Abstract
To examine the prognostic value of claudin 18.2 (CLDN18.2) and human epidermal growth factor receptor-2 (HER-2) expression in patients with resected pancreatic ductal adenocarcinoma (PDAC). This study enrolled patients who underwent surgery and were diagnosed with PDAC at Suleyman Demirel University Hospital, Turkey between 2015 and 2019. Sixty-eight patients with resected PDAC treated at a medical oncology clinic were assessed. All patients were over the age of 18 years, underwent follow-up and treatment in our unit, and had pathology slides that we could access. Clinicopathological data were obtained from medical files, including the patients’ age, sex, pathological parameters, and clinical stage according to the Eighth International Union against Cancer/American Joint Committee on Cancer. Patient survival and the period from the date of diagnosis to death were assessed in the follow-up data. There was no statistically significant difference between CLDN18.2 and HER-2 expression scores for samples and patient clinicopathological characteristics. No HER-2 expression scores of ≥2 were found in the samples. Only 25% (n = 17) of the samples had HER-2 expression scores of +1. CLDN18.2 expression was detected in 54.4% (n = 37) of the patient samples. CLDN18.2 expression scores were +1 in 30.8% (n = 21) of the patient samples, +2 in 16.2% (n = 11), and +3 in 7.4% (n = 5). When CLDN18.2 and HER-2 expression were compared, a statistically significant difference and moderate positive correlation were observed. No significant relationship between HER-2 expression and survival was observed in the survival analysis of PDAC patients; however, high CLDN18.2 expression was related to longer overall survival. Our study is the third to research CLDN18.2 expression in PDAC. HER-2 expression is low and CLDN18.2 expression is high in patients with PDAC. HER-2 expression is not related to overall survival but CLDN18.2 is related and may be used as a prognostic marker in patients with PDAC.
Keywords: claudin 18.2, HER-2, pancreatic adenocarcinoma
1. Introduction
Pancreatic cancer is the fourth most common cancer-related cause of death (4.7%), accounting for 466,000 deaths worldwide.[1] Unfortunately, the median overall survival (OS) of patients with metastatic pancreatic ductal adenocarcinoma (PDAC) is less than a year. Ductal adenocarcinomas account for 85% of all pancreatic neoplasms.[2] Therapeutic agents with molecularly focused therapies have become more prevalent in recent years. Only a small percentage of the patients (10%) were currently eligible for targeted therapy. Olaparib is effective in patients with metastatic pancreatic cancer who have BRCA1/BRCA2 germline mutations[3]; entrectinib and larotrectinib are effective with neurotrophic tyrosine receptor kinase gene fusion,[4,5] dabrafenib with BRAF mutation,[6] and alpelisib with phosphoinositide 3-kinases mutation.[7]
It is vital to identify new targets for therapeutic antibodies in PDAC. Sahin discovered a tight junction molecule claudin-18.2 (CLDN18.2) expressed in a significant percentage of primary gastric tumors and their metastases.[8] The monoclonal antibody zolbetuximab, discovered by Schnatbaum, binds to cancer cells that are CLDN18.2 positive and leads to cancer cell death by antibody-dependent cellular toxicity and complement-dependent cytotoxicity.[9] In the phase 2a MONO study, conducted by Türeci,[10] and phase 2 FAST study conducted by Sahin,[11] zolbetuximab was effective in patients with metastatic gastric and esophageal adenocarcinoma. Clinical trials of zolbetuximab in the first-line setting in combination with chemotherapy and immunotherapy, are ongoing worldwide for metastatic gastric and gastroesophageal junction cancer (NCT03505320, NCT03504397, NCT03653507).
Wöll investigated the expression of CLDN18.2 in PDAC. Primary PDAC samples were determined to be positive at a rate of 59.2%. The majority of positive samples (54.6%) had high levels of CLDN18.2 expression with staining intensities ≥+2.[12] Non-clinical pancreatic cancer models were used in a study conducted by Türeci to examine the mechanism of action and anticancer efficacy of zolbetuximab. Human pancreatic cancer cells that expressed CLDN18.2 on their surface were selectively and strongly bound by zolbetuximab.[13] The effectiveness of zolbetuximab in patients with metastatic PDAC in conjunction with nab-paclitaxel and gemcitabine is currently being investigated in a clinical trial with the identifier NCT03816163.
