Abstract
Background
In last years the role of fascia in proprioception and pain has been confirmed in numerous papers, but the real structure of fasciae is not still entirely known. To date, many studies have evaluated the elastic fibres in arteries, ligaments, lungs, epidermis and dermis, but only two studies exist about the elastic fibres in the fasciae, and they did not distinguish between superficial (in the subcutaneous tissue) and deep/muscular fasciae. The aim of the study was to assess the percentage of elastic fibres between superficial and deep fascia.
Materials and methods
Three full thickness specimens (proximal, middle and distal respectively) were taken from each of four regions of the thigh of three non‐embalmed cadavers: the anterior (Ant), the lateral (Lat), the posterior (Post) and the medial (Med) aspect. Thus, a total of 12 specimens were collected from each analysed thigh and histological Weigert Van Gieson stains was performed. Three sections per specimen were considered for the morphometric analysis.
Results
In all the specimens the superficial and deep fasciae were clearly recognizable. The difference in percentage of elastic fibres between superficial and deep fasciae in same region for all four was highly significant (p < 0.001). They are abundant in the superficial fascia than deep fascia.
Conclusions
In the light of these findings is evident that the superficial (in the subcutaneous tissue) and deep fasciae have different elasticity. This difference may improve grading of fascial dysfunction in dermatological diseases as burns, scars and lymphedema to better plan treatments.
Keywords: connective tissue, deep fascia, elastic fibres, elasticity, subcutaneous tissue, superficial fascia, thigh
1. INTRODUCTION
Nowadays several studies have confirmed the role of fasciae in proprioception and pain, 1 but the real structure of fasciae is not still entirely known. Besides, many people use the term ‘fascia’ in an indiscriminate way to reference to various types of connective tissues. For example, the term ‘fascial system’, proposed during the IV Fascia World Congress in Washington in 2015 is useful to understand the function of the global fascial net during movement, including the interconnections of fascial tissues with joint capsules, aponeuroses, tendons, ligaments and intramuscular connective tissues, but it gives also the idea that all the fasciae have the same microscopic anatomy and behaviour. Really, based on previous studies, 1 , 2 , 3 , 4 inside the fascia system at least two completely different structures could be described: superficial fascia and deep fascia. The superficial fascia is the fascial layer that organizes the subcutaneous adipose tissue, the superficial vessel and nerves and is related with the lymphatic drainage. It corresponds to the panniculus carnosus in the animals and is also named ‘stratum membranosus’ in the Terminologia Anatomica. The deep/muscular fasciae are the envelop of the muscles, they are composed by two or three layers of densely packed collagen fibres with a layer of loose connective tissue between these fibrous layers. 5 , 6 They have the functions to reduce tissue friction, protect underlying structures and most importantly transmit mechanical forces generated by muscle skeletal system at a distant, working as a bridge that connects all the elements involved in a precise movement. 7 So, surely the superficial fascia and deep fascia have different anatomical relationship and different functions, but it is not clear if they have the same histological features or not. One of the most debated question is about the fascial elasticity, which is a passive mechanical property of fascia that plays an important role in the completion of each movement. 8 The passive elastic forces of fasciae and stretch forces determine the stiffness of every muscle‐fascia unit, 9 the latter being a functional unit between the muscle and the deep/muscular fascia. Many training protocols and manual therapies have the aim to restore the fascial elasticity, 10 but really is not clear if the fascia is an elastic tissue or not. Indeed, the deep fasciae often works as a tendon, and it is well known that the tendons have a very small amount of elastic fibres. To date, while some studies have evaluated the elastic fibres in arteries, ligaments, lungs, epidermis and dermis, 11 only two studies 1 , 12 showed different percentage of elastic fibres in the fasciae, even if they did not distinguish between superficial (in subcutaneous tissue) and deep fasciae. Veronese et al. (2020) highlighted that: “dermatological diseases often affect not only the superficial part of the dermis but deepen into the hypodermis and sometime involve the muscle bundles”. 13 Several dermatological diseases, such as burns, scars, lymphedema but also aesthetic medicine could benefit from an in‐depth knowledge of the fasciae. Moreover, the superficial fascia, with its role in the wound healing by means of fascia fibroblasts, and the deep/muscular fascia with its fibrous and water component alterations, could be an important target for the therapies and the treatments. 14 , 15 , 16 , 17 The current study set out to assess the different distribution of elastic fibres between superficial fascia and deep fascia and if the elastic component varies inside the same fascia in the different areas. For this purpose, we choose the topographic region of thigh in which superficial fascia and deep fascia can be clearly distinguished.
