Abstract
Background
Effective antimicrobial treatment is key for survival in bloodstream infection (BSI), but the impact of timing of treatment remains unclear. Our aim was to assess the association between time to appropriate antimicrobial treatment and 30-day mortality in BSI patients.
Methods
This was a retrospective cohort study using electronic health record data from a large academic center in Sweden. Adult patients admitted between the years 2012 and 2019, with onset of BSI at the emergency department or general wards, were included. Pathogen-antimicrobial drug combinations were classified as appropriate or inappropriate based on reported in vitro susceptibilities. To avoid immortal time bias, the association between appropriate therapy and mortality was assessed with multivariable logistic regression analysis at pre-specified landmark times.
Results
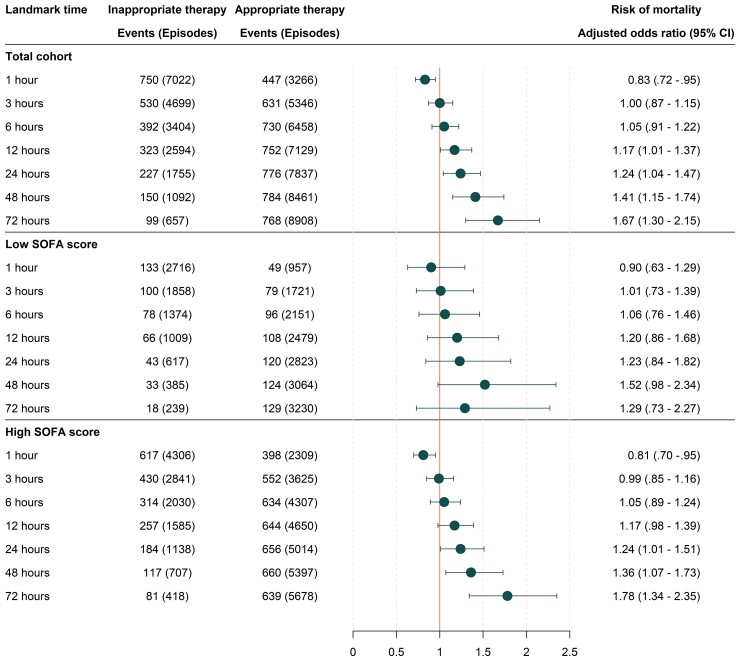
We included 10 628 BSI-episodes, occurring in 9192 unique patients. The overall 30-day mortality was 11.8%. No association in favor of a protective effect between appropriate therapy and mortality was found at the 1, 3 and 6 hours landmark after blood culture collection. At 12 hours, the risk of death increased with inappropriate treatment (adjusted odds ratio 1.17 [95% confidence interval {CI}, 1.01–1.37]) and continued to increase gradually at 24, 48, and 72 hours. Stratifying by high or low SOFA score generated similar odds ratios, with wider confidence intervals.
Conclusions
Delays in appropriate antimicrobial treatment were associated with increased 30-day mortality after 12 hours from blood culture collection, but not at 1, 3, and 6 hours, in BSI. These results indicate a benchmark for providing rapid microbiological diagnostics of blood cultures.
Keywords: bloodstream infection, antimicrobial treatment, mortality
In this cohort study of 10 628 bloodstream infection episodes, delays in appropriate antimicrobial treatment were associated with increased 30-day mortality after 12 hours from blood culture collection but not at 1, 3, and 6 hours.
Bloodstream infections (BSIs) are severe infections associated with substantial mortality [1]. Influenced by sepsis guidelines, antimicrobial therapy is usually initiated promptly before susceptibility results are available, but there is a trade-off between broadened pathogen coverage and antimicrobial overuse [2, 3]. Unnecessary broad-spectrum or combination regimens are associated with adverse effects and select for antimicrobial resistance [4, 5].
It is estimated that empirical treatment without in vitro coverage of the cultured pathogen occurs in approximately one third of infections and observational studies of BSI have shown an association between non-covering treatment and mortality [6–8]. Most studies, however, are small with substantial heterogeneity in terms of definitions, precluding direct comparisons of study findings [8]. Recently, Kadri et al published the largest retrospective cohort study to date showing an increased mortality risk with discordant antimicrobial treatment at 24 hours, but they could not assess hourly estimates due to insufficient granularity of the data [7]. Timing of empirical treatment is a crucial determinant to inform clinical management and treatment recommendations. The urgency of antimicrobials is debated, but observational studies of sepsis have prompted current guidelines to recommend antimicrobial treatment within 1–3 hours [2, 9–11]. It is unclear whether these results can be directly translated to microbiologically confirmed infections such as BSI.
Another concern with the current body of evidence for time-to-treatment with antimicrobials is insufficient account of potential biases [12, 13]. Although many studies adjust for severity of disease (indication bias), few recognize problems that arise with patients having to survive long enough to be switched to appropriate treatment (immortal time bias). Inadequate controlling for this effect may lead to an underestimation of the effect of earlier time-to-treatment.
Our aim was to assess the association between time to appropriate antimicrobial treatment based on in vitro drug-pathogen coverage and 30-day mortality in BSI, accounting for important biases.
METHODS
Study Design, Setting and Participants
A retrospective cohort study was conducted using electronic health record data from the Karolinska University Hospital in Stockholm, Sweden. This is an academic centre with 1200 beds spread over two sites. Data on demographics, hospital administrative data, comorbidities, drug administration, vital parameters, and laboratory results was collected. Mortality data was derived from the national personal data register. The study followed the STROBE guidelines for reporting observational studies.
