Abstract
Aims
To investigate whether left bundle branch area pacing (LBBAP) can reduce the risk of new-onset atrial fibrillation (AF) compared with right ventricular pacing (RVP).
Methods and results
Patients with indications for dual-chamber pacemaker implant and no history of AF were prospectively enrolled if they underwent successful LBBAP or RVP. The primary endpoint was time to the first occurrence of AF detected by pacemaker programming or surface electrocardiogram. Follow-up at clinic visit was performed and multivariate Cox regression models were applied to evaluate the effect of LBBAP on new-onset AF. The final analysis included 527 patients (mean age 65.3 ± 12.6, male 47.3%), with 257 in the LBBAP and 270 in the RVP groups. During a mean follow-up of 11.1 months, LBBAP resulted in significantly lower incidence of new-onset AF (7.4 vs. 17.0%, P < 0.001) and AF burden (3.7 ± 1.9 vs. 9.3 ± 2.2%, P < 0.001) than RVP. After adjusting for confounding factors, LBBAP demonstrated a lower hazard ratio for new-onset AF compared with RVP {hazard ratio (HR) [95% confidence interval (CI)]: 0.278 (0.156, 0.496), P < 0.001}. A significant interaction existed between pacing modalities and the percentage of ventricular pacing (VP%) (P for interaction = 0.020). In patients with VP ≥ 20%, LBBAP was associated with decreased risk of new-onset AF compared with RVP [HR (95% CI): 0.199 (0.105, 0.378), P < 0.001]. The effect of pacing modalities was not pronounced in patients with VP < 20% [HR (95% CI): 0.751 (0.309, 1.823), P = 0.316].
Conclusion
Left bundle branch area pacing demonstrated a reduced risk of new-onset AF compared with RVP. Patients with a high ventricular pacing burden might benefit from LBBAP.
Keywords: Left bundle branch area pacing, Right ventricular pacing, New-onset atrial fibrillation
What’s new.
The incidence of new-onset atrial fibrillation (AF) in patients with conventional right ventricular pacing (RVP) is nearly 2.3-fold higher than that in those with left bundle branch area pacing (LBBAP).
LBBAP was associated with a significantly reduced risk of new-onset AF by nearly 70% compared with conventional RVP.
A significant interaction between pacing modalities and ventricular pacing burden was observed. The beneficial effect of LBBAP on reducing new-onset AF after pacemaker implantation was more pronounced in patients with a high ventricular pacing burden (VP ≥ 20%).
Introduction
In clinical practice, right ventricular pacing (RVP) is a well-established pacing strategy. However, several large randomized controlled trials had demonstrated that chronic RVP was associated with a significantly increased risk of heart failure and new-onset atrial fibrillation (AF), especially in patients with a high percentage of ventricular pacing (VP%).1,2 Pacing-induced electromechanical desynchrony is one of the leading reasons for adverse clinical outcomes.3
Physiological pacing modalities, which facilitate electrical propagation via intrinsic conduction system fibres, have been the pursuit of electrophysiologists. Currently, His bundle pacing (HBP) and left bundle branch area pacing (LBBAP) are the two most common physiological pacing strategies.4 In a retrospective single-centre cohort study that enrolled 148 patients with no history of AF, Ravi et al.5 found that HBP was associated with a significantly decreased risk of new-onset AF by 47% compared with RVP. The beneficial effect of HBP on reduced risk of AF may result from improved biventricular synchrony and atrial function.6 LBBAP was first introduced by Huang et al.7 in 2017 and had been rapidly evolving worldwide. Previous prospective cohort studies have validated the long-term efficacy and safety of LBBAP.8,9 Compared with RVP, LBBAP also had a beneficial effect of improved clinical outcomes, including all-cause mortality, heart failure hospitalization (HFH), and upgrade to biventricular pacing (BiVP).10,11 However, few studies focused on whether LBBAP can reduce the risk of new-onset AF compared with RVP. Therefore, the present study was conducted to explore the effect of LBBAP on new-onset AF after pacemaker implantation compared with RVP. We hypothesized that LBBAP is associated with a reduced risk of new-onset AF when compared with the non-physiological pacing modality of RVP in patients without a history of AF after dual-chamber pacemaker implantations.
Methods
Study design and population
This study was designed as a prospective observational cohort study and conducted at Fuwai Hospital and The Second Hospital of Hebei Medical University. All patients with bradycardia and indicated for dual-chamber pacemaker implantation per the current guideline12 were consecutively enrolled if they had no prior AF history since 2019. The pacing strategies were determined by operators according to clinical practice at each hospital. The LBBAP group included all patients with successful LBBAP procedures while the RVP group included patients undergoing RV apex or septum pacing. The exclusion criteria were as follows: (i) younger than 18 years old; (ii) prior AF history or received AF catheter or surgical ablations; (iii) indicated for cardiac resynchronization therapy or implantable cardioverter defibrillator; (iv) pacemaker replacement or upgrade with existing lead; (v) moderate to severe mitral or aortic regurgitation which may necessitate cardiac surgery within 1 year; and (vi) unable to provide the written informed consent or be regularly followed up at clinic visit. This study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of both hospitals. All patients signed written informed consent for agreement of the implantation procedure and study analysis.
