Background:
Traumatic brain injury (TBI) is a major health and socioeconomic problem that affects all societies. Consciousness disorder is a common complication after TBI while there is still no effective treatment currently. The aim of this study was to investigate the protective effect of electro-acupuncture (EA) on cognitive recovery for patients with mild TBI.
Methods:
A total of 83 patients with initial Glasgow coma scale score higher than 12 points were assigned into this study. Then patients were randomly divided into 2 groups: EA group and control group (group C). Patients in group EA received EA treatment at Neiguan and Shuigou for 2 weeks. At 0 minute before EA treatment (T1), 0 minute after EA treatment (T2), and 8 weeks after EA treatment (T3), level of neuron-specific enolase (NSE), glial fibrillary acidic protein (GFAP), hypoxia inducible factor-1α (HIF-1α), and malondialdehyde were tested by enzyme-linked immunosorbent assay. The score of Montreal Cognitive Function Assessment (MoCA) and mini-mental state examination (MMSE) as well as cerebral oxygen saturation (rSO2) were detected at the same time.
Results:
Compared with the baseline at T1, the level of NSE, GFAP, HIF-1α, MDA, and rSO2 decreased, and the score of MoCA and MMSE increased in the 2 groups were significantly increased at T2–3 (P < .05). Compared with group C, the level of NSE, GFAP, HIF-1α, MDA, and rSO2 decreased, and the score of MoCA and MMSE increased were significantly increased at T2–3 in group EA; the difference were statistically significant (P < .05).
Conclusions:
EA treatment could improve the cognitive recovery for patients with mild TBI and the potential mechanism may be related to improving cerebral hypoxia and alleviating brain injury.
Keywords: cerebral oxygen metabolism, cognitive recovery, electro-acupuncture, traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) is a common and multifaceted disease that seriously endangers human health. In the United States, it is the leading cause of death and disability among people under 45 years of age, and its incidence has been on the rise for some time.[1–3] TBI-affected brain tissue has a high metabolic rate and is more susceptible to hypoxia, which can result in irreversible damage to the central nervous system.[4,5] Studies have found that more than 90% of patients with TBI are accompanied by ischemia and hypoxia of local brain tissue, which cause abnormal brain metabolism, cerebral perfusion and brain tissue damage.[6] According to Elder GA, there are approximately 558 people in every hundred thousand will suffered from TBI, and a quarter of these patients will also have cognitive impairments, such as memory, attention, thinking abilities, and executive functions.[7] Cognitive impairment after TBI may accompany patients for life, making it one of the most important socio-economic and public health problems.[8]
Over 2000 years ago, acupuncture was developed in China as a special treatment for systemic diseases through the conduction of meridians and acupoints.[9] Electro-acupuncture (EA) is a new therapeutic method developed by increasing electric current of different frequency and intensity on the basis of traditional acupuncture. Wong showed EA has a unique therapeutic effect on the treatment and rehabilitation of TBI patients.[10] The parameters of waveform, time, frequency, and intensity can be adjusted to produce different treatment effects based on the acupoint and electrical stimulation combined. The research from Liu J[11] showed EA intervention can effectively promote the recovery of consciousness after TBI with initial Glasgow coma scale score of less than 8 points, but the exact protective mechanism of EA is still unclear.
Given that this aim of our study was to explore the protective effect of EA on cognitive recovery for patients with mild TBI, so as to provide a potential choice for cognitive recovery in patients with TBI.
2. Methods
This prospective, randomized, controlled trial has been approved by the Ethics Committee of Cangzhou Central Hospital and complies with the Helsinki Declaration. Obtained written informed consent from each participating patient before randomization.
