Abstract
Noncovalent interactions between cells and environmental cues have been recognized as fundamental physiological interactions that regulate cell behavior. However, the effects of the covalent interactions between cells and biomaterials on cell behavior have not been examined. Here, we demonstrate a combined strategy based on covalent conjugation between biomaterials (collagen fibers/lipid nanoparticles) and various cells (exogenous neural progenitor cells/astrocytes/endogenous tissue-resident cells) to promote neural regeneration after spinal cord injury (SCI). We found that metabolic azido-labeled human neural progenitor cells conjugated on dibenzocyclooctyne-modified collagen fibers significantly promoted cell adhesion, spreading, and differentiation compared with noncovalent adhesion. In addition, dibenzocyclooctyne-modified lipid nanoparticles containing edaravone, a well-known ROS scavenger, could target azide-labeled spinal cord tissues or transplanted azide-modified astrocytes to improve the SCI microenvironment. The combined application of these covalent conjugation strategies in a rat SCI model boosted neural regeneration, suggesting that the covalent interactions between cells and biomaterials have great potential for tissue regeneration.
A combined strategy based on covalent conjugation between biomaterials and exogenous cells promotes spinal cord injury repair.
INTRODUCTION
Cells can respond to various geometrical, mechanical, electrical, chemical, or biological environmental cues to determine their behavior. Many studies have revealed that noncovalent (NC) interactions between cells, and between cells and matrices/various molecules are very important for regulating cell adhesion, migration, spreading, proliferation, and differentiation (1–4). NC interactions, which include hydrogen bonds, ionic bonds, van der Waals forces, and hydrophobic interactions, are weaker than covalent bond interactions. It has been thoroughly recognized that NC interactions are a dynamic and reversible inherent feature of life. In contrast to NC interactions, covalent interactions are irreversible and stronger bonds that hold atoms together by sharing electrons. Given that covalent interactions between cells are not very common in the body, their biological effects have not been extensively investigated.
Stem cell transplantation holds promise for repairing injured and degenerative tissues by differentiating into mature cells or inducing endogenous regeneration with secreted molecules (5, 6), but it is challenged by the poor retention and survival and low differentiation efficiency after transplantation (7, 8). For example, in a spinal cord injury (SCI), transplanted cells tend to be diffused at the injured SC immersed in cerebrospinal fluid and cannot survive in the adverse microenvironment caused by oxidative stress, ischemia, excitotoxicity, and inflammatory cytokine storms. Since most mammalian cells are anchorage dependent, they need to adhere to a surface to survive. In that context, biomaterials can be used to promote anchorage-dependent cell survival. The adhesion of stem cells to scaffolds can be regulated by the stiffness (9), surface topological structure (10) and porosity (11) of the scaffold, as well as the modification of integrin ligand or adhesion molecules (12). Previous studies have demonstrated that enhanced NC interactions between biomaterials and cells can improve the efficiency of stem cell transplantation in SCI (3, 5, 13). However, the dynamic reversibility of NC adhesion might be influenced by complicated environment cues, including variations in pH, ion concentration, and various enzymes (14). In contrast, covalent chemical bonds are irreversible and are more stable and robust than NC interaction. Therefore, whether covalent conjugation (CC) between biomaterials and stem cells could improve stem cell behavior after transplantation in SCI is an important scientific issue that remains to be addressed.
Tetraacetylated N-azidoacetyl-d-mannosamine (Ac4ManNAz), a well-known cell label agent, is widely used for live cell imaging (15) and glycoproteomic analysis (16). It can label cell surface glycoproteins and glycolipids with azido groups by incorporating itself into the sialic acid biosynthetic pathway. As excellent “artificial antigens,” azide groups can be introduced to the surface of the membrane at a higher density than a certain class of proteins, which greatly increases the chances of being recognized (17). In addition, as a small-molecule compound, Ac4ManNAz has only minimal interference with the conformation and functional sites of glycoproteins and glycolipids compared with biotin and aptamer (17). The azide groups on the membrane surface can form stable and robust chemical bonds by bioorthogonal ligation with the complementary group dibenzocyclooctyne (DBCO) (18). The DBCO group does not react with hydroxyls or amines, which are abundant in many proteins, and the reaction between the DBCO group and azide group is specific and fast under physiological conditions. Multiple studies for living cell surface modifications or imaging have shown that this ligation is not cytotoxic (19–21). We proposed that N3-modified stem cells and DBCO-modified scaffolds might be advantageous in the study of the effects of covalent interactions between stem cells and scaffolds.
Click chemistry enables rapid in situ cross-linking between polymer chains without toxic catalysts. Therefore, click chemistry–based hydrogels are widely used for filling of defect site (22), controlled release of drugs (23), and encapsulation of cells (24). The specific and fast covalent recognition in click chemical reactions between azide groups and DBCO group offers advantage for targeted drug delivery in complex in vivo environment. Several successful tumor-targeting therapies involving labeling tumors with azide groups to specifically bind DBCO-modified drugs have been reported (25–27). Considering that excessive reactive oxygen species (ROS) in SCI sites is one of the reasons that lead to the death of endogenous tissue cells and exogenous transplanted stem cells (28), the specific covalent recognition based on click chemical reaction between cells and nanoparticles could be useful for delivering antioxidants for SCI repair.