Procedures
Left bundle branch area pacing
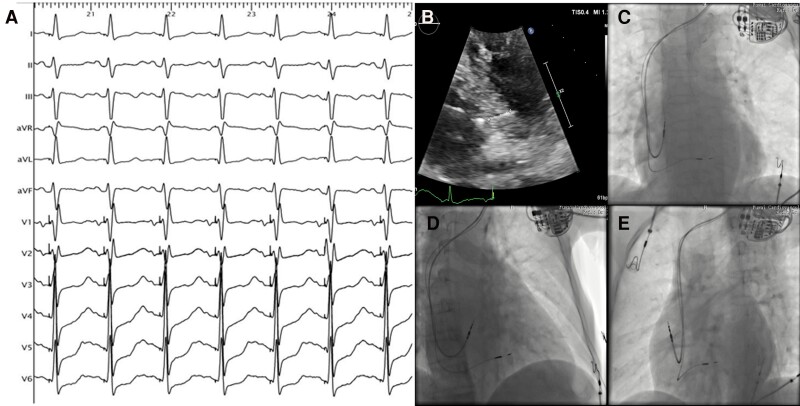
All LBBAP procedures were performed using dedicated C315 His sheath and 3830 lumen-less lead (Medtronic, Inc., Minneapolis, MN, USA) as previously described.13 Briefly, the 3830 lead was delivered through C315 His sheath in the right anterior oblique 30° fluoroscopy view. The right ventricular septal pacing at 2 V/0.4 ms was applied to identify the optimal target site, commonly 1.5–2.0 cm towards the apex from the tricuspid annulus. Then, the lead was quickly rotated clockwise into the septum until a right bundle branch block morphology of paced QRS was observed in Lead V1. LBBAP was considered to be successful when the stimulus to left ventricular activation time (Sti-LVAT) measured in Lead V5 was suddenly shortened and remained constant at high or low outputs (commonly ≤ 75 ms). Figure 1 illustrates the paced QRS complex and lead position in a patient with a successful LBBAP procedure (paced QRS duration: 120 ms; Sti-LVAT: 70 ms).
Figure 1.
Lead positioning of LBBAP. (A) Surface 12-lead ECG in a patient with successful LBBAP (paced QRS duration: 120 ms; Sti-LVAT: 70 ms); (B) location of the LBBAP lead in the ventricular septum by two-dimensional echocardiography; (C–E) Fluoroscopic imaging of LBBAP lead in different projection angles. LBBAP, left bundle branch area pacing; Sti-LVAT, stimulus to left ventricular activation time.
Right ventricular pacing
The active or passive fixation lead was inserted into the right ventricular septum or apex using a pre-shaped stylet. A fluoroscopy view of the left anterior oblique at 45° was applied to confirm the exact lead position.
Device programming
Individualized atrioventricular (AV) delay was programmed depending on the intrinsic AV interval and conduction system disease. The automatic AV search algorithm was routinely turned on in patients with sinus node dysfunction (SND) or intact AV conduction to avoid unnecessary ventricular pacing. For patients with intermittent AV block, AV delay was programmed based on intrinsic AV conduction to minimize the pacing burden. In patients with complete AV block, a default AV interval (180/150 ms quite often) was set for AV synchrony.
Study endpoints
The primary study endpoint was the time to the first occurrence of AF episodes after pacemaker implantation. New-onset AF was defined as device-detected AF episodes lasting at least 30 s on intracardiac electrogram or surface 12-lead ECG. Atrial high-frequency episodes (atrial rate ≥ 190 bpm) detected by devices were manually checked to verify the incidence of AF, which might be silent.
Data collection and follow-up
Baseline characteristics were collected, including demographics, comorbidities, prior medication history, ECG, and echocardiographic parameters. After discharge, all patients were followed up at the device clinic at 3, 6, 12 months, and annually. Pacing parameters, including capture threshold, R-wave amplitudes, and impedance, were routinely recorded. The percentage of ventricular pacing was calculated as a mean value of data from all device interrogations for each patient. If no AF episodes occurred during follow-up, the patient would be censored at the last follow-up or death; once patients suffered from clinical AF or underwent AF-related ablation procedures, the subjects were immediately censored.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median with the interquartile range depending on the data’s distribution. The means or medians are compared using Student's t-test or the Kruskal–Wallis H test. Categorical variables are expressed as frequency or percentage and compared using the χ2 or Fisher exact test. The Kaplan–Meier (KM) survival curve and log-rank test were employed to estimate cumulative event rates in all enrolled patients or subgroups stratified by VP%. Cox proportional hazards regression analysis was performed to investigate potential risk factors of postoperative new-onset AF. Baseline variables considered to be clinically relevant or that showed a univariate relationship with the outcome (P-value < 0.1) were entered into multivariate Cox regression models. The interaction between VP% and pacing modalities was also tested. A two-tailed P < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5 (Graphpad Software, Inc., San Diego, CA, USA).