2. MATERIALS AND METHODS
2.1. Macroscopic study
Anatomical studies were conducted on three nonembalmed cadavers (age ranging 55−65 years; two male, one female) without documented or anatomical evidence of thigh pathologies, obtained from the ‘Body Donation Programme’ at the Institute of Anatomy, University of Padova. 18 The thighs were dissected according to the following protocol. Three full thickness specimens (proximal, middle and distal respectively) were taken from each of four regions of the thigh: the anterior (Ant), the lateral (Lat), the posterior (Post) and the medial (Med) aspect. Thus, a total of 12 specimens were collected from each analysed thigh.
Each sample was 60 mm long and 30 mm thick, in order to obtain optimal samples to examine the microscopic anatomy of the various subcutaneous layers. Anatomic integrity was carefully respected.
2.2. Histological study
The twelve full‐thickness specimens collected from each right thigh, were mounted on cardboard to avoid deformation artifacts, fixed in 10% formalin solution, and embedded in paraffin, attention being paid to obtain full‐thickness sections from the skin to the deep layer. 10‐μm thick sections were stained with Weigert Van Gieson (Figure 1, 2, 3, 4) and three sections per specimen were considered for the morphometric analysis. All preparations were observed under a DM4500‐B light microscope (Leica Microsystems, Wetzlar, Germany) and the image of six field, at magnification of 1.6×, were recorded in full colour (24 bits) by a digital camera (DFC 480, Leica Microsystems).
FIGURE 1.
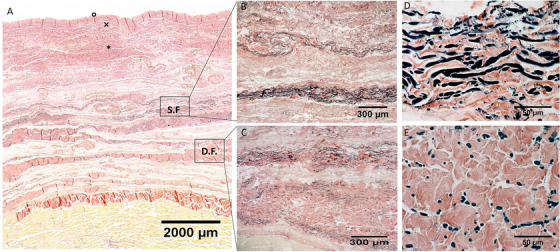
Histological images (Weigert Van Gieson staining) of anterior region (A), S.F. = Superficial Fascia; D.F. = Deep Fascia; × = papillary dermis; * = reticular dermis; ° = epidermis. (B) and (D): Superficial fascia. (C) and (E): Deep fascia
FIGURE 2.
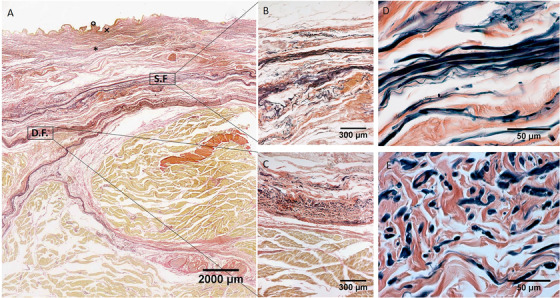
Histological images (Weigert Van Gieson staining) of medial region (A), S.F. = Superficial Fascia; D.F. = Deep Fascia; × = papillary dermis; * = reticular dermis; ° = epidermis. (B) and (D): Superficial fascia. (C) and (E): Deep fascia
FIGURE 3.
