Abstract
Post-acute sequelae of SARS-CoV-2 infection (PASC) affects a wide range of organ systems among a large proportion of patients with SARS-CoV-2 infection. Although studies have identified a broad set of patient-level risk factors for PASC, little is known about the association between “exposome”—the totality of environmental exposures and the risk of PASC. Using electronic health data of patients with COVID-19 from two large clinical research networks in New York City and Florida, we identified environmental risk factors for 23 PASC symptoms and conditions from nearly 200 exposome factors. The three domains of exposome include natural environment, built environment, and social environment. We conducted a two-phase environment-wide association study. In Phase 1, we ran a mixed effects logistic regression with 5-digit ZIP Code tabulation area (ZCTA5) random intercepts for each PASC outcome and each exposome factor, adjusting for a comprehensive set of patient-level confounders. In Phase 2, we ran a mixed effects logistic regression for each PASC outcome including all significant (false positive discovery adjusted p-value < 0.05) exposome characteristics identified from Phase I and adjusting for confounders. We identified air toxicants (e.g., methyl methacrylate), particulate matter (PM2.5) compositions (e.g., ammonium), neighborhood deprivation, and built environment (e.g., food access) that were associated with increased risk of PASC conditions related to nervous, blood, circulatory, endocrine, and other organ systems. Specific environmental risk factors for each PASC condition and symptom were different across the New York City area and Florida. Future research is warranted to extend the analyses to other regions and examine more granular exposome characteristics to inform public health efforts to help patients recover from SARS-CoV-2 infection.
Keywords: Exposome, SARS-CoV-2 infection, Long-COVID, Air pollution, Neighborhood deprivation, Built environment
Graphical abstract
Abbreviations
- PASC
post-acute sequelae of SARS-CoV-2 infection
- COVID-19
the 2019 novel coronavirus disease
- US
the United States
- ZCTA5
5-digit ZIP Code tabulation area
- CRN
clinical research network
- PCORnet
the National Patient-Centered Clinical Research Network
- PM2.5
fine particulate matter with diameters that are 2.5 μm and smaller
- CO
carbon monoxide
- SO2
sulfur dioxide
- NO2
nitrogen dioxide
sulfate
ammonium
nitrate
- OM
organic matter
- BC
black carbon
- DUST
mineral dust
- SS
sea-salt
- O3
ozone
- ACAG
The University of Washington at St. Louis Atmospheric Composition Analysis Group
- CACES
The Center for Air, Climate, & Energy Solutions
- US EPA
The United States Environmental Protection Agency
- JHU CSSE
Johns Hopkins University, Center for Systems Science and Engineering Coronavirus Resource Center
- CDC
The Centers for Disease Control and Prevention
- NATA
National Air Toxics Assessment
- USDA
US Department of Agriculture
- HUD
Department of Housing and Urban Development
- USPS
US Postal Service
- NACIS
The North American Industry Classification System
- NDVI
Normalized Difference Vegetation Index
- NDI
Neighborhood Deprivation Index
- ED
emergency department
- VIF
variance inflation factor
1. Introduction
Post-acute sequelae of SARS-CoV-2 infection (PASC) refers to ongoing, relapsing, or new symptoms occurring after the acute phase of SARS-CoV-2 infection. Approximately one in five individuals aged 18-64 and one in four individuals aged 65 or older experience potential PASC symptoms and conditions following acute SARS-CoV-2 infection (Bull-Otterson et al., 2022). Studies have identified PASC symptoms and conditions that affect multiple organ systems, including shortness of breath (Al-Aly et al., 2021; Bell et al., 2021; Taquet et al., 2021; Wang et al., 2022), fatigue (Al-Aly et al., 2021; Bell et al., 2021; Cohen et al., 2022; Shoucri et al., 2021), cognitive dysfunction (Blomberg et al., 2021; Davis et al., 2021; Taquet et al., 2021), pulmonary diseases (Cohen et al., 2022), cardiovascular diseases (Davis et al., 2021), diabetes (Cohen et al., 2022), and mental health conditions (Cohen et al., 2022; Taquet et al., 2021; Wang et al., 2022). As the number of individuals with SARS-CoV-2 infection keeps growing, understanding, treating, and preventing PASC conditions and symptoms have become a priority to help patients recover completely from SARS-CoV-2 infection.
Incidence and severity of PASC symptoms and conditions vary significantly among COVID-19 patients (Groff et al., 2021; Xie et al., 2021). A critical public health objective is to identify key factors that contribute to a higher risk of PASC symptoms and conditions following SARS-CoV-2 infection. Such evidence is important to help prioritize preventions and treatment strategies and improve health equity (Sudre et al., 2021; Yoo et al., 2022). Recent studies have identified a set of patient-level risk factors for PASC among COVID-19 patients, including female sex (Bliddal et al., 2021; Sudre et al., 2021), higher body mass index (Bliddal et al., 2021; Sudre et al., 2021), older age (Carvalho-Schneider et al., 2021; Petersen et al., 2021), preexisting comorbidities (Su et al., 2022; Thompson et al., 2022), minority race/ethnicity (Halpin et al., 2021), and severity of acute SARS-CoV-2 infection (Carvalho-Schneider et al., 2021; Sudre et al., 2021). However, little is known about the environmental characteristics associated with PASC.
Disadvantaged environmental characteristics, such as air pollution, social vulnerability, and poor built environment, have long been recognized as risk factors for viral respiratory infections (Diez Roux, 2001; Pica & Bouvier, 2012; Smith et al., 1999). A growing body of evidence has established strong associations between environmental risk factors (e.g., exposures to air pollutants and chemicals) and increased risk of incidence and mortality of SARS-CoV-2 infection (H. Hu et al., 2021; Weaver et al., 2022; Wu et al., 2020; Zhou et al., 2021). Many of these studies focused on PM2.5 and identified a strong association between short-term and long-term exposure to PM2.5 and COVID-19 mortality. However, as Contini et al., suggested, results from these studies need to be interpreted with caution when chemical, physical, and biological analyses are lacking, and some important confounders were not adjusted (Contini & Costabile, 2020). Recent research examined a limited set of environmental risk factors for PASC. For example, one study examined the association between the Social Vulnerability Index (SVI) and PASC using a sample of 1,000 COVID-19 patients from a single health system and found no differences in the likelihood of PASC between patients with higher and lower levels of SVI (Yoo et al., 2022). To date, little is known about the association between exposome—the totality of environmental exposures and the risk of PASC. The exposome concept recognizes that individuals are simultaneously exposed to a multitude of environmental factors (Wild, 2005). Variation in exposome among COVID-19 patients might explain an important, yet currently understudied, part of the variability in PASC conditions and outcomes. Leveraging two large cohorts of COVID-19 patients in the New York City metropolitan area and Florida, we aimed to identify environmental risk factors for a broader set of PASC symptoms and conditions associated with SARS-CoV-2 infection.
2. Materials and methods
2.1. Data source and setting
We conducted a retrospective cohort study using electronic health record (EHR) data from two large clinical research networks (CRNs) of PCORnet, including INSIGHT and OneFlorida+. PCORnet is a network of healthcare systems that facilitates multi-site research using EHR data. The network utilizes a common data model that fosters interoperability across participating sites. The INSIGHT CRN collects data from five academic health systems in New York City, covering a diverse patient population in the New York City Metropolitan Area (Kaushal et al., 2014). The OneFlorida+ is a partnership of 14 academic institutions and health systems across Florida, Georgia, and Alabama with longitudinal patient-level EHR data for approximately 20 million patients (Shenkman et al., 2018). Using COVID-19 patients from two regions with different social and environmental conditions helped to demonstrate the heterogeneity of exposome characteristics associated with PASC conditions.
2.2. Study sample
We identified COVID-19 positive patients as those with a positive SARS-CoV-2 PCR/antigen test or COVID-19 diagnosis (U07.1, U07.2, J12.81, B34.2, B97.2, B97.21, U04, and U04.9) between March 1st, 2020 and October 31st, 2021 in both CRNs. We included COVID-19-related diagnosis codes in addition to positive laboratory test results because patients could have received a positive SARS-CoV-2 test outside CRN affiliated health systems or at home and only a diagnosis code was observed in EHR data. We identified COVID-19 negative patients as those with a negative PCR/antigen test, no positive tests, and/or no COVID-19-related diagnosis codes during the same period. We defined the date of first positive or negative PCR/antigen test or COVID-19 diagnosis as the index date.
