SUMMARY
The heterogeneity of tissue macrophages, in health and in disease, has become increasingly transparent over the last decade. But with the plethora of data comes a natural need for organization and the design of a conceptual framework for how we can better understand the origins and functions of different macrophages. We propose that the ontogeny of a macrophage—beyond its fundamental derivation as either embryonically or bone marrow-derived, but rather inclusive of the course of its differentiation, amidst steady-state cues, disease-associated signals, and time—constitutes a critical piece of information about its contribution to homeostasis or the progression of disease.
INTRODUCTION
Since their early description as mononuclear phagocytes in both invertebrate and vertebrate species, macrophages have become increasingly important for our understanding of human health and disease. Their fundamental attributes enable them to “clean” their surroundings by phagocytosing cellular material and regulating tissue repair and maintenance. Macrophages are, therefore, key tissue sentinel cells that are present across various organs throughout the body (Wynn et al., 2013). And quite remarkably, these tissue-resident macrophages (RTMs) are involved in various complex processes, including neuro-, angio-, and osteo-genesis, and even erythropoiesis, indicative of their unique ability to adapt and contribute to their place of residence. This is largely reflective of macrophages’ plasticity that allows them to react to tissue-specific signals, while retaining the ability to execute core functions as tissue phagocytes (Lavin and Merad, 2013; Lavin et al., 2014; Gosselin et al., 2014; Amit et al., 2016; Cohen et al., 2018).
However, during disease, monocytes are recruited to inflamed tissues and differentiate into monocyte-derived macrophages (mo-macs) that, as we review below, are functionally and phenotypically distinct from RTMs. With developments in single-cell transcriptomics, we are able to appreciate—now, more than ever before—the diverse molecular programs used by mo-macs that underscore the cellular heterogeneity and pleiotropic functions of these phagocytes during development, health, and disease. The differences across these macrophage subsets and states highlights the significance of ontogeny (i.e., develop mental origin), and given that a number of parameters likely influence when and where ontogeny matters—such as the availability of certain cytokines, the balance of homeostatic cues and disease-associated signals, as well as biological time—much can be understood about the acquisition and application of the transcriptional programs that distinguish mo-macs from RTMs. Indeed, more work remains to be done to uncover the order in which these programs are acquired and how their induction further diversifies the split in differentiation trajectory of monocytes into RTMs at the steady state or mo-macs during disease. Still, the present review aims to highlight the compilation of studies thus far that have been done on this front. Toward that end, we begin with a summary of the unique, core functionalities of RTMs in different tissues, though we acknowledge that numerous reviews have already outlined—at far greater length and quite recently, too—the various species of RTMs and their tissue-specific activities (Lavin et al., 2015; DeNardo and Ruffell, 2019; Guilliams et al., 2020; Bleriot et al., 2021; Nobs and Kopf, 2021; Cox et al., 2021; Guilliams and Svedberg, 2021; Delfini et al., 2022; Guilliams and Scott, 2022; Zaman and Epelman, 2022; Aegerter et al., 2022). Then, in the latter sections of this review, we illustrate how they differ from the functional contributions of mo-macs to disease progression.
Tissue-resident macrophages are gatekeepers of homeostasis
Nearly all tissues are populated by self-maintaining pools of RTMs. Across different organs, long-lived RTMs execute a list of core responsibilities that serve to help facilitate homeostatic organ functions. Specifically, we highlight as examples the ability for RTMs to clear damaged cells or foreign bodies, to protect neuronal synapses and to prune undesired neuronal connections, to preserve the vasculature, and to form the first line of defense against invading pathogens (Figure 1).
Figure 1. Fundamental responsibilities of tissue-resident macrophages across tissues.
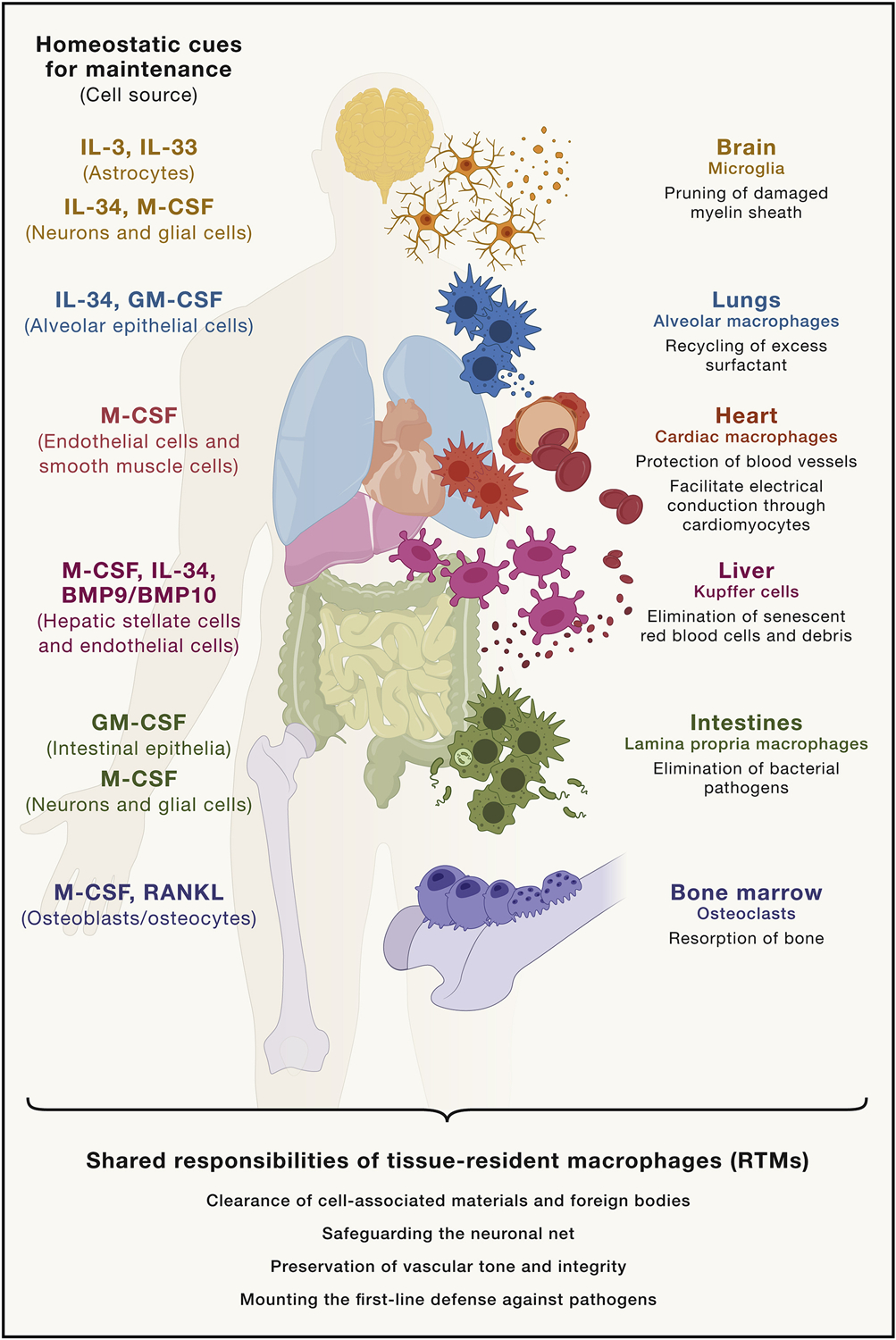
RTMs represent the primary entourage of tissue sentinel phagocytes that help maintain the tissues they inhabit. For example, osteoclasts of the bone eliminate excess bone mass, whereas other macrophages residing within the marrow and splenic macrophages facilitate the generation and elimination of new and dying red blood cells. Whether it be in the CNS, where microglia cooperatively function within neurovascular units, or in the periphery, where perivascular and cardiac macrophages support vascular integrity, RTMs help preserve the vasculature in different tissues. Microglia are also integral for the pruning of neuronal synapses, which also requires them to phagocytose any debris originating from degraded myelin. Alveolar macrophages, like other RTMs in mucosal surfaces, form the immediate innate defense to pathogens.
Clearance of cell-associated materials and foreign bodies
Equipped with long dendrites or pseudopods and extensive endolysosomal machinery, RTMs function as sessile and observant tissue surveyors, both sensing and eliminating damaged or dying stromal or epithelial cells, with the help of an armory of phagocytic receptors. To handle the metabolic strain of ingesting so much, RTMs leverage sub-cellular mechanisms that are tailored to manage high-lipid content cargo without overreacting to potentially pro-inflammatory signals (e.g., downregulating Toll-like receptors) (Roberts et al., 2017). RTMs are also able to do this by modulating the extra-cellular environment, too. In situ-imaging of the peritoneal serosa showed that peritoneal RTMs, as part of their canonical roles in clearing damage-associated tissue lesions, expand their pseudopodia to physically enclose or “cloak” the pro-inflammatory debris and sequester them away from circulating neutrophils that might otherwise react to them and elicit unnecessary harmful inflammation (Uderhardt et al., 2019).
In the lungs, alveolar macrophages recycle excess surfactant and clear eosinophilic material from the alveolar air space (Wright, 1990; Wright and Youmans, 1995; Poelma et al., 2004; Whitsett et al., 2010). Mutations that result in the failed clearance of these substances were categorically associated with defects in surfactant homeostasis, several of which involved genes important for alveolar macrophage development (Whitsett et al., 2010). For instance, mice deficient in lipoprotein lipase or with loss-of-function mutations in granulocyte-macrophage colony-stimulating factor (GM-CSF, encoded by Csf2) or its receptor (GM-CSFR) harbor dysfunctional alveolar macrophages and, as a result, develop severe pulmonary alveolar proteinosis (PAP) (Greenhill and Kotton., 2009; Todd et al., 2016). Analogously, patients with GM-CSF deficits, either due to a mutation (Suzuki et al., 2008) or autoantibodies against GM-CSF (Dranoff et al., 1994; Stanley et al., 1994; Kitamura et al., 1999; Uchida et al., 2003), are also deprived of alveolar macrophages and exhibit PAP. Supplementing these patients with inhaled GM-CSF helped recover alveolar macrophages by restoring overall GM-CSF levels, prototypically provided by type II alveolar pneumocytes that form the alveolar macrophage niche (Reed et al., 1999; Wylam, 2006; Tazawa et al., 2006, 2009; Papiris et al., 2020; Gschwend et al., 2021).
Osteoclasts residing within the endosteal niche of bone also perform a similar function, as they are critical for bone resorption (i.e., dissolving excess bone) (Martin et al., 2005). These endosteal macrophages attach to sites of critical bone mass via vitronectin and other integrins and release acid phosphatases and lysosomal proteases, within a self-made acidic seal that helps break down the hydrated proteins and calcium of the bone. Osteoclasts proceed to phagocytose that debris to clear the degraded bone matrix (Lévesque et al., 2010). Mutations in the gene encoding the vacuolar ATPase that helps generate the acidic proton gradient result in osteoclast dysfunction, resulting in abnormally high bone density, a condition known as osteopetrosis (Scimeca et al., 2000; Blin-Wakkach et al., 2006). This phenotype was also observed in Csf1 and Csf1r knockout mice (Marks and Lane, 1976; Wiktor-Jedrzejczak et al., 1990), as macrophage colony stimulating factor (M-CSF/CSF-1, encoded by Csf1) is critical for macrophage differentiation and survival. A similar phenotype was observed for patients with autosomal recessive mutations in the gene RANKL, indicating a central role for endosteal niche factors in the development and phagocytic functionality of resident osteoclasts (Cleiren et al., 2001; Sobacchi et al., 2007).
A third classic example of this core macrophage function is the role that different RTMs play while clearing cellular nuclei and debris during the development and subsequent clearance of other immune cells. This is seen in the hippocampus, in the kidney, and most frequently in the bone marrow, liver, and spleen, where bone marrow and splenic macrophages and Kupffer cells (KC) oversee the start and finish of the erythropoietic cycle, respectively (Bessis and Breton-Gorius, 1959; Sierra et al., 2010; Munro et al., 2019). Resident macrophages of the bone marrow were found to associate with the progeny of proerythro-blasts during the early stages of erythropoiesis; termed erythroblastic islands, the ejected nuclei of maturing erythrocytes were phagocytosed by these bone marrow macrophages, allowing mature reticulocytes to then enter the circulatory system (Yoffey and Yaffe, 1980; Qui et al., 1995). Later in the life cycle of erythrocytes, KC in the liver have been shown to phagocytose both dying erythrocytes and hemoglobin-containing vesicles released by aging erythrocytes (Schroit et al., 1985; Loegering et al., 1987; Willekens et al., 2005; Theurl et al., 2016). In tandem, red pulp splenic macrophages, which reside in a niche comprised of M-CSF and Wilms’ tumor-1 (WT1)-expressing reticular fibroblasts (Bellomo et al., 2020), also phagocytose senescent or damaged erythrocytes and recycle the accompanying iron (Kohyama et al., 2009; Haldar et al., 2014).
Safeguarding the neuronal net
Early studies have shown that macrophages interact with neurons in the central and peripheral nervous systems (CNS and PNS). But it is only within the past decade that we have recognized the dynamic crosstalk between these cell types that is essential for the health of the nervous system at-large (Prinz and Priller, 2014; Colonna and Butovsky, 2017). In the CNS, microglia constitute the major type of RTMs; work from our groups and others helped show that microglia arise from primitive macrophages in the yolk sac that invade the brain parenchyma and persist from early development through adulthood (Ginhoux et al., 2010; Ajami et al., 2011; Kierdorf et al., 2013; Hoeffel et al., 2015). Studies dating back as early as the 1980s used silver carbonate staining to show that microglia are important for phagocytosing dying neurons (Morgese et al., 1983; Nimmerjahn et al., 2005). Recent work indicates that microglia play more extensive roles in managing neuronal health. For instance, microglia join the neurovascular unit (NVU) to modulate blood flow and nutrient supply to neurons and other glial cells (Arnold and Betsholtz, 2013; Jolivel et al., 2015; Lou et al., 2016; Stankovic et al., 2016; Zarb et al., 2021; Delaney et al., 2021; Császar et al., 2022). Importantly, the presence and functionality of these brain RTMs from early stages of brain development have been linked to synaptic plasticity, learning, and memory (Thion et al., 2018), while in the periphery, recovery of compromised nerves has been shown to be dependent on the presence of nerve-associated macrophages, specifically those in the epineurium (epineurial macrophages) and endoneurium (endoneurial macrophages) (Mueller et al., 2001; Müller et al., 2008, 2010; Kolter et al., 2020). Orthogonal lineage-tracing experiments using Cx3cr1CreERT2:Rosa26-YFP and Cxcr4CreERT2:Rosa26-tdTomato mice showed that nerve-associated macrophages are indeed derived from embryonic precursors (De Schepper et al., 2018; Ydens et al., 2020).
Additional work has shown that this relationship is not strictly unidirectional; in fact, both CNS and PNS neurons promote macrophage survival by producing growth factors like M-CSF or IL-34, a cytokine that shares the same receptor with M-CSF (Greter et al., 2012; Wang et al., 2012; Muller et al., 2014; Kana et al., 2019). In response to neurotoxic insults, macrophages protect their neuronal allies, too; muscularis macrophages that associate with enteric neurons limit neuronal death during intestinal infections by responding to β2-adrenergic signals and engaging an arginase-1/polyamine axis (Matheis et al., 2020; Ahrends et al., 2021), highlighting that this two-way street is indeed a fundamental symbiosis for the maintenance of the PNS.
Preservation of vascular integrity
Experiments with mice that lack M-CSF also showed that angiogenesis and lymphangiogenesis are impaired, indicating a central role for macrophage in a functioning vasculature (Kubota et al., 2009). Seminal lineage-tracing studies subsequently showed that these perivascular and cardiac macrophages are RTMs that locally self-renew and collaborate to help preserve cardiac function and vascular tone in peripheral tissues (Epelman et al., 2014; Lavine et al., 2014; Lapenna et al., 2018; Chakarov et al., 2019). During the postnatal phases of development, for example, cardiac RTM not only stimulate angiogenesis and proliferation of cardiomyocytes, but they also sustain the electrical conductivity and metabolic health of the heart by eliminating cardiac-derived exophers of junk mitochondria via the phagocytic receptor MerTK (Hulsmans et al., 2017; Nicolás-Ávila et al., 2020). In the periphery, perivascular macrophages (PVMs) chaperone anastomoses downstream of induced tip cells (Fantin et al., 2010; Cattin et al., 2015; Graney et al., 2020; Vagesjö et al., 2021), regulate permeability (Hickey and Kimura, 1988; Zhang et al., 2012; He et al., 2016), phagocytose blood-borne materials, and contribute to the proteome of the tissues that the vessels supply (Serrats et al., 2010; Pinto et al., 2012). Upon CSF-1R blockade, mice exhibit significant edema (i.e., enhanced fluid retention in tissues), associated with an increase in matrix metal-loproteinases, changes to the integrin-mediated adhesion strength of vessels, and enhanced deposits of hyaluronan and proteoglycan (Evans et al., 2019; Bissinger et al., 2021), emphasizing further the role of PVMs in the preservation of vascular function.
