BACKGROUND:
Although sugammadex is well known for its use in reducing the incidence of residual neuromuscular blockade, this has not always been translated to improved clinical measures of postoperative respiratory muscle strength. Expiratory muscles play an important role in airway clearance and inspiratory muscle capacity augmentation, yet they have not been well studied. Therefore, we tested the hypothesis on whether sugammadex could enhance expiratory muscle strength recovery more completely than neostigmine in the immediate postextubation period.
METHODS:
Adult patients having microlaryngeal surgery under total intravenous anesthesia were randomized to receive sugammadex or neostigmine. The thickening fraction of internal oblique abdominal muscle (TFIO) and diaphragm excursion, respectively, reflecting expiratory and inspiratory muscle strength, were measured via ultrasonography at 3 time points: before induction (baseline), train-of-four ratio (TOFR) recovery to 0.9, and 30 minutes after postanesthesia care unit (PACU) arrival. The primary outcome was the change in TFIO from baseline to TOFR ≥0.9. The postoperative changes of diaphragm excursion from baseline, incidences of TFIO and diaphragm excursion returning to baseline levels, and the time from TOFR 0.9 to 0.95 and 1 were also measured.
RESULTS:
Among 58 patients, a significant difference in the change in TFIO from baseline to TOFR ≥0.9 between the sugammadex and neostigmine groups was observed: mean ± standard deviation, 9% ± 6% vs 16% ± 9%; difference in means: −6% (95% confidence interval [CI], −10 to −2); and adjusted P =.005 (adjusting for imbalanced variables between 2 groups). Sugammadex resulted in smaller changes in diaphragm excursion from baseline to TOFR ≥0.9 compared with neostigmine: difference in means: −0.83 cm (99.4% CI, −1.39 to −0.28 cm; Bonferroni-corrected P < .001). After 30 minutes in the postanesthesia care unit (PACU),33% of patients reversed with sugammadex versus 14% of those receiving neostigmine reached baseline TFIO levels (99.4% CI, −14 to 52; Bonferroni-corrected P > .999). The incidences of TFIO and diaphragm excursion returning to baseline were relatively low (<40%) in both groups despite TOFR reaching 1. The median time from TOFR of 0.9 to 0.95 and to 1 among patients receiving sugammadex was 7 and 10× faster than those receiving neostigmine (0.3 vs 2 minutes, Bonferroni-corrected P = .003; 0.5 vs 5.3 minutes, Bonferroni-corrected P < .001, respectively).
CONCLUSIONS:
Sugammadex provides a more complete recovery of expiratory muscle strength than neostigmine at TOFR ≥0.9. Our data suggest that the respiratory muscle strength might still be impaired despite TOFR reaching 1.
KEY POINTS.
Question: Does sugammadex enhance expiratory muscle strength recovery more completely than neostigmine in the immediate postextubation period?
Findings: Sugammadex provided more complete expiratory muscle strength recovery in the immediate postextubation period than neostigmine; however, despite train-of-four ratio (TOFR) reaching 1, strength of respiratory muscles did not fully recover in most patients 30 minutes after postanesthesia care unit (PACU) arrival, irrespective of reversal agents.
Meaning: The superiority of sugammadex to neostigmine in enhancing expiratory muscle strength recovery immediately after extubation may contribute to generating high expiratory pressures for effective coughing and secretion clearance during emergence, possibly suggesting a relationship between sugammadex and a lower incidence of adverse respiratory outcomes.
Residual neuromuscular blockade (NMB) related to neuromuscular blocking agents (NMBAs) is associated with increased postoperative pulmonary complications (PPCs).1,2 Compared with neostigmine, sugammadex reduces the incidence of residual NMB.3–5 However, the association between PPCs and sugammadex remains unclear.6–10 The return of muscle function to its preoperative level is responsible for protecting patients from PPCs. Although the clinical impression is that patients’ strength appears stronger after reversal with sugammadex compared with neostigmine, it remains controversial on the effect of different reversal drugs on the recovery of respiratory muscle strength.11–13 Cappellini et al13 reported that the recovery of diaphragmatic strength representing inspiratory muscle strength at extubation was enhanced in patients reversed with sugammadex, while other studies detected no difference between the 2 drugs when forced vital capacity was used to reflect global respiratory muscle function.11,12
While inspiratory muscle weakness causes alveolar hypoventilation leading to atelectasis development, expiratory muscle weakness is associated with ineffective cough, which is also one of the major contributors to PPCs.14,15 Contraction of the abdominal muscles during expulsive expiratory efforts not only generates high expiratory pressures for effective coughing and secretion clearance but also increases inspiratory muscle capacity.16 A recent study showed that the thickening fraction of internal oblique abdominal muscle (TFIO) reflecting expiratory muscle strength was correlated to the airway pressure generated by cough, and this reduced abdominal muscle thickening during cough was associated with a high risk of liberation failure in mechanically ventilated patients,17 implying the vital importance of the expiratory muscle function. Unfortunately, comparative studies on the postextubation recovery of expiratory muscle function are scant.
Although sugammadex reverses NMB to the train-of-four ratio (TOFR) of ≥0.9 faster than neostigmine,18,19 extubation at TOFR ≥0.9 was not associated with better pulmonary outcomes,7 whereas TOFR ≥0.95 was required to reduce PPCs.20 Eikermann et al21 suggested that extubation after TOFR = 1 might be preferable. However, the recovery time from TOFR of 0.9 to 0.95 and to 1 after using different reversal agents has not been reported yet.
Therefore, we designed this trial primarily to compare the impact of sugammadex and neostigmine on the recovery of expiratory muscle strength at TOFR ≥0.9 in patients undergoing ambulatory microlaryngeal surgery. We hypothesized that sugammadex would enhance expiratory muscle strength recovery more completely than neostigmine in the immediate postextubation period. Moreover, we hypothesized that the time from TOFR of 0.9 to 0.95 and to 1 would be shorter in patients receiving sugammadex than those receiving neostigmine.
