Abstract
Background
At present, there are many different evaluation methods for sensitive skin, including subjective, semisubjective, and objective evaluation. Various objective tests focus on assessing changes in barrier functions. It is anticipated that the ANTERA 3D®, in combination with GPSkin Barrier®, will provide better evaluation of sensitive skin.
Methods
A total of 20 subjects with sensitive skin and 20 healthy participants were recruited. Sensitive skin subjects were treated with an anti‐sensitive moisturizing tolerance‐extreme cream which has anti‐inflammatory and moisturizing effects, twice daily on the whole face for 28 days. VISIA® Skin Detector was used to record clinical images and red area. GPSkin Barrier® was used to measure TEWL and SCH. Texture, hemoglobin, and influenced area (mm2) were recorded using ANTERA 3D®. Subjects underwent skin tests and recorded changes at D0 and D28. Data were only collected from healthy participants who did not receive treatment as controls.
Results
TEWL, texture, hemoglobin, and affected area in sensitive skin group were significantly higher than those in healthy group, while SCH was significantly lower than that in healthy group (p all<0.05). After anti‐inflammatory and moisturizing treatment, the texture, hemoglobin, and affected area of sensitive skin decreased, TEWL decreased while SCH increased (p all<0.05).
Conclusions
Based on the results, the combination of the ANTERA 3D® with GPSkin Barrier® could be used as a new kind of quantitative evaluation method for the detection and diagnosis of sensitive skin.
Keywords: ANTERA 3D® , GPSkin Barrier® , quantitative evaluation, sensitive skin
1. INTRODUCTION
Sensitive skin refers to a highly reactive state of the skin under various physiological or pathological conditions. Clinical presentation of sensitive skin commonly occurs on the face, which can be manifested as facial erythema accompanied by pruritus, burning or tingling sensation, and skin tightness. 1 , 2 , 3 These symptoms can be caused by physical, chemical, spiritual, and other factors, including cutaneous hyperreactivity to cosmetics, cold, heat, and sun. 4 Sensitive skin is a common and frequently occurring skin disorder that may affect about 30–50% of the global population. Persistent itching and erythema in different areas of the face will seriously affect patients' aesthetics and quality of life. 5
At present, a number of different methods have been utilized to evaluate sensitive skin, including the subjective, the semisubjective, and objective evaluation measures. Subjective tests is mainly dependent on self‐assessment questionnaires to assess patients’ clinical progress and treatment outcomes, including the Dermatology Life Quality Index (DLQI), Sensitive Scale‑10 (SS‑10), Body Skin Discomfort Index (BSDI), and Burden of Sensitive Skin (BoSS). 6 , 7 , 8 , 9 , 10 However, due to the influence of education level, cognitive level and other factors, these questionnaires are based on subjective feeling and lack objectivity. Indeed, the prevalence of self‐declared (subjective) sensitive skin could be over 70% among adults worldwide and could be close to 40% claiming to have severe or moderate sensitive skin. 11 Yet, the prevalence is dependent on the diagnostic methods, and subjective methods based on questionnaires may overestimate prevalence. 5 Semisubjective tests could be used to diagnose sensitive skin, but are biased with impaired skin barrier, including Christensen and Kligman test, Chloroform: menthol (20:80) test, capsaicin test, dimethyl sulfoxide test, nicotine test, and histamine test. 2
Many objective tests detect changes in barrier functions. GPSkin Barrier®, a skin barrier function measurement device, which simultaneously measures both trans‐epidermal water loss (TEWL) and skin cuticle hydration (SCH) quickly and accurately. 12 It has been identified that the value of GPSkin Barrier® for the monitoring of rosacea and atopic dermatitis treatment in daily practice, 13 , 14 but few have attempted to evaluate the texture and hemoglobin of sensitive skin.
ANTERA 3D® is the latest specialized medical skin imaging and analysis equipment that analyzes shadow and light reflectance to create a three‐dimensional color image of the skin surface, allowing rapid, simple and accurate analysis and measurement of wrinkles, texture, hemoglobin, pigmentation and redness. A series of clinical studies on chloasma, acne scar, and photokeratitis were carried out by using ANTERA 3D®. 15 , 16 , 17
The value of skin texture, hemoglobin, and hemoglobin influenced area can be observed to reflect the level of inflammation. 18 In this study, we measured the degree of texture and hemoglobin using ANTERA 3D® in order to quantify our results, then explored the differences between normal skin and sensitive skin, as well as sensitive skin before and after treatment. Our goal was to determine the value of ANTERA 3D® and GPSkin Barrier® in quantitative evaluation of sensitive skin.
