Abstract
Background
When assessing long-term tuberculosis (TB) mortality, few studies addressed the impact of behavior habits and socioeconomic status. Therefore, we aimed to evaluate long-term TB mortality and risk factors while accounting for potential confounders.
Methods
This cohort study included TB survivors (n = 82 098) aged ≥20 years between 2010 and 2017, and 1:1 age- and sex-matched controls (n = 82 098). The participants were followed up for death 1 year after study enrollment until December 2018. Long-term mortality was adjusted for behavior habits (smoking, alcohol consumption, or exercise), income level, body mass index (BMI), and comorbidities.
Results
During a median of 3.7 years of follow-up, the incidence rate of mortality was significantly higher in TB survivors than those in the matched controls (18.2 vs. 8.8 per 1000 person-years, P < .001). Even after adjusting for potential confounders, the mortality risk was 1.62-fold (95% confidence interval [CI], 1.54–1.70) higher in TB survivors than those in the matched controls. In addition, the hazard of mortality in TB survivors relative to matched controls significantly increased in participants aged ≥30 years, with the highest risk in those in their 40s. Male sex (adjusted hazard ratio [HR]: 2.31; 95% CI, 2.16–2.47), smoking pack-years (HR: 1.005; 95% CI, 1.004–1.006), heavy alcohol consumption (HR: 1.12; 95% CI, 1.01–1.23), and lowest income (HR: 1.27; 95% CI, 1.18–1.37) were positively associated with increased hazards for mortality, whereas higher BMI (HR: 0.91; 95% CI, .90–.92) and regular exercise (HR: 0.82; 95% CI, .76–.88) reduced the hazards of long-term mortality in TB survivors.
Conclusions
The long-term mortality risk was significantly higher in TB survivors than those in the matched controls, even after adjusting for potential confounders.
Keywords: tuberculosis, survivors, mortality, epidemiology
The long-term mortality risk in this study was significantly higher in tuberculosis survivors than those in the matched controls, even after adjusting for potential confounders, including behavior habits, income level, body mass index, and comorbidities.
The global disease burden of tuberculosis (TB) is substantial, with an estimated 1.5 million deaths caused by TB in 2020 [1], and the impact of TB-related mortality on economic and health losses is expected to be increasing [2]. Whlie the World Health Organization mortality data estimate the number of people with TB who die before starting or during TB treatment irrespective of the cause of death in the given year, recent studies have shown that patients with TB also have higher long-term posttreatment mortality compared with the general population or age- and sex-matched group [3–9].
Potential confounders associated with survival should be accounted for when evaluating the risk of long-term mortality in TB survivors. Previous studies used standardized mortality ratio to account for confoundings [3, 4]; however, other factors such as unhealthy health behaviors (smoking habits, heavy alcohol consumption, or sedentary lifestyle), poor comorbid health status (poor nutritional status or comorbidities), and lower socioeconomic status [10–20] are worth being considered. Furthermore, we also need to consider comorbidities meticulously when evaluating long-term TB mortality. For example, chronic obstructive pulmonary disease (COPD) is more common in TB survivors than those in the general population and is associated with increased mortality [21, 22].
Hence, this study aimed to evaluate the long-term mortality of TB survivors using a population-based cohort with information on potential confounders, such as nutritional status, lifestyle habits, and comorbidities. Fortunately, South Korea has an advantage in evaluating this issue because the prevalence of TB is relatively high despite its high income [23], and it has a nationwide health screening database that contains information on these potential confounders [24].
METHODS
Data Source and Study Setting
The National Health Insurance Service (NHIS) is a mandatory universal public health insurance system that covers approximately 97% of the Korean population and provides medical aid to 3% of the lowest income bracket. The NHIS database contains a comprehensive health database consisting of the following: (1) a qualification database containing information on age, sex, income, region, and eligibility type; (2) a claim database providing general information on specifications and statements on diagnosis defined by the International Classification of Disease 10th revision (ICD-10), consultation, and prescription; (3) a health screening database; and (4) death information [25, 26].
The health screening database includes data from annual or biennial health screening examination programs for all adults provided free of charge by the Ministry of Health and Welfare. From 2009, the health screening examination included the following: anthropometric measurement including body mass index (BMI); questionnaires regarding smoking, alcohol consumption, and physical activity; blood tests such as lipid levels; and chest radiographs. The current participation rate in health screening examinations is 70%–80%, making it representative of the population. In addition, the Korean government provides anonymized health examination data to researchers for research purposes [27].
Study Population
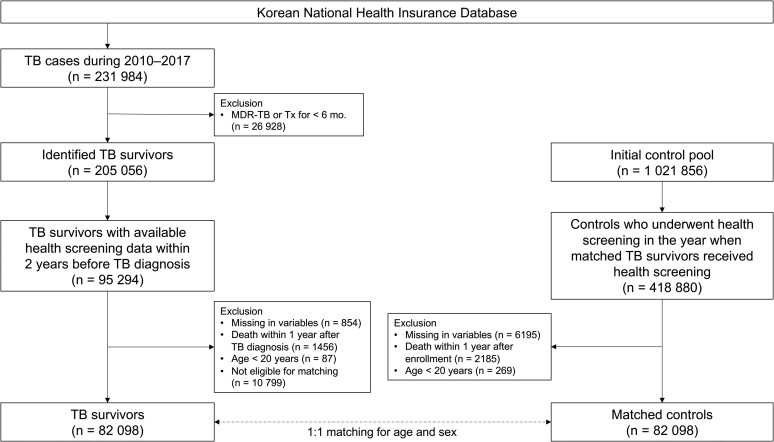
To establish TB survivors, among 231 984 patients diagnosed and treated for TB for the first time between 2010 and 2017, we excluded patients with multidrug-resistant TB or those who were treated for less than 6 months (n = 26 928) [28]. Of the 205 056 identified TB survivors, this study initially included 95 294 TB survivors who underwent health screening within 2 years before the date of TB diagnosis. After excluding 854 individuals with missing information, 1456 who died within 1 year after TB diagnosis, and 87 aged < 20 years, 92 897 TB survivors remained. The missing information indicates missing 1 of the variables, including age, sex, BMI, smoking status, alcohol consumption, physical activity, income, residential area, systolic and diastolic blood pressure, fasting blood glucose, and total cholesterol. Of the 92 897 TB survivors, 82 098 were eligible for 1:1 age and sex matching (Figure 1).
Figure 1.
Flow chart of the study population. Abbreviations: MDR, multidrug resistant; TB, tuberculosis; Tx, treatment.
