Abstract
Background
The introduction and adoption of pneumococcal conjugate vaccines (PCVs) into pediatric national immunization programs (NIPs) has led to large decreases in invasive pneumococcal disease (IPD) incidence caused by vaccine serotypes. Despite these reductions, the global IPD burden in children remains significant.
Methods
We collected serotype-specific IPD data from surveillance systems or hospital networks of all 30 high-income countries that met inclusion criteria. Data sources included online databases, surveillance system reports, and peer-reviewed literature. Percentage of serotyped cases covered were calculated for all countries combined and by PCV type in the pediatric NIP.
Results
We identified 8012 serotyped IPD cases in children <5 or ≤5 years old. PCV13 serotype IPD caused 37.4% of total IPD cases, including 57.1% and 25.2% for countries with PCV10 or PCV13 in the pediatric NIP, respectively, most commonly due to serotypes 3 and 19A (11.4% and 13.3%, respectively, across all countries). In PCV10 countries, PCV15 and PCV20 would cover an additional 45.1% and 55.6% of IPD beyond serotypes contained in PCV10, largely due to coverage of serotype 19A. In PCV13 countries, PCV15 and PCV20 would cover an additional 10.6% and 38.2% of IPD beyond serotypes contained in PCV13. The most common IPD serotypes covered by higher valency PCVs were 10A (5.2%), 12F (5.1%), and 22F and 33F (3.5% each).
Conclusions
Much of the remaining IPD burden is due to serotypes included in PCV15 and PCV20. The inclusion of these next generation PCVs into existing pediatric NIPs may further reduce the incidence of childhood IPD.
Keywords: children, invasive pneumococcal disease, pneumococcal conjugate vaccines, serotype distribution, streptococcus pneumoniae
Pneumococcal serotype distribution in children from high-income countries was assessed. Much of the burden of invasive pneumococcal disease (IPD) is due to serotypes in PCV15 and PCV20; including these pneumococcal conjugate vaccines (PCVs) into existing pediatric immunization programs may help reduce IPD incidence.
Introduction
Streptococcus pneumoniae infection has several clinical manifestations, including invasive pneumococcal disease (IPD; eg, meningitis, bacteremia, and bacteremic pneumonia) and noninvasive disease [1–3]. IPD is a leading cause of morbidity and mortality in children and adults [4], with S pneumoniae the most common cause of bacteremic pneumonia among children [5]. One hundred one unique S pneumoniae serotypes have been identified, with most being capable of causing serious diseases in humans [1, 6–8]. Pneumococcal disease is endemic globally, but pneumococcal serotype distribution varies over time, by age, clinical manifestation, and geography [1, 6].
Currently licensed vaccines for pneumococcal disease prevention in children in high-income countries include the 10- and 13-valent pneumococcal conjugate vaccines (PCV10 and PCV13). Among high-income countries, PCV10 (serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F) or PCV13 (PCV10 serotypes plus 3, 6A, and 19A) vaccination is typically included as part of the national immunization program (NIP) for children <5 years old. Pneumococcal vaccination is usually administered as a 2 + 1, 3 + 0, or 3 + 1 schedule, depending on the country (the UK recently adopted a 1 + 1 schedule) (Table 1).
Table 1.
Characteristics of Countries and Pneumococcal Vaccine Use
| Country | WHO Region | Surveillance System (Type) or Hospital Network (n hospitals) | NIP Pediatric Schedule Used During Years of Data Reported in the Analysisa | Year PCV Introduced Into Pediatric NIP | PCV Used During Years of Data Reported in the Analysis | Year PCV Use Began Relevant to Years of Data Reported in the Analysis |
|---|---|---|---|---|---|---|
| Australia | WPRO | National, passive, mandatory | 3 + 0b | 2005 | PCV13 | 2011 |
| Austria | EURO | National, passive, mandatory | 2 + 1 | 2002 | PCV10 | 2012c |
| Belgium | EURO | Sentinel, passive, voluntary | 2 + 1 | 2007 | PCV10, PCV13 | 2015 or 2016 (PCV10); 2018 or 2019 (PCV13)d |
| Canada | AMRO | Multiprovincial, passive, voluntary | 2 + 1 | 2002–2006, varies by province | PCV13 | 2010e |
| Chile | AMRO | Laboratory based | 2 + 1 | 2011 | PCV10 | 2011f |
| Czech Republic | EURO | National, active, mandatory | 3 + 1g | 2005 | PCV10, PCV13 | 2009 (PCV10); 2010 (PCV13) |
| Denmark | EURO | National, passive, mandatory | 2 + 1 | 2007 | PCV13 | 2010 |
| United Kingdom | EURO | National, passive, mandatory | 2 + 1h | 2006 | PCV13 | 2010 |
| Finland | EURO | National, passive, mandatory | 2 + 1 | 2010 | PCV10 | 2010 |
| France | EURO | National, active, voluntary | 2 + 1 | 2006 | PCV13 | 2010 |
| Germany | EURO | National, active, voluntary | 2 + 1i | 2006 | PCV13 | 2009 (PCV10), 2010 (PCV13) |
| Hong Kong | WPRO | National, passive, mandatory | 3 + 1j | 2010 | PCV13 | 2011 |
| Hungary | EURO | National, passive, voluntary | 2 + 1 | 2009 | PCV13 | 2010 |
| Ireland | EURO | National, passive, mandatory | 2 + 1 | 2008 | PCV13 | 2010 |
| Israel | EMRO | National, active, voluntary | 2 + 1 | 2009 | PCV13 | 2010k |
| Italy | EURO | National, passive, voluntary | 2 + 1 | 2005 | PCV13l | 2010 |
| Japan | WPRO | Hospital network (n = 341) | 3 + 1 | 2011 | PCV13 | 2013 |
