Abstract
Background
This study aimed to determine whether paromomycin plus miltefosine (PM/MF) is noninferior to sodium stibogluconate plus paromomycin (SSG/PM) for treatment of primary visceral leishmaniasis in eastern Africa.
Methods
An open-label, phase 3, randomized, controlled trial was conducted in adult and pediatric patients at 7 sites in eastern Africa. Patients were randomly assigned to either 20 mg/kg paromomycin plus allometric dose of miltefosine (14 days), or 20 mg/kg sodium stibogluconate plus 15 mg/kg paromomycin (17 days). The primary endpoint was definitive cure after 6 months.
Results
Of 439 randomized patients, 424 completed the trial. Definitive cure at 6 months was 91.2% (155 of 170) and 91.8% (156 of 170) in the PM/MF and SSG/PM arms in primary efficacy modified intention-to-treat analysis (difference, 0.6%; 97.5% confidence interval [CI], −6.2 to 7.4), narrowly missing the noninferiority margin of 7%. In the per-protocol analysis, efficacy was 92% (149 of 162) and 91.7% (155 of 169) in the PM/MF and SSG/PM arms (difference, −0.3%; 97.5% CI, –7.0 to 6.5), demonstrating noninferiority. Treatments were well tolerated. Four of 18 serious adverse events were study drug–related, and 1 death was SSG-related. Allometric dosing ensured similar MF exposure in children (<12 years) and adults.
Conclusions
PM/MF and SSG/PM efficacies were similar, and adverse drug reactions were as expected given the drugs safety profiles. With 1 less injection each day, reduced treatment duration, and no risk of SSG-associated life-threatening cardiotoxicity, PM/MF is a more patient-friendly alternative for children and adults with primary visceral leishmaniasis in eastern Africa.
Clinical Trials Registration
Keywords: visceral leishmaniasis, phase 3 trial, miltefosine, paromomycin, eastern Africa
With 1 fewer injection each day, shorter treatment duration, and no risk of sodium stibogluconate–associated cardiotoxicity, paromomycin plus miltefosine is a more patient-friendly alternative to sodium stibogluconate plus paromomycin for children and adults with visceral leishmaniasis in eastern Africa.
The number of cases of visceral leishmaniasis (VL), a potentially fatal parasitic disease caused by Leishmania, is decreasing, mainly due to VL elimination efforts in South Asia. Eastern Africa has the highest burden of VL globally, mainly affecting children, with numbers stagnating in recent years [1, 2].
Since 2010, sodium stibogluconate (SSG) and paromomycin (PM) combination has been the standard of care for VL in Africa [3]. It was an improvement on 30-day SSG monotherapy but entails hospitalization with painful twice-daily injections for 17 days and potentially life-threatening antimony-associated toxicities such as cardiotoxicity, hepatotoxicity, and pancreatitis.
Liposomal amphotericin B (AmBisome) is a second-line drug for rescue treatment and specific target populations. The need for a cold-chain, sterile, 2-step dilution process and well-trained personnel as well as (rare) anaphylactic reactions limit its use in eastern Africa. Alternative efficacious, safe, ideally short-course treatments suitable for use in remote areas are needed.
Miltefosine (MF), the only oral treatment for VL, has been extensively used in Asia as a 28-day monotherapy. It is well tolerated, with mainly mild to moderate gastrointestinal side effects and transient increases in liver enzymes and creatinine. Female patients of childbearing potential are required to use contraception during treatment and for 5 months afterward due to potential teratogenicity. In a phase 2 clinical trial in Kenya and Sudan [4, 5], MF (2.5 mg/kg/day) used as monotherapy or in combination with AmBisome did not reach the predefined satisfactory efficacy level of >90%. Treatment failure was associated with poor MF exposure in children (59% efficacy) [5, 6]. Allometric MF dosing in 30 children (aged 4–12 years) for 28 days in Kenya achieved increased and less-variable exposure, with a cure rate of 90.0% (95% confidence interval [CI], 73.5% to 97.9%) at 6 months follow-up [7, 8]. MF may, therefore, be an option for Africa, ideally in combination to reduce treatment duration and improve efficacy.
PM treatment of 15 mg/kg/day for 21 days showed satisfactory efficacy in Asia but only 63.8% efficacy in Africa [9] with high variability. A higher daily dose of 20 mg/kg/day for 21 days was well tolerated and resulted in a more homogeneous response, with overall efficacy of 84.3% at 6 months follow-up [10]. Side effects included reversible kidney and liver toxicity and ototoxicity.
In Asia, a 10-day combination of MF (2.5 mg/kg/day) with PM (15 mg/kg/day) has been very successful with definitive cure rates at 6 months ranging from 96.9% to 98.7% [11]. An adapted PM/MF treatment regimen is expected to achieve satisfactory efficacy in Africa.
PM/MF combination therapy could be an alternative to SSG/PM, minimizing the number of injections and removing antimonial-related toxicity. It could be an attractive option for children, a high proportion of the at-risk population, and the elderly, the most at risk of SSG-related toxicity.
We determined whether a 14-day PM/MF combination treatment is noninferior to SSG/PM in eastern African patients with primary VL.
METHODS
Trial Design
This open-label, phase 3, randomized, controlled, multicenter, noninferiority trial was conducted in hospitals at 7 sites in Kenya, Uganda, Sudan, and Ethiopia. The trial started with 2 investigational arms, PM/MF for 14 days (arm 1) and PM for 14 days/MF for 28 days (arm 2), and a third study arm, the standard-of-care SSG/PM (arm 3). Since initial enrollment was considerably slower than anticipated, 2 additional sites were included, exclusion criteria were revised to allow for recruitment of a more representative VL population (see Supplementary Material), and recruitment in arm 2 was discontinued. Therefore, comparative analysis was performed between PM/MF arm 1 and SSG/PM arm 3, whereas results for arm 2 were descriptive. The choice to prematurely discontinue arm 2 was due to practical advantages of the arm 1 regimen, that is, shorter duration, complete administration during hospitalization, and lower cost. No efficacy or safety concerns required early termination of arm 2.