To establish a control group, we initially evaluated the initial control pool (1 021 856 individuals who were approximately 1:5 matched to 205 056 TB survivors based on age and sex), which was provided by the NHIS for research purposes. Of the 1 021 856 individuals, 418 880 underwent health screening in the same year when matched TB survivors received health screening. After excluding 6195 individuals with missing information on health screening data, 2185 who died within 1 year after enrollment and 269 aged < 20 years, 410 231 controls remained. Of the 410 231 controls, 82 098 were eligible for 1:1 age and sex matching for TB survivors. The ages of study participants at the time of health screening were calculated by subtracting their birth date from the health screening date, and then the ages were changed to integers. Thereafter, the ages were exactly matched between TB survivors and controls (Figure 1).
This study was approved by the institutional review board of Hallym University Kangnam Sacred Heart Hospital (application no. 2021-12-011). The review board waived the requirement for written informed consent because the data were public and anonymized under confidentiality guidelines.
Main Exposure
The primary exposure was TB, and the inclusion criteria were defined as follows: (1) patients with at least 2 healthcare uses, including outpatient department visits, emergency department visits, or hospitalizations, with ICD-10 codes for TB (A15-19) and the specific NHIS codes for TB (V206, V246, and V000) and (2) patients who were prescribed 2 or more of the following anti-TB drugs for at least 90 days: rifampin, isoniazid, ethambutol, pyrazinamide, cycloserine, and para-aminosalicylate [29]. In Korea, to reduce the burden of TB on the nation, the specific NHIS codes for active TB are automatically applied to patients after the diagnosis of active TB, independent of the ICD-10 codes. Furthermore, additional insurance is covered for all patients with active TB (the copayment by patients is 0%–10% of total costs, whereas it is 20%–30% for other common diseases). Hence, the validity of the specific NHIS codes for active TB is strictly reviewed by the health insurance review and assessment service [24]. Additionally, TB survivors were defined as those who survived at least 1 year after the date of TB diagnosis.
TB was classified into 4 groups according to the type of TB using the ICD-10 codes: (1) pulmonary TB (A15 or A16 without any of A17, A18, and A19); (2) extrapulmonary TB (A17 or A18 without any of A15 and A16); (3) pulmonary and extrapulmonary TB (presence of A15 or A16 plus either A17 or A18); and (4) miliary or disseminated TB (A19 regardless of the presence of A15–A18).
Study Outcomes
The primary endpoint was to compare the long-term mortality risk in TB survivors versus matched controls, defined as death at least 1 year after TB diagnosis. We also aimed to identify the risk factors associated with long-term mortality in TB survivors.
Covariates
To determine the socioeconomic status, we used residence (metropolitan vs. other areas) and income level of five quintiles. Smoking status was classified as never, ex-, or current smoker. In ex- and current smokers, the cumulative smoking exposure was also evaluated as pack-years by multiplying the average cigarette consumption per day (packs) and the smoking period (years) [30]. Alcohol consumption was classified as never, mild to moderate (< 30 g of alcohol/d), and heavy drinkers (≥ 30 g of alcohol/d). Regular exercise was defined as ≥ 30 minutes of moderate physical activity ≥ 5 times per week, or ≥ 20 minutes of vigorous physical activity ≥ 3 times per week [31]. BMI was calculated as weight in kilograms divided by square of height in meters and categorized into 4 groups as recommended for Asians: (1) < 18.5 kg/m2 (underweight); (2) 18.5–22.9 kg/m2 (normal); (3) 23.0–24.9 kg/m2 (overweight); and (4) ≥ 25.0 kg/m2 (obesity) [32]. The overall comorbidity load was evaluated using the Charlson Comorbidity Index (CCI) [33]. The presence of each comorbidity was also assessed, which included hypertension, diabetes mellitus, dyslipidemia, asthma, COPD, bronchiectasis, ischemic heart disease, myocardial infarction, congestive heart failure, stroke, dementia, solid cancer, hematologic malignancy, and chronic kidney disease/end-stage renal disease.
Statistical Analysis
The baseline characteristics of TB survivors were compared with matched controls using a 2-tailed Student t test for continuous variables and a χ2 test for categorical variables. The incidence rate of death was calculated by dividing the number of incident cases by the total follow-up duration (1000 person-years). A cumulative incidence plot was used to compare the incidence of death between TB survivors and matched controls, and a log-rank test was used to evaluate significant differences between the 2 groups. Cox proportional hazards models were used to evaluate the hazard of long-term mortality in TB survivors and to explore the related factors. When analyzing the hazard of mortality relative to matched controls, demographic characteristics (age, sex, and BMI), life habits potentially affecting mortality (smoking pack-years, alcohol consumption, and regular exercise), and socioeconomic status (income level and residential area) were adjusted in model 1; CCI plus variables included in model 1 were adjusted in model 2; and all comorbidities instead of CCI plus variables included in model 1 were adjusted in model 3. When analyzing risk factors for long-term mortality in TB survivors, the previously mentioned models 2 and 3 were used for multivariable analyses.
To validate the multivariable Cox regression model for mortality, sensitivity analysis using propensity score matching was also performed. The propensity score matching included 85 635 TB survivors and 85 635 controls, matched based on demographic characteristics, life habits, socioeconomic status, and comorbidity profiles (Supplementary Table 1). Standardized mean difference > 0.1 was considered a sign of imbalance for propensity matching. In addition, we performed a sensitivity analysis using Cox proportional hazards models for prematched individuals (92 897 TB survivors and 410 231 controls) (Supplementary Table 2). In the 2 sensitivity analyses, the multivariable models adjusted the same variables used in Cox proportional hazards models for TB survivors and 1:1 age- and sex-matched controls. Statistical analyses were performed using Statistical Analysis System (SAS) version 9.4 (SAS Institute Inc., Cary, NC, USA), and P values < .05 were considered statistically significant.
RESULTS
Baseline Characteristics
The baseline characteristics of TB survivors and matched controls are summarized in Table 1. The mean age at TB diagnosis was 56.1 years, 56.9% were men, and the mean BMI was lower in the TB survivors than those in the matched controls. The proportions of current smokers (26.5% vs. 20.8%), heavy drinkers (9.3% vs. 6.7%), and those who did not exercise regularly (82.8% vs. 79.3%) were higher in TB survivors than those in the matched controls. The proportions of the lowest income quintile (18.6% vs. 17.4%) and metropolitan residents (44.4% vs. 42.9%) were also higher in the TB survivors than those in the matched controls. All comorbidities except hypertension were more frequent in TB survivors than those in the matched controls. Additionally, TB survivors showed higher CCI than those in the matched controls (mean ± standard deviation [SD], 2.3 ± 2.0 vs. 1.3 ± 1.6).