Adults (≥18 years) admitted between 1 January 2012 and 31 December 2019 with a BSI were included, with the possibility of multiple study entries. Only the first BSI episode per admission was assessed. Participants were followed for 30 days from the first blood culture and during follow-up we did not include additional BSI episodes for that patient. To capture a patient population where clinicians had a suspicion of infection and an intention-to-treat promptly, we included episodes that received antimicrobial treatment within 24 hours from blood culture collection. Since we aimed to assess the association between time to appropriate therapy and mortality, BSI episodes with appropriate treatment in the 24 hours prior to onset were excluded, except for appropriate therapy started during the hour before onset. Patients with BSI onset at the intensive care unit (ICU) or admission to the obstetric unit were excluded since structured data on drug administration and vital parameters were not available for these wards.
Bloodstream Infections
The first blood culture was regarded as the onset of the BSI and data on all significant pathogens identified within 24 hours from this blood culture was collected. Depending on whether the onset was within or beyond 48 hours after hospital admission, the BSI was classified as community-onset or hospital-onset respectively. We excluded pre-defined contaminants, based on the Centers for Disease Control and Prevention (CDC)/National Healthcare Safety Network Patient Safety Component Manual (Supplementary Appendix 2), if these were isolated in only 1 culture bottle or only 1 set (1 anaerobe and 1 aerobe blood culture bottle) if more than 1 set of blood cultures were collected within 24 hours [14]. Findings of Methicillin-resistant Staphylococcus aureus, Enterobacterales with extended-spectrum beta-lactamases production or vancomycin-resistant enterococcus were considered antimicrobial-resistant phenotypes. The local microbiology laboratory routines and use of rapid diagnostics are described in Supplementary Appendix 1. Most culture results were available to clinicians within 24–72 hours after culture collection.
Appropriate Treatment
Appropriate treatment was defined as receiving at least 1 antimicrobial for which the identified pathogen was found to be susceptible in vitro. Non-covering or no treatment was classified as inappropriate treatment. In polymicrobial infection, all identified pathogens needed to be covered by at least 1 antimicrobial drug to be classified as appropriate therapy. Antimicrobial susceptibility was inferred from disk diffusion methods, and the drugs considered for matching with susceptibility reports can be found in the Supplementary Appendix 3. Surrogate antibiograms with imputed susceptibilities were created for drugs not directly registered in the susceptibility report based on reported susceptibilities, known intrinsic resistance, expert rules, and breakpoint tables from the European Committee of Antimicrobial Susceptibility Testing (Supplementary Appendix 4), similar to previously described methods [4, 7]. BSI episodes where the susceptibility for received treatment remained undetermined after using the surrogate antibiogram were excluded for the main analysis. To avoid misclassification of treatment for patients that were admitted to the ICU without appropriate therapy within 72 hours of BSI onset (n = 181), these episodes were manually reviewed. In 122 (67%) of these episodes, a new antimicrobial agent was registered after ICU admission.
Outcome and Covariates
Mortality was defined as death due to any cause within 30 days from the onset of BSI. Comorbidities were assessed with the Charlson comorbidity index (CCI, categorized as 0, 1–2, 3–4, ≥5) based on ICD-10 codes available from 5 years before admission until 24 hours after admission [15]. Immunosuppression was assessed based on ICD-10 codes registered in the year prior to admission until 24 hours after admission [16, 17] (Supplementary Appendix 5). We classified source of infection according to the corresponding ICD-10 codes registered during the admission [4, 18]. The following categories were used: urinary, respiratory, abdominal, endocarditis, other, multifocal, or unknown. Severity of illness was assessed as worst Sequential Organ Failure Assessment (SOFA) score categorized as 0, 1, 2, 3, 4, or ≥5 points, from 24 hours before until 6 hours after onset of BSI [19]. In case of missing data, normal values were assumed. Some adaptations to the original SOFA score were made in order to deal with common missing data outside of the ICU-setting (Supplementary Appendix 6) [14, 20]. Septic shock was defined as either administration of vasopressors, or ICU admission with septic shock as the main reason for admission, within 24 hours of onset of BSI.
Statistical Analysis
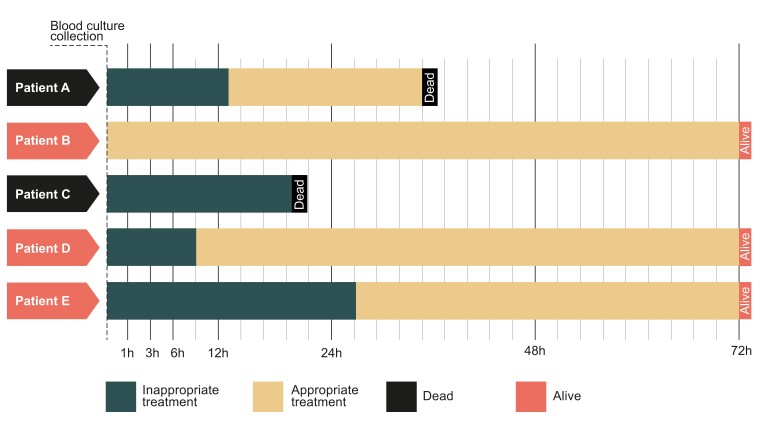
To avoid immortal time bias for inappropriate treatment, we used the landmark method with the following predefined landmark times: 1, 3, 6, 12, 24, 48, and 72 hours (Figure 1). At each time point, logistic regression analysis was used to assess the association between inappropriate therapy and 30-day mortality, presented as adjusted odds ratios (aOR). Deceased patients, as well as those with undetermined pathogen-antimicrobial drug combinations were excluded at the respective time points. We used clinical reasoning to identify confounding factors a priori. Analyses were adjusted for age, sex, CCI, immunosuppression, SOFA score, polymicrobial BSI, source of infection, calendar year and hospital-onset of BSI. Because severity of disease is known to be an important confounder in time-to-treatment studies of infections [12], the multivariable model was repeated with stratification based on the Sepsis-3 definition of SOFA score ≥2. The subgroups of patients with septic shock, the groups of most common pathogens and episodes with antimicrobial-resistant pathogens, were analysed separately. Confidence intervals (CI) are presented as the 2.5th and 97.5th percentiles. Two-sided P values < .05 were considered statistically significant. All analyses were done in R (3.6.2).
