Abstract
Objective
We analyzed predictors of SARS-CoV-2 infection and COVID-19 death among residents of long-term care facilities (LTCFs) in Sweden for the pandemic year 2020 and its different waves.
Methods
The study included 99% of Swedish LTCF residents (N = 82,488). Information on COVID-19 outcomes, sociodemographic factors, and comorbidities were obtained from Swedish registers. Fully adjusted Cox regression models were used to analyze predictors of COVID-19 infection and death.
Results
For the entirety of 2020, age, male sex, dementia, cardiovascular-, lung-, and kidney disease, hypertension, and diabetes mellitus were predictors of COVID-19 infection and death. During 2020 and the two waves, dementia remained the strongest predictor of COVID-19 outcomes, with the strongest effect on death being among those aged 65–75 years.
Conclusion
Dementia emerged as a consistent and potent predictor of COVID-19 death among Swedish residents of LTCFs in 2020. These results provide important information on predictors associated with negative COVID-19 outcomes.
Key Words: Dementia, COVID-19, Risk factors, Long-term care facilities, Longitudinal study
INTRODUCTION
Residents of long-term care facilities (LTCFs) have been severely affected by COVID-19,1 but factors associated with increased risk of infection and death among this population have remained understudied. Among individual-level factors, age, sex, impaired cognition, diabetes, and kidney disease have been associated with risk of COVID-19 outcomes.1, 2, 3 However, most previous studies have been confined to a short period or a limited sample, or both, thereby reducing the generalisability of their findings.
We aimed to fill these gaps by studying a host of individual-level predictors of SARS-CoV-2 infection and COVID-19 death using register data on almost all individuals who lived in Swedish LTCFs from March to December 2020. We analyze the first wave and the first part of the second wave separately. To account for age as a moderating factor, we also examined predictors of COVID-19 death in four age groups.
METHODS
Study Population
This Swedish study is based on data from the register of Interventions for the Elderly and Individuals with Disabilities, which comprised of 83,083 individuals with a government decision to live in LTCFs as of February 2020.4 We had access to information from registers for 82,488 of these individuals (99%), including 27,542 men and 54,946 women. A detailed description of the registers used is found in Additional file 1.
The Ethical Review Authority of Sweden approved the present study (dnr. 2020-06492 and dnr. 2021-01115).
COVID-19 Outcomes
COVID-19 death was determined using information from the Causes-of-Death Register (ICD10 codes U07.1 or U07.2).
SARS-CoV-2 infection was determined using information from the SmiNet database and the National Patient Register (NPR) (ICD10 code U07.1 or U07.2). Individuals with no information on infection in SmiNet or NPR but with COVID-19 as an underlying or contributing cause of death in the Causes-of-Death Register, were also categorized as having been infected.
Sociodemographic Factors and Comorbidities
Information on age (number of years at the end of 2020) was obtained from the register of Interventions for the Elderly and Individuals with Disabilities, educational attainment (compulsory or higher) was obtained from Statistics Sweden, and comorbidities from NPR and the Swedish Prescribed Drug Register (Additional file 2).
Statistical Analysis
Cox regressions were used to analyze individual-level factors in relation to SARS-CoV-2 infection and COVID-19 death, presented as hazard ratios (HRs) and 95% confidence intervals. The model included age, sex, education, comorbidities, as well as region, which was used as a proxy to adjust for the uneven spread of infection among the 21 regions of Sweden.4 Time at risk was calculated as time from March 1, 2020, until 1) date of COVID-19 infection and death, 2) date of death for non-COVID-19 deaths, 3) date for those who no longer had a decision to live in LTCFs, or 4) December 31, 2020, for COVID-19 free survivors.
We also performed separate analyzes for the pandemic's first wave (March 1–July 1, 2020), and the first part of the second wave (October 1–December 31, 2020).
Finally, we performed the survival analyzes in four different age groups (age 65–75, 76–85, 86–95, and 96–110 years).
RESULTS
The mean age of the 82,488 individuals who lived in Swedish LTCFs in February 2020 was 86 years (range 65–110 years). 49% had more than compulsory education, 46% had dementia, 72% had cardiovascular disease, 18% had lung disease, 7% had kidney disease, 80% had hypertension, and 20% had diabetes mellitus. Additional file 3 reports the descriptive statistics for multimorbidity.
