Abstract
Despite marked progress in the management of atrial fibrillation (AF), detecting AF remains difficult and AF-related complications cause unacceptable morbidity and mortality even on optimal current therapy. This document summarizes the key outcomes of the 8th AFNET/EHRA Consensus Conference of the Atrial Fibrillation NETwork (AFNET) and the European Heart Rhythm Association (EHRA). Eighty-three international experts met in Hamburg for 2 days in October 2021. Results of the interdisciplinary, hybrid discussions in breakout groups and the plenary based on recently published and unpublished observations are summarized in this consensus paper to support improved care for patients with AF by guiding prevention, individualized management, and research strategies. The main outcomes are (i) new evidence supports a simple, scalable, and pragmatic population-based AF screening pathway; (ii) rhythm management is evolving from therapy aimed at improving symptoms to an integrated domain in the prevention of AF-related outcomes, especially in patients with recently diagnosed AF; (iii) improved characterization of atrial cardiomyopathy may help to identify patients in need for therapy; (iv) standardized assessment of cognitive function in patients with AF could lead to improvement in patient outcomes; and (v) artificial intelligence (AI) can support all of the above aims, but requires advanced interdisciplinary knowledge and collaboration as well as a better medico-legal framework. Implementation of new evidence-based approaches to AF screening and rhythm management can improve outcomes in patients with AF. Additional benefits are possible with further efforts to identify and target atrial cardiomyopathy and cognitive impairment, which can be facilitated by AI.
Keywords: Atrial fibrillation, Artificial intelligence, Heart failure, Atrial cardiomyopathy, Cognitive function, Dementia, Outcomes, Quality of care, Cost, Research, Rhythm management, Catheter ablation, Anticoagulation, Bleeding, Research priorities, Technology, Stroke, Integrated care, Screening, AFNET, EHRA, Guidelines, Consensus statement
Introduction
Despite marked progress in the detection and management of patients with atrial fibrillation (AF), the arrhythmia remains undetected in a large proportion of patients, particularly in the elderly. Atrial fibrillation confers an important public health burden, causing high mortality and morbidity, impacting affected patients and their families, and incurring high costs to healthcare systems.1,2
Implanted devices, and more recently, wearables, as well as other consumer electronics, enable long-term continuous monitoring of biosignals, providing the ability to detect rare arrhythmias including AF. Initial results from continuous rhythm recording over years have provided insights into the natural history of AF and generated evidence on AF burden-related outcomes. It appears intuitive that more and longer atrial arrhythmias should be associated with higher complication rates, but a clear biological gradient supporting this assumption with data is missing.3 Defined screening populations as well as modes of screening and analysis are required to render AF screening beneficial and cost-effective.4,5
New data show that early rhythm control therapy, initiated after a recent clinical diagnosis of AF, can improve cardiovascular outcomes and mortality in patients with AF and cardiovascular risk factors.4 These findings will change the concept and practice of rhythm management. Rhythm management should preferably include a resolute attempt at rhythm control in patients with new AF, but also comprise rate control to prepare patients for recurrences of AF. There has also been increasing focus on cognitive function assessment in patients with AF for early detection and prevention of cognitive impairment and its negative impact on treatment compliance.6 These new data call for better integration of AF screening and early rhythm control in clinical care.
Translational research suggests a complex concept of atrial cardiomyopathy as a major driver for AF incidence and progression, as well as AF-related complications. Advances have been made in the characterization and quantification of atrial cardiomyopathy from cellular to clinical levels. Atrial dysfunction and the broad spectrum of clinical and risk factors have been used to define populations at risk of AF in primary stroke prevention as well as post-stroke cohorts. Artificial intelligence (AI)-based methods integrating multimodal information and extracting central features have shown promising first results, but legal barriers for its implementation have to be overcome while ensuring fair and secure use of data. To improve estimation of AF-related risk and to guide therapy of the arrhythmia, further translational science and clinical research is needed to better understand the different underlying mechanisms that are reflected by the electrocardiographic (ECG) pattern of AF.
Methods
The 8th AFNET/European Heart Rhythm Association (EHRA) Consensus Conference brought together 83 international interdisciplinary experts including arrhythmia and heart failure (HF) specialists, patients with AF, an AF patient organization, pharmacologists, translational scientists, general practitioners, neurologists, nurse practitioners, epidemiologists, consultants in public health medicine, clinical trialists, and health economists in Hamburg, Germany, for 2 days of intense discussion in the plenary and in breakout groups. Participants who could not travel for pandemic-related reasons participated remotely. The results of the expert discussion and their potential clinical impact are summarized here.
Atrial fibrillation screening implementation
Approaches to screening
The 2020 European Society of Cardiology (ESC) guidelines on AF management recommend opportunistic screening for AF in persons aged ≥65 years (Class I, Level B) as well as systematic screening for AF in individuals aged ≥75 years, or at high risk of stroke (Class IIa, Level B).1 Efforts to reduce the burden of AF complications by an early diagnosis have been made through opportunistic and systematic screening approaches, but also through interrogation of cardiac implantable devices and consumer devices, e.g. wearables.
The recommendation for opportunistic screening during medical visits for purposes other than screening was based on a study using pulse palpation carried out in the UK before 2005.7 New evidence emerged after the publication of the guidelines. The D2AF (Detecting and Diagnosing Atrial Fibrillation) study showed that opportunistic screening in primary care did not increase the detection of AF in individuals aged >65 years.8 In addition, no difference in AF detection was demonstrated when using a single-lead handheld ECG during regularly scheduled office visits in the larger VITAL-AF (Screening for Atrial Fibrillation Among Older Patients in Primary Care Clinics) study, except for a signal for increased detection in patients aged ≥85.9 Similar observations were made in the randomized MonDAFIS (Systematic monitoring for detection of atrial fibrillation in patients with acute ischaemic stroke) study comparing systematic ECG monitoring with additional Holter-ECG recording for up to 7 days in hospital and usual care in patients hospitalized with acute ischaemic stroke.10 These studies illustrate the improvement in AF detection in routine care, i.e. the control arms, during the last decade, possibly because of increased awareness by health professionals and the public attributable to dissemination of the importance of stroke prevention by AF detection and treatment. At the same time, these studies underline the well-known shortcomings of opportunistic AF screening that may miss high-risk parts of the population.
Systematic ECG screening could improve AF detection by including patients who do not seek medical help, as demonstrated in two large recent randomized clinical trials.11,12 In the population-based, randomized STROKESTOP study, AF screening using ECG recordings from a portable device, taken twice daily for 2 weeks, was used in individuals aged 75–76 years. This intervention resulted in a reduction of the combined outcome of mortality, stroke, and severe bleeding compared with a control group.11 In the LOOP study, AF screening in the intervention group was conducted using an implantable cardiac device, which continuously monitored heart rhythm for 3 years. In case of AF detection for >6 min, oral anticoagulant (OAC) therapy was initiated. Despite a three-fold increase in AF detection and OAC initiation, the LOOP study did not show a significant decrease in the outcome of stroke and systemic embolism compared with the control group.12
With many consumer devices integrating algorithms for detecting AF readily available, consumer-led screening is a growing trend that healthcare systems need to respond to. While the conceptual ability of AF detection using consumer electronics, e.g. smartwatches, has been shown,13–15 substantial challenges remain, including access to the technology, usability in elderly populations and in populations with relevant risk factors, data privacy, legal, data transfer issues, AF diagnosis validation, and, importantly, sound information on when to initiate treatment in the light of the potential for over-diagnosis of arrhythmias without clinical implications. Once the therapeutic implications of detecting short, rare atrial arrhythmias are understood16,17 and the usability and reliability in elderly populations has been improved, consumer electronics may be a suitable way to enhance AF screening.
Evidence-based implementation of systematic screening for atrial fibrillation
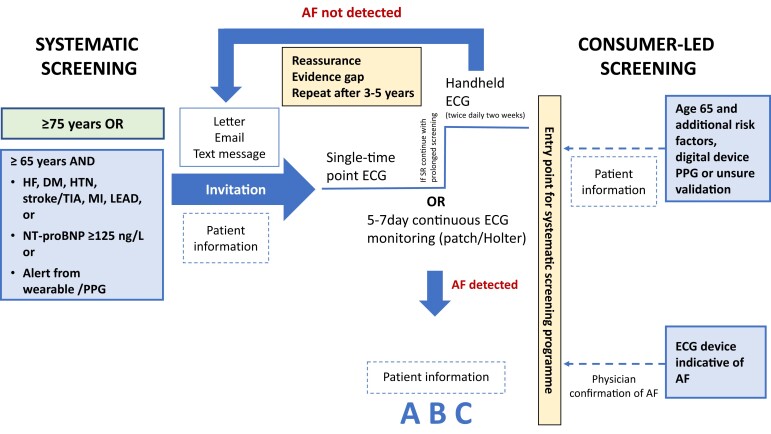
To enable the implementation of systematic screening for AF, the participants of the 8th AFNET EHRA consensus conference propose a simple, scalable, and pragmatic AF screening pathway suitable for different healthcare systems. Such a pathway may improve timely diagnosis of AF and subsequently prevent AF-related complications. At the same time, such efforts can improve research on AF screening by providing systematic evaluation of screening strategies and devices. The consensus conference experts recommend systematic screening for all individuals aged ≥75 years, and that systematic screening may be considered for individuals aged 65–74 years with additional risk factors [e.g. HF, hypertension, diabetes, previous stroke/TIA, myocardial infarction (MI), lower extremity artery disease], elevation of natriuretic peptides [N-terminal pro B-type natriuretic peptide (NT-proBNP) ≥125 ng/L], or a positive alert by a digital device using photoplethysmography (PPG, Figure 1). Individuals considered for screening could be invited by the healthcare institution responsible for the screening initiative by letter, email, phone call, or text message based on local availability and practice. Ideally, the invitation should include information on the potential benefits and harms of AF screening in the participants’ own language using simple wording.
Figure 1.
Suggested systematic screening pathway and entry of consumer-led screening into the systematic screening pathway. The scores comprise congestive HF, hypertension, age ≥75 years, DM, stroke, vascular disease, age 65–74 years, sex category (female) (CHA2DS2-VASc score) and hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR (international normalized ratio), elderly (>65 years), drugs/alcohol concomitantly (HAS-BLED). Consider HAS-BLED to minimize bleeding risk. DM, diabetes mellitus; ECG, electrocardiogram; HF, heart failure; LEAD, lower extremity arterial disease; MI, myocardial infarction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PPG, photoplethysmography; TIA, transient ischaemic attack; SR, sinus rhythm.
The ECG screening procedure should be adapted to available healthcare resources. In case of in-person screening, a single time point screen using, for example, a rhythm strip or 12-lead ECG, could be used to diagnose AF.1 If this does not show AF, 2-week twice-daily handheld ECG monitoring should be performed. Alternatively, a 5–7 day (up to 14 days) continuous ECG patch or Holter recording could be performed.18 In continuous monitoring, an AF episode lasting ≥30 s is diagnostic according to current guidelines.1 As evidence showing the benefits of screening grows (affect-eu.eu),19 discussions with regulatory authorities should be initiated to ensure reimbursement for AF screening and define the ensuing diagnostic and therapeutic work-up.
Following AF detection, a medical assessment by a medical team experienced in AF management, in an in-person or remote setting, is required to confirm the diagnosis of AF and to evaluate prognosis. The assessment will evaluate patient information, confirmation of the CHA2DS2-VASC [congestive HF, hypertension, age ≥75 years, diabetes mellitus (DM), stroke, vascular disease, age 65–74 years, sex category (female)] stroke risk score, and detection of additional concomitant cardiovascular conditions and risk factors. Subsequently, a comprehensive AF management incorporating all elements of the guideline-recommended approach (avoiding stroke, better rhythm and rate management, treatment of concomitant cardiovascular conditions), considering diagnosis and therapy of concomitant cardiovascular diseases as well as risk factors, can be initiated.1 Individuals without detected arrhythmias should be reassured. In these individuals, a timeframe for repeated screening/monitoring need to be established in different categories of pre-detection risk. The value of repeated screening and the ideal time point for follow-up (FU) using single time point ECGs are still unclear and should be investigated in future trials.20–22
Digital approaches to AF screening were rapidly developed in response to the COVID-19 pandemic and may become the preferred pathway. Systematic AF screening can be achieved using entirely digital technology: Electronic medical records or the population registry (if available) can be used to identify participants eligible for screening based on age. A letter with a link (e.g. a QR code) to an online questionnaire can be sent to confirm whether individuals are willing to participate in the screening programme. Individuals aged 65–74 years would also be invited to answer questions about comorbidities (with the help of their general practitioner if needed, Figure 1). Thereafter, a screening device, e.g. an ECG patch or a PPG-based pulse device, can be posted to participants for a recording and returned to the healthcare provider to complete the digital pathway. Alternatively, the pathway could make use of a device already accessible to the participant. If AF is detected and confirmed in the recording, participants are invited for an appointment with a healthcare professional to implement therapy as recommended according to the A–B–C {Atrial fibrillation Better Care [includes A (avoid stroke), B (better symptom control/better rhythm management), and C (cardiovascular risk factors and comorbid conditions management)]} pathway,1 which could also potentially take place as a digital appointment.