This study focused on PASC symptoms and conditions among adult patients. Patients were included if they were 20 years or older, had at least one clinical encounter 3 years to 7 days before the index date (baseline period), and had at least one encounter 31-180 days after the index date (follow-up period). This requirement was necessary to observe symptoms and conditions in the pre-test period and allow us to identify patients with incident new conditions and symptoms after SARS-CoV-2 infection. We were also able to account for baseline demographics (e.g., age and gender) and comorbidities as confounders in the analysis. We further restricted patients to those with a 5-digit residential zip-code in EHR data. We cross-walked 5-digit zip code to 5-digit zip-code tabulation areas (ZCTA5) and only included patients from a ZCTA5 with at least ten patients. eFigures 1&2 in the appendix represented the catchment areas of our sample in New York and Florida.
2.3. Defining PASC
We included 23 PASC symptoms and conditions that were identified from our previous study based on existing literature, input from clinical experts, and data-driven analytics (Zang et al., 2022). A detailed description of methods of identifying these PASC symptoms and conditions was reported separately (Zang et al., 2022). These symptoms and conditions are categorized into the following eight organ systems: nervous system (encephalopathy, dementia, cognitive problems, sleep disorders, and headache), skin (hair loss and pressure ulcer of skin), respiratory system (pulmonary fibrosis, dyspnea, and acute pharyngitis), circulatory system (pulmonary embolism, thromboembolism, chest pain, and abnormal heartbeat), blood (anemia), endocrine (malnutrition, diabetes mellitus, fluid disorders, and edema), digestive system (constipation and abdominal pain), and general signs and symptoms (malaise and fatigue and joint pain). We examined exposome characteristics associated with having at least one PASC condition or symptom in each organ system as well as characteristics associated with each individual PASC condition and symptom.
2.4. Exposome characteristics
We integrated a variety of exposome measures from multiple sources to characterize patients’ exposures to their surrounding natural, built, and social environments before acute SARS-CoV-2 infection. Table 1 presents a summary of these exposome factors, along with the corresponding data sources. To account for the heterogeneous spatiotemporal scales of these factors, area- and time-weighted averages were generated to aggregate them at the ZCTA5 level. We considered a total of 259 factors covering three domains of exposome characteristics with ten categories. A complete list of factors is in the appendix (eTable 1).
Table 1.
Summary of ZCTA5-level exposome characteristics.
| Data Source and Validation Study | Year | Original Spatial/ Temporal Scale | # of Measures | Example Measures | |
|---|---|---|---|---|---|
| Natural Environment | |||||
| PM2.5 compositions | ACAG | 2015-2017 | 0.01°/1-month | 7 | Sulfate, nitrate, ammonium |
| Criteria air pollutants | CACES | 2015-2017 | BG/1-year | 6 | PM2.5, O3, PM10, NO2, CO, SO2 |
| Air toxicants | EPA NATA/AirToxScreen | 2014-2019 | CT/1-year | 140 | Acrolein, propylene oxide |
| Built Environment | |||||
| Vacant land | US HUD | 2015-2019 | CT/3-month | 18 | Average days addresses vacant |
| Walkability | National Walkability Index | 2015 | BG/CS | 1 | Walkability Index |
| Food Access | USDA FARA | 2015, 2019 | CT/1-year | 43 | Percent of low-access population at 1 mile |
| Green Space | NASA MODIS | 2015-2019 | 1000m/1-monoth | 1 | Normalized difference vegetation index |
| Social Environment | |||||
| Neighborhood Deprivation | ACS | 2015-2019 | ZCTA5/5-year | 1 | Neighborhood deprivation index |
| Social Capital | CBP | 2015-2019 | ZCTA5/1-year | 10 | Religious, civic, and social organizations |
| Crime and Safety | UCR | 2015-2016 | County/1-year | 32 | Burglary rate, aggravated assault rate |
Notes: BG: Census Block Group; CT: Census Tract; CS: Cross-sectional; ACAG: Atmospheric Composition Analysis Group; CACES: Center for Air, Climate, & Energy Solutions; EPA: Environmental Protection Agency; NATA: National Air Toxics Assessment; HUD: Department of Housing and Urban Development; USDA: US Department of Agriculture; FARA: Food Access Research Atlas; NASA: National Aeronautics and Space Administration; MODIS: Moderate Resolution Imaging Spectroradiometer; ACS: American Community Survey; CBP: Census Business Pattern; UCR: Uniform Crime Reporting.
2.4.1. Natural environment
Natural environment factors include compositions of particulate matter with diameters that are 2.5 μm and smaller (PM2.5 compositions), criteria air pollutants, and air toxicants. These factors could increase the risk of developing PASC by directly leading to certain conditions (e.g., respiratory diseases) or making individuals more susceptible to SARS-CoV-2 infection (e.g., exacerbate infection severity) (Weaver et al., 2022).
Data on PM2.5 compositions were obtained from the University of Washington at St. Louis Atmospheric Composition Analysis Group (ACAG) (van Donkelaar et al., 2019). ACAG estimated annual PM2.5 and its compositions at a spatial resolution of 0.01 degree in longitude and latitude. The estimates were derived using data from a chemical transport model (GEOS-Chem) and satellite observations of aerosol optical depth statistically fused by geographically-weighted models that have been extensively cross-validated (van Donkelaar et al., 2019).
We obtained criteria air pollutants, such as PM10 and carbon monoxide, from the center for air, climate, & energy solutions (CACES) (S. Y. Kim et al., 2020). These measures were derived at the census block group level using data from the US environmental protection agency (EPA) regulatory monitors, land use, and satellite-derived estimates of air pollution with well-validated land use regression models (S. Y. Kim et al., 2020). Finally, we obtained air toxicant measures at the census tract level from the 2014 national air toxics assessment (NATA) and 2017-2019 Air Toxics Screening Assessment (AirToxScreen) conducted by EPA based on a national emissions inventory of outdoor air toxics sources (Logue et al., 2011). We generated time-weighted averages between 2014-2019 for each air toxicant. . These measures represent long-term exposures rather than acute exposures to hazardous air pollutants (H. Hu et al., 2021; Petroni et al., 2020).
2.4.2. Built environment
Built environment factors, including vacant land, walkability, food access, and green space, were considered. These are important determinants to various symptoms and conditions that may be associated with SARS-CoV-2 infection. For example, better access to healthy food mitigates the risk of developing diabetes associated with SARS-CoV-2 infection (Kirby et al., 2021). Green space in neighborhood could reduce the risk of developing respiratory conditions (Tischer et al., 2017).
We obtained census-tract level vacant land measures in the period of 2015-2019 from the US Department of Housing and Urban Development (Garvin et al., 2013). We used the National Walkability Index developed by EPA, which measures walkability on a scale from 1 to 20 for each census block group, with 1 indicating the least walkable block group and 20 indicating the most walkable block group (Watson et al., 2020). Food access measures were obtained from the US Department of Agriculture (USDA)’s Food Environment Atlas (United States Department of Agriculture, 2019). We used 43 food access measures at the census-tract level of 2015 and 2019. Finally, we obtained the Normalized Difference Vegetation Index (NDVI) as a measure of green space in a neighborhood (Rhew et al., 2011). NDVI is a validated measure based on remote-sensing spectral data from NASA Moderate Resolution Imaging Spectroradiometer.
2.4.3. Social environment
We measured neighborhood deprivation, social capital, and crime and safety for neighborhood social environment (Table 1 and eTable 1). These measures represent important socioeconomic conditions that are associated with individuals’ health and various conditions.
The neighborhood deprivation index (NDI) was used to characterize overall neighborhood socioeconomic status. NDI is a weighted average of 20 measures that represent seven domains of neighborhood deprivation, including poverty, occupation, housing, employment, education, racial composition, and residential stability. We extracted ZCTA5-level data for all 20 measures from the American Community Survey five-year estimates of 2015-2019 and derived NDI for New York, New Jersey, and Florida using an established method (Walker et al., 2020). Ten social capital measures were constructed based on the North American Industry Classification System (NACIS) codes using the 2015-2019 Census Business Pattern data at the ZCTA5-level (Rupasingha et al., 2006). Finally, we obtained county-level crime and safety measures from the Uniform Crime Reporting Program (Table 1 and eTable 1).
