Abstract
Background
An important window of opportunity for early-life exposures has been proposed for the development of atopic eczema and asthma.
Objective
However, it is unknown whether hay fever with a peak incidence around late school age to adolescence is similarly determined very early in life.
Methods
In the Protection against Allergy-Study in Rural Environments (PASTURE) birth cohort potentially relevant exposures such as farm milk consumption and exposure to animal sheds were assessed at multiple time points from infancy to age 10.5 years and classified by repeated measure latent class analyses (n = 769). Fecal samples at ages 2 and 12 months were sequenced by 16S rRNA. Hay fever was defined by parent-reported symptoms and/or physician’s diagnosis of hay fever in the last 12 months using questionnaires at 10.5 years.
Results
Farm children had half the risk of hay fever at 10.5 years (adjusted odds ratio [aOR] 0.50; 95% CI 0.31–0.79) than that of nonfarm children. Whereas early life events such as gut microbiome richness at 12 months (aOR 0.66; 95% CI 0.46–0.96) and exposure to animal sheds in the first 3 years of life (aOR 0.26; 95% CI 0.06–1.15) were determinants of hay fever, the continuous consumption of farm milk from infancy up to school age was necessary to exert the protective effect (aOR 0.35; 95% CI 0.17–0.72).
Conclusions
While early life events determine the risk of subsequent hay fever, continuous exposure is necessary to achieve protection. These findings argue against the notion that only early life exposures set long-lasting trajectories.
Key words: Childhood, Farm milk, Farming, Gut microbiome, Hay fever, Animal sheds
Abbreviations used: aOR, Adjusted odds ratio; GMR, Geometric mean ratio; NNT, Number needed to treat; PASTURE, Protection Against Allergy Study in Rural Environments; RMLCA, Repeated measure latent class analyses; SCFAs, Short-chain fatty acids; SPT, Skin prick test
Visual Summary
© Jemastock/ stock.adobe.com, © Fernando/ stock.adobe.com, © さしみ (sashimi)/ stock.adobe.com
What is already known about this topic? The protective effects of early-life farm exposures and gut microbiome composition on atopic diseases and asthma propose an important window of opportunity.
What does this article add to our knowledge? Early-life farm exposures also determine the risk of hay fever. However, continuous farm milk consumption is necessary for optimal prevention, thereby arguing against the notion of an early-determined trajectory governing later outcomes.
How does this study impact current management guidelines? These results emphasize the preventive potential of continuously drinking unprocessed farm milk for hay fever protection, suggesting carrying out clinical trials to test microbiologically safe cow’s milk for protection from hay fever.
Introduction
Hay fever is the most common allergic disease worldwide with prevalence between 20% and 30%.1 The high prevalence has a vast impact on several factors such as quality of life and high health care costs.2,3 Numerous epidemiological studies have shown the protective effect of early-life farm exposures and gut microbiome composition on asthma, atopy, atopic sensitization, and hay fever4, 5, 6, 7, 8, 9, 10, 11; thus, proposing an important window of opportunity for early-life farm exposures and gut microbiome composition for the protection of atopic diseases and asthma. However, it is unknown whether hay fever with a peak incidence around late school age to adolescence is only determined very early in life or whether later exposure before the onset of disease matters most.
The protective farm effect has been attributed to 2 factors; consumption of unprocessed cow’s milk, subsequently termed farm milk, and exposure to animal sheds.12, 13, 14, 15, 16 Hence, the aim of these analyses is to study the temporal pattern of these protective exposures on hay fever development using the longitudinal data from the Protection Against Allergy Study in Rural Environments (PASTURE) study. Furthermore, the role of the gut microbiome was investigated.
Methods
Study design and population
The PASTURE study is a prospective birth cohort study started in 2002 and is conducted in children from rural areas of 5 European countries (Austria, Finland, France, Germany, and Switzerland).17 The study was designed to evaluate risk and preventive factors for atopic diseases. The study was approved by the local research ethics committees in each country, and written informed consents were obtained from the children’s parents. Pregnant women were invited to participate during their third trimester of pregnancy. The children from the participating women were recruited at birth. Children of mothers living on family-run livestock farms at birth of the children were assigned to the farm group. The nonfarm group included children of mothers from the same rural areas but not living on a farm.18 Information was obtained through questionnaires in interviews or self-administered questionnaires from mothers.
Definitions of outcome
Hay fever was defined by parent-reported symptoms (itchy, runny, or blocked nose without a cold accompanied by red itchy eyes) and/or a physician’s diagnosis of hay fever in the last 12 months using questionnaires at 10.5 years. Allergen-specific immunoglobulin E and skin prick test (SPT) were assessed at 10.5 years.19 Inhalant sensitization was defined as at least 1 immunoglobulin E specific to alder, birch, hazel, plantain, mugwort, alternaria, grass, rye, Dermatophagoides(D.) pteronyssinus, D. farina, cat, dog, or horse at levels of 0.7 I UmL–1 or greater or SPT (birch, grass, alternaria, D. pteronyssinus, D. farinae, cat, or dog) of 3 mm or greater. A more stringent definition of hay fever consisting of hay fever plus inhalant sensitization at 10.5 years was used in sensitivity analyses.
Assessment of exposures
The child’s consumption of any farm milk, pasteurized, and homogenized milk subsequently termed processed milk consumption, and any exposure to animal sheds (cows, pigs, sheep, or horses) at time points 12 and 18 months and 2, 3, 4, 5, 6, and 10.5 years were assessed. In addition, maternal any farm milk consumption and animal sheds exposure was assessed during pregnancy and infant’s consumption of any farm milk, processed milk, and exposure to animal sheds (4–12 months) were obtained on a weekly basis by diary. The exposure to animal sheds was further dichotomized based on the third quartile (17 weeks) weeks spent on animal sheds as a cut-off.
Avoidance of milk or milk products was assessed at 12 and 18 months and 2, 3, 4, 5, and 6 years. In addition, information on the frequency of farm milk consumption was assessed at 18 months and 2, 3, 4, 5, 6 and 10.5 years. The frequency of processed milk consumption was assessed at 10.5 years.
DNA extraction from fecal samples and sequencing analyses
Fecal samples were collected from children’s diapers during the home visit at 2 and 12 months. The DNA was extracted from homogenized samples and bioinformatics processing were performed as previously described in detail.10 Briefly, α-diversity (ie, richness and Shannon index) was calculated as the average of multiple times rarefied samples.10 Metabolite levels of short-chain fatty acids (SCFA) were measured in fecal samples obtained from 301 children at 12 months.20,21 Two variables, butyrate and propionate scores, were created by modeling SCFA levels on the relative abundance of all bacterial genera using random forest model in the R-package ranger.