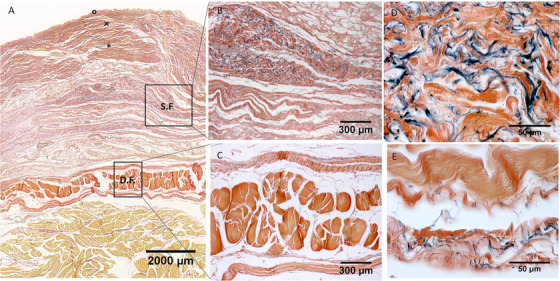
Histological images (Weigert Van Gieson staining) of lateral region (A), S.F. = Superficial Fascia; D.F. = Deep Fascia; × = papillary dermis; * = reticular dermis; ° = epidermis. (B) and (D): Superficial fascia. (C) and (E): Deep fascia
FIGURE 4.
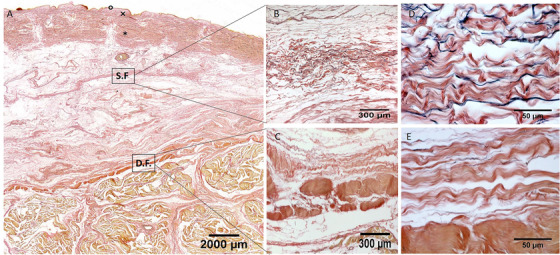
Histological images (Weigert Van Gieson staining) of posterior region (A), S.F. = Superficial Fascia; D.F. = Deep Fascia; × = papillary dermis; * = reticular dermis; ° = epidermis. (B) and (D): Superficial fascia. (C) and (E): Deep fascia
Computer‐assisted image analysis was then used to estimate the amount of elastic fibres in the superficial fascia and deep fascia (fascia lata), and the ImageJ software 19 (freely available at http://rsb.info.nih.gov/ij/) was used to perform this analysis. Briefly, to identify elastic fibres in the images from the Weigert Van Gieson‐stained sections colour deconvolution was applied. This procedure implements stain separation according to the method by Ruifrok et al. 20 and was performed by using an ImageJ plugin specifically developed by Gabriel Landini (see http://www.mecourse.com/landinig/software/software.html). This procedure leads to the generation of two images mainly containing picrofuchsine‐ and resorcin fuchsin‐stained structures, respectively. From the latter one, elastic fibre profiles can be easily discriminated by conventional thresholding methods. A region of interest was then traced to correspond to the fasciae. The total amount of elastic fibres in the fasciae was then evaluated by estimating the percent area (Area%) occupied by stained structures. Morphometric measurements of the superficial fascia and deep fascia (fascia lata) were recorded on full‐thickness specimens and analysed by Image J software. 18 The mean values and standard deviations of percentage measurements were calculated.
2.3. Statistical analysis
Results are reported as mean (±SD) values. Mann–Whitney test was performed to verify significant differences among elastic fibres in superficial and deep fascia of the same region. To detect statistical differences in elastic fibres within the same type of fascia and different regions, a nonparametric one‐way analysis of variance (ANOVA) was performed by using Kruskal–Wallis test, followed by Dunn's test for multiple comparisons. All the statistical analyses were performed by GraphPad Prism 8.4.2 software (GraphPad Software Inc., San Diego, CA, USA). p < 0.05 was always considered as the limit for statistical significance.
3. RESULTS
In all the full‐thickness specimens, constant morphology of the subcutaneous tissue was seen, organized in the following layers, beginning from the surface: skin (epidermis and dermis), superficial adipose tissue (SAT), superficial fascia, deep adipose tissue (DAT), and deep fascia (Figure 1, 2, 3, 4). Only in the posterior region the superficial fascia appeared less defined, presenting multiple layers and the elastic fibres were distributed on overall of the subcutaneous tissue. The superficial fascia (mean thickness of 146.6±31.5 μm;) was formed of various sublayers of fibro‐elastic tissue with variable points of interconnections between the layers. The mean amount of elastic fibres was 13.25%. The area% of the elastic fibres, among the various regions of superficial fascia (Ant = 13.79 ± 4.750%; Med = 12.69 ± 3.108%; Post = 13.08 ± 1.960%; Lat = 13.45 ± 3.159%) (Figure 5), is not statistically significant.
FIGURE 5.