Overexpression of the human epidermal growth factor receptor (HER-2) protein, which is a poor prognostic indicator, has been demonstrated to play a significant role in the onset and progression of breast cancer.[14] HER-2-directed therapeutic agents such as trastuzumab, pertuzumab, and lapatinib, and antibody-drug conjugates trastuzumab-emtansine and trastuzumab-deruxtecan displayed antitumor activity against breast cancer.[15–18] An antibody-drug combination trastuzumab-deruxtecan combines the humanized monoclonal antibody trastuzumab with the topoisomerase I inhibitor deruxtecan. In patients with HER-2-expressing metastatic breast cancer, it demonstrated greater clinical efficacy than the traditional anti-HER-2 therapy trastuzumab-emtansine.[19] In the phase 2 DESTINY-CRC01 clinical trial, trastuzumab deruxtecan showed clinical activity in patients with HER-2-expressing metastatic colorectal cancer.[20] Two clinical trials have investigated the clinical activity of trastuzumab-deruxtecan in patients with HER-2-expressing metastatic PDAC (NCT04482309 and NCT04639219).
We aimed to assess the expression of CLDN18.2 and HER-2, and the predictive value of both indicators for OS in patients with resected PDAC.
2. Materials and methods
The current study enrolled patients who underwent surgery and were diagnosed with PDAC at the Suleyman Demirel University Hospital, Turkey, between 2015 and 2019. This study was approved by the Ethics Committee of the School of Medicine of Suleyman Demirel University. As the investigation was retrospective, there was no need for scientific research funding. Informed consent was obtained from all the patients.
Sixty-eight patients with resected PDAC treated at a medical oncology clinic were assessed. All patients were over the age of 18 years, underwent follow-up and treatment in our unit, and had pathology slides that we could access. Clinicopathological data were obtained from medical files, including the patients’ age, sex, pathological parameters, and clinical stage according to the Eighth International Union against Cancer/American Joint Committee on Cancer. Patient survival and the period from the date of diagnosis to death were assessed in the follow-up data.
2.3. Ethical statement
The study protocol was approved by the Institutional Review Board (178-20.06.2022).
2.4. Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) 26.0 (SPSS Inc., Chicago, IL). Age and clinical characteristics were compared between patients with claudin 18.2 and HER-2 expression using the Mann–Whitney U test for individual samples. In patients with claudin 18.2 and HER-2 expression, sex, tumor size, localization, histological grade, perineural and lymphovascular invasion, necrosis, and pathological T and N stage were compared using Pearson chi-square test. The relationship between claudin 18.2 and HER-2 expression was determined by Student t test and the Spearman correlation test. Descriptive analyses were used for demographic data and the Kaplan–Meier test was used for survival analysis. OS was estimated using the Kaplan–Meier method and a log-rank test was used to compare the survival of the study groups. Multivariate analyses were performed using the Cox regression analysis. Statistical significance was set at P < .05.
3. Results
Seventy-three patients were identified, but 5 were excluded from the study because they dropped out of the follow-up. The mean age of the patients was 67.1 ± 9.95 (45.89) years. During a median follow-up of 13.3 months, 52 patients died of PDAC. Of the patients, 75% (n = 51) were male and 25% (n = 17) were female. Regarding tumor origin, 75% (n = 51) of the tumors originated in the head, 14.7% (n = 10) in the body, and 10.3% (n = 7) in the tail of the pancreas.