Figure 1.
Schematic figure illustrates the time varying exposure of appropriate treatment. Patients being switched to appropriate treatment at a later time had guaranteed survival to this time point. To avoid immortal time bias, only patients surviving to the landmark time of interest were included in the analysis. At each landmark time, exposure was classified according to the present treatment, irrespectively of if they were later switched to appropriate treatment.
Sensitivity Analyses
To assess the impact of methodological decisions, we performed predefined sensitivity analyses. (I) We excluded patients that did not receive appropriate treatment within 6 days, because there might be no indication to treat these patients. (II) To consider the group with less obvious clinical indication of early empirical treatment, we included those having no antimicrobial treatment in the first 24 hours. (III) Because patients with recurring BSI may be more likely to have appropriate treatment guided by prior culture results, we restricted analyses to only one BSI episode per patient. (IV) We restricted analyses to mono-microbial BSI and (V) assessed community-onset BSI with admission time as onset of infection, to reduce heterogeneity and account for potential variability in time to blood culture collection. (VI) The impact of immortal time bias was evaluated by including all deceased patients at each landmark time. (VII) We evaluated our definitions of pathogen-antimicrobial drug combinations by using only the original antibiogram, excluding all possible contaminants, and classifying all undetermined pathogen-antimicrobial drug combinations as either appropriate or inappropriate treatment.
RESULTS
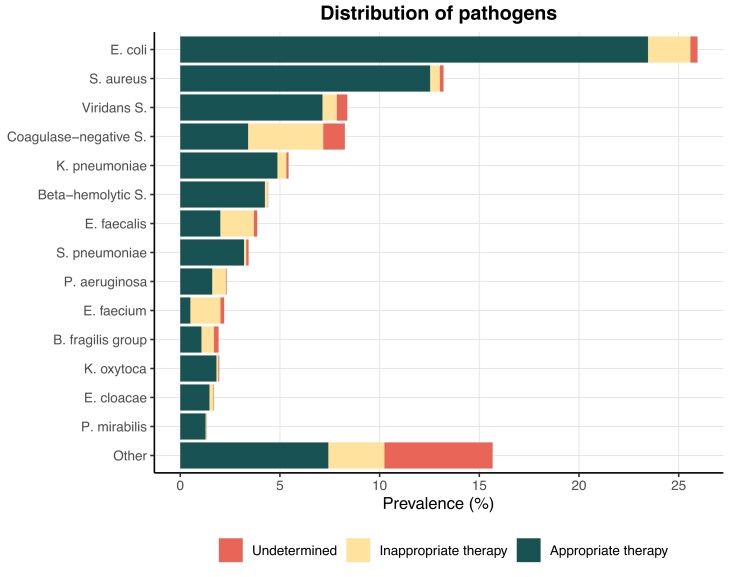
Out of 581 334 adult admissions, we were able to identify 13 511 BSI episodes. After applying exclusion criteria, 10 628 BSI episodes remained for analysis, occurring in 9192 unique patients (Figure 2). The BSI episodes corresponded to 12 223 unique pathogens of which Escherichia coli, Staphylococcus aureus, and viridans streptococci were the most prevalent pathogens (Figure 3). The most common empirical antimicrobials were cefotaxime (46.5%), piperacillin-tazobactam (35.7%), and meropenem (12.1%).
Figure 2.
Study cohort flowchart. The final cohort consisted of 10 628 BSI episodes occurring in 9192 unique patients. Abbreviations: BSI, bloodstream infection; SOFA, sequential organ failure assessment score.
Figure 3.
Overview of pathogens retrieved in blood cultures (n = 12 223) and the proportion of appropriate and inappropriate antimicrobial treatment at 24 h. The proportion of pathogen-antimicrobial drug combinations which could not be determined as either appropriate or inappropriate based on the surrogate antibiogram are shown as undetermined. Only pathogens with a prevalence >1% are shown and the remaining pathogens are grouped as “other.” Abbreviations: B. fragilis group, Bacteroides fragilis group; beta-hemolytic S., beta-hemolytic Streptococcus; coagulase-negative S., coagulase-negative Staphylococcus; E. cloacae, Enterobacter cloacae; E. faecalis, Enterococcus faecalis; E. faecium, Enterococcus faecium; E. coli, Escherichia coli; K. oxytoca, Klebsiella oxytoca; K. pneumoniae; Klebsiella pneumoniae; P. mirabilis, Proteus mirabilis; P. aeruginosa, Pseudomonas aeruginosa; S. aureus, Staphylococcus aureus; S. pneumoniae, Streptococcus pneumoniae; viridans S., viridans streptococci.
