Abstract
Background
The effects of coronavirus disease 2019 (COVID-19) remain a global public health emergency because of the ensuing economic burden and death. With robust research into vaccines, antibody treatments, and antiviral drugs for COVID-19, there is still a dearth of evidence on the role of an individual’s nutritional status on the severity of COVID-19.
Objective
This study aimed to investigate the association between selenium (Se) and zinc (Zn) status and COVID-19 severity among individuals diagnosed with COVID-19 in North Carolina.
Methods
Subjects (n = 106) were recruited remotely as part of the Nutrition and COVID-19 in North Carolina (NC-NC) study and filled out online screening questionnaires and dietary surveys. Toenail samples from 97 participants were analyzed to determine Se and Zn concentrations. To assess the severity of severe acute respiratory coronavirus (SARS-CoV)-2 infection, subjects were asked about the presence and duration of 10 commonly reported symptoms. These responses were used to calculate a COVID-19 severity index (CSI). The relationship between Se and Zn status (intake and toenail concentrations) and CSI was explored using a regression analysis.
Results
Our results showed that the median (25th, 75th percentiles) dietary Se and Zn intake from selected food sources were 65.2 μg (43.2, 112.9) and 4.3 mg (1.8, 8), respectively. Headache, cough, loss of smell or taste, and fever were reported by at least half of the participants. In stepwise regression analysis, among individuals with low Se and Zn intake (below the median), Se intake was inversely associated with increasing CSI (β = −0.66; 95% CI: −1.21, −0.11; P = 0.02).
Conclusions
Findings from this study support a potential benefit of increasing the intake of dietary Se to mitigate the severity of SARS-CoV-2 infection.
Keywords: COVID-19, inflammation, cytokine storm, selenium status, zinc status, antioxidants, dietary components, dietary screener questionnaire, toenail mineral concentrations
Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory coronavirus (SARS-CoV)-2 remains one of the largest public health emergencies globally because of the ensuing economic burden and death since its discovery in 2019 [1]. Globally, as of October 21, 2022, >6 million deaths were recorded with >1 million deaths in the United States [2].
SARS-CoV-2 infection leads to an unregulated innate immune response resulting in a hyperinflammatory state/cytokine storm [3]. For instance, higher plasma levels of pro-inflammatory cytokines were observed in patients with COVID-19 admitted to the intensive care unit than those in patients with COVID-19 not admitted to the intensive care unit [4]. This inflammatory state is linked to multiorgan damage in the kidneys, lungs, brain, and skin in severe SARS-CoV-2 infection [1]. Patients infected with the SARS-CoV-2 virus can also present mild symptoms of fever, diarrhea, changes in smell and/or taste, cough, or shortness of breath [1]. In light of this, current therapies such as corticosteroids and interleukin-6 antagonists have focused on mitigating/modulating the underlying unregulated immune response among individuals infected with SARS-CoV-2 [3].
There is increasing evidence of a link between nutritional status and risk and progression of COVID-19 through the immune function [5]. A poor eating pattern or unbalanced diet can lead to a chronic low-grade inflammatory state, and antioxidants such as selenium (Se) and zinc (Zn) may reduce the cytokine storm seen with COVID-19 [5,6]. In support of these findings, a study by Razeghi Jahromi et al. [7] showed that high Se and Zn concentration were associated with low serum C-reactive protein levels among patients with COVID-19. Se deficiency may increase the progression and severity of COVID-19 [8,9]. However, the evidence to support the role of Zn deficiency in COVID-19 is somewhat conflicting. For example, among individuals at different levels of SARS-CoV-2 infection severity (determined by respiratory distress, pneumonia, ventilator use, or death), 67% of the patients showed Se deficiency without Zn deficiency [10]. With robust research into therapeutics such as vaccines, antibody treatments, antiviral drugs [11], there is still a dearth of evidence highlighting the implications for dietary intake and nutritional status in mitigating the pro-inflammatory and oxidative stress states in COVID-19. This study aimed to investigate the association between Se and Zn intake and status and COVID-19 severity among individuals diagnosed with COVID-19 in North Carolina.
Methods
Study participants and recruitment
In November 2020, subjects were recruited remotely through mass e-mails, social media, COVID-19 testing sites, such as local pharmacies, and directly through health professionals at Moses Cone Hospital, as part of the Nutrition and COVID-19 in North Carolina (NC-NC) study. Potential subjects filled out an online screening questionnaire that included a copy of the informed consent form to read and electronically sign if they agreed to participate in the study. The informed consent contained information on the study and benefits and risks of participating in the study. The screening form was an 86-item questionnaire to determine demographics, general health history, and COVID-19 severity. Subjects were eligible for the study if they were 18 years and older, not pregnant or lactating, a resident of North Carolina, and diagnosed with COVID-19 in the last month. Subjects who qualified based on the inclusion criteria were invited through an e-mail to participate in the study by uploading a copy of a positive diagnosis with COVID-19, followed by a brief online dietary assessment. All study participants were unvaccinated for COVID-19 at the time of data collection. All surveys were developed using Research Electronic Data Capture (REDCap), an electronic data collection tool [12].