| Netherlands | EURO | Sentinel, passive, voluntary | 2 + 1 | 2006 | PCV10 | 2011 |
| New Zealand | WPRO | National, passive, mandatory | 3 + 1 | 2008 | PCV13 | 2014m |
| Norway | EURO | National, passive, mandatory | 2 + 1 | 2006 | PCV13 | 2011 |
| Panama | AMRO | Laboratory based | 2 + 1 | 2010 | PCV13 | 2011 |
| Poland | EURO | National, passive, mandatory | 2 + 1 | 2017 | PCV13 | 2017n |
| Singapore | WPRO | National, passive, mandatory | 2 + 1 | 2009 | PCV13 | 2011 |
| Slovakia | EURO | National, active, mandatory | 2 + 1 | 2009 | PCV10, PCV13 | 2010 |
| Slovenia | EURO | National, passive, mandatory | 2 + 1 | 2005 | PCV10o | 2015 |
| South Korea | WPRO | Hospital network (n = 44) | 3 + 1 | 2014 | PCV10, PCV13 | 2014 |
| Spain | EURO | Regional, passive, mandatory | 2 + 1 | 2001 | PCV13 | 2015 |
| Sweden | EURO | National, passive, mandatory | 2 + 1 | 2009 | PCV10, PCV13p | 2010 |
| United States | AMRO | Sentinel, passive, mandatory | 3 + 1 | 2001 | PCV13 | 2010 |
| Uruguay | AMRO | Laboratory-based | 2 + 1 | 2008 | PCV13 | 2010 |
Abbreviations: AMRO, Regional Office for the Americas; EMRO, Regional Office for the Eastern Mediterranean; EURO, Regional Office for Europe; NIP, National Immunization Program; PCV, pneumococcal conjugate vaccine; PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; WHO, World Health Organization; WPRO, Regional Office for the Western Pacific.
PCV schedule information accessed on International Vaccine Access Center ViewHub (https://view-hub.org/).
In Australia, a 3 + 0 schedule was used during the years of data included in this analysis (2016–2018). In 2018, Australia switched from a 3 + 0 to a 2 + 1 schedule.
In Austria, PCV10 was used during the years of data included in this analysis (2016–2018). Austria switched to PCV13 in 2020.
Belgium is divided into 2 regions that make independent PCV decisions, which can occur at different times. The Flanders region switched from PCV13 to PCV10 in July 2015. The Wallonia region (includes Brussels) switched from PCV13 to PCV10 in May 2016. Both regions switched back to PCV13 in 2018 or 2019 (Wallonia in late 2018, and Flanders in July 2019).
In Canada, PCV10 was used from 2009 to 2010 in 2 provinces.
In Chile, PCV10 was used during the years of data included in this analysis. Chile switched from PCV10 to PCV13 in 2017.
In the Czech Republic, a 3 + 1 schedule was used during the years of data included in this analysis. In 2018, they switched to a 2 + 1 schedule. Both PCV10 and PCV13 are recommended and estimates of PCV coverage do not differentiate between use of PCV10 and PCV13.
The United Kingdom switched to a 1 + 1 schedule in 2020.
In Germany, PCV13 is used primarily (market share >90%). In 2015, Germany switched from a 3 + 1 to a 2 + 1 schedule.
In Hong Kong, a 3 + 1 schedule was used during the year of data included in this analysis. They switched from a 3 + 1 to a 2 + 1 schedule in July 2019.
In Israel, PCV13 gradually replaced PCV7; a catch-up program was not implemented.
The Piemonte region of Italy switched from PCV13 to PCV10 in 2018. The other regions continue to use PCV13.
New Zealand switched from PCV7 to PCV10 in 2011, to PCV13 in 2014 (the year marking the beginning of data included in this analysis), and to PCV10 in 2017.
In Poland, serotype data from 2017 were included, the year PCV13 was included in the NIP.
Slovenia switched from PCV10 to PCV13 in 2019.
In Sweden, both PCV10 and PCV13 are recommended, and choice of PCV is determined by individual counties.
PCV use in pediatric NIPs has led to large decreases in IPD incidence caused by serotypes included in available vaccines [1]. Despite these reductions, IPD continues to cause a significant global burden in children [3]. Although existing vaccines have greatly reduced vaccine serotype disease, they have not eliminated carriage or transmission [9], leaving a remaining burden from these serotypes among unvaccinated or undervaccinated children or those with vaccine failure. More importantly, existing vaccines contain a limited number of the 101 known serotypes, and nonvaccine serotypes may replace vaccine serotypes in the upper airway, causing increased transmission and disease at a population level [7, 10–12]. The propensity to cause carriage and disease is concentrated among a relatively small number of nonvaccine serotypes [13] and to most efficiently reduce the remaining pediatric IPD burden these serotypes have been the focus of expanded valency PCVs. Recently, 2 vaccines have been licensed in adults in the United States and are in advanced clinical development for pediatric populations: 15- and 20-valent PCV (PCV15 [PCV13 serotypes plus 22F and 33F] and PCV20 [PCV15 serotypes plus 8, 10A, 11A, 12F, and 15B], respectively) [14, 15].
Robust IPD surveillance is required to monitor disease incidence and serotype distribution to inform vaccination policies, guide vaccine development, and understand vaccination impact on IPD across the population. Here we investigate the contemporary IPD serotype distribution among children in high-income countries with the goal of estimating the vaccine serotype coverage for currently available (PCV10 and PCV13) and investigational (PCV15 and PCV20) pediatric pneumococcal vaccines and describe the influence of PCVs currently used in the pediatric NIP on pediatric IPD serotype distribution.
