Background:
The pregabalin is approved for the management of persistent pain. The aim of this study is to assess the advantages and disadvantages of the use of pregabalin in eye pain management.
Methods:
The PubMed, Cochrane Library, Embase, and Web of Science databases were searched until January 2022 for randomized controlled trials. Randomized, double-blinded trials comparing pregabalin with placebo in eye pain management were included. The primary outcome was visual analog scale or numerical rating scale at acute (24 hours) and chronic (≥7 days after surgery) timepoints. The secondary outcomes were analgesic medication requirements and pregabalin-related complications (nausea, vomiting, dizziness, and headache). We also compared the effect of pregabalin on dry-eye syndrome.
Main results:
Six relevant articles were identified that studied the use of pregabalin as pain relief for photorefractive keratectomy (n = 2), laser epithelial keratomileusis (n = 1), laser-assisted in situ keratomileusis (n = 1), eyelid surgery (n = 1), and dacryocystorhinostomy (n = 1). Pregabalin was associated with a significant reduction in pain scores (95% confidence interval = −0.41 [−0.76–−0.06]) 24 hours after surgical procedures. The data were insufficient to draw conclusions regarding dry eye symptoms. Because of the high heterogeneity of outcomes regarding adverse effects, there is no conclusion regarding the safety of pregabalin in eye pain.
Conclusions:
Pregabalin reduced acute eye pain but had no significant effect on long-term analgesia after ophthalmological surgery in adults. It had no effect on dry-eye symptoms after ocular surgery. Further studies on the safety of pregabalin in eye pain management are required to draw solid conclusions.
Keywords: eye pain, pregabalin, systematic review
1. Introduction
Eye pain is one of the most distressing symptoms and complaints among ophthalmic patients. Basic eye history and eye examination are critical for diagnosing the cause of eye pain.[1] Eye pain disorders are categorized into 2 groups: nociceptive pain and neuropathic pain.[2] The former is usually caused by tissue inflammation, acute trauma, or surgery.[3] Neuropathic pain usually arises from nervous system disease.[4] These 2 kinds of pain are often found to coexist. Patients diagnosed with ocular symptoms and eye pain will further need pain management.
Pregabalin is a structural analog of γ-aminobutyric acid and is structurally related to gabapentin.[5,6] Pregabalin is a third-line agent for eye pain management.[7] It is efficient in controlling mild to moderate pain and has lower dose requirements and fewer dose-related complications.[8,9] Although many studies have evaluated the efficacy and safety of pregabalin in pain management, there has been no systematic review of eye pain management using pregabalin. This review was conducted based on existing clinical randomized controlled trials (RCTs); the primary aim was to compare the efficacy and safety of pregabalin with those of placebo in acute and chronic eye pain control, and the secondary aim was to evaluate the effect of pregabalin on the relief of dry eye symptoms after eye treatment in adults.
2. Method
This study was conducted according to the recommendations of the Cochrane group and the PRISMA guidelines for systematic reviews and meta-analyses.[10] Two authors (J.Z. and X.H.S.) performed the review and data extraction independently, and any discrepancy was resolved by consensus.
2.1. Search strategy
We searched for relevant studies in English, and there was no limitation on publication year. A systematic review of the relevant articles was conducted in 4 bibliographic databases, namely, PubMed, Cochrane Library, Embase, and Web of Science, until January 2022. Additionally, to ensure that most of the relevant studies were identified, key journals relevant to the topic were searched separately. We also searched Open Access Theses and Dissertations for any eligible unpublished studies. Pregabalin and eye pain were the search terms.
2.2. Study inclusion criteria and relevance screening
The duplicate records were removed, and a pair of authors (X.S. and X.C.) reviewed the titles and abstract of the articles independently. The exclusion criteria were as follows: the study focused on a pain model; animal experiments were used to study the effect of pregabalin; the study was a case report; the study was designed as an observational study; and all reviews and editorial comments. Moreover, the selected studies were included for analysis according to the following criteria: the study was designed as an RCT; the study focused on eye pain with no restriction to any specific age group; pain was measured as a primary outcome; the study compared pregabalin with placebo or another active treatment for pain; and the study was published in English.
For each reference identified by electronic search, 2 authors (X.S. and Y.H.) assessed eligibility according to the selection criteria defined above. References that met the inclusion criteria were retrieved in full-text format and further assessed independently by the same 2 authors. Reviewers resolved any disagreements by discussion to achieve consensus.
2.3. Data extraction
The selected studies were conducted independently by 2 reviewers (J.Z. and X.S.) based on the Cochrane Handbook for systematic reviews of interventions.[11] Data, including study characteristics (design, longitude), participants’ characteristics (age, sex, and number of patients), treatment details (dose and dosing regimen), and efficacy outcomes, were extracted from all eligible studies using a constructed data extraction form. The primary outcome was acute pain (24 hours) and chronic pain (≥7 days after surgery). The secondary outcomes were side effects and ocular symptoms and signs.