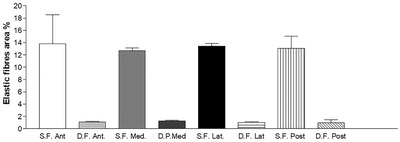
Elastic fibres area (%) of superficial fascia and deep/muscular fascia of thigh in various regions. S.F. = superficial fascia. D.F. = deep fascia. Ant = anterior; Med = medial, Lat = lateral ; Posterior = posterior
Moreover, at high magnification a precise organization of the elastic fibres was possible to recognize, their direction was both longitudinal and transversal orientations. They appeared predominantly transversal oriented in the superficial part of the superficial fascia, while they are predominantly longitudinal in the deep part.
In the deep fascia (mean thickness 804.9 ± 200.4 μm;), the mean amount of elastic fibres was 1.098%. The area% of elastic fibres in the deep fascia, among the various regions of thigh (Ant = 1.089 ± 0.8336%; Med = 1.289 ± 0.9614%; Post = 0.9683 ± 0.5255%; Lat = 1.046 ± 0.7932%) (Figure 5), was not statistically significant.
The main direction of elastic fibres in deep fascia was transversal than the longitudinal direction of the thigh. The difference in percentage of elastic fibres between superficial and deep fasciae in same region for all four was highly significant (p < 0.001).
The difference among regions, respectively for superficial fascia and deep fascia, was not statistically significant (p > 0.05).
Moreover, qualitatively there was a difference among various regions, in the superficial fascia the elastic fibres are organized as a 3D network where the transversal direction (respect to the thigh main axis) is prevalent.
In the superficial fascia, the elastic fibres formed a sort of lacework pattern, in some areas perforating the collagen bundles at different levels, framing in various direction concentric layers in a 3‐D point of view in which collagen and elastic fibres were more mixed making a network to support the lymphatic vessels (Figure 7). At higher enlargement we can recognize that elastic fibres were predominantly transversally oriented (compared the longitudinal axis of thigh) in the superficial part of the superficial fascia and longitudinally in the deep part. Moreover, also if there is not a statistical difference in the elastic fibres amount among the various regions of the deep fasciae, qualitatively there are some differences. The layered conformation is more clearly evident in the lateral region, where the deep fascia is formed by three layers: the superficial and deep ones are thin and relatively reach in elastic fibres placed transversally respect to the longitudinal axis of the thigh, the central one (corresponding to the iliotibial tract) is constituted by compact fibrous bundles almost without elastic fibres, having probably the only function of force transmission. At the contrary, the layered conformation of the deep fascia is less clear in the posterior region and the elastic fibres component was scarce and distributed above all in longitudinal direction.
FIGURE 7.
Scheme of superficial fascia, behaviour of elastic fibres in relaxation state (A) and in traction state (B)
4. DISCUSSION
Our study demonstrated that the fasciae of the thigh are well recognizable, layered structures, with a highly significant difference in the elastic fibres in superficial and deep fasciae for all four regions (Ant; Med; Post; Lat). The superficial fascia appeared as a fibroelastic layer in the middle of the subcutaneous fat tissue, mostly made up of elastic fibres. This layer splits in some points in order to envelop the superficial vessels and nerves, as great saphenous vein. In the posterior region it lost the fibrous appearance and it was filled by fat tissue, assuming an honeycomb aspect. At the contrary, the deep/muscular fascia is accompanied by poor amount of elastic fibres which are present above all in the loose connective tissue among the fibrous bundles. Consequently, it is evident that the superficial and deep fasciae have a totally different mechanical behaviour and probably have different functions.
The findings are consistent with those of the other two previous studies about the deep fasciae, in which they were clearly less numerous than the collagenous fibres. 21 , 22 Besides, we confirmed the sublayer organization of the collagen fibres in the deep fasciae already described by Benetazzo et al. 23 Basing on this microscopic anatomy of the deep fascia, we can affirm that the major amount of elastic fibres is present in the loose connective tissue between two adjacent fibrous layers and in the thin layer that define the borders of the deep fascia, and secondarily around each collagen fibre bundles, where exhibit an organization interdependent on collagen bundle arrangement.