Participants
Patients aged 4 to ≤50 years with VL symptoms and parasitological diagnosis were included unless they had relapse, severe malnutrition, severe VL, positive human immunodeficiency virus (HIV) diagnosis or a concomitant severe infection, or were women of childbearing potential unwilling to use contraception until 5 months after the end of treatment (Supplementary Material 1 lists all criteria). Approval was obtained from independent ethics committees in Sudan, Kenya, Uganda, and Ethiopia and from the Médecins Sans Frontières’ Ethics Review Board. Informed consent and assent (when applicable) were obtained per regulatory requirements in each country.
Interventions
Arm 1 comprised a once-daily intramuscular (IM) injection of 20 mg/kg/day PM for 14 days and oral MF (Impavido) twice daily for 14 days. Children who weighed <30 kg received MF allometric dosing based on sex, weight, and height [12] (Supplementary Figure 1); patients who weighed ≥30 kg to <45 kg received 100 mg/day; and patients who weighed ≥45 kg received 150 mg/day. The arm 2 (discontinued) regimen was as arm 1 but with MF for 28 days. Arm 3 was SSG 20 mg/kg/day IM/intravenous with PM 15 mg/kg/day IM for 17 days. Arm 1 and arm 3 patients were hospitalized during treatment; arm 2 patients were hospitalized for the first 14 days of treatment followed by 14 days as outpatients.
Rescue treatment was indicated in case of initial treatment failure, relapse, or intolerability (Supplementary Material 1). End-of-treatment (EOT) assessments were conducted on day 28. Patients were followed up for 6 months until day 210. Compliance was measured as described in Supplementary Material 1.
Pharmacokinetics
Pharmacokinetics measurements of MF were made in all patients in arm 1 at day 14 and in arms 1 and 2 at day 28 and day 56. Intensive pharmacokinetics sampling for both PM and MF was performed in a subset of patients at day 1 and day 14 (Supplementary Material 1).
Outcomes
The primary efficacy end point was definitive cure at 6 months follow-up, defined as absence of clinical signs and symptoms of VL at day 210 and no rescue treatment during the trial. Safety endpoints were the frequency and severity of adverse events (AEs) from treatment start to day 210 and the frequency of serious AEs and AEs that required treatment discontinuation. A secondary efficacy endpoint was initial cure at EOT (resolution of clinical signs and symptoms, negative microscopy [spleen or bone marrow], and no rescue treatment).
The pharmacokinetics end point was total and partial blood plasma exposure to PM and MF, defined as the area under the concentration-time curve for PM and the maximal concentrations (Cmax) for MF, during treatment and until end of follow-up for MF and based on full curves both on day 1 and day 14 for PM.
Sample Size
The sample size of 153 patients per arm was based on an expected efficacy at 6 months follow-up of 93% for arm 1 and 91% for arm 3, a noninferiority margin of 7%, power of 80%, and alpha of 0.025 (1-sided, no adjustment for multiplicity). A 10% provision for loss to follow-up brought the sample size to 170 per arm (arms 1 and 3).
Randomization
Patients were originally randomized centrally to treatment in a 1:1:1 allocation ratio using varying block sizes of 6, 9, or 12 patients in random order. Following discontinuation of arm 2, a 1:1 allocation ratio was used with varying block sizes of 4, 6, or 8 to assign patients to study arms 1 and 3. Randomization codes were generated through an online system.
Statistical Methods
Efficacy analysis was performed comparing treatment arm 1 with arm 3. Data for the discontinued arm 2 were described. Estimates of the proportion with initial (EOT)/definitive (day 210) cure were reported. The difference in proportions between treatment arms was presented in the modified intention-to-treat (mITT; all randomized patients receiving at least 1 dose of treatment) and in the per-protocol (PP; patients in mITT with no major protocol deviations) sets. The Blackwelder test was used to test for noninferiority. For sensitivity analysis for imputation of missing data and the set of completers, see Supplementary Table 1. Multivariate logistic regression analysis was conducted to assess the difference in odds of failure between treatment arms adjusting for country, age group, and sex.
Safety analysis was performed in the mITT set based on treatment-emergent adverse events (TEAEs). The proportion of patients with at least 1 adverse drug reaction (ADR) was compared for each treatment arm. All AEs were categorized by system organ class and preferred term according to the Medical Dictionary for Regulatory Activities versions 20.1 to 24.0.
RESULTS
Participants
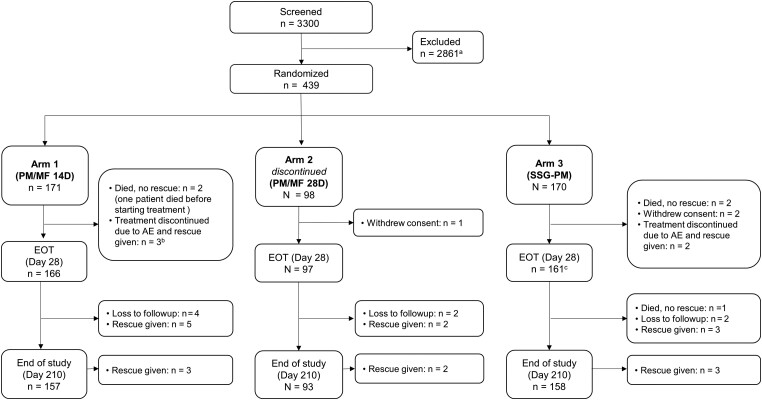
A total of 439 patients were recruited between 23 January 2018 and 17 May 2020; 171, 170, and 98 patients were assigned to arms 1, 3, and 2, respectively. There were 424 of 439 (96.6%) patients who reached EOT and 408 of 439 (92.9%) who reached the end of the study (day 210), with only 8 (1.8%) lost to follow-up (Figure 1). Apart from 1 patient in arm 1 who died before starting treatment, all patients were included in the mITT set and 416 of 439 (94.8%) patients were included in the PP set. Demographic and baseline characteristics were generally comparable between treatment arms. The median age was 11 years (interquartile range, 8.0–19.0), and 59.7% of the trial patients were aged ≤12 years. There was a higher proportion of male patients (79.7%), which is consistent with the overall VL population in the region (Table 1); 95.5% of female patients recruited were aged ≤12 years. Most patients (99.3%) presented with fever at baseline and most (80.6%) had weight loss (Supplementary Table 2).
Figure 1.