Mounting the first line of defense against pathogens
Lastly, but also perhaps most importantly, RTMs of various tissues act as the first line of defense against pathogens, representing the most effective and frequently leveraged form of cellular innate immunity. Along the skin and internal mucosal surfaces, this function is essential, as they are the most vulnerable to breach by microbes. The lungs, for instance, are patrolled by alveolar macrophages that migrate along the air-liquid-air interface to capture and contain bacteria or virally infected cells (Neupane et al., 2020). This ability is dependent on the availability of pro-differentiation factors, such as GM-CSF and its activation of the transcription factor PU.1. When deprived of this signal, mice with impaired or absent alveolar macrophages fail to eliminate Streptococcus pneumoniae during active pneumonia (LeVine et al., 1999; Deady et al., 2014), Mycobacterium tuberculosis (Gonzalez-Juarrero et al., 2005), Pseudomonas (Ballinger et al., 2006), Pneumocystis (Paine et al., 2000), and other viral agents. Salvaging alveolar macrophages with exogenous GM-CSF, however, enabled mice to respond appropriately to subsequent secondary challenges. For instance, following recovery from a non-fatal influenza infection, mice given supplemental GM-CSF were better able to clear secondary S. pneumoniae infections than control mice, indicating specific protection conferred by promoting differentiation of lung-infiltrating monocytes into alveolar macrophages during the recovery phase (Umstead et al., 2020). Ultimately, the timely function of RTMs serves to avert pathogenic, systemic inflammation, without completely compromising the innate immune response to infections. As discussed above, one method that RTMs employ is physically concealing the local sites of inflammatory response and preventing them from injudiciously recruiting an armada of inflammatory monocytes and neutrophils (Uderhardt et al., 2019). In doing so, RTMs deter what might result in unnecessary tissue destruction.
That is not to say that RTMs themselves are shielded from the inflammation caused by infections. Acute inflammation can result in the death and destruction of RTMs in a variety of scenarios; for example, alveolar macrophages may either succumb to direct infection and become necrotic or undergo an epithelioid transition as part of the granulomatous response to advanced tuberculosis (Cooper et al., 2009; Pagán et al., 2022; Cronan et al., 2016, 2021). In the liver, KC also undergo regulated necrosis during Listeria infection to elicit a microbicidal inflammatory response (Bleriot et al., 2015; Ginhoux et al., 2017). These instances of RTM death can prompt the recruitment of monocytes to fill RTM niches; and if the quality of RTM niches is still preserved (i.e., homeostatic cues remain available and the cellular sources of these cues are still present and not too damaged), the supply of new RTMs will help control the infection and appropriately engage tissue repair pathways. Below, we elaborate further why we suspect monocyte-derived RTMs are needed to preserve tissues at the steady state, in addition to effectively redirecting inflamed tissues toward resolution.
Monocyte-derived RTMs reinforce embryonically derived phagocytes in protecting tissue homeostasis
Some organs, such as the brain, will generally function with just the native pool of embryonically derived RTMs throughout a lifetime, granted that no major perturbations force RTM loss. In other tissues such as the heart, pancreas, or gut, though, genetic fate-mapping models have shown that some RTMs may also originate from circulating monocytes in response to specific cues in certain tissues, and often the proportion of monocyte-derived RTMs increases with time (Figure 2) (Bain et al., 2013, 2014). For instance, at the steady state, turnover of tissue cells associated with “natural” perturbations that do not necessarily elicit pathognomonic inflammation (e.g., aging, shifts in the microbiome) necessitates certain RTMs to be maintained by the circulating monocytes that extravasate from the vasculature (Cummings et al., 2016)). Such need for peripheral input may be driven by the inability for embryonic RTMs to keep up with the tissues’ demand for phagocytes (Barker et al., 2010), due to either a limited self-renewal capacity, their inability to access and colonize newly forming niches, or an excessive rate of tissue cell turnover. The latter is less probable, as the epidermis and lungs experience a higher rate of epithelial cell turnover than do the pancreas or heart, yet embryonically derived RTMs dominate these tissues. Therefore, other parameters likely dictate this balance between demand and supply. For example, monocyte differentiation into RTMs likely require prolonged periods of cellular interactions with the tissue microenvironment (Scott et al., 2016; Chakarov et al., 2019; Bleriot et al., 2020), so monocyte differentiation may heavily depend on biological time and the continued maintenance of niche integrity (Liu et al., 2019).
Figure 2. Composition of different tissue-resident macrophage populations at the steady state.
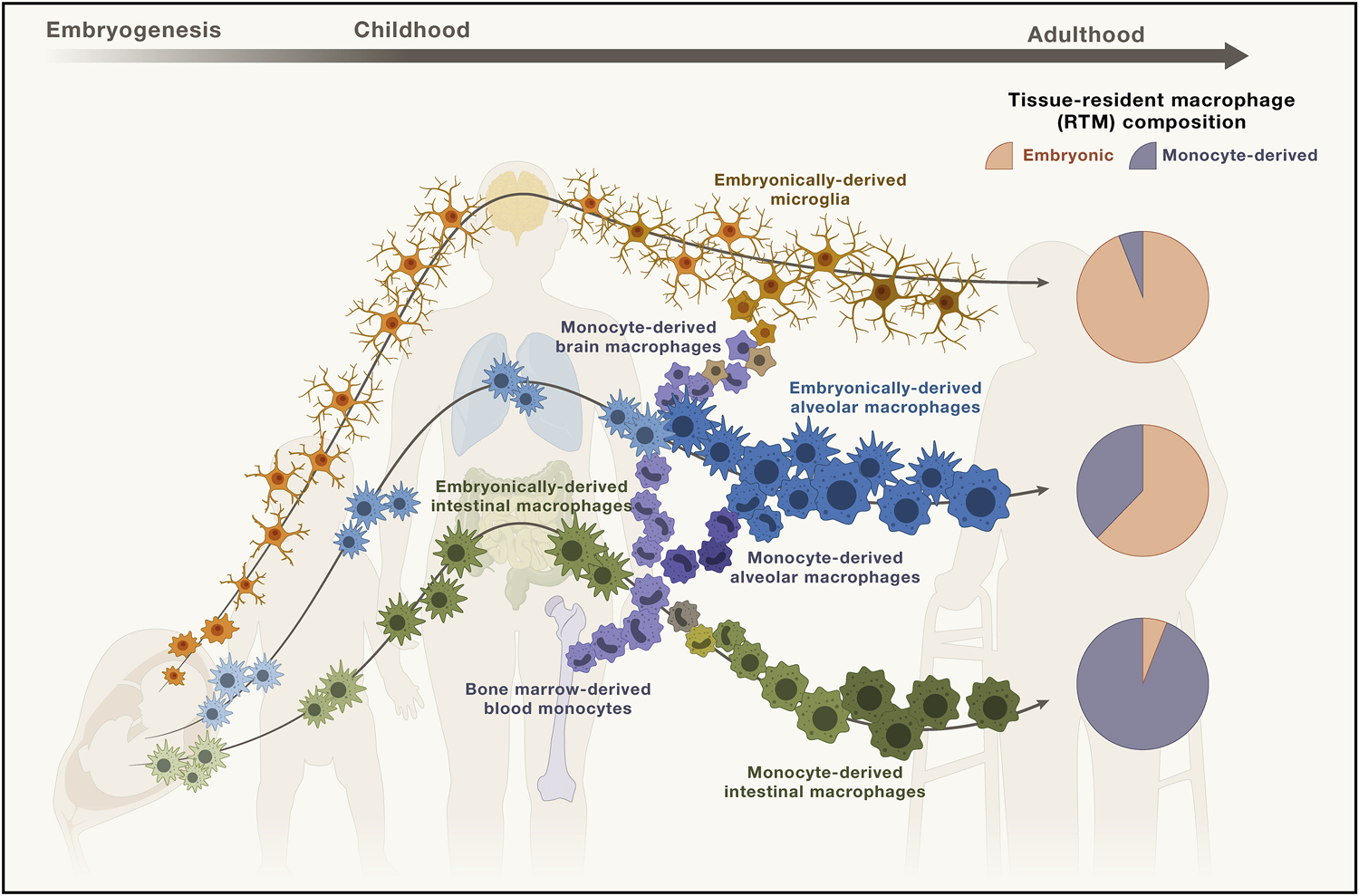
Depending on the tissue, the ontological composition of the tissue-resident macrophage (RTM) compartment varies, and here, we depict the generation and development of these RTM populations during fetal development and post-birth, based on the accepted paradigm that all RTM are embryonically derived phagocytes.
Microglia continue to self-maintain in the brain through interactions with glial cells, like astrocytes, and persist through age with minimal input from peripheral monocytes that infrequently pass the blood-brain barrier to infiltrate the brain parenchyma at the steady state.
Alveolar macrophages in the lungs are also capable of preserving their pool of embryonically derived cells during homeostasis, but unlike the brain-resident microglia, tissue-infiltrating monocytes make up an increasing proportion of alveolar macrophages over the course of aging. Therefore, a notable fraction of alveolar macrophages can be derived from monocytes.
Intestinal lamina propria macrophages are one such exception of RTMs that are largely comprised of monocyte-derived RTMs. The remarkable turnover of macrophages in the gut require input from blood monocytes.
In the brain, for instance, a number of soluble factors preserve microglial identity. These include M-CSF and IL-34 from neurons or glial cells (Greter et al., 2012; Wang et al., 2012; Kana et al., 2019), and resident microglia continue to rely on these maintenance cues to perform (e.g., rapidly accumulate around sources of foreign or damaging materials via clonal microgliosis) (Ladeby et al., 2005; Ajami et al., 2007). Another molecule, IL-3 and IL-33 from astrocytes, promotes microglial motility and encourages microglial clustering in healthy mice and around plaques in diseased mice (Vainchtein et al., 2018; McAlpine et al., 2021). Accordingly, deletion of astrocyte-derived IL-3 or microglial IL-3 receptor exacerbates plaque disease, whereas exogenous IL-3 infusion alleviates plaque burden and improves cognitive function (McAlpine et al., 2021). The decline of these signals and the accumulation of insoluble debris results in microglial dysfunction. The autophagy protein Beclin-1, for instance, regulates phagocytosis, and its expression in microglia is reduced in Alzheimer’s disease (AD), thus impairing phagocytosis of cellular debris by these cells (Lucin et al., 2013). Excessive myelin degradation overwhelms dysfunctional microglia and leads to the formation of insoluble, lipofuscin-like lysosomal bodies that further hinder their homeostatic activity (Safaiyan et al., 2016). With time, the functional exhaustion and the subsequent attrition of microglia is compensated for by a repopulation of the niche by microglial-like cells. Over the years, different studies have sought to probe the ontogeny of these cells but have yielded mixed reports. Chemical depletion of microglia using a diphtheria toxin model indicated a replenishment of the microglial niche by infiltrating monocytes (Lund et al., 2018). In contrast, fate-mapping in CCR2 and CX3CR1 reporter mice crossed to an AD model of plaque formation indicated a near exclusive repopulation of the microglial pool by resident phagocytes (Reed-Geaghan et al., 2020). But after accounting for the presence of RTMs in the different brain border regions (Masuda et al., 2022), more advanced fate-mapping models collectively point to the more modern consensus that niche-reconstituting cells in the brain are, in fact, likely comprised of both a subset of locally proliferating microglia and monocyte-derived RTMs (Bruttger et al., 2015; Askew et al., 2017) that remain transcriptionally, epigenetically, and functionally distinct from one another (Shemer et al., 2018, Silvin et al., 2022).
In the lungs, nerve-associated (CX3CR1+MHCIIhi) and perivascular (LYVE-1+) macrophages were found to be derived from embryonic progenitors but are slowly replaced by monocyte-derived cells in adults (Lim et al., 2018; Chakarov et al., 2019); these subsets of macrophages in these specific subtissular locations were identified in other tissues (Chakarov et al., 2019), suggesting that their global presence may reflect a safety feature associated with replenishing the local supply of tissue macrophages with monocyte-derived RTMs, as these cells reinforce the structural integrity and maintenance of the nervous innervation and vascularization of tissues that are so important for their health.
Macrophage ontogeny is more than just a one-time label of developmental origin
Given these insights into embryonic and monocyte-derived RTMs, we preface the following text on mo-macs with a commentary on issues of vernacular that we suspect may disrupt the community, especially because one could argue that the ontogeny of RTMs fails to matter functionally at the steady state (van de Laar et al., 2016). But, in fact, whether embryonic and monocyte-derived RTMs react to disease signals differently is not well known, and perhaps even more importantly, the ontogeny of tissue macrophages has only recently gained traction as an important metadatum and has received correspondingly little attention, particularly in disease contexts. Studies thus far, though, suggest that ontogeny does indeed matter (as we review below). Accordingly, characterization of the molecular programs used by these ontogenically distinct macrophages and how they contribute to disease pathogenesis lacks precision. So, these insights caution the potential shortcomings of underestimating our interpretation and use of macrophage ontogeny as it currently stands—strictly based on whether a macrophage, in general, is embryonically derived or bone marrow-derived—and instead emphasize the need to investigate the cues that influence monocyte-to-macrophage differentiation.
For macrophages, timely tissue cues are everything
Taking on this challenge requires a nuanced perspective on how embryonic and monocyte-derived RTMs can both feed tissue-residing reservoirs, while monocyte-derived cells recruited during disease fail to fully recapitulate the phenotypes of their RTM counterparts. Toward this end, we define homeostatic differentiation as the steady-state process of imprinting tissue-specific traits into either fetal precursors or adult monocytes that intend on becoming homeostatic RTMs. This contrasts with non-homeostatic differentiation during disease, whereby disease-associated signals drown out the homeostatic ones and skew the maturation of monocyte-derived cells toward dysregulated, often inflammatory, states. This paradigm is seemingly binary, in that both homeostatic and non-homeostatic differentiation are not likely to necessarily occur simultaneously within a single tissue, albeit our expanding understanding of the heterogeneity of distinctive topological areas within a given tissue section (e.g., cancerous tissue versus adjacent, non-involved tissue) would suggest that both types of differentiation could concurrently be permitted for recruited monocytes (Shaw et al., 2018). But by and large, what this may indicate is that timing is integral: the point at which homeostatic differentiation becomes improbable and signals that drive non-homeostatic differentiation begin to overwhelm macrophage niches may determine the degree to which specific disease-associated molecular programs are engaged by mo-macs and affect disease course.
Defining these threshold time points for each organ remains a major quest in macrophage biology. Kinetic profiling of monocyte and macrophage populations in vivo and characterizing them at the molecular level in silico are two potential approaches to the problem (Bleriot et al., 2020). For instance, liver-resident KC that survey the hepatic sinusoids at the steady state, if depleted, are replaced by monocytes that fill emptied KC niches and take on KC features (Scott et al., 2016). These monocyte-derived KC downregulate monocytic markers (e.g., Ly6C) and quickly acquire general macrophage markers (e.g., F4/80, CD64) within two days of KC depletion in a diphtheria toxin receptor model. Interestingly, though, KC markers like CLEC4F or TIMD4 were expressed weeks after engraftment, indicating that homeostatic differentiation may take more time than previously thought. Understanding how to preserve homeostatic tissue cues over this duration of time to ensure homeostatic differentiation, in the face of disease or inflammation-associated signals that might otherwise disrupt them, is a critical aim. Thus, our characterization of mo-macs in diseased tissues as “pathogenic drivers of disease” speaks to a timely need to expand our interpretation and use of cell ontogeny as more than just a notation of developmental origin or age or tissue type. This adjustment in our frame of reference will be essential for achieving our broader goal of identifying (1) disease-associated signals that fuel non-homeostatic differentiation and (2) the downstream cellular networks regulated by mo-macs to develop clinically relevant therapies.
Disease-associated signals recruit monocyte-derived cells into tissues
Canonical pro-inflammatory cytokines, such as IL-6 and IL-8, are secreted by stressed stromal cells (e.g., fibroblasts), epithelial cells, and activated immune cells. In tandem, other “red-light” signals, including canonical alarmin cytokines like IL-33, damage-associated and pathogen-associated molecular patterns (DAMPs and PAMPs) amplify the cascades that result in enhanced myelopoiesis and excess recruitment of inflammatory monocytes into tissues.
Alarmins, such as IL-33 and thymic stromal lymphopoietin (TSLP), are also released from the intracellular storage of damaged endothelial, epithelial, and fibroblastic cells, and in most instances, promote a reparative type II immune response (Liew et al., 2016; Cayrol and Girard, 2018; Andersson et al., 2018). Monocytes and mo-macs share the IL-33 receptor and respond to local alarmin signals. In the lungs, IL-33 has been shown to regulate the self-renewal of mo-macs that resemble resident alveolar macrophages during the resolutive phases of influenza infection (Dagher et al., 2020). In the spleen, in the context of erythrocyte damage, Il33−/− and Il1rl1−/− (IL-33 receptor-deficient) mice exhibit a profound decrease in both the abundance of red pulp macrophages and their erythrophagocytic potential, highlighting once more the importance of alarmin signals in the generation and function of mo-macs during disease (Lu et al., 2020).