Statistical analyses
We performed repeated measure latent class analyses (RMLCA) using data from pregnancy to 10.5 years (ie, 9 time points) were included separately for exposure to animal sheds and farm milk consumption (Figure 1). The children were allocated to specific exposure classes by their highest posterior probabilities. The analyses were done on children having data at least at 7 of the 9 assessed time points. The optimal number of exposure classes was then determined according to the Bayesian Information Criterion, and the labelling of the exposure classes was based on main features of each class.
Figure 1.
Types of exposure classes. Solution for repeated measure latent classes defined by different exposures, which are (A) exposure to animal sheds and (B) farm milk consumption in the PASTURE children. Numbers in parentheses indicate the total number of children in each class.
Further as sensitivity analyses, we repeated the farm milk RMLCA in the subgroup of children without a family history of parental asthma and/or atopy and excluding children avoiding milk or milk products at 1 to 6 years as it could introduce confounding by reverse causation (ie, a positive family history). A farm milk consumption score (see Methods; available in this article’s Online Repository at www.jaci-inpractice.org) reflecting the frequency of farm milk consumed was built and divided into quintiles. The quintiles were further categorized as low (q1), intermediate (q2–q4), and high (q5).
The associations between hay fever and potential exposures (farm milk exposure classes, animal sheds exposure classes, frequency of farm milk consumption [continuous and quintiles], frequency of processed milk consumption, SCFAs [butyrate score and propionate score] as well as gut microbiome’s richness, and Shannon index) were assessed by logistic regression. We tested the differences in relative abundance of the most common single bacterial genera at 2 and 12 months with hay fever by the Wilcoxon test.10 The associations between gut microbiome richness and farm milk consumption, processed milk consumption, and exposure to animal sheds during infancy was assessed by linear regression. The effect estimates are presented as adjusted odds ratios (aORs) for logistic regression and geometric mean ratios (GMRs; calculated by exponentiation of the regression coefficients and their 95% CIs) for linear regression along with their respective 95% CI and a P value of .05 was considered significant. The previously listed models were adjusted for centers and confounders (growing up on a farm and parental asthma and/or atopy) associated with hay fever and exposures in our study. No other confounders (ie, associated with both outcome and exposures) were found. In addition, we calculated the number needed to treat (NNT), which is the effectiveness of a treatment on an outcome using an R-script.22
Furthermore, we conducted mediation analyses to assess whether the associations between farm milk consumption and exposure to animal sheds in infancy (4–12 months) and the risk of hay fever is mediated by gut microbiome features adjusting for centers. The mediation analysis was conducted through path analysis using the maximum likelihood test to estimate the regression parameters in Mplus 8.5.23 The mediating effect is reported as the proportion of the estimated indirect effect to the total effect.
The statistical analyses were performed with SAS 9.4 software (SAS Institute, Cary, NC) and Mplus 8.5 software (Muthén & Muthén, Los Angeles, CA).
Results
Characteristics of the study population
At 10.5-year follow-up, 778 children participated in the PASTURE study and 769 have data on hay fever. Comparing the baseline characteristics between included (n = 769) and excluded children (n = 364) did not show any significant difference except for maternal age at pregnancy, maternal smoking, parental education, and premature birth (Table E1; available in this article’s Online Repository at www.jaci-inpractice.org). Data on farm milk consumption and exposure to animal sheds at least at 1 time point (from pregnancy, 12 and 18 months, and 2, 3, 4, 5, 6, and 10.5 years) were available for all these children. Of these, 769 children had information on hay fever at 10.5 years. The proportion of children growing up on a farm was 47.7%. Hay fever at 10.5 years was reported in 12.9% children. Of these, 28.9%, 36.7%, and 21.7% had asthma, eczema, and food allergy at 10.5 years, respectively (Table I). Further, 86.8% were sensitized to inhalant allergens at 10.5 years (Table I). Figure E1 (available in this article’s Online Repository at www.jaci-inpractice.org) shows the proportion of children who were consuming farm milk or were exposed to animal sheds at each time point. The consumption of farm milk by children increased from 1 to 3 years and gradually decreased after 4 years. Similarly, exposure to animal sheds also increased from 1 to 4 years and slightly decreased after 5 years.
Table I.
Description of the study population∗
| Characteristic | All (n = 769) |
Hay fever (n = 99 [12.9%]) |
No hay fever (n = 670 [87.1%]) |
P value |
|---|---|---|---|---|
| n (%)/Total | n (%)/Total | n (%)/Total | ||
| Farm child (yes)† | 367 (47.7)/768 | 31 (31.3)/99 | 336 (50.2)/670 | .0005 |
| Exposure to cats at 2 mo (yes)‡ | 199 (26.0)/767 | 19 (19.2)/99 | 180 (27.0)/668 | .11 |
| Exposure to dogs at 2 mo (yes)‡ | 147 (19.2)/766 | 17 (17.2)/99 | 130 (19.5)/667 | .68 |
| Maternal age at pregnancy (y), mean ± SD | 31.2 ± 4.5 (n = 769) | 31.4 ± 4.4 (n = 99) | 31.2 ± 4.5 (n = 670) | .52 |
| Maternal smoking (yes)§ | 96 (12.5)/766 | 16 (16.5)/97 | 80 (12.0)/669 | .25 |
| Second-hand smoking (yes)‖ | 33 (4.3)/764 | 3 (3.1)/98 | 30 (4.5)/666 | .79 |
| Parental education (yes)¶ | .13 | |||
| Low | 62 (8.1)/764 | 3 (3.1)/97 | 59 (8.9)/667 | |
| Medium | 280 (36.7/764) | 39 (40.2)/97 | 241 (36.1)/667 | |
| High | 422 (56.7)/764 | 55 (56.7)/97 | 367 (55.0)/667 | |
| Use of antibiotics during pregnancy (yes)# | 204 (27.0)/755 | 26 (26.5)/98 | 178 (27.1)/657 | .99 |
| Parental atopy (yes)∗∗ | 416 (54.4)/765 | 72 (73.5)/98 | 344 (51.6)/667 | <.0001 |
| Mode of delivery (normal) | 624 (81.9)/762 | 82 (83.7)/98 | 542 (81.6)/664 | .68 |
| Premature birth (yes)†† | 11 (1.4)/769 | 1 (1.0)/99 | 10 (1.5)/670 | .99 |
| Birth weight (kg), mean ± SD | 3.4 ± 0.44 (n = 605) | 3.4 ± 0.5 (n = 82) | 3.4 ± 0.4 (n = 523) | .81 |
| Breastfeeding 2 mo (yes)‡‡ | 711 (92.7)/767 | 90 (90.9)/99 | 621 (93.0)/668 | .41 |
| Gender (female) | 366 (47.7)/768 | 42 (42.4)/99 | 324 (48.4)/669 | .28 |
| Having siblings (yes) | 494 (64.2)/769 | 60 (60.6)/99 | 434 (64.8)/670 | .43 |
| Use of antibiotics during first y of life (wk), mean ± SD§§ | 0.03 ± 0.3 (n = 746) | 0.01 ± 0.1 (n = 97) | 0.03 ± 0.4 (n = 649) | .86 |
| Doctor‘s diagnosis of hay fever (yes) | 36 (4.7)/769 | 36 (36.4)/99 | NA | NA |
| Inhalant sensitization (IgE ≥ 0.7 kU/L or SPT ≥ 3 mm) at 10.5 y‖‖ | 259 (49.6)/522 | 66 (86.8)/76¶¶ | 193 (43.3)/446¶¶ | <.0001 |
| Concomitants## | ||||
| Asthma (yes) | 69 (9.0)/764 | 28 (28.9)/97 | 41 (6.2)/667 | <.0001 |
| Eczema (yes) | 100 (13.1)/763 | 36 (36.7)/98 | 64 (9.6)/665 | <.0001 |
| Food allergy (yes) | 41 (5.5)/746 | 21 (21.7)/97 | 20 (3.1) /649 | <.0001 |
IgE, Immunoglobulin E; NA, not applicable.