No HER-2 expression scores ≥ 2 were found in the samples. Only 25% (n = 17) of the samples had a HER-2 expression scores of + 1 (Fig. 1). There was no statistically significant difference between the HER-2 expression scores of the samples and patient clinicopathologic characteristics (Table 1). CLDN18.2 expression was detected in 54.4% (n = 37) of patient samples (Fig. 2). CLDN18.2 expression scores were +1 in 30.8% (n = 21) of the patient samples, +2 in 16.2% (n = 11), and +3 in 7.4% (n = 5). There was no statistically significant relationship between CLDN18.2 expression levels and patient clinicopathological characteristics (Table 2).
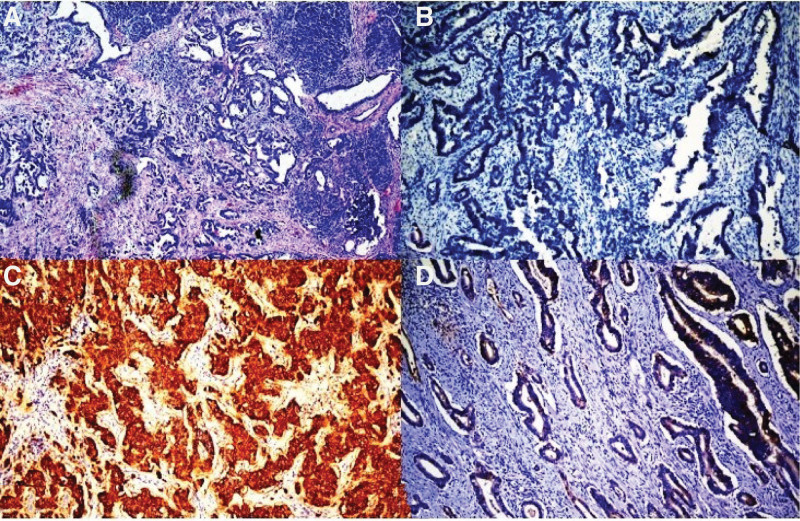
Figure 1.
Representative examples of human epidermal growth factor receptor-2 (HER-2) protein. (A) A pancreatic adenocarcinoma. (B) Pancreatic adenocarcinoma did not show expression of the HER-2 protein (score 0). (C) Breast carcinoma used as external control showed expression with HER-2 protein (score 3). (D) Pancreatic adenocarcinoma showed weak expression with HER-2 protein (score 1). (A) HE ×100; (B–D) HER-2 DAB ×100. DAB = 3,3′-diaminobenzidine, HE = hematoxylin eosin.
Table 1.
Clinicopathological classification of primary PDAC by HER-2 expression characteristics.
| HER-2 | |||||||
|---|---|---|---|---|---|---|---|
| Score 0 | Score 1 | ||||||
| n | % | n | % | n | % | P value | |
| Age | |||||||
| <60 | 16 | 23.5 | 13 | 81.3 | 38 | 73.1 | .382 |
| ≥60 | 52 | 76.5 | 3 | 18.8 | 14 | 26.9 | |
| Gender | |||||||
| Male | 51 | 75.0 | 39 | 76.5 | 12 | 70.6 | .425 |
| Female | 17 | 25.0 | 12 | 23.5 | 5 | 29.4 | |
| Grade | |||||||
| G1 | 31 | 45.6 | 21 | 41.2 | 10 | 58.8 | .232 |
| G2 | 31 | 45.6 | 25 | 49.0 | 6 | 35.3 | |
| G3 | 6 | 8.8 | 5 | 9.8 | 1 | 5.9 | |
| Localization | |||||||
| Head | 51 | 75.0 | 36 | 70.6 | 15 | 88.2 | .092 |