2.4. Risk bias
The Cochrane Collaboration’s tool was used to assess the risk of potential bias in the selected studies. The quality of the included RCTs was assessed independently by 2 researchers (H.X. and Y.H.).[11] The risk of bias tool requires reviewers to judge seven domains: methods of randomization; allocation concealment, blinding of the outcome assessors, blinding of the study participants, presence of incomplete data, selective reporting, and other sources. Each study was classified as having a high, unclear or low risk of bias. Only data from studies featuring a low or unclear risk of bias were included in our analysis.
2.5. Statistical analysis
Data analysis was completed independently by 2 authors (X.C. and Y.H.) using Review Manager v. 5.3 (The Nordic Cochrane Centre, Cochrane Collaboration, 2014, Copenhagen, Denmark). The mean difference and 95% confidence interval were used for continuous variables. The random-effects method and the fixed-effect method were used for studies with significant heterogeneity and for those without heterogeneity, respectively.
3. Results
3.1. Study selection and characteristics
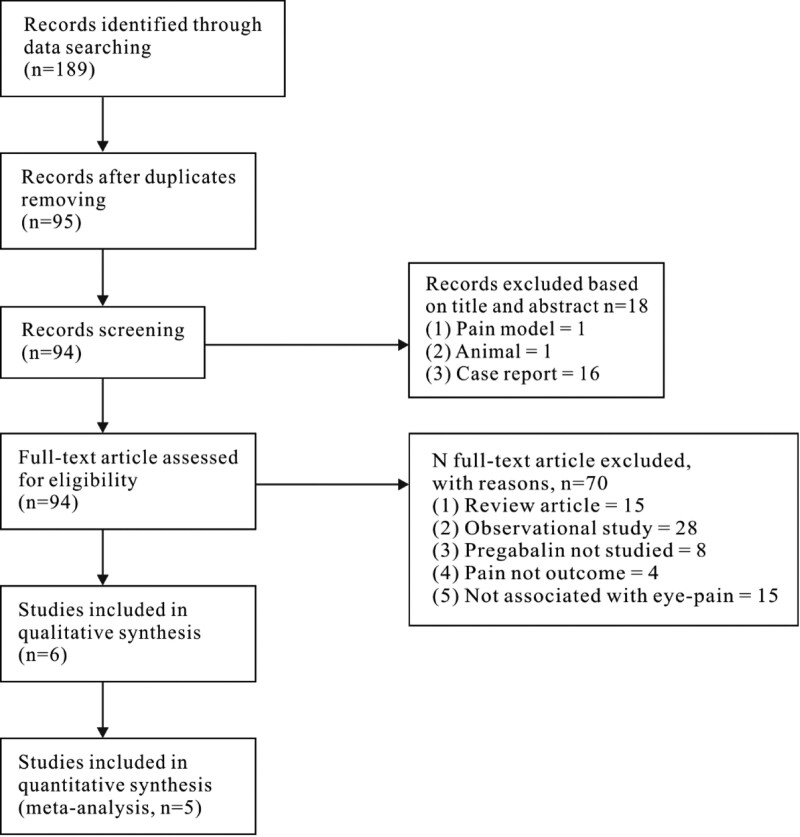
The flow chart of study identification is presented in Figure 1. A total of 189 publications relevant to search words were identified; 76 studies were deemed potentially eligible, and their full text versions were retrieved for full eligibility assessment. A total of 6 publications were included in the qualitative thesis (Table 1), with 244 patients in the pregabalin group and 238 participants in the placebo group. The study by Pakravan et al also used gabapentin as an intervention and had 50 patients in this group.[12] The characteristics of each study are summarized in Table 1. They all in eye pain relief,[13,14] and investigated the efficacy of pregabalin for laser epithelial keratomileusis (LASEK) and laser-assisted in situ keratomileusis surgery,[12,15] investigated the efficacy of pregabalin for photorefractive keratectomy (PRK),[16] investigated the efficacy of pregabalin for eyelid surgery, and[17] investigated the efficacy of pregabalin for dacryocystorhinostomy. In general, the characteristics of the interventions in all 6 studies are described in Table 2. The studies by Paik et al and Galor et al did not present primary outcomes (pain scores at 24 hours and adverse effects), but they described the effect of pregabalin on chronic eye pain at 7 days, 3 months, and 6 months.[13,14] The trial by Wei et al presented pain scores as the mean and range instead of the mean and standard deviation, which could not be used in the meta-analysis.[16] Hence, we excluded these 3 papers from the meta-analysis but kept them in the systematic review.
Figure 1.
Flow diagram of the recruitment of studies to a systematic review of the safety and efficacy of pregabalin after eye surgery.
Table 1.