The study population had a median age of 69 years, and 56.8% were female (Table 1). Most episodes were community-onset (85.3%). Relatively few episodes featured an antimicrobial-resistant pathogen (4.0%), were polymicrobial (11.5%) or included a possible contaminant species either as the culprit pathogen or in polymicrobial infection (9.9%). Appropriate therapy was administered within the first hour after blood culture collection in 3266 of 10 628 (30.7%) episodes and within 3 hours in 5353 of 10 628 (50.4%) episodes (Table 1). At 3 days after blood culture collection, 657 of 10 628 (6.2%) episodes had inappropriate therapy and 745 of 10 628 (7.0%) episodes had undetermined coverage. This group decreased to 374 of 10 628 (3.5%) with inappropriate therapy and 679 of 10 628 (6.4%) with undetermined coverage at 6 days after BSI onset. Patients receiving appropriate therapy within the first hour had high SOFA scores and a high proportion of combination therapy. In contrast, patients who received appropriate therapy after 24 hours and onward had increased proportions of antimicrobial-resistant pathogens, polymicrobial infections and potential contaminants. Septic shock was present in 608 of 10 628 (5.7%) episodes, and only 77 of 608 (12.6%) had inappropriate treatment at 12 hours.
Table 1.
Clinical Characteristics
| Variable | Time of Appropriate Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| < 1 h | 1 h–3 h | 3 h–6 h | 6 h–12 h | 12 h–24 h | 24 h–48 h | 48–72 h | >72 h | Overalla | |
| Episodes, no. (% of total) | 3266 (30.7) | 2087 (19.6) | 1127 (10.6) | 703 (6.6) | 756 (7.1) | 683 (6.4) | 517 (4.9) | 1402 (13.2) | 10 628 (100) |
| Age, median [min, max] | 68.3 [18.0, 105] | 69.3 [18.3, 103] | 69.9 [18.1, 97.7] | 67.5 [19.0, 99.5] | 68.9 [18.1, 101] | 67.7 [18.4, 98.7] | 68.4 [18.7, 99.0] | 68.2 [18.3, 101] | 68.6 [18.0, 105] |
| Female, no. (%) | 1903 (58.3%) | 1171 (56.1%) | 597 (53.0%) | 382 (54.3%) | 422 (55.8%) | 411 (60.2%) | 294 (56.9%) | 808 (57.6%) | 6034 (56.8%) |
| Immunocompromised, no. (%) | 406 (12.4%) | 301 (14.4%) | 166 (14.7%) | 90 (12.8%) | 99 (13.1%) | 133 (19.5%) | 79 (15.3%) | 200 (14.3%) | 1484 (14.0%) |
| Charlson comorbidity index, no. (%) | |||||||||
| ȃ0 | 747 (22.9%) | 553 (26.5%) | 287 (25.5%) | 218 (31.0%) | 227 (30.0%) | 133 (19.5%) | 87 (16.8%) | 328 (23.4%) | 2584 (24.3%) |
| ȃ1–2 | 1213 (37.1%) | 749 (35.9%) | 372 (33.0%) | 234 (33.3%) | 246 (32.5%) | 258 (37.8%) | 207 (40.0%) | 481 (34.3%) | 3778 (35.5%) |
| ȃ3–4 | 506 (15.5%) | 341 (16.3%) | 213 (18.9%) | 124 (17.6%) | 130 (17.2%) | 114 (16.7%) | 73 (14.1%) | 228 (16.3%) | 1759 (16.6%) |
| ȃ≥5 | 800 (24.5%) | 444 (21.3%) | 255 (22.6%) | 127 (18.1%) | 153 (20.2%) | 178 (26.1%) | 150 (29.0%) | 365 (26.0%) | 2507 (23.6%) |
| SOFA score, no. (%) | |||||||||
| ȃ0 | 419 (12.8%) | 346 (16.6%) | 190 (16.9%) | 166 (23.6%) | 185 (24.5%) | 117 (17.1%) | 86 (16.6%) | 247 (17.6%) | 1762 (16.6%) |
| ȃ1 | 538 (16.5%) | 418 (20.0%) | 241 (21.4%) | 163 (23.2%) | 163 (21.6%) | 131 (19.2%) | 89 (17.2%) | 289 (20.6%) | 2035 (19.1%) |
| ȃ2 | 589 (18.0%) | 405 (19.4%) | 241 (21.4%) | 120 (17.1%) | 167 (22.1%) | 109 (16.0%) | 100 (19.3%) | 283 (20.2%) | 2022 (19.0%) |
| ȃ3 | 507 (15.5%) | 298 (14.3%) | 164 (14.6%) | 86 (12.2%) | 82 (10.8%) | 107 (15.7%) | 100 (19.3%) | 227 (16.2%) | 1585 (14.9%) |
| ȃ4 | 490 (15.0%) | 260 (12.5%) | 132 (11.7%) | 77 (11.0%) | 83 (11.0%) | 104 (15.2%) | 78 (15.1%) | 171 (12.2%) | 1407 (13.2%) |
| ȃ≥5 | 723 (22.1%) | 360 (17.2%) | 159 (14.1%) | 91 (12.9%) | 76 (10.1%) | 115 (16.8%) | 64 (12.4%) | 185 (13.2%) | 1817 (17.1%) |
| Source of infection, no. (%) | |||||||||
| ȃUrinary | 836 (25.6%) | 544 (26.1%) | 287 (25.5%) | 164 (23.3%) | 135 (17.9%) | 160 (23.4%) | 102 (19.7%) | 215 (15.3%) | 2447 (23.0%) |
| ȃAbdominal | 231 (7.1%) | 172 (8.2%) | 100 (8.9%) | 71 (10.1%) | 78 (10.3%) | 57 (8.3%) | 36 (7.0%) | 160 (11.4%) | 914 (8.6%) |