2. MATERIALS AND METHODS
2.1. Study subjects
A total of 20 Chinese subjects with sensitive skin were recruited from the Dermatology department of Hangzhou Third People's Hospital (3 males and 17 females). The average age was 29.47 ± 7.75 years (range, 19−44 years). The duration of sensitive skin was 13 ± 7 months (range, 1−24 months). Collecting 20 healthy participants (2 males and 18 females), the average age was 29.55 ± 7.48 years (range, 18−50 years). The subjects were enrolled after receiving their written informed consent and were informed on the purpose of the study. They consented to the publication of the pictures. Their clinical manifestations were pruritus, burning, tightness, facial erythema; Lactic acid test ≥ 3 points. The exclusion criteria were as follows: (1) patients with facial skin ulcers, serious infection; (2) patients have a history of exposure to sunlight during the treatment; and (3) pregnancy, lactation.
3. INSTRUMENTS MATERIAL AND METHODS
All measurements were performed in the same room with no daylight under controlled ambient conditions (22–24°C, 50–60% relative humidity). Before the test, each subject was required to clean the face. After a 20‐min break, VISIA® (Canfield Corporation, USA) Skin Detector was used to take subjects’ left 45°, center 0°, right 45° facial positions photos, mainly recording clinical images and red area. Texture, hemoglobin, and influenced area (mm2) were recorded on both cheeks of subjects using ANTERA 3D®(Miravex, Ireland), which contains a camera for image acquisition and corresponding software for analysis of the skin. GPSkin Barrier® (GPOWER Inc, Seoul, South Korea) was used to measure subjects’ TEWL and SCH. The average values of TEWL and SCH were measured three times in each part. Each measurement was marked on the photograph and the same site was measured during follow‐up.
3.1. Measurements
Sensitive skin subjects were treated with an antisensitive moisturizing tolerance‐extreme cream, which has anti‐inflammatory and moisturizing effects (Beitaini Bio‐technological Co., China), twice daily on the whole face for 28 days. 9 Subjects accept skin test and record the changes of TEWL, SCH, texture, hemoglobin, and influenced area at D0 and D28. Healthy participants without treatment, only data were collected as control.
3.2. Statistical analysis
The data were processed and analyzed by IBM® SP sensitive skin® Statistics 19.0 software. The data from GPSkin Barrier® were compared by t‐test, differences between ANTERA 3D® values of sensitive skin subjects at D0 and healthy controls were analyzed using the Mann–Whitney U test, ANTERA 3D® differences of sensitive skin subjects at D0 and D28 were explored with the Wilcoxon signed rank test. For all statistical tests, p < 0.05 was considered statistically significant.
4. RESULTS
4.1. The result of GPSkin Barrier®
Significant differences were found in GPSkin Barrier® readings between sensitive skin subjects and controls (Table 1). Compared to controls, TEWL of sensitive skin subjects was significantly higher (p = 0.000), while SCH was significantly lower (p = 0.000). After 28 days of treatment, TEWL decreased and SCH increased in sensitive skin subjects, these changes were statistically different (p = 0.000, 0.000).
TABLE 1.
GPSkin Barrier® values of sensitive skin subjects at D0 and D28 compared to healthy controls
| Controls | Sensitive skin at D0 | Sensitive skin at D28 | |
|---|---|---|---|
| TEWL | 9.14 ± 2.30 | 16.63 ± 5.83 | 10.07 ± 2.90 |
| SCH | 34.71 ± 6.25 | 21.97 ± 10.85 | 32.20 ± 7.93 |
Values are expressed as mean ± DS.
4.2. The result of ANTERA3D®
Compared with controls, the facial erythema of sensitive skin subjects is serious, and the hemoglobin subsides obviously after treatment (Figure 1). Similarly, through ANTERA 3D®, it is found that hemoglobin and influenced area of sensitive skin subjects were significantly higher than healthy participants’ data (Table 2) (p = 0.000, 0.000). Compared to D0, both a clear trend toward decreased hemoglobin and influence area of sensitive skin subjects were seen at D28 (p = 0.000, 0.000).