2.1. Participants
Between February 2019 and July 2022, patients suffered from mild TBI in the department of neurosurgery, Cangzhou Central Hospital were eligible for the study. The inclusion/exclusion criteria for subject enrollment have been reported previously.[12] The inclusion criteria were as follows: Meet the diagnostic criteria for TBI (confirmed by MRI and brain CT examination); Patients aged 18 to 70 years old, male or female; Initial Glasgow coma scale score higher than 12 on the beginning day; MRI shows head has no obvious shift, missing, large necrosis of brain structure change and obvious brain stem (not including pyramidal tract) or thalamic lesions, each lobe lesions range cannot exceed 30% of the scope of 1 side of the brain; No primary consciousness disorder and limb functional activity disorder; All patients or their family members signed informed consent; Able to receive oral drug and EA treatment. The exclusion criteria were as follows: Cognitive impairment of patients were not induced by TBI; Suffered from TBI more than 1 years; Combined with heart, liver or kidney failure endanger the safety of life at any time; Patients were younger than 18 years or older than 70 years; Refused to receive treatment of EA or not receiving a full course of treatment; Skin infection occurs at the corresponding points of EA intervention; Women with pregnancy and lactation; History of drug or alcohol addiction; Need for any operation. The trial will be ceased if one of the following conditions appeared a serious poststroke complication arises or recurrent stroke or any other severe condition occurs leaving the patient in a critical condition.
2.2. Randomization and blinding
Eighty-three individuals completed a baseline assessment and were randomly divided into 2 groups using the random number table method: control group (group C, n = 43) and EA group (n = 40). All patients were blinded to the group allocations. The physicians who conducted EA intervention, evaluated and analyzed the patients knew nothing about the grouping.
2.3. Assessment and intervention procedures
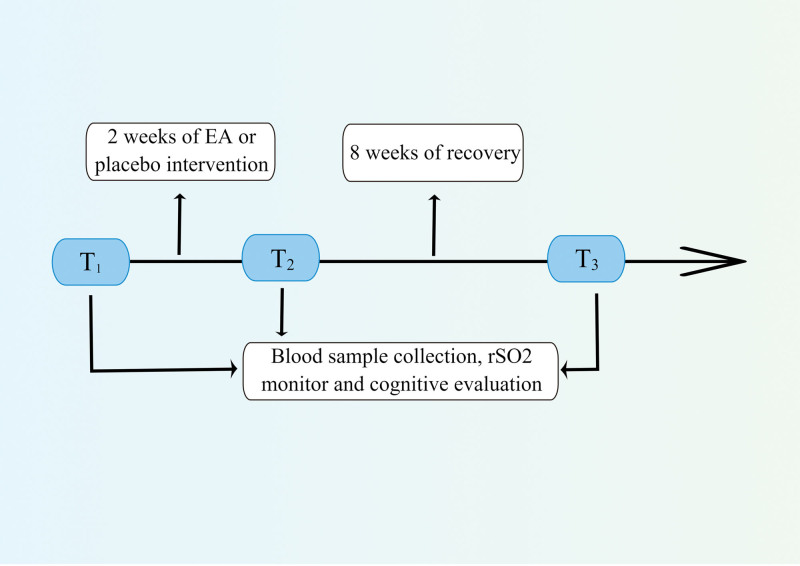
Figure 1 shows the schematic of the intervention timeline. In the 2-weeks treatment phase after eligibility, participants in both groups received conventional treatment including the prescription of coma arousal and neuroprotective medicines. In addition to conventional treatment, patients in the group EA received a 2-weeks EA treatment at Neiguan (PC 6) and Shuigou (GV 26) with disperse-dense wave, 2 Hz/100 Hz in frequency, 0.1 to 5 mA in intensity by EA stimulator instrument (Model G6805; SMIF, Shanghai, China) according to the method of previous research for 30 minutes once daily,[11] while patients in group C were not received EA treatment. Neiguan is located between the palmaris longus tendon and the flexor carpi radialis tendon, 2 inches above the wrist striation. Shuigou is located at the intersection of upper and middle 1/3 of Renzhong ditch.
Figure 1.
Schematic of the intervention timeline (drawn by Figdraw, ID:TRPWO2c2ee).