Consumer-led atrial fibrillation screening
For individuals who have screened themselves for AF using their own devices, i.e. consumer-led screening, false positive findings by the device may be more frequent than in systematic screening. The rate of false positives depends on the specifications of the device, its algorithm, and the pre-test probability in the individual. Therefore, confirmation needs to be done, taking into account individual risk and unrecognized symptoms. The large-scale Apple,13 Huawei,23 and Fitbit (NCT04380415) Heart Studies have illustrated the potential of fast, nationwide recruitment of screening participants and demonstrated low rates of false positives among participants who completed the screening. The vast majority of participants were younger than 50 years old and drop-out rates were high. The studies also showed that consumer-led AF screening leads to increased use of healthcare resources in the short term. First, arrhythmias require review and verification by a health professional. Secondly, other arrhythmia findings will trigger additional contacts with the healthcare system for verification. Thirdly, consumer-based AF screening will also be conducted by individuals without stroke risk factors. There is a lack of evidence on the implications and the benefits of screening in patients who are not at risk of stroke, and in young populations. The magnitude of the resources involved is not known and should be estimated during the evaluation of consumer-led ECG AF screening. Although the resources may be substantial, it can also be advantageous as consumer-led AF screening could be an entry point into a more systematic screening programme for patients at cardiovascular risk. Consumer-led screening engages people and gets them involved in managing and learning about AF and their health, being more adherent, focusing on prevention, and thereby has the potential to reinforce the concept of shared decision-making. Further evidence to determine the effectiveness of consumer-led AF screening would be of benefit.24
The clinical implications of AF detected by consumer-led screening are not known and dedicated studies, including outcome studies of consumer-screening led interventions, are needed. In consumer-led screening, a diagnosis of AF can be made if the individual used a device with ECG recording, and confirmation was provided by a physician after inspection of the rhythm strip.1 Digital devices can monitor the rhythm for variable durations depending on the technology used and patient adherence. Therefore, calculated AF burden will differ depending on the device used.13–15 A structured AF management and initiation of therapy should be considered on an individual basis. Recent data indicate that untreated opportunistic screening-detected AF has a similar stroke risk to clinically detected AF.21,25 Reassuringly, anticoagulation used in the LOOP study did not show an increased risk of bleeding in patients with atrial arrhythmias detected by an implantable loop recorder, but the stroke rate was lower than expected based on AF diagnosis and stroke risk.12
Knowledge gaps and hurdles
There are still important knowledge gaps, e.g. the ideal timing of repeat screening and the threshold of AF burden related to an increased stroke risk which should lead to the initiation of oral anticoagulation (and other AF therapies) in patients at risk if AF is picked up by long-term monitoring, e.g. using implanted devices. It is also unclear whether picking up irregular pulses by PPG signals could lead to a more direct recommendation of anticoagulation in patients at risk, in the future. For digital AF screening, digital health literacy, inclusiveness of minorities, and equality in access,26–28 digital pathways to enable participation, outreach initiatives, overcoming geographic distances,29 awareness of AF, and education are key. Financial coverage needs to be secured, with regard to screening, the ensuing additional visits and investigations, and the subsequent treatment. Today, it remains a privilege to advance digital AF screening when in most regions of the world, even an ECG is not broadly available and pulse palpation has to be considered as an alternative.
The growing role of rhythm management
The paradigm of rhythm management
Recent controlled trials and observational analyses demonstrated safety of rhythm control therapy.4,30–35 Combined with the effectiveness of early rhythm control,4 these findings suggest a wider use of rhythm control therapy to improve symptoms and quality of life, and provide an additional avenue to prevent outcomes such as stroke and cardiovascular death.4,36,37 While other mechanisms are still possible,32,36–38 recent data clearly support the hypothesis that these clinical effects are mediated by the lower arrhythmia burden achieved by systematic rhythm management.4,36,37 Therefore, it is timely to consider a new approach in the management of patients with clinically detected AF. This group proposes that this approach should encompass initiation of oral anticoagulation based on stroke risk, diagnosis and treatment of concomitant cardiovascular conditions and risk factors, as well as rhythm management. Goals of rhythm management include reduction of adverse outcomes (stroke, HF, hospitalization, cardiovascular mortality),4,35 symptom improvement, and improvement in quality of life. It prioritizes sinus rhythm maintenance, but retains rate control and AV-nodal therapy (ablate and pace) plus possibly cardiac resynchronization as an option when rhythm control is difficult to achieve.39
Rhythm management includes (i) rhythm and rate-controlling drugs, (ii) AF ablation, (iii) cardioversion, and/or (iv) AV-nodal therapy. Successful management requires determination of therapy safety for rhythm or rate control. Several randomized controlled trials underpin the safety of rhythm control therapy in elderly AF patients with concomitant cardiovascular diseases, including AF-CHF (Atrial Fibrillation and Congestive Heart Failure),33 CASTLE-AF (Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation),34 ATHENA (Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death from any Cause in Patients with Atrial Fibrillation/Atrial Flutter),35 and EAST-AFNET 4 (Early Rhythm-Control Therapy in Patients with Atrial Fibrillation).4 This is substantiated by analyses of electronic health records and observational data sets that do not show adverse safety signals.31,32 Thus, the safety of rhythm management has improved compared with the strategy tested in AFFIRM.40 Additional analyses of large health data sets are underway and may provide further information on the safety of early rhythm control therapy.
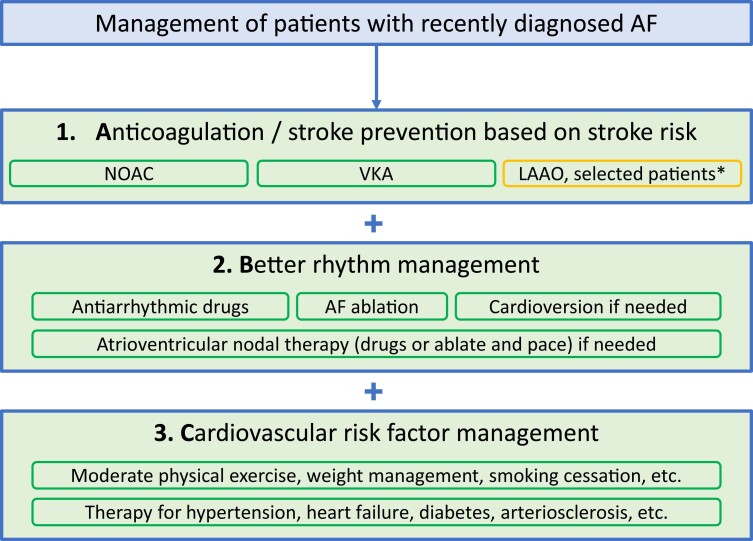
Successful rhythm management in selected patients should be embedded in an overall AF management strategy comprising proper anticoagulation (‘A’) and treatment of concomitant cardiovascular conditions and risk factors (‘C’). Reflecting on the new evidence available today, the meaning of ‘B’ in the A–B–C acronym for AF management could potentially be adapted to Better rhythm management in selected patients (Figure 2).
Figure 2.
Suggested A–B–C pathway for recently diagnosed AF. *LAAO is a potential option for selected patients with absolute contraindication for oral anticoagulation. LAAO, left atrial appendage occlusion; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist.
Attempt at restoration of sinus rhythm
Many patients are suitable for a trial of sinus rhythm restoration to reduce risk of cardiovascular events.4,35 Although the results of the EAST-AFNET 4 trial were not yet available, the potential of widely used rhythm management was already recognized in the 2020 ESC AF guidelines:1 The main recommendation on rhythm focuses on AF-related symptoms, but according to the 2020 ESC AF guidelines, rhythm management using antiarrhythmic drugs (AAD) and AF ablation are reasonable. Both usual care and systematic, early rhythm control equally improve symptoms,4,35 but cardiovascular complications are lower in patients randomized to early rhythm control,4,35 including in asymptomatic patients.36 In addition, the improved AF-related symptoms lead to better quality of life. However, it is important to manage expectations when starting rhythm management: While it is very likely that a reduced AF burden mediates the beneficial effects of rhythm management, successful rhythm management does not require complete freedom from recurrent AF and will often call for repeated intervention or adaptation of therapy.
A few patients will not be suitable for continued efforts to maintain sinus rhythm, and occasionally not even for an initial trial of restoration of sinus rhythm. Clinical examples are ‘legacy’ patients who have a very long history of AF without symptoms and in whom there is less evidence for clinical benefit of rhythm management, patients with severe atrial cardiomyopathy and atrial enlargement, patients with a recent stroke, patients who decide against rhythm management, end-of-life palliative patients, and very old patients in whom limited data are available. The decision to initiate rhythm management in such patients will need clinical acumen and shared decision-making.
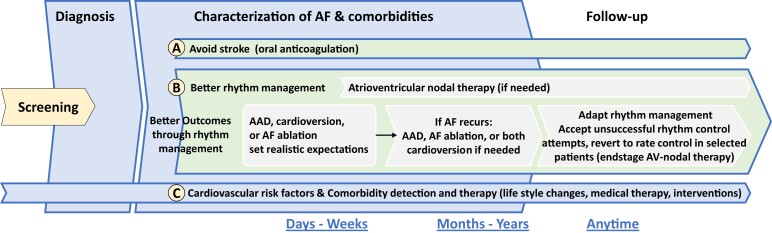
Lifelong rhythm management
When rhythm management is initiated, the concept of chronic disease management needs to be communicated and the treatment options presented (Figure 3). It is important to set out realistic treatment goals from the start.
Figure 3.
Lifelong AF management incorporating anticoagulation, rhythm management, and concomitant conditions. AAD, antiarrhythmic drugs; AF, atrial fibrillation.
Rhythm control starting with AAD can prevent AF-related outcomes as shown in ATHENA and EAST-AFNET 4.4,35 Rhythm management is a lifelong part of AF management and will typically require different treatment choices at different time points. These can include referral to AF ablation when AAD do not work,41 repeat AF ablation,30,42 or treatment with AAD after AF ablation.43 Atrial fibrillation ablation and antiarrhythmic drugs appear to have synergistic effects.43–45 Cardioversion is an important component of rhythm management although not a rhythm maintenance strategy per se, but typically a bridge to either AAD or AF ablation to achieve longer-term rhythm management. AV-nodal-directed therapies such as beta-blockers, verapamil/diltiazem, digitalis, or pacemakers have an important role in patients with chronic forms of AF and/or a high arrhythmia burden. But, based on the new evidence, an attempt at sinus rhythm restoration should be considered for many patients.
Potential of atrial fibrillation ablation therapy
Early rhythm management is effective in patients with AF and HF.37 Recent clinical trials suggest a possible preference for AF ablation in patients with HF.34,46 In patients with strong AF-related symptoms and in those in whom an optimal reduction of AF burden seems clinically important,47,48 AF ablation is also preferred, especially if done early in the course of their disease. Atrial fibrillation ablation is also more effective in improving AF-related quality of life and symptoms than antiarrhythmic drug therapy.49,50 Atrial fibrillation surgery may succeed in selected patients in whom other rhythm management options fail.
Practical implications to improve rhythm management
In view of recent data, many newly diagnosed patients with AF should have access to early rhythm management. This recommendation has major implications for training and resource provision. Rhythm management will need to be led not only by electrophysiologists, but also general cardiologists, HF specialists, and other healthcare professionals whose patients have AF. Nurse-led AF clinics may also play a role in improving access to and FU for rhythm management. Educating patients on the importance of rhythm management will be crucial. Practical next steps should include the following:
Healthcare professionals’ knowledge on prescribing and managing AAD will need to be updated and learned based on the principles laid out in AF guidelines. European Heart Rhythm Association and similar professional organizations are well placed to create new rhythm management training programmes.
Access to catheter ablation will need to be improved. This calls for simple, standardized procedures that can be delivered more widely and locally to the patient by well-trained teams. More complex procedures required for repeat ablations are probably better concentrated in specialized centres.
Access to cardioversion will need to be improved. This procedure can be done under sedation by general cardiologists, emergency physicians, and internists, as well as in specialized centres. Apart from direct current cardioversion, newer drugs such as vernakalant allow more effective rapid pharmacological cardioversion.51
Knowledge gaps and research opportunities
The tipping point at which rigorous rhythm management can improve outcomes still needs to be defined. Early rhythm control supports the hypothesis that the observed clinical effects are mediated by the lower arrhythmia burden achieved by rhythm management, but the exact mechanisms remain unclear. Furthermore, the identification of patients suitable for antiarrhythmic medication or direct ablation therapy needs to be optimized. Selective, more effective, and safer AAD may allow a tailored treatment of AF patients but requires further research. Left atrial appendage removal should be a routine part of open-heart surgery in patients with AF52 and the role of concomitant AF ablation at the time of cardiac surgery needs to be defined.
Assessment and treatment of cognitive impairment in atrial fibrillation
Atrial fibrillation may lead to cognitive impairment or dementia as a result of clinically overt and/or silent stroke. However, several pathways that are independent of stroke may also be implicated,53,54 including AF-induced systemic inflammation, chronic brain hypoperfusion,55 and side effects of AF-specific medication.56 Thus, AF significantly contributes directly to the burden of cognitive impairment and dementia for patients, and indirectly to their caregivers and society.6,57 It is likely that an integrated AF management which considers the assessment of cognitive function can reduce the risk of dementia.1 However, the potential protective role of AF treatment options for maintenance of cognitive function is not well established because the available data are derived from population-based observational studies. Fortunately, several randomized trials are ongoing (Table 1). In the absence of specific recommendations in the 2020 guidelines of the ESC,1 the lack of awareness regarding the impact of AF on cognitive function among physicians may be a key hurdle in clinical practice. Involvement of the patient, their family/caregiver(s), and primary care physician is needed. Conversely, screening for AF seems reasonable in patients presenting with dementia (Figure 4), as AF and dementia share risk factors like hypertension and diabetes, and therefore undetected AF may lead to further cognitive decline.
Table 1.