Elastic component, inside loose connective tissue, allows the fibrous layers to move with respect to the adjacent one and to return to initial position (Figure 6). They behave like a bridge among various layers and sublayers that so can be moved, stretched and brought back to the initial position. This organization is probably the one that has a fundamental role in the elastic capacity of deep fasciae, to mix their elastic capacity and of force transmission. The fibrous component transmits forces, the loose connective tissue and the elastic component allows deep fascia to adapt and to go back to the starting position, returning elastic energy in the form of plyometric energy. 24
FIGURE 6.
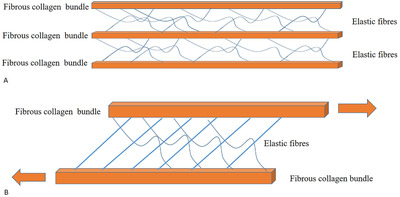
Biomechanical behaviour of elastic component, inside loose connective tissue of deep fascia. (A) Relaxed state. (B) Movement state, elastic component between fibrous collagen bundles allows them to move with respect to the adjacent one and to return to initial position
Despite the study needs to be deepened, in the light of these findings is evident the crucial role of elastic fibres in thigh's fasciae. They are abundant in the superficial fascia than deep fascia. Elastin elements influence biomechanical properties of the fascial tissues, not only as result of their extensibility but also their unique, three‐dimensional arrangement within collagen bundles, that is different between superficial and deep. From this study it is evident that the superficial fascia is more adaptable than deep fascia which has an important role in the force transmission. This also suggest a different manual approach if we want to treat the superficial or the deep fascia, as it is suggested in many physiotherapy techniques. 21 The network organization of the elastic fibres in the superficial fascia creates a sort of elastic containment for lymphatic vessels and veins like a female coolant. The elastic fibres disposition, that is more transversally oriented than longitudinal, could explain, in a translational point of view, the application of manual therapy or lymphatic drainage, being easier in transversal skin pinching than longitudinal.
Further studies are needed to understand the role of elastic fibres in the pathogenesis of different dermatological and musculoskeletal diseases, but also for the venous return, lymphatic drainage and so on. Moreover, skin tissue repair after surgical intervention can result in a broad spectrum of scar types, ranging from a fine line to a variety of abnormal and pathologic scars, which can have functional, cosmetic and psychological consequences. 25 , 26 , 27 The assessment is fundamental for monitoring scar evolution and treatment efficacy. Many devices and procedures have already been implemented for the analysis of the superficial layer. 27 , 28 , 29 A better understanding of histological features of these fascial components can explain better the involvement of these in dermatological and musculoskeletal diseases, both in structural and sometimes in functional alterations, which can affect the patient's quality of life. Our assessment may also give further insight into skin conditions associated with disrupted elastic fibres, such as cutis laxa, anetoderma and photoageing.
Finally, it is well known that in the epidermis and dermis the amount of elastic fibres decrease with aging, 30 , 31 it is consequently possible that also the superficial fascia could be affected by a progressive loss of elastic fibres over time. Further studies will be necessary to understand this aspect.
5. LIMITATIONS OF THE STUDY
The small number of cadavers included in this study and the qualitative aspect of the assessments mean that it is not possible to statistically analyse the prevalence of elastic fibres as well as to explain their possible causes, prognostic significance and therapeutic implications. Moreover, having used only corpses with an average age of 60 years old, it was not possible to quantify variation in elastic fibres between different ages.
6. CONCLUSIONS
This study added to the lack of knowledge about elastic fibres within fasciae. Our results may contribute to a better understanding of the pathogenesis of fascial disorders from superficial to deep and improve the knowledge about the difference between superficial in subcutaneous tissue and deep/muscular fascia. Accordingly, this difference may help to improve grading of fascial dysfunction in dermatological diseases and soft tissue injuries, and to better plan therapies, treatments, manual therapy, exercise and stretching.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ACKNOWLEDGEMENTS
The authors sincerely thank the people who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind's overall knowledge that can then improve patient care. Therefore, these donors and their families deserve our highest gratitude. The authors gratefully thank all the collaborators (in particular Maria Martina Sfriso) who work in the Institute of Human Anatomy, University of Padua.
