Abstract
Background
We aimed to describe the frequency of use and effectiveness of bezlotoxumab (BZX) and fecal microbiota transplantation (FMT) in patients with Clostridioides difficile infection (CDI) in real-world practice.
Methods
This was a retrospective study conducted in a university hospital in which adult patients treated with BZX or FMT from January 2018 to April 2021 were included. The primary objective was to evaluate the effectiveness of BZX and FMT in preventing early (within 8 weeks) and late (within 1 year) CDI recurrences (rCDI). A multivariate analysis of risk factors for early recurrence was performed.
Results
Of 1377 consecutive CDI episodes, 117 (8.5%) received BZX or FMT, with full information available for 100 of the episodes: 51 received BZX, and 49 received FMT. BZX was used mostly in immunosuppressed patients (66.7%) and in first episodes or first recurrences in 70.6% of the cases. FMT was prescribed only in CDI recurrences. Despite the different conditions of the patients, there were no significant differences between BZX and FMT in preventing early rCDI (19.6% vs 24.5%; P = .55) or late rCDI (9.8% vs 18.4%; P = .31). In the multivariate analysis, risk factors for recurrence were presence of ≥2 previous rCDI episodes (odds ratio [OR], 2.90; 95% CI, 1.03–8.63) and use of non-CDI antibiotics (OR, 3.45; 95% CI, 1.24–9.57).
Conclusions
BZX and FMT were infrequently used in real-world practice. Both treatments had similar effectiveness in preventing CDI recurrence despite their application to different populations.
Keywords: bezlotoxumab, Clostridioides difficile, fecal microbiota transplantation, recurrence, risk factors
Clostridioides difficile infection (CDI) represents one of the most important nosocomial infections, with recurrence rates estimated to be 15%–20% in patients after first episodes and 40%–65% in patients with previous recurrences and attributable costs ranging between €2882 and €4396 for first CDI episodes and between €10 877 and €14 023 for recurrent CDI (rCDI) episodes [1–4].
Currently, there are recommended therapies that have demonstrated reduction in rates of rCDI, such as bezlotoxumab (BZX) and fecal microbiota transplantation (FMT) [5–10]. However, the positioning of the guidelines on the indications of both treatments is not entirely clear [11, 12], and their use in real life is not well known and usually subject to the influence of cost (BZX) and treatment availability (FMT). In addition, cost and availability are limitations for both treatments in many centers. At present, real-world experience with these strategies is limited and needed.
We aimed to describe the frequency of use and the characteristics of patients with CDI episodes treated with BZX or FMT in our institution. We assessed the effectiveness of BZX and FMT in early CDI recurrences that occurred within the first 8 weeks after an episode of CDI and in late recurrences that appeared from 8 weeks to 1 year later. Second, we evaluated the global mortality and attributable mortality at 8 weeks and 1 year of follow-up in both cohorts of patients.
METHODS
This was a retrospective study conducted in a university hospital from January 2018 to April 2021. All adult patients with confirmed CDI episodes according to the European Society of Clinical Microbiology and Infectious Diseases criteria [12] were prospectively included in a database from the microbiology department. Patients who received BZX or FMT during this period were selected. For the objectives of the study, a follow-up of at least 8 weeks was required, so cases for which there were no follow-up data were excluded.
BZX was approved in 2018, and it was administered as a single dose during vancomycin treatment for CDI. Doses were calculated at 10 mg/kg as per the manufacturer indications. Vials were stored and prepared in the pharmacy department. Our hospital has significant experience with FMT, and since 2018 FMT has been performed with lyophilized oral capsules, whose elaboration has been previously described [13, 14]. Patients received a single dose of FMT after completing a course of oral vancomycin or fidaxomicin for the treatment of a CDI episode. Selection of treatment in each case was decided according to local guidelines and per the advice of infectious diseases specialists and pharmacy department.