Current studies on cognitive function and dementia in relation to AF
| Trial / Study | Intervention / Treatment | Study Details | Cognition-Related Outcome | ||
|---|---|---|---|---|---|
| Oral anticoagulation | |||||
| ARISTA NCT03839355 |
Apixaban vs. warfarin | N = 280; FU = 24 months | Primary | Change in cognitive function (using standardized neurocognitive assessment) and new silent cerebral infarcts on MRI | |
| Randomized, single blinded | |||||
| Terminated due to low enrolment | |||||
| CAF | Dabigatran vs. warfarin | N = 120; FU = 24 months | Incident dementia or moderate cognitive decline at 24 months | ||
| NCT03061006 | Prospective, randomized, open label, blinded outcome assessment trial | ||||
| Secondary endpoints: Stroke/TIA, intracranial bleed, changes from baseline scores on MMSE and Hachinski ischaemic scale | |||||
| GIRAF | Dabigatran vs. warfarin | N = 200; FU = 24 months | Neuropsychological evaluation (MoCA + NINDS-CSN-Vascular Cognitive Impairment Harmonization) after 1 year and at the end of follow-up | ||
| NCT01994265 | Prospective, randomized, open, blinded outcome assessment, efficacy study | ||||
| BRAIN-AF | Rivaroxaban vs. standard care (placebo/ASA) | N = 2280; FU = 5 years | Cognitive decline (MoCA decreased ≥ 3 points from baseline) and/or stroke/TIA | ||
| NCT02387229 | |||||
| Prospective, randomized, double blind, efficacy study | |||||
| ARTESIA neurocognitive sub-study | Apixaban vs. ASA | N = 1000 | Change of cognitive function on MoCA | ||
| Prospective, multicentre, double-blind, randomized controlled trial | |||||
| NCT01938248 | |||||
| DaRe2THINK | Non-vitamin K oral anticoagulant vs. no therapy in AF at low to intermediate stroke risk | N = 3000; FU = 5 yearsNationwide England | Cognitive outcomes are cognitive decline using the UK Biobank cognitive assessment tests yearly and at 10 years, plus a sub-study of N=160 with brain imaging at baseline and 3 years plus Cambridge Neuropsychological Test Automated Battery (CANTAB) cognitive function | ||
| NCT04700826 | |||||
| OCEAN | Rivaroxaban vs. ASA | N = 1572; FU = up to 3 years | Secondary | Changes on neuropsychological testing (MoCA and MMSE) | |
| NCT02168829 | Prospective, open label, randomized trial | ||||
| NOAH-AFNET6 | Edoxaban vs. ASA | N = 2686; FU = 24 months | Cognitive function (MoCA) at 12 and 24 months | ||
| NCT02618577 | Prospective, parallel-group, double-blind, randomized, multicentre trial | ||||
| The LOOP study | Internal loop recorder monitoring and oral anticoagulation if AF detected or standard of care | N = 6.004; FU 65 months | Cognition-related outcome | ||
| Prospective, randomized, single blinded | |||||
| NCT02036450 | |||||
| Rhythm management | |||||
| DIAL-F NCT01816308 |
AAD vs. AF ablation | N = 888; FU = 24 months | Primary | MoCA at baseline and 2 years follow-up | |
| Prospective case–control study | |||||
| AFCOG NCT04033510 |
Standard of care intervention for restoring sinus rhythm | N = 600; FU = n/a | Differences in cognitive test performance (CANTAB) while in arrhythmia vs. sinus rhythm | ||
| Single cohort study | |||||
| Comparison of Brain Perfusion in Rhythm Control and Rate Control of Persistent Atrial Fibrillation | Rhythm control (propafanone) vs. control (apixaban) | N = 200; FU = 12 months | Cognitive functions (k-MoCA) and brain perfusion (CT) | ||
| Prospective, randomized control trial | |||||
| NCT02633774 | |||||
| EAST-AFNET4 | Early, comprehensive, rhythm control therapy vs. usual care | N = 2789; FU = 60 months | Secondary | MoCA at 24 months after randomization | |
| Prospective, randomized, open, blinded outcome assessment trial | |||||
| NCT01288352 | |||||
| Other | |||||
| UK SAFER trial | AF screening vs. no screening | N = 126 000; FU = 5 years | Secondary | Cognitive function | |
| Cluster randomized trial | |||||
| SWISS-AF | Cohort study | N = 2415; FU = 30 months | Annual neurocognitive function tests (not specified) | ||
| NCT02105844 | |||||
| Prospective, observational, multicentre cohort study | |||||
| PLUG dementia trial | Cohort study | N = 60; FU = 24 months | Primary | Incident dementia as determined by a neurologist, change in cognitive test (30% decrease in Alzheimer’s Disease Assessment Scale/score of <50% or 30% change on the Disability Assessment for Dementia) | |
| NCT03091855 | Terminated due to low enrolment | ||||
Estimated patient enrolment and completion date were taken from trial registration on ClinicalTrials.gov. ARISTA, Apixaban Versus Warfarin in Reducing Rate of Cognitive Decline, Silent Cerebral Infarcts and Cerebral Microbleeds in Patients With Atrial Fibrillation; CAF, Impact of Anticoagulation Therapy on the Cognitive Decline and Dementia in Patients With Non-Valvular Atrial Fibrillation. GIRAF, Cognitive Impairment Related to Atrial Fibrillation Prevention Trial; BRAIN-AF, Blinded Randomized Trial of Anticoagulation to Prevent Ischemic Stroke and Neurocognitive Impairment in Atrial Fibrillation; ARTESIA, Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation; OCEAN, Optimal Anticoagulation for Higher Risk Patients Post-Catheter Ablation for Atrial Fibrillation Trial; DIAL-F, Cognitive Impairment in Atrial Fibrillation; AFCOG, Acute Cognitive Changes During Atrial Fibrillation Episodes; SWISS-AF, Swiss Atrial Fibrillation Cohort Study; PLUG, Dementia Trial and MRI PLUG Dementia Sub-Study.
CANTAB, Cambridge Neuropsychological Test Automated Battery; FU, follow-up; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; MRI, magnetic resonance imaging.
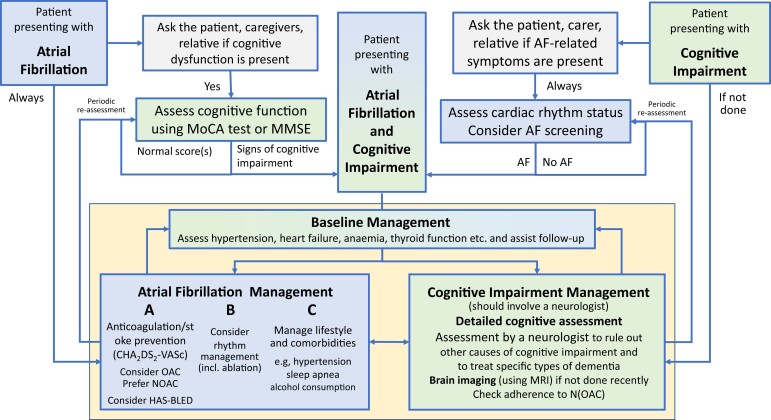
Figure 4.
Flow chart on three common presentations of AF and cognitive impairment (or dementia): (i) a patient presenting with known AF whose mental state is not the reason for the presentation, (ii) a patient with known cognitive impairment, and (iii) a patient with both, known AF and cognitive impairment. For the patient with known AF, simple tests of cognitive function may confirm the need for detailed assessment to evaluate the cause of cognitive impairment and initiate therapy for reversible causes. Similarly, patients presenting with cognitive impairment should undergo opportunistic screening for AF (pulse palpation eventually followed by an ECG) and, if AF is discovered, it should be assessed and managed initially by a specialist following the A–B–C (Atrial fibrillation Better Care [includes A (avoid stroke), B (better symptom control), C (cardiovascular risk factors and comorbid conditions management)] scheme recommended by the European Society of Cardiology. AF, atrial fibrillation; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; MRI, magnetic resonance imaging; NOAC, non-vitamin K oral anticoagulant; OAC, oral anticoagulant.
Specific aspects of management in patients with cognitive dysfunction
Cognitive dysfunction is defined as a reduction in one or more cognitive abilities, such as memory, awareness, judgement, and mental acuity, across the adult lifespan. Multiple significant cognitive impairments in memory plus one or more other cognitive defects are characteristic of dementia. As shown in Figure 4, a Mini-Mental State Examination (MMSE) or Montreal Cognitive Assessment (MoCA) test provide robust signals suggesting the presence of cognitive impairment. Such a finding should trigger a detailed cognitive assessment using an established and validated battery of tests. Furthermore, assessment by a neurologist and brain imaging using magnetic resonance imaging (MRI) can often identify the (most probable) aetiology for cognitive impairment and guide selection of disease-specific treatment.
There is clear evidence showing that anticoagulation therapy prevents strokes in patients with AF and stroke risk factors.1 Some data suggest that effective anticoagulation can preserve cognitive function in patients with AF.58 Unfortunately, cognitive dysfunction and dementia are among the factors leading to discontinuation of anticoagulation, thereby enhancing the risk of stroke and death.59,60 Moreover, cognitive dysfunction was associated with a shorter time in therapeutic range in patients treated with vitamin K antagonists.58
Evidence from the EAST-AFNET 4 trial shows that early rhythm management is associated with a lower risk of stroke,4 but there is no clear evidence that early rhythm management affects cognitive function 2 years after randomization.36 In a large retrospective cohort study using propensity score matching, AF patients who underwent catheter ablation had a reduced risk of stroke and dementia compared with non-ablation patients.61 In a population-based nationwide Korean cohort, catheter ablation was associated with a lower incidence and risk of dementia compared with AF patients who were medically managed.62 Despite potential residual confounding and bias, this could be attributed to the greater reduction of AF burden achieved by AF ablation compared with antiarrhythmic drug therapy.30 However, AF ablation also causes clinically silent white matter lesions as detected in studies using MRI.63–66 Whether or not ablation-related MRI-detected acute ischaemic brain lesions worsen cognitive function has not been demonstrated so far.54 A recent analysis of over 300 patients undergoing AF ablation and high-resolution brain MRI did not find an effect of MRI-detected lesions on cognitive function 3 months after AF ablation.67 Thus, even though it is clearly desirable that ablation-induced brain lesions are minimized, rhythm management could be considered for appropriately anticoagulated AF patients with cognitive dysfunction, given the possible reductions in stroke risk and progression to dementia (Figure 4).68
Identification and treatment of cardiovascular risk factors can reduce dementia.69 Increasing evidence points towards the importance of lifestyle modification in AF patients,70 although more data on cognitive decline and incident dementia are needed. For example, AF patients who start or continue regular exercise after the diagnosis of AF were observed to have a lower risk of dementia than persistent non-exercisers.71
Practical and research implications for cognitive dysfunction in patients with atrial fibrillation
Experts suggest that even a moderate suspicion for cognitive impairment should lead to formal cognitive function assessment in AF patients.72 In addition, patients with cognitive dysfunction/dementia should be assessed for the presence of AF, as AF has to be regarded as an independent risk factor of cognitive impairment. The presence of cognitive impairment should trigger a stepwise and interdisciplinary diagnostic pathway, aiming for optimal (non-)medical treatment to avoid the progression of cognitive dysfunction.73–76
Knowledge gaps and research opportunities
Further prospective studies addressing the interplay of cognitive (dys-)function and AF and its treatment are urgently needed, despite emerging evidence that anticoagulation is associated with a lower risk of dementia in AF patients.77,78 On the contrary, it is not known if rhythm management or therapy of concomitant conditions can reduce cognitive decline. Therefore, randomized controlled trials evaluating management strategies for AF should also evaluate the course of cognitive function. In addition, the FU duration of such interventional trials should also be adapted to adequately address cognitive decline in patients with AF. Ideally, brain imaging would be obtained to detect structural brain damage associated with cognitive decline.
Atrial (cardio)myopathies: definition, risk factors, and progression
Defining the concept
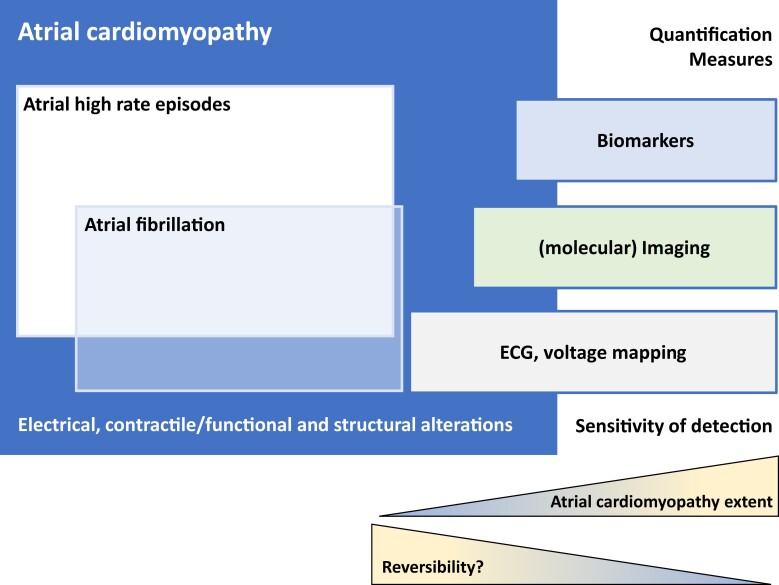
The earliest systematic characterisation of atrial (cardio)myopathy dates back to 1972.79 In line with the 2017 EHRA/HRS/APHRS/SOLAECE expert consensus,80 we consider atrial cardiomyopathy as an extension of the pathophysiological concept of AF initiation and perpetuation that comprises triggers, substrate, and modifying factors together with the genetic architecture. Atrial cardiomyopathy affects all cellular components of the atria and manifests in three main ways, where each component can be present alone or in combination with the others: electrical, contractile/functional, and structural alterations. We acknowledge that detecting all potential manifestations of atrial cardiomyopathy is not feasible yet, mainly because our diagnostic abilities of atrial imaging or electrical mapping are limited. Furthermore, the underlying pathophysiological processes are not completely known.81 For application in clinical care, atrial cardiomyopathy needs to be quantifiable and uniform definitions are a prerequisite. Several ways of measurement are possible and refined methods will improve detection. To date, it has been observed that detection increases with advanced impairment (Figure 5). Specific and sensitive non-invasive measures are not available to validate the clinical relevance of the concept of atrial cardiomyopathy. Indication-specific characterizations have to be developed, e.g. voltage mapping in invasive electrophysiology, combinations of electrocardiographic, imaging, and blood biomarker-based definitions (also see Table 2). The latter has been used as a provisional surrogate of atrial cardiomyopathy in the ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke) trial.116 In addition to clinical characteristics and imaging, circulating biomolecules reflecting general cardiovascular and atrial disease processes can help to detect and characterize atrial cardiomyopathy, e.g. natriuretic peptide, bone morphogenic protein 10, or fibroblast growth factor 23.104,117–119 Such definitions require prospective confirmation in relation to interventions and hard clinical outcomes.
Figure 5.
Atrial cardiomyopathy and its possible phenotypes: AF and atrial high rate episodes. Different potential quantification measures and their sensitivity for detection of atrial cardiomyopathy depending on the extent of impairment.
Table 2.