FIGURE 1.
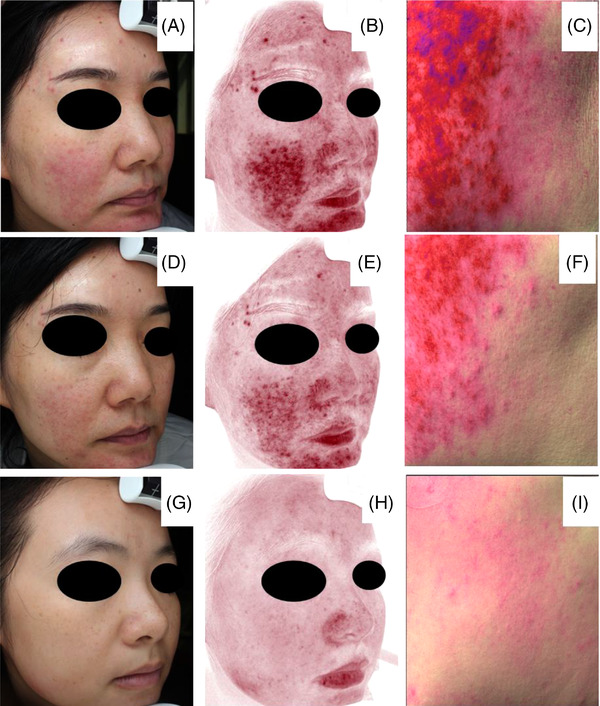
Representative images obtained by VISIA® and ANTERA 3D®. (A–C) Clinical images, erythema area, hemoglobin respectively of sensitive skin subject at D0. (D–F) Clinical images, red area, hemoglobin respectively of sensitive skin subject at D28. (G–I) Controls’ clinical images, red area, hemoglobin respectively
TABLE 2.
ANTERA 3D® values of sensitive skin subjects at D0 and D28 compared to healthy controls
| Controls | Sensitive skin at D0 | Sensitive skin at D28 | |
|---|---|---|---|
| Hemoglobin | 17.87 (15.65–29.54) | 88.27 (53.78–105.55) | 25.17 (12.21–33.14) |
| Hemoglobin influenced area (mm 2 ) | 77 (61–134.25) | 894 (598–1628.5) | 299 (139.5–407.5) |
| Texture | 6.64 (6.13–7.32) | 9.96 (7.64–10.87) | 8.94 (6.89–10.12) |
Values are expressed as median (range).
Compared with healthy participant, the skin of sensitive skin subjects appears rough (Figure 2), and the value representing skin texture is significantly increased (p = 0.000). After 28 days of treatment, the texture values of sensitive skin subjects decreased (p = 0.015).
FIGURE 2.
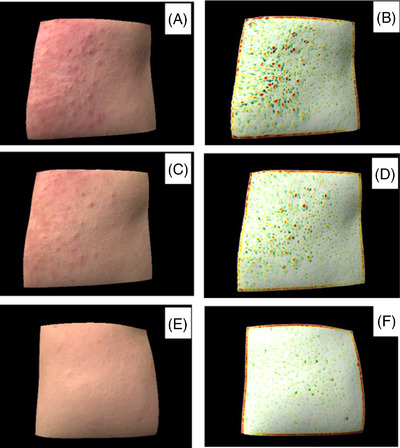
Representative 3D images and skin texture images of different populations obtained by ANTERA 3D®. (A, B) Sensitive skin subject at D0. (C, D) Sensitive skin subject at D28. (E, F) Healthy participant.
5. DISCUSSION
Sensitive skin is a common complaint syndrome, characterized by the unpleasant sensations (pruritus, burning or tingling sensation, and skin tightness), and is elicited by physical, thermal, or chemical stimuli that may not normally provoke such feelings in healthy skin. 19 Environmental factors, lifestyle factors, and physiological factors have been reported to elicit the symptoms of sensitive skin. 20 The main mechanism of sensitive skin is the skin barrier impairment, the high input of sensory nerve signal, the high reactivity of inflammation, or blood vessel and genetic factors. 21 , 22 , 23 , 24 Studies have shown that the incidence of sensitive skin in China is about 36.1%, and in Japan, Europe, and the United States, it is about 50%. 25 A high global prevalence of sensitive skin in the population and the intrusive character put a heavy burden on patients. However, specific diagnostic methods and treatment protocols are challenging due to lacking distinct international consensus and the subjectivity of sensitive skin. In all cases, there is of increasingly high interest in assessing the subjective opinions of the patients.