| ȃPulmonary | 386 (11.8%) | 233 (11.2%) | 117 (10.4%) | 66 (9.4%) | 63 (8.3%) | 57 (8.3%) | 52 (10.1%) | 202 (14.4%) | 1192 (11.2%) |
| ȃEndocarditis | 64 (2.0%) | 63 (3.0%) | 33 (2.9%) | 25 (3.6%) | 30 (4.0%) | 12 (1.8%) | 13 (2.5%) | 31 (2.2%) | 271 (2.5%) |
| ȃOther | 157 (4.8%) | 86 (4.1%) | 51 (4.5%) | 44 (6.3%) | 35 (4.6%) | 22 (3.2%) | 21 (4.1%) | 52 (3.7%) | 472 (4.4%) |
| ȃMultifocus | 175 (5.4%) | 136 (6.5%) | 79 (7.0%) | 38 (5.4%) | 48 (6.3%) | 39 (5.7%) | 32 (6.2%) | 69 (4.9%) | 620 (5.8%) |
| ȃUnknown | 1417 (43.4%) | 853 (40.9%) | 460 (40.8%) | 295 (42.0%) | 367 (48.5%) | 336 (49.2%) | 261 (50.5%) | 673 (48.0%) | 4712 (44.3%) |
| Septic shock, no. (%) | 317 (9.7%) | 101 (4.8%) | 38 (3.4%) | 28 (4.0%) | 17 (2.2%) | 27 (4.0%) | 17 (3.3%) | 50 (3.6%) | 608 (5.7%) |
| Hospital-onset BSI, no. (%) | 449 (13.7%) | 208 (10.0%) | 127 (11.3%) | 124 (17.6%) | 155 (20.5%) | 149 (21.8%) | 112 (21.7%) | 228 (16.3%) | 1565 (14.7%) |
| Resistant pathogen, no. (%) | 88 (2.7%) | 57 (2.7%) | 32 (2.8%) | 27 (3.8%) | 25 (3.3%) | 90 (13.2%) | 53 (10.3%) | 39 (2.8%) | 420 (4.0%) |
| Polymicrobial BSI, no. (%) | 259 (7.9%) | 135 (6.5%) | 80 (7.1%) | 56 (8.0%) | 66 (8.7%) | 103 (15.1%) | 97 (18.8%) | 397 (28.3%) | 1226 (11.5%) |
| Potential contaminants, no. (%) | 157 (4.8%) | 84 (4.0%) | 66 (5.9%) | 42 (6.0%) | 44 (5.8%) | 105 (15.4%) | 167 (32.3%) | 378 (27.0%) | 1054 (9.9%) |
| Combination therapy, no. (%) | 1326 (40.6) | 676 (32.4) | 303 (26.9) | 253 (36.0) | 309 (40.9) | 223 (32.7) | 188 (36.4) | 426 (30.4) | 3728 (35.1) |
| ICU admission, no. (%) | 355 (10.9) | 143 (6.9) | 66 (5.9) | 45 (6.4) | 48 (6.3) | 53 (7.8) | 34 (6.6) | 68 (4.9) | 823 (7.7) |
| 30-day mortality | 447 (13.7) | 191 (9.2) | 114 (10.1) | 54 (7.7) | 72 (9.5) | 67 (9.8) | 54 (10.4) | 172 (12.3) | 1258 (11.8) |
Abbreviations: BSI, bloodstream infection; ICU, intensive care unit; SOFA, sequential organ failure assessment score.
Please note that the sum of all individual groups is not equal to the total study population. This is due to the fact that the different columns specify the time of appropriate treatment. Since 87 patients died prior to receiving appropriate treatment they will not be represented in any of the columns. The “>72 h” group represents all episodes without appropriate therapy in the first 3 days. The “Overall” group contains the complete study population, including the 87 patients who died prior to receiving appropriate therapy.
The crude 30-day mortality was 11.8% in the overall population, 13.7% in those who received appropriate therapy within the first hour, and 25.3% in patients with septic shock. The distribution of deaths was skewed towards the first days of the 30-day follow-up interval (Supplementary Figure 1). Receiving inappropriate treatment at 1 hour was associated with lower risk of death (aOR 0.83 [95% CI, .72–.95]) (Figure 4). Inappropriate treatment after 12 hours was associated with significantly increased mortality (aOR 1.17 [95% CI, 1.01–1.37)]) and continued to increase gradually at landmark 24, 48, and 72 hours. Stratifying by high or low SOFA score generated similar odds ratios, but the confidence intervals increased. No apparent trend was observed for patients with septic shock (Supplementary Figure 2).
Figure 4.
Association of time to appropriate treatment with mortality at specified landmark times. High SOFA score was defined as ≥2 points and low SOFA score was defined as <2 points. Only patients with determined pathogen-antimicrobial drug combinations were analysed at each landmark time of interest. Associations were estimated using logistic regression with adjustments for age, sex, Charlson comorbidity index, immunosuppression, SOFA score, polymicrobial bloodstream infection, source of infection, admission year and community vs hospital-onset. Abbreviations: CI, confidence interval; SOFA, sequential organ failure assessment.