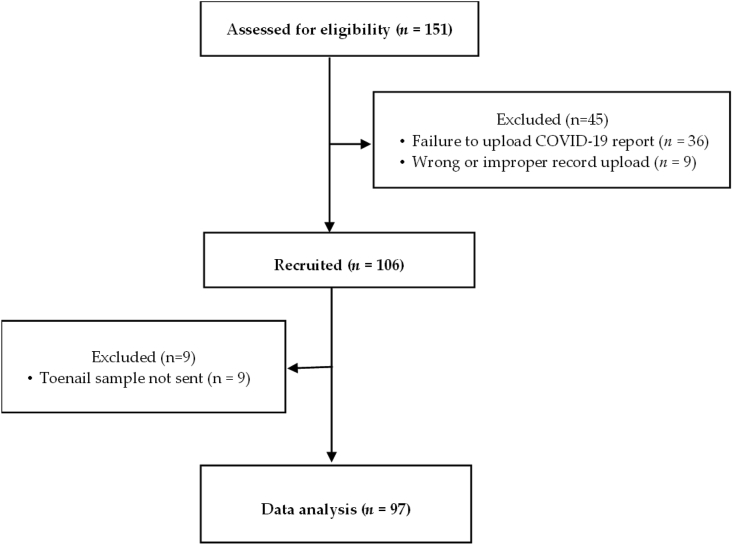
It was estimated that 103 subjects would be needed to determine a significant association between COVID-19 severity and Se and Zn intake and status in a regression model (assuming an R2 = 0.5 for a regression model with up to 7 predictors, at an α level of 0.05, with a statistical power of 0.8). To meet this sample size, we recruited 106 subjects. Details on subject recruitment are shown in Figure 1. Of the 106 subjects recruited, complete data from 97 subjects were analyzed. The protocol for this study was approved by the institutional review board of the University of North Carolina Greensboro (IRB# 21-0044) and Moses Cone Hospital, Greensboro, NC (IRB# 1654979-2).
FIGURE 1.
Study recruitment.
Study design
This study was a cross-sectional study conducted remotely to obtain information on COVID-19 severity and dietary intake data and to collect toenail samples as a biomarker of Se and Zn status from subjects. There were 5 remote contacts with participants based on continued eligibility: 1) an invitation to participate in the study and complete screening questionnaire; 2) an e-mail to upload a copy of COVID-19 diagnosis test result; 3) an e-mail to complete dietary questionnaire; 4) a toenail collection kit was mailed to the participants, and toenail samples were mailed back to our laboratory; and 5) an e-mail with electronic gift cards. Instructions for completing all records were included in the online questionnaires.
On completing all questionnaires, participants were mailed a toenail collection kit containing a stainless-steel clipper, instruction sheet with toenail collection guide (to collect at least 50 mg of sample), plastic specimen bags, and a return envelope. Toenail samples were analyzed to determine Se and Zn concentrations.
Assessment of COVID-19 status
To assess the severity of COVID-19 (SARS-CoV-2 infection), in the screening questionnaire, subjects were asked about date of diagnosis, hospitalization, ventilator use if hospitalized, and the presence and duration of 10 commonly reported COVID-19 symptoms (headache, fever, diarrhea, shortness of breath, loss of taste and/or smell, vision issues, toe and finger problems, hemoptysis, body ache, and cough) [4,13,14]. Symptom duration was categorized as follows: never, 1–3 days, 4–7 days, 8–13 days, and 14 days or more. All participants who had been diagnosed for less than 2 weeks before filling out the screening questionnaire were sent a link to a COVID-19 follow-up form to provide information on any change in the symptoms reported in the initial screening questionnaire.
Selenium and zinc and dietary intake
To estimate Se and Zn intake before diagnosis with COVID-19, eligible subjects were required to fill an online semiquantitative food frequency questionnaire, which obtained information on how often they consumed high Se and Zn source foods (≥20% of the daily value) and the typical amount they consumed in the month before COVID-19 diagnosis. Se sources included seafoods, beef, turkey, egg, ham, halibut, and chicken, whereas Zn sources included breakfast cereals, pumpkin seeds, pasta, bread, brown rice, oatmeal and grains and its sources, and cheese.
Subjects were also asked to fill out a 26-item dietary screener questionnaire (DSQ) developed by the National Cancer Institute. The DSQ asks about the frequency of consumption of selected foods and drinks in the past month. The DSQ captures daily intake of fruits and vegetables, dairy, calcium, added sugars, whole grains, fiber, and frequency of consumption of red meat and processed meat. Each of the 26 items on the screener is based on its relationship to 1 or more dietary factors of interest in the Dietary Guidelines for Americans [15].
Sample analysis
Toenail samples from all toes were collected for the measurement of Se and Zn concentrations. The samples (approximately equal parts of big and other toes) were weighed and washed twice each with acetone and rinsed with deionized water to remove any remaining nail polish and unwanted particles. The samples were digested with 1 mL of concentrated trace metal grade nitric acid (65%–70% by weight) and 1.5 mL of hydrogen peroxide (30% by volume) in a hot block at approximately 110°C until a clear solution was obtained. Then, the samples were diluted with deionized water to 10 mL. Next, 4 mL and 0.5 mL of the previous solutions were further diluted to 10 mL with deionized water for Se and Zn determination, respectively. The digested toenail samples were taken to the Department of Chemistry at Wake Forest University for analysis using a tandem ICP-MS/MS instrument (Agilent Technologies). Se and Zn were determined at the mass-to-charge ratios (m/z) 78 and 66, respectively, using single quadrupole mode (Q2). Hydrogen gas flowing at 4 mL/min in the octopole collision/reaction cell was adopted to minimize spectral interferences in Se determination. No octopole collision/reaction cell strategy was required for Zn determination. An addition and recovery experiment was performed to ensure the accuracy of the inductively coupled plasma mass spectrometry method, which provided recoveries of 105% and 98.2% for Se and Zn, respectively. The external standard calibration method was adopted in all determinations, which was performed using standard reference solutions prepared in 1% vol:vol HNO3 from adequate dilutions of 10 mg/L stock solutions of Se and Zn (SPEX CertPrep).
Statistical analysis
The primary outcome variable, COVID-19 severity index (CSI), was calculated from the duration of the 10 reported symptoms. Mean scores of 2, 5.5, 11, and 21 were allocated for 1–3 days, 4–7 days, 8–13 days, and 14 days or more, respectively, for duration of each reported symptom. The CSI score for each subject was the sum of the mean scores for the different symptoms they reported. Hence, this score ranged from 0 (no symptoms) to 210 (10 symptoms for 14 days or more). The daily intakes of Se and Zn from the semiquantitative food frequency questionnaire were calculated from the product of the reported frequencies converted to frequency per day (never/less than monthly = 0/day; 1–3×/month = 0.067/day; 1–3×/week = 0.286/day; 4–6×/week = 0.714/day; daily = 1/day), the amount of Se or Zn in a serving of food from the United States Department of Agriculture FoodData Central [16], and the individual servings consumed.