2.5. Covariates
We examined a comprehensive set of patient characteristics as potential confounders using EHR data. These included patient age (20-39 [ref.], 40-54, 55-64, 65-74, 75-84, and 85+); gender (female [ref.], male. and other/missing); race (White [ref.], Black, Asian, and other or missing); ethnicity (Hispanic [ref.], Non-Hispanic, and Missing); year-month indicators of COVID-19 positive testing (March 2020 through October 2021); baseline comorbidities; and indicators for the institutions contributing data. We used a revised list of Elixhauser comorbidities for pre-existing comorbidities, including alcohol abuse, anemia, arrythmia, asthma, cancer, chronic kidney disease, chronic pulmonary disorders, cirrhosis, coagulopathy, congestive heart failure, COPD, coronary artery disease, dementia, type 1 diabetes, type 2 diabetes, end stage renal disease on dialysis, hemiplegia, HIV, hypertension, inflammatory bowel disorder, lupus or systemic lupus erythematosus, mental health disorders, multiple sclerosis, Parkinson's disease, peripheral vascular disorders, pregnant, pulmonary circulation disorder, rheumatoid arthritis, seizure/epilepsy, severe obesity (BMI >= 40 kg/m2), and weight loss. Each comorbidity was identified using ICD-10-CM diagnosis codes. We also adjusted for hospitalization status for SARS-CoV-2 infection as a proxy for COVID-19 severity. Hospitalized patients were those with a hospitalization encounter in the day prior through the 16 days following the index test date whereas non-hospitalized patients were those with only an ambulatory or ED encounter in the day prior through the 16 days following the index test date.
2.6. Statistical analysis
For all COVID-19 positive patients, we calculated the incidence of having at least one PASC condition in each organ system (e.g., having at least one nervous PASC condition), as well as incidence of each individual PASC condition. To calculate incidence of PASC for each organ system, we first included patients without any diagnosis of PASC conditions in that organ system during the baseline period (i.e., 3 years to 7 days before the index date). Among these patients, for each organ system we identified those with at least one diagnosis of PASC conditions during the follow-up period (i.e., 31-180 days after the index date). The incidence of PASC condition of each organ system was then calculated by dividing the number of patients in step 1 by the number of patients in step 2. Incidence of each individual PASC condition was calculated using same method by including patients without any diagnosis of a given PASC condition during the baseline period and identifying those with at least one diagnosis of that PASC condition during the follow-up period.
We derived all the 259 exposome measures for ZCTA5s in New York, New Jersey, and Florida, and merged them with EHR data of INSIGHT and OneFlorida+ CRNs. We excluded measures with five or fewer unique non-zero and non-missing values, indicating little variations in these measures across ZCTA5s in our sample. This approach led to the exclusion of 63 measures in INSIGHT sample and 55 in OneFlorida+ sample (eTable 2). The remaining 196 measures in INSIGHT and 204 in OneFlorida+ were included in our analysis. We standardized all continuous measures to account for different scales of these measures and easier interpretation.
We performed a two-phase environment-wide association study based on multiple regressions using all COVID-19 positive patients (H. Hu et al., 2021; Lin et al., 2019). We started with a data engineering process including deriving exposome measures and data linkage as mentioned above. Then in the Phase 1 analysis, we ran a single regression model for each PASC outcome (including 23 individual PASC conditions and 8 PASC groups by organ system). Each regression included one exposome factor while controlling for all covariates described above. We used mixed effects logistic regressions with a random intercept for each ZCTA5. We used the false discovery rate (FDR) adjusted p values (q values) to account for multiple testing. A exposome factor was considered significant if the q-value is < 0.05.
In Phase 2, we ran a single mixed effects logistic regression with ZCTA5 random intercepts for each PASC outcome including all the significant exposome factors identified in Phase 1, adjusting for the same set of patient level covariates. We calculated the variance inflation factor (VIF) for each PASC outcome to examine multicollinearity among all significant exposome factors and excluded factors with a VIF of 10 or higher. We identified environmental risk factors for each PASC outcome as those with a statistically significant adjusted odds ratio > 1 (P < 0.05).
2.7. Secondary analysis
Exposome characteristics could be risk factors among all patients, regardless of COVID-19 status. For example, COVID-19 negative patients could also develop respiratory conditions after long-term exposures to air pollutants. We therefore performed an additional analysis to examine the excessive risk of exposome characteristics for PASC symptoms and conditions among COVID-19 positive patients compared with negative patients using the same analytic pipeline as the primary analysis. To identify exposome characteristics associated with excessive risk, we included both COVID-19 positive and negative patients and ran a single mixed effects logistic regression for each PASC outcome. Each regression included all the significant environmental risk factors identified from Phase 2 analysis, an indicator of COVID-19 status, an interaction term between each environmental risk factor and COVID-19 status, all other covariates, and ZCTA5 random intercepts. We identified exposome factors associated with excessive risk for COVID-19 positive patients if (1) the adjusted odds ratio of the interaction term between this factor and COVID-19 status > 1 and (2) the adjusted odds ratio was statistically significant (P < 0.05). All analyses were done using R.
This study was approved by the Institutional Review Boards of Weill Cornell Medicine (21-10-95-380) and University of Florida (IRB202001831).
3. Results
3.1. Patient characteristics
We included 65,472 COVID-19 patients from the INSIGHT CRN and 35,023 from the OneFlorida+ CRN (Table 2 ). OneFlorida+ had a higher proportion of patients under 65 than INSIGHT (78% vs 70%, P<0.001). Both CRNs had more female patients (60% or higher) than male patients (40% or lower). INSIGHT included a lower proportion of Black patients (18% vs 31%, P < 0.001) but a higher proportion of Hispanic patients (25% vs 17%, P < 0.001). A higher proportion of COVID-19 patients were hospitalized in OneFlorida+ than INSIGHT (25% vs 19%, P < 0.001). More patients from INSIGHT tested positive for SARS-CoV-2 in early waves of the pandemic than patients from OneFlorida+. Nearly 30% of INSIGHT patients tested positive in March to June 2020, as compared to 12% in OneFlorida+. Overall, patients from OneFlorida+ had a higher burden of baseline comorbidities compared with patients from INSIGHT (Table 2).
Table 2.