Patient disposition. aThe most common reasons for screening failure were negative parasitology (746 patients, 22.6%), laboratory abnormalities (338 patients, 13%), age <4 years or >50 years (329 patients, 12.7%), severe malnourishment (230 patients, 8.9%), or other reasons (245 patients, 9.4%). One patient died during screening. bOne additional patient discontinued the study treatment due to AEs but did not receive rescue medication and completed the study. cThree patients missed the EOT (day 28) visit but were included at later visits. Abbreviations: AE, adverse event; D, day; EOT, end of treatment; MF, miltefosine; PM, paromomycin; SSG, sodium stibogluconate.
Table 1.
Baseline Characteristics by Treatment Arm and Overall—Intention-to-Treat Set
| Parameter | Statistics | Arm 1, PM/MF 14 Days (n = 171) |
Arm 2,a PM/MF 28 Days (n = 98) |
Arm 3, SSG/PM (n = 170) |
Overall (n = 439) |
|---|---|---|---|---|---|
| Age, years | Mean (SD) | 13.5 (7.9) | 13.9 (8.0) | 13.0 (7.1) | 13.4 (7.6) |
| Median (Q1–Q3) | 10.0 (8.0–19.0) | 12.0 (8.0–20.0) | 10.0 (8.0–18.0) | 11.0 (8.0–19.0) | |
| Age category | 4 to ≤12 years, n (%) | 103 (60.2) | 54 (55.1) | 105 (61.8) | 262 (59.7) |
| >12 to 50 years, n (%) | 68 (39.8) | 44 (44.9) | 65 (38.2) | 177 (40.3) | |
| Sex | Female, n (%) | 34 (19.9) | 17 (17.3) | 38 (22.4) | 89 (20.3) |
| Male, n (%) | 137 (80.1) | 81 (82.7) | 132 (77.6) | 350 (79.7) | |
| Weight, kg | Range (Min–Max) | 11.0–71.0 | 14.0–61.0 | 11.0–60.0 | 11.0–71.0 |
| Mean (SD) | 32.7 (14.4) | 33.4 (13.7) | 31.4 (13.4) | 32.4 (13.9) | |
| Median (Q1–Q3) | 28.0 (21.0–46.0) | 32.0 (20.5–47.0) | 27.3 (19.5–43.0) | 28.0 (20.0–45.0) | |
| Height, cm | Range (Min–Max) | 93.0–190.0 | 102.0–182.0 | 92.0–190.0 | 92.0–190.0 |
| Mean (SD) | 141.0 (23.8) | 143.9 (22.8) | 139.9 (23.3) | 141.2 (23.4) | |
| Median (Q1–Q3) | 137.0 (124.0–165.0) | 141.5 (123.0–166.0) | 138.0 (121.0–163.0) | 139.0 (122.0–164.0) | |
| Nutritional status according to World Health Organization reference curves and Body mass index | N = 145 | N = 79 | N = 145 | N = 369 | |
| Moderate underweight, n (%) | 43 (29.7) | 35 (44.3) | 49 (33.8) | 127 (34.4) | |
| Normal, n (%) | 101 (69.7) | 42 (53.2) | 95 (65.5) | 238 (64.5) | |
| Overweight, n (%) | 1 (0.7) | 2 (2.5) | 1 (0.7) | 4 (1.1) | |
| Nutritional status, mid-upper arm circumference (MUAC), mm | N = 26 | N = 19 | N = 25 | N = 70 | |
| Range (Min–Max) | 160.0–260.0 | 165.0–240.0 | 168.0–255.0 | 160.0–260.0 | |
| Mean (SD) | 217.0 (23.1) | 200.1 (18.8) | 208.2 (23.3) | 209.3 (22.8) | |
| Median (Q1–Q3) | 220.0 (200.0–230.0) | 200.0 (184.0–220.0) | 205.0 (190.0–225.0) | 210.0 (190.0–223.0) | |
| Hemoglobin, g/dL | Range (Min–Max) | 5.0–12.3 | 5.1–19.7 | 5.1–13.8 | 5.0–19.7 |
| Mean (SD) | 8.0 (1.4) | 8.5 (2.2) | 8.1 (1.7) | 8.2 (1.7) | |
| Median (Q1–Q3) | 8.0 (6.9–8.9) | 8.1 (7.0–9.6) | 8.0 (6.7–9.2) | 8.0 (6.9–9.1) | |
| White blood cells, ×103/μL | Range (Min–Max) | 0.9–9.1 | 1.0–8.5 | 0.5–6.4 | 0.5–9.1 |
| Mean (SD) | 2.8 (1.3) | 2.4 (1.0) | 2.8 (1.2) | 2.7 (1.2) | |
| Median (Q1–Q3) | 2.6 (2.0–3.4) | 2.3 (1.6–3.0) | 2.6 (1.9–3.5) | 2.5 (1.9–3.3) | |
| Platelets, ×103/μL | Range (Min–Max) | 19.0–338.0 | 45.0–342.0 | 26.0–446.0 | 19.0–446.0 |
| Mean (SD) | 118.8 (56.5) | 113.9 (50.8) | 120.8 (61.7) | 118.5 (57.3) | |
| Median (Q1–Q3) | 108.0 (73.0–154.0) | 108.5 (83.0–134.0) | 105.0 (80.0–147.0) | 107.0 (79.0–146.0) | |
| Aspartate aminotransferase, U/L | Range (Min–Max) | 6.0–592.0 | 13.0–118.0 | 10.0–621.0 | 6.0–621.0 |
| Mean (SD) | 79.9 (85.3) | 51.9 (26.0) | 89.8 (94.8) | 77.5 (81.6) | |
| Median (Q1–Q3) | 58.0 (40.0–83.0) | 45.5 (31.0–68.0) | 62.5 (38.0–99.0) | 56.0 (36.0–87.0) | |
| Alanine aminotransferase, U/L | Range (Min–Max) | 0.8–473.0 | 2.0–105.0 | 6.0–397.0 | 0.8–473.0 |
| Mean (SD) | 40.1 (50.0) | 27.0 (18.9) | 46.7 (58.9) | 39.7 (49.4) | |
| Median (Q1–Q3) | 27.0 (18.0–44.0) | 22.0 (14.0–40.0) | 30.0 (18.0–48.0) | 27.0 (16.0–45.0) | |
| Creatinine, mg/dL | Range (Min–Max) | 0.1–1.5 | 0.3–1.3 | 0.1–1.4 | 0.1–1.5 |
| Mean (SD) | 0.8 (0.3) | 0.9 (0.2) | 0.7 (0.3) | 0.8 (0.3) | |
| Median (Q1–Q3) | 0.8 (0.6–1.0) | 0.8 (0.7–1.0) | 0.7 (0.6–0.9) | 0.8 (0.6–1.0) | |
| Albumin, g/L | Range (Min–Max) | 1.5–54.61 | 4.0–53.2 | 9.0–48.6 | 1.5–54.6 |
| Mean (SD) | 27.4 (7.8) | 29.9 (9.5) | 26.8 (7.1) | 27.7 (8.0) | |
| Median (Q1–Q3) | 27.0 (23.0–31.0) | 28.4 (23.6–35.4) | 26.4 (22.2–31.0) | 27.0 (23.0–31.8) | |
| Spleen size, cm | Range (Min–Max) | 0.0–22.0 | 0.0–20.0 | 0.0–24.0 | 0.0–24.0 |
| Mean (SD) | 7.6 (4.1) | 8.8 (3.9) | 7.8 (4.5) | 7.9 (4.2) | |
| Median (Q1–Q3) | 7.0 (4.0–10.0) | 8.0 (6.0–11.5) | 7.0 (4.0–11.0) | 8.0 (5.0–11.0) | |
| Liver size, cm | Range (Min–Max) | 0.0–7.0 | 0.0–8.0 | 0.0–10.0 | 0.0–10.0 |
| Mean (SD) | 1.6 (2.0) | 1.0 (1.8) | 1.5 (2.1) | 1.4 (2.0) | |
| Median (Q1–Q3) | 0.0 (0.0–4.0) | 0.0 (0.0–2.0) | 0.0 (0.0–3.0) | 0.0 (0.0–2.0) |
Abbreviations: MF, miltefosine; PM, paromomycin; Q, quartile; SD, standard deviation; SSG, sodium stibogluconate.