Results
Baseline clinical characteristics
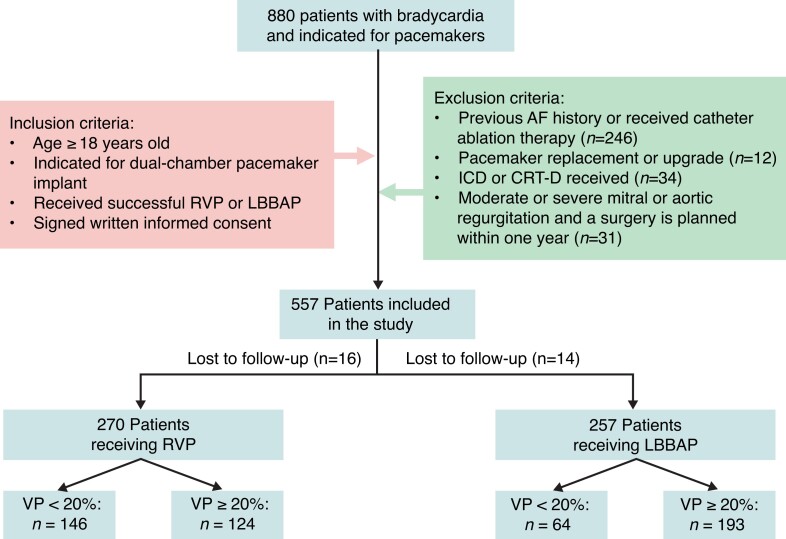
A total of 880 patients with symptomatic bradycardia and indications for permanent pacemaker implantation were continuously screened from June 2019 to November 2021. After exclusion, 557 patients were included in the final analysis (Figure 2). All patients received dual-chamber pacemakers. LBBAP was successfully achieved in 257 patients, whereas 270 patients received RVP. The mean age was 65.3 ± 12.6 years old and was significantly lower in the LBBAP group than in RVP by ∼3 years (P = 0.017). The LBBAP group demonstrated a higher prevalence of heart failure defined as left ventricular ejection fraction (LVEF) ranging from 35–50% (5.1 vs. 1.5%; P = 0.020) and lower prevalence of diabetes than RVP (15.2 vs. 22.3%; P = 0.048). Patients with wide QRS duration (left or right bundle branch block) were more common in LBBAP than the RVP group (both P < 0.001). The mean LVEF was comparable between LBBAP (62.8%) and RVP (63.1%) (P = 0.541). The LBBAP group had a higher prevalence of AV block than RVP (P < 0.001). No significant difference was observed in other clinical features between the two groups (Table 1).
Figure 2.
Flowchart of enrolled patients in the study according to inclusion and exclusion criteria. AF, atrial fibrillation; CRT-D, cardiac resynchronization therapy-defibrillator; ICD, implantable cardioverter defibrillator; LBBAP, left bundle branch area pacing; RVP, right ventricular pacing; VP%, percentage of ventricular pacing.
Table 1.
Baseline characteristics of all enrolled patients
| Variable | Overall (n = 527) | LBBAP (n = 257) | RVP (n = 270) | P-value |
|---|---|---|---|---|
| Age, years | 65.3 ± 12.6 | 63.6 ± 13.5 | 66.9 ± 11.5 | 0.017 |
| Sex (male), n (%) | 249 (47.3%) | 119 (46.3%) | 130 (48.1%) | 0.672 |
| Body mass index, kg/m2 | 24.2 ± 3.3 | 24.3 ± 3.6 | 24.0 ± 3.0 | 0.284 |
| Hypertension, n (%) | 306 (58.1%) | 142 (55.3%) | 164 (60.7%) | 0.202 |
| Diabetes mellitus, n (%) | 99 (18.8%) | 39 (15.2%) | 60 (22.3%) | 0.048 |
| Coronary artery disease, n (%) | 107 (20.3%) | 48 (18.7%) | 59 (21.9%) | 0.354 |
| Valvular heart disease, n (%) | 29 (5.5%) | 14 (5.4%) | 15 (5.6%) | 0.957 |
| Heart failure, n (%) | 17 (3.2%) | 13 (5.1%) | 4 (1.5%) | 0.020 |
| Electrocardiography | ||||
| ȃQRS duration, ms | 107.8 ± 24.5 | 111.8 ± 25.5 | 99.8 ± 20.0 | <0.001 |
| ȃLeft bundle branch block, n (%) | 37 (7.3%) | 33 (12.8%) | 4 (1.5%) | <0.001 |
| ȃRight bundle branch block, n (%) | 81 (15.4%) | 65 (25.5%) | 16 (6.5%) | <0.001 |
| Echocardiography | ||||
| ȃLeft atrial diameter, mm | 37.2 ± 5.7 | 36.9 ± 5.6 | 37.5 ± 5.9 | 0.281 |
| ȃLeft ventricular end-diastolic diameter, mm | 48.6 ± 20.7 | 47.9 ± 5.4 | 49.2 ± 8.3 | 0.473 |
| ȃLeft ventricular ejection fraction, % | 63.0 ± 5.2 | 62.8 ± 4.9 | 63.1 ± 5.4 | 0.541 |
| ACEI/ARB, n (%) | 193 (36.6%) | 83 (32.3%) | 110 (40.7%) | 0.055 |
| AAD, n (%) | 110 (20.9%) | 45 (17.5%) | 65 (24.1%) | 0.064 |
| Pacing indications | <0.001 | |||
| ȃSinus node dysfunction, n (%) | 225 (42.7%) | 64 (25.1%) | 161 (59.9%) | |
| ȃAtrioventricular block, n (%) | 299 (56.7%) | 191 (74.9%) | 108 (40.1%) | |
Data are presented as mean ± standard deviation for continuous variables and number and percentages for categorical variables.