Baseline Characteristics of COVID-19 Positive Patients from INSIGHT and OneFlorida+.
|
Demographics and baseline comorbidities |
INSIGHT (N = 65,427) | OneFlorida+ (N = 35,023) | P value |
|---|---|---|---|
| Demographics | |||
| Age categories, N (%) | |||
| 20-<40 years | 15,958 (24.4) | 11,692 (33.4) | < 0.001 |
| 40-<55 years | 15,969 (24.4) | 9,015 (25.7) | < 0.001 |
| 55-<65 years | 14,086 (21.5) | 6,507 (18.6) | < 0.001 |
| 65-<75 years | 11,136 (17.0) | 4,254 (12.1) | < 0.001 |
| 75-<85 years | 6,117 (9.3) | 2,489 (7.1) | < 0.001 |
| 85+ years | 2,161 (3.3) | 1,066 (3.0) | 0.03 |
| Sex, N (%) | |||
| Female | 39,212 (59.9) | 22,818 (65.2) | < 0.001 |
| Male | 26,215 (40.1) | 12,205 (34.8) | < 0.001 |
| Race, N (%) | |||
| Asian | 2,972 (4.5) | 477 (1.4) | < 0.001 |
| Black or African American | 11,887 (18.2) | 10,783 (30.8) | < 0.001 |
| White | 28,052 (42.9) | 17,460 (49.9) | < 0.001 |
| Other1 | 15,836 (24.2) | 5,773 (16.5) | < 0.001 |
| Missing2 | 6,680 (10.2) | 530 (1.5) | < 0.001 |
| Ethnicity, N (%) | |||
| Hispanic | 16,508 (25.2) | 5,971 (17.0) | < 0.001 |
| Non-Hispanic | 39,493 (60.4) | 23,216 (66.3) | < 0.001 |
| Other/Missing2 | 9,426 (14.4) | 5,836 (16.7) | < 0.001 |
| Hospitalized for COVID-19, N (%) | |||
| Yes | 12,698 (19.4) | 8,742 (25.0) | < 0.001 |
| Index date, N (%) | |||
| March 2020 – June 2020 | 19,017 (29.1) | 4,157 (11.9) | < 0.001 |
| July 2020 – October 2020 | 9,684 (14.8) | 9,035 (25.8) | < 0.001 |
| November 2020 – February 2021 | 23,139 (35.4) | 9,343 (26.7) | < 0.001 |
| March 2021 – June 2021 | 10,817 (16.5) | 3,916 (11.2) | < 0.001 |
| July 2021 – October 2021 | 2,770 (4.2) | 8,572 (24.5) | < 0.001 |
| Baseline comorbidities, N (%) | |||
| Alcohol Abuse | 1,153 (1.8) | 1,436 (4.1) | < 0.001 |
| Anemia | 7,027 (10.7) | 7,765 (22.2) | < 0.001 |
| Arrythmia | 8,036 (12.3) | 5,413 (15.5) | < 0.001 |
| Asthma | 6,468 (9.9) | 4,705 (13.4) | < 0.001 |
| Cancer | 5,499 (8.4) | 3,445 (9.8) | < 0.001 |
| Chronic Kidney Disease | 6,011 (9.2) | 4,265 (12.2) | < 0.001 |
| Chronic Pulmonary Disorders | 9,548 (14.6) | 7,599 (21.7) | < 0.001 |
| Cirrhosis | 749 (1.1) | 595 (1.7) | < 0.001 |
| Coagulopathy | 3,006 (4.6) | 2,653 (7.6) | < 0.001 |
| Congestive Heart Failure | 4,731 (7.2) | 4,093 (11.7) | < 0.001 |
| COPD | 2,641 (4.0) | 2,935 (8.4) | < 0.001 |
| Coronary Artery Disease | 7,790 (11.9) | 4,690 (13.4) | < 0.001 |
| Dementia | 1,294 (2.0) | 1,722 (4.9) | < 0.001 |
| Diabetes Type 1 | 575 (0.9) | 889 (2.5) | < 0.001 |
| Diabetes Type 2 | 11,799 (18.0) | 7,767 (22.2) | < 0.001 |
| End Stage Renal Disease on Dialysis | 1,741 (2.7) | 1,156 (3.3) | < 0.001 |
| Hemiplegia | 558 (0.9) | 842 (2.4) | < 0.001 |
| HIV | 917 (1.4) | 368 (1.1) | < 0.001 |
| Hypertension | 23,868 (36.5) | 14,315 (40.9) | < 0.001 |
| Hypertension and Type 1 or 2 Diabetes Diagnosis | 9,623 (14.7) | 0 (0.0) | < 0.001 |
| Inflammatory Bowel Disorder | 670 (1.0) | 486 (1.4) | < 0.001 |
| Lupus or Systemic Lupus Erythematosus | 468 (0.7) | 430 (1.2) | < 0.001 |
| Mental Health Disorders | 5,380 (8.2) | 6,942 (19.8) | < 0.001 |
| Multiple Sclerosis | 352 (0.5) | 177 (0.5) | 0.53 |
| Parkinson's Disease | 314 (0.5) | 264 (0.8) | < 0.001 |
| Peripheral vascular disorders | 3,776 (5.8) | 3,613 (10.3) | < 0.001 |
| Pregnant | 2,032 (3.1) | 2,187 (6.2) | < 0.001 |
| Pulmonary Circulation Disorder | 787 (1.2) | 1,205 (3.4) | < 0.001 |
| Rheumatoid Arthritis | 1,002 (1.5) | 802 (2.3) | < 0.001 |
| Seizure/Epilepsy | 941 (1.4) | 1,383 (3.9) | < 0.001 |
| Severe Obesity (BMI>=40 kg/m2) | 4,206 (6.4) | 4,563 (13.0) | < 0.001 |
| Weight Loss | 1,828 (2.8) | 2,809 (8.0) | < 0.001 |
Other race includes native Hawaiian or other pacific islander, American Indian or Alaska Native, multiple race, and all other races.
Missing race and ethnicity includes refuse to answer, no information, unknown, and missing values.
Notes: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
3.2. Incidence of PASC conditions and symptoms
Table 3 presents incidence of PASC symptoms and conditions in both INSIGHT and OneFlorida+ cohorts among all COVID-19 positive patients. Patients from INSIGHT had higher incidence of conditions related to nervous, respiratory, circulatory, digestive, and general signs and symptoms, and lower incidence of conditions related to blood and endocrine. Incidence of conditions related to skin was similar between two CRNs. The differences in incidence of individual PASC conditions varied. Conditions with higher relative differences between INSIGHT and OneFlorida+ included fluid and electrolyte disorders (0.5% vs 4.3%, P<0.001), hair loss (1.2% vs 0.6%, P<0.001), pressure ulcer of skin (0.6% vs 1.1%, P<0.001), and acute pharyngitis (1.3% vs 1.9%, P<0.001).
Table 3.
Incidence of New Conditions and Symptoms among COVID-19 Patients from INSIGHT and OneFlorida+.
|
PASC conditions and symptoms |
INSIGHT (%) | OneFlorida+(%) | P value |
|---|---|---|---|
| Nervous | |||
| Encephalopathy | 1.6 | 2.1 | < 0.001 |
| Dementia | 0.8 | 1.1 | < 0.001 |
| Cognitive problems | 3.5 | 3.4 | 0.49 |
| Sleep disorders | 3.5 | 3.0 | < 0.001 |
| Headache | 3.3 | 3.8 | < 0.001 |
| Any nervous condition | 9.6 | 8.1 | < 0.001 |
| Skin | |||
| Hair loss | 1.2 | 0.6 | < 0.001 |
| Pressure ulcer of skin | 0.6 | 1.1 | < 0.001 |
| Any skin conditions | 1.8 | 1.7 | 0.13 |
| Respiratory | |||
| Pulmonary fibrosis | 2.6 | 2.5 | 0.17 |
| Dyspnea | 11.4 | 9.1 | < 0.001 |
| Acute pharyngitis | 1.3 | 1.9 | < 0.001 |
| Any respiratory condition | 13.1 | 10.4 | < 0.001 |
| Circulatory | |||
| Pulmonary embolism | 0.7 | 1.0 | < 0.001 |
| Thromboembolism | 1.2 | 1.3 | 0.16 |
| Chest pain | 5.6 | 5.1 | 0.005 |
| Abnormal heartbeat | 5.0 | 4.6 | 0.02 |
| Any circulatory condition | 8.9 | 8.4 | < 0.001 |
| Blood | |||
| Anemia | 3.9 | 4.7 | < 0.001 |
| Endocrine | |||
| Malnutrition | 1.3 | 1.9 | < 0.001 |
| Diabetes mellitus | 3.0 | 2.5 | < 0.001 |
| Fluid disorders | 0.5 | 4.3 | < 0.001 |
| Edema | 6.1 | 7.6 | < 0.001 |
| Any endocrine condition | 8.8 | 9.1 | 0.25 |
| Digestive | |||
| Other constipation | 3.3 | 2.8 | < 0.001 |
| Abdominal pain | 7.8 | 8.2 | 0.07 |
| Any digestive condition | 9.3 | 8.7 | 0.008 |
| General signs and symptoms | |||
| Malaise and fatigue | 4.6 | 5.0 | 0.03 |
| Joint pain | 9.7 | 7.4 | < 0.001 |
| Any general signs and symptoms | 13.0 | 9.5 | < 0.001 |
3.3. Environmental risk factors for PASC conditions and symptoms
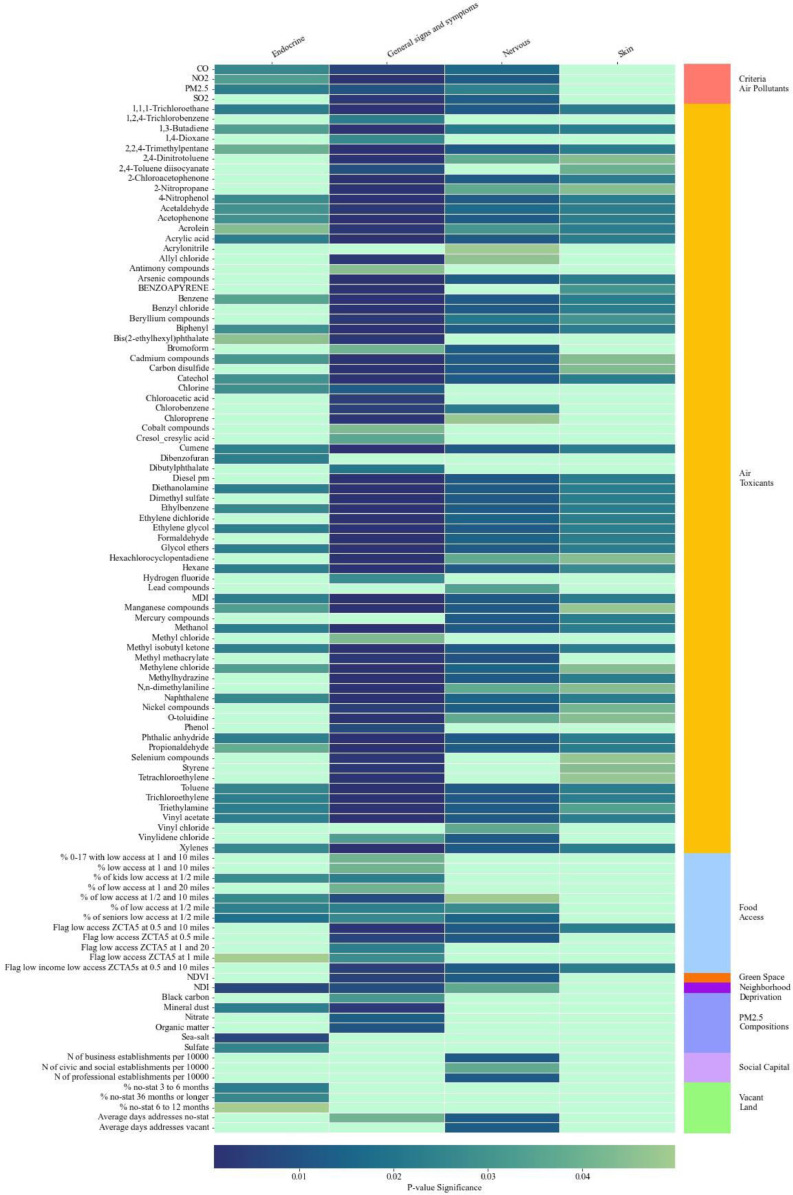
Fig. 1 presents environmental risk factors that were significantly (q < 0.05) associated with having at least one PASC condition or symptom in each organ system from the Phase 1 analysis using COVID-19 patients from INSIGHT. A large group of air toxicant factors had significant associations with PASC related to endocrine, nervous, skin, and general signs and symptoms. In addition, food access had statistically significant associations with PASC related to endocrine, nervous, and general signs and symptoms. Food access, green space, neighborhood deprivation, social capital, and vacant land were associated with PASC conditions and symptoms of endocrine and nervous.