Study and patient characteristics for randomized controlled trials identified in systematic review regarding the efficacy and safety of pregabalin in eye pain.
| Reference | Study funding | Country | Type of surgery | Sex F/M | Ages (yr) | Intervention/Comparator | Pain outcome | Dry-eye symptom | Adverse effect |
|---|---|---|---|---|---|---|---|---|---|
| Paik et al | National research foundation | Korean | Laser epithelial keratomileusis | 40/40 | Range 18–45 | Pregabalin/placebo 40/ 40 |
Chronic pain | Yes | No |
| Galor et al | Department of Veterans Affairs etc | US | Laser-assisted in situ keratomileusis | 22/21 | Mean 37.8 |
Pregabalin/placebo 21/ 22 |
Chronic pain | Yes | Yes |
| Meek et al | None declared | US | Photorefractive keratectomy | 70/60 | Mean 34.86 |
Pregabalin/placebo 67/ 63 |
Acute pain | No | Yes |
| Wei et al | Synthes/AO foundation KLS Martin and AO foundation |
US | Eyelid surgery | 23/26 | Mean 67.4 |
Pregabalin/placebo 26/ 23 |
Acute pain | No | Yes |
| Alimian et al | None declared | Iran | Dacryocystorhinostomy | 31/49 | Mean 43.25 |
Pregabalin/placebo 40/ 40 |
Acute pain | No | Yes |
| Pakravan et al | None declared | Iran | Photorefractive keratectomy | 86/64 | Mean 26.57 |
Pregabalin and gabapentin/placebo 50 and 50/ 50 |
Acute pain | No | No |
Table 2.
Invention characteristics for randomized controlled trials identified in systematic review regarding the safety and efficacy pregabalin after eye surgery.
| Reference | Intervention | Route | Daily dose | Duration of administration | Comparator |
|---|---|---|---|---|---|
| Paik et al | Pregabalin (40) | Oral | 150 mg twice per d | 12 h before surgery, fellow by treatment for 15 d | Placebo (40) |
| Galor et al | Pregabalin (21) | Oral | 150 mg twice per d | First dose prior to surgery, continue for 14 d | Placebo (22) |
| Meek et al | Pregabalin (67) | Oral | 75 mg twice per day | 2 h before surgery, continue every 12 h for 5 days | Placebo (63) |
| Wei et al | Pregabalin (26) | Oral | 150 mg | 15 min to 1 h prior to the eyelid surgery | Placebo (23) |
| Alimian et al | Pregabalin (40) | Oral | 300 mg | 1 h before the surgery | Placebo (40) |
| Pakravan et al | Pregabalin (50) | Oral | 75 mg thrice per d | 2 h after surgery and for 3 d | Placebo (50) |
| Gabapentin (50) | Oral | 300 mg thrice per d | 2 h after surgery and for 3 d |
3.2. Methodological assessment and risk of bias assessment
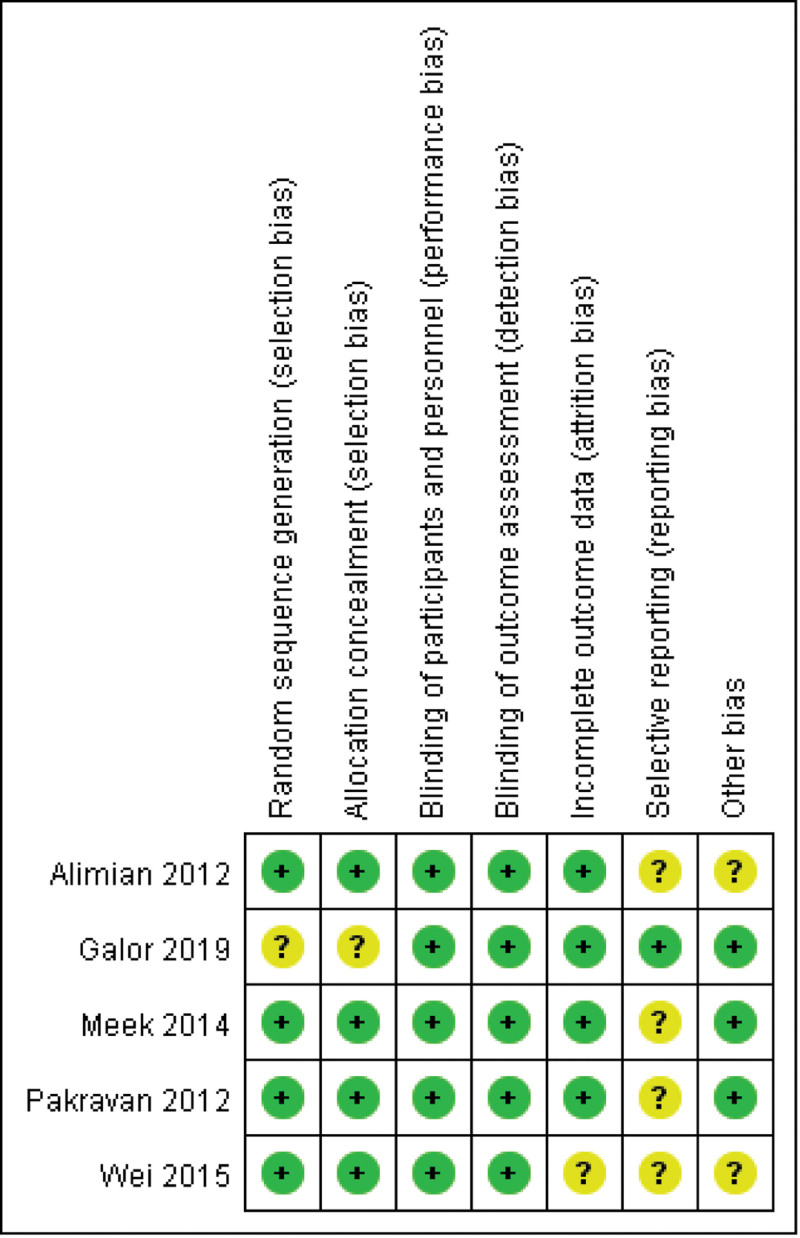
We used seven criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions to assess the quality of the RCTs included in our meta-analysis (Fig. 2). All the trials were placebo-controlled studies. Paik et al conducted a variable blocked randomization list for treatment assignments to ensure that treatment assignments were balanced, which was considered to be an unclear risk. Because of the lack of a study protocol for review, 4 studies were considered to have an unclear risk of report bias. One study was judged to be at an unclear risk regarding incomplete outcome data due to a lack of explanation about why 2 patients were not followed up. Two studies did not present baseline pain intensity, and they tended to have an unclear risk. All the studies were considered to have a low risk of methodological shortcomings. Five studies were identified as relevant, included in the meta-analysis and analyzed.[12–17] Publication bias, statistically assessed using Begg and Egger tests, showed no significant bias (P = .42 and P = .36, respectively). Statistical heterogeneity for pain scores at 24 hours was moderate (I2 = 58%).