While other DAMPs like hyaluronan and nucleic acids or PAMPs like LPS during bacterial infections contribute to the gradient of chemotactic agents that would also recruit and mold the monocyte-derived cells that infiltrate tissues (Zindel and Kubes, 2020), other non-conventional gradients may also act as trails for inflammatory blood monocytes to follow. Metabolites, for instance, can facilitate the recruitment of these cells. Early studies of atherosclerosis identified a role for oxidized cholesterol (oxysterol), not just ligands for CCR2, in the recruitment of inflammatory blood monocytes to plaque lesions and the subsequent pathogenesis of vascular disease (Staprans et al., 2000; Bensinger et al., 2008; Moore and Tabas, 2011; Calkin and Tontonoz, 2012; York and Bensinger, 2013). Similar mechanisms involving chemotactic oxysterols had also been described for dyslipidemia, neurodegenerative disease (Gamba et al., 2019; Varma et al., 2021; Griffiths et al., 2021) and cancer (Villablanca et al., 2010; Raccosta et al., 2013), all of which have also been shown to be associated with an accumulation of dysfunctional mo-macs, as we review more extensively below. At the steady state, oxysterols are present at low levels and typically modulate innate lymphoid cells and dendritic cells (DCs) in secondary and tertiary lymphoid structures via the G-protein coupled receptor (GPR183) (also known as Epstein Barr virus-induced GPCR, or EBI2) (Gatto et al., 2013; Emgård et al., 2018; Wyss et al., 2019). But in certain diseases, oxysterol levels have been shown to rise; in cancer, for instance, tumors themselves generate oxysterols. Then, oxysterol sensing subsequently recruits GPR183+ inflammatory blood monocyte-derived cells into damaged tissues. Typically, soluble DAMPs first induce oxysterol production by mo-macs via the enzyme cholesterol 25-hydroxylase (encoded by the gene CH25H) in both humans and in mice (Dang et al., 2017). This local release is also accompanied by a transient upregulation of GPR183, and the autocrine loop promotes the migratory capacity of these cells by mobilizing intracellular calcium (Hannedouche et al., 2011; Preuss et al., 2014; Rutkowska et al., 2016). It is also fed by oxysterol gradients generated by distal sites of damage, thus promoting trafficking of inflammatory blood monocyte-derived cells into injured tissues.
Decay of RTM niches and the occupation of damaged tissues by mo-macs drives disease progression
During severe, and often chronic disease, RTMs fail to meet the challenge presented by the successive, uncloaked inflammation that leads to tissue barrier activation and damage and likely their own death, as well. Likely, the sustained damage to tissues during disease results in a loss of tissue cells that are responsible for providing the steady-state cues that preserve RTM niches (Lavin et al., 2014; Okabe and Medzhitov, 2014; Amit et al., 2016). In combination with injury-associated signals, including alarmins and pro-inflammatory cytokines (Maus et al., 2006; Bosurgi et al., 2017; Minutti et al., 2017), recruited monocytes rapidly undergo non-homeostatic differentiation into mo-macs (Liu et al., 2019), with major transcriptomic changes upon exposure to the flux of disease-associated signals, oxidative species, and inflammatory byproducts (Desalegn and Pabst, 2019). Whether this event occurs in multiple waves, whether the lifespan of mo-macs changes during disease versus during resolution, and precisely when recruited mo-macs will go on to help repopulate RTMs during disease resolution are unclear. However, what is seemingly conserved across various tissues in multiple disease conditions is the observation that the broader collection of these mo-macs shapes disease (Figure 3), wherein the molecular programs that these cells engage mold the immune landscape of the local microenvironment.
Figure 3. The dynamic of ontogenically distinct RTMs at homeostasis and disease-associated mo-macs during disease.
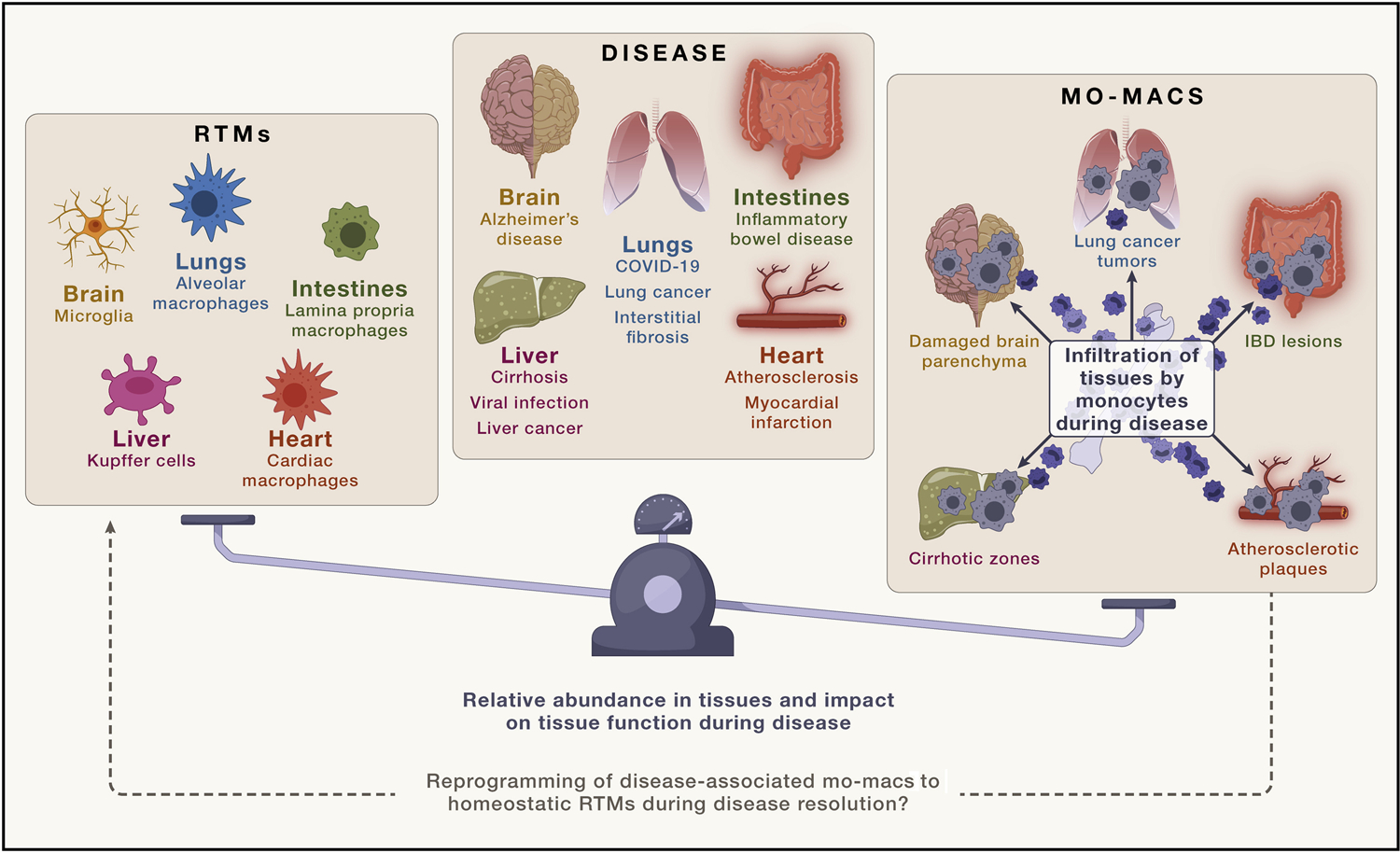
At the steady state, tissue-resident macrophages (RTMs) can be comprised of either embryonically derived RTMs or monocyte-derived RTMs, depending on the tissue. Yet, regardless of their development, the two groups are generally phenotypically indistinguishable from one another. However, during disease, injury-associated signals and disease-specific cues prompt the recruitment of blood monocytes and instigate their inflammatory differentiation into disease-associated monocyte-derived macrophages (mo-macs). Although a growing body of work illustrates their more significant contributions to disease and the functionality of tissues during that time, whether these macrophages, at the resolutive phases of disease, simply die off or revert to the bona fide RTM phenotype upon recovery of homeostatic tissue signals is not entirely clear.
We propose that this pattern of RTM depletion and repopulation by mo-macs is pathognomonic for disease and discuss this below. Understanding how these mo-macs modulate the inflammatory response to the drivers of disease will likely depend on our ability to (1) parse the transcriptomic programs that uniquely identify specific subsets of mo-macs (Figure 4) and to (2) uncouple the components that drive excessive inflammation from those that prematurely solicit an immunosuppressive wound healing or tissue-repair response. Although we assuredly fall short of reviewing all available studies, the intent of the following is to highlight recent bodies of work that provide robust evidence suggesting a unique role for mo-macs in disease onset or progression and contrasting them with their RTM counterparts that have failed to fulfill their duties in clearing tissue debris, surveilling the microenvironment, and curbing excess inflammation.
Figure 4. Transcriptional differences that distinguish disease-associated mo-macs from RTMs.
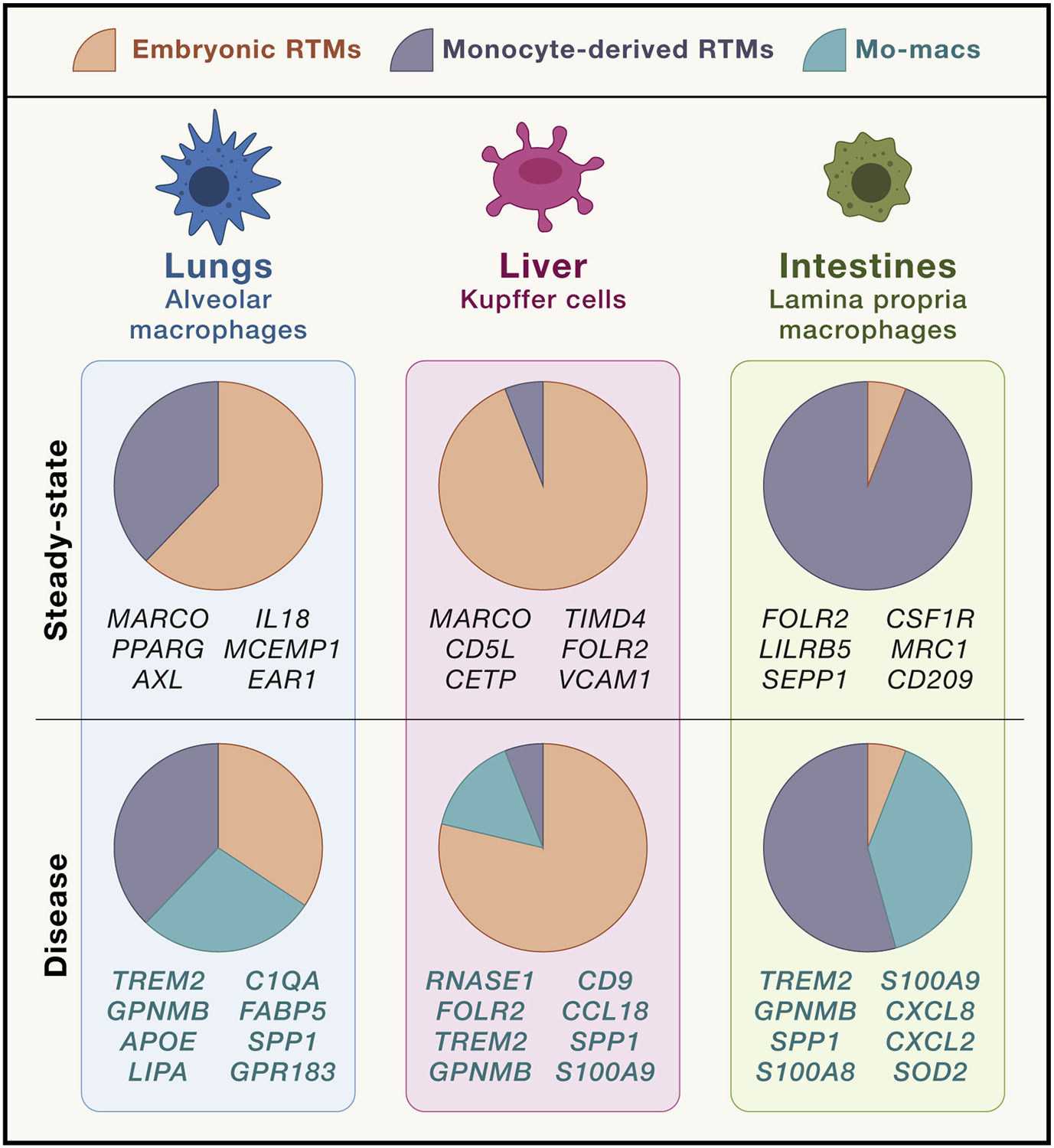
The recruitment of monocyte-derived macrophages (mo-macs) during disease reflects a wide variety of inputs, including the lack of steady-state tissue cues that encourage homeostatic differentiation of monocytes into monocyte-derived tissue-resident macrophages (RTMs) and the presence of disease-specific alarmins. As such, their transcriptomic signature distinguishes them from their tissue-resident counterparts, and what remains to be studied more extensively are the functional contributions of these molecular programs to disease progression.
Respiratory disease
The recruitment of inflammatory blood monocyte-derived cells into diseased tissues and their differentiation into mo-macs that influence disease progression can be most easily exemplified from within the COVID-19 literature. Initial characterizations of myeloid cells in the peripheral blood and in bronchoalveolar lavage samples of COVID-19 patients showed a significant expansion of immature monocytes and neutrophilia, indicative of emergency myelopoiesis in the bone marrow (Schulte-Schrepping et al., 2020; Silvin et al., 2020; Wilk et al., 2020; Grant et al., 2021; Chen et al., 2022). Detailed analyses of these cell subtypes highlighted an inflammatory phenotype, marked by the elevated expression of calprotectin and other S100 family molecules, like S100A12, occupying the lungs of SARS-CoV-2-positive patients.
Subsequent studies that more closely dissected the composition of the macrophage subset within the immune landscape of SARS-CoV-2-infected lungs demonstrated that resident alveolar macrophages are severely depleted in patients with severe disease, even more so than those with mild to moderate disease. Instead, the lungs of patients with poorer clinical outcomes were heavily infiltrated by mo-macs that failed to recapitulate the antigen presentation capacity and homeostatic wound healing properties of their RTM predecessors (Chen et al., 2022). And strikingly, convalescent patients showed a rebound of alveolar macrophages and a decline of these putatively pathogenic mo-macs in the lungs (Chen et al., 2022). Accordingly, clinical efforts to rescue resident alveolar macrophages in patients with COVID-19 with inhaled GM-CSF yielded improvement in oxygenation, highlighting the therapeutic potential of salvaging lost RTMs to counterbalance the deleterious role of dysfunctional mo-macs during disease (Bosteels et al., 2021). Beyond COVID-19, it is likely that restoring the altered balance between RTMs and inflammatory mo-macs may help treat other critical lung illnesses, representing an important and unmet clinical need. For example, in chronic lung diseases such as pulmonary fibrosis, the same imbalance between alveolar macrophages and mo-macs is also evident (Watanabe et al., 2021). Additional transcriptomic profiling characterized major differences between alveolar macrophages and mo-macs over the course of fibrosis, such as the expression of profibrotic genes by mo-macs (Misharin et al., 2017).
Intestinal disease
Early genome-wide association studies demonstrated that the dysregulation of innate immunity in genetically prone individuals drives the development of inflammatory bowel disease (IBD) and related intestinal conditions, including ileal Crohn disease (CD) and ulcerative colitis (UC) (Jostins et al., 2012). A number of genomic loci have been identified as sites of risk variants, including NOD2 (nucleotide-binding oligomerization domain protein 2), SLAMF8 (SLAM family member 8), and ATG16L1 (autophagy related 16-like protein 1). These and other IBD risk loci are strongly enriched for promoters that regulate mo-mac differentiation and have implicated these cells in the development of IBD in both pediatric and adult patients (de Lange et al., 2017; Huang et al., 2019).
The pool of resident intestinal macrophages constitutes the largest subset of myeloid cells in the intestinal tract. Recent efforts to profile myeloid cells in IBD patients suggest that dysregulation of the intestinal macrophage pool may underscore disease progression; CD patients, for example, have been found to harbor anti-GM-CSF autoantibodies that recognize post-translational glycosylation on GM-CSF, detectable even years before diagnosis (Mortha et al., 2022). Additionally, the mucosa of IBD patients are highly enriched with immature inflammatory monocytes that produce IL-23, TNF-α, IL-6, and OSM (West et al., 2017), supporting the potential pro-disease roles that recruited blood monocyte-derived cells play in IBD (Kamada et al., 2008; Ogino et al., 2013; Bernardo et al., 2018). This particular observation has been validated in multiple models of colonic inflammation (Duerr et al., 2006; Yen et al., 2006; Martin et al., 2019). But, most recently, single-cell profiling of immune cells in the inflamed and non-involved mucosa of CD patients revealed that depletion of circulating monocytes, which likely reflects their excessive recruitment at the injury site, alongside the enrichment for a cellular module, comprised of inflammatory mo-macs—transcriptionally distinct from their resident intestinal macrophage counterparts—associated with resistance to anti-TNF therapy (Martin et al., 2019). Expressing high levels of CXCL2, CXCL3, CXCL8, and SOD2, these mo-macs likely respond to pro-inflammatory IL-1 family cytokines, chemokines like CCL2 and CCL7, and alarmins (Martin et al., 2019; Waddell et al., 2021; Friedrich et al., 2021). More in-depth functional studies will likely help uncover how these mo-macs, in fact, drive IBD pathogenesis, and how we can modulate their phenotype toward a resolutive, anti-inflammatory state, since depleting them entirely may compromise the proper replenishment of resident intestinal macrophages, too. Designing the appropriate strategies to test these cells functionally will heavily depend on our ability to parse the molecular programs that define mo-mac states, as we will want to preserve any potentially beneficial properties of these mo-macs, while suppressing their harmful effects.