Fig. 1.
Significant Exposome Factors Associated with PASC Groups in Phase 1 Analysis Using INSIGHT Sample.
Notes: Figure represent significant neighborhood and environmental characteristics identified from mixed effects logistic regressions where a PASC condition is the outcome and each neighborhood and environmental characteristic is the key independent variable. All regressions controlled for patient-level covariates. A neighborhood or environmental characteristic is considered significant if the false discovery rate adjusted p value is < 0.05.
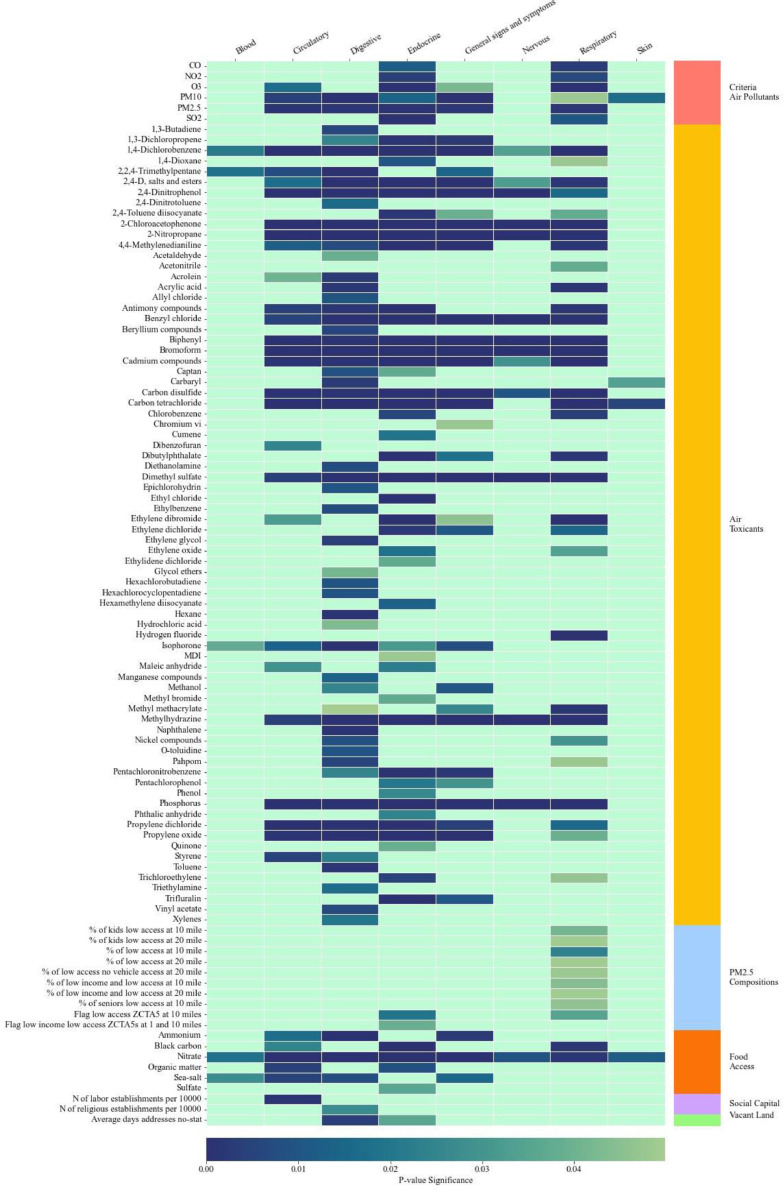
Fig. 2 presents Phase 1 results using COVID-19 patients from OneFlorida+. Blood and skin PASC were each associated with a small set of exposome characteristics. Similar with INSIGHT, a large set of criteria air pollutant and air toxicant characteristics were associated with PASC of endocrine, nervous, and general signs and symptoms. Many criteria air pollutants and air toxicants were associated with circulatory, digestive, and respiratory PASC. A smaller set of built and social environment characteristics were associated with circulatory, digestive, and endocrine PASC conditions among OneFlorida+ patients.
Fig. 2.
Significant Exposome Factors Associated with PASC Groups in Phase 1 Analysis Using OneFlorida+ Sample.
Notes: Figure represent significant neighborhood and environmental characteristics identified from mixed effects logistic regressions where a PASC condition is the outcome and each neighborhood and environmental characteristic is the key independent variable. All regressions controlled for patient-level covariates. A neighborhood and environmental characteristic is considered significant if the false discovery rate adjusted p value is < 0.05.
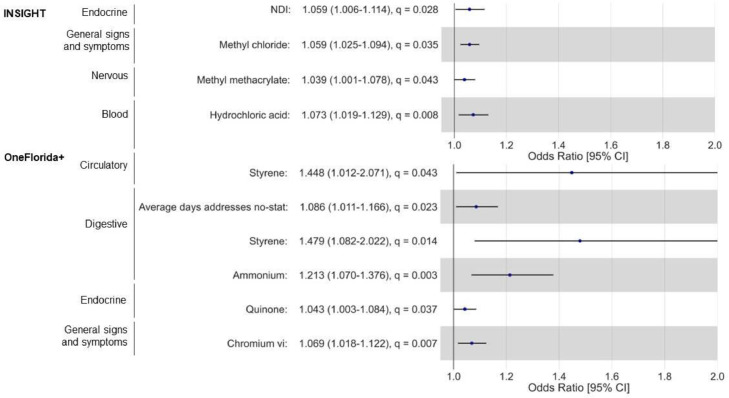
Fig. 3 presents significant environmental risk factors from Phase 2 analysis for INSIGHT and OneFlorida+. Among COVID-19 patients from INSIGHT, a higher NDI score was associated with increased odds of developing at least one endocrine PASC condition (adjusted odds ratio [aOR]: 1.06, 95% confidence interval [CI]: 1.01-1.11). In addition, certain air toxicants were associated with increased odds of developing PASC of blood, nervous, and general signs and symptoms. For example, a higher level of methyl methacrylate was associated with increased odds of developing at least one nervous PASC condition (aOR: 1.04, 95% CI: 1.01-1.08). Among patients in OneFlorida+, we identified statistically significant risk factors, including air toxicants, PM2.5 compositions, and built environment, for PASC conditions of circulatory, digestive, endocrine, and general signs and symptoms. For example, a higher level of styrene was associated with increased odds of developing at least one circulatory (aOR: 1.45, 95% CI: 1.01-2.07) or digestive (aOR: 1.48, 95% CI: 1.08-2.02) PASC condition.
Fig. 3.
Environmental Risk Factors for PASC Conditions by Organ System.
Notes: NDI: Neighborhood Deprivation Index. ORs were estimated from mixed effects logistic regressions with ZCTA5 random intercept. Each regression includes all significant neighborhood and environmental characteristics identified from phase 1 analysis for each PASC outcome, controlling for all patient-level covariates. For N of fitness and recreational sports centers per 10000 residents, the odds ratio means that a lower number of fitness and recreational sports centers was associated with increased odds of developing PASC conditions and symptoms.