| Body | 10 | 14.7 | 8 | 15.7 | 2 | 11.8 | |
| Tail | 7 | 1.3 | 7 | 13.7 | 0 | 0.0 | |
| Tumor stage | |||||||
| 1B | 5 | 7.3 | 3 | 5.9 | 2 | 11.8 | .482 |
| 1C | 17 | 25.0 | 14 | 27.5 | 3 | 17.6 | |
| 2 | 24 | 35.3 | 19 | 37.3 | 5 | 29.4 | |
| 3 | 22 | 32.4 | 15 | 29.4 | 7 | 41.2 | |
| Node stage | |||||||
| 0 | 27 | 39.7 | 22 | 43.1 | 5 | 29.4 | .326 |
| 1 | 25 | 36.8 | 18 | 35.3 | 7 | 41.2 | |
| 2 | 16 | 23.5 | 11 | 21.6 | 5 | 29.4 | |
| Clinical stage | |||||||
| IA | 13 | 19.1 | 10 | 19.6 | 3 | 17.6 | .388 |
| IB | 9 | 13.2 | 8 | 15.7 | 1 | 5.9 | |
| IIA | 5 | 7.4 | 4 | 7.8 | 1 | 5.9 | |
| IIB | 25 | 36.8 | 18 | 35.3 | 7 | 41.2 | |
| III | 16 | 23.5 | 11 | 21.6 | 5 | 29.4 | |
| PNI | |||||||
| Yes | 48 | 70.6 | 36 | 70.6 | 12 | 70.6 | .628 |
| No | 20 | 29.4 | 15 | 29.4 | 5 | 29.4 | |
| LVI | |||||||
| Yes | 39 | 57.4 | 29 | 56.9 | 10 | 58.8 | .559 |
| No | 29 | 42.6 | 22 | 43.1 | 7 | 41.2 | |
| Necrosis | |||||||
| Yes | 9 | 13.2 | 9 | 17.6 | 0 | 0.0 | .062 |
| No | 59 | 86.8 | 42 | 82.4 | 17 | 100.0 | |
| Exitus | |||||||
| Yes | 52 | 76.5 | 39 | 78.0 | 13 | 86.7 | .371 |
| No | 16 | 23.5 | 11 | 22.0 | 2 | 13 | |
Since both scores 2 and 3 are not expressed, they are not listed in the table.
HER-2 = human epidermal growth factor receptor 2, LVI = lymphovascular invasion, PDAC = pancreatic ductal adenocarcinoma, PNI = perineural invasion.
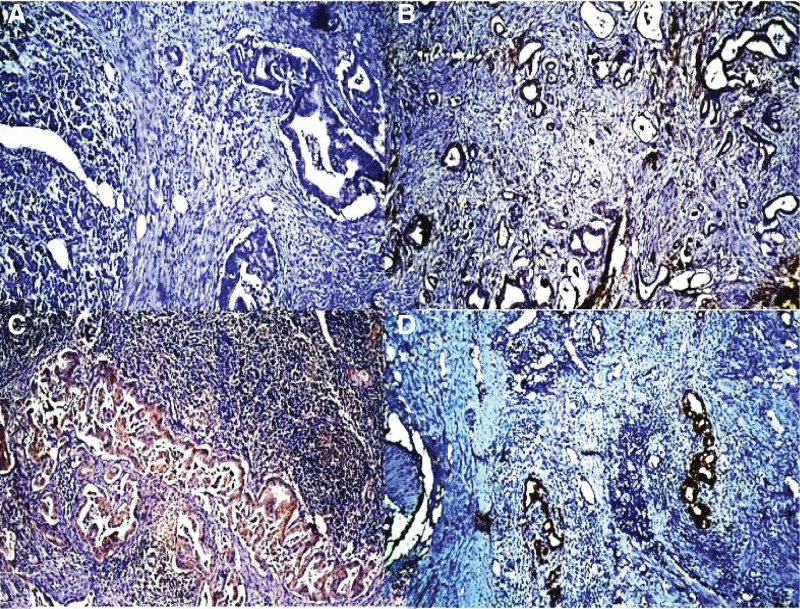
Figure 2.
Representative examples of the claudin 18.2 (CLDN18.2) protein. (A) CLDN18.2 protein negativity in pancreatic adenocarcinoma (score 0). (B) Weak membranous staining with CLDN18.2 protein (score 1). (C) Moderate membranous staining with CLDN18.2 protein (score 2). (D) Strong membranous staining with CLDN18.2 protein (score 3) (CLDN18.2 DAB ×100). DAB = 3,3′-diaminobenzidine.