AAD, antiarrhythmic drug; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; LBBAP, left bundle branch area pacing; RVP, right ventricular pacing.
Primary endpoints
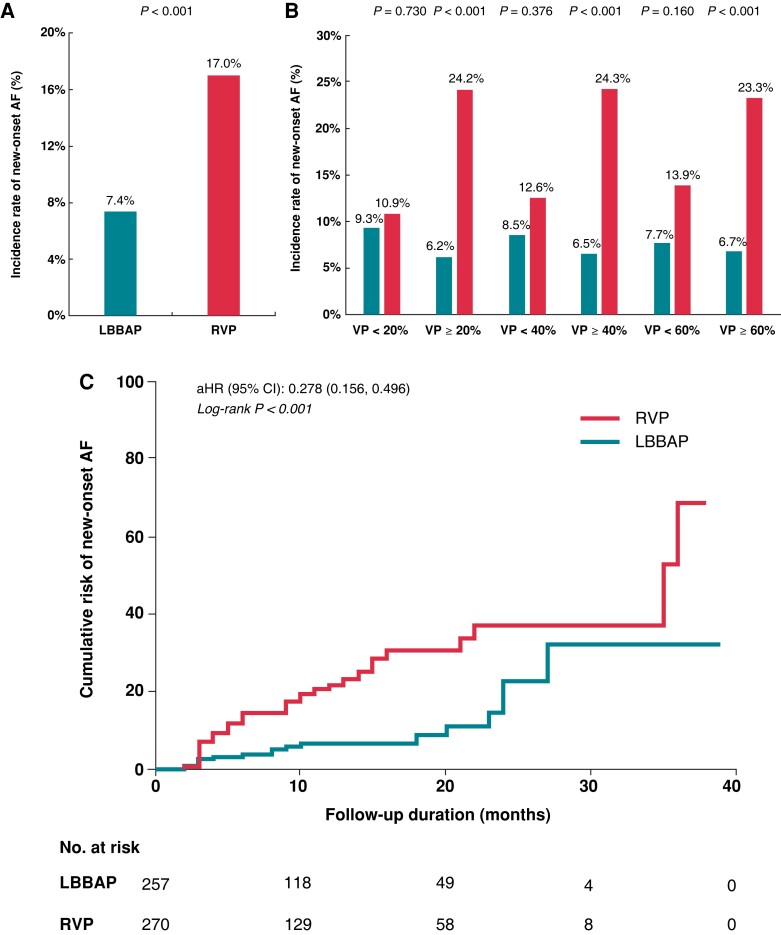
During a mean follow-up duration of 11.1 ± 7.5 months, the primary endpoint of new-onset AF occurred in 12.1% of all patients with an average AF burden of 7.7 ± 2.1%. The burden of AF was significantly lower in the LBBAP group than that in the RVP group (3.7 ± 1.9 vs. 9.3 ± 2.2%, P < 0.001). The duration of follow-up was similar in both groups, with 30 patients lost to follow-up: 10.1 ± 7.6 months for LBBAP and 12.8 ± 7.4 months for RVP (P = 0.250). The incidence rate of new-onset AF in the LBBAP group was significantly lower than RVP (7.4 vs. 17.0%, P < 0.001, Figure 3A). Figure 3B shows the incidence of new-onset AF in subgroups stratified by VP%. In patients with VP < 20%, the incidence of new-onset AF was similar between LBBAP and RVP (9.3 vs. 10.9%, P = 0.730). In patients with VP ≥ 20%, the incidence of now-onset AF was significantly lower in LBBAP than the RVP group (6.2 vs. 24.2%, P < 0.001). In patients with VP ≥ 40% or ≥ 60%, the incidence of AF remained significantly different between LBBAP and RVP (both P < 0.001).
Figure 3.
Comparison of the incidence rate and cumulative risk of new-onset AF between LBBAP and RVP. (A) The incidence rate of new-onset AF in all enrolled patients; (B) the incidence rate of new-onset AF in different subgroups stratified by VP%; (C) KM curve for cumulative risk of new-onset AF in all enrolled patients. AF, atrial fibrillation; LBBAP, left bundle branch area pacing; RVP, right ventricular pacing; VP%, percentage of ventricular pacing.
Figure 3C illustrates the KM curves for the cumulative risk of new-onset AF between two groups in all enrolled patients. LBBAP had a significantly lower cumulative risk of new-onset AF compared with RVP when adjusting other confounding factors {adjusted hazard ratio (HR) [95% confidence interval (CI)]: 0.278 (0.156, 0.496), log-rank P < 0.001}.