3.4. Environmental risk factors for individual PASC symptoms and conditions
We also identified environmental risk factors for each individual PASC condition using the same analytic strategies (eFigures 3-6). Using COVID-19 patients from INSIGHT, we found that higher level of neighborhood deprivation and criteria air pollutants, and lower availability of social capital were risk factors for developing PASC conditions. For example, a higher level of PM10 was associated with increased odds of developing abdominal pain (aOR: 1.06, 95% CI: 1.01-1.12). A higher level of neighborhood deprivation was also associated with increased odds of developing joint pain (aOR: 1.07, 95% CI: 1.02-1.12). A set of air toxicants were associated with an increased odds for anemia, cognitive problems, abnormal heartbeat, edema, and other PASC conditions. Using COVID-19 patients from OneFlorida+ identified a broader set of air toxicants, PM 2.5 compositions, and social environmental characteristics as risk factors for multiple PASC conditions. For example, limited food access was associated with an increased odds of developing cognitive problems, dementia, edema, and pulmonary fibrosis.
3.5. Excessive risk of environmental characteristics for PASC symptoms and conditions
Analyses including COVID-19 negative patients and interaction terms between environmental risk factors and COVID-19 status identified several characteristics with excessive risk for PASC among COVID-19 positive patients relative to negative patients (odds ratio of the interaction term > 1 and P < 0.05). For example, we found that a higher level of lead compounds was associated with excessive risk for nervous PASC among COVID-19 positive patients compared with negative patients (aOR: 1.05, P = 0.03). For individual PASC symptoms and conditions, a higher level of PM10 was associated with excessive risk for abnormal heartbeat among COVID-19 positive patients compared with negative patients (aOR: 1.09, P = 0.02). Full results of these analyses are available in the appendix (eTables 3-4).
4. Discussions
To our knowledge, this is the first study examining environmental risk factors for a comprehensive set of PASC symptoms and conditions. Using large and diverse COVID-19 patient samples from two CRNs, we identified ZCTA5-level risk factors from nearly 200 variables for 23 PASC conditions of eight organ systems. Risk factors for PASC symptoms and conditions were primarily concentrated on air toxicants, overall neighborhood deprivation, and PM2.5 compositions (e.g., sulfate). A few built environment characteristics, such as food access, were also associated with PASC symptoms and conditions. Our findings indicated significant heterogeneity in environmental risk factors for PASC between the New York City area and Florida.
Disadvantaged environmental characteristics can increase the risk for PASC through multiple direct and indirect pathways. Long-term exposure to air pollution can directly cause various symptoms and conditions of the central nervous system, respiratory, endocrine, and other organ systems. The association between air pollution and respiratory conditions has been well established. PM2.5 is associated with increased risk of incident asthma, COPD, and other respiratory diseases (Tiotiu et al., 2020; Z. Zhang et al., 2021). Growing numbers of studies also demonstrate associations between air pollution and nervous conditions. Air pollution is associated with metabolic abnormalities and oxidative stress in the brain (H. Kim et al., 2020; Thomson, 2019). Air pollution-induced dysfunction of the insulin signaling system can reduce cognitive function and increase the risk of dementia (H. Kim et al., 2020; Paul et al., 2020). People living in neighborhoods of greater deprivation often have fewer financial resources, lower health literacy, and higher food insecurity, leading to the development of diabetes and other conditions (M. D. Hu et al., 2021; Kurani et al., 2021). Previous studies found that COVID-19 patients are disproportionately from areas with disadvantaged neighborhood conditions (Y. Zhang et al., 2021). Addressing neighborhood and environmental vulnerability is important to help patients recover from SARS-CoV-2 infection.
Compared with the robust evidence on direct health effects of environmental risk factors, the interactions between these characteristics and SARS-CoV-2 infection are understudied and may be of great importance to address. Early evidence indicated that air pollution can modify individuals’ susceptibility to SARS-CoV-2 infection and disease severity (Chen et al., 2022; Pica & Bouvier, 2012; Weaver et al., 2022). This may be mediated by upregulation of proteins critical to viral entry and by immune system suppression from oxidative stress, epithelial damage, and pulmonary inflammation (van der Valk & In 't Veen, 2021; Weaver et al., 2022). Studies found that exposure to particulate matter can increase the expression of angiotensin-converting enzyme 2 (ACE2) and other proteins critical to SARS-CoV-2 entry into host cells (Hoffmann et al., 2020; Sagawa et al., 2021). Upregulation of proteins necessary for viral entry may lead to higher viral load and elevate the risk of severe COVID-19. Immunological impairment prior to COVID-19 infection, induced by long-term exposure to PM, NO2, and other air pollutants, may also increase the risk of COVID-19 infection and/or its severity (Weaver et al., 2022). Severe COVID-19 is associated with high inflammation and elevated levels of inflammatory cytokines, both are important pathophysiologic factors for PASC symptoms and conditions (Mehandru & Merad, 2022; Nalbandian et al., 2021). Our analyses provided important evidence for this question. Results indicated that certain exposome characteristics, particularly air toxicants, were associated with excessive risk for PASC symptoms and conditions among COVID-19 positive patients compared with negative patients.
We found considerable heterogeneity of environmental risk factors for PASC between New York City and Florida. This could be due to different exposure levels of neighborhood and environmental characteristics between the two regions (eTable 1). For example, we found that acetamide was associated with increased odds for multiple PASC conditions among patients from OneFlorida+ but not among patients in INSIGHT. Further analysis indicated that the concentration of acetamide in the catchment areas of OneFlorida+ was almost twice the concentration in the New York area. The PM10 level was 15% higher in the New York area than the areas covered by OneFlorida+. This may explain why PM10 was a risk factor for PASC among INSIGHT patients but not among OneForida+ patients. In addition, the proportion of residents with low food access was significantly higher in areas covered by OneFlorida+ than the New York area (eTable 1). Therefore, low food access was found to be a risk factor for multiple PASC conditions among patients from Florida but not in the New York area. The differential burden of preexisting comorbidities among patients in Florida may also account for the heterogeneous findings. Patients with a higher burden of pre-existing chronic conditions may be more susceptible to air pollution induced adverse health effects and therefore are at a higher risk for PASC (To et al., 2015). Other potential explanations may include variations in vaccination rate, healthcare utilization pattern, and differing courses of pandemic in these two regions. More research is needed to extend the analyses to other regions and understand reasons for heterogeneity in environmental risk factors for PASC.
This study has several major strengths. We were able to account for simultaneous exposure to multifaceted disadvantaged environmental risk factors by examining a very comprehensive set of exposome characteristics. Lack of detailed patient level data has been considered a major limitation in previous studies examining environmental risk factors and COVID-19 related outcomes (Weaver et al., 2022). Compared with previous ecologic studies relying on data aggregated at the county level, we were able to adjust for detailed patient level characteristics (e.g., demographics and pre-existing comorbidities) as potential confounders. We compared findings between two large COVID-19 patient cohorts in New York City area and Florida and demonstrated significant heterogeneity in environmental risk factors for PASC. This finding provides important implications for public health efforts to address social risk factors and help patients recover from SARS-CoV-2 infection.
Limitations of this study include: (1) we used exposome characteristics at ZCTA5 level, which may not be granular enough to estimate individuals’ exposure to risk factors. This is particularly an issue in New York City where each ZCTA5 may cover a broad geographic area and a higher number of residents. (2) Similar with many previous studies, we focused on long-term exposure to air toxicants before SARS-CoV-2 infection. Air toxicants may have changed during the pandemic. We were not able to measure this short-term acute exposure to air toxicants and other risk factors.. (3) We were not able to use a life-course approach to determine patients’ exposome due to the lack of residential history data from conception. (4) Some important potential confounders, such as vaccination status, were not adjusted due to data limitations. These residual confounders may also partially explain the heterogeneous findings between New York City and Florida. (5) We only included patients who sought care from the health systems affiliated with the two CRNs 31-180 days after SARS-CoV-2 infection. These patients may not be representative of patients in these two regions. (6) Patients who always tested negative might have had a positive test that was not captured in EHR (e.g., self-test at home). Thus, it is possible that some patients in the negative group may be tested positive at some point.
5. Conclusion
We found that multiple environmental risk factors, especially certain air pollutants and toxicants, are significantly associated with an increased risk of PASC conditions that impact multiple organ systems. These risk factors for PASC symptoms and conditions differed in the New York City area compared to Florida. Targeting interventions to reduce the burden of PASC among patients with disadvantaged exposome characteristics will help to reduce disparities of COVID-19 pandemic.