METHODS
A prospective, single-center, randomized, controlled, assessor-blinded trial, according to the Consolidated Standards of Reporting Trials (CONSORT) statement, was performed from September 2020 to February 2021 at a tertiary academic medical center. The study protocol was approved by the research ethics committee of the First Affiliated Hospital, Sun Yat-sen University in Guangzhou, China (Ref: [2020]180) and registered at http://www.chictr.org.cn/showproj.aspx?proj=55007 (ChiCTR2000033832, Principal investigator: Ying Xiao, date of registration: June 14, 2020) before the first patient was enrolled. All patients provided written informed consent.
Study Population
Patients 18 to 65 years of age scheduled for ambulatory microlaryngeal surgery were included. Exclusion criteria were as follows: (1) American Society of Anesthesiology (ASA) physical status III–V; (2) significant kidney disease (stage 4 kidney disease or higher); (3) significant liver disease (Child-Pugh B or C class); (4) history of chronic obstructive pulmonary disease; (5) known or suspected neuromuscular disease; (6) arrhythmic disease or use of antiarrhythmic drugs; (7) allergy to rocuronium, neostigmine, or sugammadex; (8) suspected difficult airway; (9) pregnancy or breastfeeding women; (10) nonparticipation; and (11) failure to cooperate with the assessment.
Randomization and Blinding
Patients were randomly allocated with a 1:1 ratio to the sugammadex group or the neostigmine group. The randomization sequence without any stratification was generated on www.randomization.com and sealed with consecutively numbered envelopes providing concealment of random allocation. The allocation was revealed to the anesthesiologist 10 minutes before the expected end of the surgery. Surgeons, outcome assessors (not directly involved in the patients’ care), and statisticians were blinded to group assignment until the final statistical analysis was completed.
Anesthetic Management
No premedication was administered. Anesthetic management was standardized in all subjects. Monitoring consisted of electrocardiogram, pulse oximetry, noninvasive blood pressure, temperature, and capnography. Depth of anesthesia was judged by raw electroencephalogram monitored by Narcotrend (Monitor Technik). Anesthesia was induced with propofol and remifentanil. The plasma-targeted concentration of propofol started at 4–6 μg·mL−1 and was delivered via a target-controlled infusion pump (Alaris PK, Cardinal Health) programmed with the Marsh model, and remifentanil was started at an infusion rate of 0.3 μg·kg−1·min−1. After calibration of TOF-Watch SX, 0.6 mg·kg−1 rocuronium was administered, and tracheal intubation was performed in the absence of train-of-four (TOF) count. During surgery, the target propofol concentration was adjusted in steps of 0.5 μg·mL−1 to keep 95% spectral edge frequency within 8–12 Hz as guided by the electroencephalogram. Remifentanil was maintained at the rate of 0.3–0.7 μg·kg−1·min−1 during the main procedure, and infusion levels were set to 0.1 μg·kg−1·min−1 toward the end of surgery. We titrated remifentanil to maintain heart rate within 10% of values after induction and systemic blood pressure within 20% of baseline measures. Hypotension was treated with a bolus of 0.3 mg metaraminol or a continuous infusion of norepinephrine, as clinically indicated. Dexamethasone (10 mg) and palonosetron (0.25 mg) were given to prevent postoperative nausea and vomiting, which was the common adverse effect of neostigmine.22 Flurbiprofen (50 mg) was administered for preventive analgesia.
Neuromuscular Monitoring
Depth of NMB was continuously monitored by acceleromyography of the adductor pollicis muscle of the left arm using TOF-Watch SX (Organon). After skin cleansing, 2 surface electrodes were positioned over the ulnar nerve at the wrist. A hand adapter that applied a constant preload to the thumb was secured to the hand with tape, and the acceleration transducer was attached to the distal phalanx of the thumb via the hand adapter. The left hand was positioned on the transport cart to prevent movement of the fingers except for the thumb during each assessment. After confirmation of loss of consciousness, TOF-Watch SX was calibrated with the built-in calibration modus (CAL 2 mode) after 5 seconds of 50-Hz tetanic stimulation preceded by a repetitive TOF stimulation for 1 minute. After calibration, a 3-minute repetitive TOF stimulation was applied to ensure a stable response (TOFR ranged from 0.9 to 1.1).23 A forced-air warming device (Bair Hugger, 3M Company) was used to maintain left-hand skin temperatures >32°C. TOFR was measured every 15 seconds throughout the procedure and up to 30 minutes after arrival at the postanesthesia care unit (PACU). Rocuronium (0.15 mg·kg−1) was administered when the posttetanic count (repeated every 2 minutes) was ≥3.
Intervention
At the end of the surgery, patients received either neostigmine (50 μg·kg−1, maximum 5 mg) combined with atropine (25 μg·kg−1, maximum 2.5 mg) or sugammadex (2 mg·kg−1) after TOF counts at least exceeding 1 and when the raw electroencephalogram showed the trend of cognitive arousal (tracking electroencephalogram changes from high-amplitude, low-frequency activity during anesthesia to low-amplitude, high-frequency activity during wakefulness). Extubation was performed in the operating room when the patient was fully awake and fulfilled clinical criteria for extubation.
Outcome Measures and Data Collection
Demographic characteristics and intraoperative data were recorded. TOF data were automatically recorded utilizing the TOF-Watch SX monitoring program, version 2.5. The effect-site concentration of propofol at the beginning of strength testing was recorded.