2.4. Sample collection and detection
Blood samples of 4 mL were collected from the 2 groups 0 minute before EA treatment (T1), 0 minute after EA treatment (T2), and 8 weeks after EA treatment (T3). After centrifugation at 3000 × g for 15 minutes, the collected serum was stored at −80°C. Serum concentrations of neuron-specific enolase (NSE) (Item No. EPX010-12335-901, Thermo Scientific, Waltham), GFAP (Item No. ab114149, Abcam, Cambridge, UK), hypoxia inducible factor-1α (Item No. ab111577, Abcam, Cambridge, UK), and malondialdehyde (Item No. ab287797, Abcam, Cambridge, UK) were detected by enzyme-linked immunosorbent assay according to the manufacturer’s instructions.
Cerebral oxygen saturation (rSO2) detector (Covidine II) was used to monitor rSO2. Two electrodes were placed on the left and right forehead, 4 cm away from the eyebrow arch. At T1–3, rSO2 were recorded.
2.5. Cognitive function evaluation
Cognitive function was assessed using the Montreal Cognitive Function Assessment (MoCA)[13] scale and the mini-mental state examination (MMSE)[14] scale at T1–3. MoCA mainly includes cognitive assessment of visual spatial executive ability, naming, memory, attention, language fluency, abstract thinking, delayed memory, orientation, etc. MMSE is a scale of cognitive function which could evaluate patients’ time-directed force, site directed force, immediate memory, attention and computing power, delayed memory, language, and visual space. MoCA and MMSE contains 30 questions, and patients will obtain 1 point if answer is correct, but 0 point if the answer is wrong. The total scores less than 27 indicate cognitive dysfunction.
2.6. Sample size estimation and statistical analyses
The sample size of the study was calculated using the G*Power program (V.3.1.9) (gpower.hhu.de/). We aimed to show a significant difference in cognitive function. According to preliminary experimental results,[12] the required sample size was thus 36 subjects per group with 80% power and a 2-tailed α error of 5%. Considering a high incidence of dropout, we decided to include 40 patients in each group at least.
SPSS 21.0 software (SPSS, Inc., Chicago, IL) was applied to statistical analysis of all experimental data, and normally distributed measurement data were represented as mean ± standard deviation. Categorical variables are analyzed by χ2 test or Fisher exact test and presented as frequencies and percentages. One-way analysis of variance was used to compare the data between groups and within groups. P < .05 was regarded as statistically significant.
3. Results
3.1. Demographic characters of patients
As shown in flow diagram of this study, there were 208 individuals evaluated for eligibility; 68 participants did not meet eligibility criteria, and of the 140 eligible participants, 57 patients declined participation. Of the patients who declined participation, 30 participants were “not interested,” 19 participants reported they worried about affecting recovery, and 8 participants reported “other reasons.” During this study period, there were no participants refused to be followed up. Finally, a total of 83 patients were enrolled in this study and assigned into 2 groups: group C (n = 43) and group EA (n = 40).
As shown in Table 1, the demographic data of the 2 groups, such as: age, gender, body mass index (BMI), education years, Glasgow coma scale score, course of disease after injury, as well as the cause of injury, and the differences were no statistically significant (P > .05).
Table 1.
Demographic data of patients between 2 groups (χ ± s).
| Characteristics | Group C (n = 43) | Group EA (n = 40) | P value |
|---|---|---|---|
| Age (yr) | 50.84 ± 6.17 | 51.92 ± 6.50 | .562 |
| Gender | .668 | ||
| Male [(n) %] | 26 (60.47%) | 25 (62.5%) | |
| Female [(n) %] | 17 (39.53%) | 15 (37.5%) | |
| BMI (kg/m2) | 25.19 ± 2.67 | 24.88 ± 2.91 | .652 |
| Education years (yr) | 8.81 ± 2.64 | 7.69 ± 2.35 | .481 |
| GCS score | 13.85 ± 1.34 | 14.02 ± 1.51 | .269 |
| Course of disease after injury (d) | 10.49 ± 2.03 | 11.35 ± 2.41 | .389 |
| Cause of injury | |||
| Car accident injury [(n) %] | 19 (44.19%) | 16 (40%) | .447 |
| Falling injury [(n) %] | 11 (25.58%) | 10 (25%) | .782 |
| Hit injury [(n) %] | 7 (16.28%) | 6 (15%) | .519 |
| Other reason [(n) %] | 6 (19.95%) | 8 (20%) | .681 |
BMI = body mass index, EA = electro-acupuncture, GCS = Glasgow coma scale.