To minimize within-cluster variances, ward hierarchical clustering was used to group similar data points into 2 groups by separating individuals with similar observations from those that were dissimilar into different clusters (healthy compared with unhealthy dietary patterns). Clustering was performed as part of a subanalysis, i.e., solely among individuals with low Se and Zn intake (less than or equal to the median) using food group data from the DSQ using whole grains, fruits, vegetables, and sugar-sweetened beverages as clustering variables.
The concentrations of Se and Zn in toenail samples were adjusted for average sample weights (before and after drying). Means and standard errors were reported for continuous variables with normal distributions; median and interquartile ranges for Se and Zn intake; and percentages for categorical variables. A stepwise linear regression analysis using the “MASS” package and the AIC method (it provides a means for linear model selection) to yield a best-fit model were used to determine the associations among CSI, dietary patterns obtained from the cluster analysis, and Se and Zn intake and their toenail concentrations, adjusting for the potential confounders, namely, age, sex, and ethnicity. The statistical significance was set at P ≤ 0.05. The R software for statistical computing was used for data analysis [17].
Results
Our results (Table 1) showed that participants were predominantly (78%) a young population (aged younger than 30 years), and most (57%) of them were Non-Hispanic Whites, followed by Non-Hispanic Blacks (21%). In addition, most participants were women (71%) who had never smoked (76%). Individuals with normal weight were approximately half of the sample; 87% reported no medical condition; and slightly over half of the participants reported some college education without a degree.
TABLE 1.
Demographic characteristics of participants diagnosed with COVID-19.
| Demographic data | N = 106 |
|---|---|
| Age (y) | 26.8 ± 1 |
| Age group (y) | |
| Young adults (<30) | 83 (78) |
| Middle adults (30–49) | 16 (15) |
| Older adults (>49) | 7 (7) |
| BMI (kg/m2) | |
| Underweight | 4 (4) |
| Normal | 49 (46) |
| Overweight | 27 (25) |
| Obese | 26 (25) |
| Sex | |
| Female | 75 (71) |
| Male | 31 (29) |
| Ethnicity | |
| Non-Hispanic Black | 22 (21) |
| Non-Hispanic White | 61 (57) |
| Hispanic | 7 (7) |
| Others1 | 16 (15) |
| Chronic disease | |
| No | 92 (87) |
| Yes | 14 (13) |
| Smoking status | |
| Never | 81 (76) |
| Former smoker | 14 (13) |
| Current smoker | 11 (11) |
| Educational status | |
| Less than or high school | 16 (15) |
| Some college but no degree | 53 (50) |
| Bachelor’s or graduate degree | 37 (35) |
| Marital status | |
| Married | 17 (16) |
| Single | 85 (80) |
| Divorced or separated | 4 (4) |
| Income ($) | |
| 1–49,999 | 88 (83) |
| 50,000–99,999 | 13 (12) |
| $100,000 and above | 5 (5) |
Values are given as n (%) or means ± SEM.
BMI, body mass index.
Others include multiracial, Asian, and Middle Eastern.
From Table 2, the mean CSI was 29.4 ± 2.3, whereas the median dietary Se and Zn intake from high food sources were 65.2 μg (43.2, 112.9 μg) and 4.3 mg (1.8, 8 mg), respectively. Participants consumed approximately 2 cups/day of total fruits and vegetables (2.3 ± 0.08 cups equivalents/day). Added sugars from sugar-sweetened beverages (7.2 ± 0.7 tsp) was almost half of the total added sugar intake (16.2 ± 0.8 tsp) consumed per day.
TABLE 2.
Dietary intake and toenail Se and Zn concentrations of participants diagnosed with COVID-19.
| Biochemical and dietary intake data | N = 106 |
|---|---|
| CSI | 29.4 ± 2.3 |
| Se intake (μg/day) | 65.2 (43.2, 112.9) |
| Zn intake (mg/day) | 4.3 (1.8, 8) |
| Calcium intake (mg/day) | 923 ± 20 |
| Fiber intake (g/day) | 16.4 ± 0.5 |
| Whole grain (oz equivalents/day) | 0.94 ± 0.09 |
| Dairy intake (cup equivalents/day) | 1.5 ± 0.04 |
| Fruit intake (cup equivalents/day) | 0.95 ± 0.09 |
| Fruit and vegetables (cup equivalents/day) | 2.3 ± 0.08 |
| Vegetables (cup equivalents/day) | 1.4 ± 0.04 |
| Added sugar (tsp equivalents/day) | 16.2 ± 0.8 |
| Added sugars from sugar-sweetened beverages (tsp equivalents/day) | 7.2 ± 0.7 |
| Red meat (day) | 0.24 ± 0.02 |
| Processed meat (day) | 0.19 ± 0.02 |
| Toenail Se concentration (μg/g)1 | 0.81 (0.73, 0.93) |
| Toenail Zn concentration (μg/g)1 | 115.9 (100.2, 137.1) |
Values are given as means ± SEM or median (25th, 75th percentile).
CSI, COVID-19 severity index.
n = 97.