Nowadays, research has been carried out to study the evaluation of sensitive skin and skin barrier. Given the subjectivity of sensitive skin, the self‐assessment questionnaire seems to be a reliable tool for diagnosing sensitive skin in clinical practice. The DLQI is a scale used in dermatology for assessing the quality of life in patients pre‐ and posttreatment. The SS‐10 is the first scale to quantify the severity of skin sensitivity. The BSDI can detect skin changes after receiving treatment. The BOSS is a useful scale to assess the burden of sensitive skin. However, since the diagnosis of sensitive skin is mainly dependent on the subjective symptoms, patients would be overdiagnosed and the prevalence of sensitive skin would be overestimated. Tests have been used to identify sensitive skin with specific sensory reactions. Stinging tests with lactic acid and capsaicin were regarded as the most commonly used and effective method to evaluate the sensitive skin and also can be used to detect the severity of sensitive skin. Each of them has significant limitations. For instance, patients with sensitive skin may respond to one stimulus that elicits stinging, such as lactic acid, but be nonresponsive to other substances.
At present, VISIA® can detect erythema area, and Mexameter MX18 can detect erythema index. Confocal Raman Microspectroscopy (CRS) is an optical device to measure the barrier function based on the principle of inelastic (Raman) scattering. 26 A SpectraCam system is identified as a robust hyperspectral imaging system quantifying total hemoglobin and oxygen saturation. 27 However, there are few instruments for detecting inflammation.
Instruments such as Tewameter TM300 and GPSkin Barrier® supported by dermatologists and cosmetic companies provide simple, noninvasive and objective tests for physiological parameters such as water loss and SCH. ANTERA 3D® uses multidirectional illumination obtained by LEDs to acquire data of hemoglobin concentrations and affected area simultaneously. The value of hemoglobin measured with ANTERA 3D® was significantly correlated with those measured by VISIA® in the red area, but the results are more comprehensive. 28 The sensitivity of ANTERA 3D® to hemoglobin determination is similar to that of Mexameter MX18, but with higher repeatability. 29 , 30 Similarly, strong uniformity was found between the erythema area from VISIA and the hemoglobin from ANTERA 3D® in our study. But through the ANTERA 3D®, we obtained accurate values of hemoglobin and influenced area, which can help us quantitatively evaluate the degree of inflammation in subjects with sensitive skin. Therefore, we used GPSkin Barrier® and ANTERA 3D® to assess differences in skin barrier and inflammation in different groups. The results showed that the TEWL, texture, hemoglobin, and influenced area of sensitive skin subjects were significantly higher than those of normal subjects, while SCH decreased, suggesting that the skin barrier of sensitive skin subjects was damaged, texture was rough, and inflammation was strong. After anti‐inflammatory and moisturizing treatment, the texture, hemoglobin, and influenced area of sensitive skin subjects decreased, TEWL decreased, while SCH increased. These changes are significant, indicating that the skin barrier of sensitive skin subjects is being repaired, the inflammatory reaction is reduced and the skin texture also becomes smoother. It also indicates that the combined application of ANTERA 3D® and GPSkin Barrier® can precisely evaluate the improvement of skin status in sensitive skin patients.
6. CONCLUSION
GPSkin Barrier® records TEWL and SCH in different skin states simply and sensitively. The ANTERA 3D® allows for accurate and rapid determination of texture and hemoglobin in normal and damaged skin. Moreover, both instruments are able to quantify the improvement in sensitive skin during treatment, thus helping to objectify the treatment effect so that it can be immediately fed back to the patient. According to experience, this is beneficial for improving treatment adherence.
In conclusion, combing the ANTERA 3D® with GPSkin Barrier® could be used as a new kind of the quantitative evaluation method for the detection and diagnosis of sensitive skin, which is expected to more objectively assess the effect of sensitive skin.
CONFLICT OF INTEREST
None declared.