3.2. NSE, GFAP, HIF-1α, and MDA levels
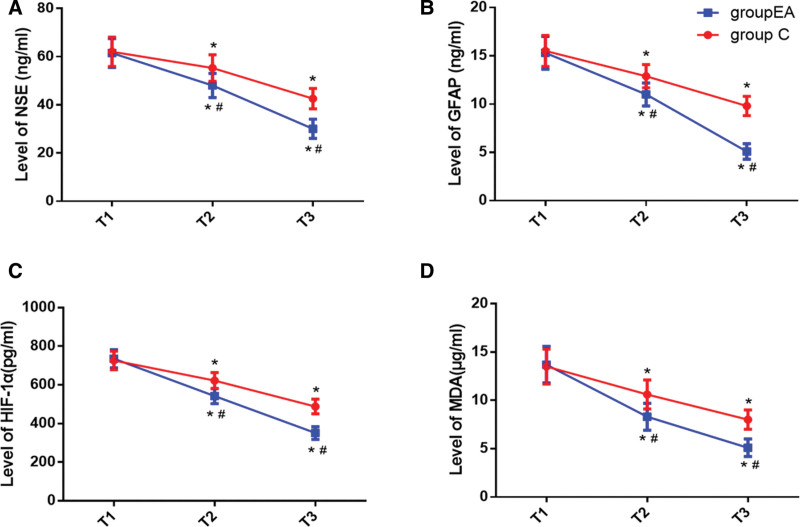
As shown in Figure 2, there was no significant difference in level of NSE, GFAP, HIF-1α and MDA between 2 groups at T1 (P > .05). Compared with the baseline at T1, the level of NSE, GFAP, HIF-1α, and MDA in both 2 groups were significantly increased at T2–3 (P < .05). The level of NSE, GFAP, HIF-1α, and MDA in group EA at T2–3 were significantly decreased compared with group C, and the difference was statistically significant (P < .05).
Figure 2.
Concentrations of serum NSE (A), GFAP (B), HIF-1α (C), and MDA (D). Compared with T1, *P < .05; compared with Group C, #P < .05. Flow diagram of study. Group C (red) and Group EA (blue). EA = electro-acupuncture, GFAP = glial fibrillary acidic protein, HIF-1α = hypoxia inducible factor-1α, MDA = malondialdehyde, NSE = neuron-specific enolase.
3.3. Cerebral oxygen saturation
Throughout the observation, the rSO2 of the 2 groups fluctuated within the normal range. As shown in Table 2, compared with T1, the values of rSO2 on 2 sides increased in 2 groups at T2–3, the values of rSO2 on 2 sides increased in group EA at T2–3 compared with group C (P < .05).
Table 2.
Comparison of cerebral oxygen saturation between 2 groups (χ ± s).
| Time | Group | Group C (n = 43) | Group EA (n = 40) | P value |
|---|---|---|---|---|
| T1 | Left | 58.22 ± 5.72 | 59.17 ± 5.52 | .374 |
| Right | 59.17 ± 4.98 | 59.55 ± 5.41 | .525 | |
| T2 | Left | 66.28 ± 6.03* | 74.68 ± 6.42*,# | .008 |
| Right | 66.92 ± 6.15* | 75.24 ± 6.64*,# | .027 | |
| T3 | Left | 74.58 ± 6.81* | 83.29 ± 7.78*,# | .016 |
| Right | 75.14 ± 6.93* | 84.12 ± 7.89*,# | .021 |
EA = electro-acupuncture, T1 = 0 min before EA treatment, T2 = 0 min after EA treatment, T3 = 8 weeks after EA treatment.