Quantification measures of atrial cardiomyopathy
| Measurement method | Parameters | References |
|---|---|---|
| X-ray | Dilated atrium | |
| Surface electrocardiogramme | Prolonged P-waves, abnormal P-waves, P-wave terminal force in ECG lead V1, sinoatrial node dysfunction, atrial ectopy | 82–86 |
| ECGi, electrocardiographic imaging | Abnormal activation, prolonged activation time, slowing of conduction velocity | 87,88 |
| Echocardiogramme | Dilated atrium, reduced contractility, reservoir function, conduit function, emptying fraction, left atrial strain imaging | 89–95 |
| CT scan | Abnormal atrial function, fatty infiltration, morphology: atrial sphericity, left atrial appendage shape | 96 |
| MRI | Dilated atrium, decreased contractility, abnormal morphology: sphericity, fibrosis (late gadolinium enhancement) | 97–103 |
| Blood biomarkers | Natriuretic peptides—atrial dilatation, myocyte stretch | 104–107 |
| Fibroblast growth factor—fibrosis | ||
| Bone morphogenic protein-10—atrial remodelling | ||
| Fatty acid binding protein 3—atrial metabolic disturbance | ||
| C-reactive protein, Interleukin-6—inflammation | ||
| Factor VIII, von Willebrand factor, Fibrinopeptide A—pre-thrombotic state, hypercoagulability | ||
| Electrophysiological 3-D mapping | Low voltage in sinus rhythm, prolonged atrial activation time, decreased conduction velocity | 108–114 |
| Biopsy | Fibrosis, fatty infiltration, collagen accumulation, amyloid depositions, endocardial remodelling, vascular rarefaction, molecular imaging | 115 |
Summary of potential methods used to quantify atrial cardiomyopathy.
Atrial cardiomyopathy disease progression
Although atrial cardiomyopathy is not precisely defined, nor the different stages of diseases are described in detail, the prevailing idea is that atrial cardiomyopathy progresses: electrical manifestations are ion channel remodelling that affects action potential duration and the development of low-voltage areas in the left atrium, causing AF to evolve from self-limiting to more persistent AF types. Contractile/functional manifestations include increased atrial size and loss of contractile function, as well as deterioration of conduit and reservoir functions of the atria.120 Progression of structural manifestations is characterized by cardiomyocyte hypertrophy, atrial fibrosis, fatty infiltration,121 atrial dilatation, and amyloid depositions.122 It can be assumed that early stages of atrial cardiomyopathy may be largely reversible, but with this progression of disease, these manifestations will become more permanent and less reversible. This idea aligns with the recent findings of EAST-AFNET44 which showed that early rhythm management in AF, i.e. within the first year of diagnosis, is beneficial for reducing the risk of common cardiovascular outcomes and in patients with detectable ventricular dysfunction.37
Atrial MRI has been proposed as a tool to quantify atrial cardiomyopathy and to assess the risk of recurrent AF after catheter ablation. Initial MRI studies (Delayed-Enhancement MRI Determinant of Successful Radiofrequency Catheter Ablation of Atrial Fibrillation, DECAAF 1) have demonstrated the correlation between the amount of fibrosis and AF recurrence.123 The multicentre results of Delayed-Enhancement MRI Determinant of Successful Radiofrequency Catheter Ablation of Atrial Fibrillation (DECAAF II) and Isolation of Pulmonary Veins With the Aid of Magnetic Resonance Imaging (ALICIA)124 trials could not demonstrate that this increased risk is modifiable by AF ablation. It remains unclear to what extent currently available MRI algorithms detect atrial cardiomyopathy that is related to low-voltage areas.125–127 In DECAAF II, the attempt to show the benefit of a fibrosis-guided individualized ablation strategy failed.
In addition, the extent of atrial cardiomyopathy has been related to the risk for cerebrovascular events.128,129 Signals for atrial cardiomyopathy could potentially be detected in community settings based on quantification of circulating cardiovascular biomolecules. Whether the concept of atrial cardiomyopathy will be adopted in clinical practice depends on its impact on patient management and whether the following central knowledge gaps can be sufficiently addressed.
Atrial cardiomyopathy and atrial fibrillation
Quantifying atrial cardiomyopathy and AF in patients may increase understanding of the temporal disconnect between AF and stroke.130 It seems likely that structural remodelling is influenced by the presence of cardiovascular risk factors and diseases as atrial remodelling occurs before the development of clinically detected AF.131 The atrial cardiomyopathy is aggravated by AF episodes and by concomitant cardiovascular conditions and risk factors. The concept of atrial cardiomyopathy may also be of value for patient communication and education to emphasize the importance of continuous risk factor and lifestyle management together with long-term use of medical therapies or FU visits because of the presence of atrial cardiomyopathy. It helps to avoid the notion that with the temporal freedom of AF, further preventive management may no longer be necessary.
A favourable clinical outcome currently is most likely achieved by optimal AF treatment (see earlier sections).
Potential practical implications
Atrial cardiomyopathy is a valuable emerging concept to explain the nature of AF and its management to patients.
The interactions between atrial cardiomyopathy and AF require quantification in patients, initially in research settings.
Better tools to objectively quantify atrial cardiomyopathy are needed. Electrocardiographic and electroanatomical mapping, modern imaging modalities, circulating biomolecules, and innovative combined analytical methods may become useful to provide such quantification.
Further potential clinical applications are provided in Table 3.
Table 3.
Potential clinical implications of atrial cardiomyopathy
|
| Progression, slowing progression |
|
|
|
|
|
|
|
| Potential future clinical implications |
|
|
|
|
|
|
|
|
ARCADIA, AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke; CAMERA-MRI, Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction; DECAAF, Delayed-Enhancement MRI-Guided Ablation versus Conventional Catheter Ablation of Atrial Fibrillation; EAST, Early treatment of atrial fibrillation for stroke prevention trial; MRI, magnetic resonance imaging; OAC, oral anticoagulant; OCEAN, Optimal anti-Coagulation for Enhanced-risk patients post-catheter Ablation for atrial fibrillation; RACE3, Routine Versus Aggressive Upstream Rhythm Control for Prevention of Early Atrial Fibrillation in Heart Failure.
Knowledge gaps and research opportunities
To investigate the central atrial cardiomyopathy hypothesis, we need basic, translational, and clinical research to more precisely define atrial cardiomyopathy, its risk factors, and progression. Clinically available accurate quantification of atrial cardiomyopathy is required to translate the concept of atrial cardiomyopathy into clinically meaningful management strategies. Furthermore, practical definitions of atrial cardiomyopathy are required for clinical trials and need to be tested for meaningful outcomes including hard endpoints.
Structuring high-quality care
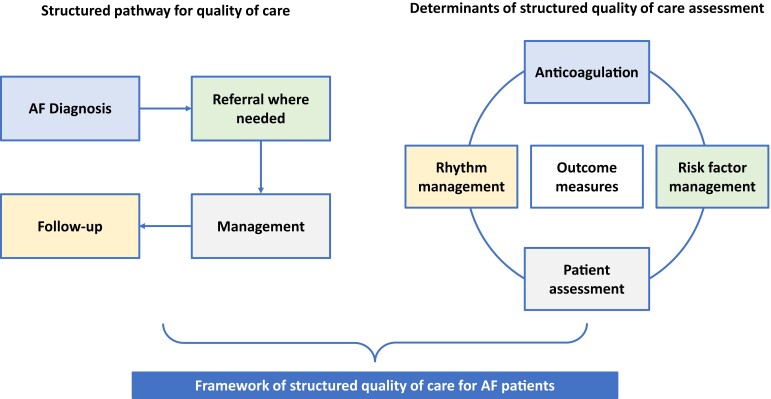
Structured quality of care for AF patients includes both having a structured pathway for delivering optimal quality of care for patients and a structured assessment of quality indicators (Figure 6). Quality of care encompasses (i) diagnosis, (ii) referral where needed, (iii) management, and (iv) FU. The main areas of quality assessment for AF diagnosis and management are: patient assessment (baseline and FU), anticoagulation therapy, rhythm management, and patient-reported outcome measures, as recently outlined by an ESC and EHRA-led international task force.138 In addition to treatment domains and outcomes, patient-reported outcomes are important measures to quantify quality of care.139
Figure 6.
Framework to deliver quality of care detailing a structured pathway for quality of care (left) and determinants of structured quality of care assessment (right).
The entire assessment of each patient should be structured, including detection and management of comorbidities, risk factors and lifestyle changes, anticoagulation and stroke prevention, and rhythm management.1 The upcoming EU-funded projects EHRA-PATHS140 (EHRA-PATHS, No. 847770) and AFFIRMO141 (AFFIRMO, No. 899871) work to develop a framework for how to improve AF management through a holistic, inclusive, and personalized care pathway centred on patients.142 Continuous FU and regular re-evaluation of the process and the quality indicators of care are needed. This structured approach should be clearly defined, interprofessional, transparent, and easy to implement. Responsibilities need to be clear, taking into account differences between various countries and local healthcare organizations. Nurses and general practitioners can have a certain autonomy in this structured approach; however, they should work in coordination with an expert in AF, e.g. a cardiologist.
An important quality measure is adherence to defined, structured care pathways.143 However, there is a knowledge gap on how to measure and ensure adherence to such pathways, which should be addressed by new research. The quality of care should be regularly evaluated, e.g. yearly, but again, there is a gap of knowledge on the optimal time interval for reassessment.
In addition to components of care and clinical outcomes, patient-reported outcomes are useful to assess the quality of care.144–146 These can be measured using scales capturing general health-related quality of life.138 Several validated tools such as EQ-5D-5L (European Quality of Life 5 Dimensions 5 Level)147,148 or SF-12 (short form survey)149 are available to measure it. Atrial fibrillation–specific quality-of-life questionnaires are validated, available,150,151 and recommended by the International Consortium of Healthcare Outcome Measures (ICHOM) for AF.144 Follow-up measurement of quality of life with the same tool is required to compare trends for evaluation and decision-making. Patient-reported experience measures such as patient satisfaction, subjective experiences (e.g. symptom control) or objective experiences (e.g. waiting time to appointment), and treatment burden are indicators to identify the quality of care.152
Education should be provided in an appropriate and understandable way for patients and their caregivers and can improve outcomes.148,153–155 Education of patients and continued medical education is needed to maintain care pathways, to achieve quality indicators, and to implement new technical developments.156 Knowledge gaps and unmet needs should be identified and addressed.157,158 An ESC and EHRA-funded project, STEEER-AF [STEEER-AF Project Study (escardio.org)] is currently evaluating the impact of an educational programme for HCPs on outcomes in patients with AF.159
Objective information on AF, its consequences, and its management should be available on websites, smartphone apps, or in print for patients and relatives, ideally available in all relevant local languages. Unbiased resources and reliable sources endorsed by scientific societies increase confidence, effectiveness, and trustworthiness.160 Health and digital literacy should be taken into account to ensure optimal education of patients and relatives and avoid potential adverse effects.157,161,162 Professional organizations such as EHRA and the ESC have an important role in the generation of such information.
Practical implications
Measuring the quality of care in patients with AF is required to improve it.
Outcomes measures capturing the efficacy and safety of therapy are known and should be measured.
The quality of care delivery can be estimated by measuring adherence to evidence-based recommendations in guidelines, possibly through audits.
In addition to these measures, patient-reported outcomes and patient experience are important domains for a comprehensive estimation of quality of care.
Knowledge gaps and research opportunities
The development of evidence-based educational interventions to improve knowledge and skills of healthcare providers needs to be fostered. Objective and easily ascertainable measures are required to ensure adherence to care pathways. The frequency of their re-evaluation needs to be determined. New care pathways should be developed to detect, address, and FU multimorbidity and polypharmacy in the often older and multimorbid patients with AF. Multidisciplinary and patient-centred approaches need to be designed. Further potential issues and barriers in the guideline-adherent management of AF at the patient and healthcare provider level will need to be identified and addressed.
Artificial intelligence in the management of atrial fibrillation
Artificial intelligence comprises methods that enable a computer to learn from data with two main subcategories: the supervised and the unsupervised learning methods. In supervised learning, the output or target is defined (e.g. recognition of a sinus rhythm or AF on the ECG). The learning process uses labelled data sets to solve classification and regression problems. In unsupervised learning, there is no prediction of any output or need for labelled data. Instead, raw data are modelled with the goal of identifying patterns and structures, and to cluster similar variables. Nowadays, unsupervised deep neural networks are the most popular method for identification of hidden signatures of diseases. However, it requires large data sets, and generates its own logical process, creating a black box that requires complex explanatory analysis methods.
There has been a massive increase of AI in the field of AF, with 5298 articles on AF and AI indexed in Pubmed since 2016.163 This trend is driven by two main factors. First, the electrical and imaging signals, which are the two pillars for AF assessment, are well-suited data for machine learning approaches. Secondly, the creation of large data sets, such as the Computing in Cardiology challenge 2017/PhysioNet,164 facilitated the deployment of studies on AI.
Artificial intelligence technologies have been used primarily in studies to improve AF detection, and, to a lesser extent, in studies to predict incident and recurrent AF in patients undergoing rhythm control therapy, or response to drug treatment in multimorbid patients.165 For example, deep neural networks have been used to detect AF based on ECG and pulse plethysmography signals.166–168 They improved the performance of AF identification based on such combinations of signals as compared to traditional AF detection algorithms.
Recent studies have implemented explanatory elements and allowed understanding of ECG features which can be used to better detect AF. Both, RR interval irregularity and lack of P-waves contribute to AF detection equally.169 Artificial intelligence algorithms also can predict impeding AF within the next 31 days based on a single standard 12-channel ECG in sinus rhythm using a deep learning convolutional network with an area under the curve of 0.87 for the detection of AF based on ECGs in sinus rhythm.170 When using multiple ECGs within the same time window, the area under the curve increased to 0.90. The same algorithm was subsequently used to predict incident AF in an independent cohort of elderly patients with a mean FU of 7.4 years.171 The performance was comparable with the clinical AF risk scores combining clinical information, ECG parameters, and blood pressure measurements. Taken together, these results demonstrate the predictive strength contained in the ECG. Such algorithms might be used to identify patients at risk for AF and in whom monitoring for AF might be initiated. Table 4 lists the ongoing registered trials using AI for AF care.
Table 4.