Table 1.
Baseline Characteristics of the Study Participants
| Total (N = 164 196) | Matched Controls (n = 82 098) | TB Survivors (n = 82 098) | |
|---|---|---|---|
| Age, y | 56.1 ± 15.4 | 56.1 ± 15.4 | 56.1 ± 15.4 |
| ȃ20–29 | 8656 (5.3) | 4328 (5.3) | 4328 (5.3) |
| ȃ30–39 | 18 178 (11.1) | 9089 (11.1) | 9089 (11.1) |
| ȃ40–49 | 29 274 (17.8) | 14 637 (17.8) | 14 637 (17.8) |
| ȃ50–59 | 36 260 (22.1) | 18 130 (22.1) | 18 130 (22.1) |
| ȃ60–69 | 32 428 (19.8) | 16 214 (19.8) | 16 214 (19.8) |
| ȃ70–79 | 32 192 (19.6) | 16 096 (19.6) | 16 096 (19.6) |
| ȃ≥ 80 | 7208 (4.4) | 3604 (4.4) | 3604 (4.4) |
| Sex | |||
| ȃMale | 93 510 (56.9) | 46 755 (56.9) | 46 755 (56.9) |
| ȃFemale | 70 686 (43.1) | 35 343 (43.1) | 35 343 (43.1) |
| BMI, kg/m2 | 23.0 ± 3.3 | 23.9 ± 3.2 | 22.2 ± 3.1 |
| ȃ<18.5 | 11 063 (6.7) | 2820 (3.4) | 8243 (10.0) |
| ȃ18–22.9 | 73 115 (44.5) | 30 091 (36.7) | 43 024 (52.4) |
| ȃ23–24.9 | 37 205 (22.7) | 20 825 (25.4) | 16 380 (20.0) |
| ȃ≥25 | 42 813 (26.1) | 28 362 (34.5) | 14 451 (17.6) |
| Smoking status | |||
| ȃNever smoker | 96 576 (58.8) | 49 570 (60.4) | 47 006 (57.3) |
| ȃEx-smoker | 28 701 (17.5) | 15 417 (18.8) | 13 284 (16.2) |
| ȃCurrent smoker | 38 919 (23.7) | 17 111 (20.8) | 21 808 (26.5) |
| Smoking pack-years | 8.0 ± 14.3 | 7.2 ± 13.1 | 8.9 ± 15.3 |
| Alcohol consumption | |||
| ȃNone | 92 907 (56.6) | 45 969 (55.9) | 46 938 (57.2) |
| ȃMild | 58 157 (35.4) | 30 667 (37.4) | 27 490 (33.5) |
| ȃHeavy | 13 132 (8.0) | 5462 (6.7) | 7670 (9.3) |
| Regular exercise | |||
| ȃNo | 133 129 (81.1) | 65 115 (79.3) | 68 014 (82.8) |
| ȃYes | 31 067 (18.9) | 16 983 (20.7) | 14 084 (17.2) |
| Income | |||
| ȃQuintile 1 (lowest) | 29 485 (18.0) | 14 253 (17.4) | 15 232 (18.6) |
| ȃQuintile 2 | 24 242 (14.8) | 11 313 (13.8) | 12 929 (15.8) |
| ȃQuintile 3 | 28 951 (17.6) | 13 733 (16.7) | 15 218 (18.5) |
| ȃQuintile 4 | 35 808 (21.8) | 17 867 (21.8) | 17 941 (21.9) |
| ȃQuintile 5 | 45 710 (27.8) | 24 932 (30.3) | 20 778 (25.2) |
| Residential area | |||
| ȃMetropolitan | 71 648 (43.6) | 35 204 (42.9) | 36 444 (44.4) |
| ȃOther area | 92 548 (56.4) | 46 894 (57.1) | 45 654 (55.6) |
| Comorbidities | |||
| ȃDiabetes mellitus | 30 568 (18.6) | 12 841 (15.6) | 17 727 (21.6) |
| ȃHypertension | 52 432 (31.9) | 26 875 (32.7) | 25 557 (31.1) |
| ȃDyslipidemia | 48 138 (29.3) | 22 590 (27.5) | 25 548 (31.1) |
| Respiratory disease | |||
| ȃAsthma | 28 236 (17.2) | 8563 (10.4) | 19 673 (24.0) |
| ȃCOPD | 25 468 (15.5) | 6077 (7.4) | 19 391 (23.6) |
| ȃBronchiectasis | 5133 (3.1) | 456 (0.6) | 4677 (5.7) |
| Cardiovascular disease | |||
| ȃIschemic heart disease | 13 154 (8.0) | 5945 (7.2) | 7209 (8.8) |
| ȃMyocardial infarction | 1054 (0.6) | 413 (0.5) | 641 (0.8) |
| ȃCongestive heart failure | 3994 (2.4) | 1421 (1.7) | 2573 (3.1) |
| ȃStroke | 1260 (0.8) | 456 (0.6) | 804 (1.0) |
| ȃCKD or ESRD | 11 878 (7.2) | 5678 (6.9) | 6200 (7.6) |
| Malignancy | |||
| ȃSold cancer | 6962 (4.2) | 2437 (3.0) | 4525 (5.5) |
| ȃHematologic malignancy | 237 (0.1) | 56 (0.1) | 181 (0.2) |
| Dementia | 2111 (1.3) | 996 (1.2) | 1115 (1.4) |
| Charlson Comorbidity Index | 1.8 ± 1.9 | 1.3 ± 1.6 | 2.3 ± 2.0 |
| ȃ0 | 51 384 (31.3) | 34 964 (42.6) | 16 420 (20.0) |
| ȃ1 | 37 292 (22.7) | 18 797 (22.9) | 18 495 (22.5) |
| ȃ2 | 28 048 (17.1) | 12 292 (15.0) | 15 756 (19.2) |
| ȃ≥3 | 47 472 (28.9) | 16 045 (19.5) | 31 427 (38.3) |
Data are presented as number (%) and mean ± standard deviation.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; ESRD, end-stage renal disease; TB, tuberculosis.