P < .05, compared with T1.
#P < .05, compared with group C.
3.4. Cognitive function
As shown in Table 3, the score of MoCA and MMSE increased at T2 and T3 in both 2 groups compared with T1 (P < .05). Compared with, group C, the score of MoCA and MMSE increased at T2 and T3 in group EA (P < .05).
Table 3.
Comparison of cognitive function score between 2 groups (χ ± s).
| Time | Group C (n = 43) | Group EA (n = 40) | P value | |
|---|---|---|---|---|
| T1 | MoCA | 15.74 ± 2.66 | 16.02 ± 2.79 | .618 |
| MMSE | 16.89 ± 2.35 | 17.25 ± 2.58 | .592 | |
| T2 | MoCA | 18.06 ± 2.34* | 20.55 ± 2.18*,# | .014 |
| MMSE | 18.67 ± 2.20* | 20.13 ± 2.470*,# | .026 | |
| T3 | MoCA | 19.15 ± 2.13* | 22.47 ± 2.64*,# | .008 |
| MMSE | 20.33 ± 2.05* | 22.99 ± 2.15*,# | .019 |
EA = electro-acupuncture, T1 = 0 min before EA treatment, T2 = 0 min after EA treatment, T3 = 8 weeks after EA treatment, MMSE = mini-mental state examination, MoCA = Montreal Cognitive Function Assessment.
P < .05, compared with T1.
#P < .05, compared with group C.
4. Discussion
With the characteristics of high energy consumption, high metabolism, and high oxygen consumption, the brain is very sensitive to ischemia and hypoxia. But the TBI destroy the normal homeostasis of the brain and the automatic regulation of cerebral blood flow which easily lead to cognitive impairment. Previous study showed EA treatment can effectively promote the recovery of consciousness after TBI, but the potential mechanism is still unclear. In the present research, we found that EA treatment could improve the cognitive recovery for patients with mild TBI and the potential mechanism may be related to improving cerebral hypoxia and alleviating brain injury.
There is a close relationship between TBI and cerebral oxygen metabolism disorder.[15] Verweij BH et al found that body energy metabolism, especially cerebral oxygen metabolism was impaired after TBI, which induced a series of adverse events, such as cognitive dysfunction.[16] Cerebral metabolism is highly dependent on cerebral blood flow and brain oxygen supply,[17] and Khellaf A et al have shown that the brain tissue ischemia and hypoxia rate of TBI patients after TBI can reach 92%.[18] Partly because head trauma can directly cause primary brain damage, such as brain contusion, subarachnoid hemorrhage and cerebrovascular injury, which arouse damage to the blood-brain barrier and the dysfunction of cerebrovascular autonomic regulation, resulting in insufficient brain tissue perfusion, reduced oxygen supply, and cerebral tissue ischemia and hypoxia.[19,20] And professor Salehi had also pointed out that there would emerge cerebral vasospasm and vascular regulation disorders after brain injury, which reduced cerebral perfusion to regions of the brain.[21] On the other hand, by causing brain tissue energy metabolism disorder, excitatory transmitter release, cell apoptosis and other secondary brain damage, aggravates cerebral edema and cerebral microcirculation disorders.[22] The interaction between the 2 forms a vicious circle of increased intracranial pressure, decreased cerebral perfusion pressure, and nerve cell ischemia and hypoxia. Rockswold SB et al found that improvement of cerebral oxygen metabolism could decrease markers of oxidative metabolism in relatively uninjured brain as well as pericontusional tissue, reduced intracranial hypertension.[23] LuY et al found that hyperbaric oxygen treatment can improve the prognosis of TBI patients by improving cerebral oxygen metabolism.[24] Therefore, the intervention of cerebral oxygen metabolism is the key point of TBI treatment.