METHODS
Country Selection Criteria and Data Collection
High-income countries were selected according to the World Bank classification and retained for the analysis if they reported IPD cases for children from a national, regional, or sentinel surveillance or a country hospital network. Information on IPD cases was retrieved from each selected country from either a publicly available source (eg, surveillance report, online database), or a peer-reviewed publication through a targeted search on PubMed. Inclusion was not restricted to countries already using PCV in infant NIPs; however, for countries that had introduced a PCV into the NIP, IPD cases had to be reported after pediatric PCV introduction to meet inclusion criteria. Countries were excluded from analysis if they reported <20 IPD cases overall during the specified inclusion period.
For each included country, we collected information on the type of surveillance systems or hospital network; PCV type if any used in the NIP during the reporting period; PCV schedule; and PCV introduction date into the pediatric NIP (Table 1). The following criteria were applied to determine which data to include in analysis: (1) if more than 1 data source for IPD cases was available, then the source with the largest number of cases reported was selected, and (2) cases from the 3 most recent years of data available were selected (if fewer than 3 years were available, then all available data were selected; Supplementary Table 1). Data were excluded if: (1) the source of isolates was not specified, (2) nonsterile and sterile site isolates were not differentiated, or (3) IPD cases were not reported by serotype and age groups.
Data Analysis
The percentage of IPD associated with individual serotypes or pooled PCV serotypes was calculated for the reporting period for each country (excluding missing and non-typeable cases from the denominator). Average percentages of IPD were calculated for individual serotypes and pooled PCV serotypes. To describe the impact of pediatric PCV use on pediatric serotype distribution, IPD percentages were calculated for countries stratified by PCV type, pediatric PCV use duration in the NIP, and World Health Organization (WHO) region (the Regional Office for the Americas [AMRO], the Regional Office for the Eastern Mediterranean [EMRO], the Regional Office for Europe [EURO], and the Regional Office for the Western Pacific [WPRO]). Serotypes were pooled according to their inclusion in pneumococcal vaccines already licensed or in development or their potential for cross-reacting with PCV serotypes: PCV10 serotypes (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F, plus cross-reacting 6A), PCV13 serotypes (PCV10 serotypes, including 6A, and 3 and 19A plus cross-reacting 6C), PCV15 serotypes (PCV13 serotypes, 22F and 33F), and PCV20 serotypes (PCV15 serotypes plus 8, 10A, 11A, 12F, and 15B, plus cross-reacting 15C).
RESULTS
IPD Serotype Distribution in Children From High-Income Countries
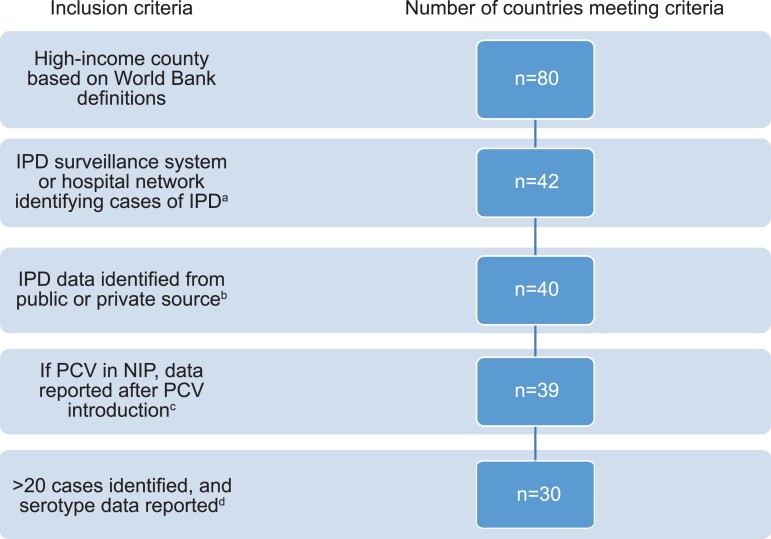
Overall, 30 high-income countries met the inclusion criteria (Figure 1, Supplementary Table 1). Twenty-eight (93%) countries reported data for all children <5 years old or ≤5 years old (2 reported data for children <2 years old). Data reporting periods ranged from 2014–2015 to 2017–2019. In 28 countries (93%), IPD serotype distribution data were generated from a surveillance system, and in the remaining 2 countries, from a hospital network (Table 1). Twenty-two countries (73%) had IPD data available from at least 3 years, 6 countries had data available from 2 years, 1 country had data available from 1 year, and 1 country (South Korea) reported aggregated serotyped IPD data for 4 years combined (Supplementary Table 2).
Figure 1.
Characteristics of countries meeting inclusion criteria for analysis. an = 38 excluded due to no surveillance system or hospital network reporting IPD cases. bn = 2 excluded (Taiwan and Portugal). cn = 1 excluded (Croatia). dn = 9 excluded (Cyprus, Estonia, Greece, Iceland, Luxembourg, Latvia, Lithuania, Malta, and Switzerland). Abbreviations: IPD, invasive pneumococcal disease; NIP, national immunization program; PCV, pneumococcal conjugate vaccine.