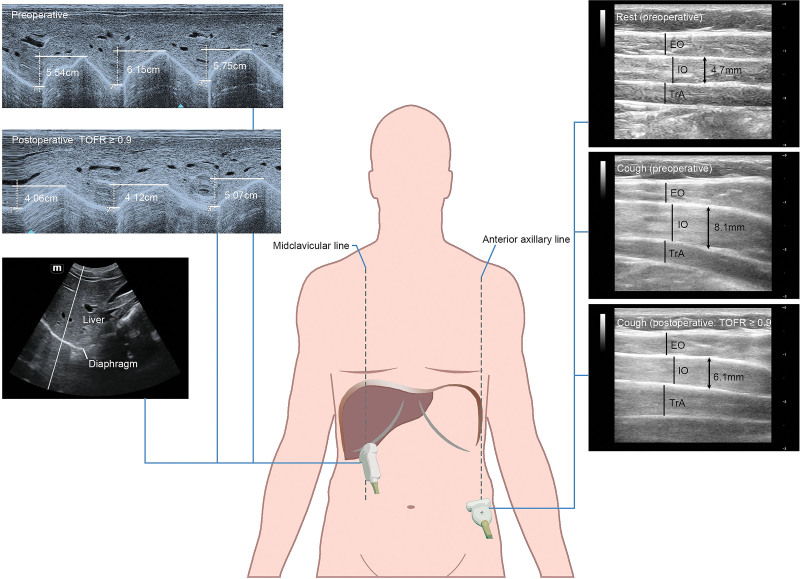
TFIO and diaphragm excursion (DIA), reflecting the expiratory and inspiratory muscle strength, respectively, were measured via ultrasonography (Mindray ME7, Mindray Bio-medical Electronics Co Ltd) at 3 predefined time points: before induction (baseline levels), TOFR ≥0.9 (postextubation), and after 30 minutes in the PACU. One outcome assessor, who had been trained to be skilled in the measurements of TFIO and DIA with the guidance of an ultrasonographic specialist, took charge of all participant assessments. Before testing, the outcome assessor coached patients on cooperating with the procedure. The patient was in a semirecumbent position (with the head of the bed elevated at an angle of 45° with a goniometer) during evaluations. As the technique might be effort-dependent, each measurement was performed 3 times, and the highest value was recorded.
During the assessment of expiratory muscle strength, an L14-6Ns linear probe was positioned on the right anterior axillary line, midway between the inferior border of the rib cage and the iliac crest, perpendicular to the abdominal wall. The different lateral abdominal wall muscles would be relatively easy to identify as hypoechogenic layers enclosed by fascial sheaths. The thickness of the internal oblique muscle was measured at end-inspiratory and the maximum contraction when the patient was told to cough with maximum strength. TFIO was calculated as the magnitude of thickness increased during coughing with maximum strength (TFIO = [end-coughing thickness − end-inspiratory thickness]/[end-inspiratory thickness] × 100%).17,24
Diaphragmatic ultrasound was performed during maximum sniff breathing with a C5-1s convex transducer positioned below the right costal arch at the midclavicular line by angling the ultrasound beam cranially and perpendicular to the diaphragmatic dome.25 B-mode was initially used to visualize the diaphragm as an echogenic line between the interface of the lung and liver and was then changed to M-mode to measure DIA on the vertical axis, tracing from the baseline to the point of a maximum height of inspiration on the graph. The sweep speed was adjusted to 25 mm/s to obtain a minimum of 3 respiratory cycles within one image (Figure 1).
Figure 1.
Diaphragmatic sniff ultrasound and abdominal wall muscle ultrasound: techniques and views. TOFR indicates train-of-four ratio.
Our primary outcome was the change in TFIO from baseline (ΔTFIO) to TOFR ≥0.9. Secondary outcomes included: (1) ΔTFIO to 30 minutes in the PACU; (2) change in DIA from baseline (ΔDIA) to TOFR ≥0.9; (3) ΔDIA to 30 minutes in the PACU; (4) the incidence of TFIO returning to baseline value at both TOFR ≥0.9 and after 30 minutes in the PACU; (5) the incidence of DIA returning to baseline at both TOFR ≥0.9 and after 30 minutes in the PACU; (6) time from TOFR of 0.9 to 0.95; and (7) time from TOFR of 0.9 to 1. We also recorded adverse events including nausea and vomiting, muscle weakness, airway obstruction requiring intervention, hypoxia and reintubation during PACU stay, and return to the emergency department or hospital readmission for respiratory complications within 7 days of surgery.
Statistical Analysis
All analyses were conducted based on intent-to-treat. The normality of data distribution was determined using the Shapiro-Wilk test. Continuous data were reported as mean ± standard deviation (SD) or median (interquartile range [IQR]). Categorical variables were reported as numbers (percentages).
Absolute standardized differences (ASDs) were used to measure the balance between the 2 groups. Variables were considered as imbalanced if the ASD was >0.2.26 We compared sugammadex versus neostigmine on ΔTFIO to TOFR ≥0.9 (the primary outcome) and ΔDIA to TOFR ≥0.9 with 2-sample t tests. Differences in means with 95% confidence intervals (CIs) were reported. ΔTFIO and ΔDIA to 30 minutes in the PACU, as well as time from TOFR of 0.9 to 0.95 and to 1 in sugammadex and neostigmine groups were compared using Mann-Whitney U tests. CIs for median differences were calculated using Hodges–Lehmann estimates. The incidence of postoperative TFIO and DIA returning to baseline values was compared using the Pearson χ2 test or the Fisher exact test, as appropriate. Differences in percentage with the outcome and 95% CI were reported. Considering that some variables might be imbalanced between 2 randomized groups, we also did a multivariable adjustment for the primary finding by adding the imbalanced variables (ASD >0.2) into a linear regression model.