Risk factors of new-onset AF
Table 2 presents the univariate analysis of baseline clinical features and potential predisposing factors for the development of AF. Age was associated with a 3% increased risk of new-onset AF [HR (95% CI): 1.030 (1.008, 1.053), P = 0.008]. LBBAP was associated with a lower risk of new-onset AF by 66% compared with RVP [HR (95% CI): 0.343 (0.198, 0.593), P < 0.001]. Both coronary artery disease (CAD) and left atrial diameter (LAD) at baseline showed a trend towards increased risk of new-onset AF without statistical significance. The percentage of ventricular pacing did not show a significant association with new-onset AF in univariate analysis.
Table 2.
Univariate analysis of new-onset AF after pacemaker implantation
| Variable | Univariate analysis | |
|---|---|---|
| HR (95% CI) | P-value | |
| Age | 1.030 (1.008, 1.053) | 0.008 |
| Male vs. female | 1.364 (0.833, 2.233) | 0.218 |
| Body mass index | 0.885 (0.819, 1.056) | 0.145 |
| Hypertension | 1.081 (0.658, 1.776) | 0.759 |
| Diabetes mellitus | 1.146 (0.623, 2.108) | 0.661 |
| Coronary artery disease | 1.658 (0.950, 2.893) | 0.075 |
| Valvular heart disease | 1.956 (0.781, 4.899) | 0.152 |
| Heart failure | 0.921 (0.225, 3.772) | 0.909 |
| QRS duration | 1.003 (0.987, 1.019) | 0.717 |
| LAD | 1.033 (0.996, 1.072) | 0.080 |
| LVEDD | 0.997 (0.973, 1.023) | 0.845 |
| LVEF | 0.982 (0.937, 1.028) | 0.438 |
| ACEI/ARB | 1.137 (0.675, 1.915) | 0.629 |
| AAD | 1.596 (0.917, 2.780) | 0.198 |
| SND vs. AVB | 0.892 (0.542, 1.470) | 0.655 |
| LBBAP vs. RVP | 0.343 (0.198, 0.593) | <0.001 |
| VP% | 1.002 (0.997, 1.008) | 0.443 |
| VP ≥ 20% | 1.442 (0.857, 2.427) | 0.168 |
| VP ≥ 40% | 1.232 (0.748, 2.030) | 0.413 |
| AP% | 0.996 (0.988, 1.005) | 0.412 |
AAD, antiarrhythmic drug; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; AP%, percentage of atrial pacing; AVB, atrioventricular block; LAD, left atrial diameter; LBBAP, left bundle branch area pacing; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; RVP, right ventricular pacing; SND, sinus node dysfunction; VP%, percentage of ventricular pacing; .
Multivariate Cox regression models were applied to further explore independent risk factors of new-onset AF in Table 3. Variables with a P-value of <0.1 in univariate analysis (such as age, CAD, LAD at baseline, pacing strategies) and that were clinically relevant (such as VP%) were entered into multivariate regression models. In Model 1, after adjusting other confounding factors, LBBAP was independently associated with a lower risk of new-onset AF compared with RVP [adjusted HR (95% CI): 0.294 (0.163, 0.532), P < 0.001]. When included as a continuous variable, VP% significantly increased the risk of new-onset AF by 0.7% for per 1% increase [adjusted HR (95% CI): 1.007 (1.001, 1.013), P = 0.018]. In Models 2 and 3, VP% was included as a categorical variable in the analyses. VP ≥ 20% significantly increased the risk of new-onset AF by 106.8% compared with those with VP < 20% [adjusted HR (95% CI): 2.068 (1.195, 3.579), P = 0.009]. VP ≥ 40% was also an independent risk factor of new-onset AF in Model 3 [adjusted HR (95% CI): 1.880 (1.103, 3.205), P = 0.020]. The protective effect of LBBAP remained consistent in all three models. The risk of new-onset AF was significantly decreased by ∼73% in the LBBAP group compared with RVP [adjusted HR (95%CI): 0.272 (0.154, 0.481), P < 0.001].
Table 3.
Multivariate Cox regression analysis for risk factors of new-onset AF
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 1.017 (0.994, 1.040) | 0.154 | 1.015 (0.992, 1.038) | 0.198 | 1.016 (0.993, 1.039) | 0.174 |
| CAD | 1.495 (0.839, 2.664) | 0.173 | 1.501 (0.844, 2.671) | 0.167 | 1.509 (0.847, 2.687) | 0.162 |
| LAD | 1.022 (0.981, 1.065) | 0.297 | 1.021 (0.980, 1.065) | 0.313 | 1.024 (0.983, 1.066) | 0.260 |
| VP% | 1.007 (1.001, 1.013) | 0.018 | – | – | – | – |
| VP ≥ 20% | – | – | 2.068 (1.195, 3.579) | 0.009 | – | – |
| VP ≥ 40% | – | – | – | – | 1.880 (1.103, 3.205) | 0.020 |
| LBBAP vs. RVP | 0.294 (0.163, 0.532) | <0.001 | 0.272 (0.154, 0.481) | <0.001 | 0.299 (0.166, 0.539) | <0.001 |
CAD, coronary artery disease; LAD, left atrial diameter; LBBAP, left bundle branch area pacing; RVP, right ventricular pacing; VP%, percentage of ventricular pacing.
Model 1: VP% adjusted as a numerical variable.