CRediT authorship contribution statement
Yongkang Zhang: Conceptualization, Methodology, Writing – original draft, Writing – original draft. Hui Hu: Conceptualization, Methodology, Writing – original draft. Vasilios Fokaidis: Data curation, Formal analysis, Visualization, Writing – original draft. Colby Lewis V: Data curation, Formal analysis, Visualization, Writing – original draft. Jie Xu: Data curation, Formal analysis, Visualization, Writing – original draft. Chengxi Zang: Data curation, Writing – original draft. Zhenxing Xu: Data curation, Writing – original draft. Fei Wang: Writing – original draft. Michael Koropsak: Writing – original draft, Project administration. Jiang Bian: Methodology, Writing – original draft. Jaclyn Hall: Methodology, Writing – original draft. Russell L. Rothman: Writing – original draft. Elizabeth A. Shenkman: Writing – original draft. Wei-Qi Wei: Writing – original draft. Mark G. Weiner: Writing – original draft, Funding acquisition. Thomas W. Carton: Writing – original draft, Funding acquisition, Project administration. Rainu Kaushal: Writing – original draft, Funding acquisition.
Declaration of Competing Interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research was funded by NIH Agreement Other Transactions Authority (OTA). OT2HL161847 (contract no. EHR-01–21) is part of the Researching COVID to Enhance Recovery (RECOVER) research program.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.envadv.2023.100352.
Appendix. Supplementary materials
Data availability
The data that has been used is confidential.
References
- Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- Bell M.L., Catalfamo C.J., Farland L.V., Ernst K.C., Jacobs E.T., Klimentidis Y.C., Jehn M., Pogreba-Brown K. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: Results from the Arizona CoVHORT. PLoS. One. 2021;16(8) doi: 10.1371/journal.pone.0254347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliddal S., Banasik K., Pedersen O.B., Nissen J., Cantwell L., Schwinn M., Tulstrup M., Westergaard D., Ullum H., Brunak S., Tommerup N., Feenstra B., Geller F., Ostrowski S.R., Gronbaek K., Nielsen C.H., Nielsen S.D., Feldt-Rasmussen U. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci. Rep. 2021;11(1):13153. doi: 10.1038/s41598-021-92045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B., Mohn K.G., Brokstad K.A., Zhou F., Linchausen D.W., Hansen B.A., Lartey S., Onyango T.B., Kuwelker K., Saevik M., Bartsch H., Tondel C., Kittang B.R., Bergen C.-R.G., Cox R.J., Langeland N. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021;27(9):1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull-Otterson L., Baca S., Saydah S., Boehmer T.K., Adjei S., Gray S., Harris A.M. Post–COVID Conditions Among Adult COVID-19 Survivors Aged 18–64 and≥ 65 Years—United States, March 2020–November 2021. Morb. Mortal. Wkly. Rep. 2022;71(21):713. [Google Scholar]
- Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., Flament T., Ferreira-Maldent N., Bruyere F., Stefic K., Gaudy-Graffin C., Grammatico-Guillon L., Bernard L. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Sidell M.A., Huang B.Z., Chow T., Eckel S.P., Martinez M.P., Gheissari R., Lurmann F., Thomas D.C., Gilliland F.D., Xiang A.H. Ambient Air Pollutant Exposures and COVID-19 Severity and Mortality in a Cohort of COVID-19 Patients in Southern California. Am. J. Respir. Crit. Care. Med. 2022 doi: 10.1164/rccm.202108-1909OC. [DOI] [PubMed] [Google Scholar]
- Cohen K., Ren S., Heath K., Dasmarinas M.C., Jubilo K.G., Guo Y., Lipsitch M., Daugherty S.E. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2022;376 doi: 10.1136/bmj-2021-068414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini D., Costabile F. Does air pollution influence COVID-19 outbreaks? Atmosphere. 2020;11(4):377. [Google Scholar]
- Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re'em Y., Redfield S., Austin J.P., Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux A.V. Investigating neighborhood and area effects on health. Am. J. Public. Health. 2001;91(11):1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin E., Branas C., Keddem S., Sellman J., Cannuscio C. More Than Just An Eyesore: Local Insights And Solutions on Vacant Land And Urban Health. J. Urban. Health. 2013;90(3):412–426. doi: 10.1007/s11524-012-9782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff D., Sun A., Ssentongo A.E., Ba D.M., Parsons N., Poudel G.R., Lekoubou A., Oh J.S., Ericson J.E., Ssentongo P., Chinchilli V.M. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA. Netw. Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., Walshaw C., Kemp S., Corrado J., Singh R., Collins T., O'Connor R.J., Sivan M. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021;93(2):1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Zheng Y., Wen X., Smith S.S., Nizomov J., Fishe J., Hogan W.R., Shenkman E.A., Bian J. An external exposome-wide association study of COVID-19 mortality in the United States. Sci. Total. Environ. 2021;768 doi: 10.1016/j.scitotenv.2020.144832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M.D., Lawrence K.G., Bodkin M.R., Kwok R.K., Engel L.S., Sandler D.P. Neighborhood Deprivation, Obesity, and Diabetes in Residents of the US Gulf Coast. Am. J. Epidemiol. 2021;190(2):295–304. doi: 10.1093/aje/kwaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal R., Hripcsak G., Ascheim D.D., Bloom T., Campion T.R., Jr., Caplan A.L., Currie B.P., Check T., Deland E.L., Gourevitch M.N., Hart R., Horowitz C.R., Kastenbaum I., Levin A.A., Low A.F., Meissner P., Mirhaji P., Pincus H.A., Scaglione C., Nyc C. Changing the research landscape: the New York City Clinical Data Research Network. J. Am. Med. Inform. Assoc. 2014;21(4):587–590. doi: 10.1136/amiajnl-2014-002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Kim W.H., Kim Y.Y., Park H.Y. Air Pollution and Central Nervous System Disease: A Review of the Impact of Fine Particulate Matter on Neurological Disorders. Front. Public. Health. 2020;8 doi: 10.3389/fpubh.2020.575330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Bechle M., Hankey S., Sheppard L., Szpiro A.A., Marshall J.D. Concentrations of criteria pollutants in the contiguous U.S., 1979 - 2015: Role of prediction model parsimony in integrated empirical geographic regression. PLoS. One. 2020;15(2) doi: 10.1371/journal.pone.0228535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J.B., Bernard D., Liang L. The Prevalence of Food Insecurity Is Highest Among Americans for Whom Diet Is Most Critical to Health. Diabetes. Care. 2021;44(6):e131–e132. doi: 10.2337/dc20-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurani S.S., Lampman M.A., Funni S.A., Giblon R.E., Inselman J.W., Shah N.D., Allen S., Rushlow D., McCoy R.G. Association Between Area-Level Socioeconomic Deprivation and Diabetes Care Quality in US Primary Care Practices. JAMA. Netw. Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.38438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Jiang R., Wu J., Wei S., Yin L., Xiao X., Hu S., Shen Y., Ouyang G. Sorption properties of hydrophobic organic chemicals to micro-sized polystyrene particles. Sci. Total. Environ. 2019;690:565–572. doi: 10.1016/j.scitotenv.2019.06.537. [DOI] [PubMed] [Google Scholar]
- Logue J.M., Small M.J., Robinson A.L. Evaluating the national air toxics assessment (NATA): Comparison of predicted and measured air toxics concentrations, risks, and sources in Pittsburgh, Pennsylvania. Atmos. Environ. 2011;45(2):476–484. doi: 10.1016/j.atmosenv.2010.09.053. [DOI] [Google Scholar]
- Mehandru S., Merad M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022;23(2):194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K.C., Haan M., Yu Y., Inoue K., Mayeda E.R., Dang K., Wu J., Jerrett M., Ritz B. Traffic-Related Air Pollution and Incident Dementia: Direct and Indirect Pathways Through Metabolic Dysfunction. J. Alzheimers. Dis. 2020;76(4):1477–1491. doi: 10.3233/JAD-200320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.S., Kristiansen M.F., Hanusson K.D., Danielsen M.E., B A.S., Gaini S., Strom M., Weihe P. Long COVID in the Faroe Islands: A Longitudinal Study Among Nonhospitalized Patients. Clin. Infect. Dis. 2021;73(11):e4058–e4063. doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroni M., Hill D., Younes L., Barkman L., Howard S., Howell I.B., Mirowsky J., Collins M.B. Hazardous air pollutant exposure as a contributing factor to COVID-19 mortality in the United States. Environ. Res. Lett. 2020;(9):15. doi: 10.1088/1748-9326/abaf86. ARTN 0940a9. [DOI] [Google Scholar]