Table 2.
Clinicopathological classification of primary PDAC by CLDN18.2 expression characteristics.
| CLDN18.2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | Score 3 | P value | |||||
| n | % | n | % | n | % | n | % | ||
| Age | |||||||||
| <60 | 7 | 43.8 | 4 | 25 | 3 | 18.8 | 2 | 12.5 | .479 |
| ≥60 | 24 | 46.2 | 17 | 32.7 | 8 | 15.4 | 3 | 5.8 | |
| Gender | |||||||||
| Male | 20 | 64.5 | 18 | 85.7 | 9 | 81.8 | 4 | 80 | .185 |
| Female | 11 | 35.5 | 3 | 14.3 | 2 | 18.2 | 1 | 20 | |
| Grade | |||||||||
| G1 | 16 | 51.6 | 9 | 42.9 | 4 | 36.4 | 2 | 40 | .633 |
| G2 | 12 | 38.7 | 10 | 47.6 | 6 | 54.5 | 3 | 60 | |
| G3 | 3 | 9.7 | 2 | 9.5 | 1 | 9.1 | 0 | 0 | |
| Localization | |||||||||
| Head | 21 | 67.7 | 16 | 76.2 | 9 | 81.8 | 5 | 100 | .067 |
| Body | 5 | 16.1 | 3 | 14.3 | 2 | 18.2 | 0 | 0 | |
| Tail | 5 | 16.1 | 2 | 9.5 | 0 | 0 | 0 | 0 | |
| Tumor stage | |||||||||
| 1B | 8 | 25.8 | 3 | 14.3 | 4 | 36.4 | 2 | 40 | .731 |
| 1C | 4 | 12.9 | 0 | 0 | 1 | 9.1 | 0 | 0 | |
| 2 | 9 | 29 | 9 | 42.9 | 4 | 36.4 | 2 | 40 | |
| 3 | 10 | 32.3 | 9 | 42.9 | 2 | 18.2 | 1 | 20 | |
| Node stage | |||||||||
| 0 | 13 | 41.9 | 9 | 42.9 | 5 | 45.5 | 0 | 0 | .579 |
| 1 | 10 | 32.3 | 7 | 33.3 | 5 | 45.5 | 3 | 60 | |
| 2 | 8 | 25.8 | 5 | 23.8 | 1 | 9.1 | 2 | 40 | |
| Clinical stage | |||||||||
| IA | 6 | 19.4 | 3 | 14.3 | 4 | 36.4 | 0 | 0 | .645 |
| IB | 4 | 12.9 | 4 | 19 | 1 | 9.1 | 0 | 0 | |
| IIA | 3 | 9.7 | 2 | 9.5 | 0 | 0 | 0 | 0 | |
| IIB | 10 | 32.3 | 7 | 33.3 | 5 | 45.5 | 3 | 60 | |
| III | 8 | 25.8 | 5 | 23.8 | 1 | 9.1 | 2 | 40 | |
| PNI | |||||||||
| Yes | 24 | 77.4 | 13 | 61.9 | 8 | 72.7 | 3 | 60 | .41 |
| No | 7 | 22.6 | 8 | 38.1 | 3 | 27.3 | 2 | 40 | |
| LVI | |||||||||
| Yes | 17 | 54.8 | 12 | 57.1 | 6 | 54.5 | 4 | 80 | .48 |
| No | 14 | 45.2 | 9 | 42.9 | 5 | 45.5 | 1 | 20 | |
| Necrosis | |||||||||
| Yes | 4 | 12.9 | 3 | 18.2 | 2 | 18.2 | 0 | 0 | .799 |
| No | 27 | 87.1 | 18 | 81.8 | 9 | 81.8 | 5 | 100 | |
| Exitus | |||||||||
| Yes | 24 | 82.8 | 17 | 81 | 8 | 80 | 3 | 60 | .361 |
| No | 5 | 17.2 | 4 | 19 | 2 | 20 | 2 | 40 | |
CLDN18.2 = claudin 18.2, LVI = lymphovascular invasion, PDAC = pancreatic ductal adenocarcinoma, PNI = perineural invasion.