Patient demographics including age, sex, nursing home residence, and medical conditions were recorded. Variables accounting for the CDI episode in which the indication of BZX or FMT was established were the type of episode (first episode, first recurrence, and second or further recurrences) and the severity of clinical presentation (nonsevere, severe, or severe-complicated) according to Infectious Diseases Society of America criteria [11]. A microbiological diagnosis of CDI was made on direct stool samples by a 2-step algorithm: first immunochromatography detecting GDH and A + B toxin and then polymerase chain reaction (PCR) confirmation (GeneXpert, Cepheid, Sunnyvale, CA, USA). In addition, toxigenic cultures were made for all stool samples. Other microbiological variables included were the toxin B cycle threshold (Ct) determined by reverse transcription PCR (RT-PCR; GeneXpert). Presumptive 027 strains were confirmed by molecular PCR ribotyping [15]. Clinical variables were obtained from the electronic health record system and microbiological variables from the microbiology department database.
We planned a multivariate analysis of the risk factors associated with early rCDI. We also prespecified several subgroup analyses in patients treated with BZX or FMT in the same numerical episode (first recurrence and second or further recurrences), in patients with immunosuppression conditions, in patients aged >65 years, and in patients who received systemic non-CDI antibiotics.
Definitions
Early recurrent CDI episode was defined as a microbiologically confirmed CDI episode in the 8 weeks following treatment either with BZX or FMT.
Late rCDI episode was defined as the rCDI appearing 8 weeks to 1 year later.
Immunosuppression conditions included the following: solid organ transplantation (SOT), hematopoietic stem cell transplantation (HSCT), solid organ malignancies, hematologic malignancies, HIV infection, and receipt of other immunosuppressive treatments.
Use of non-CDI antibiotics was recorded if patients received systemic antibiotics up to 8 weeks after the administration of BZX or FMT to treat infections other than CDI.
Mortality attributable to a CDI episode was recorded if deaths were directly related to the CDI episode or caused by other pathological processes.
Statistical Analysis
Quantitative variables were expressed as medians with interquartile ranges (IQRs). Qualitative variables were expressed as frequencies and percentages. Univariate analysis of data comparisons was performed using the unpaired t test for normally distributed continuous variables and using the Mann-Whitney test for non–normally distributed variables. Categorical variables were compared using the χ2 test or using the Fisher exact test when the χ2 test was not appropriate. Adjusted odds ratios (ORs) were computed using multivariate logistic regression analysis. Stepwise logistic regression analysis included variables with a P value <.05 in the univariate analysis or that were clinically relevant. All statistical analyses were performed using IBM SPSS Statistics for Windows (version 25.0; IBM Corp, Armonk, NY, USA).
Patient Consent
The local institutional review boards and ethics committees approved the project with code number MICRO.HGUGM.2019-021. Due to the noninterventional and retrospective nature of the analysis, we were exempted from requesting informed consent from the patients.
RESULTS
From January 2018 to April 2021, there were 1377 CDI episodes at our institution. Of these, 117 episodes (8.5%) were treated either with BZX or FMT. Three cases were excluded from the analysis because sufficient data were not available in the patients’ clinical records, and a further 14 episodes were excluded because there were no data about outcomes. Finally, 100 CDI episodes were analyzed: 51 episodes were treated with BZX, and 49 were treated with FMT.
Bezlotoxumab Experience
The clinical characteristics of the 51 CDI episodes that were treated BZX are summarized in Table 1. The median age of the 51 cases (IQR) was 73 (62–82) years, and 32 patients (62.7%) were female. Thirty-four patients (66.7%) had immunosuppression. The median Charlson comorbidity index score (IQR) was 5 (3–8). BZX was administered on first episodes in 35.3%, on first recurrences in 35.3%, and on second or further recurrences in 29.4%. Overall, 45.0% of the CDI episodes were clinically severe. Regarding the outcome, there were 10 (19.6%) early recurrences within 8 weeks and 5 (9.8%) late CDI episodes within 1 year of follow-up. The mortality rate was 7.8% in the first 8-week period and 27.5% in the 1-year follow-up period. None of the deaths were CDI related.
Table 1.