Hepatic disease
In the human body, the liver is most heavily burdened with metabolism-related demands, and the tissue-resident pool of KC are crucial for fulfilling these demands. And like other RTMs, KC maintenance and functionality are dependent on their topological orientation in hepatic sinusoids and on the availability of homeostatic signals such as M-CSF. Interactions with neighboring stromal cells, such as endothelial cells and fibroblasts, also contribute to KC maintenance (Bonnardel et al., 2019; Guilliams et al., 2022), but the accumulation of oxidative stressors and lipotoxic substances induce hepatocyte death, steatosis, and fibrosis, resulting in the decay of KC niches and the progression of liver damage toward more advanced stages of cirrhosis (Nakagawa et al., 2014; Grohmann et al., 2018; Sun et al., 2020). Homeostatic cues, such as the crosstalk between hepatic stellate cells and KCs via BMP9/BMP10-ALK1, has been shown to be important for inducing liver X receptor (LXR) signaling in KC to promote the expression of lineage-determining factors in tissue-infiltrating monocytes that fill vacant KC niches (Sakai et al., 2019; Bonnardel et al., 2019; Guilliams et al., 2022; Zhao et al., 2022). Notably, however, these mo-macs generated after an injury to the liver still failed to fully recapitulate the phenotype of bona fide embryonic RTMs, resulting in an impaired response to the subsequent lipotoxic burden of non-alcoholic steatohepatitis (Tran et al., 2020).
Different markers have been identified to distinguish these disease-associated mo-macs from KC, such as osteopontin (Spp1) (Remmerie et al., 2020). These recruited mo-macs have commonly been referred to as hepatic lipid-associated macrophages (LAMs), as they are typically enriched with lipid droplets within the cytosolic space (Morgan et al., 2021) and resemble the lipid-laden phagocytes that are enriched in the adipose tissues of obese individuals (Jaitin et al., 2019; Worthmann and Heeren, 2020; Chen et al., 2021). Analogous populations of mo-macs, also expressing TREM2 and other genes involved in lipid metabolism, were enriched in cirrhotic livers (Ramachandran et al., 2019), indicating a potentially unique role in progressive liver disease. Interestingly, in contrast to the orientation of steady-state KC, these TREM2+ macrophages are located peri-centrally in zones of steatosis, suggesting that inflammatory blood monocytes are not directly recruited to vacant KC niches, but are rather pulled to distinct locations in obese livers (Guilliams et al., 2022), likely reflecting key functional differences between RTMs and mo-macs that still need to be unraveled.
Neurodegenerative disease
Multiple sclerosis.
Infiltration of the CNS by inflammatory monocytes has been linked to multiple diseases of neuro-inflammation, such as multiple sclerosis (MS). In the experimental autoimmune encephalomyelitis model of MS, infiltrating monocytes triggered disease progression (Ajami et al., 2011). The injury to oligodendrocytes and gray matter astrocytes resulted in IL-33 release, prompting a recruitment of monocytes to the CNS (Gadani et al., 2015). Local GM-CSF promoted an inflammatory response in infiltrating monocytes that resulted in tissue damage in EAE mice. Deleting the CSF2 receptor in circulating monocytes phenocopied EAE resistance observed in complete Csf2rb-deficient mice (Croxford et al., 2015). Using single-cell transcriptomics, a specific subset of CXCL10-expressing monocytes was identified as the pathogenic subset of monocyte-derived cells in the spinal cord that drives EAE progression (Giladi et al., 2020). Interestingly, inflammatory monocytes from the bone marrow, but not this subset of CXCL10+ monocytes, were recruited into inflamed tissues, where they relied on licensing by IFN-ɣ and GM-CSF to differentiate into phagocytic mo-macs of the inflamed CNS (Amorim et al., 2022).
Alzheimer’s disease.
Similar phenomena of RTM dysfunction and replacement by inflammatory mo-macs have been observed for neurodegenerative diseases, such as AD, too. Foundational studies on the disease primarily involved whole-genome profiling of patients and murine models of AD, and identified at-risk genomic loci that are uniquely active in microglia (Lambert et al., 2013; Wightman et al., 2021). Loss-of-function mutations in TREM2, for instance, confer significant susceptibility for developing AD. Genome-wide survival analysis also showed that a single nucleotide polymorphism (SNP) in the locus of SPI1, encoding the myeloid transcription factor PU.1, also associates with AD risk, implicating a now widely explored role for dysfunctional microglial innate immunity in disease pathogenesis (Guerreiro et al., 2013; Sims et al., 2017; Huang et al., 2017). TREM2 itself recognizes multiple molecules and signals via the DAP12 adaptor protein (Daws et al., 2003; Cannon et al., 2012). Accordingly, screening of the proteomic metabolome in AD-afflicted brains highlighted a link between microglial dysfunction and lipid dysregulation (Loewendorf et al., 2015).
Broader immune profiling of myeloid cells in the CNS of AD patients and mice has shown that the composition of the immune landscape is more heterogeneous and complex than just the phenotypic decline of resident microglia. The use of single-cell omics enabled the identification of a unique microglial signature that defines a subset of microglia, termed disease-associated microglia (DAM), that (1) are uniquely enriched in Trem2 and other molecules involved in lipid metabolism, and (2) are associated with limiting neurodegeneration in AD-transgenic mice (Keren-Shaul et al., 2017; Zhou et al., 2020). These findings indicate that a portion of surviving microglia engage molecular programs that aim to compensate for the loss of ulterior microglial functions that underscore AD pathology. Enrichment of DAM in AD brains is associated with reduced disease burden, and therapeutically activating TREM2 attenuated AD in mice (Wang et al., 2020).
However, more recent works have shown that inflammatory mo-macs actually contribute significantly to the pool of TREM2-engaging DAM-like phagocytes during AD pathogenesis, representing a shift in our understanding of DAMs as an embryonically derived population of microglia. These disease-inflammatory mo-macs (DIMs) express comparable levels of TREM2 and were a primary source of TNF-α in diseased brains of AD patients and mice (Silvin et al., 2022). Interestingly, these phagocytes were preferentially organized in the hippocampus, amygdala, and frontal cortex of murine AD brains, whereas DIMs were principally located in the leptomeninges of AD patients. Collectively, though, these findings underscore the clinical efficacy demonstrated for Enbrel/etanercept, an anti-TNF antibody from rheumatoid arthritis trials, in managing AD (Butchart et al., 2015; Chou et al., 2016). And though reducing excess inflammation is likely therapeutic for reducing damage done to native microglial niches and the surrounding glia, parsing the molecular programs that uniquely identify the mo-macs will help us be more precise about what targets actually modulate the cell-intrinsic phenotype of this compartment, representing a more directed strategy that addresses the root of pathogenic inflammation in AD.
Cardiovascular disease
The vasculature is patrolled and protected by circulating monocytes and perivascular macrophages. The preservation of the cardiovascular system is, therefore, dependent on the proper function of these phagocytes. But, in atherosclerotic disease, inflammatory monocytes become more abundant in unobstructed vessels and will go on to accumulate within early plaques (Swirski et al., 2006, 2007; Tacke et al., 2007). Notably, kinetic studies of monocyte ingress and egress from injured myocardial tissue indicated rapid monocyte to mo-mac turnover (Kircher et al., 2008; Leuschner et al., 2012). Intravital imaging showed that the development of layers of inflammatory mo-macs underscores the growth of atherosclerotic plaques, with the luminal layers representing newly arrived inflammatory monocytes, and the deepest layers occupied mo-macs that destabilize the endothelial floor of advanced plaques (Williams et al., 2018). Single-cell RNA-sequencing (scRNA-seq) of these cells in atherosclerotic plaques revealed that the mo-macs express TREM2, resembling the lipid-laden macrophages found in AD lesions (Cochain et al., 2018; Depuydt et al., 2020). Accordingly, the loss of chemokine receptors such as CCR2 and CX3CR1 on monocytes alleviated plaque burden (Saederup et al., 2008; Combadiere et al., 2008). In line with these observations, inhibiting monocyte migration and suppressing monocyte differentiation into mo-macs also reduced plaques in Apoe-deficient mice (Potteaux et al., 2011; Cao et al., 2015). These observations clearly demonstrate a central role for inflammatory mo-macs in the progression of vascular disease.
Neoplastic disease
Although tumor-associated macrophages (TAMs) have become more seriously studied within the recent decade, few have gone on to specify functional roles for the different subtypes of TAMs that are present in tumor lesions. Over the past few years, a series of seminal studies have demonstrated how ontogenically distinct macrophages play distinct roles in tumor progression and in modulating anti-tumor immunity. Single-cell technologies have given a snapshot of these properties to better appreciate (1) the heterogeneity of RTMs and mo-macs in tumor tissues, (2) their localization in the tumor topography, and (3) the molecular programs that are conserved among tumor-enriched mo-macs across multiple cancer types. The results from these efforts have shown that the roles of RTMs and mo-macs recruited in response to inflammation and tumor-derived cues are often stage-specific and spatially restricted.
Recent results have revealed that both RTMs and mo-macs populate tumor lesions and engage different molecular programs. In both lung and pancreatic adenocarcinoma lesions, RTMs were shown to play a role in tumor inception (Zhu et al., 2017; Casanova-Acebes et al., 2021; Baer et al., 2022); whereas in non-small cell lung cancer (NSCLC), alveolar macrophages facilitate epithelial-mesenchymal transition in tumor cells and activate regulatory T cells and fibroblasts, all of which promote tumor progression and invasiveness (Casanova-Acebes et al., 2021). Interestingly, though, these RTMs are reorganized to the periphery of tumors and are replaced by a massive accumulation of mo-macs (Loyher et al., 2018; Casanova-Acebes et al., 2021). The tumor-infiltrating pool of mo-macs has been profiled in a variety of cancers (Cheng et al., 2021; Mulder et al., 2021; Nalio Ramos et al., 2022), and subsets of them have been shown to universally engage the lipid-associated TREM2 molecular program (Lavin et al., 2017; Molgora et al., 2020; Katzenelenbogen et al., 2020; Binnewies et al., 2021; Leader et al., 2021). Interestingly, analogous programs have been shown to be present in chronically inflamed lesions and AD, as discussed earlier.
Another mo-mac program that is seemingly common in tumor lesions involves the expression of SPP1, encoding the glycoprotein osteopontin (Mulder et al., 2021; Leader et al., 2021). In a large cohort of 35 NSCLC patients, SPP1+ mo-macs were found to closely associate with CXCL13-expressing T cells and IgG plasma cells, the sum of which were termed the “lung cancer immune activation module” or LCAM (Leader et al., 2021). Strikingly, the enrichment for LCAM positively correlated with a number of prognostic clinical metadata, including tumor mutational burden, presence of driver mutations, and response to immune checkpoint blockade (Leader et al., 2021). Such findings motivate the need for an understanding of the precise functional roles that these tumor-infiltrating mo-macs play to determine how to best target them, whether it be through modulating their phenotype or depleting them. This task becomes especially integral for the development of new myeloid-targeting therapies. In subcutaneous murine models of sarcoma and colon carcinoma, TREM2 deficiency or therapeutic antibody-based blockade of TREM2 restricted tumor growth and synergized with PD-L1 blockade in a CD8 T cell-dependent manner (Molgora et al., 2020; Katzenelenbogen et al., 2020), indicating a pathogenic role for TREM2-expressing mo-macs in tumor progression.
However, growing evidence suggests that the pro-tumorigenic contributions of TREM2 may be tissue specific. For instance, in the liver, while TREM2+ mo-macs accumulated with progressive stages of liver injury (i.e., steatosis, cirrhosis, to advanced hepatocellular carcinoma [HCC]) (Ramachandran et al., 2019; Sharma et al., 2020), TREM2 deficiency enhanced tumor growth in mice with HCC, suggesting that the TREM2 program is a tissue-dependent molecular network with both pathogenic and protective potential (Perugorria et al., 2019; Esparza-Baquer et al., 2021). Also in HCC, with additional reports suggesting that other mo-mac programs are, in fact, similar to those used by early macrophages during fetal development, questions concerning the fetal-like programming of tumor-infiltrating mo-macs and their functional purpose during tumorigenesis are also highly intriguing (Sharma et al., 2020). Overall, although mo-macs associate with progressive disease in cancer, the exact functions of the molecular programs that they engage must be probed individually to determine their utility as therapeutic targets.
Concluding remarks
The onset of disease often instigates inflammation that results in cell stress and death of exposed cell types, including RTMs, in the affected microenvironment. In tandem, the recruitment of circulating monocytes likely refills available and vacant RTM niches, as previously defined (Guilliams et al., 2020; Guilliams and Svedberg, 2021), with the likely goal of fulfilling the unmet needs of the damaged tissue, such as the clearance of pathogens and the removal of damaged cells. But unlike the macrophages that seeded the tissue in its developmental stages during embryo-genesis, these mo-macs encounter a much more distinct milieu, reacting to inflammatory and disease-specific cues that skew their differentiation and prompt the expression of distinct repertoires of molecular programs that may further drive disease, as we have discussed. Therefore, it seems the recruitment of mo-macs comes at a price: their maturation in the context of a dysregulated or damaged tissue microenvironment results in the non-homeostatic differentiation of tissue-infiltrating monocytes into mo-macs that enter cell states that may impair tissue healing and instead promote damage and fibrosis. Accordingly, these observations emphasize the importance of refining our use of macrophage ontogeny and developmental pathways, taking into account both the kinetics of monocyte recruitment and differentiation and how that affects their ability to possibly revert to “unconventional” monocyte-derived RTMs upon disease resolution (Figure 5).
Figure 5. Overview of the dynamic among RTMs and disease-associated mo-macs in tissues and leveraging it for translational science.
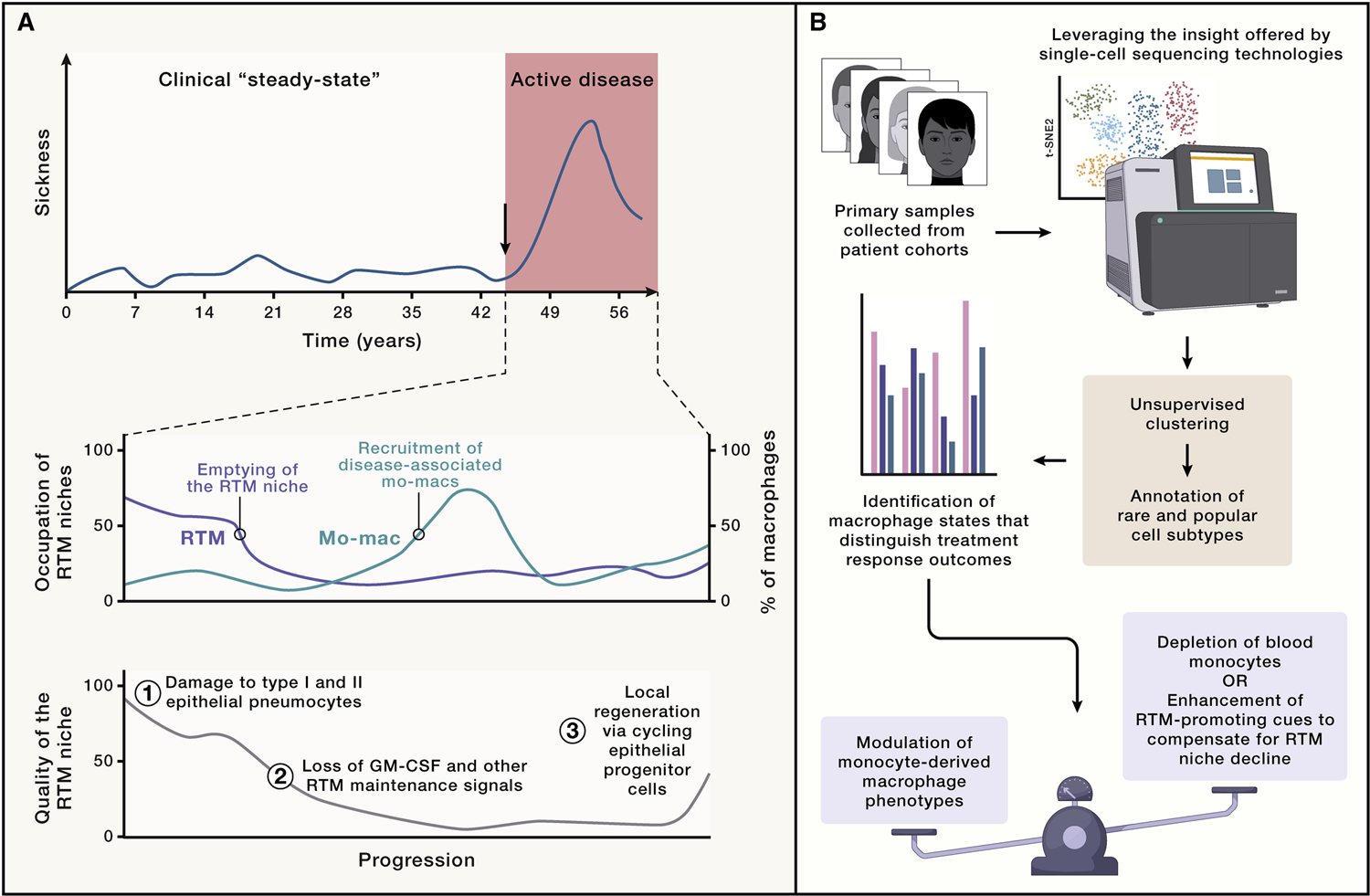
(A) Delineating the occupational dynamic of tissues by tissue-resident macrophages (RTMs) (blue) and monocyte-derived macrophages (mo-macs) generated during disease (red) will not only elucidate how their functional differences drive disease in humans but also at what points during disease would response to certain therapies be most appreciated. (B) In order to do so, insight must be driven by precise, scientific questions with translational potential. The advent and continued use of single-cell technologies enable the scientific community to do that, though other methods of highly rigorous profiling efforts could do the same. Broadly, considering the uncertainty of whether mo-macs at disease resolution help revive the local pool of RTMs and the fact that cell states among mo-macs are wide-ranging and diverse, modulating their phenotype—as opposed to trying to deplete them entirely or outcompete them by attempting to forcibly expand surviving RTMs during disease—will be the more practical outlook on new therapeutic designs.