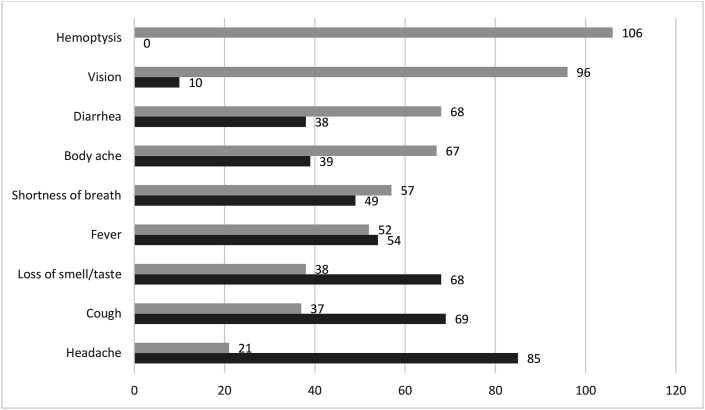
Four symptoms were reported by at least half of the participants (Figure 2). These symptoms were headache, cough, loss of smell and/or taste, and fever. Most participants (80%) reported having a headache during diagnosis with COVID-19, whereas hemoptysis (coughing up blood) was not reported by any participant (Figure 2).
FIGURE 2.
Number of participants who reported COVID-19 symptoms.
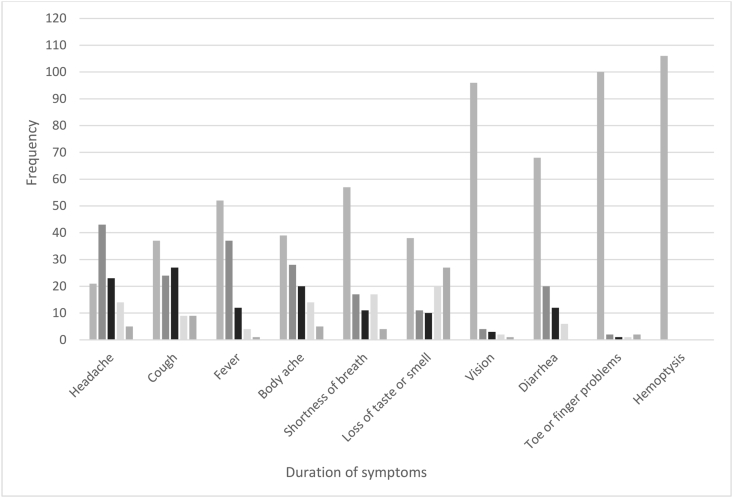
Most study participants reported that they had no symptom or symptoms lasted for 1–3 days (Figure 3). Among those who reported headaches, ∼43% reported symptoms lasted for 1–3 days. For symptoms that lasted the longest, among participants who reported a loss of smell and/or taste, 27% reported symptoms lasted for 14 days or more, followed by cough (9%), headache (5%), body ache (5%), and shortness of breath (4%).
FIGURE 3.
Duration of symptoms reported by participants.
Age, sex, and ethnicity were adjusted for in regression analysis predicting CSI (Table 3). Age was positively associated with CSI, whereas sex was inversely associated with CSI among men compared with that in women. In model 1, when ethnicity and age were included as confounders for all participants, Se status (intake and concentration) was not significant. This was similar to the results from a stepwise regression analysis performed on all participants (model 2). However, in model 4, when a subanalysis was performed among individuals with Se and Zn intake below the median, Se intake was inversely associated with CSI (β: −0.66; 95% CI: − 1.21, −0.11; P = 0.02).
TABLE 3.
Association among CSI, Se and Zn Intake, and toenail concentrations among individuals with low Se and Zn intake (less than or equal to the median).
| Predictors | Model 1 (n = 97) |
Model 2 (n = 97) |
Model 3 (n = 34) |
Model 4 (n = 34) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimates | CI | P | Estimates | CI | P | Estimates | CI | P | Estimates | CI | P | |
| (Intercept) | 14.64 | −16.61 to 45.88 | 0.354 | 16.15 | 2.76–29.53 | 0.019∗ | 34.71 | −43.14 to 112.55 | 0.367 | 59.14 | 34.55–83.73 | <0.001∗ |
| Age (y) | 0.61 | 0.13–1.09 | 0.013∗ | 0.61 | 0.16–1.06 | 0.008∗ | 0.69 | −0.47 to 1.85 | 0.229 | |||
| Sex | ||||||||||||
| Female | RG | RG | RG | RG | ||||||||
| Male | −10.43 | −21.42 to 0.55 | 0.062∗ | −11.76 | −22.00 to −1.53 | 0.025∗ | −9.54 | −31.33 to 12.26 | 0.376 | |||
| Ethnicity | ||||||||||||
| Non-Hispanic White | RG | RG | RG | RG | ||||||||
| Hispanic | 16.09 | −6.46 to 38.64 | 0.160 | 49.13 | −5.88 to 104.15 | 0.078 | ||||||
| Non-Hispanic Black | 0.20 | −13.13 to 13.52 | 0.977 | 5.66 | −27.06 to 38.37 | 0.724 | ||||||
| Others1 | 1.71 | −12.51 to 15.94 | 0.811 | 2.31 | −23.88 to 28.50 | 0.857 | ||||||
| Zn intake (mg) | 0.01 | −0.53 to 0.55 | 0.974 | 1.13 | −8.37 to 10.63 | 0.808 | ||||||
| Se intake (μg) | −0.02 | −0.07 to 0.03 | 0.448 | −0.53 | −1.31 to 0.26 | 0.182 | −0.66 | −1.21 to −0.11 | 0.021∗ | |||
| Zn concentration (μg/g) | 0.03 | −0.10 to 0.16 | 0.619 | 0.01 | −0.46 to 0.48 | 0.955 | ||||||
| Se concentration (μg/g) | −2.72 | −28.63 to 23.20 | 0.836 | −4.24 | −79.76 to 71.29 | 0.909 | ||||||
| R2 | 0.145 | 0.116 | 0.327 | 0.156 | ||||||||
P values are based on results from the multiple regression analysis (p ≤ 0.05∗). 1 Others include multiracial, Asian, and Middle Eastern. Model 1: adjusted for age, sex, ethnicity, Zn intake, Se intake, Zn concentration, and Se concentration; model 2: adjusted for age, sex, ethnicity, Zn intake, Se intake, Zn concentration, and Se concentration in a stepwise regression analysis; model 3: adjusted for age, sex, ethnicity, Zn intake, Se intake, Zn concentration, and Se concentration among individuals with low Se and Zn intake (below the median); and model 4: adjusted for age, sex, ethnicity, Zn intake, Se intake, Zn concentration, and Se concentration in stepwise regression analysis among individuals with low Se and Zn intake (below the median).