Comparison Between Bezlotoxumab- and Fecal Microbiota Transplantation–Treated CDI Episodes
| Variables | BZX (n = 51) | FMT (n = 49) | P |
|---|---|---|---|
| Baseline characteristics | |||
| • Age, median (IQR), y | 73 (62–82) | 79 (70–88) | .13 |
| • Cases older than 65 y | 36 (70.6) | 41 (83.7) | .12 |
| • Sex (females) | 32 (62.7) | 34 (69.4) | .48 |
| • Nursing home residence | 5 (9.8) | 3 (6.1) | .49 |
| • Immunosuppression | 34 (66.7) | 9 (18.4) | <.01 |
| Solid malignancies | 12 (23.5) | 3 (6.1) | |
| Hematologic malignancies | 9 (17.6) | 1 (2.0) | |
| HSCT | 3 (5.9) | 0 | |
| SOT | 11 (21.6) | 0 | |
| Other conditions | 2 (3.9) | 5 (10.2) | |
| • Inflammatory bowel disease | 4 (7.8) | 5 (10.2) | .68 |
| • Dialysis | 10 (19.6) | 3 (6.1) | .04 |
| • Congestive heart failure | 9 (17.6) | 20 (40.8) | .01 |
| • Charlson index score, median (IQR) | 5 (3–8) | 5 (4–6) | .77 |
| • Proton pump inhibitors | 2 (3.9) | 19 (38.8) | <.01 |
| Clinical presentation | |||
| • First episode | 18 (35.3) | 0 | <.01 |
| • First recurrence | 18 (35.3) | 18 (36.7) | .88 |
| • ≥2 recurrences | 15 (29.4) | 31 (63.3) | <.01 |
| • Nonsevere | 28 (54.9) | 39 (79.6) | <.01 |
| • Severe | 23 (45.0) | 10 (20.4) | .01 |
| • B toxin Ct, median (IQR) | 24 (22–29) | 23 (22–26) | .12 |
| • 027 presumptive strains | 1 (1.9) | 0 | .51 |
| • 027 ribotype confirmed strains | 0 | 0 | 1 |
| Previous CDI treatments | |||
| • Metronidazole | 10 (19.6) | 14 (28.6) | .29 |
| • Vancomycin | 29 (56.9) | 45 (91.8) | <.01 |
| • Vancomycin tapering | 3 (5.9) | 17 (34.7) | <.01 |
| • Fidaxomicin | 4 (7.8) | 10 (20.4) | .08 |
| • Fecal microbiota transplantation | 2 (3.9) | 6 (12.2) | .16 |
| • Bezlotoxumab | 0 | 1 (2.0) | .49 |
| Evolution | |||
| • Recurrences at 8 wk | 10 (19.6) | 12 (24.5) | .55 |
| • Later recurrences | 5 (9.8) | 9 (18.4) | .31 |
| • Non-CDI antibiotics after BZX/FMT | 22 (43.1) | 8 (16.3) | <.01 |
| • Global mortality at 8 wk | 4 (7.8) | 3 (6.1) | .73 |
| • Global mortality 8 wk to 1 y | 14 (27.5) | 7 (14.3) | .10 |
| • Attributable mortality to CDI | 0 | 2 (4.1) | .24 |
| • Attributable mortality to CHF | 1 (2.0) | 2 (4.1) | .46 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BZX, bezlotoxumab; CDI, Clostridioides difficile infection; CHF, congestive heart failure; Ct, cycle threshold; FMT, fecal microbiota transplantation; HSCT, hematopoietic stem cell transplantation; rCDI, recurrent CDI; SOT, solid organ transplantation.
Fecal Microbiota Transplantation Experience
The median age of the 49 CDI patients treated with FMT (IQR) was 79 (70–88) years, and 69.4% were female (Table 1). The median Charlson comorbidity index score (IQR) was 5 (4–6). Patients received FMT in cases either with first recurrences (36.7%) or with several CDI recurrences (63.3%), which clinically were predominantly nonsevere (79.6%). Most of the patients had previously been treated with oral vancomycin, and 10 cases (20.4%) had received previous treatment with fidaxomicin. As for the outcome, 12 (24.5%) cases presented early CDI recurrences at 8 weeks, and a further 9 (18.4%) cases developed late rCDI. Three (6.1%) patients died during the following 8-week period, and a further 7 (14.3%) patients died within the year of follow-up. Two of the deaths were considered attributable to CDI episodes.