Model 2: VP% adjusted as a categorical variable with 20% set as a cut-off value.
Model 3: VP% adjusted as a categorical variable with 40% set as a cut-off value.
Interaction between the percentage of ventricular pacing and pacing modalities
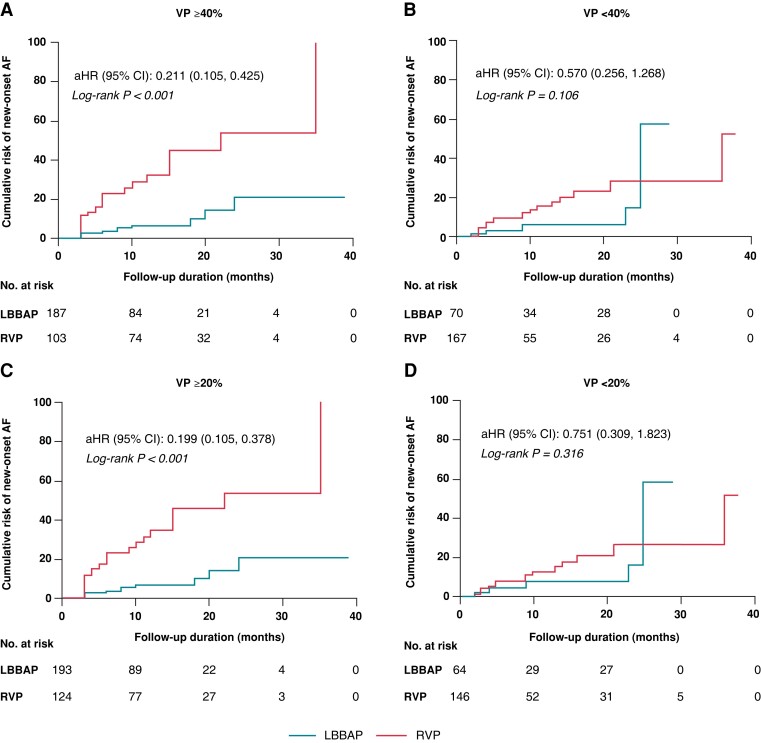
The KM survival curves for the primary endpoint were stratified by VP% to further explore the potential interaction between VP% and pacing strategies (Figure 4). Figure 4A and B demonstrates that LBBAP was significantly related with decreased risk of new-onset AF in subgroups with VP ≥ 40% [adjusted HR (95% CI): 0.211 (0.105, 0.425), P < 0.001] compared with no difference in VP < 40% [adjusted HR (95% CI): 0.570 (0.256, 1.268), P = 0.106]. There was no interaction between pacing modalities and VP% (using 40% as a cut-off value) (P for interaction = 0.074). In Figure 4C and D, the cumulative risk of new-onset AF was significantly reduced in LBBAP vs. RVP in the VP ≥ 20% group [adjusted HR (95% CI): 0.199 (0.105, 0.378), P = 0.009]. The beneficial effect of LBBAP was not significant in the VP < 20% group [adjusted HR (95% CI): 0.751 (0.309, 1.823), P = 0.316]. There was a significant interaction between pacing modalities and VP% (using 20% as a cut-off value) (P for interaction = 0.020).
Figure 4.
Kaplan–Meier curves for new-onset AF in subgroups stratified by VP%. (A and B) Kaplan–Meier curves for new-onset AF in subgroups with VP ≥ 40% and <40%. There was no interaction between pacing modalities and VP% (using 40% as a cut-off value) (P for interaction = 0.074); (C and D) Kaplan–Meier curves for new-onset AF in subgroups with VP ≥ 20% and <20%. A significant interaction existed between pacing modalities and VP% (using 20% as a cut-off value) (P for interaction = 0.020). AF, atrial fibrillation; LBBAP, left bundle branch area pacing; RVP, right ventricular pacing; VP%, percentage of ventricular pacing.
Discussion
In this prospective cohort study, which included the largest sample size to date, we demonstrated that (i) the incidence of new-onset AF in the LBBAP group was nearly 2.3-fold lower than that in RVP during a mean follow-up duration of nearly 12 months; (ii) after adjusting for confounding factors predisposing to AF, only LBBAP was an independent protective factor for decreasing the risk of new-onset AF; (iii) there was a significant interaction between pacing modalities and VP%. The beneficial effect of LBBAP was more pronounced in patients with VP ≥ 20%.
The deleterious effect of RVP on cardiac function and risk of new-onset of AF has been widely established. In the MOde Selection Trial (MOST), the incidence rate of new-onset AF was 21% in patients with SND and receiving dual-chamber pacemaker (DDD). The risk of AF was linearly correlated with VP% in the DDD group [HR (95% CI): 1.010 (1.002, 1.018) for each 1% increase in VP%].1 Subsequently, studies also demonstrated that VP% was related to the occurrence of persistent AF.2 Therefore, minimizing the ventricular pacing burden of RVP is one option for reducing the risk of AF occurrence. Also, patients with SND might be more susceptible to AF due to diseased sinoatrial node and increased automaticity of atrial tissue. However, for those patients with SND and no previous history of AF, the natural history of the disease might be interrupted if they receive timely pacemaker implantation to restore heart rates with normal AV conduction and ventricular synchrony. Thus, given the effect of pacing therapy, the risk of new-onset AF in patients with SND and no history of AF may not be significantly different from those with AV block.