RG, reference group.
In a subanalysis limited to individuals with low Se and Zn intake (less than or equal to the median), cluster analysis (whole grains, fruits, and vegetables, and added sugars as clustering variables) revealed that individuals in the healthy dietary pattern group consumed significantly higher calcium (934 and 853 mg), Se (55.8 and 28.8 μg), and Zn (2.4 and 1.3 mg), higher total added sugars (19.5 and 13.3 tsp), and added sugars from sugar-sweetened beverages (9.9 and 4.9 tsp daily) compared to those in the unhealthy dietary pattern group (Table 4), respectively.
TABLE 4.
Cluster analysis comparing dietary intake and toenail concentrations of Se and Zn between healthy and unhealthy dietary patterns among individuals with low Se and Zn (less than or equal to the median).1
| CSI and food group intake | Group 1 (n = 22), unhealthy dietary pattern | Group 2 (n = 14), healthy dietary pattern | P |
|---|---|---|---|
| Se intake (μg)2 | 28.8 | 55.8 | <0.001∗ |
| Zn intake (mg)2 | 1.3 | 2.4 | 0.007∗ |
| Whole grain (oz equivalents/day)2 | 0.7 | 1.3 | 0.1 |
| Fruit and vegetables (cup equivalents/day)2 | 2.3 | 2.3 | 0.4 |
| Added sugars (tsp equivalents/day)2 | 13.3 | 19.5 | 0.003∗ |
| CSI | 35.6 | 21.3 | 0.18 |
| Dairy intake (cups equivalents/day) | 1.3 | 1.5 | 0.02∗ |
| Se concentration (μg/g) | 0.8 | 0.9 | 0.03∗ |
| Zn concentration (μg/g) | 116.3 | 117.8 | 0.88 |
| Fiber (g/day) | 16 | 15.7 | 0.33 |
| Fruit intake (cup equivalents/day) | 0.82 | 0.89 | 0.12 |
| Vegetables (cup equivalents/day) | 1.4 | 1.5 | 0.4 |
| Added sugars from sugar-sweetened beverages (tsp equivalents/day) | 4.9 | 9.9 | 0.03∗ |
| Calcium (mg/day) | 853 | 934 | 0.04∗ |
| Red meat frequency (day) | 0.1 | 0.2 | 0.2 |
| Processed meat frequency (day) | 0.1 | 0.2 | 0.21 |
CSI, COVID-19 severity index. p≤0.05∗
Values represent mean CSI and dietary intake.
Variables used in the cluster analysis.
Next, age and sex were adjusted for in a regression analysis predicting CSI (Table 5). In model 1, a healthy dietary pattern was not significantly correlated with CSI (β: −12.91; 95% CI: 27.92–2.09; P = 0.09). However, in model 2, an inverse association that trended toward significance was observed in the stepwise regression analysis between a healthy dietary pattern and CSI (using 6 symptoms common to the participants) (β:−13.88; 95% CI: −28.46 to 0.70; P = 0.06) among individuals with Se and Zn intake at or below the median.
TABLE 5.
Association between CSI and dietary patterns among individuals with low Se and Zn intake (less than or equal to the median)
| Predictors | Model 1 (n = 36) |
Model 2 (n = 36) |
||||
|---|---|---|---|---|---|---|
| Estimates | CI | P | Estimates | CI | P | |
| (Intercept) | 23.86 | −2.95 to 50.67 | 0.079 | 31.71 | 22.30–41.13 | <0.001∗ |
| Dietary pattern | ||||||
| Unhealthy dietary pattern | RG | RG | ||||
| Healthy dietary pattern | −12.91 | −27.92 to 2.09 | 0.089 | −13.88 | −28.46 to 0.70 | 0.061 |
| Sex | ||||||
| Female | RG | RG | ||||
| Male | −5.77 | −23.52 to 11.97 | 0.512 | |||
| Age | 0.32 | −0.58 to 1.23 | 0.472 | |||
| R2 | 0.124 | 0.099 | ||||
P values are based on results from the multiple regression analysis (p≤0.05∗). Model 1: adjusted for dietary pattern (healthy and unhealthy), sex, and age; model 2: adjusted for dietary pattern (healthy and unhealthy), sex, and age in a stepwise regression analysis among individuals with Se and Zn intake at or below the median.
RG, reference group.
Discussion
COVID-19 has resulted in >6 million deaths globally as of September 2022 [18]. Research shows that a cytokine storm plays a key role in COVID-19 pathology [4,19]. The cytokine storm results in oxidative stress and hyperinflammation, leading to tissue fibrosis, pneumonia, and lung injury [20]. Consequently, COVID-19 can manifest a range of asymptomatic to fatal forms with variations in symptom duration [21,22]. In this study of predominantly young adults, most participants were asymptomatic or reported mild symptoms (1–3 days), as shown by a mean CSI of 29.4 (range: 0–210). Participants commonly reported headaches (85%), followed by cough (69%), loss of smell and/or taste (68%), fever (54%), and shortness of breath (49%). Two other studies [23,24] have reported fever followed by cough or fatigue as the most prevalent COVID-19 symptoms. Other studies [24,25] also reported other COVID-19 symptoms such as loss of taste, sore throat, and body ache. In this study, approximately one-third of individuals who reported a loss of smell and/or taste mentioned that symptoms continued for 14 days or more. This is similar to findings where anosmia (loss of smell), a prevalent symptom of long COVID, may persist for 28 days [21] or beyond 12 weeks [25].