- Pica N., Bouvier N.M. Environmental factors affecting the transmission of respiratory viruses. Curr. Opin. Virol. 2012;2(1):90–95. doi: 10.1016/j.coviro.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhew I.C., Vander Stoep A., Kearney A., Smith N.L., Dunbar M.D. Validation of the normalized difference vegetation index as a measure of neighborhood greenness. Ann. Epidemiol. 2011;21(12):946–952. doi: 10.1016/j.annepidem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupasingha A., Goetz S.J., Freshwater D. The production of social capital in US counties. J. Socio. Econ. 2006;35(1):83–101. [Google Scholar]
- Sagawa T., Tsujikawa T., Honda A., Miyasaka N., Tanaka M., Kida T., Hasegawa K., Okuda T., Kawahito Y., Takano H. Exposure to particulate matter upregulates ACE2 and TMPRSS2 expression in the murine lung. Environ. Res. 2021;195 doi: 10.1016/j.envres.2021.110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkman E., Hurt M., Hogan W., Carrasquillo O., Smith S., Brickman A., Nelson D. OneFlorida Clinical Research Consortium: Linking a Clinical and Translational Science Institute With a Community-Based Distributive Medical Education Model. Acad. Med. 2018;93(3):451–455. doi: 10.1097/ACM.0000000000002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoucri S.M., Purpura L., DeLaurentis C., Adan M.A., Theodore D.A., Irace A.L., Robbins-Juarez S.Y., Khedagi A.M., Letchford D., Harb A.A., Zerihun L.M., Lee K.E., Gambina K., Lauring M.C., Chen N., Sperring C.P., Mehta S.S., Myers E.L., Shih H., Zucker J.E. Characterising the long-term clinical outcomes of 1190 hospitalised patients with COVID-19 in New York City: a retrospective case series. BMJ. Open. 2021;11(6) doi: 10.1136/bmjopen-2021-049488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.R., Corvalan C.F., Kjellstrom T. How much global ill health is attributable to environmental factors? Epidemiology. 1999;10(5):573–584. https://www.ncbi.nlm.nih.gov/pubmed/10468437 [PubMed] [Google Scholar]
- Su Y., Yuan D., Chen D.G., Ng R.H., Wang K., Choi J., Li S., Hong S., Zhang R., Xie J., Kornilov S.A., Scherler K., Pavlovitch-Bedzyk A.J., Dong S., Lausted C., Lee I., Fallen S., Dai C.L., Baloni P., Heath J.R. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881–895. doi: 10.1016/j.cell.2022.01.014. e820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., Pujol J.C., Klaser K., Antonelli M., Canas L.S., Molteni E., Modat M., Jorge Cardoso M., May A., Ganesh S., Davies R., Nguyen L.H., Drew D.A., Astley C.M., Steves C.J. Attributes and predictors of long COVID. Nat. Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Dercon Q., Luciano S., Geddes J.R., Husain M., Harrison P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS. Med. 2021;18(9) doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E.J., Williams D.M., Walker A.J., Mitchell R.E., Niedzwiedz C.L., Yang T.C., Huggins C.F., Kwong A.S.F., Silverwood R.J., Di Gessa G., Bowyer R.C.E., Northstone K., Hou B., Green M.J., Dodgeon B., Doores K.J., Duncan E.L., Williams F.M.K., Open S.C., Steves C.J. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022;13(1):3528. doi: 10.1038/s41467-022-30836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson E.M. Air Pollution, Stress, and Allostatic Load: Linking Systemic and Central Nervous System Impacts. J. Alzheimers. Dis. 2019;69(3):597–614. doi: 10.3233/JAD-190015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiotiu A.I., Novakova P., Nedeva D., Chong-Neto H.J., Novakova S., Steiropoulos P., Kowal K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public. Health. 2020;(17):17. doi: 10.3390/ijerph17176212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer C., Gascon M., Fernandez-Somoano A., Tardon A., Lertxundi Materola A., Ibarluzea J., Ferrero A., Estarlich M., Cirach M., Vrijheid M., Fuertes E., Dalmau-Bueno A., Nieuwenhuijsen M.J., Anto J.M., Sunyer J., Dadvand P. Urban green and grey space in relation to respiratory health in children. Eur. Respir. J. 2017;(6):49. doi: 10.1183/13993003.02112-2015. [DOI] [PubMed] [Google Scholar]
- To T., Feldman L., Simatovic J., Gershon A.S., Dell S., Su J., Foty R., Licskai C. Health risk of air pollution on people living with major chronic diseases: a Canadian population-based study. BMJ. Open. 2015;5(9) doi: 10.1136/bmjopen-2015-009075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture. (2019). Food Environment Atlas. Retrieved 05/01 from https://www.ers.usda.gov/foodatlas/.
- van der Valk J.P.M., In 't Veen J. The Interplay Between Air Pollution and Coronavirus Disease (COVID-19) J. Occup. Environ. Med. 2021;63(3):e163–e167. doi: 10.1097/JOM.0000000000002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar A., Martin R.V., Li C., Burnett R.T. Regional Estimates of Chemical Composition of Fine Particulate Matter Using a Combined Geoscience-Statistical Method with Information from Satellites, Models, and Monitors. Environ. Sci. Technol. 2019;53(5):2595–2611. doi: 10.1021/acs.est.8b06392. [DOI] [PubMed] [Google Scholar]
- Walker A.F., Hu H., Cuttriss N., Anez-Zabala C., Yabut K., Haller M.J., Maahs D.M. The Neighborhood Deprivation Index and Provider Geocoding Identify Critical Catchment Areas for Diabetes Outreach. J. Clin. Endocrinol. Metab. 2020;(9):105. doi: 10.1210/clinem/dgaa462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Foer D., MacPhaul E., Lo Y.C., Bates D.W., Zhou L. PASCLex: A comprehensive post-acute sequelae of COVID-19 (PASC) symptom lexicon derived from electronic health record clinical notes. J. Biomed. Inform. 2022;125 doi: 10.1016/j.jbi.2021.103951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K.B., Whitfield G.P., Thomas J.V., Berrigan D., Fulton J.E., Carlson S.A. Associations between the National Walkability Index and walking among US Adults - National Health Interview Survey, 2015. Prev. Med. 2020;137 doi: 10.1016/j.ypmed.2020.106122. ARTN 106122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A.K., Head J.R., Gould C.F., Carlton E.J., Remais J.V. Environmental Factors Influencing COVID-19 Incidence and Severity. Annu. Rev. Public. Health. 2022;43:271–291. doi: 10.1146/annurev-publhealth-052120-101420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild C.P. Complementing the genome with an "exposome": the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer. Epidemiol. Biomarkers. Prev. 2005;14(8):1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath M.B., Braun D., Dominici F. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci. Adv. 2020;(45):6. doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Bowe B., Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat. Commun. 2021;12(1):6571. doi: 10.1038/s41467-021-26513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.M., Liu T.C., Motwani Y., Sim M.S., Viswanathan N., Samras N., Hsu F., Wenger N.S. Factors associated with post-acute sequelae of SARS-CoV-2 (PASC) after diagnosis of symptomatic COVID-19 in the inpatient and outpatient setting in a diverse cohort. J. Gen. Intern. Med. 2022 doi: 10.1007/s11606-022-07523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang, C., Zhang, Y., Xu, J., Bian, J., Morozyuk, D., Schenck, E., Khullar, D., Nordvig, A. S., Shenkman, E., Rothman, R. L., Block, J. P., Lyman, K., Weiner, M., Carton, T. W., Wang, F., & Kaushal, R. (2022). Understanding Post-Acute Sequelae of SARS-CoV-2 Infection through Data-Driven Analysis with Longitudinal Electronic Health Records: Findings from the RECOVER Initiative. doi: 10.1101/2022.05.21.22275420. [DOI]
- Zhang Y., Khullar D., Wang F., Steel P., Wu Y., Orlander D., Weiner M., Kaushal R. Socioeconomic variation in characteristics, outcomes, and healthcare utilization of COVID-19 patients in New York City. PLoS. One. 2021;16(7) doi: 10.1371/journal.pone.0255171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Weichenthal S., Kwong J.C., Burnett R.T., Hatzopoulou M., Jerrett M., van Donkelaar A., Bai L., Martin R.V., Copes R., Lu H., Lakey P., Shiraiwa M., Chen H. A population-based cohort study of respiratory disease and long-term exposure to iron and copper in fine particulate air pollution and their combined impact on reactive oxygen species generation in human lungs. Environ. Sci. Technol. 2021;55(6):3807–3818. doi: 10.1021/acs.est.0c05931. [DOI] [PubMed] [Google Scholar]
- Zhou X., Josey K., Kamareddine L., Caine M.C., Liu T., Mickley L.J., Cooper M., Dominici F. Excess of COVID-19 cases and deaths due to fine particulate matter exposure during the 2020 wildfires in the United States. Sci. Adv. 2021;(33):7. doi: 10.1126/sciadv.abi8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.