The categorical variables are presented as frequency (percentage) and the continuous variables as mean. The tests for differences between the groups are χ2 or Fischer exact test for categorical variables and Mann-Whitney U test for continuous variables.
Farm child was defined as “Children of mothers living on family-run livestock farms were assigned to the farm group. The nonfarm group included children of mothers from the same rural areas but not living on a farm.
Exposure to pets at 2 mo (cats and dogs) was defined by asking “If you have cats?,” “If you have dogs?,” and “If they stay indoors in the house?”
Maternal smoking during pregnancy was defined using the following questions, “Have you in your life smoked more than 5 packs of cigarettes?” Or “Have you quit smoking in the meantime?” and if yes, “Was it during this pregnancy?” Smoking by father, “Have you in your life smoked more than 5 packs of cigarettes?” Or “Do you still smoke?”
Second-hand smoking “How many cigarettes are on average per day were smoked in your house by other people?” If > 1 then second-hand smoking was defined as 1; else 0.
Parental education was defined as low (<10 y), medium (10 y) and high (>10 y).
Use of antibiotics during pregnancy was defined by asking, “Have you taken antibiotics since the beginning of pregnancy?” Or “Have you taken any antibiotics during this pregnancy?”
Parental atopy was defined as doctor’s diagnosis of hay fever, atopic dermatitis, or asthma ever in mother or father.
Child was defined as premature if the child was born before the completion of 37 wk of pregnancy.
Breastfeeding at 2 months (yes or no) was defined by asking, “If you have ever breastfed?”
Use of antibiotics by a child during the first year of life was defined as, “Total no. of weeks with antibiotics ingested.”
Inhalant sensitization was defined as at least 1 IgE specific to alder, birch, hazel, plantain, mugwort, alternaria, grass, rye, Dermatophagoides pteronyssinus, D, farina, cat, dog, or horse at levels ≥ 0.7I UmL–1 or SPT (birch, grass, alternaria, D. pteronyssinus, D. farinae, cat, or dog) ≥ 3mm. Serum-specific IgE and SPT was not measured in the Austrian study center; hence, only subsample n = 522 was included.
Asthma was defined as a physician’s diagnosis of asthma or recurrent obstructive bronchitis established until 10.5 y. Eczema and food allergy were defined as physician diagnoses at least once until 10.5 y.
Temporal pattern of the farm-related exposures on hay fever
Children growing up on a farm had half the risk of hay fever than nonfarm children (aOR 0.50; 95% CI 0.31–0.79; P value .003).
As a first step, we analyzed the temporal pattern of exposure to animal sheds (“continuous exposure to animal sheds,” “only early exposure to animal sheds,” “only late exposure to animal sheds,” and “no exposure to animal sheds”; Figure 1, A) on hay fever development. Of these categories, “only early exposure to animal sheds” showed an inverse association when compared with “no exposure to animal sheds” which however did not reach statistical significance (aOR 0.26; 95% CI 0.06–1.15; P value .08) (Table E2; available in this article’s Online Repository at www.jaci-inpractice.org). When adjusting this model for consumption of farm milk exposure classes, the results remained unchanged (Table E2).
We then analyzed the temporal pattern of consumption of farm milk in similar categories “continuous consumption of farm milk,” “only early consumption of farm milk,” “only late consumption of farm milk,” and “no consumption of farm milk” (Figure 1, B). The strongest inverse association was found for the “continuous consumption of farm milk” compared with “no consumption of farm milk” (aOR 0.35; 95% CI 0.17–0.72; P value .004) exposure class (Figure 2 and Table E3; available in this article’s Online Repository at www.jaci-inpractice.org). In contrast, “only early consumption of farm milk” showed no significant effect on hay fever. The inverse association of “continuous consumption of farm milk” compared with “no consumption of farm milk” was still observed when using the stringent definition of hay fever (aOR 0.41; 95% CI 0.17–0.97; P value .04) (Figure E2; available in this article’s Online Repository at www.jaci-inpractice.org) or incident hay fever at 10.5 years (aOR 0.39; 95% CI 0.15–0.99; P value .05; data not shown). Because confounding by reverse causation might have biased our findings, we ran a sensitivity analysis in the subgroup of children without a family history of parental asthma and/or atopy and excluded children avoiding milk or milk products at 1 to 6 years. This did not change the inverse association with hay fever (aOR 0.21; 95% CI 0.06–0.78; P value .02; data not shown).
Figure 2.
Associations of farm milk exposure classes with hay fever at 10.5 years. Models are adjusted for centers, growing up on a farm, and parental atopy. The forest plot represents the aOR with 95% CI.