Studies have proposed a link between nutritional deficiencies of vitamin C, D, and E, Se, and Zn to potentiate the severity of COVID-19 [26]. In this study, we found that among individuals with COVID-19 with a low Se and Zn intake (below the median), Se intake was inversely associated with CSI. Although this finding is supported by results from other studies, [7,[27], [28], [29]] which supports a link between Se deficiency and COVID-19 severity, samples of most of these studies were older adults, which makes this study of predominantly adults younger than 30 years unique. It is established in research that low levels of serum Se may inhibit Se incorporation into selenoproteins, glutathione peroxidase, and selenoprotein P [27]. Potential mechanisms through which Se via selenoproteins act may be as follows: through T-cell proliferation through the innate immune response, mitigating the pro-inflammatory response by interleukin-6, and increased DNA synthesis [30]. Low Se intake coupled with low levels of selenoprotein mRNAs caused by SARS-CoV-2 may also synergistically reduce selenoproteins, leading to elevated infection severity [31]. In addition, redox-active metabolites of dietary selenium beyond that needed for selenoprotein biosynthesis may also play a key role in minimizing COVID-19 severity [30]. The association between Se and viral infections are observed among Se-deficient individuals [27,28]. This may be the reason we did not observe an association between selenium intake and CSI among all participants because the recommended dietary allowance of 55 μg [32] was exceeded in our study with a median Se intake of 65 μg. Se intake at 55 and 105 μg/day corresponds to maximal glutathione peroxidase 1 and selenoprotein P activity, respectively [30]. Similar to our study findings, Zhang et al. [30] showed that suboptimal intake below a mean Se intake of 65 μg/day may increase COVID-19 severity.
Toenail Se may be a key surrogate marker or predictor of selenium exposure [[33], [34], [35]]. However, toenail selenium is a long-term marker (at least 3 months) of an individual’s micronutrient status compared with Se intake, which represents a short-term or recent micronutrient status [36]. In this study, although we retroactively asked about COVID-19 diagnosis, the severity index was ascertained from individuals diagnosed with COVID-19 at 1–2 months before study enrollment, which may have accounted for the lack of association between toenail micronutrient status and CSI. Moreover, because this was a retrospective study, toenail status may be confounded by use of Se stores during COVID-19 illness of the participants, that is, toenail Se status may potentially reflect elevated body Se usage during SARS-CoV-2 infection. These may in part be the reason we observed no association between dietary Se intake (excludes supplement intake) and toenail Se concentration (R = −0.09; P = 0.4).
Nutrients such as Zn may also act against viral pathogenesis and a hyper-nflammatory state [20]. Zn functions as part of the innate immune system, and in SARS-CoV-2 infection, Zn stabilizes the host cell membrane preventing viral entry by inhibiting RNA synthesis and proteolytic processing [37,38]. Another supporting mechanism of action of Zn is that ongoing inflammatory processes coupled with suboptimal Zn intake leads to a decrease in its redistribution and a consequent heightened susceptibility to infection [39]. However, the effect of Zn on infections or diseases remains inconclusive [40]. Hypozincemia may increase risk of severe SARS-CoV-2 infection [41], and some studies show that Zn supplementation may be effective in decreasing the severity and progression of COVID-19 symptoms [42,43]. For instance, in a study by Gordon and Hardigan [44], oral Zn supplementation (10, 25, or 50 mg) in the study group resulted in 7 times decreased odds of developing symptomatic COVID-19 compared with the control group of COVID-19 symptomatic individuals receiving no supplementation. On the contrary, Sobczyk and Gaunt [45] reported contrasting findings where genetically predicted zinc concentration had no effect on SARS-CoV-2 infection, its severity, and hospitalization because of COVID-19. Notably, in these studies assessing micronutrient status among individuals with COVID-19, plasma concentrations in lieu of toenail concentrations were used. Although this may muddy the mode of comparison between the studies, toenail trace metal concentrations have been shown to adequately reflect a long-term nutrient status [36]. In this study, among individuals with toenail Se and Zn concentration below the median, we observed an inverse association between Zn intake and CSI. However, this association only trended toward significance (P = 0.06; results not shown). The reason we may not have observed a significant inverse association between Zn intake and COVID-19 severity may have been because study participants were not Zn deficient. Although the mean Zn intake (4.3 mg) noted in this study was well below the recommended dietary allowance for Zn, toenail Zn concentration was 116 μg/g, similar to the findings by Golabi et al. [43] of plasma zinc concentration of 114 μg/dL among outpatients with noninfected COVID-19 (asymptomatic, recovering from COVID-19, or reporting negative reverse transcription-polymerase chain reaction test). It has been elucidated that SARS-COV-2 is able to use Zn for their own functions or to modify the function of angiotensin-converting enzyme 2 receptors, which may decrease the serum levels of Zn among individuals with COVID-19 [46,47].
Aside micronutrients, other anti-inflammatory dietary approaches have been elucidated in COVID-19 progression and severity. Plant foods such as fruits and vegetables may confer antiviral benefits and as such, reduce risk and severity of COVID infection because they prevent viral replication, enhance antibody production against influenza virus, and improve T-cell function [40,48]. Based on the 2020–2025 Dietary Guidelines for Americans, adults consuming a 2000-kcal diet are recommended to consume 2 cups/day of fruits and 2.5 cups/day of vegetables [49], which are higher than the mean fruit (1 cup/day) and vegetable (1.4 cups/day) intake observed in this study. Although fruit and vegetable intakes were low among all participants, no significant differences were observed in fruit and vegetable intake between the groups characterized as healthy and unhealthy in the cluster analysis using food components from the DSQ (results not shown). However, in subanalysis, among individuals with low Se and Zn intake (less than or equal to the median), fruit intake was lower (1 and 0.8 cup/day, respectively; P = 0.04; results not shown) compared to individuals with Se and Zn intake above the median. In addition, among participants with Se and Zn intake at or below the median, a healthy dietary pattern was characterized by higher dairy, Ca, Se, and Zn intake than an unhealthy dietary pattern, while a trend toward an inverse association was observed between a healthy dietary pattern and CSI.