Sensitivity analyses pointed to robustness of the main findings with no major changes of the point estimates induced by methodological decisions (Supplementary Figure 3). Using admission time as onset of infection in community-onset BSI resulted in a similar but delayed increase in mortality over the different landmark times (Supplementary Figure 4). In analyses excluding patients that did not receive appropriate treatment within 6 days (n = 9377), the association of time-to-treatment and mortality remained or strengthened (Supplementary Table 1 and Supplementary Figure 5). This finding indicates that patients where clinicians chose not to cover all pathogens beyond this time point had lower mortality and may not be representative of the general BSI population. Analyses were also repeated for the most common pathogens in monomicrobial BSI, but the statistical power was insufficient to draw any firm conclusions from these results (Supplementary Figure 6). In BSI caused by antimicrobial-resistant pathogens (n = 420), the aOR was higher at all time points, indicating increased mortality risk with inappropriate treatment, but the confidence intervals were wide (Supplementary Table 2 and Supplementary Figure 7).
DISCUSSION
In this large observational cohort study of 10 628 BSI episodes, inappropriate antimicrobial treatment at 12 hours after blood culture collection was associated with increased 30-day mortality in those who were alive by 12 hours. The point estimates were similar regardless of high or low SOFA score at onset. Due to the relatively small number of septic shock patients, of which most received appropriate treatment within the first hours, our study was underpowered to draw meaningful conclusions in this subgroup. These results call into question the promotion of aggressive broad spectrum empirical treatment within 3 hours when BSI is suspected, unless there is suspected septic shock or bacterial meningitis [11, 21]. A broader time window for initiating appropriate treatment enables the diagnostic work-up to better guide empirical therapy but also gives a benchmark for introducing novel biomarkers, rapid diagnostic methods or workflows in clinical microbiological laboratories.
Our results show a weaker association for time to antibiotics in BSI than studies in sepsis have suggested [10, 22]. The main argument for failure to show a convincing effect of prompt antimicrobial administration is the lack of biological plausibility, namely, uncertainty regarding onset time zero, which may range from hours to several days in patients arriving to the emergency department [23]. As healthcare is easily accessible for virtually the whole population in Sweden, it is possible that patients present themselves earlier compared to other settings, affecting the impact of antibiotic timing. Furthermore, our study included BSI patients, with insufficient power to assess the most critically ill, where previous studies have reported the greatest benefit of early adequate treatment [11, 21]. It may also be that other supportive care measures are of equal or greater benefit, such as intravenous fluids, respiratory support, or adequate infectious source control [24]. Several studies in sepsis, including 2 randomized controlled trials, have also failed to show benefit of prompt treatment, although they generally did not account for the appropriateness of antimicrobial therapy [25–33]. Yet even when time zero is easier to identify, studies have not demonstrated a convincing benefit of early antibiotics. Hranjec et al found that a more conservative antimicrobial policy at a surgical ICU did not lead to increased mortality and was even associated with better patient outcomes [34].
Studies have also reported an association between early appropriate treatment in BSI and mortality [35–39]. A meta-analysis from 2010 showed a significant reduction in all-cause mortality with the use of appropriate empirical antibiotic treatment [40]. However, there was substantial heterogeneity, high risk of bias and variable effects in the included studies. One of the main problems with non-randomized studies of appropriate treatment in infections are indication bias, particularly the fact that critically ill patients both have a higher likelihood of receiving broader antimicrobial therapy and a higher risk of death [41]. To account for this, we adjusted for several factors associated with disease severity, but even so we found higher mortality risk associated with receiving appropriate treatment within the first hour, indicating residual confounding. As expected, antimicrobial-resistant pathogens were overrepresented among patients with delayed appropriate treatment, but the overall prevalence of resistance was low compared to other European and US centers and the impact of inappropriate therapy may be different in high-resistance settings.
Another important methodological aspect is immortal time bias, but this has generally not been acknowledged in time-to-treatment studies of sepsis or BSI. Immortal time bias in pharmacoepidemiology is mainly recognized when the guaranteed survival for 1 of the exposures is long, but similar effects also apply to shorter periods of days in patients with critical illness [13]. Reviewing some of the largest or most cited research on time-to-antimicrobials in sepsis and BSI, we could not find any clear statements that this effect had been accounted for in the analyses [7, 10, 42]. This may have led to biased estimates, which needs to be considered when interpreting the results. To account for immortal time bias in this study, we used landmark analysis, which more clearly displays different time thresholds compared to alternative methods, such as cox regression with a time-varying predictor variable [43]. On the contrary, in landmark analysis the results can only be generalized to patients surviving to the specified landmark time. Additionally, because we did not model the effect of time explicitly, it is possible that non-proportionality of the association between appropriate therapy and mortality are overlooked. The immortal time bias was not large in this cohort, but even so it biased the results toward a small underestimation of the mortality risk of inappropriate treatment (Supplementary Figure 3).
Our study has limitations, mainly related to its observational nature. Even though internal validity was ensured by access to a large and complete hospital population, our results need to be confirmed in external data sets, also including BSI with onset in the ICU and BSI in high-resistance settings. Generalization of our results to a septic population should also be done with caution as we have not accounted for baseline SOFA scores in our stratified analysis. We acknowledge that our definition of appropriate therapy is narrow since it was only based on in vitro susceptibility. As with many similar studies, we could not account for dosing, the route of administration or treatment duration, which may have affected patient outcomes. However, this effect would most likely not be differential based on initial appropriate treatment or not and thus limit the bias. Because the study population was large, it was not feasible to assess if adequate source control measures were taken, for which thorough manual chart review would have been necessary. Similar to previous studies, episodes where the susceptibility for received treatment remained undetermined after using the surrogate antibiogram were excluded in the main analyses [4]. However, sensitivity analyses could not demonstrate any major alterations of the main results based on this missing data. As with all non-randomized treatment studies, we cannot exclude that residual confounding affected our findings despite our efforts to control for this. Finally, our findings need to be interpreted on a population-level and effects in individual patients may be different.