With the publication of new data on macrophage heterogeneity and function at the steady state and during disease, it remains essential for the field to approach two principal questions: (1) how the molecular diversity of cell states is modulated by the balance of homeostatic tissue signals and disease-associated alarmins, and (2) how it is influenced by the persistence, disappearance, dysregulation, and neo-formation of subtissular niches. Harnessing our findings to these questions will be crucial for our study of disease, where we actually find the greatest diversity of macrophage cell states, some of which are conserved across tissues (Mulder et al., 2021). And it will be equally important to synthesize our understanding of how both niche-based education of embryonic or monocyte-derived RTMs, and the lack thereof for mo-macs occupying disease-afflicted niches, can be modulated to select for disease-resolving programs. The identification of conserved mo-mac programs across disease states and across multiple tissues, most popularly the TREM2 program, speaks to the broader translational potential in studying these programs, as they might reveal candidate targets that may be ideal for therapeutic modulation. This approach to studying macrophage biology requires a more informed and evolved take on ontogeny, one that accounts for the functional distinction between homeostatic RTMs and mo-macs generated in response to disease signals. Doing so would present the opportunity to be more precise with how we target pathogenic macrophage programs in a tissue-specific manner. In addition to genetic fate-mapping models, such as the Ms4a3Cre model (Liu et al., 2019) or the binary-transgenic system (Kim et al., 2021), other classic methods that reveal ontogeny can be helpful, including parabiosis or bone marrow transplants. This task does also require information about (1) intrinsic properties of tissues (i.e., handling of metabolic burden, stromal cell composition, etc.), (2) the homeostatic duties of RTMs that are relegated to them at the steady state, and (3) whether mo-macs recruited in response to injury appropriately meet the demands of those responsibilities. The sum of all these factors will likely dictate whether certain programs—even if they are engaged by mo-macs across multiple tissues—play protective or pathogenic roles in disease.
Therefore, several pressing questions remain. The first is concerned with where embryonic and monocyte-derived RTMs localize in tissues, i.e., whether monocyte-derived RTMs fully migrate to and occupy the exact same niches as their embryonic counterparts, and what their respective contributions are to homeostasis. And in the contexts of disease-related injury, it remains important for us to determine whether being monocyte-derived imprints certain epigenetic memories into monocyte-derived RTMs that prompt them to react differently to injurious cues or damage to their niche long after acquiring RTM phenotypes. One possibility is that soluble disease-associated signals influence the education and phenotype of myeloid progenitor cells in the bone marrow during disease inception, resulting in the epigenetic imprinting of monocyte-derived cells that distinguish them from epigenetic hallmarks of RTMs (Netea et al., 2016). Alternatively, mo-macs may be imprinted locally at tissue sites of disease, though the likelihood of this option is likely to be more dependent on the lifespan of mo-macs in different disease contexts and tissues, which still requires more work to be done, as previously mentioned. Both scenarios would also require us to better understand how disease-associated cues may impact monocyte phenotypes before they even enter the circulation, whereby education within the marrow influences the activity of monocytes once they begin to migrate (Askenase et al., 2015).
Ultimately, it will be crucial for us to identify reliable markers that distinguish the subsets of RTMs and the disease-driven pool of mo-macs. And with that information, we can better characterize the lifespan of mo-macs that are recruited during disease and determine how that may inform our insight into their functional contribution and relevance to different stages of disease progression. For instance, we could imagine that initial waves of monocyte-derived cells involve an elevated phagocytic activity and lipidic remodeling of the microenvironment, with latter waves bringing in monocytes that attempt to synthesize resolutive cues and promote tissue repair (Kratofil et al., 2022), which may or may not actually be beneficial to the tissue at that particular point in time, depending on the context. Alternatively, the converse may be true; earlier waves of monocyte-derived cells that manage to properly differentiate and resolve disease could inculcate an environment that is advantageous for responding to secondary insults (Machiels et al., 2017; Aegerter et al., 2020). Therefore, a growing body of literature has begun revealing a host-protective function of mo-macs, but as previously stated, when and why these properties manifest remain crucial pieces of information that must be elucidated.
An expanded understanding of this kind of dynamic would then be additive to characterizing the kinetic heterogeneity of monocyte-derived cells that are recruited early versus later in the course of the disease. It would help guide therapeutic intervention at different stages of diseases, especially if mo-macs from the more recent waves of recruitment might be more susceptible to homeostatic cues and be more “easily convinced” to reform RTM niches to their pre-disease state; in sum, they would probably benefit more from that extra therapeutic push needed to be beneficial for disease resolution than older mo-macs that might be more difficult to reprogram. Accordingly, both descriptive profiling and functional studies are needed to appreciate the value and utility of this type of macrophage heterogeneity to the fullest extent. In doing so, we will be armed with a clearer and practical understanding of how myeloid programs can be targeted therapeutically in a tissue-specific and disease-specific manner.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Aegerter H, Kulikauskaite J, Crotta S, Patel H, Kelly G, Hessel EM, Mack M, Beinke S, and Wack A (2020). Influenza-induced monocyte-derived alveolar macrophages confer prolonged antibacterial protection. Nat. Immunol 21, 145–157. 10.1038/s41590-019-0568-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aegerter H, Lambrecht BN, and Jakubzick CV (2022). Biology of lung macrophages in health and disease. Immunity 55, 1564–1580. 10.1016/j.immuni.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrends T, Aydin B, Matheis F, Classon CH, Marchildon F, Furtado GC, Lira SA, and Mucida D (2021). Enteric pathogens induce tissue tolerance and prevent neuronal loss from subsequent infections. Cell 184, 5715–5727.e12. 10.1016/j.cell.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, and Rossi FMV (2007). Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci 10, 1538–1543. 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, McNagny KM, and Rossi FMV (2011). Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci 14, 1142–1149. 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Amit I, Winter DR, and Jung S (2016). The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat. Immunol 17, 18–25. 10.1038/ni.3325. [DOI] [PubMed] [Google Scholar]
- Amorim A, De Feo D, Friebel E, Ingelfinger F, Anderfuhren CD, Krishnarajah S, Andreadou M, Welsh CA, Liu Z, Ginhoux F, et al. (2022). IFNγ and GM-CSF control complementary differentiation programs in the monocyte-to-phagocyte transition during neuroinflammation. Nat. Immunol 23, 217–228. 10.1038/s41590-021-01117-7. [DOI] [PubMed] [Google Scholar]
- Andersson P, Yang Y, Hosaka K, Zhang Y, Fischer C, Braun H, Liu S, Yu G, Liu S, Beyaert R, et al. (2018). Molecular mechanisms of IL-33-mediated stromal interactions in cancer metastasis. JCI Insight 3, 122375. 10.1172/jci.insight.122375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold T, and Betsholtz C (2013). The importance of microglia in the development of the vasculature in the central nervous system. Vasc. Cell 5, 4. 10.1186/2045-824x-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askenase MH, Han SJ, Byrd AL, Morais da Fonseca D, Bouladoux N, Wilhelm C, Konkel JE, Hand TW, Lacerda-Queiroz N, Su XZ, et al. (2015). Bone-Marrow-Resident NK Cells Prime Monocytes for Regulatory Function during Infection. Immunity 42, 1130–1142. 10.1016/j.immuni.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, et al. (2017). Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep. 18, 391–405. 10.1016/j.celrep.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JM, Zuo C, Kang L-I, de la Lastra AA, Borcherding NC, Knolhoff BL, Bogner SJ, Zhu Y, Lewis MA, Zhang N, et al. (2022). Pancreas Resident Macrophage-Induced Fibrosis Has Divergent Roles in Pancreas Inflammatory Injury and PDAC. [DOI] [PMC free article] [PubMed]
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, and Mowat AM (2013). Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6, 498–510. 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, and Mowat AM (2014). Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol 15, 929–937. 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger MN, Paine R, Serezani CHC, Aronoff DM, Choi ES, Stand-iford TJ, Toews GB, and Moore BB (2006). Role of Granulocyte Macrophage Colony-Stimulating Factor during Gram-Negative Lung Infection with Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol 34, 766–774. 10.1165/rcmb.2005-0246oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Bartfeld S, and Clevers H (2010). Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell 7, 656–670. 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Bellomo A, Mondor I, Spinelli L, Lagueyrie M, Stewart BJ, Brouilly N, Malissen B, Clatworthy MR, and Bajénoff M (2020). Reticular Fibroblasts Expressing the Transcription Factor WT1 Define a Stromal Niche that Maintains and Replenishes Splenic Red Pulp Macrophages. Immunity 53, 127–142.e7. e7. 10.1016/j.immuni.2020.06.008. [DOI] [PubMed] [Google Scholar]
- Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, and Tontonoz P (2008). LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 134, 97–111. 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo D, Marin AC, Fernández-Tomé S, Montalban-Arques A, Carrasco A, Tristán E, Ortega-Moreno L, Mora-Gutiérrez I, Díaz-Guerra A, Caminero-Fernández R, et al. (2018). Human intestinal pro-inflammatory CD11chighCCR2+ CX3CR1+ macrophages, but not their tolerogenic CD11c- CCR2- CX3CR1− counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol. 11, 1114–1126. 10.1038/s41385-018-0030-7. [DOI] [PubMed] [Google Scholar]
- Bessis MC, and Breton-Gorius J (1959). Ferrtin and ferruginous micelles in normal erythroblasts and hypochromic hypersideremic anemias. Blood 14, 423–432. [PubMed] [Google Scholar]
- Binnewies M, Pollack JL, Rudolph J, Dash S, Abushawish M, Lee T, Jahchan NS, Canaday P, Lu E, Norng M, et al. (2021). Targeting TREM2 on tumor-associated macrophages enhances immunotherapy. Cell Rep. 37. 10.1016/j.celrep.2021.109844. [DOI] [PubMed] [Google Scholar]
- Bissinger S, Hage C, Wagner V, Maser IP, Brand V, Schmittnaegel M, Jegg AM, Cannarile M, Watson C, Klaman I, et al. (2021). Macrophage depletion induces edema through release of matrix-degrading proteases and proteoglycan deposition. Sci. Transl. Med 13, eabd4550. 13. 10.1126/scitranslmed.abd4550. [DOI] [PubMed] [Google Scholar]
- Bleriot C, Dupuis T, Jouvion G, Eberl G, Disson O, and Lecuit M (2015). Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity 42, 145–158. 10.1016/j.immuni.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Blériot C, Chakarov S, and Ginhoux F (2020). Determinants of Resident Tissue Macrophage Identity and Function. Immunity 52, 957–970. 10.1016/j.immuni.2020.05.014. [DOI] [PubMed] [Google Scholar]
- Blériot C, Barreby E, Dunsmore G, Ballaire R, Chakarov S, Ficht X, De Simone G, Andreata F, Fumagalli V, Guo W, et al. (2021). A subset of Kupffer cells regulates metabolism through the expression of CD36. Immunity 54, 2101–2116.e6. 10.1016/j.immuni.2021.08.006. [DOI] [PubMed] [Google Scholar]
- Blin-Wakkach C, Breuil V, Quincey D, Bagnis C, and Carle GF (2006). Establishment and characterization of new osteoclast progenitor cell lines derived from osteopetrotic and wild type mice. Bone 39, 53–60. 10.1016/j.bone.2005.12.078. [DOI] [PubMed] [Google Scholar]
- Bonnardel J, T’Jonck W, Gaublomme D, Browaeys R, Scott CL, Martens L, Vanneste B, De Prijck S, Nedospasov SA, Kremer A, et al. (2019). Stellate Cells, Hepatocytes, and Endothelial Cells Imprint the Kupffer Cell Identity on Monocytes Colonizing the Liver Macrophage Niche. Immunity 51, 638–654.e9. 10.1016/j.immuni.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosteels C, Damme KV, De Leeuw E, and Declercq J Early treatment with inhaled GM-CSF improves oxygenation and anti-viral immunity in COVID-19 induced lung injury–a randomized clinical trial
- Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, et al. (2017). Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076. 10.1126/science.aai8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruttger J, Karram K, Wörtge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y, et al. (2015). Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity 43, 92–106. 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Butchart J, Brook L, Hopkins V, Teeling J, Püntener U, Culliford D, Sharples R, Sharif S, McFarlane B, Raybould R, et al. (2015). Etanercept in Alzheimer disease: A randomized, placebo-controlled, double-blind, phase 2 trial. Neurology 84, 2161–2168. 10.1212/wnl.0000000000001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkin AC, and Tontonoz P (2012). Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol 13, 213–224. 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JP, O’Driscoll M, and Litman GW (2012). Specific lipid recognition is a general feature of CD300 and TREM molecules. Immunogenetics 64, 39–47. 10.1007/s00251-011-0562-4. [DOI] [PubMed] [Google Scholar]
- Cao M, Shikama Y, Anzai M, and Kimura J (2015). Impaired Neutrophil Migration Resulting from Mir-34a and Mir-155 Overexpressed in Neutrophils from Myelodysplastic Syndrome Patients. Blood 126, 999. 10.1182/blood.v126.23.999.999. [DOI] [Google Scholar]
- Casanova-Acebes M, Dalla E, Leader AM, LeBerichel J, Nikolic J, Morales BM, Brown M, Chang C, Troncoso L, Chen ST, et al. (2021). Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature 595, 578–584. 10.1038/s41586-021-03651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattin A-L, Burden J, Van Emmenis L, Mackenzie F, Hoving J, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg L, Quereda V, et al. (2015). Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 162, 1127–1139. 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol C, and Girard J-P (2018). Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev 281, 154–168. 10.1111/imr.12619. [DOI] [PubMed] [Google Scholar]
- Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J, Zhang X-M, Foo S, Nakamizo S, Duan K, et al. (2019). Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, eaau0964. 10.1126/science.aau0964. [DOI] [PubMed] [Google Scholar]
- Chen Q, Lai SM, Xu S, Tan Y, Leong K, Liu D, Tan JC, Naik RR, Barron AM, Adav SS, et al. (2021). Resident macrophages restrain pathological adipose tissue remodeling and protect vascular integrity in obese mice. EMBO Rep. 22, e52835. 10.15252/embr.202152835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ST, Park MD, Del Valle DM, Buckup M, Tabachnikova A, Thompson RC, Simons NW, Mouskas K, Lee B, Geanon D, D’Souza D, Dawson T, Marvin R, Nie K, Zhao Z, LeBerichel J, Chang C, Jamal H, Akturk G, Chaddha U, Mathews K, Acquah S, Brown SA, Reiss M, Harkin T, Feldmann M, Powell CA, Hook JL, Kim-Schulze S, Rahman AH, Brown BD, Mount Sinai COVID-19 Biobank Team, Gnjatic S, Kenigsberg E, Charney AW, and Merad M (2022). A shift in lung macrophage composition is associated with COVID-19 severity and recovery. Sci. Transl. Med 14, eabn5168. 10.1126/scitranslmed.abn5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, Qin S, Zhang L, Ouyang H, Du P, et al. (2021). A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184, 792–809.e23. 10.1016/j.cell.2021.01.010. [DOI] [PubMed] [Google Scholar]
- Chou RC, Kane M, Ghimire S, Gautam S, and Gui J (2016). Treatment for Rheumatoid Arthritis and Risk of Alzheimer’s Disease: A Nested Case-Control Analysis. CNS Drugs 30, 1111–1120. 10.1007/s40263-016-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleiren E, Bénichou O, Van Hul E, Gram J, Bollerslev J, Singer FR, Beaverson K, Aledo A, Whyte MP, Yoneyama T, et al. (2001). Albers-Schönberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum. Mol. Genet 10, 2861–2867. 10.1093/hmg/10.25.2861. [DOI] [PubMed] [Google Scholar]
- Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE, and Zernecke A (2018). Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ Res. 122, 1661–1674. 10.1161/CIRCRESAHA.117.312509. [DOI] [PubMed] [Google Scholar]