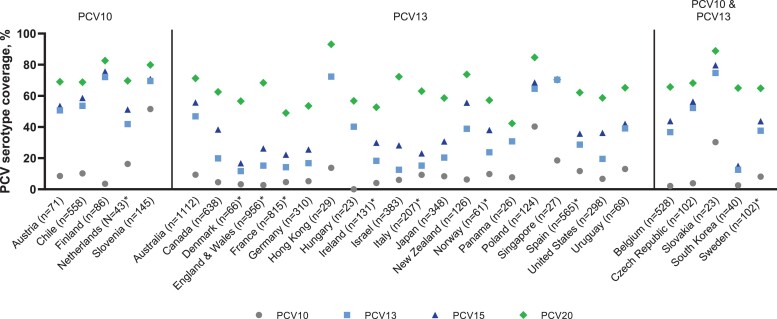
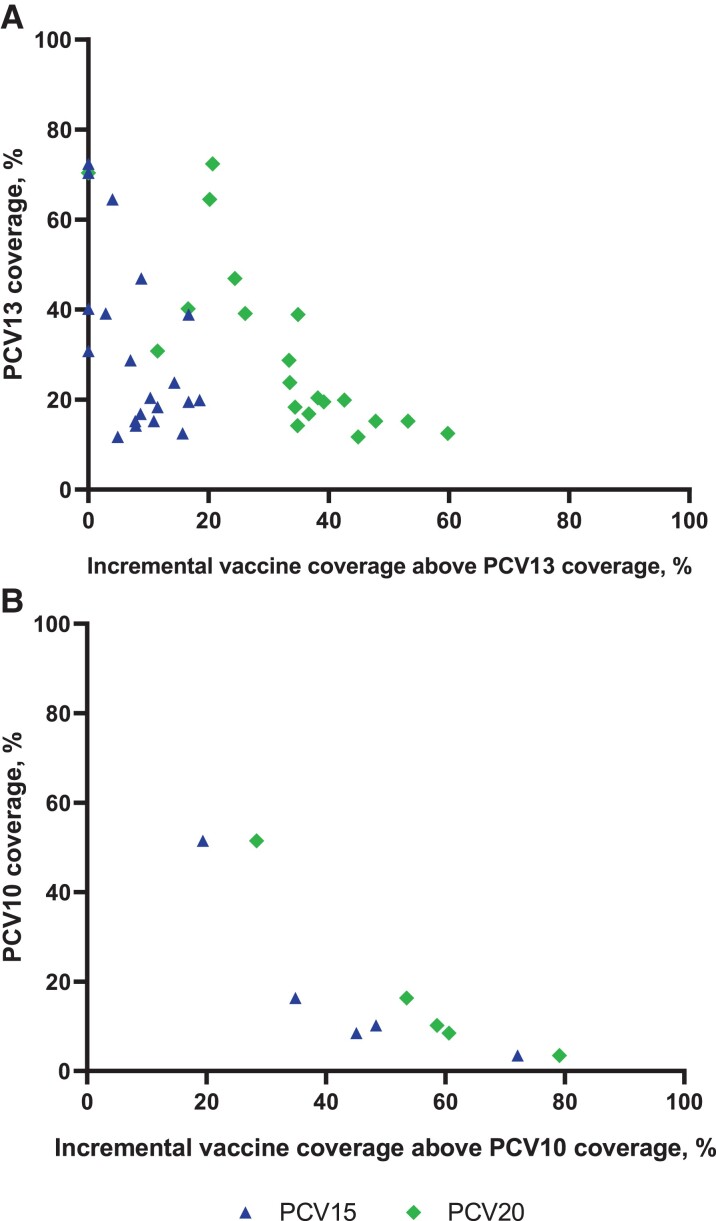
In children <5 years old, 8012 IPD cases had a serotype identified (Supplementary Table 2). The number of cases in countries ranged from 23 (Hungary and Slovakia) to 1112 (Australia). Overall, average coverage of IPD was 10.7% (min: 0%, max: 51.5%) for PCV10, 37.4% (min: 11.7%, max: 74.7%) for PCV13, 44.5% (min: 15.0%, max: 79.6%) for PCV15, and 66.5% (min: 42.3%, max: 93.1%) for PCV20 (Supplementary Table 2). Across all countries, the most commonly reported serotypes were 19A (13.3%), 3 (11.4%), 10A (5.2%), and 12F (5.1%). Although considerable variability among countries was seen, in general, a high percentage of IPD cases was caused by the aggregate serotypes not included in PCV10 and PCV13 but included in PCV15 and PCV20 (Figure 2, Supplementary Table 2). The incremental coverage of PCV15 and PCV20 beyond either PCV10 or PCV13 was inversely related to IPD serotype coverage of PCV10 or PCV13 (Figure 3A, Figure 3B).
Figure 2.
Percentages of cases caused by vaccine-specific serotypes (PCV serotype coverage) among countries with PCV10 and PCV13 use in children <5 years old. n represents the number of cases reported in each country. *Estimates of PCV20 coverage for children <5 years old are weighted averages of the <1- and 1–4-year data. For full details and source data, see Supplementary Table 2. Abbreviations: PCV, pneumococcal conjugate vaccine; PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PCV15, 15-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine.
Figure 3.
PCV13 coverage versus the incremental coverage of PCV15 or PCV20 beyond PCV13 coverage (A) and beyond PCV10 coverage (B). Abbreviations: PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PCV15, 15-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine.
Impact of Pediatric PCV Use on Pediatric IPD Serotype Distribution
All countries had introduced a PCV into the pediatric NIP: 20 (66%) had introduced PCV13, 5 (17%) PCV10, and 5 (17%) PCV10 and PCV13. The majority of countries administered PCV in a schedule with 2 infant and 1 toddler doses (2 + 1) (n = 23; 77%); introduced the current PCV into the pediatric NIP ≥3 years before the beginning of data reporting period included in this analysis (n = 24; 80%); and were located in the EURO region of the WHO (n = 18; 60%) (Table 1, Supplementary Table 2).