All statistical analyses were performed using R software (version 3.5.3, R Foundation for Statistical Computing) and GraphPad Prism (version 9.0.0 for Mac OS, GraphPad Software). The Bonferroni corrections were applied to control the type I error at 0.05 when assessing the treatment effect on 9 secondary outcomes. As such, the Bonferroni-corrected CIs for the secondary outcomes were 99.4% (ie, [1 – 0.05/9] × 100%), and the corrected P values were equal to the raw P values multiplied by 9. Throughout, a 2-sided P value of <.05 was considered statistically significant.
As no previous studies have assessed the effect of reversal drugs on ΔTFIO from baseline to TOFR ≥0.9 (the primary outcome), the sample size was calculated based on our preliminary data (unpublished), which showed that the mean (SD) ΔTFIO from baseline to TOFR ≥0.9 was 24% (15%) in patients receiving neostigmine at our institute. Assuming that a 50% reduction (24% to 12%) in ΔTFIO from baseline to TOFR ≥0.9 in the sugammadex group was clinically relevant, 26 patients were required per group, given an SD of 15%, an alpha error of .05, and a power of 80%. We planned to enroll a total of 60 patients when considering a dropout rate of 15%.
RESULTS
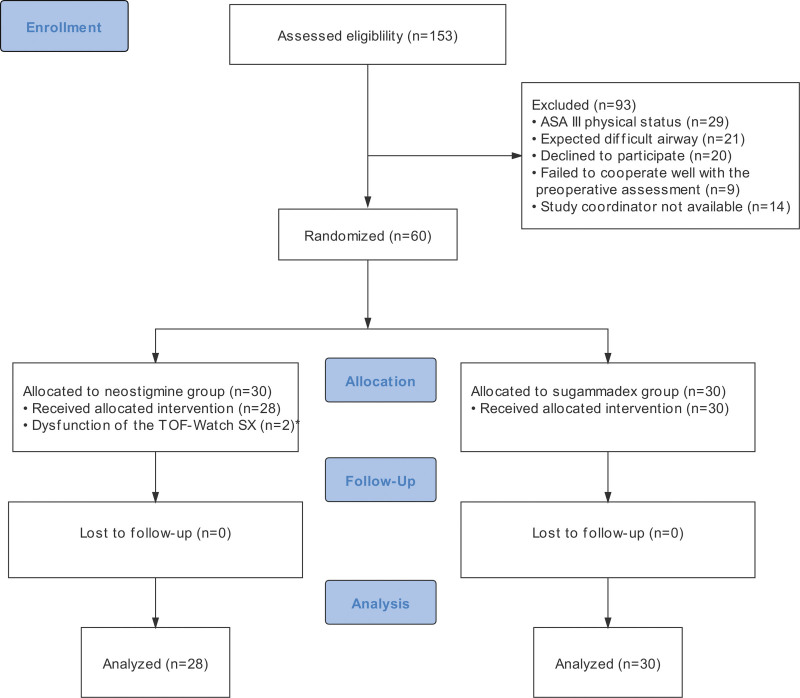
A total of 153 patients undergoing microlaryngeal surgery were screened from September 2020 to February 2021, of whom 60 patients were randomly allocated (1:1) to the neostigmine or sugammadex group (Figure 2). Two patients in the neostigmine group were excluded because of the dysfunction of the TOF-Watch device, leaving 28 patients in the neostigmine group and 30 patients in the sugammadex group in the final analysis. Demographics and baseline characteristics are summarized in Table 1. Except for the ASA physical status and intraoperative remifentanil consumption, characteristics were balanced (ASD <0.2) between the 2 groups.
Figure 2.
CONSORT diagram showing the flow of participants through each stage of the randomized trial. *We failed to capture the neuromuscular monitoring data due to dysfunction of the TOF-Watch device at the beginning of anesthesia in 2 patients allocated to the neostigmine group. ASA indicates American Society of Anesthesiology; CONSORT, Consolidated Standards of Reporting Trials; TOFR, train-of-four ratio.
Table 1.
Baseline Characteristics in Patients Receiving Neostigmine or Sugammadex
| Factors | Sugammadex (n = 30) | Neostigmine (n = 28) | ASDa |
|---|---|---|---|
| Age, y | 44 ± 11 | 44 ± 12 | 0.006 |
| BMI, kg.m−1 | 23.3 ± 3.5 | 22.8 ± 2.3 | 0.170 |
| Sex, female, n (%) | 10 (33) | 7 (25) | 0.184 |
| ASA physical status, n (%) | 0.265 | ||
| I | 9 (30) | 12 (43) | |
| II | 21 (70) | 16 (57) | |
| Intraoperative propofol, mg.kg−1.h−1 | 6.6 (5.0–7.7) | 6.4 (5.1–7.5) | 0.198 |
| Intraoperative remifentanil, μg.kg−1.min−1 | 0.43 ± 0.12 | 0.39 ± 0.08 | 0.421 |
| Intraoperative rocuronium, mg.kg−1 | 0.9 (0.7–1.2) | 0.9 (0.7–1.1) | 0.021 |
| Surgery duration, min | 34 (22–48) | 27 (19–39) | 0.153 |
| Propofol CeTEST, μg.mL−1 | 0.7 (0.6–0.9) | 0.7 (0.7–0.8) | 0.144 |
Variables are present as n (%), means ± SDs, or medians (IQR), as appropriate. Variables with an ASD >0.2 were considered imbalanced.
Abbreviations: ASA, American Society of Anesthesiology; ASD, absolute standardized difference; BMI, body mass index; CeTEST, effect-site concentration at the beginning of postoperative muscle strength testing; IQR, interquartile range; SD, standard deviation.
Variables were considered imbalanced if the ASD was >0.2.