We next assessed the association of the frequency of farm milk consumption (ie, whether frequently drinking farm milk has a dose-response effect on hay fever). The highest compared with the lowest quintile of farm milk consumption was inversely associated with hay fever (aOR 0.37; 95% CI 0.16–0.84; P value .02), whereas the intermediate group (q2–q4 aOR 0.63; 95% CI 0.37–1.10; P value .10) showed a similarly inverse but nonsignificant association. Similar results were obtained when using frequency of farm milk consumption score as a continuous variable (data not shown).
We further investigated whether consumption of processed milk shows similar effects as consumption of farm milk (Figure E3, A; available in this article’s Online Repository at www.jaci-inpractice.org). Consumption of “high farm and low processed milk” was inversely associated with hay fever (aOR 0.24; 95% CI 0.09–0.66; P value .006); however, the consumption of processed milk attenuated the farm milk effect when both farm milk and processed milk were consumed (“mixed consumption of farm and processed milk” [aOR 0.43; 95% CI 0.19–0.96; P value .04]) (Figure E3, B; available in this article’s Online Repository at www.jaci-inpractice.org and Table E3). Furthermore, daily consumption of shop milk at 10.5 years showed association in a positive direction with hay fever (Figure E4; available in this article’s Online Repository at www.jaci-inpractice.org).
In addition, NNT calculated in our study was 7.14 (ie, 7 children would have to drink farm milk continuously from pregnancy by mothers until 10.5 years in order to prevent hay fever in 1 child).
Effect of the early-life gut microbiome on hay fever
We investigated the role of the early-life gut microbiome by relating bacterial composition, richness, Shannon index (at 2 and 12 months) and SCFA to hay fever.
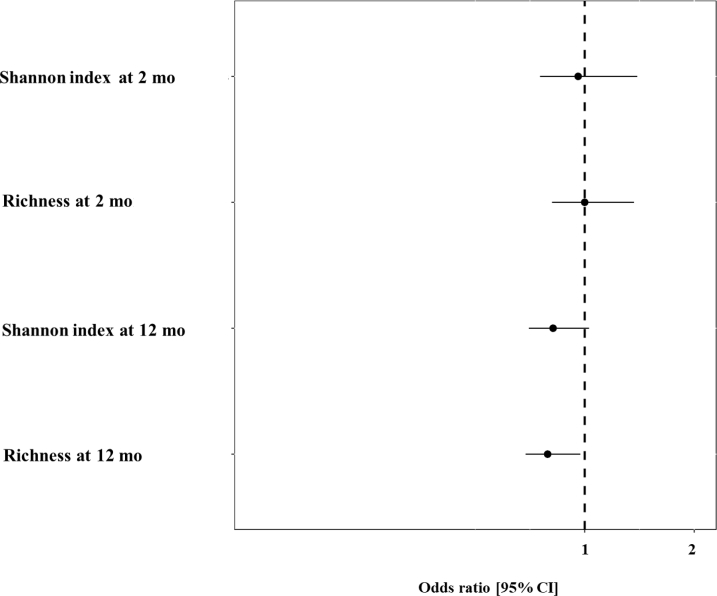
We did not find any significant differences in relative abundance of most common bacterial genera at 2 and 12 months with subsequent hay fever at 10.5 year (data not shown). Also, richness and Shannon index of bacteria at 2 months were not associated with hay fever at 10.5 years (Figure 3). However, the bacterial richness of the gut microbiome at 12 months was inversely associated with hay fever (aOR 0.66; 95% CI 0.46–0.96; P value .03; Figure 3). The Shannon index at 12 months also showed an inverse nonsignificant trend for hay fever (aOR 0.71; 95% CI 0.49–1.04; P value .08; Figure 3). The SCFAs butyrate (aOR 1.00; 95% CI 0.92–1.09; P value .99) and propionate scores (aOR 0.97; 95% CI 0.90–1.05; P value .50) were, in turn, not associated with hay fever (data not shown). We reasoned that consumption of milk and exposure to animal sheds may shape the gut microbiome, in particular its richness. Consumption of farm milk (adjusted GMR [aGMR] 1.20; 95% CI 1.03–1.40; P value .02) and exposure to animal sheds (aGMR 1.19; 95% CI 1.01–1.40; P value .04) in the first year of life increased gut microbiome richness (Figure 4). In turn, no association was observed for consumption of processed milk (Figure 4). Because both farm milk consumption and exposure to animal sheds during infancy (4–12 months) showed significant associations with gut microbiome richness at 12 months, we performed a mediation analysis including unexposed and children exposed to both in infancy. The mediation analysis revealed that part (18.4%) of the total protective effect of farm milk consumption and exposure to animal sheds in the first year of life on hay fever was mediated by gut microbiome richness (P value .03; Figure 5). The number of children only being exposed to animal sheds or farm milk, respectively, was too low to allow separate mediation analyses.
Figure 3.
Association of gut microbiome richness, and Shannon index at months 2 (hay fever/total: 59/439) and 12 (hay fever/total: 79/633) with hay fever at 10.5 years. Models are adjusted for centers, growing up on a farm, and parental atopy. The association with hay fever is shown as aOR per interquartile range of the probability along with 95% CI.
Figure 4.
Association of consumption of farm milk (n = 624), consumption of processed milk (n = 624), and exposure to animal sheds (n = 617) with richness at 12 months. Models are adjusted for centers, growing up on a farm, and parental atopy. The forest plot represents the aGMRs with 95% CI.
Figure 5.
Mediation analysis of the protective effect of consumption of farm milk and exposure to animal sheds in infancy on hay fever mediated by gut microbiome richness at 12 months adjusting for centers (n = 466). The figure shows the direct (β1), indirect (β2), and total (β) effects as well as their respective 95% CI from the path model. The proportion of the mediated (indirect) effect was 18.4%.
Discussion
In the PASTURE birth cohort, the continuous consumption of farm milk up to 10.5 years, but neither the only early nor the only late exposure alone, was significantly associated with reduced risk of hay fever at 10.5 years. In contrast, exposure to animal sheds only exerted a trend toward protection early in life. Both exposures, farm milk and animal sheds, early in life increased gut microbiome richness at 12 months, which partly explained the protective effect of these exposures on hay fever.