Incidence Rate and Risk of Mortality in TB Survivors Versus Matched Controls
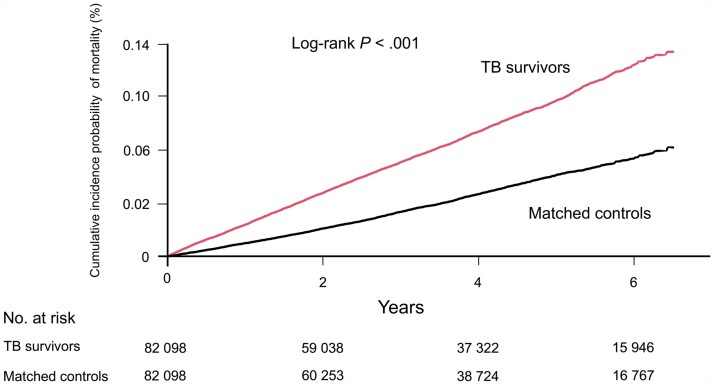
During a median 3.7 (interquartile range, 1.8–5.6) years of follow-up duration, the incidence rate of long-term mortality was significantly higher in the TB survivors than those in the matched controls (18.2 vs. 8.8 per 1000 person-years, P < .001) (Table 2). In line with this result, there was a significant difference in cumulative incidence probability of mortality between TB survivors and matched controls (log-rank P < .001) (Figure 2). The risk of mortality was 1.62-fold (95% confidence interval [CI], 1.54–1.70) higher in the TB survivors than those in the matched controls. Furthermore, the risk of mortality in the TB survivors relative to matched controls significantly increased in those aged ≥ 30 years, with the highest risk in those in their 40 s (adjusted hazard ratio [HR]: 2.80; 95% CI, 2.09–3.76) (Table 2). Additionally, the propensity score-matching analysis also depicted a higher mortality risk in TB survivors than those in the matched controls (adjusted HR: 1.40; 95% CI, 1.34–1.46) (Supplementary Tables 1 and 2).
Table 2.
Incidence Rate and Risk of Mortality in TB Survivors Versus Matched Controls
| No. at Risk | No. of Deaths | Follow-up Duration (PY) | IR (/1000 PY) | Hazard Ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||||
| Overall | Matched controls | 82 098 | 2738 | 312 552 | 8.8 | Reference | Reference | Reference | Reference | |
| TB survivors | 82 098 | 5549 | 304 971 | 18.2 | 2.08 (1.99–2.18) | 1.86 (1.78–1.95) | 1.55 (1.47–1.62) | 1.62 (1.54–1.70) | ||
| Age group, y | 20–29 | Control | 4328 | 7 | 19 160 | 0.4 | Reference | Reference | Reference | Reference |
| TB | 4328 | 9 | 19 155 | 0.5 | 1.29 (.48–3.46) | 1.17 (.44–3.14) | 1.05 (.39–2.83) | 1.11 (.41–2.98) | ||
| 30–39 | Control | 9089 | 23 | 38 043 | 0.6 | Reference | Reference | Reference | Reference | |
| TB | 9089 | 48 | 37 960 | 1.3 | 2.09 (1.27–3.44) | 1.82 (1.11–2.99) | 1.62 (.98–2.66) | 1.71 (1.04–2.80) | ||
| 40–49 | Control | 14 637 | 57 | 57 904 | 1.0 | Reference | Reference | Reference | Reference | |
| TB | 14 637 | 212 | 57 463 | 3.7 | 3.75 (2.80–5.03) | 3.13 (2.34–4.20) | 2.64 (1.97–3.54) | 2.80 (2.09–3.76) | ||
| 50–59 | Control | 18 130 | 198 | 69 221 | 2.9 | Reference | Reference | Reference | Reference | |
| TB | 18 130 | 669 | 68 047 | 9.8 | 3.44 (2.94–4.03) | 2.83 (2.41–3.32) | 2.31 (1.97–2.71) | 2.44 (2.08–2.86) | ||
| 60–69 | Control | 16 214 | 464 | 60 593 | 7.7 | Reference | Reference | Reference | Reference | |
| TB | 16 214 | 1272 | 58 375 | 21.8 | 2.86 (2.57–3.18) | 2.47 (2.22–2.75) | 2.01 (1.81–2.24) | 2.10 (1.88–2.34) | ||
| 70–79 | Control | 16 096 | 1400 | 57 645 | 24.3 | Reference | Reference | Reference | Reference | |
| TB | 16 096 | 2511 | 54 605 | 46.0 | 1.91 (1.76–2.03) | 1.68 (1.58–1.80) | 1.40 (1.31–1.50) | 1.45 (1.35–1.55) | ||
| ≥ 80 | Control | 3604 | 589 | 9986 | 59.0 | Reference | Reference | Reference | Reference | |
| TB | 3604 | 828 | 9366 | 88.4 | 1.51 (1.36–1.68) | 1.38 (1.24–1.53) | 1.16 (1.05–1.29) | 1.22 (1.10–1.36) | ||
| Sex | Male | Control | 46 755 | 1823 | 175 380 | 10.4 | Reference | Reference | Reference | Reference |
| TB | 46 755 | 3979 | 169 469 | 23.5 | 2.26 (2.14–2.39) | 2.01 (1.89–2.12) | 1.66 (1.57–1.76) | 1.73 (1.63–1.84) | ||
| Female | Control | 35 343 | 915 | 137 172 | 6.7 | Reference | Reference | Reference | Reference | |
| TB | 35 343 | 1570 | 135 502 | 11.6 | 1.74 (1.60–1.89) | 1.56 (1.46–1.72) | 1.33 (1.22–1.44) | 1.40 (1.28–1.52) | ||
Data are presented as numbers or ratios with 95% CI, as appropriate. Model 1 was adjusted for age, sex, body mass index (continuous variable), smoking pack-years (continuous variable), alcohol consumption, regular exercise, income level, and residential area. Model 2 was adjusted for the Charlson Comorbidity Index (continuous variable) in addition to the variables included in model 1. Model 3 was adjusted for comorbidities (diabetes mellitus, hypertension, dyslipidemia, asthma, chronic obstructive pulmonary disease, bronchiectasis, ischemic heart disease, myocardial infarction, congestive heart failure, stroke, dementia, solid cancer, hematologic malignancy, and chronic kidney disease/end-stage renal disease), in addition to the variables included in model 1.
Abbreviations: CI, confidence interval; IR, incidence rate; PY, person-years; TB, tuberculosis.
Figure 2.
Cumulative incidence probability (%) of mortality in tuberculosis (TB) survivors versus matched controls. Year 0 indicates 1 year after TB diagnosis in TB survivors and 1 year after the time of being matched in matched controls, respectively.