Figure 2.
Quality summary of the pregabalin randomized controlled trials for each methodological quality item of each included study.
3.3. Pain-associated outcomes
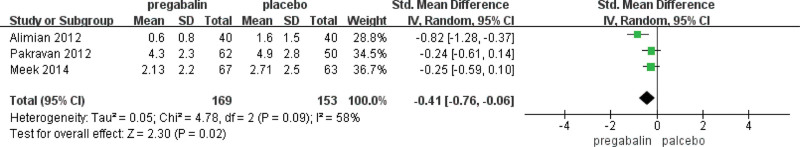
We summarized 6 trials on the use of different treatments to manage eye pain (Table 3). They studied acute and chronic eye pain by using the visual analog scale or numerical rating scale. Two trials focused on the chronic pain effect.[17,18] A study by Wei et al showed that pregabalin reduced acute pain scores in histograms but had no specific data. Therefore, 3 trials were included in the quantitative analysis of ocular pain relief at 24 hours. Meta-analysis showed that pregabalin significantly reduced pain scores by 0.41 points (95% confidence interval −0.76–−0.06, P = .02, Fig. 3).
Table 3.
Efficacy of pregabalin versus comparators in systematic review regarding the safety and efficacy pregabalin after eye surgery.
| Reference | Primary outcome | Intervention | Comparator | Significant (P value) |
|---|---|---|---|---|
| Paik et al | Pain scores using VAS from baseline to post-operation 7 d, mean (SD) | 0.15 (0.37) | 1.5 (2.89) | P = .044 |
| Galor et al | Ocular pain intensity using NRS at 3-mo, mean (SD) | 0.85 (0.27) | 0.27 (0.55) | Not significant |
| Sf-MPQ sensory at 3-mo, mean (SD) | 0.65 (1.23) | 0.36 (0.66) | Not significant | |
| Sf-MPQ affective at 3-mo, mean (SD) | 0.30 (0.66) | 0.18 (0.39) | Not significant | |
| NPSI-eye at 3-mo, mean (SD) | 2.7 (4.55) | 1.86 (4.07) | Not significant | |
| Ocular pain intensity using NRS at 6-mo, mean (SD) | 1.10 (1.48) | 0.38 (0.97) | Not significant | |
| NPSI-eye, mean (SD) | 2.81 (4.07) | 3.14 (5.85) | Not significant | |
| Meek et al | Pain intensity from VAS at day 1 AM, mean (SD) | 6.32 (9.14) | 10.22 (15.27) | Not significant |
| Pain intensity from VAS at day 1 PM, mean (SD) | 21.32 (21.95) | 2.09 (25.03) | Not significant | |
| Pain intensity from VAS at day 2 AM, mean (SD) | 23.49 (21.31) | 29.27 (25.1) | Not significant | |
| Pain intensity from VAS at day 2 PM, mean (SD) | 26.14 (22.79) | 25.78 (26.66) | Not significant | |
| Consumption of total rescue pain medication at day 1, mean | 1.7 | 2.4 | <0.03 | |
| Consumption of total rescue pain medication at day 2, mean | 1.7 | 2.6 | <0.025 | |
| Wei et al | Pain scores from VAS after 1–2 h of surgery, mean (range) | 12.9 (0–73) | 29.4 (0–94) | Patients in the pregabalin group reported pain score 5.5 points lower compared with the placebo group (P = .0307). |
| Pain scores from VAS after 2–4 h of surgery, mean (range) | 14.0 (0–48) | 25.6 (0–76) | ||
| Pain scores from VAS after 8–12 h of surgery, mean (range) | 10.8 (0–63) | 20.6 (0–97) | ||
| Pain scores from VAS after 20–28 h of surgery, mean (range) | 11.3 (0–80) | 9.7 (0–69) | ||
| Pain scores from VAS after 36–48 h of surgery, mean (range) | 11.0 (0–57) | 2.1 (0–10) | ||
| Alimian et al | Pain intensity from VAS at time of recovery, mean (SD) | 3.2 (1.5) | 5.1 (1.5) | <0.001 |
| Require for opioid to relieve pain, n (%) | 7 (17.5%) | 21 (52.5%) | P = .001 | |
| Pakravan et al | Pain intensity from VAS at day 1 AM, mean (SD) | Pregabalin: 4 (2.4) gabapentin 3.9 (2.4) |
4.7 (3) | Not significant |
| Pain intensity from VAS at day 1 PM, mean (SD) | Pregabalin: 4.3 (2.3) gabapentin: 4.3 (2.7) |
4.9 (2.8) | Not significant | |
| Pain intensity from VAS at day 2 AM, mean (SD) | Pregabalin: 2.2 (1.8) gabapentin: 2.2 (2.3) |
3.5 (3) | Not significant | |
| Pain intensity from VAS at day 2 PM, mean (SD) | Pregabalin: 2 (1.9) gabapentin: 2.1 (2.4) |
2.7 (2.6) | Not significant | |
| Consumption of rescue pain medication, mean (SD) | Pregabalin: 7.9 (5.2) gabapentin: 9.0 (4.1) |
10.3 (5.6) | Not significant |
SD = standard deviation, Sf-MPQ = short form McGill pain questionnaire, NPSI = neuropathic pain symptom inventory-eye, NRS = numerical rating scale, VAS = visual analogue scale.