The human gut microbiome composition plays an important role in shaping the development of the immune system.24 There is some evidence that the gut microbiome diversity in the first years of life may protect from atopic sensitization. In the population-based CHILD cohort, the Shannon index at 3 months was associated with protection from atopic sensitization at 1 year.8 However, in a Swedish study, the Shannon index in early infancy was not associated with allergic rhinoconjunctivitis and SPT at 7 years.25 Our analyses likewise do not confirm this very early window of opportunity because gut microbiome richness and Shannon index at 2 months was unrelated to hay fever development.
In contrast, gut microbiome richness at 1 year was inversely associated with hay fever at 10.5 years. We have previously shown in the PASTURE cohort in agreement with others that the compositional structure of the gut microbiome undergoes very significant changes from early age, when most infants are breastfed, to 12 months, when most foods have been introduced into a child’s diet.10,11 Nevertheless, an inverse association of gut microbiome richness at 1 year with an outcome much later in life at 10.5 years may seem surprising. This long-term association may be attributable to an earlier onset of disease. In fact, 4.6%, 5.9%, and 6.7% of children with data on hay fever at 10.5 years had already reported symptoms and/or a diagnosis of hay fever at 4, 5, and 6 years, respectively. Furthermore, early alterations of the composition of the gut microbiome may shape its subsequent development toward an adult-like compositional structure in the first 3 years of life.26 Unfortunately, no fecal samples have been collected at later time points in the PASTURE cohort.
The production of the SCFAs butyrate and propionate measured at 12 months has been reported previously as determinants of protection against atopic sensitization at 6 years.20 In our study, no relation between the SCFAs butyrate and propionate with hay fever was found. Furthermore, no association with single taxa was seen. Thus, different facets of the early development of the gut microbiome composition may matter for different clinical outcomes.
Of the environmental exposures investigated in these analyses, the continuous, but neither the early nor the late, consumption of farm milk was seen to protect from hay fever development. Moreover, a dose-response effect was found corroborating the strength of the observation. Interestingly, this protective effect was partly mediated by gut microbiome richness, which may suggest that a continued exposure to unprocessed cow’s milk may increase gut microbiome richness beyond 12 months and thereby confer its protective effect.
Continuous exposure also implies repeated exposures. The novel concept of trained immunity may lend itself to mechanistic speculations because phenomena like lipopolysaccharides tolerance are based on the necessity of repeated rather than single exposures.27
A potential explanation for the differential effect of unprocessed versus processed cow’s milk is grounded in the observation that most farm children drink their milk unboiled. In fact, too few children received only boiled (ie, heat-treated farm milk) over the study period to allow meaningful stratified analyses. A number of population-based and experimental studies have stressed the potential importance of heat treatment of cow’s milk for the loss of protective effects.16,28, 29, 30, 31 Whether alterations of the milk microbiome or denaturation and loss of function of milk (whey) proteins underlie these findings awaits further elucidation.
Exposure to animal sheds during early years showed an inverse, albeit nonsignificant effect on hay fever. This is in contrast to previous farm studies showing stronger effects.12,32 The discrepancy might be attributable to important differences in the definition of exposure to animal sheds used in the PASTURE study, which only assessed exposure to any animal sheds without differentiating between cows, pigs, sheep, and horses. The nature of animal exposure may matter, however. Whereas exposure to cow sheds showed a significant protective effect on hay fever and asthma,12 sheep sheds and keeping of hares and rabbits were risk factors for wheezing and asthma, respectively, in the PARSIFAL farm study.33
The main strength of this study is its longitudinal design, which enabled us to assess the exposures at several time points before the assessment of the outcome. Excluding children with parental asthma and/or atopy and who were avoiding milk or milk products showed similar inverse associations with hay fever, consequently arguing against confounding by reverse causation. An elevated risk of diarrhea and farm milk consumption at 10.5 years was not observed (data not shown). The results of the present study show protective association of continuous consumption farm milk on hay fever. However, one of the potential caveats of the observation study is finding causality. Hence, the Milk Against Respiratory Tract Infections and Asthma (MARTHA), an ongoing interventional trial, is being carried out to evaluate the preventive effect of minimally treated (ie, only pasteurized and, thus, microbiologically safe) cow’s milk on upper respiratory tract infections and allergy.34 Further, the NNT in our study was 7; however, this study is not a randomized placebo-controlled double-blind trial, and thus, numbers must be taken with some caution. One of the drawbacks of the study is the missing data on hay fever at 10.5 years. However, comparing the baseline characteristics between included and excluded children did not show any significant difference except for maternal age at pregnancy, maternal smoking, parental education, and premature birth. However, adjusting for these variables did not change the results (data not shown). Another drawback is the small number in the “only early” and “only late” exposure groups that shows protective nonstatistical significant effect on hay fever. However, using the RMLCA approach, our study could identify these small groups manifesting that these types of habits (ie, farm milk consumption or exposure to animal sheds) do exist. We performed a post hoc power calculation using SAS and considering α of 0.05 (2-sided). For our sample size of 650 (ie, in the exposure groups “continuous consumption of farm milk” and “no consumption of farm milk”), the power of study is over 80% assuming the response probabilities ranging from 0.02 to 0.18 for having hay fever in children who consume farm milk and unadjusted odds ratio of 0.24. Thus, our study was well powered to detect a relatively strong effect of farm milk consumption on hay fever.
In summary, the results of the present study demonstrate that continuous exposure of the main determinant (ie, farm milk consumption) but neither only early nor only late exposure alone conferred protection from hay fever development. The early compositional structure of the gut microbiome at 1 year, but not 2 months, did, however, in part mediate this protective effect. One might speculate that continuous consumption of unprocessed cow’s milk may also increase gut microbiome richness at later ages, but we do not have data to support this notion. Overall, the findings presented herein do not support the notion of an early-determined trajectory in which only early exposures in the first months of life would govern later outcomes. These results emphasize the preventive potential of continuously drinking unprocessed farm milk for hay fever protection. However, the risks associated with raw cow’s milk consumption prohibit its recommendation for daily life. The results of the MARTHA trial, however, will shed light on potential side effects.34 Further clinical trials based on the present results are warranted.