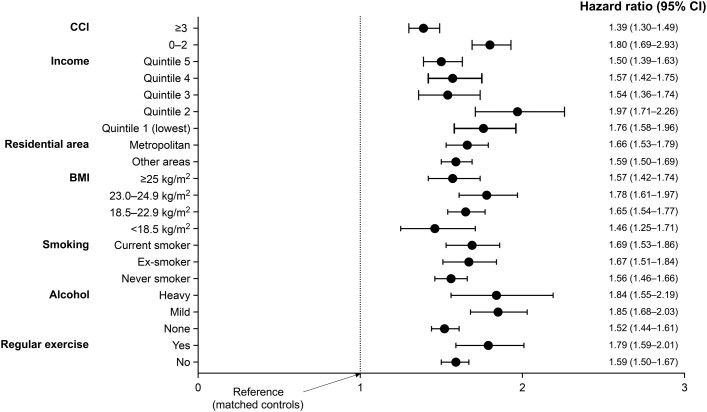
Regardless of sex, BMI group, smoking status, alcohol consumption, regular exercise, residential area, income level, and CCI category, the hazard of mortality was significantly higher in the TB survivors than those in the matched controls (Table 2 and Figure 3).
Figure 3.
Increased mortality in TB survivors versus matched controls, stratified analysis according to CCI, income, residential area, BMI, smoking status, alcohol consumption, and regular exercise. Cox proportional hazard model was adjusted for age, sex, body mass index, smoking status, alcohol consumption, regular exercise, income, and comorbidities (diabetes mellitus, hypertension, dyslipidemia, asthma, chronic obstructive pulmonary disease, bronchiectasis, ischemic heart disease, myocardial infarction, congestive heart failure, stroke, dementia, solid cancer, hematologic malignancy, and chronic kidney disease/end-stage renal disease). Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; TB, tuberculosis.
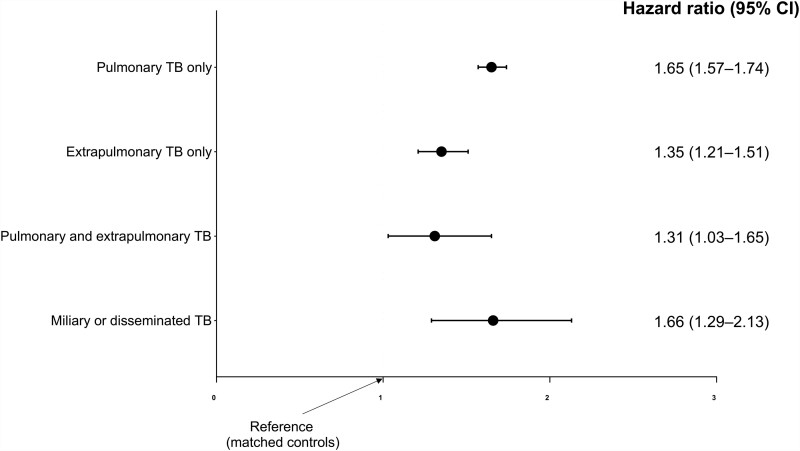
Type of TB and Hazard of Mortality
The relationship between the type of TB and hazard of long-term mortality in TB survivors versus the matched controls are summarized in Figure 4. The hazard of long-term mortality in the TB survivors relative to the matched controls was the highest in individuals who had miliary or disseminated TB (adjusted HR: 1.66; 95% CI, 1.29–2.13), followed by those with pulmonary TB only (adjusted HR: 1.65; 95% CI, 1.57–1.74), those with extrapulmonary TB only (adjusted HR: 1.35; 95% CI, 1.21–1.51), and those with pulmonary and extrapulmonary TB (adjusted HR: 1.31; 95% CI, 1.03–1.65).
Figure 4.
The impact of each type of TB on long-term TB mortality compared with matched controls are presented as hazard ratios and 95% CI. Cox proportional hazard model was adjusted for age, sex, body mass index, smoking status, alcohol consumption, regular exercise, income, and comorbidities (diabetes mellitus, hypertension, dyslipidemia, asthma, chronic obstructive pulmonary disease, bronchiectasis, ischemic heart disease, myocardial infarction, congestive heart failure, stroke, dementia, solid cancer, hematologic malignancy, and chronic kidney disease/end stage renal disease). Abbreviations: CI, confidence interval; TB, tuberculosis.
Risk Factors Associated With Long-term Mortality Among TB Survivors
Table 3 summarizes the risk factors for mortality in TB survivors. Male sex (adjusted HR in model 3: 2.31; 95% CI, 2.16–2.47), smoking pack-years (adjusted HR in model 3: 1.005; 95% CI, 1.004–1.006), heavy alcohol consumption (adjusted HR in model 3: 1.12; 95% CI, 1.01–1.23), lowest income (adjusted HR in model 3: 1.27; 95% CI, 1.18–1.37), non-metropolitan residence (adjusted HR: 1.09 in model 3; 95% CI, 1.03–1.16), and higher CCI (adjusted HR in model 2: 1.15; 95% CI, 1.14–1.17) were associated with an increased hazard of long-term mortality. On the other hand, higher BMI and regular exercise were associated with decreased hazard of mortality. Similar risk factors for mortality were identified among matched controls (Supplementary Table 3).
Table 3.