- Cohen M, Giladi A, Gorki A-D, Solodkin DG, Zada M, Hladik A, Miklosi A, Salame T-M, Halpern KB, David E, et al. (2018). Lung Single-Cell Signaling Interaction Map Reveals Basophil Role in Macrophage Imprinting. Cell 175, 1031–1044.e18. 10.1016/j.cell.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Colonna M, and Butovsky O (2017). Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol 35, 441–468. 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, and Mallat Z (2008). Combined Inhibition of CCL2, CX3CR1, and CCR5 Abrogates Ly6Chi and Ly6Clo Monocytosis and Almost Abolishes Atherosclerosis in Hypercholesterolemic Mice. Circulation 117, 1649–1657. 10.1161/circulationaha.107.745091. [DOI] [PubMed] [Google Scholar]
- Cooper AM (2009). Cell-Mediated Immune Responses in Tuberculosis. Annu. Rev. Immunol 27, 393–422. 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N, Pokrovskii M, Vicario R, and Geissmann F (2021). Origins, Biology, and Diseases of Tissue Macrophages. Annu. Rev. Immunol 39, 313–344. 10.1146/annurev-immunol-093019-111748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan M, Beerman R, Rosenberg A, Saelens J, Johnson M, Oehlers S, Sisk D, Jurcic Smith K, Medvitz N, Miller S, et al. (2016). Macrophage Epithelial Reprogramming Underlies Mycobacterial Granuloma Formation and Promotes Infection. Immunity 45, 861–876. 10.1016/j.immuni.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan MR, Hughes EJ, Brewer WJ, Viswanathan G, Hunt EG, Singh B, Mehra S, Oehlers SH, Gregory SG, Kaushal D, and Tobin DM (2021). A non-canonical type 2 immune response coordinates tuberculous granuloma formation and epithelialization. Cell 184, 1757–1774.e14. 10.1016/j.cell.2021.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxford A, Lanzinger M, Hartmann F, Schreiner B, Mair F, Pelczar P, Clausen B, Jung S, Greter M, and Becher B (2015). The Cytokine GM-CSF Drives the Inflammatory Signature of CCR2+ Monocytes and Licenses Autoimmunity. Immunity 43, 502–514. 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Császár E, Lénárt N, Cserép C, Környei Z, Fekete R, Pósfai B, Balázsfi D, Hangya B, Schwarcz AD, Szabadits E, et al. (2022). Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J. Exp. Med 219, e20211071. 10.1084/jem.20211071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings RJ, Barbet G, Bongers G, Hartmann BM, Gettler K, Muniz L, Furtado GC, Cho J, Lira SA, and Blander JM (2016). Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature 539, 565–569. 10.1038/nature20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher R, Copenhaver AM, Besnard V, Berlin A, Hamidi F, Maret M, Wang J, Qu X, Shrestha Y, Wu J, et al. (2020). IL-33-ST2 axis regulates myeloid cell differentiation and activation enabling effective club cell regeneration. Nat. Commun 11, 4786. 10.1038/s41467-020-18466-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang EV, McDonald JG, Russell DW, and Cyster JG (2017). Oxysterol Restraint of Cholesterol Synthesis Prevents AIM2 Inflammasome Activation. Cell 171, 1057–1071.e11. 10.1016/j.cell.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, and Seaman WE (2003). Pattern Recognition by TREM-2: Binding of Anionic Ligands. J. Immunol 171, 594–599. 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- De Lange, Moutsianas L, and Lamb Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, Voytyuk I, Schmidt I, Boeckx B, Dierckx de Casterlé I, et al. (2018). Self-Maintaining Gut Macrophages Are Essential for Intestinal Homeostasis. Cell 175, 400–415. 10.1016/j.cell.2018.07.048. [DOI] [PubMed] [Google Scholar]
- Deady LE, Todd EM, Davis CG, Zhou JY, Topcagic N, Edelson BT, Ferkol TW, Cooper MA, Muenzer JT, and Morley SC (2014). L-plastin is essential for alveolar macrophage production and control of pulmonary pneumococcal infection. Infect. Immun 82, 1982–1993. 10.1128/iai.01199-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney C, Farrell M, Doherty CP, Brennan K, O’Keeffe E, Greene C, Byrne K, Kelly E, Birmingham N, Hickey P, et al. (2021). Attenuated CSF-1R signalling drives cerebrovascular pathology. EMBO Mol. Med 13, e12889. 10.15252/emmm.202012889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfini M, Stakenborg N, Viola MF, and Boeckxstaens G (2022). Macrophages in the gut: Masters in multitasking. Immunity 55, 1530–1548. 10.1016/j.immuni.2022.08.005. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, and Ruffell B (2019). Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol 19, 369–382. 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt MA, Prange KH, Slenders L,Örd T, Elbersen D, Boltjes A, de Jager SC, Asselbergs FW, de Borst GJ, Aavik E, et al. (2020). Micro-anatomy of the Human Atherosclerotic Plaque by Single-Cell Transcriptomics. Circ. Res 127, 1437–1455. 10.1161/circresaha.120.316770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desalegn G, and Pabst O (2019). Inflammation triggers immediate rather than progressive changes in monocyte differentiation in the small intestine. Nat. Commun 10, 3229. 10.1038/s41467-019-11148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, and Mulligan RC (1994). Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 264, 713–716. 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. (2006). A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463. 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emgård J, Kammoun H, García-Cassani B, Chesné J, Parigi SM, Jacob J-M, Cheng H-W, Evren E, Das S, Czarnewski P, et al. (2018). Oxysterol Sensing through the Receptor GPR183 Promotes the Lymphoid-Tissue-Inducing Function of Innate Lymphoid Cells and Colonic Inflammation. Immunity 48, 120–132.e8. 10.1016/j.immuni.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S, Lavine K, Beaudin A, Sojka D, Carrero J, Calderon B, Brija T, Gautier E, Ivanov S, Satpathy A, et al. (2014). Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation. Immunity 40, 91–104. 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza-Baquer A, Labiano I, Sharif O, Agirre-Lizaso A, Oakley F, Rodrigues PM, Zhuravleva E, O’Rourke CJ, Hijona E, Jimenez-Agüero R, et al. (2021). TREM-2 defends the liver against hepatocellular carcinoma through multifactorial protective mechanisms. Gut 70, 1345–1361. 10.1136/gutjnl-2019-319227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R, Flores-Borja F, Nassiri S, Miranda E, Lawler K, Grigoriadis A, Monypenny J, Gillet C, Owen J, Gordon P, et al. (2019). Integrin-mediated macrophage adhesion promotes lymphovascular dissemination in breast cancer. Cell Rep. 27, 1967–1978.e4. 10.1016/j.celrep.2019.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, and Ruhrberg C (2010). Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829–840. 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M, Pohin M, Jackson MA, Korsunsky I, Bullers SJ, Rue-Albrecht K, Christoforidou Z, Sathananthan D, Thomas T, Ravindran R, et al. (2021). IL-1-driven stromal–neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat. Med 27, 1970–1981. 10.1038/s41591-021-01520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani S, Walsh J, Smirnov I, Zheng J, and Kipnis J (2015). The Glia-Derived Alarmin IL-33 Orchestrates the Immune Response and Promotes Recovery following CNS Injury. Neuron 85, 703–709. 10.1016/j.neuron.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamba P, Staurenghi E, Testa G, Giannelli S, Sottero B, and Leonarduzzi G (2019). A Crosstalk Between Brain Cholesterol Oxidation and Glucose Metabolism in Alzheimer’s Disease. Front Neurosci. 13. 10.3389/fnins.2019.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto D, Wood K, Caminschi I, Murphy-Durland D, Schofield P, Christ D, Karupiah G, and Brink R (2013). The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nat. Immunol 14, 446–453. 10.1038/ni.2555. [DOI] [PubMed] [Google Scholar]
- Giladi A, Wagner LK, Li H, Dörr D, Medaglia C, Paul F, Shemer A, Jung S, Yona S, Mack M, et al. (2020). Cxcl10+ monocytes define a pathogenic subset in the central nervous system during autoimmune neuroinflammation. Nat. Immunol 21, 525–534. 10.1038/s41590-020-0661-1. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. (2010). Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science 330, 841–845. 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Bleriot C, and Lecuit M (2017). Dying for a Cause: Regulated Necrosis of Tissue-Resident Macrophages upon Infection. Trends Immunol. 38, 693–695. 10.1016/j.it.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Juarrero M, Hattle JM, Izzo A, Junqueira-Kipnis AP, Shim TS, Trapnell BC, Cooper AM, and Orme IM (2005). Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J. Leukoc. Biol 77, 914–922. 10.1189/jlb.1204723. [DOI] [PubMed] [Google Scholar]
- Gosselin D, Link VM, Romanoski C, Fonseca G, Eichenfield D, Spann N, Stender J, Chun H, Garner H, Geissmann F, and Glass C (2014). Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340. 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graney PL, Ben-Shaul S, Landau S, Bajpai A, Singh B, Eager J, Cohen A, Levenberg S, and Spiller KL (2020). Macrophages of diverse phenotypes drive vascularization of engineered tissues. Sci. Adv 6, eaay6391. 10.1126/sciadv.aay6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RA, Morales-Nebreda L, Markov NS, Swaminathan S, Querrey M, Guzman ER, Abbott DA, Donnelly HK, Donayre A, Goldberg IA, et al. (2021). Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature 590, 635–641. 10.1038/s41586-020-03148-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill SR, and Kotton DN (2009). Pulmonary Alveolar Proteinosis. Chest 136, 571–577. 10.1378/chest.08-2943. [DOI] [PubMed] [Google Scholar]
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kündig T, Frei K, Ginhoux F, Merad M, and Becher B (2012). Stroma-Derived Inter-leukin-34 Controls the Development and Maintenance of Langerhans Cells and the Maintenance of Microglia. Immunity 37, 1050–1060. 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths WJ, Abdel-Khalik J, Moore SF, Wijeyekoon RS, Crick PJ, Yutuc E, Farrell K, Breen DP, Williams-Gray CH, Theofilopoulos S, et al. (2021). The Cerebrospinal Fluid Profile of Cholesterol Metabolites in Parkinson’s Disease and Their Association With Disease State and Clinical Features. Front. Aging Neurosci 13, 685594. 10.3389/fnagi.2021.685594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann M, Wiede F, Dodd GT, Gurzov EN, Ooi GJ, Butt T, Rasmiena AA, Kaur S, Gulati T, Goh PK, et al. (2018). Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC. Cell 175, 1289–1306.e20. 10.1016/j.cell.2018.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwend J, Sherman SPM, Ridder F, Feng X, Liang H-E, Locksley RM, Becher B, and Schneider C (2021). Alveolar macrophages rely on GM-CSF from alveolar epithelial type 2 cells before and after birth. J. Exp. Med 218, e20210745. 10.1084/jem.20210745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, et al. (2013). TREM2 Variants in Alzheimer’s Disease. N. Engl. J. Med 368, 117–127. 10.1056/nejmoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, and Svedberg FR (2021). Does tissue imprinting restrict macrophage plasticity? Nat. Immunol 22, 118–127. 10.1038/s41590-020-00849-2. [DOI] [PubMed] [Google Scholar]
- Guilliams M, Thierry GR, Bonnardel J, and Bajenoff M (2020). Establishment and Maintenance of the Macrophage Niche. Immunity 52, 434–451. 10.1016/j.immuni.2020.02.015. [DOI] [PubMed] [Google Scholar]
- Guilliams M, Bonnardel J, Haest B, Vanderborght B, Wagner C, Remmerie A, Bujko A, Martens L, Thoné T, Browaeys R, et al. (2022). Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell 185, 379–396.e38. 10.1016/j.cell.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, and Scott CL (2022). Liver macrophages in health and disease. Immunity 55, 1515–1529. 10.1016/j.immuni.2022.08.002. [DOI] [PubMed] [Google Scholar]
- Haldar M, Kohyama M, So AL, Kc W, Wu X, Briseño C, Satpathy A, Kretzer N, Arase H, Rajasekaran N, et al. (2014). Heme-Mediated SPI-C Induction Promotes Monocyte Differentiation into Iron-Recycling Macrophages. Cell 156, 1223–1234. 10.1016/j.cell.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, Guerini D, Baumgarten BU, Roggo S, Wen B, et al. (2011). Oxysterols direct immune cell migration via EBI2. Nature 475, 524–527. 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Mack JJ, Güç E, Warren CM, Squadrito ML, Kilarski WW, Baer C, Freshman RD, McDonald AI, Ziyad S, et al. (2016). Perivascular macrophages limit permeability. Arterioscler. Thromb. Vasc. Biol 36, 2203–2212. 10.1161/atvbaha.116.307592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF, and Kimura H (1988). Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo 239 (4837), 290–292. 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hoeffel G, Chen J, Lavin Y, Low D, Almeida F, See P, Beaudin A, Lum J, Low I, Forsberg E, et al. (2015). C-Myb+ Erythro-Myeloid Progenitor-Derived Fetal Monocytes Give Rise to Adult Tissue-Resident Macrophages. Immunity 42, 665–678. 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KL, Marcora E, Pimenova AA, Di Narzo AF, Kapoor M, Jin SC, Harari O, Bertelsen S, Fairfax BP, Czajkowski J, et al. (2017). A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer’s disease. Nat. Neurosci 20, 1052–1061. 10.1038/nn.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Chen Z, Geng L, Wang J, Liang H, Cao Y, Chen H, Huang W, Su M, Wang H, et al. (2019). Mucosal Profiling of Pediatric-Onset Colitis and IBD Reveals Common Pathogenics and Therapeutic Pathways. Cell 179, 1160–1176.e24. 10.1016/j.cell.2019.10.027. [DOI] [PubMed] [Google Scholar]
- Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G, et al. (2017). Macrophages Facilitate Electrical Conduction in the Heart. Cell 169, 510–522.e20. 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, et al. (2019). Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 178, 686–698.e14. 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivel V, Bicker F, Binamé F, Ploen R, Keller S, Gollan R, Jurek B, Birkenstock J, Poisa-Beiro L, Bruttger J, et al. (2015). Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 129, 279–295. 10.1007/s00401-014-1372-1. [DOI] [PubMed] [Google Scholar]
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, et al. (2012). Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease 491, 119–124. 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, Sakuraba A, Kitazume MT, Sugita A, Koganei K, et al. (2008). Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-g axis. J. Clin. Invest [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana V, Desland FA, Casanova-Acebes M, Ayata P, Badimon A, Nabel E, Yamamuro K, Sneeboer M, Tan IL, Flanigan ME, et al. (2019). CSF-1 controls cerebellar microglia and is required for motor function and social interaction. J. Exp. Med 216, 2265–2281. 10.1084/jem.20182037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenelenbogen Y, Sheban F, Yalin A, Yofe I, Svetlichnyy D, Jaitin DA, Bornstein C, Moshe A, Keren-Shaul H, Cohen M, et al. (2020). Coupled scRNA-Seq and Intracellular Protein Activity Reveal an Immunosuppressive Role of TREM2 in Cancer. Cell 182, 872–885.e19. 10.1016/j.cell.2020.06.032. [DOI] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, et al. (2017). A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169, 1276–1290. 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Hölscher C, et al. (2013). Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci 16, 273–280. 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- Kim J-S, Kolesnikov M, Peled-Hajaj S, Scheyltjens I, Xia Y, Trzebanski S, Haimon Z, Shemer A, Lubart A, Van Hove H, et al. (2021). A Binary Cre Transgenic Approach Dissects Microglia and CNS Border-Associated Macrophages. Immunity 54, 176–190.e7. 10.1016/j.immuni.2020.11.007. [DOI] [PubMed] [Google Scholar]
- Kircher MF, Grimm J, Swirski FK, Libby P, Gerszten RE, Allport JR, and Weissleder R (2008). Noninvasive In Vivo Imaging of Monocyte Trafficking to Atherosclerotic Lesions. Circulation 117, 388–395. 10.1161/circulationaha.107.719765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Kanegasaki S, Yamada Y, and Nakata K (1999). Idiopathic Pulmonary Alveolar Proteinosis as an Autoimmune Disease with Neutralizing Antibody against Granulocyte/Macrophage Colony-Stimulating Factor. J. Exp. Med 190, 875–880. 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama M, Ise W, Edelson BT, Wilker PR, Hildner K, Mejia C, Frazier WA, Murphy TL, and Murphy KM (2009). Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature 457, 318–321. 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter J, Kierdorf K, and Henneke P (2020). Origin and Differentiation of Nerve-Associated Macrophages. J. Immunol 204, 271–279. 10.4049/jimmunol.1901077. [DOI] [PubMed] [Google Scholar]