His bundle pacing is theoretically the most physiological pacing modality and has been associated with reduced risk for the combined endpoint of death, HFH, or upgrade to BiVP compared with RVP.14 The beneficial effect of HBP on the decreased risk of new-onset AF has also been reported when compared with RV apical pacing [HR (95% CI): 0.28 (0.16–0.48), P = 0.0001)].15 The incidence of AF is significantly lower in patients with HBP than those with RV septal pacing or apical pacing (16.9 vs. 25.7 vs. 28.0%, P = 0.049) during a mean follow-up duration of 58.5 months, and no significant difference was observed between RV septal and apical pacing. In another study of patients without a history of persistent/permanent AF,5 HBP also demonstrated a lower risk of new-onset AF than RVP.
Left bundle branch area pacing, a physiological pacing form alternative to HBP, has been developed rapidly in recent years. Compared with HBP, LBBAP showed a better pacing threshold and sensing amplitude, similar paced QRS duration, and lower risk of increased capture threshold or loss of capture.16 Compared with RVP, LBBAP manifested better LV electromechanical synchrony and less events of HFH or upgrade to BiVP.10 In another large prospective observational study,11 LBBAP was an independent protective factor for the composite endpoint of all-cause mortality, HFH, and upgrade to BiVP compared with RVP [HR (95% CI): 0.46 (0.306–0.695), P < 0.001)]. A retrospective cohort study reported a lower incidence of new-onset AF in patients with LBBAP than that of RVP (5.2 vs. 18.1%).17 Our prospective observational study confirmed the beneficial effect of LBBAP on new-onset AF in a relatively larger sample size (total of 527 patients, and 257 patients with LBBAP). The positive interaction between pacing modalities and ventricular pacing burden validated the more pronounced beneficial effect of LBBAP in patients with VP > 20%. This might be explained by the significantly lower incidence of new-onset AF in LBBAP than RVP group [0/7 (0%) vs. 5/21(23.8%)] in the subgroup with VP 20–40%. The beneficial effect of LBBAP in subgroup with VP < 40% was exaggerated by the significant difference between LBBAP and RVP in patients with VP 20–40%, which led to more overlap in 95% CI and no statistically significant interaction between VP < 40% and ≥40%. Because the sample size of this subgroup was small, the interaction between VP burden and LBBAP or RVP should be investigated in future large sample size studies.
Left atrial (LA) function, described by the reservoir, conduit, and booster roles, is vulnerable to LV mechanic function.18 The LA passive emptying fraction is easily affected by RVP3 with increased LA volumes. Right ventricular pacing significantly increased LV electromechanical delay and intra-LV dyssynchrony with a higher LA volume pre-atrial contraction, minimal volume, and lower passive and total emptying fraction than HBP.6 LBBAP may result in increased LA strain or strain rate.19 Our previous study also found a decreased LAD after LBBAP when compared with RVP in patients with persistent AF and high VP%.20 Medium or long-term echocardiographic studies were required to evaluate whether LBBAP can improve LA function and facilitate LA remodelling.
Limitations
The main limitation of the study is the non-randomized controlled study design. Large sample size, multicentre, prospective randomized controlled trials are warranted to validate the superiority of LBBAP over RVP in reducing the risk of new-onset AF. Our study comprises the largest sample size to date, and the main results may help select appropriate pacing strategies in clinical practice. Second, we only enrolled patients without a history of AF, which made it inability to explore the effect of LBBAP vs. RVP on AF progression in patients with paroxysmal AF. Third, other confounding factors predisposing to AF (such as intrinsic PR interval and atrial fibrosis) were not adjusted in regression models and may partially influence the reliability of results. Finally, an AF episode lasting more than 30 s in this study has limited prognostic value for clinical outcomes. Future studies are needed to investigate the impact of LBBAP on much longer duration of AF episodes due to their deleterious effect.
Conclusions
The risk of new-onset AF after dual-chamber pacemaker implantation might be reduced in patients with LBBAP when compared with RVP. This kind of effect seems to be more pronounced in patients with a VP burden of ≥20%. The superiority of LBBAP over RVP in reducing the risk of new-onset AF needs to be confirmed in future large sample randomized controlled studies.
Contributor Information
Haojie Zhu, Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Peking Union Medical College, No. 167, Beilishi Road, Xicheng District, Beijing 100037, China.
Xiaofei Li, Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Peking Union Medical College, No. 167, Beilishi Road, Xicheng District, Beijing 100037, China.
Zhao Wang, Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Peking Union Medical College, No. 167, Beilishi Road, Xicheng District, Beijing 100037, China.
Qian Liu, Department of Cardiology, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China.
Bingqian Chu, Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Peking Union Medical College, No. 167, Beilishi Road, Xicheng District, Beijing 100037, China.
Yan Yao, Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Peking Union Medical College, No. 167, Beilishi Road, Xicheng District, Beijing 100037, China.
Zhimin Liu, Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Peking Union Medical College, No. 167, Beilishi Road, Xicheng District, Beijing 100037, China.