Prospective trials on AI-based methods listed currently under clinicaltrials.gov
| Intended use of AI method | Study title | No. participants | Country |
|---|---|---|---|
| AI-guided patient selection for AF catheter ablation | AI-PAFA | 340 | South Korea |
| NCT04997824 | |||
| AI-based AF detection referring to heart rhythm monitoring with wearable devices | WB-AF | 100 | Finland |
| NCT04917653 | |||
| AI-enabled ECG-based screening tool for AF | BEAGLE | 1000 | USA |
| NCT04208971 | |||
| Predicting patient-level new onset atrial fibrillation | Re-use of CPRD-GOLD and CPRD-AURUM172 | 140 000 | UK |
| AI-enhanced performance of Apple watch in ablation/cardioversion patients | AI-AW | 200 | France |
| NCT05045456 | |||
| Prevention of stroke and sudden cardiac death by AI-enhanced one-channel ECGs | PRICE | 10 000 | Germany |
| NCT04637230 | |||
| Home-based AF screening with handheld one-lead ECG recording | HUATUO-AF | 1740 | Hong Kong |
| NCT04523649 | |||
| Correlation between cardiovascular disease and individualized differences | Artificial intelligence with deep learning and genes on cardiovascular disease | 5000 | Taiwan |
| NCT03877614 | |||
| Remote monitoring of AF recurrence using mHealth technology | REMOTE-AF | 35 | UK |
| NCT05037136 | |||
| Accuracy of cardiac arrhythmias and conduction disorders diagnosis using a smartwatch | Accuracy of cardiac arrhythmias and conduction disorders diagnosis using a smartwatch | 110 | Brazil |
| NCT04437914 | |||
| Arrhythmia classification for shockable cardiac rhythms | AI-ECG | 25 458 | USA |
| NCT03662802 | |||
| Differentiate stroke subtypes and predict source in acute ischemic stroke | Validation of 3D simulations in embolic stroke | 100 | UK |
| NCT05055960 | |||
| Multimodality imaging for diagnosis and outcomes evaluation of multicenter patients with heart failure with preserved ejection fraction and AF | Diagnosis and OutcoMes evaluAtIoN of Multicenter Patients With HFpEF Using Multimodality Imaging | 1000 + 430 | China |
| NCT04602338 |
Estimated patient enrolment and completion date were taken from trial registration on ClinicalTrials.gov. AI-PAFA Trial, Artificial Intelligence Guided Patient Selection for Atrial Fibrillation Catheter Ablation: Randomized Clinical Trial; WB-AF, Portable Measurement Methods Combined With Artificial Intelligence in Detection of Atrial Fibrillation; BEAGLE, Batch Enrollment for AI-Guided Intervention to Lower Neurologic Events in Unrecognized AF; AI-AW, Observational Clinical Investigation of EKG Diagnostic Performance of the Apple Watch Augmented With an AI Algorithm; PRICE, Prevention of Stroke and Sudden Cardiac Death by Recording of 1-Channel Electrocardiograms; HUATUO-AF, Home-Based SolUtion for Remote Atrial Fibrillation Screening to PrevenT RecUrrence StrOke; REMOTE-AF, Remote Monitoring of AF Recurrence Using mHealth Technology; AI-ECG, Development of a Novel Convolution Neural Network for Arrhythmia Classification.
Although these algorithms perform well, the deep neural network studies often have not provided sufficient insights into how exactly the algorithm is working and how a diagnostic decision is taken—leaving uncertainties on independent verification and generalizability, jeopardizing its incorporation into daily clinical practice.
Knowledge gaps and research opportunities
Artificial intelligence benefits from large, high-quality data sets, where data constitution and access represent a major challenge for both research and clinical translation. First, different data sets are governed by different data protection rules which limit accessibility, especially in Europe. Data sets are owned and controlled by diverse stakeholders (e.g. private companies that might develop and commercialize AI tools; academic institutions that might also develop, compare, and implement them). Strict legal frameworks for data protection and use (e.g. General Data Protection Regulation and Good Clinical Practice) apply to the use of medical data. For reproducibility, data accessibility is crucial for validation of algorithm-generated predictions. Good data accessibility is also required including scientific transparency in accordance with the International Committee of Medical Journal Editors (ICMJE). In this context, it should be required that all prospective clinical trials and registries using AI are listed in official trial registries.
Unfortunately, the current complexity and disorganization of processes for data sharing and circulation between academic partners within Europe and beyond are a major handicap for progress in AI in medicine. Although the European Union (EU) has been vocal about data strategies (Strategy for Data | Shaping Europe’s digital future (europa.eu)) and FAIR (Findability, Accessibility, Interoperability, and Reuse) guiding principles for scientific data and digital assets management and stewardship, the principles outlined in these strategies often conflict with other European and National regulations, e.g. EU general data protection regulation, at the individual project level. In particular, there is also the fundamental aspect of the medical responsibility when using tools for prediction and aid in medical decisions that needs to be evaluated in context with the updated medical device regulation (MDR).
Steps to facilitate clinical implementation of artificial intelligence
The results of AI-based investigations and applications will become increasingly available. Beyond formal regulatory approval of such applications by the respective authorities, their performance and use will need to be compared in the context of the current state of art of AF care. Such context is typically generated by the medical community and their professional societies such as the ESC and EHRA. Understanding the methodology and implications of AI-based AF detection methods requires specific knowledge, involving expertise in the fields of informatics, computational networks, biostatistics, legal and medico-legal implications, and medicine. Additional questions need to be addressed, such as the reimbursement of these machine learning derived medical devices which likely requires a health economic analysis comparing costs of current practice with an AI supported pathway. Cardiology societies like ESC and EHRA are encouraged to establish and promote working groups equipped with such expertise to generate informed guidance on the sharing and use of AI-related data and algorithms, devices, and products to their society members and the medical community.
Summary
During the 8th Joint AFNET/EHRA Consensus Conference, we have clarified current AF screening and management strategies, especially an update on the role of rhythm management, with clear implications for clinical practice. Research gaps in clinical and translational science have been identified and tasks and mandates for the ESC and EHRA suggested.
Main outcomes of the 8th Joint AFNET/EHRA Consensus Conference:
New evidence supports the implementation of systematic screening for AF to achieve long-term reduction in a combined outcome of mortality, stroke, and severe bleeding.
Rhythm management, including a consideration of an attempt at rhythm control, should be part of the initial treatment in patients with recently diagnosed AF.
As AF can be regarded as a risk factor for stroke and cognitive decline, attention should be paid to cognitive function, which is a cornerstone for compliance with AF treatment.
The concept of atrial cardiomyopathy requires better and objective tools for its quantification and research to align cardiomyopathy and AF.
Defining and measuring the quality of care in terms of process, patient experience, and outcomes will be important to improve it.
Artificial intelligence has the potential to unravel the complex pathophysiology leading to atrial cardiomyopathy and AF, early AF detection, prognosis, and precision treatment, but its role, circumstances of its application, and the optimal methods need to be defined.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
We wish to thank all participants of the 8th AFNET/EHRA Consensus Conference and the staff of AFNET, EHRA, and ESC for the excellent organization of the conference.
Contributor Information
Renate B Schnabel, Atrial Fibrillation Network (AFNET), Muenster, Germany; Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Centre for Cardiovascular Research (DZHK) partner site Hamburg/Kiel/Lübeck, Hamburg, Germany.
Elena Andreassi Marinelli, Daiichi Sankyo Europe, Munich, Germany.
Elena Arbelo, Arrhythmia Section, Cardiology Department, Hospital Clinic, Universitat de Barcelona, Barcelona, Spain; IDIBAPS, Institut d'Investigació August Pi i Sunyer, Barcelona, Spain; CIBERCV, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares, Madrid, Spain.
Giuseppe Boriani, Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Polyclinic of Modena, Modena, Italy.
Serge Boveda, Cardiology—Heart Rhythm Management Department, Clinique Pasteur, 45 Avenue de Lombez, 31076 Toulouse, France; Universiteit Ziekenhuis, Vrije Universiteit Brussel (VUB), Brussels, Belgium.
Claire M Buckley, School of Public Health, University College Cork, Cork, Ireland.
A John Camm, Cardiology Clinical Academic Group, Molecular and Clinical Sciences Institute, St. George's University of London, London, UK.
Barbara Casadei, RDM, Division of Cardiovascular Medicine, British Heart Foundation Centre of Research Excellence, NIHR Oxford Biomedical Research Centre, University of Oxford, Oxford, UK.
Winnie Chua, Institute of Cardiovascular Sciences, University of Birmingham, Birmingham, UK.
Nikolaos Dagres, Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Leipzig, Germany.
Mirko de Melis, Medtronic Bakken Research Center, Maastricht, The Netherlands.
Lien Desteghe, Research Group Cardiovascular Diseases, University of Antwerp, Antwerp, Belgium; Department of Cardiology, Antwerp University Hospital, Antwerp, Belgium; Faculty of Medicine and Life Sciences, Hasselt University, Hasselt, Belgium; Heart Centre Hasselt, Jessa Hospital, Hasselt, Belgium.
Søren Zöga Diederichsen, Department of Cardiology, Copenhagen University Hospital—Rigshospitalet, Copenhagen, Denmark.
David Duncker, Hannover Heart Rhythm Center, Department of Cardiology and Angiology, Hannover Medical School, Hannover, Germany.
Lars Eckardt, Atrial Fibrillation Network (AFNET), Muenster, Germany; Division of Electrophysiology, Department of Cardiology and Angiology, Münster, Germany.
Christoph Eisert, Preventicus, Jena, Germany.
Daniel Engler, Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Centre for Cardiovascular Research (DZHK) partner site Hamburg/Kiel/Lübeck, Hamburg, Germany.
Larissa Fabritz, Atrial Fibrillation Network (AFNET), Muenster, Germany; Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Centre for Cardiovascular Research (DZHK) partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; Institute of Cardiovascular Sciences, University of Birmingham, Birmingham, UK; University Center of Cardiovascular Science Hamburg, Hamburg, Germany.
Ben Freedman, Heart Research Institute, The University of Sydney, Sydney, Australia.
Ludovic Gillet, Roche Diagnostics, Basel, Switzerland.
Andreas Goette, Atrial Fibrillation Network (AFNET), Muenster, Germany; St Vincenz Hospital, Paderborn, Germany.
Eduard Guasch, Arrhythmia Section, Cardiology Department, Hospital Clinic, Universitat de Barcelona, Barcelona, Spain; IDIBAPS, Institut d'Investigació August Pi i Sunyer, Barcelona, Spain; CIBERCV, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares, Madrid, Spain.
Jesper Hastrup Svendsen, Department of Cardiology, Copenhagen University Hospital—Rigshospitalet, Copenhagen, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Stéphane N Hatem, IHU ICAN, Sorbonne-Universite, Paris, France.
Karl Georg Haeusler, Atrial Fibrillation Network (AFNET), Muenster, Germany; Department of Neurology, Universitätsklinikum Würzburg, Würzburg, Germany.
Jeff S Healey, Population Health Research Institute, McMaster University Hamilton, ON, Canada.
Hein Heidbuchel, Research Group Cardiovascular Diseases, University of Antwerp, Antwerp, Belgium; Department of Cardiology, Antwerp University Hospital, Antwerp, Belgium.
Gerhard Hindricks, Atrial Fibrillation Network (AFNET), Muenster, Germany; Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Leipzig, Germany.
F D Richard Hobbs, Oxford Primary Care, University of Oxford, Oxford, UK.
Thomas Hübner, Preventicus, Jena, Germany.
Dipak Kotecha, University of Birmingham & University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK.
Michael Krekler, Bristol Myers Squibb, New York, NY, USA.
Christophe Leclercq, Department of Cardiology, University Rennes, Rennes, France.
Thorsten Lewalter, Atrial Fibrillation Network (AFNET), Muenster, Germany; Hospital Munich South, Department of Cardiology, Munich, Germany; Department of Cardiology, University of Bonn, Bonn, Germany.
Honghuang Lin, Department of Medicine, University of Massachusetts Medical School, Worcester, MA, USA.
Dominik Linz, Department of Cardiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht, Maastricht, The Netherlands; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Gregory Y H Lip, Liverpool Centre for Cardiovascular Science, University of Liverpool and Liverpool Heart & Chest Hospital, Liverpool, UK; Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
Maja Lisa Løchen, Department of Community Medicine, UiT The Arctic University of Norway, Tromsø, Norway.
Wim Lucassen, Amsterdam UMC (location AMC), Department General Practice, Amsterdam, The Netherlands.
Katarzyna Malaczynska-Rajpold, Royal Brompton Hospital, London, UK.
Steffen Massberg, Department of Cardiology, University Hospital, LMU Munich, Munich, Germany; German Centre for Cardiovascular Research (DZHK), partner site: Munich Heart Alliance, Munich, Germany.
Jose L Merino, Arrhythmia & Robotic EP Unit, La Paz University Hospital, IDIPAZ, Madrid, Spain.
Ralf Meyer, Medtronic, Dublin, Ireland.
Lluıs Mont, Arrhythmia Section, Cardiology Department, Hospital Clinic, Universitat de Barcelona, Barcelona, Spain; IDIBAPS, Institut d'Investigació August Pi i Sunyer, Barcelona, Spain; CIBERCV, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares, Madrid, Spain.
Michael C Myers, Bristol Myers Squibb, New York, NY, USA.
Lis Neubeck, Arrhythmia & Robotic EP Unit, La Paz University Hospital, IDIPAZ, Madrid, Spain.
Teemu Niiranen, Medtronic, Dublin, Ireland; Centre for Cardiovascular Health Edinburgh Napier University, Edinburgh, UK.
Michael Oeff, Atrial Fibrillation Network (AFNET), Muenster, Germany.
Jonas Oldgren, University of Turku and Turku University Hospital, Turku, Finland.
Tatjana S Potpara, National Institute for Health and Welfare, Helsinki, Finland.
George Psaroudakis, Uppsala Clinical Research Center and Department of Medical Sciences, Uppsala University, Uppsala, Sweden.
Helmut Pürerfellner, School of Medicine, Belgrade University, Cardiology Clinic, University Clinical Centre of Serbia, Belgrade, Serbia.
Ursula Ravens, Atrial Fibrillation Network (AFNET), Muenster, Germany; Bayer AG, Leverkusen, Germany.
Michiel Rienstra, Ordensklinikum Linz, Elisabethinen, Cardiological Department, Linz, Austria.
Lena Rivard, Institute of Experimental Cardiovascular Medicine, University Hospital Freiburg, Freiburg, Germany.
Daniel Scherr, Department of Cardiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Ulrich Schotten, Atrial Fibrillation Network (AFNET), Muenster, Germany; Montreal Heart Institute, University of Montreal, Montreal, Canada.
Dipen Shah, Division of Cardiology, Medical University of Graz, Graz, Austria.