In conclusion, delays in appropriate antimicrobial treatment were associated with increased 30-day mortality after 12 hours from blood culture collection, but not at 1, 3, and 6 hours, in BSI. These results indicate a benchmark for providing rapid microbiological diagnostics of blood cultures.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Jasper Van Heuverswyn, Department of Medicine, Solna, Division of Infectious Diseases, Karolinska Institutet, Stockholm, Sweden.
John Karlsson Valik, Department of Medicine, Solna, Division of Infectious Diseases, Karolinska Institutet, Stockholm, Sweden; Department of Infectious Diseases, Karolinska University Hospital, Stockholm, Sweden.
Suzanne Desirée van der Werff, Department of Medicine, Solna, Division of Infectious Diseases, Karolinska Institutet, Stockholm, Sweden; Department of Infectious Diseases, Karolinska University Hospital, Stockholm, Sweden.
Pontus Hedberg, Department of Medicine, Solna, Division of Infectious Diseases, Karolinska Institutet, Stockholm, Sweden; Department of Infectious Diseases, Karolinska University Hospital, Stockholm, Sweden.
Christian Giske, Clinical microbiology, Karolinska University Hospital, Stockholm, Sweden; Division of Clinical Microbiology, Department of Laboratory Medicine, Karolinska Institutet, Stockholm, Sweden.
Pontus Nauclér, Department of Medicine, Solna, Division of Infectious Diseases, Karolinska Institutet, Stockholm, Sweden; Department of Infectious Diseases, Karolinska University Hospital, Stockholm, Sweden.
Notes
Acknowledgments . The authors thank Fredrik Granath at the Department of Medicine Solna at Karolinska Institutet for valuable statistics input. They also thank Katie Isitt at the Department of Medicine Solna at Karolinska Institutet for proofreading.
Financial support. The work was supported by grants from VINNOVA under the project name PLATINEA (grant number 2021-02699); Region Stockholm ALF-agreement (grant number FoUI-954788); Region Stockholm Health Medicine Technology (grant number FoUI-966658); and the Swedish Research Council (grant number 2021-02271). J. V. H. was supported by grants from Karolinska Institutet and ESCMID Young Scientist travel grant. J. K. V. was supported by Region Stockholm (combined clinical residency and PhD training program). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1. Laupland KB, Lyytikäinen O, Søgaard M, et al. . The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect 2013; 19:465–71. [DOI] [PubMed] [Google Scholar]
- 2. Evans L, Rhodes A, Alhazzani W, et al. . Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021; 47:1181–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leibovici L, Paul M, Ezra O. Ethical dilemmas in antibiotic treatment. J Antimicrob Chemother 2012; 67:12–6. [DOI] [PubMed] [Google Scholar]
- 4. Rhee C, Kadri SS, Dekker JP, et al. . Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw open 2020; 3:e202899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect 2016; 22:416–22. [DOI] [PubMed] [Google Scholar]
- 6. Kariv G, Paul M, Shani V, Muchtar E, Leibovici L. Benchmarking inappropriate empirical antibiotic treatment. Clin Microbiol Infect 2013; 19:629–33. [DOI] [PubMed] [Google Scholar]
- 7. Kadri SS, Lai YL, Warner S, et al. . Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis 2021; 21:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marquet K, Liesenborgs A, Bergs J, Vleugels A, Claes N. Incidence and outcome of inappropriate in-hospital empiric antibiotics for severe infection: a systematic review and meta-analysis. Crit Care 2015; 19:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seymour CW, Gesten F, Prescott HC, et al. . Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 376:2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrer R, Martin-Loeches I, Phillips G, et al. . Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour. Crit Care Med 2014; 42:1749–55. [DOI] [PubMed] [Google Scholar]
- 11. Nauclér P, Huttner A, van Werkhoven CH, et al. . Impact of time to antibiotic therapy on clinical outcome in patients with bacterial infections in the emergency department: implications for antimicrobial stewardship. Clin Microbiol Infect 2021; 27:175–81. [DOI] [PubMed] [Google Scholar]
- 12. McGregor JC, Rich SE, Harris AD, et al. . A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin Infect Dis 2007; 45:329–37. [DOI] [PubMed] [Google Scholar]
- 13. Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 2008; 167:492–9. [DOI] [PubMed] [Google Scholar]
- 14. Valik JK, Ward L, Tanushi H, et al. . Validation of automated sepsis surveillance based on the sepsis-3 clinical criteria against physician record review in a general hospital population: observational study using electronic health records data. BMJ Qual Saf 2020; 29:735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quan H, Li B, Couris CM, et al. . Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173:676–82. [DOI] [PubMed] [Google Scholar]
- 16. Agency for Healthcare Research and Quality . AHRQ QI ICD-10-CM/PCS Specification v2020 Prevention Quality Indicators Appendices. Appendix C: Immunocompromised State Diagnosis and Procedure Codes. Available at: https://www.qualityindicators.ahrq.gov/Downloads/M. Accessed April 2021.
- 17. Organisation, for Economic Co-operation and Developments (OECD) . Definitions for Health Care Quality Indicators: 2014–2015 HCQI Data Collection. Available at: https://www.oecd.org/els/health-systems/Definition. Accessed April 2021.