Recruitment into arm 2 was discontinued.
Compliance
Full (90%–110%) compliance to all treatments was >95% during hospitalization and 90% (to MF) during home treatment in arm 2.
Efficacy
Definitive cure at 6 months follow-up was achieved in 155 of 170 (91.2%) patients in arm 1 and 156 of 170 (91.8%) in arm 3 in the mITT set. The resulting difference in efficacy was 0.6% (97.5% CI, –6.2 to 7.4). Noninferiority was not demonstrated for a predefined margin of 7%, as the upper limit of the 97.5% CI of 7.4% exceeded slightly the noninferiority margin of 7%. In the PP set, 149 of 162 (92.0%) patients in arm 1 and 155 of 169 (91.7%) in arm 3 achieved definitive cure at 6 months follow-up and PM/MF was noninferior to SSG/PM (difference in efficacy = –0.3%; 97.5% CI, –7.0 to 6.5; Table 2).
Table 2.
Primary Efficacy Outcome of Definite Cure With Primary Imputation by Treatment Arm
| Analysis Set | Statistics | Arm 1, PM/MF 14 Days |
Arm 3, SSG/PM |
|---|---|---|---|
| Modified intention-to-treata | N | 170 | 170 |
| Number cured | 155 | 156 | |
| Efficacy, % | 91.2 | 91.8 | |
| Difference in efficacyb (97.5% CI) | 0.6 (−6.2 to 7.4) | … | |
| Per-protocolc | N | 162 | 169 |
| Number cured | 149 | 155 | |
| Efficacy, % | 92.0 | 91.7 | |
| Difference in efficacyb (97.5% CI) | −0.3 (−7.0 to 6.5) | … |
Abbreviations: CI, confidence interval; MF, miltefosine; PM, paromomycin; SSG, sodium stibogluconate.
Primary analysis.
Difference in efficacy from SSG/PM, that is, efficacy of SSG/PM minus efficacy of PM/MF 14 days.
Sensitivity analysis.
Efficacy was similar between the 2 arms by age group, although the study was not powered for subgroup analysis. Definitive cure was achieved more often in patients aged 4 to ≤12 years (94.1% and 95.2% in arms 1 and 3, respectively) than in patients aged >12 to 50 years (86.8% and 86.2% in arms 1 and 3, respectively).
Logistic regression analysis showed no statistically significant difference in the odds of treatment failure between arms 1 and 3 after adjusting for age, sex, and country (odds ratio of 1.04; 95% CI, .5 to 2.3; P = .926). Reasons for treatment failure requiring rescue medication were relapse (3.6%), AE leading to treatment discontinuation (1.1%), 1 case of initial failure, and 1 case of post-kalazar dermal leishmaniasis (PKDL) with mucosal involvement.
In arm 2, 92 of 98 (93.9%) patients (97.5% CI, 86.1 to 98.1) in the mITT set achieved definite cure. Initial cure at EOT was achieved in 164 of 170 (96.5%) patients in arm 1 and 159 of 166 (95.8%) in arm 3 in the mITT set. The resulting difference in efficacy was –0.7% (97.5% CI, –5.4 to 4.0); similar results were obtained in the PP set.
PKDL
A total of 28 patients presented with PKDL after treatment, 5 of 170 (2.9%) in arm 1 and 23 of 170 (13.5%) in arm 3. All PKDL cases were reported in Sudan and Ethiopia where the frequency of PKDL in VL patients was significantly lower in arm 1 than in arm 3 (5 of 114, 4.4% and 23 of 110, 20.9%, respectively; P = .0002).
Safety
At least 1 TEAE occurred in 54.6% of patients, mostly during the treatment period, with 52.4%, 64.3%, and 51.2% in arms 1, 2, and 3, respectively (Table 3). The most common severe or life-threatening AEs were anemia (3.7%) and aspartate aminotransferase increase (2.5%). At least 1 ADR was reported in 30.6% of patients, the most common being MF-related vomiting (13.5%), injection site pain (10.3%), and PM-related hypoacusis (5.0%) (Table 4). Although the frequency of ADRs was higher in the PM/MF arms, most of the ADRs were mild and moderate vomiting events associated with MF, and only 1 patient discontinued treatment due to severe vomiting. ADRs suggesting SSG-related cardiac toxicity were reported in 6.5% of patients in the SSG/PM arm. Common Terminology Criteria for Adverse Events grade ≥3 ADRs were reported in 6.4% of patients, with 4.1% in arm 2 and 7.1% in both arms 1 and 3, the most common being increases in aspartate (2.5%) and alanine aminotransferases (0.7%) and prolonged electrocardiogram QT (0.7%; Table 5). Nonserious ADRs that led to treatment discontinuation were reported in 6 patients (1.4%). Serious AEs occurred in 11 patients (2.5%), with 2.9%, 3.1%, and 1.8% in arms 1, 2, and 3, respectively. These included 1 ADR of SSG-related cardiotoxicity and 3 cases of infections that led to death in 4 patients (0.9%). Two patients not included in the mITT population died of internal abdominal bleeding, possibly due to complications from splenic aspiration before study drug administration. Serious ADRs of acute kidney injury were reported in 1 patient in arm 1 and 1 patient in arm 2 related to PM and MF and to MF, respectively. The patient in arm 1 also had a serious ADR of bilateral deafness related to PM that was ongoing at the end of the study (Table 4).