Risk Factors for Long-term Mortality in TB Survivors
| No. at Risk (N= 82 098) | No. of Deaths | Follow-up Duration (PY) | IR (/1000 PY) | Hazard Ratio (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | ||||||
| Type of TB | Extrapulmonary TB | 11 054 | 359 | 42 722 | 8.4 | Reference | Reference | Reference |
| Pulmonary TB | 68 801 | 5054 | 252 764 | 20.0 | 2.38 (2.14–2.65) | 1.30 (1.17–1.45) | 1.24 (1.11–1.38) | |
| Pulmonary and extrapulmonary TB | 1561 | 73 | 6934 | 10.5 | 1.25 (.97–1.61) | 1.04 (.81–1.33) | 0.99 (.77–1.27) | |
| Miliary or disseminated TB | 682 | 63 | 2551 | 24.7 | 2.95 (2.26–3.86) | 1.33 (1.01–1.73) | 1.27 (.97–1.67) | |
| Sex | Female | 35 343 | 1570 | 135 502 | 11.6 | Reference | Reference | Reference |
| Male | 46 755 | 3979 | 169 469 | 23.5 | 2.03 (1.91–2.15) | 2.33 (2.18–2.50) | 2.31 (2.16–2.47) | |
| Age group, y | 20–29 | 4328 | 9 | 19 155 | 0.5 | 0.13 (.07–.25) | 0.59 (.30–1.19) | 0.51 (.26–1.02) |
| 30–39 | 9089 | 48 | 37 960 | 1.3 | 0.34 (.25–.47) | 0.79 (.57–1.10) | 0.73 (.52–1.01) | |
| 40–49 | 14 637 | 212 | 57 463 | 3.7 | Reference | Reference | Reference | |
| 50–59 | 18 130 | 669 | 68 047 | 9.8 | 2.67 (2.29–3.12) | 1.07 (.89–1.28) | 1.13 (.94–1.36) | |
| 60–69 | 16 214 | 1272 | 58 375 | 21.8 | 5.93 (5.13–6.86) | 1.01 (.80–1.29) | 1.09 (.86–1.39) | |
| 70–79 | 16 096 | 2511 | 54 605 | 46.0 | 12.63 (10.98–14.54) | 1.09 (.80–1.50) | 1.19 (.86–1.63) | |
| ≥ 80 | 3604 | 828 | 9366 | 88.4 | 24.83 (21.35–28.88) | 1.21(.82–1.79) | 1.30 (.88–1.92) | |
| Body mass index, kg/m2 | 82 098 | … | … | … | 0.94 (.93–.943) | 0.91 (.906–.92) | 0.91 (.90–.92) | |
| Smoking pack-years | 82 098 | … | … | … | 1.017 (1.016–1.019) | 1.005 (1.004–1.007) | 1.005 (1.004–1.006) | |
| Alcohol consumption | None | 46 938 | 3722 | 173 933 | 21.4 | Reference | Reference | Reference |
| Mild | 27 490 | 1262 | 102 871 | 12.3 | 0.57 (.54–.61) | 0.87 (.81–.93) | 0.88 (.83–.95) | |
| Heavy | 7670 | 565 | 28 167 | 20.1 | 0.94 (.86–1.03) | 1.10 (.997–1.21) | 1.12 (1.01–1.23) | |
| Regular exercise | No | 68 014 | 4708 | 254 294 | 18.5 | Reference | Reference | Reference |
| Yes | 14 084 | 841 | 50 677 | 16.6 | 0.90 (.83–.97) | 0.82 (.76–.89) | 0.82 (.76–.88) | |
| Income level | Quintile 1 (lowest) | 15 232 | 1142 | 55 508 | 20.6 | 0.94 (.87–1.01) | 1.27 (1.17–1.37) | 1.27 (1.18–1.37) |
| Quintile 2 | 12 929 | 762 | 48 643 | 15.7 | 0.71 (.65–.78) | 1.24 (1.14–1.35) | 1.24 (1.14–1.36) | |
| Quintile 3 | 15 218 | 854 | 57 332 | 14.9 | 0.68 (.62–.74) | 1.12 (1.03–1.21) | 1.13 (1.04–1.23) | |
| Quintile 4 | 17 941 | 1123 | 67 541 | 16.6 | 0.76 (.70–.82) | 1.04 (.96–1.12) | 1.04 (.96–1.12) | |
| Quintile 5 | 20 778 | 1668 | 75 946 | 22.0 | Reference | Reference | Reference | |
| Residential area | Metropolitan | 36 444 | 2011 | 137 786 | 14.6 | Reference | Reference | Reference |
| Other areas | 45 654 | 3538 | 167 185 | 21.2 | 1.45 (1.37–1.53) | 1.10 (1.04–1.16) | 1.09 (1.03–1.16) | |
| Charlson Comorbidity Index | 82 098 | … | … | … | 1.30 (1.29–1.32) | 1.15 (1.14–1.17) | … | |
Data are presented as numbers or ratios with 95% CI, as appropriate.
Model 1 was adjusted for age, sex, body mass index (continuous variable), smoking pack-years (continuous variable), alcohol consumption, regular exercise, income level, residential area, and Charlson Comorbidity Index (continuous variable). Model 2 was adjusted for age, sex, body mass index (continuous variable), smoking pack-years (continuous variable), alcohol consumption, regular exercise, income level, residential area, and comorbidities (diabetes mellitus, hypertension, dyslipidemia, asthma, chronic obstructive pulmonary disease, bronchiectasis, ischemic heart disease, myocardial infarction, congestive heart failure, stroke, dementia, solid cancer, hematologic malignancy, and chronic kidney disease/end-stage renal disease).
Abbreviations: CI, confidence interval; BMI, body mass index; IR, incidence rate; PY, person-years; TB, tuberculosis.
DISCUSSION
During a median follow-up duration of 3.7 years after 1 year of TB diagnosis, the mortality risk was 1.62-fold higher in TB survivors than those in the age- and sex-matched controls, even considering potential confounders. This result highlights the long-standing effect of TB on mortality despite TB treatment completion. The relative hazard of long-term mortality in TB survivors compared with matched controls was more evident in those aged ≥ 40 years.
Regarding long-term mortality in TB survivors, a previous meta-analysis showed that patients with TB had a 2.91-fold standardized mortality ratio for all-cause mortality compared with the controls [3]. This figure compares with our unadjusted HR of 2.1 (matched for only age and sex). Furthermore, the relatively higher mortality risk can be explained because 6 of the 10 countries included in the previous studies were performed in low- and middle-income countries [3]. Taking advantage of the relatively high prevalence of TB in Korea despite its high income, one of the major strengths of this study is that it provides updated information on long-term mortality in TB survivors living in a high-income country. Unexpectedly, this study indicates that the long-term mortality in TB survivors is still high, even in a high-income country. The results of this study might be helpful in developing health policies to reduce long-term TB morbidity and mortality in high-income countries with low-to-intermediate TB burden.
Our study also has the advantage of minimizing bias affecting long-term mortality by adjusting for potential confounders. In this study, the confounders included nutritional status, personal habits (smoking status, alcohol consumption, and regular exercise), socioeconomic status (income and residential area), and comorbidities. Although previous studies also considered some of the factors, these studies did not comprehensively include all those factors when analyzing long-term mortality in TB survivors [3, 4, 6, 25, 34]. As expected, our study showed that the adjustment for potential confounders reduced the estimated risk of long-term mortality (from an unadjusted HR of 2.1 to a fully adjusted HR of 1.6), which suggests that the previously reported estimates of long-term mortality risk of TB might have been overestimated. Additionally, by including patients with TB who survived 1 year after treatment completion, we minimized the direct effect of TB itself or treatment-related complications on mortality, which was not considered in some previous studies [34].