Figure 3.
Pain scores 24 hours after surgery in pregabalin trials.
Paik et al and Galor et al also studied the chronic effect of pregabalin on pain relief.[13,14] A study by Paik showed that patients took pregabalin 150 mg twice daily for 14 days. The ocular pain scores were significantly reduced compared with the placebo group for 1 week. While Galor’s study had no similar result, they showed that pregabalin did not have an effect on pain for up to 3 and 6 months. Two trials did not investigate pain at similar times, and 3 and 6 months was much longer than 1 week. Therefore, the chronic effect of pregabalin needs to be further studied to reach a solid conclusion.
Three trials investigated the effect of pregabalin in the consumption of rescue pain medication postoperatively. Meek et al showed that there was a significant decrease in the consumption of total rescue pain medication per patient on postoperative Days 1 and 2 in the pregabalin group.[15] Pakravan et al reported a similar result, although the consumption of rescue medication in the pregabalin group did not reach statistical significance.[12] Alimian et al reported that patients in the pregabalin group were less likely to require opioids than those in the placebo group.[17]
3.4. Ocular symptoms and sign-associated outcomes
Paik et al and Galor et al studied dry eye symptoms after keratomileusis.[13,14] A trial by Galor et al showed that perioperative pregabalin did not reduce the frequency of dry-eye symptoms at a 6-month follow-up after laser-associated in situ keratomileusis. Paik evaluated dry-eye symptoms at 1, 3, and 6 months; there was no significant difference between the pregabalin and placebo groups. They both used the ocular surface disease index, dry eye questionnaire 5, and tear breakup time index to evaluate dry-eye symptoms (Table 4). Paik et al also tested corneal nerve regeneration by assessing the subbasal nerve plexus, and there was no significant difference between the 2 groups in nerve fiber density, fiber length and nerve branch density.
Table 4.
Proportion of patients about ocular symptoms and signs.
| Reference | Outcome measure | Intervention | Comparator | Significant |
|---|---|---|---|---|
| Paik et al | OSDI at 1-mo, mean | 19.46 | 24.12 | Not significant |
| DEQ-5 at 1-mo, mean | 8.27 | 8.54 | Not significant | |
| Nerve fiber density, mean (SD) | 8.40 (3.10) | 7.70 (2.70) | Not significant | |
| Nerve fiber length, mean (SD) | 3.18 (1.23) | 2.60 (1.03) | Not significant | |
| Nerve branch density, mean (SD) | 9.45 (4.25) | 7.35 (2.42) | Not significant | |
| Galor et al | DEQ-5 at 3-mo, mean (SD) | 6.6 (3.9) | 4.7 (4.4) | Not significant |
| Change in DEQ5 from baseline, mean (SD) | 1.1 (3.9) | 0.4 (4.0) | Not significant | |
| OSDI at 3-mo, mean (SD) | 11.9 (11.5) | 11.0 (16.6) | Not significant | |
| Change in OSDI from baseline, mean (SD) | −0.01 (15.6) | 2.0 (15.1) | Not significant | |
| TBUT at 6-mo, seconds, mean (SD) | 8.36 (2.46) | 9.05 (5.93) | Not significant | |
| Schirmer score at 6-mo, mm, mean (SD) | 15.45 (8.17) | 15.05 (8.64) | Not significant |
DEQ-5 = dry eye questionnaire 5, OSDI = ocular surface disease index, SD = standard deviation, TBUT = tear breakup time.