Primary Analysis
As shown in Table 2, sugammadex versus neostigmine resulted in smaller changes in TFIO from baseline to TOFR ≥0.9 (mean ± SD, 9% ± 6% vs 16% ± 9%), with a difference in means of −6% (95% CI, −10 to −2; adjusted, P = .005). The difference remained significant (P = .005) after multivariable adjustment for imbalanced characteristics (ie, ASA physical status and intraoperative remifentanil consumption).
Table 2.
Comparison of Sugammadex and Neostigmine on Primary and Secondary Outcomes
| Outcomes | Sugammadex (n = 30) | Neostigmine (n = 28) | Multiple Testing Correcteda | |
|---|---|---|---|---|
| Difference (Corrected CI)b | P value | |||
| Primary outcome | ||||
| ΔTFIO to TOFR ≥0.9, % | 9 ± 6 | 16 ± 9 | −6 (−10 to −2)c,d | .005d |
| Secondary outcomes | ||||
| Changes in respiratory muscle strength from baseline to postoperatively | ||||
| ΔTFIOto PACU 30 min, % | 4 (–2 to 11) | 5 (4–10) | −2 (−9 to 5)e | >.999 |
| ΔDIA to TOFR ≥0.9, cm | 0.45 ± 0.74 | 1.28 ± 0.73 | −0.83 (−1.39 to −0.28)c | <.001 |
| ΔDIA to PACU 30 min, cm | 0.16 (−0.10 to 0.34) | 0.33 (0.01–0.58) | −0.17 (−0.60 to 0.06)e | .234 |
| Incidence of respiratory muscle strength returning to baseline level | ||||
| TFIO at TOFR ≥0.9 | 2 (7%) | 0 (0) | 7% (−9 to 23)f | >.999 |
| TFIO at PACU 30 min | 10 (33%) | 4 (14%) | 19% (−14 to 52)f | >.999 |
| DIA at TOFR ≥0.9 | 9 (30%) | 0 (0) | 30% (4–56)f | .018 |
| DIA at PACU 30 min | 11 (37%) | 8 (29%) | 8% (−29 to 45)f | >.999 |
| Neuromuscular monitoring data | ||||
| TOFR of 0.9–0.95, min | 0.3 (0.0–0.5) | 2.0 (0.3–2.5) | −1.8 (−2.3 to −0.3)e | .003 |
| TOFR of 0.9–1, min | 0.5 (0.2–0.8) | 5.3 (2.8–7.7) | −4.8 (−6.5 to −2.8)e | <.001 |
Variables are present as n (%), means ± SDs, or medians (IQR), as appropriate.
Abbreviations: ΔDIA, changes in diaphragm excursion from baseline to TOFR ≥0.9 or 30 min after PACU arrival; ΔTFIO, changes in thickening fraction of the internal oblique muscle from baseline to TOFR ≥0.9 or 30 min after PACU arrival; CI, confidence interval; DIA, diaphragm excursion; IQR, interquartile range; PACU, postanesthesia care unit; SD, standard deviation; TFIO, thickening fraction of the internal oblique muscle; TOFR, train-of-four ratio.
The CIs and P values for secondary outcomes were corrected for multiple testing using the Bonferroni method. The 9 hypotheses for all secondary outcomes were regarded as a family for correction. The corrected CI indicates the 99.4% CI (ie, [1 – 0.05/9] × 100%), and the corrected P value is equal to the raw P value multiplied by 9.
Difference indicates the difference in means, the median difference, or the difference in percentage.
Difference in means; tested with 2-sample t tests.
Adjusting for imbalanced variables (ie, ASA physical status and intraoperative remifentanil dose) using linear regression.
Median difference using Hodges–Lehmann estimator; tested with Mann-Whitney U test.
Difference in percentage; tested with the Pearson χ2 test or the Fisher exact test, as appropriate. We also calculated the available relative risk and its 95% CI using the Koopman asymptotic score for the sugammadex compared with the neostigmine group: TFIO after 30 min in the PACU: 2.33 (0.89–6.50); DIA after 30 min in the PACU: 1.28 (0.62–2.73).
Secondary Analyses
ΔDIA to TOFR ≥0.9 was smaller in the sugammadex group than in the neostigmine group (mean ± SD; 0.45 ± 0.74 cm vs 1.28 ± 0.73 cm), with a difference in means of −0.83 cm (99.4% CI, −1.39 to −0.28 cm; Bonferroni-corrected P < .001; Table 2). After 30 minutes in the PACU, neither a difference of the change in TFIO nor DIA was observed between the sugammadex and neostigmine groups after the Bonferroni correction (Table 2). No difference was found in TOFR at the initiation of strength testing for the sugammadex group and the neostigmine group (median [IQR], 1.06 [1.01–1.09] vs 1.05 [1.03–1.13]).
At TOFR ≥0.9, TFIO and DIA returning to the baseline levels were only observed in patients receiving sugammadex. Nine (30%) patients in the sugammadex group returned to baseline DIA level versus none of the patients in the neostigmine group (Table 2). Despite TOFR ≥0.9, only 2 (7%) patients in the sugammadex group recovered to baseline TFIO level. After 30 minutes in the PACU,33% of patients reversed with sugammadex versus 14% of those receiving neostigmine reached baseline TFIO levels (99.4% CI, −14 to 52; Bonferroni-corrected P > .999). Even at this time point, when all patients had the return of TOFR to 1, the incidences of TFIO and DIA returning to baseline levels were rather low in both groups (<40%), although there was no statistically significant difference between the sugammadex and the neostigmine groups. The baseline values of TFIO and DIA and their postoperative recovery (percentage of baseline values) are reported in Supplemental Digital Content 1, Table 1, http://links.lww.com/AA/E45.
