inc.
Supplementary Material
Contributor Information
Holly Yu, Pfizer Inc, Collegeville, Pennsylvania, USA.
Tamuno Alfred, Pfizer Inc, New York, New York, USA.
Jennifer L Nguyen, Pfizer Inc, New York, New York, USA.
Jingying Zhou, Pfizer Inc, Peapack, New Jersey, USA.
Margaret A Olsen, Washington University School of Medicine, St Louis, Missouri, USA.
References
- 1. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015; 372:1539–48. [DOI] [PubMed] [Google Scholar]
- 2. Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med 1994; 330:257–62. [DOI] [PubMed] [Google Scholar]
- 3. Keller JM, Surawicz CM. Clostridium difficile infection in the elderly. Clin Geriatr Med 2014; 30:79–93. [DOI] [PubMed] [Google Scholar]
- 4. Simor AE, Bradley SF, Strausbaugh LJ, Crossley K, Nicolle LE; SHEA Long-Term-Care Committee. Clostridium difficile in long-term-care facilities for the elderly. Infect Control Hosp Epidemiol 2002; 23:696–703. [DOI] [PubMed] [Google Scholar]
- 5. Kim JH, Toy D, Muder RR. Clostridium difficile infection in a long-term care facility: hospital-associated illness compared with long-term care-associated illness. Infect Control Hosp Epidemiol 2011; 32:656–60. [DOI] [PubMed] [Google Scholar]
- 6. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:987–94. [DOI] [PubMed] [Google Scholar]
- 7. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 2002; 97:1769–75. [DOI] [PubMed] [Google Scholar]
- 9. Guh AY, Mu Y, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med 2020; 382:1320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Centers for Disease Control and Prevention . 2018 Annual report for the Emerging Infections Program for Clostridioides difficile infection. Available at: https://www.cdc.gov/hai/eip/Annual-CDI-Report-2018.html. Accessed 10 February 2022.
- 11. Zhang D, Prabhu VS, Marcella SW. Attributable healthcare resource utilization and costs for patients with primary and recurrent Clostridium difficile infection in the United States. Clin Infect Dis 2018; 66:1326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 2012; 55(Suppl 2):S88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vindigni SM, Surawicz CM. C. difficile infection: changing epidemiology and management paradigms. Clin Transl Gastroenterol 2015; 6:e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shorr AF, Zilberberg MD, Wang L, Baser O, Yu H. Mortality and costs in Clostridium difficile infection among the elderly in the United States. Infect Control Hosp Epidemiol 2016; 37:1331–6. [DOI] [PubMed] [Google Scholar]
- 15. Kaiser Family Foundation . A dozen facts about Medicare Advantage in 2020. Available at: https://www.kff.org/medicare/issue-brief/a-dozen-facts-about-medicare-advantage-in-2020/. Accessed 12 July 2021.
- 16. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 17. Nanwa N, Kwong JC, Krahn M, et al. The economic burden of hospital-acquired Clostridium difficile infection: a population-based matched cohort study. Infect Control Hosp Epidemiol 2016; 37:1068–78. [DOI] [PubMed] [Google Scholar]
- 18. Nanwa N, Sander B, Krahn M, et al. A population-based matched cohort study examining the mortality and costs of patients with community-onset Clostridium difficile infection identified using emergency department visits and hospital admissions. PLoS One 2017; 12:e0172410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shrestha MP, Bime C, Taleban S. Decreasing Clostridium difficile–associated fatality rates among hospitalized patients in the United States: 2004–2014. Am J Med 2018; 131:90–6. [DOI] [PubMed] [Google Scholar]
- 20. Garg SK, Obaitan I, Sarvepalli S, Anugwom CM, Pardi DS, Khanna S. Clostridium difficile infection in the emergency department. J Clin Gastroenterol 2020; 54:350–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.