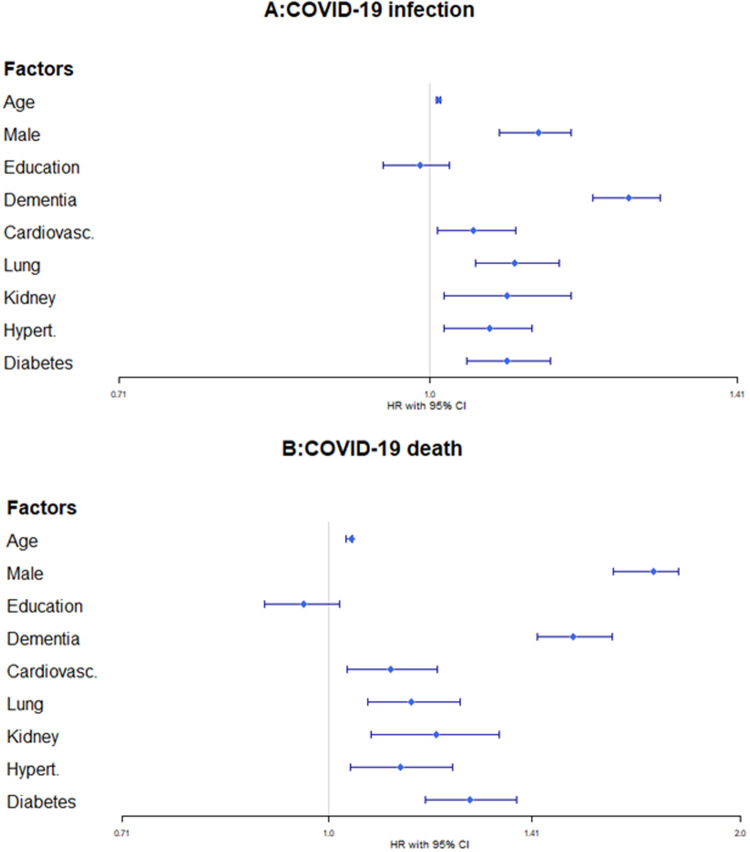
During a mean follow-up of 248 days (SD 97 days; 20,460,428 person-days), 11,595 residents were diagnosed with COVID-19 and 4,072 died from it. For the year of 2020, all individual-level factors, apart from education, were associated with an increased risk of infection and death (Fig. 1 ). Dementia and male sex emerged as the strongest predictors of infection (dementia: HR 1.25, 95% CI 1.20–1.29; male sex: HR 1.13, 95% CI 1.08–1.17) and death (dementia: HR 1.51, 95% CI 1.42–1.61; male sex: HR 1.73, 95% CI 1.62–1.80).
FIGURE 1.
Hazard ratios (HR) and 95% Confidence intervals (CI) of the relationship between individual-level factors (age, sex, education, dementia, cardiovascular disease, lung disease, kidney disease, hypertension, and diabetes) and risk of [A] SARS-CoV-2 infection (N=82,488, n events =11,595) and [B] COVID-19 death (N = 82,488, n events = 4,072) in a fully adjusted Cox regression model (using R's survival and survminer packages), including region of residence as a proxy for infection spread. The proportional hazard assumption was met for all analyzes.
Table 1 reports the results of the analyzes for two waves of the pandemic. Dementia emerged as the only consistent predictor of both infection (first wave: HR 1.09, 95% CI 1.04–1.15; second wave: HR 1.21, 95% CI 1.14–1.28) and death (first wave: HR 1.25, 95% CI 1.161–1.356; second wave: HR 1.56, 95% CI 1.399–1.741), and male sex as a consistent predictor of death in each of the waves (first wave: HR 1.33, 95% CI 1.23–1.44; second wave: HR 1.81, 95% CI 1.62–2.03).
TABLE 1.