Risk Factors for Recurrence Following BZX or FMT
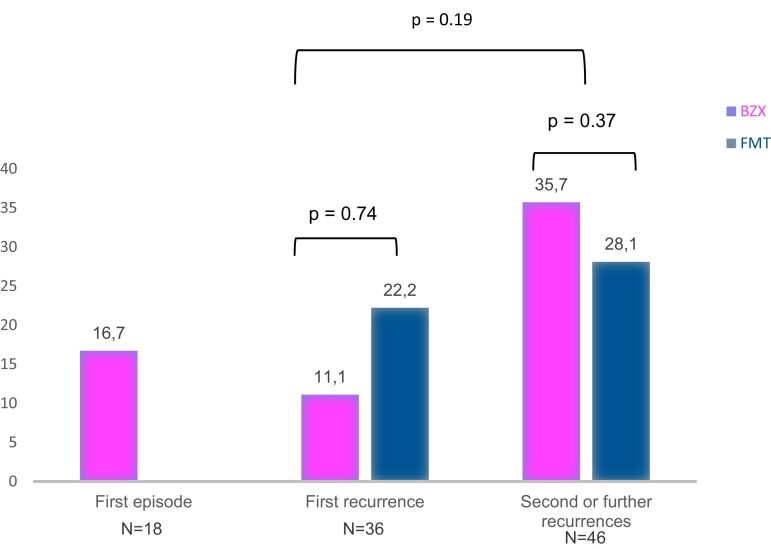
Table 2 describes the comparison between cases with early recurrences following therapy with BZX or FMT (n = 22) and cases without early recurrences (n = 78). The recurrence group had been treated for second or further rCDI episodes (63.6% in the recurrence group vs 41.0% in the no recurrence group; P = .05) and presented more frequent prescriptions of systemic non-CDI antibiotics in the following 8 weeks (50.0% vs 24.4%; P = .02). Frequencies of recurrences at 8 weeks according to the type of episode were as follows: 16.7% in patients treated for their first episode, 16.7% in those treated for their first recurrence, and 30.4% for cases treated after 2 or more rCDI episodes (Figure 1). In the multivariate analysis, risk factors for recurrence included age >65 years, presence of ≥2 previous rCDI episodes, and use of systemic non-CDI antibiotics. The factors associated with recurrences at 8 weeks were presence of ≥2 previous rCDI episodes (OR, 2.90; 95% CI, 1.03–8.63; P = .04) and use of non-CDI antibiotics (OR, 3.45; 95% CI, 1.24–9.57; P = .01) (Table 3).
Table 2.
Comparison Between Cases With Recurrence at 8 Weeks and Cases Without Recurrences
| Variables | Recurrence (n = 22) | No Recurrence (n = 78) | P |
|---|---|---|---|
| Baseline characteristics | |||
| • Age, median (IQR), y | 83 (66–89) | 74 (66–84) | .16 |
| • Cases older than 65 y | 17 (77.3) | 60 (71.4) | .97 |
| • Sex (females) | 17 (77.3) | 49 (62.8) | .20 |
| • Nursing home residence | 2 (9.1) | 6 (7.7) | .83 |
| • Immunosuppression | 8 (36.4) | 35 (44.9) | .47 |
| • Inflammatory bowel disease | 2 (9.1) | 7 (8.9) | .98 |
| • Dialysis | 2 (9.1) | 11 (14.1) | .53 |
| • Chronic heart failure | 7 (31.8) | 22 (28.2) | .79 |
| • Charlson index score, median (IQR) | 6 (3–8) | 5 (3–6) | .60 |
| • Proton pump inhibitors | 4 (18.2) | 10 (12.8) | .71 |
| Clinical presentation | |||
| • First episode | 2 (9.1) | 12 (15.4) | .45 |
| • First recurrence | 6 (27.3) | 30 (38.5) | .33 |
| • ≥2 rCDIs | 14 (63.6) | 32 (41.0) | .05 |
| • Nonsevere | 14 (63.6) | 53 (67.9) | .70 |
| • Severe | 8 (36.4) | 24 (30.8) | .61 |
| • B toxin Ct, median (IQR) | 22 (20–27) | 24 (22–27) | .16 |
| Previous CDI treatments | |||
| • Metronidazole | 4 (18.2) | 20 (25.6) | .46 |
| • Vancomycin | 19 (86.4) | 55 (70.5) | .31 |
| • Vancomycin tapering | 7 (31.8) | 13 (16.7) | .11 |
| • Fidaxomycin | 4 (18.2) | 10 (12.8) | .52 |
| CDI strategy treatment | |||
| • Bezlotoxumab | 10 (45.5) | 41 (52.6) | .55 |
| • Fecal microbiota transplantation | 12 (54.5) | 37 (47.4) | .55 |
| Other treatments | |||
| Non-CDI antibiotics after BZX/FMT | 11 (50.0) | 19 (24.4) | .02 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CDI, clostridioides difficile infection; Ct, cycle threshold; FMT, fecal microbiota transplantation; rCDI, recurrent CDI.