- Kratofil RM, Shim HB, Shim R, Lee WY, Labit E, Sinha S, Keenan CM, Surewaard BGJ, Noh JY, Sun Y, et al. (2022). A monocyte-leptin-angiogenesis pathway critical for repair post-infection. Nature 609, 166–173. 10.1038/s41586-022-05044-x. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, and Suda T (2009). M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J. Exp. Med 206, 1089–1102. 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Laar L, Saelens W, De Prijck S, Martens L, Scott C, Van Isterdael G, Hoffmann E, Beyaert R, Saeys Y, Lambrecht B, and Guilliams M (2016). Yolk Sac Macrophages, Fetal Liver, and Adult Monocytes Can Colonize an Empty Niche and Develop into Functional Tissue-Resident Macrophages. Immunity 44, 755–768. 10.1016/j.immuni.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, and Finsen B (2005). Microglial cell population dynamics in the injured adult central nervous system. Brain Res. Rev 48, 196–206. 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, et al. (2013). Meta-analysis of 74, 046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet 45, 1452–1458. 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapenna A, De Palma M, and Lewis CE (2018). Perivascular macrophages in health and disease. Nat. Rev. Immunol 18, 689–702. 10.1038/s41577-018-0056-9. [DOI] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, and Amit I (2014). Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326. 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Kobayashi S, Leader A, Amir E-AD, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, et al. (2017). Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 169. 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, and Merad M (2013). Macrophages: gatekeepers of tissue integrity. Cancer Immunol Res. 1. 10.1158/2326-6066.CIR-13-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Mortha A, Rahman A, and Merad M (2015). Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol 15, 731–744. 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, and Mann DL (2014). Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. USA 111, 16029–16034. 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader AM, Grout JA, Maier BB, Nabet BY, Park MD, Tabachnikova A, Chang C, Walker L, Lansky A, Le Berichel J, et al. (2021). Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification. Cancer Cell 39, 1594–1609.e12. 10.1016/j.ccell.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, et al. (2012). Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med 209, 123–137. 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque J-P, Helwani FM, and Winkler IG (2010). The endosteal “osteoblastic” niche and its role in hematopoietic stem cell homing and mobilization. Leukemia 24, 1979–1992. 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Reed JA, Kurak KE, Cianciolo E, and Whitsett JA (1999). GM-CSF–deficient mice are susceptible to pulmonary group B streptococcal infection. J. Clin. Invest 103, 563–569. 10.1172/jci5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Girard J-P, and Turnquist HR (2016). Interleukin-33 in health and disease. Nat. Rev. Immunol 16, 676–689. 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- Lim HY, Lim SY, Tan CK, Thiam CH, Goh CC, Carbajo D, Chew SHS, See P, Chakarov S, Wang XN, et al. (2018). Hyaluronan Receptor LYVE-1-expressing Macrophages Maintain Arterial Tone through Hyaluronan-Mediated Regulation of Smooth Muscle Cell Collagen. Immunity 49, 326–341.e7. 10.1016/j.immuni.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gu Y, Chakarov S, Bleriot C, Kwok I, Chen X, Shin A, Huang W, Dress RJ, Dutertre C-A, et al. (2019). Fate Mapping via Ms4a3-Expression History Traces Monocyte-Derived Cells. Cell 178, 1509–1525.e19. 10.1016/j.cell.2019.08.009. [DOI] [PubMed] [Google Scholar]
- Loegering DJ, Commins LM, Minnear FL, Gary LA, and Hill LA (1987). Effect of Kupffer cell phagocytosis of erythrocytes and erythrocyte ghosts on susceptibility to endotoxemia and bacteremia. Infect. Immun 55, 2074–2080. 10.1128/iai.55.9.2074-2080.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou N, Takano T, Pei Y, Xavier AL, Goldman SA, and Nedergaard M (2016). Purinergic receptor P2RY12-dependent microglial closure of the injured blood–brain barrier. Proc. Natl. Acad. Sci. USA 113, 1074–1079. 10.1073/pnas.1520398113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewendorf AI, Fonteh AN, Harrington MG, and Csete ME (2015). Inflammation in Alzheimer’s Disease: Cross-talk between Lipids and Innate Immune Cells of the Brain. Journal of Immunological Research 2, 1022. [Google Scholar]
- Loyher PL, Hamon P, Laviron M, Meghraoui-Kheddar A, Goncalves E, Deng Z, Torstensson S, Bercovici N, Baudesson de Chanville C, Combadière B, et al. (2018). Macrophages of distinct origins contribute to tumor development in the lung. J. Exp. Med 215, 2536–2553. 10.1084/jem.20180534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Basatemur G, Scott IC, Chiarugi D, Clement M, Harrison J, Jugdaohsingh R, Yu X, Newland SA, Jolin HE, et al. (2020). Interleukin-33 Signaling Controls the Development of Iron-Recycling Macrophages. Immunity 52, 782–793.e5. 10.1016/j.immuni.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin K, O’Brien C, Bieri G, Czirr E, Mosher K, Abbey R, Mastroeni D, Rogers J, Spencer B, Masliah E, and Wyss-Coray T (2013). Microglial Beclin 1 Regulates Retromer Trafficking and Phagocytosis and Is Impaired in Alzheimer’s Disease. Neuron 79, 873–886. 10.1016/j.neuron.2013.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund H, Pieber M, Parsa R, Han J, Grommisch D, Ewing E, Kular L, Needhamsen M, Espinosa A, Nilsson E, et al. (2018). Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat. Commun 9, 4845. 10.1038/s41467-018-07295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiels B, Dourcy M, Xiao X, Javaux J, Mesnil C, Sabatel C, Desmecht D, Lallemand F, Martinive P, Hammad H, et al. (2017). A gamma-herpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat. Immunol 18, 1310–1320. 10.1038/ni.3857. [DOI] [PubMed] [Google Scholar]
- Marks SC, and Lane PW (1976). Osteopetrosis, a New Recessive Skeletal Mutation on Chromosome 12 of the Mouse. J. Hered 67, 11–18. 10.1093/oxfordjournals.jhered.a108657. [DOI] [PubMed] [Google Scholar]
- Martin JC, Chang C, Boschetti G, Ungaro R, Giri M, Grout JA, Gettler K, Chuang L-S, Nayar S, Greenstein AJ, et al. (2019). Single-Cell Analysis of Crohn’s Disease Lesions Identifies a Pathogenic Cellular Module Associated with Resistance to Anti-TNF Therapy. Cell 178, 1493–1508.e20. e20. 10.1016/j.cell.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Sims NA, and Sims NA (2005). Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol. Med 11, 76–81. 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Masuda T, Amann L, Monaco G, Sankowski R, Staszewski O, Krueger M, Del Gaudio F, He L, Paterson N, Nent E, et al. (2022). Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature 604, 740–748. 10.1038/s41586-022-04596-2. [DOI] [PubMed] [Google Scholar]
- Matheis F, Muller PA, Graves CL, Gabanyi I, Kerner ZJ, Costa-Borges D, Ahrends T, Rosenstiel P, and Mucida D (2020). Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell 180, 64–78.e16. 10.1016/j.cell.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, Seeger W, Welte T, and Lohmeyer J (2006). Resident Alveolar Macrophages Are Replaced by Recruited Monocytes in Response to Endotoxin-Induced Lung Inflammation. Am. J. Respir. Cell Mol. Biol 35, 227–235. 10.1165/rcmb.2005-0241oc. [DOI] [PubMed] [Google Scholar]
- McAlpine CS, Park J, Griciuc A, Kim E, Choi SH, Iwamoto Y, Kiss MG, Christie KA, Vinegoni C, Poller WC, et al. (2021). Astrocytic interleukin-3 programs microglia and limits Alzheimer’s disease. Nature 595, 701–706. 10.1038/s41586-021-03734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minutti CM, Jackson-Jones LH, García-Fojeda B, Knipper JA, Sutherland TE, Logan N, Ringqvist E, Guillamat-Prats R, Ferenbach DA, Artigas A, et al. (2017). Local amplifiers of IL-4Rα-mediated macrophage activation promote repair in lung and liver. Science 356, 1076–1080. 10.1126/science.aaj2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, Chen C-I, Anekalla KR, Joshi N, Williams KJ, et al. (2017). Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med 214, 2387–2404. 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgora M, Esaulova E, Vermi W, Hou J, Chen Y, Luo J, Brioschi S, Bugatti M, Omodei AS, Ricci B, et al. (2020). TREM2 Modulation Remodels the Tumor Myeloid Landscape Enhancing Anti-PD-1 Immunotherapy. Cell 182, 886–900.e17. 10.1016/j.cell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K, and Tabas I (2011). Macrophages in the Pathogenesis of Atherosclerosis. Cell 145, 341–355. 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PK, Huynh K, Pernes G, Miotto PM, Mellett NA, Giles C, Meikle PJ, Murphy AJ, and Lancaster GI (2021). Macrophage polarization state affects lipid composition and the channeling of exogenous fatty acids into endogenous lipid pools. J. Biol. Chem 297, 101341. 10.1016/j.jbc.2021.101341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgese VJ, Elliott EJ, and Muller KJ (1983). Microglial movement to sites of nerve lesion in the leech CNS. Brain Res. 272, 166–170. 10.1016/0006-8993(83)90375-x. [DOI] [PubMed] [Google Scholar]
- Mortha A, Remark R, Del Valle DM, Chuang L-S, Chai Z, Alves I, Azevedo C, Gaifem J, Martin J, Petralia F, et al. (2022). Neutralizing anti-GM-CSF autoantibodies recognize posttranslational glycosylations on GM-CSF years prior to diagnosis and predict complicated Crohn’s Disease. Gastroenterology 163, 659–670. 10.1053/j.gastro.2022.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Wacker K, Ringelstein EB, Hickey WF, Imai Y, and Kiefer R (2001). Rapid Response of Identified Resident Endoneurial Macrophages to Nerve Injury. Am. J. Pathol 159, 2187–2197. 10.1016/s0002-9440(10)63070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder K, Patel AA, Kong WT, Piot C, Halitzki E, Dunsmore G, Khalilnezhad S, Irac SE, Dubuisson A, Chevrier M, et al. (2021). Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity 54, 1883–1900.e5. 10.1016/j.immuni.2021.07.007. [DOI] [PubMed] [Google Scholar]
- Muller P, Koscsó B, Rajani G, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, et al. (2014). Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 1210. 10.1016/j.cell.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Müller M, Wacker K, Getts D, Ringelstein EB, and Kiefer R (2008). Further evidence for a crucial role of resident endoneurial macrophages in peripheral nerve disorders: Lessons from acrylamide-induced neuropathy. Glia 56, 1005–1016. 10.1002/glia.20674. [DOI] [PubMed] [Google Scholar]
- Müller M, Leonhard C, Krauthausen M, Wacker K, and Kiefer R (2010). On the longevity of resident endoneurial macrophages in the peripheral nervous system: a study of physiological macrophage turnover in bone marrow chimeric mice. J. Peripher. Nerv. Syst 15, 357–365. 10.1111/j.1529-8027.2010.00295.x. [DOI] [PubMed] [Google Scholar]
- Munro DA, Wineberg Y, Tarnick J, Vink CS, Li Z, Pridans C, Dzierzak E, Kalisky T, Hohenstein P, and Davies JA (2019). Macrophages restrict the nephrogenic field and promote endothelial connections during kidney development. Elife 8, e43271. 10.7554/elife.43271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek M, Seki E, Hidalgo J, et al. (2014). ER Stress Cooperates with Hypernutrition to Trigger TNF-Dependent Spontaneous HCC Development. Cancer Cell 26, 331–343. 10.1016/j.ccr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalio Ramos R, Missolo-Koussou Y, Gerber-Ferder Y, Bromley CP, Bugatti M, Núñez NG, Tosello Boari J, Richer W, Menger L, Denizeau J, et al. (2022). Tissue-resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer. Cell 185, 1189–1207. 10.1016/j.cell.2022.02.021. [DOI] [PubMed] [Google Scholar]
- Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, O’Neill LAJ, and Xavier RJ (2016). Trained immunity: A program of innate immune memory in health and disease. Science 352, aaf1098. 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupane AS, Willson M, Chojnacki AK, Vargas E Silva Castanheira F, Morehouse C, Carestia A, Keller AE, Peiseler M, DiGiandomenico A, Kelly MM, et al. (2020). Patrolling Alveolar Macrophages Conceal Bacteria from the Immune System to Maintain Homeostasis. Cell 183, 110–125.e11. e11. 10.1016/j.cell.2020.08.020. [DOI] [PubMed] [Google Scholar]
- Nicolás-Ávila JA, Lechuga-Vieco AV, Esteban-Martínez L, Sánchez-Díaz M, Díaz-García E, Santiago DJ, Rubio-Ponce A, Li JL, Balachander A, Quintana JA, et al. (2020). A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 183, 94–109.e23. 10.1016/j.cell.2020.08.031. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, and Helmchen F (2005). Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 308, 1314–1318. 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nobs SP, and Kopf M (2021). Tissue-resident macrophages: guardians of organ homeostasis. Trends Immunol. 42, 495–507. 10.1016/j.it.2021.04.007. [DOI] [PubMed] [Google Scholar]
- Ogino T, Nishimura J, Barman S, Kayama H, Uematsu S, Okuzaki D, Osawa H, Haraguchi N, Uemura M, Hata T, et al. (2013). Increased Th17-Inducing Activity of CD14+ CD163low Myeloid Cells in Intestinal Lamina Propria of Patients With Crohn’s Disease. Gastroenterology 145, 1380–1391.e1. 10.1053/j.gastro.2013.08.049. [DOI] [PubMed] [Google Scholar]
- Okabe Y, and Medzhitov R (2014). Tissue-Specific Signals Control Reversible Program of Localization and Functional Polarization of Macrophages. Cell 157, 832–844. 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán AJ, Lee LJ, Edwards-Hicks J, Moens CB, Tobin DM, Busch-Nentwich EM, Pearce EL, and Ramakrishnan L (2022). mTOR-regulated mitochondrial metabolism limits mycobacterium-induced cytotoxicity. Cell 185, 3720–3738. 10.1016/j.cell.2022.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine R, Preston AM, Wilcoxen S, Jin H, Siu BB, Morris SB, Reed JA, Ross G, Whitsett JA, and Beck JM (2000). Granulocyte-Macrophage Colony-Stimulating Factor in the Innate Immune Response toPneumocystis cariniiPneumonia in Mice. J. Immunol 164, 2602–2609. 10.4049/jimmunol.164.5.2602. [DOI] [PubMed] [Google Scholar]
- Papiris SA, Griese M, and Manali ED (2020). Inhaled GM-CSF for Pulmonary Alveolar Proteinosis. N. Engl. J. Med 382, 197. 10.1056/NEJMc1914606. [DOI] [PubMed] [Google Scholar]
- Perugorria MJ, Esparza-Baquer A, Oakley F, Labiano I, Korosec A, Jais A, Mann J, Tiniakos D, Santos-Laso A, Arbelaiz A, et al. (2019). Non-parenchymal TREM-2 protects the liver from immune-mediated hepatocellular damage. Gut 68, 533–546. 10.1136/gutjnl-2017-314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, and Rosenthal NA (2012). An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One 7, e36814. 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelma DLH, Zimmermann LJ, van Cappellen WA, Haitsma JJ, Lach-mann B, and van Iwaarden JF (2004). Distinct effects of SP-B and SP-C on the uptake of surfactant-like liposomes by alveolar cells in vivo and in vitro. Am. J. Physiol. Lung Cell Mol. Physiol 287, L1056–L1065. 10.1152/ajplung.00054.2004. [DOI] [PubMed] [Google Scholar]
- Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, and Randolph GJ (2011). Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J. Clin. Invest 121, 2025–2036. 10.1172/jci43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss I, Ludwig M-G, Baumgarten B, Bassilana F, Gessier F, Seuwen K, and Sailer AW (2014). Transcriptional regulation and functional characterization of the oxysterol/EBI2 system in primary human macrophages. Biochem. Biophys. Res. Commun 446, 663–668. 10.1016/j.bbrc.2014.01.069. [DOI] [PubMed] [Google Scholar]
- Prinz M, and Priller J (2014). Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci 15, 300–312. 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Qiu LB, Dickson H, Hajibagheri N, and Crocker PR (1995). Extruded erythroblast nuclei are bound and phagocytosed by a novel macrophage receptor. Blood 85, 1630–1639. 10.1182/blood.v85.6.1630.bloodjournal8561630. [DOI] [PubMed] [Google Scholar]