Moritz F Sinner, Atrial Fibrillation Network (AFNET), Muenster, Germany; Amsterdam UMC (location AMC), Department General Practice, Amsterdam, The Netherlands; Royal Brompton Hospital, London, UK.
Rüdiger Smolnik, Daiichi Sankyo Europe, Munich, Germany.
Gerhard Steinbeck, Atrial Fibrillation Network (AFNET), Muenster, Germany; MUMC+, Maastricht, The Netherlands.
Daniel Steven, Atrial Fibrillation Network (AFNET), Muenster, Germany; University Hospital of Geneva, Cardiac Electrophysiology Unit, Geneva, Switzerland.
Emma Svennberg, Center for Cardiology at Clinic Starnberg, Starnberg, Germany.
Dierk Thomas, Atrial Fibrillation Network (AFNET), Muenster, Germany; University Hospital Cologne, Heart Center, Department of Electrophysiology, Cologne, Germany; Karolinska Institutet, Department of Medicine Huddinge, Karolinska University Hospital, Stockholm, Sweden; Department of Cardiology, Medical University Hospital, Heidelberg, Germany.
Mellanie True Hills, HCR (Heidelberg Center for Heart Rhythm Disorders), Medical University Hospital Heidelberg, Heidelberg, Germany.
Isabelle C van Gelder, DZHK (German Center for Cardiovascular Research), partner site Heidelberg/Mannheim, Heidelberg, Germany.
Burcu Vardar, Uppsala Clinical Research Center and Department of Medical Sciences, Uppsala University, Uppsala, Sweden.
Elena Palà, StopAfib.org, American Foundation for Women’s Health, Decatur, TX, USA.
Reza Wakili, Atrial Fibrillation Network (AFNET), Muenster, Germany; Department of Cardiology, University Medical Center Groningen, University of Groningen, The Netherlands.
Karl Wegscheider, Atrial Fibrillation Network (AFNET), Muenster, Germany; German Centre for Cardiovascular Research (DZHK) partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; Neurovascular Research Laboratory, Vall d’Hebron Institute of Research (VHIR), Autonomous University of Barcelona, Barcelona, Spain.
Mattias Wieloch, Department of Cardiology and Vascular Medicine, Westgerman Heart and Vascular Center, University of Duisburg-Essen, Essen, Germany; Institute of Medical Biometry and Epidemiology, University Medical Center Hamburg-Eppendorf, Germany.
Stephan Willems, Atrial Fibrillation Network (AFNET), Muenster, Germany; German Centre for Cardiovascular Research (DZHK) partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; Department of Coagulation Disorders, Skane University Hospital, Lund University, Malmö, Sweden.
Henning Witt, Sanofi, Paris, France.
André Ziegler, Roche Diagnostics, Basel, Switzerland.
Matthias Daniel Zink, Asklepios Hospital St Georg, Department of Cardiology and Internal Intensive Care Medicine, Faculty of Medicine, Semmelweis University Campus Hamburg, Hamburg, Germany.
Paulus Kirchhof, Atrial Fibrillation Network (AFNET), Muenster, Germany; Department of Cardiology, University Heart & Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Centre for Cardiovascular Research (DZHK) partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; Institute of Cardiovascular Sciences, University of Birmingham, Birmingham, UK.
Funding
The 8th AFNET/EHRA Consensus Conference was co-financed by AFNET and European Heart Rhythm Association (EHRA), and received additional financial support from the AFFECT-EU (Digital, risk-based screening for atrial fibrillation in the European community) consortium (affect-eu.eu). AFFECT-EU receives funding from the European Union’s Horizon 2020 research and innovation Programme under grant agreement No. 847770. Industry participants paid an attendance fee for the conference and provided an industry perspective during the discussions at the meeting, but had no involvement in the writing process.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Nabauer M, Oeff M, Gerth A, Wegscheider K, Buchholz A, Haeusler KGet al. Prognostic markers of all-cause mortality in patients with atrial fibrillation: data from the prospective long-term registry of the German Atrial Fibrillation NETwork (AFNET). Europace 2021;23:1903–12. [DOI] [PubMed] [Google Scholar]
- 3. Singer DE, Ziegler PD, Koehler JL, Sarkar S, Passman RS. Temporal association between episodes of atrial fibrillation and risk of ischemic stroke. JAMA Cardiol 2021;6:1364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 5. Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J, et al. Screening for atrial fibrillation: a report of the AF-SCREEN international collaboration. Circulation 2017;135:1851–67. [DOI] [PubMed] [Google Scholar]
- 6. Rivard L, Friberg L, Conen D, Healey JS, Berge T, Boriani G, et al. Atrial fibrillation and dementia: a report from the AF-SCREEN international collaboration. Circulation 2022;145:392–409. [DOI] [PubMed] [Google Scholar]
- 7. Fitzmaurice DA, Hobbs FDR, Jowett S, Mant J, Murray ET, Holder R, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ 2007;335:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uittenbogaart SB, Verbiest-van Gurp N, Lucassen WAM, Winkens B, Nielen M, Erkens PMG, et al. Opportunistic screening versus usual care for detection of atrial fibrillation in primary care: cluster randomised controlled trial. BMJ 2020;370:m3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lubitz SA, Atlas SJ, Ashburner JM, Lipsanopoulos ATT, Borowsky LH, Guan W, et al. Screening for atrial fibrillation in older adults at primary care visits: the VITAL-AF randomized controlled trial. medRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haeusler KG, Kirchhof P, Kunze C, Tutuncu S, Fiessler C, Malsch C, et al. Systematic monitoring for detection of atrial fibrillation in patients with acute ischaemic stroke (MonDAFIS): a randomised, open-label, multicentre study. Lancet Neurol 2021;20:426–36. [DOI] [PubMed] [Google Scholar]
- 11. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021;398:1498–506. [DOI] [PubMed] [Google Scholar]
- 12. Svendsen JH, Diederichsen SZ, Hojberg S, Krieger DW, Graff C, Kronborg C, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet 2021;398:1507–16. [DOI] [PubMed] [Google Scholar]
- 13. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, et al. Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol 2020;75:1523–34. [DOI] [PubMed] [Google Scholar]
- 15. Hermans ANL, Gawalko M, Dohmen L, van der Velden RMJ, Betz K, Duncker D, et al. Mobile health solutions for atrial fibrillation detection and management: a systematic review. Clin Res Cardiol 2021:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirchhof P, Blank BF, Calvert M, Camm AJ, Chlouverakis G, Diener HC, et al. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH-AFNET 6) trial. Am Heart J 2017;190:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lopes RD, Alings M, Connolly SJ, Beresh H, Granger CB, Mazuecos JB, et al. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J 2017;189:137–45. [DOI] [PubMed] [Google Scholar]
- 18. Hermans ANL, Gawalko M, Pluymaekers N, Dinh T, Weijs B, van Mourik MJW, et al. Long-term intermittent versus short continuous heart rhythm monitoring for the detection of atrial fibrillation recurrences after catheter ablation. Int J Cardiol 2021;329:105–12. [DOI] [PubMed] [Google Scholar]
- 19. Engler D, Heidbuchel H, Schnabel RB. Digital, risk-based screening for atrial fibrillation in the European community-the AFFECT-EU project funded by the European Union. Eur Heart J 2021;42:2625–7. [DOI] [PubMed] [Google Scholar]
- 20. Aronsson M, Svennberg E, Rosenqvist M, Engdahl J, Al-Khalili F, Friberg L, et al. Designing an optimal screening program for unknown atrial fibrillation: a cost-effectiveness analysis. Europace 2017;19:1650–6. [DOI] [PubMed] [Google Scholar]
- 21. Sun W, Freedman B, Martinez C, Wallenhorst C, Yan BP. Atrial fibrillation detected by single time-point handheld electrocardiogram screening and the risk of ischemic stroke. Thromb Haemost 2021;122:286–94. [DOI] [PubMed] [Google Scholar]
- 22. Nagata Y, Yamagami T, Nutbeam D, Freedman B, Lowres N. Incremental yield of ECG screening repeated annually over 4 years in an adult Japanese population without prior atrial fibrillation: a retrospective cohort study. BMJ Open 2020;10:e035650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–75. [DOI] [PubMed] [Google Scholar]
- 24. Kaleschke G, Hoffmann B, Drewitz I, Steinbeck G, Naebauer M, Goette A, et al. Prospective, multicentre validation of a simple, patient-operated electrocardiographic system for the detection of arrhythmias and electrocardiographic changes. Europace 2009;11:1362–8. [DOI] [PubMed] [Google Scholar]
- 25. Zink MD, Mischke KG, Keszei AP, Rummey C, Freedman B, Neumann G, et al. Screen-detected atrial fibrillation predicts mortality in elderly subjects. EP Europace 2021;23:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stavrakis S, Elkholey K, Lofgren MM, Asad ZUA, Stephens LD, Freedman B. Screening for atrial fibrillation in american indian adults in a tribal primary care clinic. J Am Heart Assoc 2021;10:e020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gwynn J, Gwynne K, Rodrigues R, Thompson S, Bolton G, Dimitropoulos Y, et al. Atrial fibrillation in indigenous australians: a multisite screening study using a single-lead ECG device in aboriginal primary health settings. Heart Lung Circ 2021;30:267–74. [DOI] [PubMed] [Google Scholar]
- 28. Lowres N, Freedman B. Time to develop guidelines for screening and management of atrial fibrillation in Indigenous Australians. Med J Aust 2020;212:212–3. [DOI] [PubMed] [Google Scholar]
- 29. Engdahl J, Holmen A, Svennberg E, Friberg L, Frykman-Kull V, Al-Khalili F, et al. Geographic and socio-demographic differences in uptake of population-based screening for atrial fibrillation: the STROKESTOP I study. Int J Cardiol 2016;222:430–5. [DOI] [PubMed] [Google Scholar]
- 30. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noseworthy PA, Gersh BJ, Kent DM, Piccini JP, Packer DL, Shah ND, et al. Atrial fibrillation ablation in practice: assessing CABANA generalizability. Eur Heart J 2019;40:1257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim D, Yang PS, You SC, Sung JH, Jang E, Yu HT, et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: nationwide cohort study. BMJ 2021;373:n991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667–77. [DOI] [PubMed] [Google Scholar]
- 34. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 35. Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009;360:668–78. [DOI] [PubMed] [Google Scholar]
- 36. Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns H, et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J 2021;43:1219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rillig A, Magnussen C, Ozga AK, Suling A, Brandes A, Breithardt G, et al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation 2021;144:845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Metzner A, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns H, et al. Anticoagulation, therapy of concomitant conditions, and early rhythm control therapy: a detailed analysis of treatment patterns in the EAST - AFNET 4 trial. Europace 2021;24:552–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brignole M, Pentimalli F, Palmisano P, Landolina M, Quartieri F, Occhetta E, et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J 2021;42:4731–9. [DOI] [PubMed] [Google Scholar]
- 40. Steinberg JS, Sadaniantz A, Kron J, Krahn A, Denny DM, Daubert J, et al. Analysis of cause-specific mortality in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004;109:1973–80. [DOI] [PubMed] [Google Scholar]
- 41. Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–40. [DOI] [PubMed] [Google Scholar]
- 42. Brachmann J, Lewalter T, Kuck KH, Andresen D, Willems S, Spitzer SG, et al. Long-term symptom improvement and patient satisfaction following catheter ablation of supraventricular tachycardia: insights from the German ablation registry. Eur Heart J 2017;38:1317–26. [DOI] [PubMed] [Google Scholar]
- 43. Darkner S, Chen X, Hansen J, Pehrson S, Johannessen A, Nielsen JB, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J 2014;35:3356–64. [DOI] [PubMed] [Google Scholar]
- 44. Fabritz L, Crijns H, Guasch E, Goette A, Hausler KG, Kotecha D, et al. Dynamic risk assessment to improve quality of care in patients with atrial fibrillation: the 7th AFNET/EHRA Consensus Conference. Europace 2021;23:329–44. [DOI] [PubMed] [Google Scholar]
- 45. Duytschaever M, Demolder A, Phlips T, Sarkozy A, El Haddad M, Taghji P, et al. PulmOnary vein isolation With vs. without continued antiarrhythmic Drug trEatment in subjects with Recurrent Atrial Fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J 2018;39:1429–1437. [DOI] [PubMed] [Google Scholar]
- 46. Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation 2021;143:1377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. [DOI] [PubMed] [Google Scholar]
- 48. Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani S, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316–24. [DOI] [PubMed] [Google Scholar]
- 49. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blomstrom-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kenneback G, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA 2019;321:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roy D, Pratt CM, Torp-Pedersen C, Wyse DG, Toft E, Juul-Moller S, et al. Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation 2008;117:1518–25. [DOI] [PubMed] [Google Scholar]
- 52. Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma M, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–91. [DOI] [PubMed] [Google Scholar]
- 53. Dagres N, Chao TF, Fenelon G, Aguinaga L, Benhayon D, Benjamin EJ, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on arrhythmias and cognitive function: what is the best practice? Europace 2018;20:1399–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dietzel J, Haeusler KG, Endres M. Does atrial fibrillation cause cognitive decline and dementia? Europace 2018;20:408–19. [DOI] [PubMed] [Google Scholar]
- 55. Gardarsdottir M, Sigurdsson S, Aspelund T, Gardarsdottir VA, Forsberg L, Gudnason V, et al. Improved brain perfusion after electrical cardioversion of atrial fibrillation. Europace 2020;22:530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim D, Yang PS, Yu HT, Kim TH, Jang E, Sung JH, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a population-based cohort. Eur Heart J 2019;40:2313–23. [DOI] [PubMed] [Google Scholar]