3.5. Adverse effects
Adverse effect outcomes from pregabalin were reported by 4 of 5 retained studies (Table 5). Due to the heterogeneity of the study outcome of adverse effects, side effects were not included in the conclusion. However, the original results for each study were described in this review. Three trials reported that the number of adverse events in the pregabalin group was greater than that in the placebo group,[14–16] and 1 trial had opposite results.[17] However, these differences in each study were not statistically significant. In Galor’s study, they reported that subjects in the pregabalin group had a higher frequency of tiredness and dizziness than those in the control group (P < .05, Table 5). Alimian et al reported that patients in the pregabalin treatment group had a lower frequency of nausea (P < .05, Table 5). Nausea and dizziness were the most commonly reported individual adverse effects, often reported in between 3.8% and 12.5% and 7.7% and 29% of patients in the pregabalin group.
Table 5.
Proportion of patients about adverse effects with pregabalin versus comparator in systematic review regarding the safety and efficacy pregabalin after eye surgery.
| Reference | Primary outcome | Intervention | Comparator | Significant |
|---|---|---|---|---|
| Galor et al | Total side effect, n (%) | 13 (62%) | 10 (46%) | Not significant |
| Tiredness, n (%) | 8 (38%) | 2 (9%) | P = .03 | |
| Dizziness, n (%) | 6 (29%) | 1 (5%) | P = .05 | |
| Headache, n (%) | 3 (14%) | 3 (14%) | Not significant | |
| Nausea, n (%) | 1 (5%) | 2 (9%) | Not significant | |
| Dry mouth, n (%) | 3 (14%) | 0 (0%) | Not significant | |
| Constipation, n (%) | 3 (14%) | 1 (5%) | Not significant | |
| Bloating, n (%) | 3 (14%) | 4 (18%) | Not significant | |
| High or elevated mood, n (%) | 4 (19%) | 1 (5%) | Not significant | |
| Meek et al | Total adverse events, n | 39 | 33 | Not significant |
| Dizziness/lightheadedness, n | 10 | 2 | Not significant | |
| Nausea, n | 3 | 9 | Not significant | |
| Somnolence, n | 9 | 5 | Not significant | |
| Rhinorrhea/ congestion, n | 7 | 7 | Not significant | |
| Wei et al | Total adverse events, n (%) | 9 (34.6%) | 4 (17.4%) | Not significant |
| Sleepiness, n (%) | 4 (15.4%) | 0 | Not significant | |
| Dizziness, n (%) | 2 (7.7%) | 2 (8.7%) | Not significant | |
| Nausea, n (%) | 1 (3.8%) | 2 (8.7%) | Not significant | |
| Headache, n (%) | 2 (7.7%) | 0 | Not significant | |
| Alimian et al | Total adverse events, n (%) | 6 (15%) | 22 (55%) | Not described |
| Nausea, n (%) | 5 (12.5%) | 17 (47%) | P = .03 | |
| Vomiting, n (%) | 1 (2.5%) | 5 (12.5%) | Not significant |
4. Discussion
Eye pain is a danger signal for ocular conditions, either in a “quiet eye or “red eye.”[18,19] Many causes trigger eye pain, and neurologists must identify these causes. Moreover, these patients also need effective pain management to relieve pain. Increasing studies of the analgesic efficacy and safety of pregabalin in the management of pain have been investigated, and they included both acute and chronic pain.[20–22] In our review, 6 relevant RCTs in eye pain were identified. Two clinical trials focused on postoperative pain (dacryocystorhinostomy and eyelid surgery). Four other trials studied corneal surgery, such as PRK, LASEK and laser-assisted in situ keratomileusis. They all investigated the effect of pregabalin on pain management, and 4 trials studied the adverse effects in the pregabalin and placebo groups. Therefore, we synthesized eye pain scores after treatment between the pregabalin and placebo groups from the included studies.
Pregabalin is an anticonvulsant medication approved for reducing neuropathic pain in various diseases.[23–25] Pregabalin manages pain by diminishing hyperalgesia and central sensitization.[26,27] Clinical trials have shown that pregabalin can relieve acute and persistent postoperative pain.[28,29] Currently, oral administration of pregabalin has already been used in managing ocular pain,[30] but there is no conclusion about the efficacy and safety of pregabalin in eye pain management. Our findings suggest that pregabalin may reduce acute eye pain within 24 hours after surgery (Fig. 3).
Orally administered pregabalin has maximal oral bioavailability and a half-life of 6.3 hours.[31] Although effective pain control is dependent on the timing of analgesic administration, these trials orally administered pregabalin before or 1 to 2 hours after surgery. It seemed that the difference in dosing time did not affect the analgesic effect of pregabalin. Paik et al and Galor et al investigated the effect of pregabalin on chronic pain.[13,14] Paik et al tested the pain intensity at 7 days postoperation, and pregabalin significantly reduced pain compared with placebo.[13] However, Galor et al tested ocular pain at 3 and 6 months, and there was no difference in pain control, suggesting that a single administration of pregabalin may not be adequate to reduce long-term pain. Pregabalin was not able to suppress the entire afferent barrage of nociceptive signals or chemical mediators originating from the cornea and affect the central nervous system, leading to sensitization and chronicity of pain.[32] However, they did not test at a similar time, and the 7-week and 3- or 6-month durations were quite different regarding the effect of analgesics. Hence, the effect of chronic pain management with pregabalin on cornel injury needs further study.