- Raccosta L, Fontana R, Maggioni D, Lanterna C, Villablanca EJ, Panic-cia A, Musumeci A, Chiricozzi E, Trincavelli ML, Daniele S, et al. (2013). The oxysterol–CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J. Exp. Med 210, 1711–1728. 10.1084/jem.20130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P, Dobie R, Wilson-Kanamori JR, Dora EF, Henderson BEP, Luu NT, Portman JR, Matchett KP, Brice M, Marwick JA, et al. (2019). Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518. 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JA, Ikegami M, Cianciolo ER, Lu W, Cho PS, Hull W, Jobe AH, and Whitsett JA (1999). Aerosolized GM-CSF ameliorates pulmonary alveolar proteinosis in GM-CSF-deficient mice. Am. J. Physiol. Lung Cell Mol. Physiol 276, L556–L563. 10.1152/ajplung.1999.276.4.l556. [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Croxford AL, Becher B, and Landreth GE (2020). Plaque-associated myeloid cells derive from resident microglia in an Alzheimer’s disease model. J. Exp. Med 217, e20191374. 10.1084/jem.20191374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmerie A, Martens L, Thoné T, Castoldi A, Seurinck R, Pavie B, Roels J, Vanneste B, De Prijck S, Vanhockerhout M, et al. (2020). Osteopontin Expression Identifies a Subset of Recruited Macrophages Distinct from Kupffer Cells in the Fatty Liver. Immunity 53, 641–657.e14. 10.1016/j.immuni.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, and Barton GM (2017). Tissue-Resident Macrophages Are Locally Programmed for Silent Clearance of Apoptotic Cells. Immunity 47, 913–927.e6. 10.1016/j.immuni.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowska A, O’Sullivan SA, Christen I, Zhang J, Sailer AW, and Dev KK (2016). The EBI2 signalling pathway plays a role in cellular crosstalk between astrocytes and macrophages. Sci. Rep 6, 25520. 10.1038/srep25520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saederup N, Chan L, Lira SA, and Charo IF (2008). Fractalkine Deficiency Markedly Reduces Macrophage Accumulation and Atherosclerotic Lesion Formation in CCR2 −/− Mice. Circulation 117, 1642–1648. 10.1161/circulationaha.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, Edinger AL, Jung S, Rossner MJ, and Simons M (2016). Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci 19, 995–998. 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M, Troutman TD, Seidman JS, Ouyang Z, Spann NJ, Abe Y, Ego KM, Bruni CM, Deng Z, Schlachetzki JC, et al. (2019). Liver-Derived Signals Sequentially Reprogram Myeloid Enhancers to Initiate and Maintain Kupffer Cell Identity. Immunity 51, 655–670.e8. 10.1016/j.immuni.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroit AJ, Madsen JW, and Tanaka Y (1985). In vivo recognition and clearance of red blood cells containing phosphatidylserine in their plasma membranes. J. Biol. Chem 260, 5131–5138. 10.1016/s0021-9258(18)89189-x. [DOI] [PubMed] [Google Scholar]
- Schulte-Schrepping J, Reusch N, Paclik D, Babler K, Schlickeiser S, Zhang B, Krämer B, Krammer T, Brumhard S, Bonaguro L, et al. ; Deutsche COVID-19 OMICS Initiative DeCOI (2020). Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 182, 1419–1440.e23. 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimeca J-C, Franchi A, Trojani C, Parrinello H, Grosgeorge J, Robert C, Jaillon O, Poirier C, Gaudray P, and Carle GF (2000). The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic (oc/oc) mutants. Bone 26, 207–213. 10.1016/s8756-3282(99)00278-1. [DOI] [PubMed] [Google Scholar]
- Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, Lippens S, Abels C, Schoonooghe S, Raes G, et al. (2016). Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 7. 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrats J, Schiltz JC, García-Bueno B, van Rooijen N, Reyes TM, and Sawchenko PE (2010). Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron 65, 94–106. 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Seow JJW, Dutertre C-A, Pai R, Blériot C, Mishra A, Wong RMM, Singh GSN, Sudhagar S, Khalilnezhad S, et al. (2020). Onco-fetal Reprogramming of Endothelial Cells Drives Immunosuppressive Macrophages in Hepatocellular Carcinoma. Cell 183, 377–394.e21. 10.1016/j.cell.2020.08.040. [DOI] [PubMed] [Google Scholar]
- Shaw TN, Houston SA, Wemyss K, Bridgeman HM, Barbera TA, Zangerle-Murray T, Strangward P, Ridley AJ, Wang P, Tamoutounour S, et al. (2018). Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J. Exp. Med 215, 1507–1518. 10.1084/jem.20180019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer A, Grozovski J, Tay TL, Tao J, Volaski A, Süß P, Ardura-Fabregat A, Gross-Vered M, Kim J-S, David E, et al. (2018). Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat. Commun 9, 5206. 10.1038/s41467-018-07548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Over-street-Wadiche LS, Tsirka SE, and Maletic-Savatic M (2010). Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell 7, 483–495. 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvin A, Chapuis N, Dunsmore G, Goubet A-G, Dubuisson A, Derosa L, Almire C, Hénon C, Kosmider O, Droin N, et al. (2020). Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell 182, 1401–1418.e18. 10.1016/j.cell.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvin A, Uderhardt S, Piot C, Da Mesquita S, Yang K, Geirsdottir L, Mulder K, Eyal D, Liu Z, Bridlance C, et al. (2022). Dual ontogeny of disease-associated microglia and disease inflammatory macrophages in aging and neurodegeneration. Immmunity 55, 1448–1465. 10.1016/j.immuni.2022.07.004. [DOI] [PubMed] [Google Scholar]
- Sims R, van der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, Kunkle BW, Boland A, Raybould R, et al. (2017). Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet 49, 1373–1384. 10.1038/ng.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobacchi C, Frattini A, Guerrini MM, Abinun M, Pangrazio A, Susani L, Bredius R, Mancini G, Cant A, Bishop N, et al. (2007). Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat. Genet 39, 960–962. 10.1038/ng2076. [DOI] [PubMed] [Google Scholar]
- Dudvarski Stankovic N, Teodorczyk M, Ploen R, Zipp F, and Schmidt MHH (2016). Microglia–blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol. 131, 347–363. 10.1007/s00401-015-1524-y. [DOI] [PubMed] [Google Scholar]
- Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, and Dunn AR (1994). Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc. Natl. Acad. Sci. USA 91, 5592–5596. 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans I, Pan X-M, Rapp JH, Grunfeld C, and Feingold KR (2000). Oxidized Cholesterol in the Diet Accelerates the Development of Atherosclerosis in LDL Receptor– and Apolipoprotein E–Deficient Mice. Arterioscler. Thromb. Vasc. Biol 20, 708–714. 10.1161/01.atv.20.3.708. [DOI] [PubMed] [Google Scholar]
- Sun X, Seidman JS, Zhao P, Troutman TD, Spann NJ, Que X, Zhou F, Liao Z, Pasillas M, Yang X, et al. (2020). Neutralization of Oxidized Phospholipids Ameliorates Non-alcoholic Steatohepatitis. Cell Metabol. 31, 189–206.e8. e8. 10.1016/j.cmet.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Sakagami T, Rubin BK, Nogee LM, Wood RE, Zimmerman SL, Smolarek T, Dishop MK, Wert SE, Whitsett JA, et al. (2008). Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J. Exp. Med 205, 2703–2710. 10.1084/jem.20080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, and Weissleder R (2006). Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc. Natl. Acad. Sci. USA 103, 10340–10345. 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, and Pittet MJ (2007). Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest 117, 195–205. 10.1172/jci29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et al. (2007). Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest 117, 185–194. 10.1172/jci28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa R, Nakata K, Inoue Y, and Nukiwa T (2006). Granulocyte-macrophage colony-stimulating factor inhalation therapy for patients with idiopathic pulmonary alveolar proteinosis: a pilot study; and long-term treatment with aerosolized granulocyte-macrophage colony-stimulating factor: a case report. Respirology 11, S61–S64. 10.1111/j.1440-1843.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- Tazawa R, Trapnell BC, Inoue Y, Arai T, Takada T, Nasuhara Y, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, et al. (2009). Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 181, 1345–1354. 10.1164/rccm.200906-0978OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurl I, Hilgendorf I, Nairz M, Tymoszuk P, Haschka D, Asshoff M, He S, Gerhardt LMS, Holderried TAW, Seifert M, et al. (2016). On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat. Med 22, 945–951. 10.1038/nm.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion MS, Ginhoux F, and Garel S (2018). Microglia and early brain development: an intimate journey. Science 362, 185–189. 10.1126/science.aat0474. [DOI] [PubMed] [Google Scholar]
- Todd EM, Zhou JY, Szasz TP, Deady LE, D’Angelo JA, Cheung MD, Kim AHJ, and Morley SC (2016). Alveolar macrophage development in mice requires L-plastin for cellular localization in alveoli. Blood 128, 2785–2796. 10.1182/blood-2016-03-705962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S, Baba I, Poupel L, Dussaud S, Moreau M, Gélineau A, Marcelin G, Magréau-Davy E, Ouhachi M, Lesnik P, et al. (2020). Impaired Kupffer Cell Self-Renewal Alters the Liver Response to Lipid Overload during Non-alcoholic Steatohepatitis. Immunity 53, 627–640.e5. 10.1016/j.immuni.2020.06.003. [DOI] [PubMed] [Google Scholar]
- Uchida K, Nakata K, Trapnell BC, Hamano E, Mikami A, Matsushita I, Seymour JF, Oh-Eda M, Ishige I, Kitamura T, et al. (2003). High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood 103, 1089–1098. 10.1182/blood-2003-05-1565. [DOI] [PubMed] [Google Scholar]
- Uderhardt S, Martins AJ, Tsang JS, Lämmermann T, and Germain RN (2019). Resident Macrophages Cloak Tissue Microlesions to Prevent Neutrophil-Driven Inflammatory Damage. Cell 177, 541–555.e17. 10.1016/j.cell.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umstead TM, Hewage EK, Mathewson M, Beaudoin S, Chroneos ZC, Wang M, and Halstead ES (2020). Lower respiratory tract delivery, airway clearance, and preclinical efficacy of inhaled GM-CSF in a postinfluenza pneumococcal pneumonia model. Am. J. Physiol. Lung Cell Mol. Physiol 318, L571–L579. 10.1152/ajplung.00296.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, Liddelow SA, Nguyen PT, Nakao-Inoue H, Dorman LC, et al. (2018). Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 359, 1269–1273. 10.1126/science.aal3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vågesjö E, Parv K, Ahl D, Seignez C, Herrera Hidalgo C, Giraud A, Leite C, Korsgren O, Wallén H, Juusola G, et al. (2021). Perivascular Macrophages Regulate Blood Flow Following Tissue Damage. Circ. Res 128, 1694–1707. 10.1161/circresaha.120.318380. [DOI] [PubMed] [Google Scholar]
- Varma VR, Büşxra Lüleci H, Oommen AM, Varma S, Blackshear CT, Griswold ME, An Y, Roberts JA, O’Brien R, Pletnikova O, et al. (2021). Abnormal brain cholesterol homeostasis in Alzheimer’s disease—a targeted metabolomic and transcriptomic study. Npj Aging and Mechanisms of Disease 7, 11. 10.1038/s41514-021-00064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, Sanvito F, Ponzoni M, Valentinis B, Bregni M, et al. (2010). Tumor-mediated liver X receptor-α activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat. Med 16, 98–105. 10.1038/nm.2074. [DOI] [PubMed] [Google Scholar]
- Waddell A, Vallance JE, Fox S, and Rosen MJ (2021). IL-33 is produced by colon fibroblasts and differentially regulated in acute and chronic murine colitis. Sci. Rep 11, 9575. 10.1038/s41598-021-89119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, and Colonna M (2012). IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immuno 13, 753–760. 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Mustafa M, Yuede CM, Salazar SV, Kong P, Long H, Ward M, Siddiqui O, Paul R, Gilfillan S, et al. (2020). Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med 217, e20200785. 10.1084/jem.20200785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Markov NS, Lu Z, Piseaux Aillon R, Soberanes S, Runyan CE, Ren Z, Grant RA, Maciel M, Abdala-Valencia H, et al. (2021). Resetting proteostasis with ISRIB promotes epithelial differentiation to attenuate pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 118. e2101100118. 10.1073/pnas.2101100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S, Coccia M, Görtz D, This S, Stockenhuber K, et al. (2017). Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med 23, 579–589. 10.1038/nm.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett JA, Wert SE, and Weaver TE (2010). Alveolar Surfactant Homeostasis and the Pathogenesis of Pulmonary Disease. Annu. Rev. Med 61, 105–119. 10.1146/annurev.med.60.041807.123500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman DP, Jansen IE, Savage JE, Shadrin AA, Bahrami S, Holland D, Rongve A, Børte S, Winsvold BS, Drange OK, et al. (2021). A genome-wide association study with 1, 126, 563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet 53, 1276–1282. 10.1038/s41588-021-00921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr., Ahmed-Ansari A, Sell KW, Pollard JW, and Stanley ER (1990). Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA 87, 4828–4832. 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martínez-Colón GJ, McKechnie JL, Ivison GT, Ranganath T, Vergara R, Hollis T, et al. (2020). A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med 26, 1070–1076. 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willikens FLA, Werre JM, Kruijt JK, Roerdinkholder-Stoelwinder B, Groenen-Döpp YAM, van den Bos AG, Bosman GJCGM, and van Berkel TJC Blood 105, 2141–2145. [DOI] [PubMed] [Google Scholar]
- Williams JW, Martel C, Potteaux S, Esaulova E, Ingersoll MA, Elvington A, Saunders BT, Huang LH, Habenicht AJ, Zinselmeyer BH, et al. (2018). Limited Macrophage Positional Dynamics in Progressing or Regressing Murine Atherosclerotic Plaques-Brief Report. Arterioscler Thromb Vasc Biol. 38, 1702–1710. 10.1161/ATVBAHA.118.311319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthmann A, and Heeren J (2020). TREM2-Positive Lipid-Associated Macrophages (LAMs) Control White Adipose Tissue Remodeling and Metabolic Adaptation in Obesity. Immunometabolism 2, e200014. 10.20900/immunometab20200014. [DOI] [Google Scholar]
- Wright JR (1990). Clearance and recycling of pulmonary surfactant. Am. J. Physiol. Lung Cell Mol. Physiol 259, L1–L12. 10.1152/ajplung.1990.259.2.l1. [DOI] [PubMed] [Google Scholar]
- Wright JR, and Youmans DC (1995). Degradation of surfactant lipids and surfactant protein A by alveolar macrophages in vitro. Am. J. Physiol. Lung Cell Mol. Physiol 268, L772–L780. 10.1152/ajplung.1995.268.5.l772. [DOI] [PubMed] [Google Scholar]
- Wylam ME (2006). Aerosol granulocyte-macrophage colony-stimulating factor for pulmonary alveolar proteinosis. Eur. Respir. J 27, 585–593. 10.1183/09031936.06.00058305. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, and Pollard JW (2013). Macrophage biology in development, homeostasis and disease. Nature 496, 445–455. 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss A, Raselli T, Perkins N, Ruiz F, Schmelczer G, Klinke G, Moncsek A, Roth R, Spalinger MR, Hering L, et al. (2019). The EBI2-oxysterol axis promotes the development of intestinal lymphoid structures and colitis. Mucosal Immunol. 12, 733–745. 10.1038/s41385-019-0140-x. [DOI] [PubMed] [Google Scholar]
- Ydens E, Amann L, Asselbergh B, Scott CL, Martens L, Sichien D, Mossad O, Blank T, De Prijck S, Low D, et al. (2020). Profiling peripheral nerve macrophages reveals two macrophage subsets with distinct localization, transcriptome and response to injury. Nat. Neurosci 23, 676–689. 10.1038/s41593-020-0618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, Mckenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. (2006). IL-23 is essential for T cell–mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest 116, 1310–1316. 10.1172/jci21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoffey JM, and Yaffe P (1980). The phagocytic central reticular cell of the erythroblastic island in rat bone marrow: changes in hypoxia and rebound. J Reticuloendothel Soc. 28, 37–47. [PubMed] [Google Scholar]
- York AG, and Bensinger SJ (2013). Subverting sterols: rerouting an oxysterol-signaling pathway to promote tumor growth. J. Exp. Med 210, 1653–1656. 10.1084/jem.20131335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman R, and Epelman S (2022). Resident cardiac macrophages: Heterogeneity and function in health and disease. Immunity 55, 1549–1563. 10.1016/j.immuni.2022.08.009. [DOI] [PubMed] [Google Scholar]
- Zarb Y, Sridhar S, Nassiri S, Utz SG, Schaffenrath J, Maheshwari U, Rushing EJ, Nilsson KPR, Delorenzi M, Colonna M, et al. (2021). Microglia control small vessel calcification via TREM2. Sci. Adv 7, eabc4898. 10.1126/sciadv.abc4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Dai M, Fridberger A, Hassan A, DeGagne J, Neng L, Zhang F, He W, Ren T, Trune D, et al. (2012). Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc. Natl. Acad. Sci. USA 109, 10388–10393. 10.1073/pnas.1205210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Yang F, Wang Y, Li S, Li Y, Hou F, Yang W, Liu D, Tao Y, Li Q, et al. (2022). ALK1 signaling is required for the homeostasis of Kupffer cells and prevention of bacterial infection. J. Clin. Invest 132, e150489. 10.1172/JCI150489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Song WM, Andhey PS, Swain A, Levy T, Miller KR, Poliani PL, Cominelli M, Grover S, Gilfillan S, et al. (2020). Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat Med. 26, 131–142. 10.1038/s41591-019-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Herndon JM, Sojka DK, Kim K-W, Knolhoff BL, Zuo C, Cull-inan DR, Luo J, Bearden AR, Lavine KJ, et al. (2017). Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity 47, 597. 10.1016/j.immuni.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindel J, and Kubes P (2020). DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu Rev Pathol. 15, 493–518. 10.1146/annurev-pathmechdis-012419-032847. [DOI] [PubMed] [Google Scholar]