Pirri C, Fede C, Petrelli L, Guidolin D, Fan C, De Caro R, Stecco C. Elastic Fibres in the subcutaneous tissue: Is there a difference between superficial and muscular fascia? A cadaver study. Skin Res Technol. 2022;28:21–27. 10.1111/srt.13084
REFERENCES
- 1. Abu‐Hijleh MF, Roshier AL, Al‐Shboul Q, Dharap AS, Harris PF. The membranous layer of superficial fascia: Evidence for its widespread distribution in the body. Surg Radiol Anat. 2006;28 (6):606–19. [DOI] [PubMed] [Google Scholar]
- 2. Stecco C, Porzionato A, Macchi V, Tiengo C, Parenti A, Aldegheri A, et al. Histological characteristics of the deep fascia of the upper limb. Ital J Anat Embryol. 2006;111:105–10. [PubMed] [Google Scholar]
- 3. Stecco C, Gagey O, Belloni A, Pozzuolia A, Porzionatoc A, Macchi V, et al. Anatomy of the deep fascia of the upper limb. Second part: study of innervation. Morphologie. 2007;91:38–43. [DOI] [PubMed] [Google Scholar]
- 4. Stecco C, Pirri C, Fede C, Fan C, Giordani F, Stecco L, et al. Dermatome and fasciatome. Clin Anat. 2019;32:896–902. [DOI] [PubMed] [Google Scholar]
- 5. Stecco C. Functional atlas of the human fascial system. London, UK: Elsevier Health Sciences; 2015. [Google Scholar]
- 6. Pirri C, Fede C, Petrelli L, Guidolin D, Fan C, De Caro R, et al. An anatomical comparison of the fasciae of the thigh: 450 A macroscopic, microscopic and ultrasound imaging study. J Anat. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eng CM, Pancheri FQ, Lieberman DE, Biewener AA, Dorfmann L. Directional differences in the biaxial material properties of fascia lata and the implications for fascia function. Ann Biomed Eng. 2014;42:1224–37. [DOI] [PubMed] [Google Scholar]
- 8. Liu CL, Zhou JP, Sun PT, Chen B‐Z, Zhang J, Tang C‐Z, et al. Influence of Different Knee and Ankle Ranges of Motion on the Elasticity of Triceps Surae Muscles, Achilles Tendon, and Plantar Fascia. Sci Rep. 2020;10(1):6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zwirner J, Ondruschka B, Scholze M, Schulze‐Tanzil G, Hammer N. Mechanical Properties of Native and Acellular Temporal Muscle Fascia for Surgical Reconstruction and Computational Modelling Purposes. J Mech Behav Biomed Mater. 2020;108:103833. [DOI] [PubMed] [Google Scholar]
- 10. Chaitow L. Fascial dysfunction manual therapy approaches – Second edition. Pencaitland, UK: Handspring Publishing; 2018. [Google Scholar]
- 11. Baldwin AK, Simpson A, Steer R, Cain SA, Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. 2013;15:e8. [DOI] [PubMed] [Google Scholar]
- 12. Stecco A, Gilliar W, Hill R, Fullerton B, Stecco C. The Anatomical and Functional Relation Between Gluteus Maximus and Fascia Lata. J Bodyw Mov Ther. 2013;17(4):512–7. [DOI] [PubMed] [Google Scholar]
- 13. Veronese S, Picelli A, Smania N, Sbarbati A. Hypodermis involvement in skin disorders: Imaging and functional imaging diagnostic tools. Skin Res Technol. 2020. [DOI] [PubMed] [Google Scholar]