Sociodemographic Factors and Comorbidities in Relation to SARS-CoV-2 Infection and COVID-19 Death Among Residents of Long-term Care Facilities During the First and Second Waves
| The First Wave |
||||
|---|---|---|---|---|
| A: SARS-CoV-2 Infection N = 19,691, n Cases = 6,230 |
B: COVID-19 Death N =16,486, n cases = 2,686 |
|||
| HR (CI) | p-Values | HR (CI) | p-Values | |
| Agea | 0.99 (0.987–0.993) | <0.001 | 1.00 (0.999–1.010) | 0.08 |
| Sex, male | 0.99 (0.939–1.047) | 0.8 | 1.33 (1.232–1.444) | <0.001 |
| Education, ≥compulsory | 0.95 (0.902–0.999) | 0.04 | 0.92 (0.853–0.998) | 0.04 |
| Dementia | 1.09 (1.036–1.146) | <0.001 | 1.25 (1.161–1.356) | <0.001 |
| Cardiovascular disease | 0.91 (0.856–0.966) | 0.002 | 0.92 (0.835–1.006) | 0.07 |
| Lung disease | 1.00 (0.937–1.065) | 0.9 | 0.97 (0.879–1.071) | 0.5 |
| Kidney disease | 0.94 (0.862–1.035) | 0.2 | 0.98 (0.863–1.117) | 0.8 |
| Hypertension | 1.05 (0.977–1.119) | 0.2 | 1.09 (0.979–1.211) | 0.1 |
| Diabetes mellitus | 1.01 (0.952–1.081) | 0.65 | 1.14 (1.035–1.248) | 0.007 |
|
The Second Wave |
||||
|
C: SARS-CoV-2 Infection |
D: COVID-19 Death |
|||
| Agea | 1.01 (1.008–1.015) | <0.001 | 1.04 (1.037–1.053) | <0.001 |
| Sex, male | 1.10 (1.034–1.166) | 0.002 | 1.81 (1.616–2.026) | <0.001 |
| Education, ≥compulsory | 1.02 (0.96–1.078) | 0.5 | 1.02 (0.915–1.139) | 0.7 |
| Dementia | 1.21 (1.14–1.279) | <0.001 | 1.56 (1.399–1.741) | <0.001 |
| Cardiovascular disease | 1.09 (1.017–1.159) | 0.01 | 1.25 (1.094–1.438) | 0.001 |
| Lung disease | 1.14 (1.063–1.222) | <0.001 | 1.36 (1.193–1.550) | <0.001 |
| Kidney disease | 1.01 (0.899–1.131) | 0.9 | 1.09 (0.891–1.338) | 0.4 |
| Hypertension | 1.05 (0.975–1.128) | 0.2 | 1.12 (0.963–1.303) | 0.1 |
| Diabetes mellitus | 1.08 (1.006–1.158) | 0.03 | 1.24 (1.085–1.416) | 0.002 |
Hazard ratios (HR), their 95% confidence intervals (CI) and associated p-values are estimated using fully adjusted Cox regression models (using R's survival and survminer packages), including region of residence as a proxy for infection spread. p-values are from z-statistics (z=coef/se(coef)). The proportional hazard assumption was met for all analyzes, i.e., analyzes of SARS-CoV-2 infection by factors during the first [A] and second [C] waves, and COVID-19 death by factors during the first [B] and second [D] waves.
Age is the number of years.
Additional file 4 reports results of the analyzes by age group. It shows that for those aged 65–75 years, dementia was the strongest predictor (HR 2.24, 95% CI 2.77–2.82), while it was male sex for the remaining age groups (76–85 years: HR 1.77, 95% CI 1.57–1.98; 86–95 years: HR 1.79, 95% CI 1.63–1.95; 96–110 years: HR 1.66, 95% CI 1.35–2.05).
Additional file 5 shows the results on individual-level factors in relation to risk of non-COVID-19 deaths.
DISCUSSION
In this study, using data on almost all individuals who lived in Swedish LTCFs in 2020, we found that age, male sex, dementia, cardiovascular-, lung-, and kidney disease, hypertension, and diabetes mellitus predicted COVID-19 infection and death. Dementia revealed itself as a consistent and potent predictor of COVID-19 infection and death, with the strongest effect on death among those aged 65–75 years. To the best of our knowledge, this is the first study on residents of nursing homes with such large sample.
Our findings that age, male sex, and all six comorbidities predicted COVID-19 infection and death are in line with results from previous studies.1, 2, 3 By contrast, a study of 80 LTCFs in Spain from the first wave did not find an effect from cardiovascular and lung diseases on COVID-19 death.5 One reason for the discrepant results could be differences in follow-up time (Spanish study: the first wave vs our study: the year 2020). Support for this comes from our finding that cardiovascular disease was associated with reduced risk COVID-19 outcomes in the first wave.
Furthermore, our finding that dementia was a particularly potent predictor of infection and death compared to other comorbidities is also in line with previous studies.1 , 3 , 6 A possible explanation for increased risk of infection among individuals with dementia could be their increased requirement for assistance with daily routines, resulting in greater exposure to infection from care personnel and/or relatives.7 In Sweden, visitation restrictions were removed on October 1, 2020, which, in part, could explain the higher predictive value of dementia on COVID-19 death in the second wave compared to the first. Also, the atypical clinical presentation of infection in people with dementia (e.g., delirium and worsening of the general condition), could explain the strong effect of dementia on COVID-19 death, since these symptoms may have hindered early detection and treatment.8
Interestingly, we found that dementia was the strongest predictor of death among those aged 65–75 years, which could be due to the characteristics of early onset dementia (e.g., faster progression in early compared to late onset).9 Contrarily, a UK Biobank study reported a higher prediction of COVID-19 death among those aged ≥80 years.6 A reasons for the divergent results could be that we used a population of LTCF residents examined for the whole year of 2020, whereas the UK Biobank study used a community-living cohort examined between March 16 and August 24, 2020.