There are potential mechanisms that can explain the higher mortality in TB survivors compared with matched controls. First, higher rates of cardiovascular mortality in TB survivors than those of their counterparts may explain the higher mortality rate. Monocyte/macrophages, lymphocytes, and cytokines involved in cell-mediated immune responses against Mycobacterium tuberculosis are the main drivers of atherogenesis [35]. In the same vein, a previous epidemiologic study revealed that patients with pulmonary or extrapulmonary TB had an increased subsequent rate of acute myocardial infarction [36]. Furthermore, the risk remained elevated after several years from the initial diagnosis of TB [36]. Second, higher rates of respiratory diseases such as COPD among TB survivors than those in the matched controls may explain the higher mortality. In agreement with our findings, a history of pulmonary TB has been identified as a significant comorbid condition of COPD [37, 38], which is associated with a higher risk for all-cause mortality [21]. Considering up to 39% of patients with COPD were never smokers [39], the prevalent COPD among TB survivors may hamper long-term survival regardless of smoking status.
Our study showed that lower BMI, which might indicate poor nutritional status, was associated with higher long-term mortality in TB survivors. Low BMI has a substantial impact on TB development [24, 40], and weight loss during TB treatment is associated with increased mortality [41]. In addition, TB survivors are known to have lower BMI than those without TB [42]. However, the long-term effect of low BMI on mortality in TB survivors is not well known, and our study provided informative data showing the persistent long-term effect of low BMI on mortality in TB survivors. In this regard, timely interventions to improve the nutritional status of TB survivors using various strategies may be essential [43].
There are limitations to this study that should be acknowledged. First, 10 799 TB survivors were not matched to controls. Because clinical differences exist between unmatched and matched TB survivors (Supplementary Table 4), there might be a selection bias. However, despite some differences in clinical characteristics, 2 sensitivity analyses by propensity score matching and Cox proportional hazards models for prematched individuals (all 92 897 TB survivors and 410 231 controls) showed similar results (Supplementary Table 2). Accordingly, we carefully suggest that the effect of unmatched TB survivors did not have a significant impact on our final results. Second, this study lacked clinical information on TB severity, such as cavitation and acid-fast bacilli smear results, affecting long-term mortality among TB survivors. Third, the causes of mortality were not available for our dataset; thus, we could not explore whether the causes of mortality in TB survivors and their controls were different. Fourth, this study was conducted in Korea. The factors affecting mortality in TB survivors might be different in other countries, particularly in countries with low socioeconomic indices. Although this limits the generalization of our study results, our study results would help develop health policies to reduce long-term TB mortality in high socioeconomic countries.
In conclusion, the mortality risk was 1.62-fold higher in TB survivors than those in the age- and sex-matched controls, even considering potential confounders. This result highlights the longstanding effect of TB on mortality despite TB treatment completion. Furthermore, the hazard of long-term mortality in TB survivors was consistently higher than those in the matched controls in all stratified analyses except for age; the hazard was significant in the older age group.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. This study was performed using the National Health Insurance System database, and the results do not necessarily represent the opinion of the National Health Insurance Corporation.
Disclaimer. The funders had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Financial support. This work was supported by the National Research Foundation of Korea grant funded by the Ministry of Science, ICT and Future Planning (nos. 2020R1F1A1070468 and 2021M3E5D1A01015176 to H. L.) and the Ministry of Education (no. 2021R1I1A3052416 to H. C.).
Supplementary Material
Contributor Information
Hayoung Choi, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, Seoul, Republic of Korea.
Kyungdo Han, Department of Statistics and Actuarial Science, Soongsil University, Seoul, Republic of Korea.
Jin-Hyung Jung, Department of Biostatistics, College of Medicine, Catholic University of Korea, Seoul, Republic of Korea.
Sang Hyun Park, Department of Biostatistics, College of Medicine, Catholic University of Korea, Seoul, Republic of Korea.
Sang Hyuk Kim, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, Seoul, Republic of Korea.
Hyung Koo Kang, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Republic of Korea.
Jang Won Sohn, Division of Pulmonary Medicine and Allergy, Department of Internal Medicine, Hanyang Medical Center, Hanyang University College of Medicine, Seoul, Republic of Korea.
Dong Wook Shin, Department of Family Medicine/Supportive Care Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea; Department of Clinical Research Design & Evaluation, Samsung Advanced Institute for Health Science & Technology (SAIHST), Sungkyunkwan University, Seoul, Republic of Korea; Center for Wireless and Population Health System, University of San Deigo, San Diego, California, USA.
Hyun Lee, Division of Pulmonary Medicine and Allergy, Department of Internal Medicine, Hanyang Medical Center, Hanyang University College of Medicine, Seoul, Republic of Korea.
References
- 1. World Health Organization . Global tuberculosis report 2021. Geneva: World Health Organization, 2021. [Google Scholar]
- 2. Silva S, Arinaminpathy N, Atun R, Goosby E, Reid M. Economic impact of tuberculosis mortality in 120 countries and the cost of not achieving the Sustainable Development Goals tuberculosis targets: a full-income analysis. Lancet Glob Health 2021; 9:e1372-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romanowski K, Baumann B, Basham CA, Ahmad Khan F, Fox GJ, Johnston JC. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2019; 19:1129–37. [DOI] [PubMed] [Google Scholar]
- 4. Ranzani OT, Rodrigues LC, Bombarda Set al. Long-term survival and cause-specific mortality of patients newly diagnosed with tuberculosis in São Paulo state, Brazil, 2010–15: a population-based, longitudinal study. Lancet Infect Dis 2020; 20:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Selvaraju S, Thiruvengadam K, Watson B, et al. Long-term survival of treated tuberculosis patients in comparison to a general population in South India: a matched cohort study. Int J Infect Dis 2021; 110:385–93. [DOI] [PubMed] [Google Scholar]
- 6. Osman M, Welte A, Dunbar R, et al. Morbidity and mortality up to 5 years post tuberculosis treatment in South Africa: a pilot study. Int J Infect Dis 2019; 85:57–63. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y, Zheng Y, Chen J, et al. Tuberculosis-associated mortality and its risk factors in a district of Shanghai, China: a retrospective cohort study. Int J Tuberc Lung Dis 2018; 22:655–60. [DOI] [PubMed] [Google Scholar]