- 57. Malavasi VL, Zoccali C, Brandi MC, Micali G, Vitolo M, Imberti JF, et al. Cognitive impairment in patients with atrial fibrillation: implications for outcome in a cohort study. Int J Cardiol 2021;323:83–9. [DOI] [PubMed] [Google Scholar]
- 58. Flaker GC, Pogue J, Yusuf S, Pfeffer MA, Goldhaber SZ, Granger CB, et al. Atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events I. Cognitive function and anticoagulation control in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2010;3:277–83. [DOI] [PubMed] [Google Scholar]
- 59. Orkaby AR, Ozonoff A, Reisman JI, Miller DR, Zhao S, Rose AJ. Continued use of warfarin in veterans with atrial fibrillation after dementia diagnosis. J Am Geriatr Soc 2017;65:249–56. [DOI] [PubMed] [Google Scholar]
- 60. Wilke T, Groth A, Pfannkuche M, Harks O, Fuchs A, Maywald U, et al. Real life anticoagulation treatment of patients with atrial fibrillation in Germany: extent and causes of anticoagulant under-use. J Thromb Thrombolysis 2015;40:97–107. [DOI] [PubMed] [Google Scholar]
- 61. Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS, et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:839–45. [DOI] [PubMed] [Google Scholar]
- 62. Kim D, Yang PS, Sung JH, Jang E, Yu HT, Kim TH, et al. Less dementia after catheter ablation for atrial fibrillation: a nationwide cohort study. Eur Heart J 2020;41:4483–93. [DOI] [PubMed] [Google Scholar]
- 63. Herm J, Schurig J, Martinek MR, Holtgen R, Schirdewan A, Kirchhof P, et al. MRI-detected brain lesions in AF patients without further stroke risk factors undergoing ablation - a retrospective analysis of prospective studies. BMC Cardiovasc Disord 2019;19:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kirchhof P, Haeusler KG, Blank B, De Bono J, Callans D, Elvan A, et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J 2018;39:2942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Herm J, Fiebach JB, Koch L, Kopp UA, Kunze C, Wollboldt C, et al. Neuropsychological effects of MRI-detected brain lesions after left atrial catheter ablation for atrial fibrillation: long-term results of the MACPAF study. Circ Arrhythm Electrophysiol 2013;6:843–50. [DOI] [PubMed] [Google Scholar]
- 66. Hohnloser SH, Camm J, Cappato R, Diener HC, Heidbuchel H, Mont L, et al. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial. Eur Heart J 2019;40:3013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Haeusler KG, Eichner FA, Heuschmann PU, Fiebach JB, Engelhorn T, Blank B, et al. MRI-detected brain lesions and cognitive function in atrial fibrillation patients undergoing left atrial catheter ablation in the randomized AXAFA-AFNET 5 trial. Circulation 2021;145:906–15. [DOI] [PubMed] [Google Scholar]
- 68. Friberg L, Andersson T, Rosenqvist M. Less dementia and stroke in low-risk patients with atrial fibrillation taking oral anticoagulation. Eur Heart J 2019;40:2327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim D, Yang PS, Jang E, Tae Yu H, Kim TH, Uhm JS, et al. Blood pressure control and dementia risk in midlife patients with atrial fibrillation. Hypertension 2020;75:1296–304. [DOI] [PubMed] [Google Scholar]
- 70. Lee SR, Choi EK, Ahn HJ, Han KD, Oh S, Lip GYH. Association between clustering of unhealthy lifestyle factors and risk of new-onset atrial fibrillation: a nationwide population-based study. Sci Rep 2020;10:19224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lim J, Lee SR, Choi EK, Han KD, Jung JH, Ahn HJ, et al. Exercise and the risk of dementia in patients with newly diagnosed atrial fibrillation: a nationwide population-based study. J Clin Med 2021;10:3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rivard L, Friberg L, Conen D, Healey JS, Berge T, Boriani G, et al. Atrial fibrillation and dementia: a report from the AF-SCREEN International Collaboration. Circulation 2022;145(5):392-409. doi:10.1161/CIRCULATIONAHA.121.055018. Epub 2022 Jan31. Erratum in: Circulation. 2022 Apr 19;145:e842. [DOI] [PubMed]
- 73. Sundermann EE, Maki P, Biegon A, Lipton RB, Mielke MM, Machulda M, et al. Sex-specific norms for verbal memory tests may improve diagnostic accuracy of amnestic MCI. Neurology 2019;93:e1881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fredriksen-Goldsen KI, Jung H, Kim HJ, Petros R, Emlet C. Disparities in subjective cognitive impairment by sexual orientation and gender in a national population based study of U.S. adults, 2013-2018. J Aging Health 2021:8982643211046466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kiselica AM, Johnson E, Lewis KR, Trout K. Examining racial disparities in the diagnosis of mild cognitive impairment. Appl Neuropsychol Adult 2021:1–8. doi: 10.1080/23279095.2021.1976778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sachs BC, Chelune GJ, Rapp SR, Couto AM, Willard JJ, Williamson JD, et al. Robust demographically-adjusted normative data for the Montreal Cognitive Assessment (MoCA): results from the systolic blood pressure intervention trial. Clin Neuropsychol 2021:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. Association of anticoagulant therapy with risk of dementia among patients with atrial fibrillation. EP Europace 2021;23:184–95. [DOI] [PubMed] [Google Scholar]
- 78. Lee SR, Choi EK, Park SH, Jung JH, Han KD, Oh S, et al. Comparing warfarin and 4 direct oral anticoagulants for the risk of dementia in patients with atrial fibrillation. Stroke 2021;52:3459–68. [DOI] [PubMed] [Google Scholar]
- 79. Nagle RE, Smith B, Williams DO. Familial atrial cardiomyopathy with heart block. Br Heart J 1972;34:205. [PubMed] [Google Scholar]
- 80. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016;18:1455–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fabritz L, Guasch E, Antoniades C, Bardinet I, Benninger G, Betts TR, et al. Expert consensus document: defining the major health modifiers causing atrial fibrillation: a roadmap to underpin personalized prevention and treatment. Nat Rev Cardiol 2016;13:230–7. [DOI] [PubMed] [Google Scholar]
- 82. Kamel H, Bartz TM, Elkind MSV, Okin PM, Thacker EL, Patton KK, et al. Atrial cardiopathy and the risk of ischemic stroke in the CHS (Cardiovascular Health Study). Stroke 2018;49:980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jadidi A, Muller-Edenborn B, Chen J, Keyl C, Weber R, Allgeier J, et al. The duration of the amplified sinus-P-wave identifies presence of left atrial low voltage substrate and predicts outcome after pulmonary vein isolation in patients with persistent atrial fibrillation. JACC Clin Electrophysiol 2018;4:531–43. [DOI] [PubMed] [Google Scholar]
- 84. Nagel C, Luongo G, Azzolin L, Schuler S, Dössel O, Loewe A. Non-invasive and quantitative estimation of left atrial fibrosis based on P waves of the 12-Lead ECG—a large-scale computational study covering anatomical variability. J Clin Med 2021;10:1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ooie T, Wakisaka O, Hujita T, Urakabe Y, Kaneko M, Miyamoto N, et al. A specific combination of P wave duration and morphology accurately predicts the presence of left atrial low voltage area in patients with atrial fibrillation. J Electrocardiol 2020;63:173–80. [DOI] [PubMed] [Google Scholar]
- 86. Schreiber T, Kahler N, Tscholl V, Nagel P, Blaschke F, Landmesser U, et al. Correlation of P-wave properties with the size of left atrial low voltage areas in patients with atrial fibrillation. J Electrocardiol 2019;56:38–42. [DOI] [PubMed] [Google Scholar]
- 87. Eichenlaub M, Mueller-Edenborn B, Lehrmann H, Minners J, Nairn D, Loewe A, et al. Non-invasive body surface electrocardiographic imaging for diagnosis of atrial cardiomyopathy. Europace 2021;23:2010–9. [DOI] [PubMed] [Google Scholar]
- 88. Cochet H, Dubois R, Yamashita S, Al Jefairi N, Berte B, Sellal JM, et al. Relationship between fibrosis detected on late gadolinium-enhanced cardiac magnetic resonance and re-entrant activity assessed with electrocardiographic imaging in human persistent atrial fibrillation. JACC Clin Electrophysiol 2018;4:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Russo C, Jin Z, Homma S, Rundek T, Elkind MSV, Sacco RL, et al. LA phasic volumes and reservoir function in the elderly by real-time 3D echocardiography: normal values, prognostic significance, and clinical correlates. JACC Cardiovasc Imaging 2017;10:976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Eichenlaub M, Mueller-Edenborn B, Minners J, Allgeier M, Lehrmann H, Allgeier J, et al. Echocardiographic diagnosis of atrial cardiomyopathy allows outcome prediction following pulmonary vein isolation. Clin Res Cardiol 2021;110:1770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation 1995;92:835–41. [DOI] [PubMed] [Google Scholar]
- 92. Froehlich L, Meyre P, Aeschbacher S, Blum S, Djokic D, Kuehne M, et al. Left atrial dimension and cardiovascular outcomes in patients with and without atrial fibrillation: a systematic review and meta-analysis. Heart 2019;105:1884–91. [DOI] [PubMed] [Google Scholar]
- 93. Tiwari S, Lochen ML, Jacobsen BK, Hopstock LA, Nyrnes A, Njolstad I, et al. CHA2DS2-VASc score, left atrial size and atrial fibrillation as stroke risk factors in the Tromso Study. Open Heart 2016;3:e000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pathan F, Sivaraj E, Negishi K, Rafiudeen R, Pathan S, D'Elia N, et al. Use of atrial strain to predict atrial fibrillation after cerebral ischemia. JACC Cardiovasc Imaging 2018;11:1557–65. [DOI] [PubMed] [Google Scholar]
- 95. Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol 2020;76:1051–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. D'Ambrosio G, Romano S, Alothman O, Frommhold M, Borisov G, El Garhy M, et al. Computed tomography-derived left atrial volume index, sex, and age to predict the presence and the extent of left atrial low-voltage zones in patients with atrial fibrillation: the ZAQ score. J Cardiovasc Electrophysiol 2020;31:895–902. [DOI] [PubMed] [Google Scholar]
- 97. Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol 2011;57:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bisbal F, Gomez-Pulido F, Cabanas-Grandio P, Akoum N, Calvo M, Andreu D, et al. Left atrial geometry improves risk prediction of thromboembolic events in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2016;27:804–10. [DOI] [PubMed] [Google Scholar]
- 99. Azemi T, Rabdiya VM, Ayirala SR, McCullough LD, Silverman DI. Left atrial strain is reduced in patients with atrial fibrillation, stroke or TIA, and low risk CHADS(2) scores. J Am Soc Echocardiogr 2012;25:1327–32. [DOI] [PubMed] [Google Scholar]
- 100. Obokata M, Negishi K, Kurosawa K, Tateno R, Tange S, Arai M, et al. Left atrial strain provides incremental value for embolism risk stratification over CHA(2)DS(2)-VASc score and indicates prognostic impact in patients with atrial fibrillation. J Am Soc Echocardiogr 2014;27:709–16.e4. [DOI] [PubMed] [Google Scholar]
- 101. Shen MJ, Arora R, Jalife J. Atrial myopathy. JACC Basic Transl Sci 2019;4:640–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Benito EM, De Luna AB, Baranchuk A, Mont L. Extensive atrial fibrosis assessed by late gadolinium enhancement cardiovascular magnetic resonance associated with advanced interatrial block electrocardiogram pattern. Europace 2017;19:377. [DOI] [PubMed] [Google Scholar]
- 103. Bisbal F, Guiu E, Calvo N, Marin D, Berruezo A, Arbelo E, et al. Left atrial sphericity: a new method to assess atrial remodeling. Impact on the outcome of atrial fibrillation ablation. J Cardiovasc Electrophysiol 2013;24:752–9. [DOI] [PubMed] [Google Scholar]
- 104. Chua W, Purmah Y, Cardoso VR, Gkoutos GV, Tull SP, Neculau G, et al. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur Heart J 2019;40:1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Packer M. Characterization, pathogenesis, and clinical implications of inflammation-related atrial myopathy as an important cause of atrial fibrillation. J Am Heart Assoc 2020;9:e015343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shingu Y, Takada S, Yokota T, Shirakawa R, Yamada A, Ooka T, et al. Correlation between increased atrial expression of genes related to fatty acid metabolism and autophagy in patients with chronic atrial fibrillation. PLoS One 2020;15:e0224713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dong Q, Li S, Wang W, Han L, Xia Z, Wu Y, et al. FGF23 regulates atrial fibrosis in atrial fibrillation by mediating the STAT3 and SMAD3 pathways. J Cell Physiol 2019;234:19502–19510. [DOI] [PubMed] [Google Scholar]
- 108. Seewoster T, Kosich F, Sommer P, Bertagnolli L, Hindricks G, Kornej J. Prediction of low-voltage areas using modified APPLE score. Europace 2021;23:575–80. [DOI] [PubMed] [Google Scholar]
- 109. Huo Y, Gaspar T, Pohl M, Sitzy J, Richter U, Neudeck S, et al. Prevalence and predictors of low voltage zones in the left atrium in patients with atrial fibrillation. EP Europace 2018;20:956–62. [DOI] [PubMed] [Google Scholar]
- 110. Kishima H, Mine T, Fukuhara E, Takahashi S, Ishihara M. Is the abnormal conduction zone of the left atrium a precursor to a low voltage area in patients with atrial fibrillation? J Cardiovasc Electrophysiol 2020;31:2874–82. [DOI] [PubMed] [Google Scholar]
- 111. Caixal G, Althoff T, Garre P, Alarcon F, NunezGarcia M, Benito EM, et al. Proximity to the descending aorta predicts regional fibrosis in the adjacent left atrial wall: aetiopathogenic and prognostic implications. EP Europace 2021;23:1559–67. [DOI] [PubMed] [Google Scholar]
- 112. Muller P, Makimoto H, Dietrich JW, Fochler F, Nentwich K, Krug J, et al. Association of left atrial low-voltage area and thromboembolic risk in patients with atrial fibrillation. EP Europace 2018;20:f359–65. [DOI] [PubMed] [Google Scholar]
- 113. Canpolat U. Left atrial low-voltage area as a novel indicator of cerebrovascular events in atrial fibrillation. EP Europace 2018;20:f459. [DOI] [PubMed] [Google Scholar]
- 114. Nery PB, Al Dawood W, Nair GM, Redpath CJ, Sadek MM, Chen L, et al. Characterization of low-voltage areas in patients with atrial fibrillation: insights from high-density intracardiac mapping. Can J Cardiol 2018;34:1033–40. [DOI] [PubMed] [Google Scholar]
- 115. Ezeani M, Hagemeyer CE, Lal S, Niego B. Molecular imaging of atrial myopathy: towards early AF detection and non-invasive disease management. Trends Cardiovasc Med 2020;32:20–31. [DOI] [PubMed] [Google Scholar]