Figure 1.
Percentage of recurrences at 8 weeks following BZX or FMT for each type of episode. Abbreviations: BZX, bezlotoxumab; FMT, fecal microbiota transplantation.
Table 3.
Multivariate Analysis of Risk Factors of Recurrence at 8 Weeks
| Factor | OR | 95%CI | P |
|---|---|---|---|
| Age >65 y | 0.74 | 0.21–2.53 | .63 |
| ≥2 rCDIs | 2.90 | 1.03–8.63 | .04 |
| Non-CDI antibiotics after FMT/bezlotoxumab | 3.45 | 1.24–9.57 | .01 |
Abbreviations: CDI, clostridioides difficile infection; FMT, fecal microbiota transplantation; rCDI, recurrent CDI.
Subgroup Analyses
Subgroup analyses comparing the rate of recurrences of BZX and FMT in patients treated for their first recurrence or further recurrences, in patients with immunosuppression, or in patients older than age 65 years showed no significant differences between these 2 treatments. There was a nonsignificant trend toward more recurrences in the subgroup of patients who received FMT and were treated with non-CDI systemic antibiotics, in contrast to those who were treated with BZX (62.5% vs 27.3%: OR, 2.29; 95% CI, 0.55–9.63; P = .07) (Table 4).
Table 4.
Subgroup Analysis
| Variable | rCDI at 8 Weeks, No. (%) | P |
|---|---|---|
| First CDI recurrence | FMT, 4/18 (22.2) Bezlotoxumab, 2/18 (11.1) |
.74 |
| ≥2 rCDIs | FMT, 9/32 (28.1) Bezlotoxumab, 5/14 (35.7) |
.37 |
| Immunosuppression | FMT, 2/9 (22.2) Bezlotoxumab, 6/34 (17.6) |
.79 |
| Age >65 y | FMT, 11/41 (26.8) Bezlotoxumab, 6/36 (16.7) |
.39 |
| Non-CDI antibiotics | FMT, 5/8 (62.5) Bezlotoxumab, 6/22 (27.3) |
.07 |
Abbreviations: CDI, clostridioides difficile infection; FMT, fecal microbiota transplantation; rCDI, recurrent CDI.
DISCUSSION
Our work highlights several important facts: First, BZX and FMT were infrequently used in real-world practice (8.5% in our series) and in very different clinical scenarios, as BZX was more frequently assigned to patients with immunosuppression and for first and severe episodes, while FMT was indicated in cases with several prior CDI recurrences. Second, despite these differences, both strategies of treatment had equivalent effectiveness, assessed in terms of early and late rCDI episodes. Third, BZX and FMT appeared to be more effective in the treatment of first recurrences compared with second or further rCDI episodes.
The efficacy of BZX and FMT has been evaluated in clinical trials and in real-life series, both proving a reduction in the risk of CDI recurrence [8–10, 16, 17]. However, there are no studies that have directly compared the efficacy between these 2 treatment strategies. In addition, the indications established for each of these treatments based on recent guidelines, in our experience, make it difficult to know if these strategies are equivalent. Nevertheless, our results suggest that when prescribed they present similar effectiveness in preventing early recurrences. At 1-year follow-up, BZX had a trend toward fewer late CDI episodes compared with FMT (9.8% vs 18.4%; P = .31), but we believe that this effect could be partially explained by the higher non-CDI-related mortality rate observed in the BZX group at 1 year of follow-up (27.5% vs 14.3%; P = .10).
The positioning of the guidelines based on the design of these studies and the availability of one or the other treatment in hospitals mean that the strategies for preventing relapsing episodes of CDI are heterogeneous. Current recommendations advocate the use of BZX for first recurrences and FMT for second or subsequent recurrences [11, 12]. In this regard, a key aspect to consider is the cost-effectiveness of each strategy. Recently, a predictive model demonstrated that FMT is the most cost-effective therapy for treating first recurrent CDI episodes [18]. Our results emphasize the need to reevaluate the indications for justification in current guidelines that do not consider FMT as an alternative therapy in the first rCDI episodes.