A strength of this study is the sample population. It was predominantly made up of younger adults (mean age of 27 years), with no underlying chronic condition, thereby adding to the existing literature on the nutritional status of individuals with COVID-19 living with no existing comorbidities. Studies supporting similar findings from our study sample older adults, who, owing to existing comorbidities, may have a faster progression and increased severity of COVID-19. Moreover, several studies use serum or plasma concentrations of Se and Zn in assessing COVID-19 severity, making our study one of the few studies that assessed toenail concentrations of these minerals as biomarkers of nutrient status. This is crucial because toenail concentrations of trace metals may reflect previous exposure of 3–12 months compared with blood concentrations of trace elements, which may be tightly regulated [36]. Hence, there is a need for large prospective observational studies to further evaluate the association between toenail micronutrient status (at multiple time points) and COVID-19 severity. Limitations of our study include an inability to draw a cause-and-effect relationship between dietary Se and COVID-19 and minimal information on COVID-19 variants during data collection.
There is evidence to support a higher COVID-19 cure and lower mortality rate among selenium-deficient individuals than those among selenium-adequate individuals, when supplemented with Se [30,50,51]. Hence, although Se-adequate individuals may not benefit from additional selenium intake, mainly owing to the narrow range from beneficial to adverse outcomes, findings from this study support a potential benefit from improved selenium intake among individuals consuming a low-selenium diet to mitigate COVID-19 severity.
Funding
This research was supported by the North Carolina Policy Collaboratory at the University of North Carolina at Chapel Hill with funding from the North Carolina Coronavirus Relief Fund established and appropriated by the North Carolina General Assembly and in part by the National Science Foundation’s Major Research Instrumentation Program (NSF MRI, grant CHE-1531698).
Author disclosures
All authors report no conflicts of interest.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Seth Armah reports financial support was provided by North Carolina Policy Collaboratory.
Acknowledgments
The authors’ responsibilities were as follows—DYL, MTP, SMA: designed the research; DYL, MTP, SMA, GLD: conducted the research; DYL, MTP, SMA: analyzed the data; DYL: wrote the paper; DYL, MTP, SMA, GLD: had primary responsibility for the final content; and all authors: read and approved the final version of the manuscript.
Data Availability
The data described in the manuscript, code book, and analytic code will be made available on request pending application and approval.
References
- 1.CDC, COVID-19 and your health [Internet] Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html 2020 [cited 2022 Jan 13]. Available from:
- 2.CDC, COVID data tracker [Internet]. Centers for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker 2020 [cited 2021 Jul 2]. Available from:
- 3.Gustine J.N., Jones D. Immunopathology of hyperinflammation in COVID-19. Am. J. Pathol. 2021;191:4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James P.T., Ali Z., Armitage A.E., Bonell A., Cerami C., Drakesmith H., et al. The role of nutrition in COVID-19 susceptibility and severity of disease: a systematic review. J. Nutr. 2021;151:1854–1878. doi: 10.1093/jn/nxab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Araújo Morais A.H., de Souza Aquino J., da Silva-Maia J.K., de Lima Vale S.H., Maciel B.L.L., Passos T.S. Nutritional status, diet and viral respiratory infections: perspectives for severe acute respiratory syndrome coronavirus 2. Br. J. Nutr. 2021;125:851–862. doi: 10.1017/S0007114520003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Razeghi Jahromi S., Moradi Tabriz H., Togha M., Ariyanfar S., Ghorbani Z., Naeeni S., et al. The correlation between serum selenium, zinc, and COVID-19 severity: an observational study. BMC Infect. Dis. 2021;21:899. doi: 10.1186/s12879-021-06617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fakhrolmobasheri M., Mazaheri-Tehrani S., Kieliszek M., Zeinalian M., Abbasi M., Karimi F., et al. COVID-19 and selenium deficiency: a systematic review. Biol. Trace Elem. Res. 2022;200:3945–3946. doi: 10.1007/s12011-021-02997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khatiwada S., Subedi A. A mechanistic link between selenium and coronavirus disease 2019 (COVID-19) Curr. Nutr. Rep. 2021;10:125–136. doi: 10.1007/s13668-021-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Im J.H., Je Y.S., Baek J., Chung M.-H., Kwon H.Y., Lee J.-S. Nutritional status of patients with COVID-19. Int. J. Infect. Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majumder J., Minko T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 2021;23:14. doi: 10.1208/s12248-020-00532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patridge E.F., Bardyn T.P. Research electronic data capture (REDCap) J. Med. Libr. Assoc. 2018;106:142–144. [Google Scholar]
- 13.Perico L., Benigni A., Casiraghi F., Ng L.F.P., Renia L., Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021;17:46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ish P., Sen M.K., Gupta N. In reference to anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130:E502. doi: 10.1002/lary.28832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute Dietary screener questionnaire in the NHANES 2009-10: background/EGRP/DCCPS/NCI/NIH. https://epi.grants.cancer.gov/nhanes/dietscreen/ [Internet], 2009 [cited 2021 Mar 16]. Available from:
- 16.USDA Food and Nutrient Database for Dietary Studies, USDA; Beltsville, MD: 2010. FoodData Central [Internet].https://fdc.nal.usda.gov/ [Internet] [cited 2022 Aug 23]. Available from: [Google Scholar]
- 17.R Core Team, Vienna, Austria; 1993. Available from: https://www.R-project.org/.