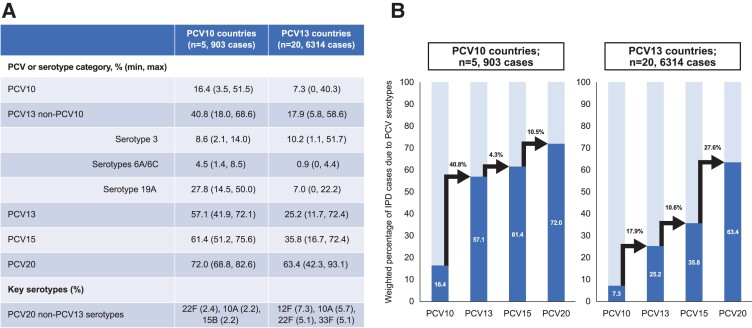
For countries with PCV13 in the pediatric NIP, serotypes contained in PCV10, PCV13, PCV15, and PCV20 caused 7.3%, 25.2%, 35.8%, and 63.4% of IPD cases, respectively. The most common serotypes overall in PCV13 countries were 3 (10.2%; min: 1.1%, max: 51.7%), 12F (7.3%; min: 0%, max: 28.7%), 19A (7.0%; min: 0%, max: 22.2%), and 10A (5.7%; min: 0%, max: 13.5%) (Figure 2, Supplementary Tables 2 and 3).
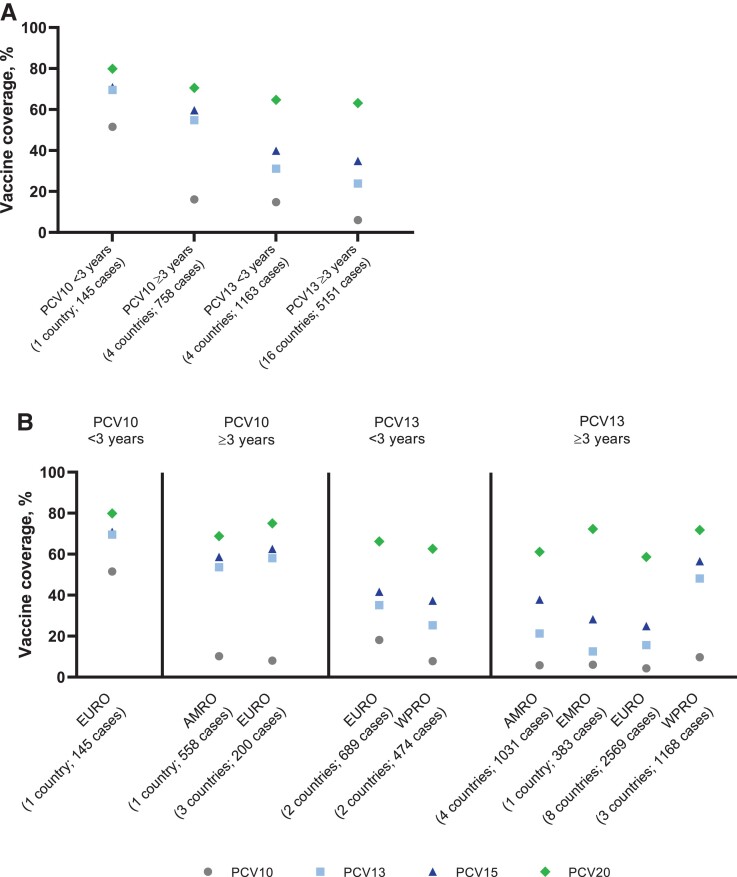
For countries with PCV10 in the pediatric NIP, serotypes contained in PCV10, PCV13, PCV15, and PCV20 caused 16.4%, 57.1%, 61.4%, and 72.0% of IPD, respectively. The most common serotypes in PCV10 countries were 19A (27.8%; min: 14.5%, max: 50.0%), 3 (8.6%; min: 2.1%, max: 14.1%), and 14 (5.1%; min: 0%, max: 18.2%) (Figure 2, Supplementary Tables 2 and 3). The relatively similar coverage from PCV13, PCV15, and PCV20 in countries with PCV10 in the NIP likely results from the high proportion of disease caused by serotype 19A (not included in PCV10) and the subsequent lower proportion due to other serotypes (Figure 4).
Figure 4.
PCV serotype coverage, by type of PCV in the pediatric NIP (A) and absolute incremental increase in PCV serotype coverage, by type pf PCV in the pediatric NIP (B). Arrows show absolute incremental increase in PCV serotype coverage among sequential PCV formulations. Abbreviations: NIP, national immunization program; PCV, pneumococcal conjugate vaccine; PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PCV15, 15-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine.
When PCV choice in the pediatric NIP was stratified further by PCV duration in the pediatric NIP and by WHO region, substantial variability in the percentages of IPD covered by PCVs was observed (Figure 5A and 5B, and Supplementary Table 4). In general, shorter duration of PCV use in the NIP corresponded with a larger share of IPD due to the original serotypes covered by the PCV in the NIP, especially for countries using PCV10 in the NIP.
Figure 5.
Percentages of IPD cases caused by vaccine-specific serotypes (PCV serotype coverage) by duration of PCV use (A) and duration of PCV use and WHO region (B). Abbreviations: AMRO, Regional Office for the Americas; EMRO, Regional Office for the Eastern Mediterranean; EURO, Regional Office for Europe; IPD, invasive pneumococcal disease; PCV, pneumococcal conjugate vaccine; PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PCV15, 15-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine; WHO, Word Health Organization; WPRO, Regional Office for the Western Pacific.