EA has an protective effect on the nervous system and immune system, which has analgesic effect, immune regulation, and Organ function protection.[25,26] Regional rSO2 is a commonly used indicator for monitoring rSO2 in clinical practice, which can timely and accurately reflect the balance between oxygen supply and oxygen demand in patients’ brain tissue, and the decrease of rSO2 index indicates insufficient cerebral tissue perfusion.[27] The study from Ning JQ showed EA stimulation can increase rSO2 levels in patients with diabetes.[28] The results of this study showed that EA treatment could decrease rSO2 level and increase the score of MoCA and MMSE, indicating that EA treatment could improve the recovery of consciousness after TBI by decreasing cerebral oxygen metabolism.
HIF-1α is a common transcription factor closely related to hypoxia in humans and other mammals, but it is easy to be degraded under the condition of normal oxygen and hardly be detected.[29] Only under hypoxia conditions can HIF-1α be expressed stably and the abnormal expression of it is involved in the development of ischemic and hypoxic diseases. A large number of domestic and foreign studies have found that the levels of HIF-1α and its mRNA in the body are significantly increased after brain injury, which promoted the cerebral vascular repair and regeneration.[30,31] TBI can make patients in a continuous stress state, increase brain oxygen consumption, stimulate the body to release a large number of inflammatory factors, cause brain edema, and aggravate brain hypoxia. Cerebral ischemia and hypoxia can promote the accumulation of a large number of oxygen free radicals and toxic substances in the body, membrane lipid peroxidation reaction, significantly increase the content of oxidative stress products. MDA is the end product of the peroxidation of free radicals and lipids, and is a sensitive index of damage.[32] It can reflect the content of oxygen free radicals and the degree of oxidative damage. The results of the current study showed that, compared with group C, the serum HIF-1α and MDA concentrations of patients in group EA at T2–3 were decreased, suggesting that EA could reduce cerebral oxidative stress.
TBI can directly cause cerebral hemorrhage to damage the blood-brain barrier, and can also through calcium overload, oxygen free radical damage, inflammatory factor release and other secondary damage to increase the blood-brain barrier permeability, so as to enable macromolecular substances to pass through the barrier.[33] NSE and GFAP are important neurobiological markers of brain injury. NSE is mainly found in nerve tissue and neuroendocrine tissue. It is a marker of neuronal cell body damage and a sensitive indicator of nerve cell death. GFAP is a highly specific marker of astrocytes and central nervous system. It is currently the most stable biological indicator of TBI, and its plasma concentration is not affected by multiple injuries.[34] Under physiological conditions, NSE and GFAP are very low in plasma, however, when the structural and functional integrity of nerve cells is destroyed and the permeability of the blood-brain barrier increases, its plasma concentration increased significantly. In the present study, the serum NSE and GFAP concentrations of patients in group EA decreased at T2–3 compared with group C, suggesting that EA can protect neurons and improve brain injury in patients with TBI.
It is undeniable that there are still some limitations in the current study. In this study, we only collected the blood sample at the intervention stage and 8 weeks after intervention, frequency of assessment needs to be increased and the follow-up time needs to be extended to obtain more accurate results.
5. Conclusion
In conclusion, EA treatment could improve the cognitive recovery for patients with mild TBI and the potential mechanism may be related to improving cerebral hypoxia and alleviating brain injury.
Acknowledgment
The authors would like to thank all the members of Department of Neurosurgery, Cangzhou Central Hospital for their great help and support.
Author contributions
Conceptualization: Haokun Jia.
Data curation: Yonghan Chen, Yi Wang, Hao Jiang.
Formal analysis: Yonghan Chen, Yi Wang, Linwei Jia, Hao Jiang.
Investigation: Linwei Jia, Yaohui Tian.
Methodology: Linwei Jia, Yaohui Tian.
Writing – original draft: Haokun Jia.
Writing – review & editing: Haokun Jia.