Except for the trials by Alimian et al and Wei et al, the other trials included ocular surgical procedures that are used to correct refractive error, including PRK, laser-assisted in situ keratomileusis surgery and LASEK, but only 2 trials reported ocular symptoms.[13,14] These surgeries induce direct injury to the cornea and nerve endings of the treated corneal area.[33] While visual outcomes after surgery tend to be excellent, a potential side effect of the procedures is the onset of dry eye symptoms.[34,35] These studies used several measurements to test dry eye symptoms, such as ocular surface disease index, dry eye questionnaire 5, TUBT, and Schirmer scores. These 2 studies reported that there was no significant difference between the pregabalin and placebo groups at any point in the follow-up period. Paik et al also investigated the effect of pregabalin on corneal nerve regeneration, and they reported that there was no statistically significant difference between the pregabalin and control groups by assessing the subbasal nerve plexus, including nerve fiber length and nerve branch density. These 2 trials provided similar results regarding the relationship between pregabalin administration and the rate of dry eye symptoms at the 3- or 6-month follow-up after refractive surgery, which suggested that pregabalin may have no effect on dry eye symptoms after ocular surgery.
The data regarding the prevalence of adverse effects appeared relatively consistent, with dizziness nausea being the most common adverse effect of pregabalin.[36] Due to the high heterogeneity of the included studies and the variability in the assessment of the adverse effect outcomes, pooling of the outcome data was not attempted, and the results are presented for each individual study (Table 5). Except for the study of Alimian et al, the other 3 studies reported that patients in the pregabalin group had a higher frequency of side effects, although the difference did not reach statistical significance.[14–16] Patients had a higher rate of side effects prolonged with administration of pregabalin.[37] A meta-analysis showed that administering pregabalin in doses over 300 mg before surgery significantly alleviated pain in the first 24-hour period after surgery, while increasing the dose significantly amplified the side effects resulting from pregabalin.[38] However, Alimian and colleagues’ results showed that a single oral dose of pregabalin at 300 mg per dose is more effective than doses under 300 mg or in divided doses compared to other studies; these authors suggested that the type of surgery is an important factor and that in minor to moderate surgeries, pregabalin has its maximal effect without any side effects.[17]
There were several limitations to our study. First, the current review included only English language published studies, which induced bias if relevant studies were reported in other languages. Thus, more RCTs with larger sample sizes are urgently needed. Second, only 2 studies reported ocular symptoms, including dry eye and ocular fiber regeneration, after pregabalin administration. Third, the timing and dosage of pregabalin administration was inconsistent across trials, making it difficult to draw a conclusion about the safety of pregabalin in eye pain management. The optimal dose and best stage of pregabalin administration remain to be confirmed by future RCTs. In conclusion, this review found a paucity of data supporting the clinical use of pregabalin as a prophylactic or treatment option for eye pain.
Author contributions
Conceptualization: Hui Xu.
Data curation: Xingying Chen.
Formal analysis: Yanyan He.
Methodology: Yanyan He.
Project administration: Xiaohua Shen.
Supervision: Jia Zhu.
Writing – original draft: Xiaohua Shen.
Writing – review & editing: Jia Zhu.
Abbreviations:
- LASEK
- laser epithelial keratomileusis
- PRK
- photorefractive keratectomy
- RCTs
- randomized controlled trials
This research was financially supported by the Basic Public Welfare Planning Project of Zhejiang Province, China (LGD20H090007), and the Sci-Tech Planning Project of Jiaxing, China (2021AY10049).
No informed consent statement was required for this review study.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Shen X, Chen X, He Y, Xu H, Zhu J. Efficacy and safety of pregabalin in eye pain: A systematic review. Medicine 2023;102:6(e32875).
Contributor Information
Xiaohua Shen, Email: s_xppx@163.com.
Xingying Chen, Email: 269221227@qq.com.
Yanyan He, Email: 249614187@qq.com.
Hui Xu, Email: taihouxu@126.com.