Table 3.
Summary of Treatment-Emergent Adverse Events—Modified Intention-to-Treat Set
| Arm 1, PM/MF 14 Days |
Arm 2, PM/MF 28 Daysa |
Arm 3, SSG/PM |
Overall | |
|---|---|---|---|---|
| Description | (n = 170) | (n = 98) | (n = 170) | (n = 438) |
| Any treatment-emergent adverse event (TEAE) | 89 (52.4) | 63 (64.3) | 87 (51.2) | 239 (54.6) |
| ȃAny at least severe TEAEb | 26 (15.3) | 14 (14.3) | 18 (10.6) | 58 (13.2) |
| ȃAny treatment-emergent serious adverse event (TESAE) | 5 (2.9) | 3 (3.1) | 3 (1.8) | 11 (2.5) |
| ȃAny TEAE leading to death | 1 (0.6) | 0 (0.0) | 3 (1.8) | 4 (0.9) |
| ȃAny TEAE leading to treatment discontinuation | 4 (2.4) | 0 (0.0) | 2 (1.2) | 6 (1.4) |
| Any treatment-emergent ADR | 59 (34.7) | 44 (44.9) | 31 (18.2) | 134 (30.6) |
| ȃAny at least severe treatment-emergent ADRb | 12 (7.1) | 4 (4.1) | 12 (7.1) | 28 (6.4) |
| ȃAny treatment-emergent serious ADR | 1 (0.6) | 1 (1.0) | 1 (0.6) | 3 (0.7) |
| ȃAny treatment-emergent ADR leading to death | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.2) |
| ȃAny treatment-emergent ADR leading to treatment discontinuation | 4 (2.4) | 0 (0.0) | 2 (1.2) | 6 (1.4) |
Data are presented as n (%) of patients with at least 1 event.
Abbreviations: ADR, adverse drug reaction; MF, miltefosine; PM, paromomycin; SSG, sodium stibogluconate; TEAE, treatment-emergent adverse events.
Recruitment into arm 2 was discontinued.
Events with a severity classification as severe (Common Terminology Criteria for Adverse Events [CTCAE] grade 3), life-threatening (CTCAE grade 4), or death (CTCAE grade 5).
Table 4.
Summary of Treatment-Emergent Adverse Drug Reactions by Treatment Arm: by System Organ Class and Preferred Term—Modified Intention-to-Treat Set
| Treatment Arm | |||||
|---|---|---|---|---|---|
| PM/MF (14 Days) | PM/MF (28 Days)a | SSG/PM | Total | ||
| System Organ Class | Preferred Term | n = 170 | n = 98 | n = 170 | n = 438 |
| Any treatment-emergent adverse drug reaction | … | 59 (34.7) [108] | 44 (44.9) [77] | 31 (18.2) [46] | 134 (30.6) [231] |
| Blood and lymphatic system disorders | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.6) [1] | 1 (0.2) [1] | |
| Neutropenia | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.6) [1] | 1 (0.2) [1] | |
| Cardiac disorders | … | 0 (0.0) [0] | 0 (0.0) [0] | 4 (2.4) [4] | 4 (0.9) [4] |
| Arrhythmia | 0 (0.0) [0] | 0 (0.0) [0] | 3 (1.8) [3] | 3 (0.7) [3] | |
| Sinus arrhythmia | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.6) [1] | 1 (0.2) [1] | |
| Ear and labyrinth disorders | … | 9 (5.3) [15] | 11 (11.2) [17] | 3 (1.8) [4] | 23 (5.3) [36] |
| Hypoacusis | 8 (4.7) [14] | 11 (11.2) [17] | 3 (1.8) [4] | 22 (5.0) [35] | |
| Deafness bilateral | 1 (0.6) [1] | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Gastrointestinal disorders | … | 40 (23.5) [59] | 26 (26.5) [37] | 1 (0.6) [1] | 67 (15.3) [97] |
| Vomiting | 34 (20.0) [48] | 25 (25.5) [35] | 0 (0.0) [0] | 59 (13.5) [83] | |
| Gastritis | 4 (2.4) [4] | 1 (1.0) [1] | 0 (0.0) [0] | 5 (1.1) [5] | |
| Abdominal pain | 2 (1.2) [2] | 1 (1.0) [1] | 0 (0.0) [0] | 3 (0.7) [3] | |
| Dyspepsia | 2 (1.2) [2] | 0 (0.0) [0] | 0 (0.0) [0] | 2 (0.5) [2] | |
| Nausea | 1 (0.6) [1] | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Abdominal pain upper | 1 (0.6) [1] | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Gastrointestinal inflammation | 1 (0.6) [1] | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Pancreatitis | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.6) [1] | 1 (0.2) [1] | |
| General disorders and administration site conditions | … | 17 (10.0) [17] | 14 (14.3) [14] | 14 (8.2) [14] | 45 (10.3) [45] |
| Injection site pain | 17 (10.0) [17] | 14 (14.3) [14] | 14 (8.2) [14] | 45 (10.3) [45] | |
| Hepatobiliary disorders | … | 2 (1.2) [2] | 0 (0.0) [0] | 0 (0.0) [0] | 2 (0.5) [2] |
| Hepatitis | 1 (0.6) [1] | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Drug-induced liver injury | 1 (0.6) [1] | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Injury, poisoning, and procedural complications | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.6) [1] | 1 (0.2) [1] | |
| Cardiotoxicity | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.6) [1] | 1 (0.2) [1] | |
| Investigations | 11 (6.5) [12] | 6 (6.1) [7] | 16 (9.4) [20] | 33 (7.5) [39] | |
| Aspartate aminotransferase increased | 6 (3.5) [6] | 1 (1.0) [1] | 6 (3.5) [6] | 13 (3.0) [13] | |
| Blood creatinine increased | 4 (2.4) [4] | 3 (3.1) [3] | 3 (1.8) [3] | 10 (2.3) [10] | |
| Electrocardiogram QT prolonged | 0 (0.0) [0] | 0 (0.0) [0] | 6 (3.5) [7] | 6 (1.4) [7] | |
| Alanine aminotransferase increased | 0 (0.0) [0] | 2 (2.0) [2] | 2 (1.2) [2] | 4 (0.9) [4] | |
| Blood bilirubin increased | 1 (0.6) [1] | 1 (1.0) [1] | 1 (0.6) [1] | 3 (0.7) [3] | |
| Liver function test increased | 1 (0.6) [1] | 0 (0.0) [0] | 1 (0.6) [1] | 2 (0.5) [2] | |
| Musculoskeletal and connective tissue disorders | … | 0 (0.0) [0] | 1 (1.0) [1] | 0 (0.0) [0] | 1 (0.2) [1] |
| Myalgia | 0 (0.0) [0] | 1 (1.0) [1] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Renal and urinary disorders | … | 3 (1.8) [3] | 1 (1.0) [1] | 1 (0.6) [1] | 5 (1.1) [5] |
| Acute kidney injury | 3 (1.8) [3] | 1 (1.0) [1] | 1 (0.6) [1] | 5 (1.1) [5] | |
Data are presented as n (%) of patients with at least 1 event and number [n] of events.