The median time from TOFR = 0.9 to TOFR = 0.95 was 0.3 minutes with sugammadex versus 2 minutes with neostigmine, with an estimated median difference of −1.8 minutes (99.4% CI, −2.3 to −0.3 minutes; Bonferroni-corrected P = .003; Table 2). Regarding the recovery from TOFR of 0.9 to 1, sugammadex was much faster than neostigmine (0.5 [0.2–0.8] minutes vs 5.3 [2.8–7.7] minutes), with a median difference of −4.8 minutes (99.4% CI, −6.5 to −2.8 minutes; Bonferroni-corrected P < .001).
Adverse Events
Adverse events during PACU stay were only observed in the neostigmine group, including 1 patient complaining of unpleasant symptoms of muscle weakness and 2 experiencing stomach aches. Nausea and vomiting did not occur in either group. None of the enrolled patients developed respiratory complications during PACU stay or within 7 days after surgery, irrespective of treatment allocation.
DISCUSSION
In the present study, patients reversed with sugammadex showed an enhanced recovery of both the expiratory and the inspiratory muscle strength immediately after extubation (at TOFR ≥0.9) compared with patients reversed with neostigmine. A similar trend was observed in inspiratory muscles, as reported by Cappellini et al.13 A TOFR ≥0.9 is currently accepted as an indicator of sufficient recovery of neuromuscular recovery,27 although roughly 75% of postsynaptic acetylcholine receptors were being occupied by NMBAs.28,29 Therefore, if the same amount of receptors are occupied in both groups, what could be the reason for their differences? Our data revealed that the median recovery time from TOFR = 0.9 to TOFR = 1 was 10× faster when reversed with sugammadex than with neostigmine (0.5 vs 5.3 minutes). These data indicated that although we started strength measurement at TOFR = 0.9, what we actually measured was not “real 0.9” but TOFR >1 in the sugammadex group, as it usually took at least 3 minutes to complete all measurements. During this process, sugammadex might have freed more acetylcholine receptors than neostigmine, leading to different degrees of neuromuscular block recovery, which is in line with Schepens’ study showing that the electromyographic activity of the diaphragm was higher during recovery from NMB after reversal with sugammadex compared with neostigmine.30 This improved respiratory muscle function may contribute to the increased ability to clear secretions and reexpand the collapsed alveolar, potentially leading to a lower incidence of early adverse respiratory outcomes.31
We failed to show differences in respiratory muscle strength recovery between groups after 30 minutes in the PACU. This result is consistent with the study by Abola et al12; their investigation showed that postoperative strength measured by incentive spirometry 30 minutes after reversal with sugammadex or neostigmine was similar. The sensitivity of the current methods used to detect respiratory muscle strength may have been inadequate to discriminate a difference in the neuromuscular recovery between groups. Another reason may be that more acetylcholine receptors are freed over time, leading to more complete neuromuscular recovery and diminishing the differences between groups. A study with a larger sample size may provide the power needed to detect a difference in muscle strength recovery at this time point. To uncover this difference at such low levels of residual NMB, fade after high-frequency tetanic (200 Hz) stimulation might be useful32 but painful and therefore unethical to use in patients.
Even 30 minutes after arrival in the PACU and all patients reached TOFR of 1, strength of respiratory muscles was not fully restored in most patients even though respiratory muscles are considered to be more resistant to muscle relaxants than peripheral muscles.33 This persistent respiratory muscle dysfunction may be related in part to impaired neuromuscular transmission. After all, roughly 70% of acetylcholine receptors remain occupied by the NMBAs even at TOFR of 1.29 However, depressed peripheral chemoreceptor activity at TOFR = 1 may compromise respiratory muscle function by inhibiting respiratory arousal.34,35
Previous works considered that the clinical importance of the reduction in the measured respiratory variables at TOFR of 0.6 or higher was negligible.33 However, NMBA administration has been well known to be associated with an increased risk of PPCs,7 and tracheal extubation in patients with a TOFR >0.95, rather than >0.9, reduced the adjusted risk of PPCs, implying that more complete neuromuscular recovery is required for PPC prevention.20 Thus, the impaired respiratory muscle strength at TOFR = 0.9 may have significant clinical impact. Eikermann et al21 demonstrated that even when TOFR was fully recovered to 1, acceptable recovery of “forced inspiratory volume in 1 second,” which was most useful for lung reexpansion, was only seen in 73% of measurements in conscious volunteers. In our study, we reported that expiratory muscle strength during a cough was impaired even after TOFR of 1. This partial impairment of muscular activity after TOFR >0.9 might lead to development of PPCs through weakened contraction of ventilatory muscles with formation of atelectasis and inability to cough. The results of our study may partially explain the mechanism by which sugammadex may contribute to a reduction in PPCs10 and lend support to the Postanaesthesia Pulmonary Complications After Use of Muscle Relaxants (POPULAR) study, strengthening the evidence that NMBAs affect pulmonary outcomes despite TOFR >0.9.7
Our study has some limitations. First, both measures (TFIO during cough and DIA during maximum sniff breathing) were volitional tests and partially dependent on patient cooperation. Submaximal efforts may result in reduced values during these effort-dependent tests. This may hinder our ability to detect a difference between groups. Nevertheless, previous work has demonstrated that the reproducibility of TFIO measurements is likely sufficient to discriminate between patients with varying levels of abdominal muscle function and associated different clinical outcomes.17 Considering that pain and respiratory muscle injury may significantly affect the evaluation of respiratory muscle performance, we chose microlaryngeal surgery. We did this because postoperative pain is negligible and possibly eliminates the lingering effects of long-acting opioids on strength measurements. In addition, the effect site of propofol concentration during testing was not different (approximately 0.7 μg·mL−1 in both groups) and was below the threshold of what diminishes muscle strength (1.2 μg·mL−1),36 potentially ruling out the effect of sedatives on strength measurements. Second, the nonnormalized TOFR may overestimate neuromuscular recovery.37 However, this reflects clinical daily practice, as clinicians tend to apply the automatically calculated TOFR provided by monitoring devices to determine the presence of residual NMB.