The possibility of a synergistic effect when combining these strategies is unknown. Even the possibility of applying these treatments after a course of fidaxomicin is poorly understood. In a clinical trial comparing the use of SER-109 with standard treatment in patients with multiple rCDIs, the subgroup that previously received fidaxomicin had the lowest recurrence rates [19]. However, in our series we cannot establish the effect of the combination of fidaxomicin with either FMT or BZX because its use was very infrequent due to our policy for its indication; in our center in that period, it was approved only for patients with second or further recurrences. Further research with high-quality clinical randomized trials is needed in order to more accurately answer all these questions.
In the multivariate analysis, we found that risk factors for CDI recurrence after BZX or FMT were cases treated for their second or further recurrence and the use of systemic non-CDI antibiotics afterwards. Our results are in concordance with previous publications and emphasize the need for preventive strategies for rCDI, including a close and specific antimicrobial stewardship intervention to avoid the use of inadequate antibiotics in these patients [20]. Finally, we also observed an improvement in recurrence rates when these therapies were administrated earlier in the course of disease (first episodes and/or first recurrences). These data are in accordance with previous publications [21].
The limitations of our work were the retrospective design, the heterogeneity of patients selected in each arm of treatment, and the lower number of patients included. However, this is one of the largest studies to compare these therapeutic strategies. The absence of a comparative control group made it difficult to estimate the effectiveness of BZX and FMT. Nevertheless, the recurrence rate in both groups is in agreement with the results obtained in previous studies [8–10, 16, 17]. We also cannot establish if these treatments were effective in CDI episodes caused by 027 ribotypes, because in our series there were no 027-confirmed strains. Finally, late recurrences, defined as those diagnosed between 8 weeks and 1 year of follow-up, could be due to reinfections and not relapses, as no typing was performed to verify it. Strengths included the large series with real-world experience in both strategies and the detailed analysis with a multivariate analysis for risk factors associated with recurrence and a subgroup analysis of the patients with higher risk of rCDI.
CONCLUSIONS
In our experience, BZX and FMT were infrequently used in real-world practice but had similar effectiveness in preventing recurrent C. difficile episodes, despite application to different populations.
Acknowledgments
We are grateful for the collaboration of Silvia Vázquez, who is an essential part of the team in the elaboration of the FMT capsules. We would also like to thank Jose Maria Bellón for his advice on statistics analysis and data presentation.
Financial support . This study was partially funded by Fondo de Investigaciones Sanitarias (FIS), Research Project number PI20/01381, Merck & Co., Inc., Project IISP 58817, and by the European Regional Development Fund (FEDER; “A way of making Europe”).
Author contributions. S.V. designed the study, coordinated the study, collected the data, analyzed the data, interpreted the results, and wrote the article. S.H., P.M., C.R, M.V., E.R., A.A.U., L.A., M.M., M.O., M.K., E.C., and E.B. collected the data, interpreted the results, and critically reviewed the article.
Contributor Information
Sofía de la Villa, Clinical Microbiology and Infectious Diseases Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain; Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain.
Sergio Herrero, Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain; Pharmacy Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain.
Patricia Muñoz, Clinical Microbiology and Infectious Diseases Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain; Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain; Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain; CIBER Enfermedades Respiratorias, CIBERES (CB06/06/0058), Madrid, Spain.
Carmen Rodríguez, Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain; Pharmacy Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain.
Maricela Valerio, Clinical Microbiology and Infectious Diseases Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain; Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain; Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain.
Elena Reigadas, Clinical Microbiology and Infectious Diseases Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain; Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain; Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain.
Ana Álvarez-Uría, Clinical Microbiology and Infectious Diseases Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain; Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain.
Luis Alcalá, Clinical Microbiology and Infectious Diseases Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain; Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain.
Mercedes Marín, Clinical Microbiology and Infectious Diseases Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain; Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain.
María Olmedo, Clinical Microbiology and Infectious Diseases Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain; Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain.
Martha Kestler, Clinical Microbiology and Infectious Diseases Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain; Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain; Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain.
Esther Chamorro, Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain; Pharmacy Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain.