Pasture Study Group Members
The members of the PASTURE study group are Johanna Theodorou (Dr. von Hauner Children‘s Hospital, Ludwig Maximilians University Munich, Munich, Germany; Member of the German Center for Lung Research, DZL, Germany), Andreas Böck (Dr. von Hauner Children’s Hospital, Ludwig Maximilians University Munich, Munich, Germany), Harald Renz (Institute of Laboratory Medicine, Philipps University of Marburg, Marburg, Germany; Department of Clinical Immunology and Allergology, Laboratory of Immunopathology, Sechenov University, Moscow, Russia), Petra I. Pfefferle (Comprehensive Biobank Marburg CBBM, Fachbereich Medizin der Philipps Universität Marburg, Marburg, Germany), Jon Genuneit (Pediatric Epidemiology, Medical Faculty, Leipzig University, Leipzig, Germany), Michael Kabesch (Department of Pediatric Pneumology and Allergy, University Children's Hospital Regensburg [KUNO] at the Hospital St. Hedwig of the Order of St. John, University of Regensburg, Regensburg, Germany), Marjut Roponen (Department of Environmental and Biological Sciences, University of Eastern Finland, Kuopio, Finland), and Lucie Laurent (University of Besanҫon, Department of Respiratory Disease, UMR/CNRS6249 Chrono-environment, University Hospital, Besanҫon, France).
Acknowledgments
We thank David A. Mills, PhD, Diana Taft, PhD , and Karen Kalanetra, PhD from the Department of Food Science & Technology, University of California, Davis, for their contribution in the generation of the original microbiome sequence and analysis.
Footnotes
The PASTURE study was supported by the European Commission (research grants QLK4-CT-2001-00250, FOOD-CT-2006-31708 and KBBE-2007-2-2-06), the European Research Council (grant 250268), Deutsche Forschungsgemeinschaft, German Center for Lung Research (DZL), Kühne Foundation, French National Programme for Hospital Research, EVO- and VTR-funding, the Academy of Finland (grant 139021, 287675, 338679, 339666), the Yrjö Jahnsson Foundation, and EU, Academy of Finland and smaller grants from Finnish foundations supporting medical research (Juho Vainio Foundation, Päivikki and Sakari Sohlberg Foundation, Finnish Cultural Foundation). The current analyses were supported by the Bavarian State Ministry for Health and Care.
Conflicts of interest: S. Pechlivanis reports support for attending the virtual European Respiratory Society (ERS) International Congress 2021. C. Roduit reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from ALK, University of Bern. C. Skevaki reports grants from Universities Giessen and Marburg Lung Center, German Center for Lung Research, University Hospital Giessen and Marburg research funding, Deutsche Forschungsgemeinschaft (DFG) (SFB1021 [C04], KFO 309 [P10], SK 317/1-1 [Project No. 428518790]), TransMIT, Mead Johnson Nutrition; consulting fees from Hycor Biomedical, Bencard Allergie, Thermo Fisher Scientific; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Hycor Speaker Bureau; support for attending meetings and/or travel from Hycor, Bencard Allergy; participation on a Data Safety Monitoring Board or Advisory Board: Hycor Biomedical, Bencard Allergy; leadership or fiduciary role from ESGREV, DZL ALLIANCE; and receipt of equipment, materials, drugs, medical writing, gifts, or other services from Bencard Allergie. C. Barnig reports support for attending meetings/travel from GlaxoSmithKline (GSK), Sanofi, Chiesi, Novartis, and AstraZeneca. M. J. Ege reports support for the present manuscript from German Center for Lung Research (DZL); grants or contracts from any entity: Dutch Longfonds; patents planned, issued, or pending: EP000001964570B1, US000009950017B2, EP000002361632B1; receipt of materials: milk powder from Friesland Campina. B. Schaub reports support for the present manuscript from DFG, and EU; grants/contracts from DFG, and EU. A. Divaret-Chauveau reports support for the present manuscript from French National Program for Hospital Research; grants/contracts from French public agency ANSES, Don du Souffle for the PASTURE 16-year Visit, Fondation du Souffle for the PASTURE 16-year visit, Novartis, ARAIRLOR (Association Régionale d’Aide aux Insuffisants Respiratoires de Lorraine); consultant fees from Stallergens, ALK, Aimmune Therapeutics; payment/honoria from Aimmune Therapeutics (speaker at symposium at the European Academy of Allergy and Clinical Immunology [EAACI] Congress in 2019 and at the French Congress of Allergy in 2019) and Novartis; attending meetings from Mead Johnson for Pediatric Allergy and Asthma Meeting 2019, Nutricia for EAACI Congress 2019, Aimmune Therapeutics for EAACI Congress 2019, Novartis for the French Congress of Allergy 2019; and stock from Essilor Luxottica. R. Lauener reports support for the present manuscript from Kühne Foundation, and EU European Commission (research grant QLK4-CT-2001-00250). A. M. Karvonen reports support for the present manuscript from EVO- and VTR-funding, the Academy of Finland (grants 139021, 287675, 338679, and 339666), the Juho Vainio Foundation, the Yrjö Jahnsson foundation, Päivikki and Sakari Sohlberg foundation, and the Finnish Cultural Foundation. J. Pekkanen reports support for the present manuscript from EU, Academy of Finland and smaller grants from Finnish foundations supporting medical research (Juho Vainio Foundation, Päivikki and Sakari Sohlberg Foundation, and the Finnish Cultural Foundation. E. von Mutius reports support for the present manuscript: from Funding PASTURE study, FORALLVENT study, EFRAIM study and Leibniz prize; grants from Bavarian State Ministry of Health and Care for “URS Study,” January 2021, German Federal Ministry of Education and Research (BMBF) Deutsches Zentrum für Lungenforschung [German Center for Lung Research], January 2021, Forschungsvorhaben: Rolle der mikrobiellen Umweltexposition für den Schutz vor Heuschnupfen und Asthma Research project: the role of the microbial environmental exposure for the protection against hay fever and asthma Bayerisches Staatsministerium für Gesundheit und Pflege Bavarian State Ministry of Health and Care, January 2020; royalties or licenses from Elsevier GmbH, Georg Thieme Verlag, Springer-Verlag GmbH, Elsevier Ltd., Springer Nature Group; consulting fees from Chinese University of Hong Kong, European Commission, HiPP GmbH & Co KG, AstraZeneca, Imperial