- 8. Fox GJ, Nguyen VN, Dinh NS, et al. Post-treatment mortality among patients with tuberculosis: a prospective cohort study of 10 964 patients in Vietnam. Clin Infect Dis 2019; 68:1359–66. [DOI] [PubMed] [Google Scholar]
- 9. Miller TL, Wilson FA, Pang JW, et al. Mortality hazard and survival after tuberculosis treatment. Am J Public Health 2015; 105:930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tocque K, Bellis MA, Beeching NJ, Syed Q, Remmington T, Davies PDO. A case-control study of lifestyle risk factors associated with tuberculosis in Liverpool, North-West England. Eur Respir J 2001; 18:959. [DOI] [PubMed] [Google Scholar]
- 11. Lienhardt C, Fielding K, Sillah J, et al. Investigation of the risk factors for tuberculosis: a case–control study in three countries in West Africa. Int J Epidemiol 2005; 34:914–23. [DOI] [PubMed] [Google Scholar]
- 12. Marais BJ, Lönnroth K, Lawn SD, et al. Tuberculosis comorbidity with communicable and non-communicable diseases: integrating health services and control efforts. Lancet Infect Dis 2013; 13:436–48. [DOI] [PubMed] [Google Scholar]
- 13. Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JDH. The social determinants of tuberculosis: from evidence to action. Am J Public Health 2011; 101:654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viana PVS, Paiva NS, Villela DAM, Bastos LS, de Souza Bierrenbach AL, Basta PC. Factors associated with death in patients with tuberculosis in Brazil: competing risks analysis. PLoS One 2020; 15:e0240090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tocque K, Convrey RP, Bellis MA, Beeching NJ, Davies PD. Elevated mortality following diagnosis with a treatable disease: tuberculosis. Int J Tuberc Lung Dis 2005; 9:797–802. [PubMed] [Google Scholar]
- 16. Christensen AS, Roed C, Andersen PH, Andersen AB, Obel N. Long-term mortality in patients with pulmonary and extrapulmonary tuberculosis: a Danish nationwide cohort study. Clin Epidemiol 2014; 6:405–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blöndal K, Rahu K, Altraja A, Viiklepp P, Rahu M. Overall and cause-specific mortality among patients with tuberculosis and multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2013; 17:961–8. [DOI] [PubMed] [Google Scholar]
- 18. Kolappan C, Subramani R, Kumaraswami V, Santha T, Narayanan PR. Excess mortality and risk factors for mortality among a cohort of TB patients from rural south India. Int J Tuberc Lung Dis 2008; 12:81–6. [PubMed] [Google Scholar]
- 19. Shuldiner J, Leventhal A, Chemtob D, Mor Z. Mortality after anti-tuberculosis treatment completion: results of long-term follow-up. Int J Tuberc Lung Dis 2016; 20:43–8. [DOI] [PubMed] [Google Scholar]
- 20. Wang XH, Ma AG, Han XX, et al. Survival and associated mortality risk factors among post-treatment pulmonary tuberculosis patients in the northwest of China. Eur Rev Med Pharmacol Sci 2015; 19:2016–25. [PubMed] [Google Scholar]
- 21. Park HY, Kang D, Lee H, et al. Impact of chronic obstructive pulmonary disease on mortality: a large national cohort study. Respirology 2020; 25:726–34. [DOI] [PubMed] [Google Scholar]
- 22. Menezes AM, Hallal PC, Perez-Padilla R, et al. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur Respir J 2007; 30:1180–5. [DOI] [PubMed] [Google Scholar]
- 23. Choi H. Nosocomial exposure to tuberculosis: a snapshot of South Korea. Korean J Intern Med 2021; 36:1061–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi H, Yoo JE, Han K, et al. Body mass index, diabetes, and risk of tuberculosis: a retrospective cohort study. Front Nutr 2021; 8:739766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shin DW, Cho B, Guallar E. Korean national health insurance database. JAMA Intern Med 2016; 176:138. [DOI] [PubMed] [Google Scholar]
- 26. Choi H, Han K, Yang B, Shin DW, Sohn JW, Lee H. Female reproductive factors and incidence of non-tuberculous mycobacterial pulmonary disease among postmenopausal women in Korea. Clin Infect Dis 2022; 75:1397–404. [DOI] [PubMed] [Google Scholar]
- 27. Shin DW, Cho J, Park JH, Cho B. National General Health Screening Program in Korea: history, current status, and future direction. Precision Future Med 2022; 6:9–31. [Google Scholar]
- 28. Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016; 63:e147-e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee CH, Kim K, Hyun MK, Jang EJ, Lee NR, Yim JJ. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax 2013; 68:1105–13. [DOI] [PubMed] [Google Scholar]
- 30. Yang B, Han K, Kim B, et al. Association between smoking status and incident non-cystic fibrosis bronchiectasis in young adults: a nationwide population-based study. J Per Med 2022; 12:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anton SD, Duncan GE, Limacher MC, Martin AD, Perri MG. How much walking is needed to improve cardiorespiratory fitness? An examination of the 2008 Physical Activity Guidelines for Americans. Res Q Exerc Sport 2011; 82:365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies . Lancet 2004; 363:157–63. [DOI] [PubMed] [Google Scholar]
- 33. Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract 2010; 11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park SC, Kang MJ, Han CH, et al. Long-term mortality of patients with tuberculosis in Korea. Int J Tuberc Lung Dis 2020; 24:492–8. [DOI] [PubMed] [Google Scholar]
- 35. Huaman MA, Henson D, Ticona E, Sterling TR, Garvy BA. Tuberculosis and cardiovascular disease: linking the epidemics. Trop Dis Travel Med Vaccines 2015; 1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chung WS, Lin CL, Hung CT, et al. Tuberculosis increases the subsequent risk of acute coronary syndrome: a nationwide population-based cohort study. Int J Tuberc Lung Dis 2014; 18:79–83. [DOI] [PubMed] [Google Scholar]
- 37. Park HY, Kang D, Shin SH, et al. Pulmonary tuberculosis and the incidence of lung cancer among patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2021; 19:640–8. [DOI] [PubMed] [Google Scholar]
- 38. Hwang YI, Kim JH, Lee CY, et al. The association between airflow obstruction and radiologic change by tuberculosis. J Thorac Dis 2014; 6:471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou Y, Wang C, Yao W, et al. COPD in Chinese nonsmokers. Eur Respir J 2009; 33:509–18. [DOI] [PubMed] [Google Scholar]
- 40. Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 2010; 39:149–55. [DOI] [PubMed] [Google Scholar]
- 41. Bernabe-Ortiz A, Carcamo CP, Sanchez JFet al. Weight variation over time and its association with tuberculosis treatment outcome: a longitudinal analysis. PLoS One 2011; 6:e18474-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ko Y, Kim C, Park YB, Mo EK, Moon JW. Changes in nutritional status in pulmonary tuberculosis: longitudinal changes in BMI according to acid-fast bacilli smear positivity. J Clin Med 2020; 9:4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Akkerman OW, Ter Beek L, Centis R, et al. Rehabilitation, optimized nutritional care, and boosting host internal milieu to improve long-term treatment outcomes in tuberculosis patients. Int J Infect Dis 2020; 92s:S10–s4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.