The reported results should be addressed in light of the study's limitations. First, due to data limitations we did not control for structural-level factors related to LTCFs (e.g., facility size or personnel characteristics) that may have affected the results. Nevertheless, in a companion study with a more restricted sample, in which structural-level factors were controlled for, the results were similar to those presented here.4 Also, the inclusion of structural-level factors would better address the uneven distribution of the virus spread. However, given the lack of structural-level data on all of the individuals in the present study, we used region as a proxy for community spread, which has been found to be a good predictor of infection rates.1 , 4 , 7 Second, although Swedish health registers are reliable and specific in detecting cases of dementia, these sources underestimate the number of dementia cases.10 This could explain the rather low proportion of individuals with dementia in our study. Third, in studies including individuals at higher ages and examining diseases in late life, competing risk of death due to causes other than COVID-19 may have affected the results. However, the use of risk-time in Cox regressions partly adjusts for this. Also, we found that all predictors of COVID-19 death also predicted death due to other causes, which most likely attenuated the reported associations.
Based on a large sample of the residents of Swedish nursing homes, this study highlights the link between dementia and the increased risk of severe COVID-19 outcomes for older people in LTCFs in the prevaccine period of the pandemic and may inform decisions on effective risk management and clinical care during pandemics, as well as healthcare policy planning in the future.
AUTHOR CONTRIBUTIONS
Najar, Broms, Nistotskaya, & Dahlström: Concept and design; Najar, Broms: Acquisition and analysis of data; Najar, Broms, Nistotskaya, & Dahlström: Interpretation of data; Najar & Nistotskaya: Preparation of manuscript; Najar, Broms, Nistotskaya, & Dahlström: Final approval. Broms, Nistotskaya, & Dahlström: Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
DATA STATEMENT
The data has not been previously presented orally or by poster at scientific meetings.
DISCLOSURES
Najar, Broms, Nistotskaya, and Dahlström have received payment from the Swedish Government's Corona commission, for writing the report “Driftsform, personalsammansättning och storlek: Om strukturella faktorer och risk att smittas av och dö i COVID-19 vid särskilt boende för äldre i Sverige” (Broms et al.) during the period March 1, 2021, until September 3, 2021.
The work was supported by the Swedish Research Council for Health, Working Life and Welfare [grant number STYB-2018/0011].
The authors thank Adam Altmejd, Evelina Björkegren, Torsten Persson, and Olof Östergren för their indispensable support in the preparation and completion of the data, which was provided by the research program “Ett forskningsprogram om COVID-19 i Sverige: Smittspridning, bekämpning och effekter på individer och samhälle” at Stockholm University.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.jagp.2023.01.027.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.Mehta HB, Li S, Goodwin JS. Risk factors associated with SARS-CoV-2 infections, hospitalization, and mortality among US nursing home residents. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DS, Ma S, Chu A, et al. Predictors of mortality among long-term care residents with SARS-CoV-2 infection. J Am Geriatr Soc. 2021;69:3377–3388. doi: 10.1111/jgs.17425. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Jiao Y, Graham DJ, et al. Risk factors for COVID-19 deaths among elderly nursing home medicare beneficiaries in the prevaccine period. J Infect Dis. 2022;225:567–577. doi: 10.1093/infdis/jiab515. [DOI] [PubMed] [Google Scholar]
- 4.Broms R, Dahlström, C., Najar, J., et al: Driftsform, personalsammansättning och storlek: Om strukturella faktorer och risk att smittas av och dö i covid-19 vid särskilt boende för äldre i Sverige, 2021
- 5.Meis-Pinheiro U, Lopez-Segui F, Walsh S, et al. Clinical characteristics of COVID-19 in older adults. A retrospective study in long-term nursing homes in Catalonia. PLoS One. 2021;16 doi: 10.1371/journal.pone.0255141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahira AC, Verjovski-Almeida S, Ferreira ST. Dementia is an age-independent risk factor for severity and death in COVID-19 inpatients. Alzheimers Dement. 2021;17:1818–1831. doi: 10.1002/alz.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin AT, Jylhävä J, Religa D, et al. COVID-19 prevalence and mortality in longer-term care facilities. Eur J Epidemiol. 2022;37:227–234. doi: 10.1007/s10654-022-00861-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchetti A, Rozzini R, Guerini F, et al. Clinical presentation of COVID19 in dementia patients. J Nutr Health Aging. 2020;24:560–562. doi: 10.1007/s12603-020-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tábuas-Pereira M, Baldeiras I, Duro D, et al. Prognosis of early-onset vs. late-onset mild cognitive impairment: comparison of conversion rates and its predictors. Geriatrics (Basel) 2016;1 doi: 10.3390/geriatrics1020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzuto D, Feldman AL, Karlsson IK, et al. Detection of dementia cases in Two Swedish health registers: a validation study. J Alzheimers Dis. 2018;61:1301–1310. doi: 10.3233/JAD-170572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.