Abbreviations: MF, miltefosine; PM, paromomycin; SSG, sodium stibogluconate.
Recruitment into arm 2 was discontinued.
Table 5.
Treatment-Emergent Adverse Drug Reactions classified as Common Terminology Criteria for Adverse Events ≥ Grade 3 by System Organ Class and Preferred Term—Modified Intention-to-Treat Set
| System Organ Class | Preferred Term | Arm 1, PM/MF 14 Days |
Arm 2, PM/MF 28 Daysa |
Arm 3, SSG/PM |
Overall |
|---|---|---|---|---|---|
| (n = 170) | (n = 98) | (n = 170) | (n = 438) | ||
| Any at least severeb treatment-emergent adverse drug reaction | 12 (7.1) [13] | 4 (4.1) [6] | 12 (7.1) [13] | 28 (6.4) [32] | |
| Investigations | 8 (4.7) [8] | 3 (3.1) [4] | 10 (5.9) [10] | 21 (4.8) [22] | |
| Aspartate aminotransferase increased | 6 (3.5) [6] | 1 (1.0) [1] | 4 (2.4) [4] | 11 (2.5) [11] | |
| Alanine aminotransferase increased | 0 (0.0) [0] | 2 (2.0) [2] | 1 (0.6) [1] | 3 (0.7) [3] | |
| Electrocardiogram QT prolonged | 0 (0.0) [0] | 0 (0.0) [0] | 3 (1.8) [3] | 3 (0.7) [3] | |
| Blood bilirubin increased | 1 (0.6) [1] | 0 (0.0) [0] | 1 (0.6) [1] | 2 (0.5) [2] | |
| Liver function test increased | 1 (0.6) [1] | 0 (0.0) [0] | 1 (0.6) [1] | 2 (0.5) [2] | |
| Blood creatinine increased | 0 (0.0) [0] | 1 (1.0) [1] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Ear and labyrinth disorders | 1 (0.6) [1] | 1 (1.0) [1] | 1 (0.6) [1] | 3 (0.7) [3] | |
| Hypoacusis | 0 (0.0) [0] | 1 (1.0) [1] | 1 (0.6) [1] | 2 (0.5) [2] | |
| Deafness bilateral | 1 (0.6) [1] | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Hepatobiliary disorders | 2 (1.2) [2] | 0 (0.0) [0] | 0 (0.0) [0] | 2 (0.5) [2] | |
| Hepatitis | 1 (0.6) [1] | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Drug-induced liver injury | 1 (0.6) [1] | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Renal and urinary disorders | 1 (0.6) [1] | 1 (1.0) [1] | 0 (0.0) [0] | 2 (0.5) [2] | |
| Acute kidney injury | 1 (0.6) [1] | 1 (1.0) [1] | 0 (0.0) [0] | 2 (0.5) [2] | |
| Blood and lymphatic system disorders | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.6) [1] | 1 (0.2) [1] | |
| Neutropenia | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.6) [1] | 1 (0.2) [1] | |
| Gastrointestinal disorders | 1 (0.6) [1] | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Vomiting | 1 (0.6) [1] | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.2) [1] | |
| Injury, poisoning, and procedural complications | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.6) [1] | 1 (0.2) [1] | |
| Cardiotoxicity | 0 (0.0) [0] | 0 (0.0) [0] | 1 (0.6) [1] | 1 (0.2) [1] |
Data are presented as n (%) of patients with at least 1 event and number [n] of events.
Abbreviations: MF, miltefosine; PM, paromomycin; SSG, sodium stibogluconate.
Recruitment into arm 2 was discontinued.
Events with a severity classification as severe (Common Terminology Criteria for Adverse Events [CTCAE] grade 3), life-threatening (CTCAE grade 4), or death (CTCAE grade 5).
Pharmacokinetics
PM exposure increased from day 1 to day 14, possibly due to decreased PM clearance over time [13]. PM AUC0–24 (area under the concentration-time curve) appeared higher in adolescents/adults (aged >12 years) than in children (aged ≤12 years), while Cmax was similar. One patient had a 10-fold higher PM exposure on day 14, probably due to renal failure resulting from acute kidney injury, which led to ototoxicity and bilateral deafness. Allometric MF dosing gave relatively similar MF exposures in children and adolescents/adults. Median day 28 MF concentrations in arm 2 and achievement of the pharmacokinetic target in children were higher than previously reported [12]. Detailed results of PK analysis and modeling will be presented separately.