- 14. Stone Ii R, Natesan S, Kowalczewski CJ, Mangum LH, Clay NE, Clohessy RM, et al. Advancements in regenerative strategies through the continuum of burn care. Front Pharmacol. 2018;9:672–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang D, Christ S, Correa‐Gallegos D, Ramesh P, Gopal SK, Wannemacher J, et al. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N‐cadherin. Nat Commun. 2020;11(1):5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hellström M, Hellström S, Engström‐Laurent A, Bertheim U. The structure of the basement membrane zone differs between keloids, hypertrophic scars and normal skin: a possible background to an impaired function. J Plast Reconstr Aesthet Surg. 2014;67(11):1564–72. [DOI] [PubMed] [Google Scholar]
- 17. Ud‐Din S, Foden P, Stocking K, Mazhari M, Al‐Habba S, Baguneid M, et al. Objective assessment of dermal fibrosis in cutaneous scarring, using optical coherence tomography, high‐frequency ultrasound and immunohistomorphometry of human skin. Br J Dermatol. 2019;181(4):722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Porzionato A, Macchi V, Stecco C, Mazzi A, Rambaldo A, Sarasin G, et al. Quality management of Body Donation Program at the University of Padova. Anat Sci Educ. 2012;5(5):264–72. [DOI] [PubMed] [Google Scholar]
- 19. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruifrok AC, Katz RL, Johnston DA. Comparison of quantification of histochemical staining by hue‐saturation‐intensity (HSI) transformation and color‐deconvolution. Appl Immunohisto Mol Morphol. 2003;11:85–91. [DOI] [PubMed] [Google Scholar]
- 21. Stecco L, Stecco A. Manipolazione fasciale per le disfunzioni interne ‐ Parte pratica. Piccin; 2015. [Google Scholar]
- 22. Szotek S, Dawidowicz J, Eyden B, Matysiak N, Czogalla A, Dudzik G, et al. Morphological Features of Fascia Lata in Relation to Fascia Diseases. Ultrastruct Pathol. 2016;40(6):297–310. [DOI] [PubMed] [Google Scholar]
- 23. Benetazzo L, Bizzego A, De Caro R, Frigo G, Guidolin D, Stecco C. 3D reconstruction of the crural and thoracolumbar fasciae. Surg Radiol Anat. 2011;33(10):855–62. [DOI] [PubMed] [Google Scholar]
- 24. Pavan PG, Pachera P, Stecco C, Natali AN. Biomechanical Behavior of Human Crural Fascia in Anterior and Posterior Regions of the Lower Limb. Med Biol Eng Comput. 2015; 53(10):951‐9. [DOI] [PubMed] [Google Scholar]
- 25. Mustoe TA, Cooter RD, Gold MH, Richard Hobbs FD, Ramelet A‐A, Shakespeare PG, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–71. [DOI] [PubMed] [Google Scholar]
- 26. Vercelli S, Ferriero G, Sartorio F, Stissi V, Franchignoni F. How to assess postsurgical scars: a review of outcome measures. Disabil Rehabil. 2009;31:2055‐63. [DOI] [PubMed] [Google Scholar]
- 27. Pirri C, Stecco A, Fede C, De Caro R, Stecco C, Özçakar L. Ultrasound imaging of a scar on the knee: Sonopalpation for fascia and subcutaneous tissues. Eur J Transl Myol. 2020;30(1):8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosicka K, Mierzejewska‐Krzyżowska B, Mrówczyński W. Comparison of different MyotonPRO probes for skin stiffness evaluation in young women. Skin Res Technol. 2020;26:1‐8. [DOI] [PubMed] [Google Scholar]
- 29. Kim MA, Kim EJ, Lee HK. Use of SkinFibrometer® to measure skin elasticity and its correlation with Cutometer® and DUB® Skinscanner. Skin Res Technol. 2018;24(3):466–71. [DOI] [PubMed] [Google Scholar]
- 30. Roten SV, Bhat S, Bhawan J. Elastic fibers in scar tissue. J Cutan Pathol. 1996;23:37–42. [DOI] [PubMed] [Google Scholar]
- 31. Starcher B, Aycock RL, Hill CH. Multiple roles for elastic fibers in the skin. J Histochem Cytochem. 2005; 53:431–43. [DOI] [PubMed] [Google Scholar]