Ruiqin Xie, Department of Cardiology, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China.
Xiaohan Fan, Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Peking Union Medical College, No. 167, Beilishi Road, Xicheng District, Beijing 100037, China.
Funding
This research was funded by National Natural Science Foundation of China (NSFC) (grant number 81970284) and Chinese Academy of Medical Sciences Innovation Found for Medical Sciences (CIFMS) (grant number 2020-I2M-C&T-B-007).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KLet al. . Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–7. [DOI] [PubMed] [Google Scholar]
- 2. Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick Det al. . Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med 2007;357:1000–8. [DOI] [PubMed] [Google Scholar]
- 3. Xie J-M, Fang F, Zhang Q, Chan JY-S, Yip GW-K, Sanderson JEet al. . Left atrial remodeling and reduced atrial pump function after chronic right ventricular apical pacing in patients with preserved ejection fraction. Int J Cardiol 2012;157:364–9. [DOI] [PubMed] [Google Scholar]
- 4. Vijayaraman P, Bordachar P, Ellenbogen KA. The continued search for physiological pacing: where are we now? J Am Coll Cardiol 2017;69:3099–114. [DOI] [PubMed] [Google Scholar]
- 5. Ravi V, Beer D, Pietrasik GM, Hanifin JL, Ooms S, Ayub MTet al. . Development of new-onset or progressive atrial fibrillation in patients with permanent HIS bundle pacing versus right ventricular pacing: results from the RUSH HBP registry. J Am Heart Assoc 2020;9:e018478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pastore G, Aggio S, Baracca E, Fraccaro C, Picariello C, Roncon Let al. . Hisian area and right ventricular apical pacing differently affect left atrial function: an intra-patients evaluation. Europace 2014;16:1033–9. [DOI] [PubMed] [Google Scholar]
- 7. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou Xet al. . A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol 2017;33:1736.e1–3. [DOI] [PubMed] [Google Scholar]
- 8. Su L, Wang S, Wu S, Xu L, Huang Z, Chen Xet al. . Long-term safety and feasibility of left bundle branch pacing in a large single-center study. Circ Arrhythm Electrophysiol 2021;14:e009261. [DOI] [PubMed] [Google Scholar]
- 9. Zhu H, Wang Z, Li X, Yao Y, Liu Z, Fan X. Medium- and long-term lead stability and echocardiographic outcomes of left bundle branch area pacing compared to right ventricular pacing. J Cardiovasc Dev Dis 2021;8:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Zhang J, Qiu C, Wang Z, Li H, Pang Ket al. . Clinical outcomes in patients with left bundle branch area pacing vs. right ventricular pacing for atrioventricular block. Front Cardiovasc Med 2021;8:685253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma PS, Patel NR, Ravi V, Zalavadia DV, Dommaraju S, Garg Vet al. . Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: results from the Geisinger-Rush conduction system pacing registry. Heart Rhythm 2022;19:3–11. [DOI] [PubMed] [Google Scholar]
- 12. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. . 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Europace 2022;24:71–164. [DOI] [PubMed] [Google Scholar]
- 13. Wang Z, Zhu H, Li X, Yao Y, Liu Z, Fan X. Comparison of procedure and fluoroscopy time between left bundle branch area pacing and right ventricular pacing for bradycardia: the learning curve for the novel pacing strategy. Front Cardiovasc Med 2021;8:695531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdelrahman M, Subzposh FA, Beer D, Durr B, Naperkowski A, Sun Het al. . Clinical outcomes of his bundle pacing compared to right ventricular pacing. J Am Coll Cardiol 2018;71:2319–30. [DOI] [PubMed] [Google Scholar]
- 15. Pastore G, Zanon F, Baracca E, Aggio S, Corbucci G, Boaretto Get al. . The risk of atrial fibrillation during right ventricular pacing. Europace 2016;18:353–8. [DOI] [PubMed] [Google Scholar]
- 16. Hua W, Fan X, Li X, Niu H, Gu M, Ning Xet al. . Comparison of left bundle branch and His bundle pacing in bradycardia patients. JACC Clin Electrophysiol 2020;6:1291–9. [DOI] [PubMed] [Google Scholar]
- 17. Ravi V, Sharma PS, Patel NR, Dommaraju S, Zalavadia DV, Garg Vet al. . New-onset atrial fibrillation in left bundle branch area pacing compared with right ventricular pacing. Circ Arrhythm Electrophysiol 2022;15:e010710. [DOI] [PubMed] [Google Scholar]
- 18. Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 1999;100:427–36. [DOI] [PubMed] [Google Scholar]
- 19. Liu Q, Yang J, Bolun Z, Pei M, Ma B, Tong Qet al. . Comparison of cardiac function between left bundle branch pacing and right ventricular outflow tract septal pacing in the short-term: a registered controlled clinical trial. Int J Cardiol 2021;322:70–6. [DOI] [PubMed] [Google Scholar]
- 20. Wang Z, Zhu H, Li X, Yao Y, Liu Z, Fan X. Left bundle branch area pacing versus right ventricular pacing in patients with persistent atrial fibrillation requiring ventricular pacing. Pacing Clin Electrophysiol 2021;44:2024–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.