DISCUSSION
In this study, we showed that a PM/MF combination achieved similar cure rates at 6 months follow-up as the standard-of-care SSG/PM in adult and pediatric patients with VL in eastern Africa. Noninferiority was demonstrated in the PP set but was narrowly missed in the mITT population. This is the first time a new combination treatment has achieved a cure rate of 91% in eastern Africa, exceeding that of MF monotherapy (72%), AmBisome and SSG (87%), and AmBisome and MF (77%) combination therapies in the Leishmaniasis East Africa Platform (LEAP) 0208 trial [5]. As expected, definitive cure rates as high as 99%, as for PM/MF in India [11, 14], were not achieved, probably due to differences in parasite susceptibility and higher genetic diversity of the African parasite population or to host factors such as immunological response [15], since drug exposure appeared adequate.
In our study, MF exposure in children aged ≤12 years was similar to that in adults, confirming the LEAP 0714 trial conclusion that allometric MF dosing is more suitable for children and leads to a satisfactorily high cure rate [7, 8].
Changes made to allow inclusion of a population more representative of VL patients in eastern Africa (exclusion criterion modified from body mass index to mid-upper arm circumference (MUAC) for Ethiopia, change in the laboratory abnormalities criterion) proved appropriate, as there was no apparent difference in efficacy before and after the protocol amendment.
The higher proportion of ADRs in arms 1 and 2 can mostly be attributed to MF intolerability; however, 6.5% of patients who received SSG/PM experienced SSG-related cardiotoxicity, including 1 patient with a fatal cardiac treatment-emergent serious adverse event (TESAE). PM/MF was generally well tolerated, with ADRs as expected given the drug safety profiles [16]. MF-related vomiting was usually a single episode, with only 1 patient discontinuing treatment. A patient with a TESAE of acute kidney injury had considerably increased PM plasma levels, hypothesized to have contributed to a subsequent serious ADR of bilateral deafness. This exceptional and unfortunate case shows the importance of monitoring renal function during treatment with PM.
PM/MF is more patient-friendly than SSG/PM with 1 less injection per day and a 3-day shorter treatment and hospitalization duration. This is particularly important since most VL patients are children. Additionally, this alternative treatment removes the risk of SSG-associated life-threatening cardiotoxicity.
The frequency of PKDL in patients from Ethiopia and Sudan was significantly lower in the PM/MF arm (4.4%) than the SSG/PM arm (20.9%). PM/MF would thus support VL control programs by reducing potential transmission reservoirs.
SSG/PM efficacy in this trial was 91.8%, similar to that previously described [10]. Nevertheless, a retrospective analysis of SSG/PM efficacy in MSF routine treatment in South Sudan (2001–2018) indicates a trend of increased relapse rates, not explained by changes in patient characteristics, compliance with treatment, or other factors [17]. A prospective study will be performed to better characterize response to treatment in South Sudan. A potential reduction in SSG/PM treatment effectiveness is another reason to bring alternative combination treatments to eastern Africa.
The preponderance of male patients in this trial is consistent with the particularly low proportion of women of childbearing potential (10% to 19%) among VL patients in eastern Africa [2]. The requirement for a pregnancy test and contraception during treatment and for 5 months afterward probably contributed to the very low number of adolescent and adult female patients who participated in the study and is an access barrier for this population, especially in Sudan and the West Pokot region. Possible strategies to minimize this barrier should be sought through qualitative research in reproductive health. In addition, access plans in endemic countries may include collection of effectiveness and safety information within routine VL care for this population and the elderly.
Life-threatening hemorrhage is a well-known complication of splenic aspiration, occurring in about 0.1% of individuals [3]. The 2 deaths associated with this procedure during screening highlights the need to validate and replace current methods with noninvasive parasitological diagnostics and tests of cure for VL.
In conclusion, our results demonstrate that a 14-day PM/MF regimen for VL has a similar efficacy to the standard of care for patients in eastern Africa. This alternative treatment regimen is associated with a lower frequency of PKDL, has no risk of SSG-associated life-threating cardiotoxicity, and is more patient-friendly.
For the longer term, a pipeline of new chemical entities aims to deliver oral treatments that will respond to patients’ needs as highlighted in the World Health Organization Neglected Tropical Diseases (NTD) Roadmap [18].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Ahmed M Musa, Institute of Endemic Diseases, University of Khartoum, Khartoum, Sudan.
Jane Mbui, Centre for Clinical Research, Kenya Medical Research Institute, Nairobi, Kenya.
Rezika Mohammed, Leishmaniasis Research and Treatment Center, University of Gondar, Gondar, Ethiopia.
Joseph Olobo, Department of Immunology and Molecular Biology, Leishmaniasis Unit, College of Health Sciences, Makerere University, Kampala, Uganda.
Koert Ritmeijer, Médecins sans Frontières, OCA, Amsterdam, The Netherlands.
Gabriel Alcoba, Médecins sans Frontières, OCG, Geneva, Switzerland.
Gina Muthoni Ouattara, Drugs for Neglected Diseases Initiative, Nairobi, Kenya.
Thaddaeus Egondi, Drugs for Neglected Diseases Initiative, Nairobi, Kenya.
Prossy Nakanwagi, Drugs for Neglected Diseases Initiative, Nairobi, Kenya.
Truphosa Omollo, Drugs for Neglected Diseases Initiative, Nairobi, Kenya.
Monique Wasunna, Drugs for Neglected Diseases Initiative, Nairobi, Kenya.
Luka Verrest, Department of Pharmacy & Pharmacology, The Netherlands Cancer Institute–Antoni van Leeuwenhoek Hospital, Amsterdam, The Netherlands.
Thomas P C Dorlo, Department of Pharmacy & Pharmacology, The Netherlands Cancer Institute–Antoni van Leeuwenhoek Hospital, Amsterdam, The Netherlands.
Brima Musa Younis, Institute of Endemic Diseases, University of Khartoum, Khartoum, Sudan.
Ali Nour, Institute of Endemic Diseases, University of Khartoum, Khartoum, Sudan.
Elmukashfi Taha Ahmed Elmukashfi, Institute of Endemic Diseases, University of Khartoum, Khartoum, Sudan.
Ahmed Ismail Omer Haroun, Institute of Endemic Diseases, University of Khartoum, Khartoum, Sudan.
Eltahir A G Khalil, Institute of Endemic Diseases, University of Khartoum, Khartoum, Sudan.
Simon Njenga, Centre for Clinical Research, Kenya Medical Research Institute, Nairobi, Kenya.
Helina Fikre, Leishmaniasis Research and Treatment Center, University of Gondar, Gondar, Ethiopia.
Tigist Mekonnen, Leishmaniasis Research and Treatment Center, University of Gondar, Gondar, Ethiopia.