College London, OM Pharma, ALK-Abello Arzneimittel GmbH; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Massachusetts Medical Society, Springer-Verlag GmbH, Elsevier Ltd., Böhringer Ingelheim International GmbH, ERS, Universiteit Utrecht, Faculteit Diergeneeskunde, Universität Salzburg, Springer Medizin Verlag GmbH, Japanese Society of Pediatric Allergy and Clinical Immunology (JSPACI), Klinikum Rechts der Isar, University of Colorado, Paul-Martini-Stiftung, Astra Zeneca, Imperial College London, Children’s Hospital Research Institute of Manitoba, Kompetenzzentrum für Ernährung (Kern), OM Pharma S.A., Swedish Pediatric Society for Allergy and Lung Medicine, Chinese College of Allergy and Asthma (CCAA), ALK-Abello Arzneimittel GmbH, Abbott Laboratories, Deutscher Apotheker Verlag GmbH & Co. KG; support for attending meetings and/or travel from Verein zur Förderung der Pneumologie am Krankenhaus Großhansdorf e.V., Pneumologie Developpement, Mondial Congress & Events GmbH & Co. KG, American Academy of Allergy, Asthma & Immunology (AAAAI), Imperial College London, Margaux Orange, Volkswagen Stiftung, Böhringer Ingelheim International GmbH, ERS, Universiteit Utrecht, Faculteit Diergeneeskunde, Österreichische Gesellschaft f. Allergologie u. Immunologie, Massachusetts Medical Society, OM Pharma S. A., Hanson Wade Ltd., iKOMM GmbH, DSI Dansk Borneastma Center, American Thoracic Society, HiPP GmbH & Co KG, Universiteit Utrecht, Faculteit Bètawetenschappen; patents planned, issued or pending: patent No. PCT/EP2019/085016 (Barn dust extract for the prevention and treatment of diseases) pending (Barn dust extract for the prevention and treatment of diseases) pending, royalties paid to ProtectImmun for patent EP2361632 (Specific environmental bacteria for the protection from and/or the treatment of allergic, chronic inflammatory and/or autoimmune disorders, granted on March 19, 2014), and patents EP1411977 (Composition containing bacterial antigens used for the prophylaxis and the treatment of allergic diseases, granted on April 18, 2007), EP1637147 (Stable dust extract for allergy protection, granted on December 10, 2008), and EP 1964570 (Pharmaceutical compound to protect against allergies and in flammatory diseases, granted on November 21, 2012) licensed to ProtectImmun; Patent EP21189353.2. 2021. von Mutius E, Rankl B, Bracher F, Müller C, Walker A, Hauck SM, Merl-Pham J, inventors; Proteins identified from bran dust extract for the prevention and treatment of diseases; Patent PCT/US2021/016918, 2021; Martinez FD, Vercelli D, Snyder SA, von Mutius E, Pivniouk V, Marques dos Santos M, inventors; Therapeutic fractions and proteins from asthma-protective farm dust; participation on a Data Safety Monitoring Board or Advisory Board: member of the EXPANSE (funded by European Commission) Scientific Advisory Board, Member of the BEAMS External Scientific Advisory Board (ESAB), member of the Editorial Board of The Journal of Allergy and Clinical Immunology: In Practice; member of the Scientific Advisory Board of the Children’s Respiratory and Environmental Workgroup (CREW); member of the International Scientific & Societal Advisory Board (ISSAB) of Utrecht Life Sciences (ULS), University of Utrecht; member of External Review Panel of the Faculty of Veterinary Science, University of Utrecht; member of the Selection Committee for the Gottfried Wilhelm Leibniz Programme (DFG); member of the International Advisory Board of Asthma UK Centre for Applied Research (AUKCAR); member of the International Advisory Board of The Lancet Respiratory Medicine, Member of the Scientific Advisory Board of the CHILD (Canadian Healthy Infant Longitudinal Development) study, McMaster University, Hamilton, Canada; Asthma UK Centre for Applied Research, Pediatric Scientific Advisory Board Iceland. J. G. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from the journal Pediatric Allergy and Immunology (co-owned by Wiley and EAACI); personal honorarium for serving as Associate Editor. M.K. reports grants or contracts from European Union, German Ministry of Education and Research, German Research Foundation, Infectopharm; consulting fees from Bionorica, Sanofi, Novartis, Bencard; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from ERS, EAACI, ATS, Novartis, Glaxo, Chiesi, Sanofi, Nutricia, Hipp, and Allergopharma; patents planned, issued, or pending Method for testing a subject thought to have or to be predisposed to asthma’ European patent application 5 EP07301135.5. The rest of the authors declare that they have no relevant conflicts of interest.
Contributor Information
Sonali Pechlivanis, Email: sonali.pechlivanis@helmholtz-muenchen.de.
the PASTURE Study Group:
Johanna Theodorou, Andreas Böck, Harald Renz, Petra I. Pfefferle, Jon Genuneit, Michael Kabesch, Marjut Roponen, and Lucie Laurent
Supplementary Data
References
- 1.World Allergy Organization (WAO) 2021. World Allergy Week 2016. Pollen Allergies—Adapting to a Changing Climate.https://www.worldallergy.org/UserFiles/file/waw16-slide-set.pdf Accessed October 12, 2022. [Google Scholar]
- 2.Tkacz J.P., Rance K., Waddell D., Aagren M., Hammerby E. Real-world evidence costs of allergic rhinitis and allergy immunotherapy in the commercially insured United States population. Curr Med Res Opin. 2021;37:957–965. doi: 10.1080/03007995.2021.1903848. [DOI] [PubMed] [Google Scholar]
- 3.Zuberbier T., Lotvall J., Simoens S., Subramanian S.V., Church M.K. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2) LEN review. Allergy. 2014;69:1275–1279. doi: 10.1111/all.12470. [DOI] [PubMed] [Google Scholar]
- 4.von Mutius E., Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10:861–868. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 5.Genuneit J. Exposure to farming environments in childhood and asthma and wheeze in rural populations: a systematic review with meta-analysis. Pediatr Allergy Immunol. 2012;23:509–518. doi: 10.1111/j.1399-3038.2012.01312.x. [DOI] [PubMed] [Google Scholar]
- 6.Abrahamsson T.R., Jakobsson H.E., Andersson A.F., Bjorksten B., Engstrand L., Jenmalm M.C. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129:434–440. doi: 10.1016/j.jaci.2011.10.025. e1-2. [DOI] [PubMed] [Google Scholar]
- 7.Azad M.B., Konya T., Guttman D.S., Field C.J., Sears M.R., HayGlass K.T., et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015;45:632–643. doi: 10.1111/cea.12487. [DOI] [PubMed] [Google Scholar]