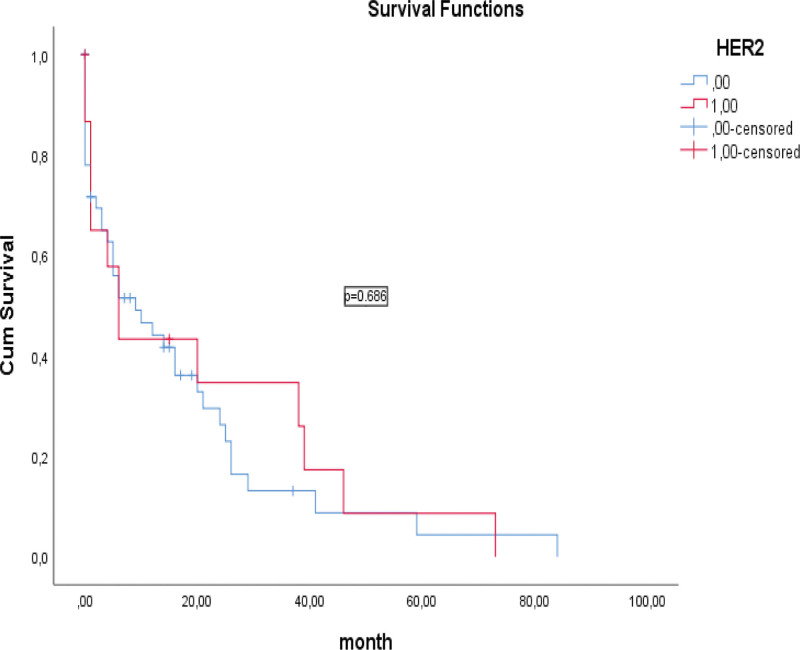
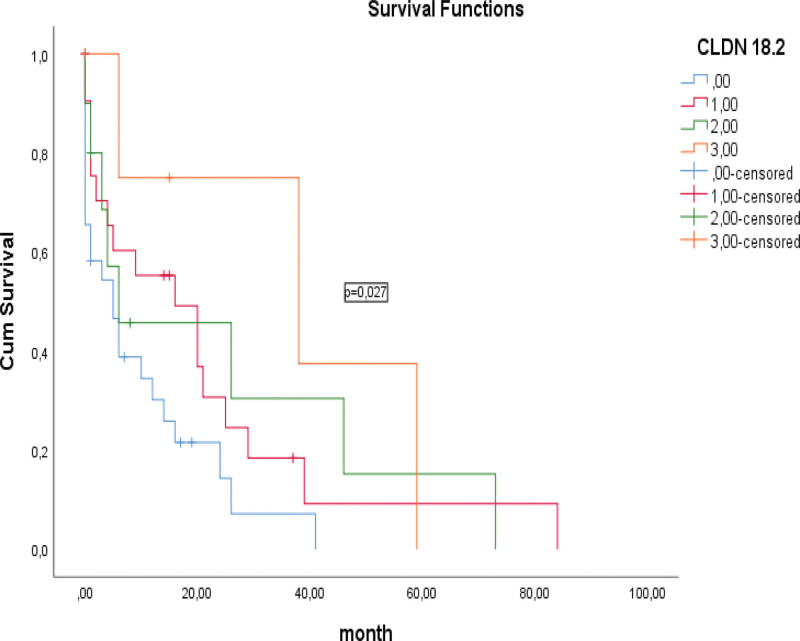
When CLDN18.2 and HER-2 expression were compared, a statistically significant difference and moderate positive correlation was observed (P < .0001, R = 0.346). No statistically significant relationship was observed between HER-2 expression and OS in the survival analysis of patients (16.6 vs 20.1 months; P = .686; Fig. 3). However, a statistically significant relationship was observed between CLDN18.2 expression and OS (9.8 vs 37.9 months; P = .027; Fig. 4). According to the CLDN18.2 scoring scheme, patients with CLDN18.2 IHC score of 3 had better survival than those with CLDN18.2 IHC score of 0, 1 and 2.