Dagnew Mersha, Médecins sans Frontières, Abdurafi, Ethiopia.
Kasaye Sisay, Médecins sans Frontières, Abdurafi, Ethiopia.
Patrick Sagaki, Amudat Hospital, Amudat Karamoja Sub-region, Uganda.
Jorge Alvar, Drugs for Neglected Diseases Initiative, Geneva, Switzerland.
Alexandra Solomos, Drugs for Neglected Diseases Initiative, Geneva, Switzerland.
Fabiana Alves, Drugs for Neglected Diseases Initiative, Geneva, Switzerland.
Notes
Acknowledgments. The authors thank the patients involved in this study and their families and communities, without whom this work would not have been possible; all co-investigators, nurses, laboratory personnel, and hospital administrators who allowed the authors to conduct the study in their respective study sites; and staff at the 5 Leishmaniasis East Africa Platform (LEAP) sites and 2 Doctors Without Borders (Médecins Sans Frontières, MSF) sites: Kacheliba in Kenya; Amudat in Uganda; Doka, Umelkher, and Tabarakallah (MSF) in Sudan; and Gondar and Abdurafi (MSF) in Ethiopia. The authors are thankful to the DNDi clinical team members, Samuel Tesema, Ayub Mpoya, Bonface Kaunyangi Mwarama, Millicent Aketch, and Lilian Were, and the Data Management and Biostatistics Department, as well as the local consultant clinical monitors in Sudan and Ethiopia. They also thank the Data and Safety Monitoring Board members and Louise Burrows (DNDi) who drafted the manuscript.
Financial support. This work was supported by the European and Developing Countries Clinical Trials Partnership (supported by the European Union); the Dutch Ministry of Foreign Affairs (Directeur-generaal Internationale Samenwerking, DGIS), the Netherlands; the Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) through KfW, Germany; and other private individuals and foundations. DNDi also thanks UK aid, Médecins sans Frontières International, and the Swiss Agency for Development and Cooperation (SDC) for supporting its overall mission. T. D. was supported by the Dutch Research Council (Nederlandse organisatie voor wetenschappelijk onderzoek, NWO/ZonMw; project 91617140).
Sponsorship. DNDi sponsored this clinical trial. F. A., J. A., T. E., G. M. O., T. O., A. S., M. W., and P. N. acknowledge employment by DNDi. H. F., J. M., R. M., S. N., A. N., A. I. O. H., A. M., E. T. A. E., T. M., P. S., J. O., B. M. Y., and T. A. acknowledge that DNDi sponsored this trial and payments were made to their institutions for the implementation of the work and provision of study materials.
References
- 1. World Health Organization . Global leishmaniasis surveillance: 2019–2020, a baseline for the 2030 roadmap. Wkly Epidemiol Rec 2021; 35:401–20. [Google Scholar]
- 2. Harhay MO, Olliaro PL, Vaillant M, et al. Who is a typical patient with visceral leishmaniasis? Characterizing the demographic and nutritional profile of patients in Brazil, East Africa, and South Asia. Am J Trop Med Hyg 2011; 84:543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization , Technical Report Series 949. Control of the leishmaniases. Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010:1–186.
- 4. Omollo R, Alexander N, Edwards T, et al. Safety and efficacy of miltefosine alone and in combination with sodium stibogluconate and liposomal amphotericin B for the treatment of primary visceral leishmaniasis in East Africa: study protocol for a randomized controlled trial. Trials 2011; 12:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wasunna M, Njenga S, Balasegaram M, et al. Efficacy and safety of AmBisome in combination with sodium stibogluconate or miltefosine and miltefosine monotherapy for African visceral leishmaniasis: phase II randomized trial. PLoS Negl Trop Dis 2016; 10:e0004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dorlo TPC, Kip AE, Younis BM, et al. Visceral leishmaniasis relapse hazard is linked to reduced miltefosine exposure in patients from eastern Africa: a population pharmacokinetic/pharmacodynamic study. J Antimicrob Chemother 2017; 72:3131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mbui J, Olobo J, Omollo R, et al. Pharmacokinetics, safety, and efficacy of an allometric miltefosine regimen for the treatment of visceral leishmaniasis in eastern African children: an open-label, phase II clinical trial. Clin Infect Dis 2019; 68:1530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palic S, Kip AE, Beijnen JH, et al. Characterizing the non-linear pharmacokinetics of miltefosine in paediatric visceral leishmaniasis patients from eastern Africa. J Antimicrob Chemother 2020; 75:3260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hailu A, Musa A, Wasunna M, et al. Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: a multicentre, open-label, randomized trial. PLoS Negl Trop Dis 2010; 4:e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Musa A, Khalil E, Hailu A, et al. Sodium stibogluconate (SSG) & paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: a randomised controlled trial. PLoS Negl Trop Dis 2012; 6:e1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sundar S, Sinha PK, Rai M, et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet 2011; 377:477–86. [DOI] [PubMed] [Google Scholar]
- 12. Dorlo TPC, Huitema ADR, Beijnen JH, De Vries PJ. Optimal dosing of miltefosine in children and adults with visceral leishmaniasis. Antimicrob Agents Chemother 2012; 56:3864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verrest L, Wasunna M, Kokwaro G, et al. Geographical variability in paromomycin pharmacokinetics does not explain efficacy differences between eastern African and Indian visceral leishmaniasis patients. Clin Pharmacokinet 2021; 60:1463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goyal V, Mahajan R, Pandey K, et al. Field safety and effectiveness of new visceral leishmaniasis treatment regimens within public health facilities in Bihar, India. PLoS Negl Trop Dis 2018; 12:e0006830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alves F, Bilbe G, Blesson S, et al. Recent development of visceral leishmaniasis treatments: successes, pitfalls, and perspectives. Clin Microbiol Rev 2018; 31:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paladin Therapeutics . Highlights of prescribing information: Impavido (revised March 2014). 2014. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Accessed 2 May 2022.
- 17. Naylor-Leyland G, Collin SM, Gatluak F, et al . The increasing incidence of visceral leishmaniasis relapse in South Sudan: a retrospective analysis of field patient data from 2001-2018. PLoS Negl Trop Dis 2022; 16:e0010696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization . Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030. 2020. Available at: https://www.who.int/publications/i/item/9789240010352. Accessed 8 June 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.