- 8.Boutin R.C.T., Sbihi H., Dsouza M., Malhotra R., Petersen C., Dai D., et al. Mining the infant gut microbiota for therapeutic targets against atopic disease. Allergy. 2020;75:2065–2068. doi: 10.1111/all.14244. [DOI] [PubMed] [Google Scholar]
- 9.Chen C.C., Chen K.J., Kong M.S., Chang H.J., Huang J.L. Alterations in the gut microbiotas of children with food sensitization in early life. Pediatr Allergy Immunol. 2016;27:254–262. doi: 10.1111/pai.12522. [DOI] [PubMed] [Google Scholar]
- 10.Depner M., Taft D.H., Kirjavainen P.V., Kalanetra K.M., Karvonen A.M., Peschel S., et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat Med. 2020;26:1766–1775. doi: 10.1038/s41591-020-1095-x. [DOI] [PubMed] [Google Scholar]
- 11.Stokholm J., Blaser M.J., Thorsen J., Rasmussen M.A., Waage J., Vinding R.K., et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun. 2018;9:141. doi: 10.1038/s41467-017-02573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Illi S., Depner M., Genuneit J., Horak E., Loss G., Strunz-Lehner C., et al. Protection from childhood asthma and allergy in Alpine farm environments—the GABRIEL Advanced Studies. J Allergy Clin Immunol. 2012;129:1470–1477.e6. doi: 10.1016/j.jaci.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Loss G., Apprich S., Waser M., Kneifel W., Genuneit J., Buchele G., et al. The protective effect of farm milk consumption on childhood asthma and atopy: the GABRIELA study. J Allergy Clin Immunol. 2011;128:766–773. doi: 10.1016/j.jaci.2011.07.048. e4. [DOI] [PubMed] [Google Scholar]
- 14.Waser M., Michels K.B., Bieli C., Floistrup H., Pershagen G., von Mutius E., et al. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin Exp Allergy. 2007;37:661–670. doi: 10.1111/j.1365-2222.2006.02640.x. [DOI] [PubMed] [Google Scholar]
- 15.Braun-Fahrlander C., von Mutius E. Can farm milk consumption prevent allergic diseases? Clin Exp Allergy. 2011;41:29–35. doi: 10.1111/j.1365-2222.2010.03665.x. [DOI] [PubMed] [Google Scholar]
- 16.Loss G., Depner M., Ulfman L.H., van Neerven R.J., Hose A.J., Genuneit J., et al. Consumption of unprocessed cow's milk protects infants from common respiratory infections. J Allergy Clin Immunol. 2015;135:56–62. doi: 10.1016/j.jaci.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 17.von Mutius E., Schmid S., Group P.S. The PASTURE project: EU support for the improvement of knowledge about risk factors and preventive factors for atopy in Europe. Allergy. 2006;61:407–413. doi: 10.1111/j.1398-9995.2006.01009.x. [DOI] [PubMed] [Google Scholar]
- 18.Hose A.J., Depner M., Illi S., Lau S., Keil T., Wahn U., et al. Latent class analysis reveals clinically relevant atopy phenotypes in 2 birth cohorts. J Allergy Clin Immunol. 2017;139:1935–1945.e12. doi: 10.1016/j.jaci.2016.08.046. [DOI] [PubMed] [Google Scholar]
- 19.Chauveau A., Dalphin M.L., Mauny F., Kaulek V., Schmausser-Hechfellner E., Renz H., et al. Skin prick tests and specific IgE in 10-year-old children: agreement and association with allergic diseases. Allergy. 2017;72:1365–1373. doi: 10.1111/all.13148. [DOI] [PubMed] [Google Scholar]
- 20.Roduit C., Frei R., Ferstl R., Loeliger S., Westermann P., Rhyner C., et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. 2019;74:799–809. doi: 10.1111/all.13660. [DOI] [PubMed] [Google Scholar]
- 21.Dostal A., Baumgartner J., Riesen N., Chassard C., Smuts C.M., Zimmermann M.B., et al. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: a randomised, placebo-controlled intervention trial in South African children. Br J Nutr. 2014;112:547–556. doi: 10.1017/S0007114514001160. [DOI] [PubMed] [Google Scholar]
- 22.Pareto A. 2015. How to Calculate the Number Needed to Treat (NNT) from Cohen’s d or Hedges’g. Accessed October 12, 2022. https://rpubs.com/RatherBit/78905. [Google Scholar]
- 23.Muthén L.K., Muthén B.O. 7th ed. Muthén & Muthén; Los Angeles, CA: 1998–2012. (Mplus User’s Guide). [Google Scholar]
- 24.Tanaka M., Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. 2017;66:515–522. doi: 10.1016/j.alit.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Abrahamsson T.R., Jakobsson H.E., Andersson A.F., Bjorksten B., Engstrand L., Jenmalm M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44:842–850. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 26.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Netea M.G., Dominguez-Andres J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbring S., Verheijden K.A.T., Diks M.A.P., Leusink-Muis A., Hols G., Baars T., et al. Raw cow's milk prevents the development of airway inflammation in a murine house dust mite-induced asthma model. Front Immunol. 2017;8:1045. doi: 10.3389/fimmu.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbring S., Wolf J., Ayechu-Muruzabal V., Diks M.A.P., Alhamwe B.A., Alhamdan F., et al. Raw cow's milk reduces allergic symptoms in a murine model for food allergy—a potential role for epigenetic modifications. Nutrients. 2019;11:1721. doi: 10.3390/nu11081721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brick T., Schober Y., Bocking C., Pekkanen J., Genuneit J., Loss G., et al. ω-3 Fatty acids contribute to the asthma-protective effect of unprocessed cow's milk. J Allergy Clin Immunol. 2016;137:1699–1706.e13. doi: 10.1016/j.jaci.2015.10.042. [DOI] [PubMed] [Google Scholar]
- 31.Sozanska B. Raw cow's milk and its protective effect on allergies and asthma. Nutrients. 2019;11:469. doi: 10.3390/nu11020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riedler J., Braun-Fahrlander C., Eder W., Schreuer M., Waser M., Maisch S., et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 33.Ege M.J., Frei R., Bieli C., Schram-Bijkerk D., Waser M., Benz M.R., et al. Not all farming environments protect against the development of asthma and wheeze in children. J Allergy Clin Immunol. 2007;119:1140–1147. doi: 10.1016/j.jaci.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 34.Brick T., Hettinga K., Kirchner B., Pfaffl M.W., Ege M.J. The beneficial effect of farm milk consumption on asthma, allergies, and infections: from meta-analysis of evidence to clinical trial. J Allergy Clin Immunol Pract. 2020;8:878–889. doi: 10.1016/j.jaip.2019.11.017. e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.