DISCUSSION
The introduction of PCVs into NIPs around the world has resulted in substantial reductions in IPD in children caused by serotypes included in PCV10 and PCV13 [3, 16, 17]. This is likely due to the direct protection against IPD offered by routine pediatric NIPs in these countries in combination with indirect protection of undervaccinated children or those who did not respond to vaccine. However, as corroborated by other studies and supported in the results of this analysis, serotypes contained in existing vaccines continue to cause a substantial burden of IPD, including serotype 19A (particularly in countries using PCV10 in the NIP but also to a lower degree in countries using PCV13 in the NIP) and serotype 3 in all countries [12, 17]. These data illustrate the limits of relying on cross-protection for some serotypes (eg, serotype 19F for 19A) and suggest that some serotypes may require more robust or different immunologic response than those induced by current conjugate vaccine formulations to reduce carriage acquisition or density and thus to provide population-based protection [18]. These factors are compounded by suboptimal vaccine uptake that has been reported in several countries [19], which may contribute to the persistent burden of vaccine-type IPD in children.
Serotype 19A was the most common serotype in countries using PCV10, which does not include 19A in its formulation. It was also frequently detected in countries using PCV13 but at a lower frequency. Consistent evidence of PCV13 effectiveness against serotype 19A disease has been reported among directly vaccinated persons [20, 21]. These data are corroborated with evidence of significant declines in serotype 19A IPD in countries that introduced PCV13 into the pediatric NIP [11, 22]. Despite high population PCV coverage in these countries, residual 19A IPD persists among children [22]. This evidence of ongoing transmission may indicate that higher antibody concentrations are needed to protect against carriage.
Furthermore, interpretation of data for serotype 19A may require knowing details about the nuances of vaccine use in certain countries. For example, Singapore and New Zealand reported that 22.2% of IPD was due to serotype 19A; however, New Zealand has alternated between PCV10 and PCV13 use, and data from Belgium indicate serotype 19A rebounded rapidly in association with a switch from PCV13 to PCV10 [23]. Australia also reported a relatively high contribution from serotype 19A but used a 3 + 0 schedule during the data reporting period, which may control transmission less well than a schedule with a post-infancy booster. The high proportions of IPD due to serotype 19A from Panama and Singapore are based on 4 and 6 serotype 19A cases among 26 and 27 total IPD cases, respectively, and thus may represent the consequences of a small denominator. Other large fluctuations between countries in serotype distribution may have similar explanations.
Serotype 3 was also one of the most common serotypes identified in countries with short-term and long-term use of PCV13 or PCV10 in the pediatric NIP. Pre-licensure immunogenicity data of PCV13 indicate a robust immune response and functional antibody activity for all serotypes including serotype 3, particularly after the booster dose [24]. Countries that introduced PCV13 into the pediatric NIP, assessed its effectiveness against IPD, and that were included in a meta-analysis together reported an effectiveness estimate of 63.5% (95% confidence interval [CI]: 37.3%, 89.7%) [25]. However, trends in serotype 3 IPD incidence among unvaccinated age cohorts indicate that initial evidence of indirect protection have begun to reverse [25]. Several explanations exist. Protection from serotype 3 carriage may require higher antibody levels than those induced by PCV13, and the unique capsular structure of serotype 3 may require activation of alternative immunological pathways [26]. In some areas, particularly the United Kingdom, the rebound in serotype 3 has been predominantly due to a new clade 2, which demonstrates higher levels of antibiotic resistance and alterations in subcapsular genes [27]. If these characteristics of clade 2 are driving the lower ability of PCV13 to protect against carriage, then it is possible a similar effect will be seen with any vaccine targeting the serotype 3 capsule antigen.
For several reasons, the additional serotypes targeted by expanded valency PCV15 and PCV20 have become an increasing global concern [10, 13, 16, 28–32]. First, these serotypes are now responsible for a high proportion of IPD and related deaths [10, 16, 28, 29]. Data from Public Health England reported that after PCV13 introduction, serotypes 8, 11A, 15B, 15C, 22F, and 33F were together responsible for 20% of IPD-related deaths in children in England and Wales [31]. Second, some serotypes such as 8, 10A, 15B/15C, 22F, and 33F have been shown to cause more severe clinical manifestations such as meningitis compared with other serotypes including some PCV13 serotypes (1, 3, 4, 9V, 14, and 19A) [13, 33]. Third, some serotypes have started to become endemic or cause outbreaks. For example, an outbreak reported in Winnipeg, Canada, led to a doubling of IPD rates, predominantly by serotype 12F, which previously accounted for very few cases each year [34]. Similarly, following the introduction of PCV13 in Israel’s NIP, the previously rare serotype 12F increased in frequency, becoming the country’s most prevalent serotype causing IPD [35]. Finally, several PCV20-unique serotypes, including 11A, 12F, 15B/15C, 22F, and 33F, have been associated with IPD strains bearing antibiotic resistance genes [30, 32, 34, 36].
Our study reported a high proportion of IPD due to PCV15 and PCV20 serotypes in children <5 years old. Similar trends were also observed in older adults as reported in our companion manuscript. Both PCV15 and PCV20 have recently been licensed for use in adults in the United States and clinical studies in children have shown promising immunogenicity and safety data [14, 15, 37, 38]. In general, PCV20 offers a substantial coverage advantage compared to all other existing or investigational PCV vaccines, due to its ability to cover against serotype 19A (especially in PCV10 countries) and its additional 5 unique serotypes, which are becoming increasingly important.