- 18. Fawcett N, Young B, Peto L, et al. . ‘Caveat emptor’: the cautionary tale of endocarditis and the potential pitfalls of clinical coding data-an electronic health records study. BMC Med 2019; 17:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vincent J-L, Moreno R, Takala J, et al. . The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–10. [DOI] [PubMed] [Google Scholar]
- 20. Valik JK, Mellhammar L, Sundén-Cullberg J, et al. . Peripheral oxygen saturation facilitates assessment of respiratory dysfunction in the sequential organ failure assessment score with implications for the sepsis-3 criteria. Crit Care Med 2022; 50:e272–83. [DOI] [PubMed] [Google Scholar]
- 21. Rhee C, Chiotos K, Cosgrove SE, et al. . Infectious Diseases Society of America Position Paper: recommended revisions to the national severe sepsis and septic shock early management bundle (SEP-1) sepsis quality measure. Clin Infect Dis 2021; 72:541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu VX, Fielding-Singh V, Greene JD, et al. . The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med 2017; 196:856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singer M. Antibiotics for sepsis: does each hour really count, or is it incestuous amplification? Am J Respir Crit Care Med 2017; 196:800–2. [DOI] [PubMed] [Google Scholar]
- 24. López-Cortés LE, Del Toro MD, Gálvez-Acebal J, et al. . Impact of an evidence-based bundle intervention in the quality-of-care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis 2013; 57:1225–33. [DOI] [PubMed] [Google Scholar]
- 25. Fitzpatrick JM, Biswas JS, Edgeworth JD, et al. . Gram-negative bacteraemia; a multi-centre prospective evaluation of empiric antibiotic therapy and outcome in English acute hospitals. Clin Microbiol Infect 2016; 22:244–51. [DOI] [PubMed] [Google Scholar]
- 26. Puskarich MA, Trzeciak S, Shapiro NI, et al. . Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 2011; 39:2066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Groot B, Ansems A, Gerling DH, et al. . The association between time to antibiotics and relevant clinical outcomes in emergency department patients with various stages of sepsis: a prospective multi-center study. Crit Care 2015; 19:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alam N, Oskam E, Stassen PM, et al. . Prehospital antibiotics in the ambulance for sepsis: a multicentre, open label, randomised trial. Lancet Respir Med 2018; 6:40–50. [DOI] [PubMed] [Google Scholar]
- 29. Lambregts MMC, Wijnakker R, Bernards AT, Visser LG, Cessie SL, de Boer MGJ. Mortality after delay of adequate empiric antimicrobial treatment of bloodstream infection. J Clin Med 2020; 9:1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoon YK, Park DW, Sohn JW, et al. . Effects of inappropriate empirical antibiotic therapy on mortality in patients with healthcare-associated methicillin-resistant Staphylococcus aureus bacteremia: a propensity-matched analysis. BMC Infect Dis 2016; 16:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seok H, Song J, Jeon JH, et al. . Timing of antibiotics in septic patients: a prospective cohort study. Clin Microbiol Infect 2020; 26:1495–500. [DOI] [PubMed] [Google Scholar]
- 32. Babich T, Naucler P, Valik JK, et al. . Risk factors for mortality among patients with Pseudomonas aeruginosa bacteraemia: a retrospective multicentre study. Int J Antimicrob Agents 2020; 55:105847. [DOI] [PubMed] [Google Scholar]
- 33. Kaasch AJ, Rieg S, Kuetscher J, et al. . Delay in the administration of appropriate antimicrobial therapy in Staphylococcus aureus bloodstream infection: a prospective multicenter hospital-based cohort study. Infection 2013; 41:979–85. [DOI] [PubMed] [Google Scholar]
- 34. Hranjec T, Rosenberger LH, Swenson B, et al. . Aggressive versus conservative initiation of antimicrobial treatment in critically ill surgical patients with suspected intensive-care-unit-acquired infection: a quasi-experimental, before and after observational cohort study. Lancet Infect Dis 2012; 12:774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Falcone M, Bassetti M, Tiseo G, et al. . Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit Care 2020; 24:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baltas I, Stockdale T, Tausan M, et al. . Impact of antibiotic timing on mortality from gram-negative bacteraemia in an English district general hospital: the importance of getting it right every time. J Antimicrob Chemother 2021; 76:813–9. [DOI] [PubMed] [Google Scholar]
- 37. Corl KA, Zeba F, Caffrey AR, et al. . Delay in antibiotic administration is associated with mortality among septic shock patients with Staphylococcus aureus bacteremia. Crit Care Med 2020; 48:525–32. [DOI] [PubMed] [Google Scholar]
- 38. Lee C-C, Lee C-H, Hong M-Y, Tang H-J, Ko W-C. Timing of appropriate empirical antimicrobial administration and outcome of adults with community-onset bacteremia. Crit Care 2017; 21:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cain SE, Kohn J, Bookstaver PB, Albrecht H, Al-Has MN. Stratification of the impact of inappropriate empirical antimicrobial therapy for gram-negative bloodstream infections by predicted prognosis. Antimicrob Agents Chemother 2015; 59:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 2010; 54:4851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schuttevaer R, Alsma J, Brink A, et al. . Appropriate empirical antibiotic therapy and mortality: conflicting data explained by residual confounding. PLoS One 2019; 14:e0225478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumar A, Roberts D, Wood KE, et al. . Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–96. [DOI] [PubMed] [Google Scholar]
- 43. Morgan CJ. Landmark analysis: a primer. J Nucl Cardiol 2019; 26:391–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.