In conclusion, sugammadex enhances expiratory muscle strength recovery more completely than neostigmine immediately after extubation. Further evidence of the relationship between the treatment allocation and expiratory muscle strength recovery beyond 30 minutes after extubation is needed. Our data suggested that respiratory muscle strength might still be impaired despite TOFR reaching 1.
DISCLOSURES
Name: Chanyan Huang, MD.
Contribution: This author helped with data acquisition for the study and with obtaining study funding, wrote and revised the manuscript, and approved the final version to be published.
Name: Xuan Wang, MD.
Contribution: This author helped with study design and data acquisition, wrote the manuscript, and approved the final version to be published.
Name: Shaowei Gao, MD.
Contribution: This author helped with data analysis and manuscript preparation, and approved the final version to be published.
Name: Wei Luo, MD.
Contribution: This author helped with data collection and approved the final version of the manuscript to be published.
Name: Xu Zhao, MD.
Contribution: This author helped with statistical analysis, and approved the final version of the manuscript to be published.
Name: Qian Zhou, MD.
Contribution: This author helped with statistical analysis, and approved the final version of the manuscript to be published.
Name: Wenqi Huang, PhD.
Contribution: This author helped with study design, and approved the final version of the manuscript to be published.
Name: Ying Xiao, PhD.
Contribution: This author helped with study design, data collection, and data interpretation; obtained study funding; revised the manuscript; and approved the final version to be published.
This manuscript was handled by: Ken B. Johnson, MD.
Supplementary Material
GLOSSARY
- ASA
- American Society of Anesthesiology
- ASD
- absolute standardized difference
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DIA
- diaphragm excursion
- IQR
- interquartile range
- NMB
- neuromuscular blockade
- NMBAs
- neuromuscular blocking agents
- PACU
- postanesthesia care unit
- POPULAR
- Postanaesthesia Pulmonary Complications After Use of Muscle Relaxants
- PPCs
- postoperative pulmonary complications
- SD
- standard deviation
- TFIO
- thickening fraction of internal oblique abdominal muscle
- TOF
- train-of-four
- TOFR
- train-of-four ratio
Funding: This study was supported by Wu Jieping Medical Foundation (No. 320.6750.2020-21-11).
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
Clinical trial information: ChiCTR identifier ChiCTR2000033832.
C. Huang and X. Wang contributed equally and share first authorship.
REFERENCES
- 1.Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesth Analg. 2008;107:130–137. [DOI] [PubMed] [Google Scholar]
- 2.Cammu G. Residual neuromuscular blockade and postoperative pulmonary complications: what does the recent evidence demonstrate? Curr Anesthesiol Rep. 2020;10:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brueckmann B, Sasaki N, Grobara P, et al. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: a randomized, controlled study. Br J Anaesth. 2015;115:743–751. [DOI] [PubMed] [Google Scholar]
- 4.Togioka BM, Yanez D, Aziz MF, Higgins JR, Tekkali P, Treggiari MM. Randomised controlled trial of sugammadex or neostigmine for reversal of neuromuscular block on the incidence of pulmonary complications in older adults undergoing prolonged surgery. Br J Anaesth. 2020;124:553–561. [DOI] [PubMed] [Google Scholar]
- 5.Murphy GS, Avram MJ, Greenberg SB, et al. Neuromuscular and clinical recovery in thoracic surgical patients reversed with neostigmine or sugammadex. Anesth Analg. 2021;133:435–444. [DOI] [PubMed] [Google Scholar]
- 6.Kheterpal S, Vaughn MT, Dubovoy TZ, et al. Sugammadex versus neostigmine for reversal of neuromuscular blockade and postoperative pulmonary complications (STRONGER): a multicenter matched cohort analysis. Anesthesiology. 2020;132:1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirmeier E, Eriksson LI, Lewald H, et al. ; POPULAR Contributors. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med. 2019;7:129–140. [DOI] [PubMed] [Google Scholar]
- 8.Li G, Freundlich RE, Gupta RK, et al. Postoperative pulmonary complications’ association with sugammadex versus neostigmine: a retrospective registry analysis. Anesthesiology. 2021;134:862–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause M, McWilliams SK, Bullard KJ, et al. Neostigmine versus sugammadex for reversal of neuromuscular blockade and effects on reintubation for respiratory failure or newly initiated noninvasive ventilation: an interrupted time series design. Anesth Analg. 2020;131:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledowski T, Szabó-Maák Z, Loh PS, et al. Reversal of residual neuromuscular block with neostigmine or sugammadex and postoperative pulmonary complications: a prospective, randomised, double-blind trial in high-risk older patients. Br J Anaesth. 2021;127:316–323. [DOI] [PubMed] [Google Scholar]
- 11.Alday E, Muñoz M, Planas A, Mata E, Alvarez C. Effects of neuromuscular block reversal with sugammadex versus neostigmine on postoperative respiratory outcomes after major abdominal surgery: a randomized-controlled trial. Can J Anaesth. 2019;66:1328–1337. [DOI] [PubMed] [Google Scholar]
- 12.Abola RE, Romeiser J, Rizwan S, Lung B, Gupta R, Bennett-Guerrero E. A randomized-controlled trial of sugammadex versus neostigmine: impact on early postoperative strength. Can J Anaesth. 2020;67:959–969. [DOI] [PubMed] [Google Scholar]