FUNDING
This study was funded by the National Natural Science Foundation of China (82003322), Natural Science Foundation of Zhejiang Province (LY21H110002), Zhejiang Science and Technology Plan of Traditional Chinese Medicine (2021ZQ075) and Hangzhou Medical and Health Science and Technology Project (A20210359).
Anqi S, Xiukun S, Ai'e X. Quantitative evaluation of sensitive skin by ANTERA 3D® combined with GPSkin Barrier® . Skin Res Technol. 2022;28:840–845. 10.1111/srt.13213
DATA AVAILABILITY STATEMENT
Researched data are not shared.
REFERENCES
- 1. Misery L, Ständer S, Szepietowski JC, et al. Definition of sensitive skin: an expert position paper from the special interest group on sensitive skin of the international forum for the study of itch. Acta Derm Venereol. 2017;97(1):4‐6. [DOI] [PubMed] [Google Scholar]
- 2. Do LHD, Azizi N, Maibach H. Sensitive skin syndrome: an update. Am J Clin Dermatol. 2020;21(3):401‐9. [DOI] [PubMed] [Google Scholar]
- 3. Berardesca E, Farage M, Maibach H. Sensitive skin: an overview. Int J Cosmet Sci. 2013;35(1):2‐8. [DOI] [PubMed] [Google Scholar]
- 4. Misery L, Loser K, Ständer S. Sensitive skin. J Eur Acad Dermatol Venereol. 2016;30(Suppl 1):2‐8. [DOI] [PubMed] [Google Scholar]
- 5. Misery L, Morisset S, Séité S, et al. Relationship between sensitive skin and sleep disorders, fatigue, dust, sweating, food, tobacco consumption or female hormonal changes: results from a worldwide survey of 10 743 individuals. J Eur Acad Dermatol Venereol. 2021;35(6):1371‐6. [DOI] [PubMed] [Google Scholar]
- 6. Polena H, Chavagnac‐Bonneville M, Misery L, Sayag M. Burden of sensitive skin (BoSS) questionnaire and current perception threshold: use as diagnostic tools for sensitive skin syndrome. Acta Derm Venereol. 2021;101(11):adv00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Segot‐Chicq E, Salah S, Jullien M, Portal N, Deschodt C, Gagnebien D. Defining and validating a Body Skin Discomfort Index (BSDI). Int J Cosmet Sci. 2018;40(5):488‐93. [DOI] [PubMed] [Google Scholar]
- 8. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210‐6. [DOI] [PubMed] [Google Scholar]
- 9. Misery L, Jean‐Decoster C, Mery S, Georgescu V, Sibaud V. A new ten‐item questionnaire for assessing sensitive skin: the Sensitive Scale‐10. Acta Derm Venereol. 2014;94(6):635‐9. [DOI] [PubMed] [Google Scholar]
- 10. Linder MD. A questionnaire for assessment of the burden posed by sensitive skin—a step forward to provide solid ground for an important concept. J Eur Acad Dermatol Venereol. 2018;32(12):2051‐2. [DOI] [PubMed] [Google Scholar]
- 11. Kim YR, Cheon HI, Misery L, Taieb C, Lee YW. Sensitive skin in Korean population: an epidemiological approach. Skin Res Technol. 2018;24(2):229‐34. [DOI] [PubMed] [Google Scholar]
- 12. Grinich EE, Shah AV, Simpson EL. Validation of a novel smartphone application‐enabled, patient‐operated skin barrier device. Skin Res Technol. 2019;25(5):612‐7. [DOI] [PubMed] [Google Scholar]
- 13. Grinich EE, Topham C, Haynes D, Chung J, Latour E, Simpson EL. Validation of a novel patient‐operated device for measuring skin barrier function in atopic dermatitis. Skin Res Technol. 2021;27(5):824‐30. [DOI] [PubMed] [Google Scholar]
- 14. Logger JGM, Driessen RJB, de Jong EMGJ, van Erp PEJ. Value of GPSkin for the measurement of skin barrier impairment and for monitoring of rosacea treatment in daily practice. Skin Res Technol. 2021;27(1):15‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cantisani C, Paolino G, Corsetti P, Bottoni U, Didona D, Calvieri S. Evaluation of Ingenolmebutate efficacy for the treatment of actinic keratosis with Antera 3D camera. Eur Rev Med Pharmacol Sci. 2015;19(1):92‐7. [PubMed] [Google Scholar]