Emilio Bouza, Instituto de Investigación Sanitaria Gregorio Marañón, Madrid, Spain; Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain; CIBER Enfermedades Respiratorias, CIBERES (CB06/06/0058), Madrid, Spain.
References
- 1. Song JH, Kim YS. Recurrent Clostridium difficile infection: risk factors, treatment, and prevention. Gut Liver 2019; 13:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bouza E, Cobo J, Rodríguez-Hernández MJ, et al. . Economic burden of recurrent Clostridioides difficile infection in adults admitted to Spanish hospitals. A multicentre retrospective observational study. Rev Esp Quimioter 2021; 34:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asensio A, Di Bella S, Lo Vecchio A, et al. . The impact of Clostridium difficile infection on resource use and costs in hospitals in Spain and Italy: a matched cohort study. Int J Infect Dis 2015; 36:31–8. [DOI] [PubMed] [Google Scholar]
- 4. Bouza E. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin Microbiol Infect 2012; 18:5–12. [DOI] [PubMed] [Google Scholar]
- 5. Louie TJ, Miller MA, Mullane KM, et al. . Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–31. [DOI] [PubMed] [Google Scholar]
- 6. Cornely OA, Crook DW, Esposito R, et al. . Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012; 12:281–9. [DOI] [PubMed] [Google Scholar]
- 7. Al Momani LA, Abughanimeh O, Boonpheng B, Gabriel JG, Young M. Fidaxomicin vs vancomycin for the treatment of a first episode of Clostridium difficile infection: a meta-analysis and systematic review. Cureus 2018; 10:e2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilcox MH, Gerding DN, Poxton IR, et al. . Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017; 376:305–17. [DOI] [PubMed] [Google Scholar]
- 9. van Nood E, Vrieze A, Nieuwdorp M, et al. . Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368:407–15. [DOI] [PubMed] [Google Scholar]
- 10. Cammarota G, Masucci L, Ianiro G, et al. . Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther 2015; 41:835–43. [DOI] [PubMed] [Google Scholar]
- 11. McDonald LC, Gerding DN, Johnson S, et al. . Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Prehn J, Reigadas E, Vogelzang EH, et al. . European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect 2021; 27:S1–21. [DOI] [PubMed] [Google Scholar]
- 13. Reigadas E, Bouza E, Olmedo M, et al. . Faecal microbiota transplantation for recurrent Clostridioides difficile infection: experience with lyophilized oral capsules. J Hosp Infect 2020; 105:319–24. [DOI] [PubMed] [Google Scholar]
- 14. Reigadas E, Olmedo M, Valerio M, et al. . Fecal microbiota transplantation for recurrent Clostridium difficile infection: experience, protocol, and results. Rev Esp Quimioter 2018; 31:411–8. [PMC free article] [PubMed] [Google Scholar]
- 15. Stubbs SL, Brazier JS, O'Neill GL, Duerden BI. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J Clin Microbiol 1999; 37:461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Du C, Luo Y, Walsh S, Grinspan A. Oral fecal microbiota transplant capsules are safe and effective for recurrent Clostridioides difficile infection: a systematic review and meta-analysis. J Clin Gastroenterol 2021; 55:300–8. [DOI] [PubMed] [Google Scholar]
- 17. Johnson TM, Molina KC, Howard AH, et al. . Real-world comparison of bezlotoxumab to standard of care therapy for prevention of recurrent Clostridioides difficile infection in patients at high risk for recurrence. Clin Infect Dis 2022; 74:1572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aby ES, Vaughn BP, Enns EA, Rajasingham R. Cost-effectiveness of fecal microbiota transplantation for first recurrent Clostridioides difficile infection. Clin Infect Dis 2022; 75:1602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feuerstadt P, Louie TJ, Lashner B, et al. . SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med 2022; 386:220–9. [DOI] [PubMed] [Google Scholar]
- 20. Tariq R, Hayat M, Pardi D, Khanna S. Predictors of failure after fecal microbiota transplantation for recurrent Clostridioides difficile infection: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 2021; 40:1383–92. [DOI] [PubMed] [Google Scholar]
- 21. Hengel RL, Ritter TE, Nathan RV, et al. . Real-world experience of bezlotoxumab for prevention of Clostridioides difficile infection: a retrospective multicenter cohort study. Open Forum Infect Dis 2020; 7:ofaa097. doi: 10.1093/ofid/ofaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]