- 116. Kamel H, Longstreth WT Jr, Tirschwell DL, Kronmal RA, Broderick JP, Palesch YY, et al. The Atrial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke randomized trial: rationale and methods. Int J Stroke 2019;14:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Reyat JS, Chua W, Cardoso VR, Witten A, Kastner PM, Kabir SN, et al. Reduced left atrial cardiomyocyte PITX2 and elevated circulating BMP10 predict atrial fibrillation after ablation. JCI Insight 2020;5:e139179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chua W, Law JP, Cardoso VR, Purmah Y, Neculau G, Jawad-Ul-Qamar M, et al. Quantification of fibroblast growth factor 23 and N-terminal pro-B-type natriuretic peptide to identify patients with atrial fibrillation using a high-throughput platform: a validation study. PLoS Med 2021;18:e1003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Brady P, Chua W, Nehaj F, Connolly DL, Khashaba A, Purmah Y, et al. Interactions between atrial fibrillation and natriuretic peptide in predicting heart failure hospitalization or cardiovascular death. J Am Heart Assoc 2022;11:e022833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Arndt M, Lendeckel U, Rocken C, Nepple K, Wolke C, Spiess A, et al. Altered expression of ADAMs (A Disintegrin And Metalloproteinase) in fibrillating human atria. Circulation 2002;105:720–5. [DOI] [PubMed] [Google Scholar]
- 121. Chilukoti RK, Giese A, Malenke W, Homuth G, Bukowska A, Goette A, et al. Atrial fibrillation and rapid acute pacing regulate adipocyte/adipositas-related gene expression in the atria. Int J Cardiol 2015;187:604–13. [DOI] [PubMed] [Google Scholar]
- 122. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on Atrial cardiomyopathies: definition, characterisation, and clinical implication. J Arrhythm 2016;32:247–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 124. Bisbal F, Benito E, Teis A, Alarcón F, Sarrias A, Caixal G, et al. Magnetic resonance imaging-guided fibrosis ablation for the treatment of atrial fibrillation: the ALICIA trial. Circ Arrhythm Electrophysiol 2020;13:e008707. [DOI] [PubMed] [Google Scholar]
- 125. Sugumar H, Prabhu S, Voskoboinik A, Young S, Gutman SJ, Wong GR, et al. Atrial remodeling following catheter ablation for atrial fibrillation-mediated cardiomyopathy: long-term follow-up of CAMERA-MRI Study. JACC Clin Electrophysiol 2019;5:681–8. [DOI] [PubMed] [Google Scholar]
- 126. Caixal G, Alarcon F, Althoff TF, Nunez-Garcia M, Benito EM, Borras R, et al. Accuracy of left atrial fibrosis detection with cardiac magnetic resonance: correlation of late gadolinium enhancement with endocardial voltage and conduction velocity. EP Europace 2021;23:380–8. [DOI] [PubMed] [Google Scholar]
- 127. Jadidi AS, Cochet H, Shah AJ, Kim SJ, Duncan E, Miyazaki S, et al. Inverse relationship between fractionated electrograms and atrial fibrosis in persistent atrial fibrillation: combined magnetic resonance imaging and high-density mapping. J Am Coll Cardiol 2013;62:802–12. [DOI] [PubMed] [Google Scholar]
- 128. Akoum N, Fernandez G, Wilson B, McGann C, Kholmovski E, Marrouche N. Association of atrial fibrosis quantified using LGE-MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2013;24:1104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. King JB, Azadani PN, Suksaranjit P, Bress AP, Witt DM, Han FT, et al. Left atrial fibrosis and risk of cerebrovascular and cardiovascular events in patients with atrial fibrillation. J Am Coll Cardiol 2017;70:1311–21. [DOI] [PubMed] [Google Scholar]
- 130. Healey JS, Gladstone DJ, Swaminathan B, Eckstein J, Mundl H, Epstein AE, et al. Recurrent stroke with rivaroxaban compared with aspirin according to predictors of atrial fibrillation: secondary analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurol 2019;76:764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gorenek B, Pelliccia A, Benjamin EJ, Boriani G, Crijns HJ, Fogel RI, et al. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Eur J Prev Cardiol 2017;24:4–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Marrouche NF, Greene T, Dean JM, Kholmovski EG, Boer LM, Mansour M, et al. Efficacy of LGE-MRI-guided fibrosis ablation versus conventional catheter ablation of atrial fibrillation: the DECAAF II trial: study design. J Cardiovasc Electrophysiol 2021;32:916–24. [DOI] [PubMed] [Google Scholar]
- 133. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol 2017;70:1949–61. [DOI] [PubMed] [Google Scholar]
- 134. McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol 2014;7:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R, et al. Prevention and regressive effect of weight-loss and risk factor modification on atrial fibrillation: the Reverse-AF study. EP Europace 2018;20:1929–35. [DOI] [PubMed] [Google Scholar]
- 136. Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brugemann J, et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J 2018;39:2987–96. [DOI] [PubMed] [Google Scholar]
- 137. Verma A, Ha ACT, Kirchhof P, Hindricks G, Healey JS, Hill MD, et al. The optimal anti-coagulation for enhanced-risk patients post-catheter ablation for atrial fibrillation (OCEAN) trial. Am Heart J 2018;197:124–32. [DOI] [PubMed] [Google Scholar]
- 138. Arbelo E, Aktaa S, Bollmann A, D’Avila A, Drossart I, Dwight J, et al. Quality indicators for the care and outcomes of adults with atrial fibrillation: task force for the development of quality indicators in atrial fibrillation of the European Heart Rhythm Association (EHRA) of the European Society of Cardiology (ESC): developed in collaboration with the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin-American Heart Rhythm Society (LAHRS). EP Europace 2021;23:494–5. [DOI] [PubMed] [Google Scholar]
- 139. Anker SD, Agewall S, Borggrefe M, Calvert M, Jaime Caro J, Cowie MR, et al. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014;35:2001–9. [DOI] [PubMed] [Google Scholar]
- 140. Heidbuchel H, Van Gelder IC, Desteghe L. ESC and EHRA lead a path towards integrated care for multimorbid atrial fibrillation patients: the Horizon 2020 EHRA-PATHS project. Eur Heart J 2021;43:1450–2. [DOI] [PubMed] [Google Scholar]
- 141. Johnsen SP, Proietti M, Maggioni AP, Lip GYH. A multinational European network to implement integrated care in elderly multimorbid atrial fibrillation patients: the AFFIRMO Consortium. Eur Heart J 2022;43:2916–8. [DOI] [PubMed] [Google Scholar]
- 142. World Health Organization . WHO global strategy on integrated people-centred health services 2016-2026. In Draft for Consultation. Placing People and Communities at the Centre of Health Services. Geneva, Switzerland: World Health Organization; 2015, 1–18. [Google Scholar]
- 143. Vitolo M, Lane DA, Boriani G, Lip GYH. The importance of adherence and persistence with oral anticoagulation treatment in patients with atrial fibrillation. Eur Heart J Cardiovasc Pharmacother 2020;7:f81–3. [DOI] [PubMed] [Google Scholar]
- 144. Seligman WH, Das-Gupta Z, Jobi-Odeneye AO, Arbelo E, Banerjee A, Bollmann A, et al. Development of an international standard set of outcome measures for patients with atrial fibrillation: a report of the International Consortium for Health Outcomes Measurement (ICHOM) atrial fibrillation working group. Eur Heart J 2020;41:1132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Barmano N, Walfridsson U, Walfridsson H, Karlsson J-E. Structured care of patients with atrial fibrillation improves guideline adherence. J Atr Fibrillation 2016;9:1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kornowski R. Patient reported outcomes measures (PROMS) in cardiovascular disease. Eur Heart J Qual Care Clin Outcomes 2021:qcab051. doi: 10.1093/ehjqcco/qcab051. [DOI] [PubMed] [Google Scholar]
- 147. Brüggenjürgen B, Schliephacke T, Darius H, De CR, Le HJ, Reimitz PE, et al. Health state in patients with atrial fibrillation on new oral anticoagulants as assessed with the new Eq-5d-5l questionnaire at baseline and 12-month follow-up: prefer in AF registry. Value Health 2014;17:A493. [DOI] [PubMed] [Google Scholar]
- 148. Humphries B, Cox JL, Parkash R, Thabane L, Foster GA, MacKillop J, et al. Patient-reported outcomes and patient-reported experience of patients with atrial fibrillation in the IMPACT-AF clinical trial. J Am Heart Assoc 2021;10:e019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kochhauser S, Joza J, Essebag V, Proietti R, Koehler J, Tsang B, et al. The impact of duration of atrial fibrillation recurrences on measures of health-related quality of life and symptoms. Pacing Clin Electrophysiol 2016;39:166–72. [DOI] [PubMed] [Google Scholar]
- 150. Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, et al. Development and validation of the atrial fibrillation effect on quality-of-life (AFEQT) questionnaire in patients with atrial fibrillation. Circ Arrhyth Electrophysiol 2011;4:15–25. [DOI] [PubMed] [Google Scholar]
- 151. Kotecha D, Ahmed A, Calvert M, Lencioni M, Terwee CB, Lane DA. Patient-reported outcomes for quality of life assessment in atrial fibrillation: a systematic review of measurement properties. PLoS One 2016;11:e0165790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Cornelis C, Hartog SJD, Bastemeijer CM, Roozenbeek B, Nederkoorn PJ, Berg-Vos RMVD. Patient-reported experience measures in stroke care. Stroke 2021;52:2432–5. [DOI] [PubMed] [Google Scholar]
- 153. Vinereanu D, Lopes RD, Bahit MC, Xavier D, Jiang J, Al-Khalidi HR, et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet 2017;390:1737–46. [DOI] [PubMed] [Google Scholar]
- 154. Zhang L, Gallagher R, Lowres N, Orchard J, Freedman SB, Neubeck L. Using the ‘Think Aloud’ technique to explore quality of life issues during standard quality-of-life questionnaires in patients with atrial fibrillation. Heart Lung Circ 2017;26:150–6. [DOI] [PubMed] [Google Scholar]
- 155. Lin R, Gallagher R, Spinaze M, Najoumian H, Dennis C, Clifton-Bligh R, et al. Effect of a patient-directed discharge letter on patient understanding of their hospitalisation. Int Med J 2014;44:851–7. [DOI] [PubMed] [Google Scholar]
- 156. Goette A, Auricchio A, Boriani G, Braunschweig F, Terradellas JB, Burri H, et al. EHRA White Paper: knowledge gaps in arrhythmia management—status 2019. EP Europace 2019;21:993–4. [DOI] [PubMed] [Google Scholar]
- 157. Jensen MT, Treskes RW, Caiani EG, Casado-Arroyo R, Cowie MR, Dilaveris P, et al. ESC working group on e-cardiology position paper: use of commercially available wearable technology for heart rate and activity tracking in primary and secondary cardiovascular prevention—in collaboration with the European Heart Rhythm Association, European Association of Preventive Cardiology, Association of Cardiovascular Nursing and Allied Professionals, Patient Forum, and the Digital Health Committee. Eur Heart J Digi Health 2021;2:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gawałko M, Duncker D, Manninger M, van der Velden RMJ, Hermans ANL, Verhaert DVM, et al. The European TeleCheck-AF project on remote app-based management of atrial fibrillation during the COVID-19 pandemic: centre and patient experiences. EP Europace 2021;23:1003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bunting KV, Van Gelder IC, Kotecha D. STEEER-AF: a cluster-randomized education trial from the ESC: the STEEER-AF trial is designed by the European Society of Cardiology (ESC) to see if better education for healthcare professionals can improve how patients are treated and how AF is managed. Eur Heart J 2020;41:1952–4. [DOI] [PubMed] [Google Scholar]
- 160. Duncker D, Svennberg E, Deharo JC, Costa FM, Kommata V. The ‘afibmatters.org’ educational website for patients with atrial fibrillation from the European Heart Rhythm Association. EP Europace 2021;23:1693–7. [DOI] [PubMed] [Google Scholar]
- 161. Frankel DS, Parker SE, Rosenfeld LE, Gorelick PB. HRS/NSA 2014 survey of atrial fibrillation and stroke: gaps in knowledge and perspective, opportunities for improvement. Heart Rhythm 2015;12:e105–13. [DOI] [PubMed] [Google Scholar]
- 162. Boriani G, Maisano A, Bonini N, Albini A, Imberti JF, Venturelli A, et al. Digital literacy as a potential barrier to implementation of cardiology tele-visits after COVID-19 pandemic: the INFO-COVID survey. J Geriatr Cardiol 2021;18:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Olier I, Ortega-Martorell S, Pieroni M, Lip GYH. How machine learning is impacting research in atrial fibrillation: implications for risk prediction and future management. Cardiovasc Res 2021;117:1700–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Clifford GD, Liu C, Moody B, Lehman LH, Silva I, Li Q, et al. AF classification from a short single lead ECG recording: the PhysioNet/Computing in Cardiology Challenge 2017. Comput Cardiol (2010) 2017;44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Karwath A, Bunting KV, Gill SK, Tica O, Pendleton S, Aziz F, et al. Redefining β-blocker response in heart failure patients with sinus rhythm and atrial fibrillation: a machine learning cluster analysis. Lancet 2021;398:1427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ding EY, Marcus GM, McManus DD. Emerging technologies for identifying atrial fibrillation. Circ Res 2020;127:128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Tison GH, Sanchez JM, Ballinger B, Singh A, Olgin JE, Pletcher MJ, et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol 2018;3:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hannun AY, Rajpurkar P, Haghpanahi M, Tison GH, Bourn C, Turakhia MP, et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med 2019;25:65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Jo YY, Cho Y, Lee SY, Kwon JM, Kim KH, Jeon KH, et al. Explainable artificial intelligence to detect atrial fibrillation using electrocardiogram. Int J Cardiol 2021;328:104–10. [DOI] [PubMed] [Google Scholar]
- 170. Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019;394:861–7. [DOI] [PubMed] [Google Scholar]
- 171. Christopoulos G, Graff-Radford J, Lopez CL, Yao X, Attia ZI, Rabinstein AA, et al. Artificial intelligence-electrocardiography to predict incident atrial fibrillation: a population-based study. Circ Arrhythm Electrophysiol 2020;13:e009355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gulliford MC, Sun X, Anjuman T, Yelland E, Murray-Thomas T. Comparison of antibiotic prescribing records in two UK primary care electronic health record systems: cohort study using CPRD GOLD and CPRD Aurum databases. BMJ Open 2020;10:e038767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.