Abbreviations:
- EA
- electro-acupuncture
- GFAP
- glial fibrillary acidic protein
- HIF-1α
- hypoxia inducible factor-1α
- MDA
- malondialdehyde
- MMSE
- mini-mental state examination
- MoCA
- Montreal Cognitive Function Assessment
- NSE
- neuron-specific enolase
- rSO2
- cerebral oxygen saturation
- TBI
- traumatic brain injury
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This study was funded by Hebei Medical Science Research Project (20232123).
The authors have no conflicts of interest to disclose.
This study was approved by the institutional review board of the Cangzhou Central Hospital in compliance with the Helsinki and declaration and consent were waived for its retrospective nature.
How to cite this article: Jia H, Chen Y, Wang Y, Jia L, Tian Y, Jiang H. The neuroprotective effect of electro-acupuncture on cognitive recovery for patients with mild traumatic brain injury: A randomized controlled clinical trial. Medicine 2023;102:6(e32885).
Contributor Information
Yonghan Chen, Email: chenyh1970@126.com.
Yi Wang, Email: 336984156@qq.com.
Linwei Jia, Email: aizai2010@163.com.
Yaohui Tian, Email: yaohuicangzhou@163.com.
Hao Jiang, Email: 485632211@qq.com.
References
- [1].Galgano M, Toshkezi G, Qiu X, et al. Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant. 2017;26:1118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kochanek PM, Berger RP, Bayir H, et al. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury:diagnosis, prognosis, probing mechanisms and therapeutic decision making. Curr Opin Crit Care. 2008;14:135–41. [DOI] [PubMed] [Google Scholar]
- [3].Morgan M, Lockwood A, Steinke D, et al. Pharmacotherapy regiments among patients with posttraumatic stress disorder and mild traumatic brain injury. Psychiatr Serv. 2012;63:182–5. [DOI] [PubMed] [Google Scholar]
- [4].Vigil FA, Bozdemir E, Bugay V, et al. Prevention of brain damage after traumatic brain injury by pharmacological enhancement of KCNQ (Kv7, “M-type”) K+ currents in neurons. J Cereb Blood Flow Metab. 2020;40:1256–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hellewell SC, Yan EB, Agyapomaa DA, et al. Post-traumatic hypoxia exacerbates brain tissue damage: analysis of axonal injury and glial responses. J Neurotrauma. 2010;27:1997–2010. [DOI] [PubMed] [Google Scholar]
- [6].Ahmed AI, Bullock MR, Dietrich WD. Hypothermia in traumatic brain injury. Neurosurg Clin N Am. 2016;27:489–97. [DOI] [PubMed] [Google Scholar]
- [7].Elder GA. Update on TBI and cognitive impairment in military veterans. Curr Neurol Neurosci Rep. 2015;15:68. [DOI] [PubMed] [Google Scholar]
- [8].Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. 2016;20:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhuang Y, Xing JJ, Li J, et al. History of acupuncture research. Int Rev Neurobiol. 2013;111:1–23. [DOI] [PubMed] [Google Scholar]
- [10].Wong V, Cheuk DK, Lee S, et al. Acupuncture for acute management and rehabilitation of traumatic brain injury. Cochrane Database Syst Rev. 2013;10:CD007700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu J, Xue X, Wu Y, et al. Efficacy and safety of electro-acupuncture treatment in improving the consciousness of patients with traumatic brain injury: study protocol for a randomized controlled trial. Trials. 2018;19:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu T, Lu Y, Yu J, et al. Effect of auricular electroacupuncture combined with body acupuncture in improving the consciousness of patients after traumatic brain injury: study protocol for a randomized controlled trial. Medicine (Baltim). 2019;98:e16587e16587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hobson J. The Montreal cognitive assessment (MoCA). Occup Med (Lond). 2015;65:764–5. [DOI] [PubMed] [Google Scholar]
- [14].Li H, Jia J, Yang Z. Mini-mental state examination in elderly Chinese: a population-based normative study. J Alzheimers Dis. 2016;53:487–96. [DOI] [PubMed] [Google Scholar]