References
- [1].Lee AG, Al-Zubidi N, Beaver HA, et al. An update on eye pain for the neurologist. Neurol Clin. 2014;32:489–505. [DOI] [PubMed] [Google Scholar]
- [2].Ebrahimiadib N, et al. Ocular neuropathic pain: an overview focusing on ocular surface pains. Clin Ophthalmol. 2020;14:2843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brooks J, Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathways. J Anat. 2005;207:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Treede RD. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain. 2019;160:19–27. [DOI] [PubMed] [Google Scholar]
- [5].Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007;73:137–50. [DOI] [PubMed] [Google Scholar]
- [6].Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–36. [DOI] [PubMed] [Google Scholar]
- [7].Dieckmann G, Goyal S, Hamrah P. Neuropathic corneal pain: approaches for management. Ophthalmology. 2017;124(11S):S34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chew M, Ma G, Xie R, et al. Population pharmacokinetics of pregabalin extended-release in healthy volunteers and patients with postherpetic neuralgia, fibromyalgia, and partial-onset seizures. J Clin Pharmacol. 2019;59:1527–42. [DOI] [PubMed] [Google Scholar]
- [9].Chizh BA, Göhring M, Tröster A, et al. Effects of oral pregabalin and aprepitant on pain and central sensitization in the electrical hyperalgesia model in human volunteers. Br J Anaesth. 2007;98:246–54. [DOI] [PubMed] [Google Scholar]
- [10].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700–b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cumpston M. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pakravan M. Pregabalin and gabapentin for post-photorefractive keratectomy pain: a randomized controlled trial. Eur J Ophthalmol. 2012;22(Suppl 7):106S106–113. [DOI] [PubMed] [Google Scholar]
- [13].Paik DW, Lim DH, Chung TY. Effects of taking pregabalin (Lyrica) on the severity of dry eye, corneal sensitivity and pain after laser epithelial keratomileusis surgery. Br J Ophthalmol. 2022;106:474–9. [DOI] [PubMed] [Google Scholar]
- [14].Galor A. Pregabalin failed to prevent dry eye symptoms after laser-assisted in situ keratomileusis (LASIK) in a randomized pilot study. J Clin Med. 2019;8:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Meek JM, Rosbolt MB, Taylor KR, et al. Pregabalin versus placebo in postoperative pain relief of patients’ status post photorefractive keratectomy: a double-masked, randomized, prospective study. J Ocul Pharmacol Ther. 2014;30:527–32. [DOI] [PubMed] [Google Scholar]
- [16].Wei LA. Perioperative pregabalin for attenuation of postoperative pain after eyelid surgery. Ophthalmic Plast Reconstr Surg. 2015;31:132–5. [DOI] [PubMed] [Google Scholar]
- [17].Alimian M. Effects of single-dose pregabalin on postoperative pain in dacryocystorhinostomy surgery. Anesth Pain Med. 2012;2:72–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Narayana S, McGee S. Bedside diagnosis of the “Red Eye”: a systematic review. Am J Med. 2015;128:1220–1224.e1. [DOI] [PubMed] [Google Scholar]
- [19].Kulenkamp J, McClelland CM, Lee MS. Eye pain in the white and quiet eye. Curr Opin Ophthalmol. 2020;31:483–8. [DOI] [PubMed] [Google Scholar]
- [20].Derry S. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1:CD007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moore RA. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;8:CD00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Reddi D. Preventing chronic postoperative pain. Anaesthesia. 2016;71(Suppl 1):64–71. [DOI] [PubMed] [Google Scholar]
- [23].Taguchi T, Nakano S, Nozawa K. Effectiveness of pregabalin treatment for neuropathic pain in patients with spine diseases: a pooled analysis of two multicenter observational studies in Japan. J Pain Res. 2021;14:757–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rafiullah M, Siddiqui K. Pharmacological treatment of diabetic peripheral neuropathy: an update. CNS Neurol Disord Drug Targets. 2022;21:884–900. [DOI] [PubMed] [Google Scholar]
- [25].Cross AL, Viswanath O, Sherman A. Pregabalin, in StatPearls. 2021: Treasure Island (FL). [Google Scholar]
- [26].Buvanendran A, Kroin JS, Kari M, et al. Can a single dose of 300 mg of pregabalin reach acute antihyperalgesic levels in the central nervous system? Reg Anesth Pain Med. 2010;35:535–8. [DOI] [PubMed] [Google Scholar]
- [27].Lee C, Lee HW, Kim JN. Effect of oral pregabalin on opioid-induced hyperalgesia in patients undergoing laparo-endoscopic single-site urologic surgery. Korean J Anesthesiol. 2013;64:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rai AS, Khan JS, Dhaliwal J, et al. Preoperative pregabalin or gabapentin for acute and chronic postoperative pain among patients undergoing breast cancer surgery: A systematic review and meta-analysis of randomized controlled trials. J Plast Reconstr Aesthet Surg. 2017;70:1317–28. [DOI] [PubMed] [Google Scholar]
- [29].Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152:691–7. [DOI] [PubMed] [Google Scholar]
- [30].Small LR. Oral gabapentinoids and nerve blocks for the treatment of chronic ocular pain. Eye Contact Lens. 2020;46:174–81. [DOI] [PubMed] [Google Scholar]
- [31].Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010;49:661–9. [DOI] [PubMed] [Google Scholar]
- [32].Vadivelu N. Preventive analgesia for postoperative pain control: a broader concept. Local Reg Anesth. 2014;7:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bech F, González-González O, Artime E, et al. Functional and morphologic alterations in mechanical, polymodal, and cold sensory nerve fibers of the cornea following photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2018;59:2281–92. [DOI] [PubMed] [Google Scholar]
- [34].Rabina G. The association between preoperative dry eye symptoms and postoperative discomfort in patients underwent photorefractive keratectomy. J Ophthalmol. 2019;2019:7029858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bower KS, Sia RK, Ryan DS, et al. Chronic dry eye in photorefractive keratectomy and laser in situ keratomileusis: manifestations, incidence, and predictive factors. J Cataract Refract Surg. 2015;41:2624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mathieson S, Maher CG, McLachlan AJ, et al. Trial of pregabalin for acute and chronic sciatica. N Engl J Med. 2017;376:1111–20. [DOI] [PubMed] [Google Scholar]
- [37].Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb. 2017;47:310–3. [DOI] [PubMed] [Google Scholar]
- [38].Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth. 2011;106:454–62. [DOI] [PubMed] [Google Scholar]