Figure 3.
Kaplan–Meier curves of overall survival based on staining for HER-2. HER-2 = human epidermal growth factor receptor-2.
Figure 4.
Kaplan–Meier curves of overall survival based on staining for claudin 18.2 (CLDN18.2).
4. Discussion
This research is crucial because it seeks to determine whether highly fatal pancreatic cancer has receptors to allow treatment with targeted drugs and whether the target receptors can predict prognosis.
In this study, there was no HER-2 ≥ 2+ overexpression, and only 25% (n = 17) of the samples had HER-2 expression scores of +1. No relationship was found between the clinicopathological characteristics of the study patients and HER-2 expression and no significant relationship was present between HER-2 expression and OS. Few studies researched HER-2 expression in PDAC, and the results are conflicting. Bittoni researched HER-2 expression in 91 PDAC patient samples and detected no HER-2 3+ expression, and only 1 (1.1%) 1+ sample[21] which is consistent with the current study. Aumayr detected HER-2 + 2 or +3 expression in 10.3% of 87 PDAC cases.[22] These contradictory results could be explained by variations in the immunohistochemistry methodology.
There was no correlation between HER-2 expression and OS in PDAC patients. HER-2 expression was not prognostic in patients with resected PDAC, but 2 clinical trials with identification numbers NCT04482309 and NCT04639219 will provide answers about whether HER-2 expression is predictive.
The question of whether PDAC samples express CLDN18.2 and if IMAB362 (zolbetuximab) can be a therapeutic option for PDAC patients is one that Wöll study is the first to address in the literature.[12]
CLDN18.2 expression was detected in 54.4% (n = 37), and ≥2+ in 23.5% (n = 16) of samples from patients with PDAC. In the first published study, Sahin evaluated the expression of CLDN18.2 in 10 PDAC samples. In that study, CLDN18.2 expression was present in 80% (8) and 2+ in 60% (6) of the samples.[8] Our study included more patients (n = 68) with PDAC, and the number of patients may be the source of this variation. In the second published study, 174 PDAC samples were examined, and CLDN18.2 expression was detected in 59.2% (n = 103) and ≥2+ cells in 54.6% (n = 95).[12] The CLDN18.2 expression in our study is consistent with the study by Wöll.[12] We detected no relationship between clinicopathological characteristics of PDAC patients and CLDN18.2 expression in samples. However, Wöll detected higher CLDN18.2 expression in the lymph node metastasis positive group.[12] This discordance may be due to differences in the postoperative tumor and node stages of the patients.
In our study, there was a statistically significant relationship between CLDN18.2 expression and OS. Higher CLDN18.2 expression was associated with longer OS. CLDN18.2 expression is prognostic in operated PDAC patients. This data is new in the literature, and our study is the first to examine OS and CLDN18.2 expression in PDAC patients.
There was no relationship between HER-2 and CLDN18.2 expression in PDAC cases. Additional studies with a larger number of patients are needed to define the effect of CLDN18.2 expression on OS. The limitations of our study include the relatively small number of patients analyzed and the retrospective nature of the study. However clinical trials with identification number NCT03816163 will show the prognostic effect of CLDN18.2 expression and the effectiveness of zolbetuximab in PDAC patients.
5. Conclusion
Our study is the third to investigate CLDN18.2 expression in PDAC. HER-2 expression is low and CLDN18.2 expression is high in patients with PDAC. HER-2 expression is not related to OS, but CLDN18.2 is and may be used as a prognostic marker in patients with PDAC.
Acknowledgments
The authors acknowledge the research coordinators who helped conduct this study.
Author contributions
Conceptualization: Erkan Kayikcioglu, Ramazan Oğuz Yüceer.
Data curation: Erkan Kayikcioglu.
Formal analysis: Erkan Kayikcioglu.