Here, we report combined strategies based on covalent interactions between cells and biomaterials to promote neural regeneration in rat SCI model (Fig. 1). First, the human neural progenitor cells (NPCs) were metabolically labeled by Ac4ManNAz, and the amino groups of the longitudinally aligned collagen fibers (LACFs) were modified by DBCO groups. We found that metabolic azide-modified NPCs could be covalently conjugated on DBCO-LACF, and CC promoted cell adhesion, achieved prolonged cell retention on the scaffolds, and induced cells to spread along the direction of fibers. We also found that CC promoted NPC differentiation compared with traditional NC adhesion. Subsequently, a targeted drug delivery strategy based on covalent recognition between cells and nanoparticles was established to deliver a neuroprotective agent, edaravone (Eda), to attenuate oxidative stress at the SCI sites. After directly injecting Ac4ManNAz into the spared SC right after an injury, the intravenously delivered DBCO-modified lipid nanoparticles containing Eda massively accumulated in the injured SC within a 10-day therapeutic window and significantly promoted the survival of exogenous implanted NPCs conjugated on collagen fibers. Furthermore, to avoid the potential secondary damage of tissues from direct injection, NPCs conjugated on DBCO-LACF were assembled into a collagen conduit containing N3-labeled human astrocytes to form an engineered spinal cord–like transplant with drug-guided function. Given that N3-labeled astrocytes not only can specifically covalently recognize intravenously injected DBCO-modified lipid nanoparticles containing Eda but also have neurotrophic function for neuronal maturation and neural synapse and circuit formation, the implantation of engineered spinal cord–like transplant significantly promotes cell survival, axon regeneration, and angiogenesis after SCI. This suggests that combined strategies based on CC between biomaterials (collagen fibers or lipid nanoparticles) and various cells (exogenous NPCs or astrocytes or endogenous tissue-resident cells) are promising for neural regeneration.
Fig. 1. Schematic illustration of covalent interactions between cell and biomaterials regulating cell behavior and drug targeting for boosting neural regeneration in SCI.
(A) CC between donor NPCs and scaffolds. (B) Implantation of NCCL and covalent recognition between Eda-Lips and endogenous N3-modified SC cells. (C) Implantation of NCCL and covalent recognition between Eda-Lips and exogenous N3-modified astrocytes.
RESULTS
DBCO-LACF and N3-modified NPCs were prepared and characterized
To build covalent interactions between NPCs and LACFs, N-hydroxysuccinimide (NHS)–Sulfo-DBCO was used to convert amino groups of LACF into DBCO groups (Fig. 2A). The chemical characteristics of DBCO-LACFs were verified by 1H nuclear magnetic resonance (NMR). The characteristic peaks of DBCO were detected at 7.0 to 8.0 parts per million (ppm), indicating the successful modification of DBCO-LACFs (Fig. 2B). The degrees of the DBCO incorporation were determined via ultraviolet-visible (UV-vis) absorption spectroscopy after LACFs were treated with different concentrations of NHS-Sulfo-DBCO. The absorbance at 310 nm confirmed that the amino group on the scaffold surface was converted to DBCO in a dose-dependent manner (Fig. 2, C and D). To identify the distribution of DBCO groups on the surface of the scaffold, the scaffolds were incubated with N3-Cy3, and the results showed that DBCO groups were evenly distributed on the surface of the scaffold (Fig. 2E). Scanning electron microscopy (SEM) images showed that DBCO modification did not destroy the longitudinally aligned topological structure of LACFs (Fig. 2F). To detect the impact of the DBCO modification on the mechanical properties of LACFs, the stretching strain test was performed and the results showed that the maximum tensile strength and elongation at break of DBCO-LACFs were 258.1 ± 4.9 MPa and 20.8 ± 0.93%, respectively, while those of LACFs were 228.2 ± 4.1 MPa and 25.1 ± 1.3% (Fig. 2G). In addition, the Young’s modulus of DBCO-LACFs (1807.1 ± 209.1 MPa) was significantly higher than that of LACFs (1258.7 ± 50.6 MPa) (Fig. 2H).
Fig. 2. Preparation and characterization of DBCO-LACF and N3-modified NPCs.