- [15].Ragan DK, McKinstry R, Benzinger T, et al. Alterations in cerebral oxygen metabolism after traumatic brain injury in children. J Cereb Blood Flow Metab. 2013;33:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Verweij BH, Amelink GJ, Muizelaar JP. Current concepts of cerebral oxygen transport and energy metabolism after severe traumatic brain injury. Prog Brain Res. 2007;161:111–24. [DOI] [PubMed] [Google Scholar]
- [17].Ng SY, Lee AYW. Traumatic brain injuries: pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019;13:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Khellaf A, Khan DZ, Helmy A. Recent advances in traumatic brain injury. J Neurol. 2019;266:2878–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Silverberg ND, Iaccarino MA, Panenka WJ, et al. Management of concussion and mild traumatic brain injury: a synthesis of practice guidelines. Arch Phys Med Rehabil. 2020;101:382–93. [DOI] [PubMed] [Google Scholar]
- [20].Lin CM, Lin MC, Huang SJ, et al. A prospective randomized study of brain tissue oxygen pressure-guided management in moderate and severe traumatic brain injury patients. Biomed Res Int. 2015;2015:1529580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Salehi A, Zhang JH, Obenaus A. Response of the cerebral vasculature following traumatic brain injury. J Cereb Blood Flow Metab. 2017;37:2320–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hiebert JB, Shen Q, Thimmesch AR, et al. Traumatic brain injury and mitochondrial dysfunction. Am J Med Sci. 2015;350:132–8. [DOI] [PubMed] [Google Scholar]
- [23].Rockswold SB, Rockswold GL, Zaun DA, et al. A prospective, randomized Phase II clinical trial to evaluate the effect of combined hyperbaric and normobaric hyperoxia on cerebral metabolism, intracranial pressure, oxygen toxicity, and clinical outcome in severe traumatic brain injury. J Neurosurg. 2013;118:1317–28. [DOI] [PubMed] [Google Scholar]
- [24].Lu Y, Zhou X, Cheng J, et al. Early intensified rehabilitation training with hyperbaric oxygen therapy improves functional disorders and prognosis of patients with traumatic brain injury. Adv Wound Care (New Rochelle). 2021;10:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xin YY, Wang JX, Xu AJ. Electroacupuncture ameliorates neuroinflammation in animal models. Acupunct Med. 2022;40:474–83. [DOI] [PubMed] [Google Scholar]
- [26].Zhang R, Lao L, Ren K, et al. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sun H, Zheng M, Wang Y, et al. Brain tissue partial pressure of oxygen predicts the outcome of severe traumatic brain injury under mild hypothermia treatment. Neuropsychiatr Dis Treat. 2016;12:2125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ning JQ, Luo JS, Ding LL, et al. The effect of electroacupuncture preconditioning on regional cerebral oxygen saturation levels in elderly patients with diabetes. Diabetes Metab Syndr Obes. 2022;15:2117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Khan M, Khan H, Singh I, et al. Hypoxia inducible factor-1 alpha stabilization for regenerative therapy in traumatic brain injury. Neural Regen Res. 2017;12:696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Khan M, Dhammu TS, Baarine M, et al. GSNO promotes functional recovery in experimental TBI by stabilizing HIF-1α. Behav Brain Res. 2018;340:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Higashida T, Kreipke CW, Rafols JA, et al. The role of hypoxia-inducible factor-1α, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J Neurosurg. 2011;114:92–101. [DOI] [PubMed] [Google Scholar]
- [32].Langley B, Ratan RR. Oxidative stress-induced death in the nervous system: cell cycle dependent or independent? J Neurosci Res. 2004;7:621–9. [DOI] [PubMed] [Google Scholar]
- [33].O’Keeffe E, Kelly E, Liu Y, et al. Dynamic blood-brain barrier regulation in mild traumatic brain injury. J Neurotrauma. 2020;37:347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sharma R, Laskowitz DT. Biomarkers in traumatic brain injury. Curr Neurol Neurosci Rep. 2012;12:560–9. [DOI] [PubMed] [Google Scholar]