Investigation: Erkan Kayikcioglu, Ramazan Oğuz Yüceer.
Methodology: Erkan Kayikcioglu, Ramazan Oğuz Yüceer.
Resources: Erkan Kayikcioglu.
Validation: Erkan Kayikcioglu.
Writing – original draft: Erkan Kayikcioglu, Ramazan Oğuz Yüceer.
Writing – review & editing: Erkan Kayikcioglu, Ramazan Oğuz Yüceer.
Abbreviations:
- CLDN18.2
- claudin 18.2
- HER-2
- human epidermal growth factor receptor-2
- IHC
- immunohistochemical
- OS
- overall survival
- PDAC
- pancreatic ductal adenocarcinoma
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Kayikcioglu E, Yüceer RO. The role of claudin 18.2 and HER-2 in pancreatic cancer outcomes Medicine 2023;102:6(e32882).
References
- [1].Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- [2].Schlitter AM, Konukiewitz B, Kasajima A, et al. [Ductal adenocarcinoma of the pancreas: subtypes and molecular pathology]. Pathologe. 2021;42:464–71. Das duktale Adenokarzinom des Pankreas: Subtypen und Molekularpathologie. [DOI] [PubMed] [Google Scholar]
- [3].Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Drilon A, Siena S, Ou SI, et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017;7:400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Singh RR, O’Reilly EM. New treatment strategies for metastatic pancreatic ductal adenocarcinoma. Drugs. 2020;80:647–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ebrahimi S, Hosseini M, Shahidsales S, et al. Targeting the Akt/PI3K signaling pathway as a potential therapeutic strategy for the treatment of pancreatic cancer. Curr Med Chem. 2017;24:1321–31. [DOI] [PubMed] [Google Scholar]
- [8].Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14:7624–34. [DOI] [PubMed] [Google Scholar]
- [9].Schnatbaum K, Schmoldt HU, Daneschdar M, et al. Peptide microarrays enable rapid mimotope optimization for pharmacokinetic analysis of the novel therapeutic antibody IMAB362. Biotechnol J. 2014;9:545–54. [DOI] [PubMed] [Google Scholar]
- [10].Türeci O, Sahin U, Schulze-Bergkamen H, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol. 2019;30:1487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sahin U, Türeci O, Manikhas G, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32:609–19. [DOI] [PubMed] [Google Scholar]
- [12].Wöll S, Schlitter AM, Dhaene K, et al. Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int J Cancer. 2014;134:731–9. [DOI] [PubMed] [Google Scholar]
- [13].Türeci Ӧ, Mitnacht-Kraus R, Wöll S, et al. Characterization of zolbetuximab in pancreatic cancer models. Oncoimmunology. 2019;8:e1523096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Han SH, Ryu KH, Kwon AY. The prognostic impact of HER2 genetic and protein expression in pancreatic carcinoma-HER2 protein and gene in pancreatic cancer. Diagnostics (Basel). 2021;11:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Johnston SRD, Hegg R, Im SA, et al. Phase III, randomized study of dual Human Epidermal Growth Factor Receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-positive, hormone receptor-positive metastatic breast cancer: updated results of ALTERNATIVE. J Clin Oncol. 2021;39:79–89. [DOI] [PubMed] [Google Scholar]
- [17].Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cortés J, Kim SB, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–54. [DOI] [PubMed] [Google Scholar]
- [20].Siena S, Di Bartolomeo M, Raghav K, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22:779–89. [DOI] [PubMed] [Google Scholar]
- [21].Bittoni A, Mandolesi A, Andrikou K, et al. HER family receptor expression and prognosis in pancreatic cancer. Int J Biol Markers. 2015;30:327e327–332. [DOI] [PubMed] [Google Scholar]
- [22].Aumayr K, Soleiman A, Sahora K, et al. HER2 gene amplification and protein expression in pancreatic ductal adenocarcinomas. Appl Immunohistochem Mol Morphol. 2014;22:146–52. [DOI] [PubMed] [Google Scholar]