- 16. Kandil SM, Soliman II, Diab HM, Bedair NI, Mahrous MH, Abdou EM. Magnesium ascorbyl phosphate vesicular carriers for topical delivery; preparation, in‐vitro and ex‐vivo evaluation, factorial optimization and clinical assessment in melasma patients. Drug Deliv. 2022;29(1):534‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Araco A, Araco F. Preliminary prospective and randomized study of highly purified polynucleotide vs placebo in treatment of moderate to severe acne scars. Aesthet Surg J. 2021;41(7):NP866–74. [DOI] [PubMed] [Google Scholar]
- 18. Kollias N, Gillies R, Muccini JA, Uyeyama RK, Phillips SB, Drake LA. A single parameter, oxygenated hemoglobin, can be used to quantify experimental irritant‐induced inflammation. J Invest Dermatol. 1995;104(3):421‐4. [DOI] [PubMed] [Google Scholar]
- 19. Richters R, Falcone D, Uzunbajakava N, Verkruysse W, van Erp P, van de Kerkhof P. What is sensitive skin? A systematic literature review of objective measurements. Skin Pharmacol Physiol. 2015;28(2):75‐83. [DOI] [PubMed] [Google Scholar]
- 20. Wollenberg A, Gimenez‐Arnau A. Sensitive skin: a relevant syndrome, be aware. J Eur Acad Dermatol Venereol. 2022;36(Suppl 5):3‐5. [DOI] [PubMed] [Google Scholar]
- 21. Zheng Y, Liang H, Li Z, Tang M, Song L. Skin microbiome in sensitive skin: the decrease of Staphylococcus epidermidis seems to be related to female lactic acid sting test sensitive skin. J Dermatol Sci. 2020;97(3):225‐8. [DOI] [PubMed] [Google Scholar]
- 22. Yatagai T, Shimauchi T, Yamaguchi H, et al. Sensitive skin is highly frequent in extrinsic atopic dermatitis and correlates with disease severity markers but not necessarily with skin barrier impairment. J Dermatol Sci. 2018;89(1):33‐9. [DOI] [PubMed] [Google Scholar]
- 23. Jiang W‐C, Zhang H, Xu Y, et al. Cutaneous vessel features of sensitive skin and its underlying functions. Skin Res Technol. 2020;26(3):431‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Misery L, Weisshaar E, Brenaut E, et al. Pathophysiology and management of sensitive skin: position paper from the special interest group on sensitive skin of the International Forum for the Study of Itch (IFSI). J Eur Acad Dermatol Venereol. 2020;34(2):222‐9. [DOI] [PubMed] [Google Scholar]
- 25. Farage MA. The prevalence of sensitive skin. Front Med (Lausanne). 2019;6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richters RJ, Falcone D, Uzunbajakava NE, et al. Sensitive skin: assessment of the skin barrier using confocal raman microspectroscopy. Skin Pharmacol Physiol. 2017;30(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nkengne A, Robic J, Seroul P, Gueheunneux S, Jomier M, Vie K. SpectraCam®: a new polarized hyperspectral imaging system for repeatable and reproducible in vivo skin quantification of melanin, total hemoglobin, and oxygen saturation. Skin Res Technol. 2018;24(1):99‐107. [DOI] [PubMed] [Google Scholar]
- 28. Linming F, Wei H, Anqi L, et al. Comparison of two skin imaging analysis instruments: the VISIA®from Canfield vs the ANTERA 3D®CS from Miravex. Skin Res Technol. 2018;24(1):3‐8. [DOI] [PubMed] [Google Scholar]
- 29. Matias AR, Ferreira M, Costa P, Neto P. Skin colour, skin redness and melanin biometric measurements: comparison study between Antera(®) 3D, Mexameter(®) and Colorimeter(®). Skin Res Technol. 2015;21(3):346‐62. [DOI] [PubMed] [Google Scholar]
- 30. Ruccia F, Zoccali G, Cooper L, Rosten C, Nduka C. A three‐dimensional scar assessment tool for keloid scars: volume, erythema and melanin quantified. Skin Res Technol. 2021;27(6):1007‐16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Researched data are not shared.