The analysis presented herein has several limitations. First, although IPD is one of the most readily available sources of serotype data from many countries, IPD may be underdiagnosed in some countries due to differences in blood culture sampling rates and practices [39]. Consequently, serotype distribution of IPD overall may not fully capture the serotype distribution in mild IPD (where blood cultures are not sought) or the most severe IPD (where children may die before blood cultures are obtained). Second, analyses were limited by reporting proportions instead of rates (precluding true conclusions on the burden). Third, as an alternative to assessing the impact of population PCV coverage on serotype distribution, time since PCV introduction into the pediatric NIP was assessed, which may not be an accurate proxy of coverage. Fourth, observation periods several years in the past, reporting of only severe disease in some surveillance systems, overrepresenting serotype distribution in meningitis, adjustments in the NIPs (change in the vaccination schedule, switch or addition of PCV13 to PCV10), improvement of vaccine uptake, or the spread of genetic lineages after the reporting period affect the likelihood to report the current serotype distribution. A more thorough and comprehensive analysis may be required to ascertain the full serotype distribution of S pneumoniae in high-income countries.
Finally, IPD only represents a small proportion of total pneumococcal vaccine-preventable disease burden [37]. A large burden of pneumococcal disease is non-invasive, including pneumococcal acute otitis media and nonbacteremic community-acquired pneumonia [37, 38]. The latter issue is particularly problematic since no serotype-specific test currently exists and no data exist to indicate whether pediatric IPD serotype distribution is predictive of non-bacteremic pneumonia serotype distribution. A recent study in Burkina Faso suggests that a serotype-specific urinary antigen detection test may work in children as has been shown in adults, but additional studies are needed [40]. To account for the full public health impact of currently existing or future investigational vaccines in reducing the burden caused by S pneumoniae, in addition to IPD, similar PCV serotype coverage analyses need to be conducted for acute otitis media and pneumonia.
CONCLUSIONS
Routine PCV10 and PCV13 pediatric immunization programs have reduced IPD in children <5 years old in high-income countries. The remaining IPD burden due to PCV10 and PCV13 serotypes may be due to several factors, including vaccination schedule, suboptimal vaccination coverage, and lower effectiveness against certain vaccine serotypes. Strengthening existing pediatric immunization programs and optimizing vaccine uptake may further reduce PCV13-serotype disease. Furthermore, as much of the remaining IPD burden is due to serotypes included in PCV15 and PCV20, their inclusion into existing pediatric immunization programs may help further reduce the incidence of pneumococcal disease in children from high-income countries.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Lindsay R Grant, Pfizer Inc, Collegeville, PA, USA.
Mary P E Slack, School of Medicine and Dentistry, Griffith University, Southport, Queensland, Australia.
Christian Theilacker, Pfizer Pharma GmbH, Berlin, Germany.
Jelena Vojicic, Pfizer Canada ULC, Montreal, Canada.
Stephane Dion, Pfizer Canada ULC, Montreal, Canada.
Ralf-Rene Reinert, Pfizer Inc, Paris, France.
Luis Jodar, Pfizer Inc, Collegeville, PA, USA.
Bradford D Gessner, Pfizer Inc, Collegeville, PA, USA.
Notes
Acknowledgments. This work was sponsored by Pfizer Inc. Editorial/medical writing support was provided by Dr Sheena Hunt and Dr Srividya Ramachandran of ICON (Blue Bell, Pennsylvania, USA) and was funded by Pfizer Inc. The authors thank José Suaya for contributions to this research.
Financial support. This work was funded by Pfizer Inc.
References
- 1. World Health Organization . Pneumococcal vaccines WHO position paper—2012. Wkly Epidemiol Rec 2012; 87:129–44. [PubMed] [Google Scholar]
- 2. Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect 2014; 20:45–51. [DOI] [PubMed] [Google Scholar]
- 3. Wahl B, O'Brien KL, Greenbaum A, et al. . Burden of Streptococcus pneumoniae and Haemophilus influenzae type B disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health 2018; 6:e744–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Randle E, Ninis N, Inwald D. Invasive pneumococcal disease. Arch Dis Child Educ Pract Ed 2011; 96:183–90. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Pneumonia: key facts. Available at: https://www.who.int/news-room/fact-sheets/detail/pneumonia. Accessed 28 June 2019.
- 6. Kalin M. Pneumococcal serotypes and their clinical relevance. Thorax 1998; 53:159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pimenta F, Moiane B, Gertz R, et al. . New pneumococcal serotype 15D. J Clin Microbiol 2021; 59:e00329-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention . Streptococcus pneumoniae. Available at: https://www.cdc.gov/pneumococcal/clinicians/streptococcus-pneumoniae.html. Accessed 11 April 2022.
- 9. Mosser JF, Grant LR, Millar EV, et al. . Nasopharyngeal carriage and transmission of Streptococcus pneumoniae in American Indian households after a decade of pneumococcal conjugate vaccine use. PLoS One 2014; 9:e79578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hausdorff WP, Hanage WP. Interim results of an ecological experiment—conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother 2016; 12:358–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis 2015; 15:535–43. [DOI] [PubMed] [Google Scholar]
- 12. Asner SA, Agyeman PKA, Gradoux E, et al. . Burden of Streptococcus pneumoniae sepsis in children after introduction of pneumococcal conjugate vaccines: a prospective population-based cohort study. Clin Infect Dis 2019; 69:1574–80. [DOI] [PubMed] [Google Scholar]
- 13. van Hoek AJ, Andrews N, Waight PA, George R, Miller E. Effect of serotype on focus and mortality of invasive pneumococcal disease: coverage of different vaccines and insight into non-vaccine serotypes. PLoS One 2012; 7:e39150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenberg D, Hoover PA, Vesikari T, et al. . Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Vaccine 2018; 36:6883–91. [DOI] [PubMed] [Google Scholar]
- 15. U.S. National Institutes of Health . Trial to evaluate the safety and immunogenicity of a multivalent pneumococcal vaccine in healthy infants. NCT03512288. Available at: https://clinicaltrials.gov/ct2/show/NCT03512288. Accessed 14 October 2020.