- 13.Cappellini I, Ostento D, Loriga B, Tofani L, De Gaudio AR, Adembri C. Comparison of neostigmine vs. sugammadex for recovery of muscle function after neuromuscular block by means of diaphragm ultrasonography in microlaryngeal surgery: a randomised controlled trial. Eur J Anaesthesiol. 2020;37:44–51. [DOI] [PubMed] [Google Scholar]
- 14.Pennati F, LoMauro A, D’Angelo MG, Aliverti A. Non-invasive respiratory assessment in Duchenne muscular dystrophy: from clinical research to outcome measures. Life (Basel). 2021;11:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoser B, Fong E, Geberhiwot T, et al. Maximum inspiratory pressure as a clinically meaningful trial endpoint for neuromuscular diseases: a comprehensive review of the literature. Orphanet J Rare Dis. 2017;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi ZH, Jonkman A, de Vries H, et al. Expiratory muscle dysfunction in critically ill patients: towards improved understanding. Intensive Care Med. 2019;45:1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiber AF, Bertoni M, Coiffard B, et al. Abdominal muscle use during spontaneous breathing and cough in patients who are mechanically ventilated: a bi-center ultrasound study. Chest. 2021;160:1316–1325. [DOI] [PubMed] [Google Scholar]
- 18.Farag E, Rivas E, Bravo M, et al. Sugammadex versus neostigmine for reversal of rocuronium neuromuscular block in patients having catheter-based neurointerventional procedures: a randomized trial. Anesth Analg. 2021;132:1666–1676. [DOI] [PubMed] [Google Scholar]
- 19.Gaszynski T, Szewczyk T, Gaszynski W. Randomized comparison of sugammadex and neostigmine for reversal of rocuronium-induced muscle relaxation in morbidly obese undergoing general anaesthesia. Br J Anaesth. 2012;108:236–239. [DOI] [PubMed] [Google Scholar]
- 20.Blobner M, Hunter JM, Meistelman C, et al. Use of a train-of-four ratio of 0.95 versus 0.9 for tracheal extubation: an exploratory analysis of POPULAR data. Br J Anaesth. 2020;124:63–72. [DOI] [PubMed] [Google Scholar]
- 21.Eikermann M, Groeben H, Hüsing J, Peters J. Accelerometry of adductor pollicis muscle predicts recovery of respiratory function from neuromuscular blockade. Anesthesiology. 2003;98:1333–1337. [DOI] [PubMed] [Google Scholar]
- 22.Koyuncu O, Turhanoglu S, Ozbakis Akkurt C, et al. Comparison of sugammadex and conventional reversal on postoperative nausea and vomiting: a randomized, blinded trial. J Clin Anesth. 2015;27:51–56. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs-Buder T, Claudius C, Skovgaard LT, Eriksson LI, Mirakhur RK, Viby-Mogensen J; 8th International Neuromuscular Meeting. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand. 2007;51:789–808. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber AF, Sabatini U, Vorona S, Bertoni M, Piva S, Goligher E. Measuring abdominal muscle function by abdominal muscle thickening on ultrasound: reproducibility, validity and normal range values. Eur Respir J. 2019;54:OA5367. [Google Scholar]
- 25.Matamis D, Soilemezi E, Tsagourias M, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 2013;39:801–810. [DOI] [PubMed] [Google Scholar]
- 26.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. SAS Global Forum. 2012;335:1–6. [Google Scholar]
- 27.Naguib M, Brull SJ, Kopman AF, et al. Consensus statement on perioperative use of neuromuscular monitoring. Anesth Analg. 2018;127:71–80. [DOI] [PubMed] [Google Scholar]
- 28.Paton WD, Waud DR. The margin of safety of neuromuscular transmission. J Physiol. 1967;191:59–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waud BE, Waud DR. The relation between the response to “train-of-four” stimulation and receptor occlusion during competitive neuromuscular block. Anesthesiology. 1972;37:413–416. [DOI] [PubMed] [Google Scholar]
- 30.Schepens T, Cammu G, Saldien V, et al. Electromyographic activity of the diaphragm during neostigmine or sugammadex-enhanced recovery after neuromuscular blockade with rocuronium: a randomised controlled study in healthy volunteers. Eur J Anaesthesiol. 2015;32:49–57. [DOI] [PubMed] [Google Scholar]
- 31.Moon TS, Reznik S, Pak T, et al. Sugammadex versus neostigmine for reversal of rocuronium-induced neuromuscular blockade: a randomized, double-blinded study of thoracic surgical patients evaluating hypoxic episodes in the early postoperative period. J Clin Anesth. 2020;64:109804. [DOI] [PubMed] [Google Scholar]
- 32.Dubois PE, Mitchell J, Regnier M, Passeraub PA, Moreillon F, d’Hollander AA. The interest of 100 versus 200 Hz tetanic stimulations to quantify low levels of residual neuromuscular blockade with mechanomyography: a pilot study. J Clin Monit Comput. 2022;36:1131–1137. [DOI] [PubMed] [Google Scholar]
- 33.Ali HH, Wilson RS, Savarese JJ, Kitz RJ. The effect of tubocurarine on indirectly elicited train-of-four muscle response and respiratory measurements in humans. Br J Anaesth. 1975;47:570–574. [DOI] [PubMed] [Google Scholar]
- 34.Broens SJL, Boon M, Martini CH, et al. Reversal of partial neuromuscular block and the ventilatory response to hypoxia: a randomized controlled trial in healthy volunteers. Anesthesiology. 2019;131:467–476. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki N, Meyer MJ, Eikermann M. Postoperative respiratory muscle dysfunction: pathophysiology and preventive strategies. Anesthesiology. 2013;118:961–978. [DOI] [PubMed] [Google Scholar]
- 36.Tsai PF, Matsuura N, Kaneko Y, Ichinohe T. Propofol dose-dependently increases bite force during sedation. J Oral Maxillofac Surg. 2011;69:2746–2752. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki T, Fukano N, Kitajima O, Saeki S, Ogawa S. Normalization of acceleromyographic train-of-four ratio by baseline value for detecting residual neuromuscular block. Br J Anaesth. 2006;96:44–47. [DOI] [PubMed] [Google Scholar]