- 18.WHO WHO coronavirus (COVID-19) dashboard. https://covid19.who.int [Internet], 2020 [cited 2022 Sep 5]. Available from:
- 19.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karki K., Bhandari L. Potential role of nutrients in immune boosting and aiding against COVID19 pathogenesis. J. Stress Physiol. Biochem. 2022;18:5–16. [Google Scholar]
- 21.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 23.Alimohamadi Y., Sepandi M., Taghdir M., Hosamirudsari H. Determine the most common clinical symptoms in COVID-19 patients: a systematic review and meta-analysis. J. Prev. Med. Hyg. 2020;61:E304–E312. doi: 10.15167/2421-4248/jpmh2020.61.3.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amin M.d.T., Hasan M., Bhuiya N.M.M.A. Prevalence of COVID-19 associated symptoms, their onset and duration, and variations among different groups of patients in Bangladesh. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.738352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian A., Nirantharakumar K., Hughes S., Myles P., Williams T., Gokhale K.M., et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022;28:1706–1714. doi: 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zabetakis I., Lordan R., Norton C., Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12:1466. doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moghaddam A., Heller R.A., Sun Q., Seelig J., Cherkezov A., Seibert L., et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12:2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J., Taylor E.W., Bennett K., Saad R., Rayman M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020;111:1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieliszek M., Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19), Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J., Saad R., Taylor E.W., Rayman M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Huang J., Sun Y., Stubbs D., He J., Li W., et al. SARS-CoV-2 suppresses mRNA expression of selenoproteins associated with ferroptosis, endoplasmic reticulum stress and DNA synthesis. Food Chem. Toxicol. 2021;153 doi: 10.1016/j.fct.2021.112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Office of Dietary Supplements, Selenium [Internet]. [cited 2018 Jan 8]. Available from: https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/.
- 33.Ovaskainen M.L., Virtamo J., Alfthan G., Haukka J., Pietinen P., Taylor P.R., et al. Toenail selenium as an indicator of selenium intake among middle-aged men in an area with low soil selenium. Am. J. Clin. Nutr. 1993;57:662–665. doi: 10.1093/ajcn/57.5.662. [DOI] [PubMed] [Google Scholar]
- 34.Longnecker M.P., Stram D.O., Taylor P.R., Levander O.A., Howe M., Veillon C., et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology. 1996;7:384–390. doi: 10.1097/00001648-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Satia J.A., King I.B., Morris J.S., Stratton K., White E. Toenail and plasma levels as biomarkers of selenium exposure. Ann. Epidemiol. 2006;16:53–58. doi: 10.1016/j.annepidem.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Gutiérrez-González E., García-Esquinas E., de Larrea-Baz N.F., Salcedo-Bellido I., Navas-Acien A., Lope V., et al. Toenails as biomarker of exposure to essential trace metals: a review. Environ. Res. 2019;179 doi: 10.1016/j.envres.2019.108787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seyed Hosseini E., Riahi Kashani N., Nikzad H., Azadbakht J., Hassani Bafrani H., Haddad Kashani H. The novel coronavirus Disease-2019 (COVID-19): mechanism of action, detection and recent therapeutic strategies. Virology. 2020;551:1–9. doi: 10.1016/j.virol.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar A., Kubota Y., Chernov M., Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19, Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wessels I., Rolles B., Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front. Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling V., Zabetakis I. The role of an anti-inflammatory diet in conjunction to COVID-19. Diseases. 2021;9:76. doi: 10.3390/diseases9040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasui Y., Yasui H., Suzuki K., Saitou T., Yamamoto Y., Ishizaka T., et al. Analysis of the predictive factors for a critical illness of COVID-19 during treatment—relationship between serum zinc level and critical illness of COVID-19. Int. J. Infect. Dis. 2020;100:230–236. doi: 10.1016/j.ijid.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jothimani D., Kailasam E., Danielraj S., Nallathambi B., Ramachandran H., Sekar P., et al. COVID-19: poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golabi S., Adelipour M., Mobarak S., Piri M., Seyedtabib M., Bagheri R., et al. The association between vitamin D and zinc status and the progression of clinical symptoms among outpatients infected with SARS-CoV-2 and potentially non-infected participants: a cross-sectional study. Nutrients. 2021;13:3368. doi: 10.3390/nu13103368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon A.M., Hardigan P.C. A Case-Control Study for the Effectiveness of Oral Zinc in the Prevention and Mitigation of COVID-19. Frontiers in Medicine. 2021;8:756707. doi: 10.3389/fmed.2021.756707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sobczyk M.K., Gaunt T.R. The effect of circulating zinc, selenium, copper and vitamin K1 on COVID-19 outcomes: a Mendelian randomization study. Nutrients. 2022;14:233. doi: 10.3390/nu14020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Name J.J., Souza A.C.R., Vasconcelos A.R., Prado P.S., Pereira C.P.M. Zinc, vitamin D and vitamin C: perspectives for COVID-19 with a focus on physical tissue barrier integrity. Front. Nutr. 2020;7 doi: 10.3389/fnut.2020.606398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heller R.A., Sun Q., Hackler J., Seelig J., Seibert L., Cherkezov A., et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2020;38 doi: 10.1016/j.redox.2020.101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merino J., Joshi A.D., Nguyen L.H., Leeming E.R., Mazidi M., Drew D.A., et al. Diet quality and risk and severity of COVID-19: a prospective cohort study. Gut. 2021;70:2096–2104. doi: 10.1136/gutjnl-2021-325353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.U.S. Department of Agriculture, U.S. Department of Health and Human Services . 9th Edition. 2020-2025. Dietary guidelines for Americans.DietaryGuidelines.gov Available from: [Google Scholar]
- 50.Zhang J., W. Taylor E., Bennett K., Rayman M.P. Does atmospheric dimethyldiselenide play a role in reducing COVID-19 mortality? Gondwana Res. 2023;114:87–92. doi: 10.1016/j.gr.2022.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demircan K., Chillon T.S., Bracken T., Bulgarelli I., Campi I., Du Laing G., et al. Association of COVID-19 mortality with serum selenium, zinc and copper: six observational studies across Europe. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data described in the manuscript, code book, and analytic code will be made available on request pending application and approval.