- 16. Moore MR, Link-Gelles R, Schaffner W, et al. . Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pilishvili T. Impact of PCV13 on invasive pneumococcal disease (IPD) burden and the serotype distribution in the U.S. Centers for Disease Control; 2018. [Google Scholar]
- 18. Dagan R, Patterson S, Juergens C, et al. . Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis 2013; 57:952–62. [DOI] [PubMed] [Google Scholar]
- 19. Johns Hopkins Bloomberg School of Public Health International Vaccine Access Center (IVAC) . VIEW-hub report: global vaccine introduction and implementation. Available at: https://view-hub.org/sites/default/files/2020-05/VIEW-hub_Report_Mar2020.pdf. Accessed 19 October 2020.
- 20. Moore MR, Link-Gelles R, Schaffner W, et al. . Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med 2016; 4:399–406. [DOI] [PubMed] [Google Scholar]
- 21. Berman-Rosa M, O'Donnell S, Barker M, Quach C. Efficacy and effectiveness of the PCV-10 and PCV-13 vaccines against invasive pneumococcal disease. Pediatrics 2020; 145:e20190377. [DOI] [PubMed] [Google Scholar]
- 22. Steens A, Bergsaker MA, Aaberge ISet al. . Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine 2013; 31:6232–8. [DOI] [PubMed] [Google Scholar]
- 23. Desmet S, Lagrou K, Wyndham-Thomas C, et al. . Dynamic changes in paediatric invasive pneumococcal disease after sequential switches of conjugate vaccine in Belgium: a national retrospective observational study. Lancet Infect Dis 2021; 21:127–36. [DOI] [PubMed] [Google Scholar]
- 24. Yeh SH, Gurtman A, Hurley DC, et al. . Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics 2010; 126:e493–e505. [DOI] [PubMed] [Google Scholar]
- 25. Sings HL, De Wals P, Gessner BD, et al. . Effectiveness of 13-valent pneumococcal conjugate vaccine against invasive disease caused by serotype 3 in children: a systematic review and meta-analysis of observational studies. Clin Infect Dis 2019; 68:2135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luck JN, Tettelin H, Orihuela CJ. Sugar-coated killer: serotype 3 pneumococcal disease. Front Cell Infect Microbiol 2020; 10:613287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Groves N, Sheppard CL, Litt D, et al. . Evolution of streptococcus pneumoniae serotype 3 in England and wales: a major vaccine evader. Genes (Basel) 2019; 10:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS One 2017; 12:e0177113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen R, Cohen JF, Chalumeau M, Levy C. Impact of pneumococcal conjugate vaccines for children in high- and non-high-income countries. Expert Rev Vaccines 2017; 16:625–40. [DOI] [PubMed] [Google Scholar]
- 30. Metcalf BJ, Gertz RE Jr, Gladstone RA, et al. . Strain features and distributions in pneumococci from children with invasive disease before and after 13-valent conjugate vaccine implementation in the USA. Clin Microbiol Infect 2016; 22:e9–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oligbu G, Collins S, Sheppard CL, et al. . Childhood deaths attributable to invasive pneumococcal disease in England and Wales, 2006–2014. Clin Infect Dis 2017; 65:308–14. [DOI] [PubMed] [Google Scholar]
- 32. Tomczyk S, Lynfield R, Schaffner W, et al. . Prevention of antibiotic-nonsusceptible invasive pneumococcal disease with the 13-valent pneumococcal conjugate vaccine. Clin Infect Dis 2016; 62:1119–25. [DOI] [PubMed] [Google Scholar]
- 33. Quesada M G, Yang Y, Bennett JC, et al. . Serotype distribution of remaining pneumococcal meningitis in the mature PCV10/13 period: findings from the PSERENADE project. Microorganisms 2021; 9:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schillberg E, Isaac M, Deng X, et al. . Outbreak of invasive Streptococcus pneumoniae serotype 12F among a marginalized inner-city population in Winnipeg, Canada, 2009–2011. Clin Infect Dis 2014; 59:651–7. [DOI] [PubMed] [Google Scholar]
- 35. Rokney A, Ben-Shimol S, Korenman Z, et al. . Emergence of Streptococcus pneumoniae serotype 12F after sequential introduction of 7- and 13-valent vaccines, Israel. Emerg Infect Dis 2018; 24:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee JK, Yun KW, Choi EHet al. . Changes in the serotype distribution among antibiotic resistant carriage Streptococcus pneumoniae isolates in children after the introduction of the extended-valency pneumococcal conjugate vaccine. J Korean Med Sci 2017; 32:1431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thompson A, Lamberth E, Severs J, et al. . Phase 1 trial of a 20-valent pneumococcal conjugate vaccine in healthy adults. Vaccine 2019; 37:6201–7. [DOI] [PubMed] [Google Scholar]
- 38. Stacey HL, Rosen J, Peterson JT, et al. . Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV-15) compared to PCV-13 in healthy older adults. Hum Vaccin Immunother 2019; 15:530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hausdorff WP, Siber G, Paradiso PR. Geographical differences in invasive pneumococcal disease rates and serotype frequency in young children. Lancet 2001; 357:950–2. [DOI] [PubMed] [Google Scholar]
- 40. Bountogo M, Sanogo B, Pride MW, et al. . Application of a pneumococcal serotype-specific urinary antigen detection test for identification of pediatric pneumonia in Burkina Faso. Pediatr Infect Dis J 2021; 40:418–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.