(A) Schematic illustration of preparation of DBCO-LACF using NHS-Sulfo-DBCO. (B) 1H-NMR spectrum of LACF and DBCO-LACF. (C) UV-vis spectra of DBCO-LACF prepared with different doses of NHS-Sulfo-DBCO. (D) Quantification of DBCO group of DBCO-LACF prepared with different doses of NHS-Sulfo-DBCO based on the absorbance value at 310 nm of UV-vis spectra. (E) Confocal microscopy image of DBCO-LACF incubated with azide-Cy3. (F) SEM image of DBCO-LACF and LACF. Scale bar, 100 μm. (G) Representative stress-strain curves of DBCO-LACF and LACF. (H) Young’s modulus values for LACF and LACF-DBCO (n = 3 independent samples, two-tailed t test). (I) Schematic illustration of N3 modification on NPCs using Ac4ManNAz. (J) Immunofluorescence images of Nestin and Sox2 in NPCs. Scale bar, 100 μm. (K) DBCO-Cy3 staining of NPCs treated with various concentrations of Ac4ManNAz. Scale bar, 50 μm. (L) Quantitative analysis of the Cy3 fluorescence intensity in (J) [n = 5 biologically independent samples, one-way analysis of variance (ANOVA) and Tukey’s test]. (M) Live/dead staining for NPCs treated with various concentrations of Ac4ManNAz. Scale bar, 50 μm. (N) Quantitative analysis of NPC viability in (L) (n = 4 biologically independent samples, one-way ANOVA and Tukey’s test). Error bars, means ± SD. , **P < 0.01. A.U., arbitrary units.
NPCs derived from the human fetal SC that highly expressed Sox2 and Nestin were used in this study (Fig. 2, I and J). DBCO-Cy3 was used to detect the efficiency of N3 modification on NPCs after adding Ac4ManNAz to the culture medium at different concentrations for 48 hours. The fluorescence intensity suggested that the efficiency of metabolic labeling was Ac4ManNAz dose dependent (Fig. 2, K and L). Because doses higher than 25 μM led to a decrease in NPC viability, as shown by live/dead staining, a dose of 12.5 μM was used in subsequent experiments to achieve safe and efficient labeling of NPCs (Fig. 2, M and N).
CC between DBCO-LACFs and N3-modified NPCs enhances retention, differentiation, and oriented axon growth of NPCs
The adhesion effects of N3-modified NPCs on collagen or DBCO-modified collagen were first evaluated (Fig. 3A). After seeding NPCs for 2 and 6 hours, the number of NPCs on collagen-DBCO was about 2.6- and 2-fold higher than that on collagen, respectively (Fig. 3, B and C). CC has no significant effect on the proliferation of NPCs (fig. S1, A and B). Hence, CC was able to capture more cells than NC interactions. Live/dead staining after 1 and 3 days of culture showed that there was no significant difference in cell viability between the CC and NC groups, which was also confirmed in a CCK-8 experiment (Fig. 3D and fig. S1, C and D).
Fig. 3. CC between DBCO-LACF and N3-modified NPCs enhanced NPC retention, differentiation, and oriented axon growth of NPCs.
(A) Schematic of evaluating the adhesion and differentiation of CC between NPCs and collagen. (B) FDA staining of NPCs after attaching for 2 or 6 hours by NC or CC interaction. Scale bar, 100 μm. (C) Quantification of attached cell number in (B) (n = 5 biologically independent samples, two-tailed t test). (D) Live/dead staining for NPCs seeded on collagen by CC or NC. Scale bar, 50 μm. (E) Heat map of RNA-seq data illustrating the expression level of differentiation-related genes of CC and NC group after differentiation for 1, 7, and 14 days. The standardized abundance of transcripts is color-coded according to the scale bar. (F) Results of qPCR analyses of Map2, GFAP, DCX, and TNF expression levels of NPCs in CC and NC group (n = 4 biologically independent samples, two-tailed t test). (G) Immunofluorescence images of Map2+ or GFAP+ cells derived from NPCs in the CC and NC groups. Scale bar, 50 μm. (H) Schematic illustration of covalent cell-scaffold conjugation. (I) FDA staining of NPCs in CC or NC group for 1, 5, and 10 days. Scale bars, 250 and 100 μm. (J) 2D FFT image analysis of oriented axon growth of NPCs. (K) SEM images of NPCs attached on scaffold by CC or NC interaction for 10 days. Scale bar, 10 μm. Error bars, means ± SD. **P < 0.01.
Next, to explore the effect of CC on the fate of NPCs, NPCs or N3-modified NPCs were adhered on collagen or DBCO-modified collagen, respectively, and were cultured in differentiation medium for 1, 7, and 14 days. By analyzing the gene expression using RNA sequencing (RNA-seq), we found that cell proliferation decreased in both groups following the cell differentiation. The expression of apoptosis-related genes slightly increased in the NC group on day 14 but not in the CC group, which might be attributed to the fact that the weak interaction between collagen and NPCs in the NC group could not well support the long-term cell culture in vitro (fig. S2A). In addition, the expression levels of marker genes of neurons and astrocytes were obviously up-regulated in the CC group (Fig. 3E). The quantitative real-time polymerase chain reaction (qPCR) was performed on NPCs that differentiated for 14 days in CC or NC group to analyze expression levels of MAP2, DCX, GFAP, and TNF. Compared with the NC groups, DCX and MAP2 were significantly up-regulated and TNF was significantly down-regulated in CC group, consistent with the results of RNA-seq analysis data (Fig. 3F). The results of immunofluorescence staining showed that the proportion of Map2+ cells in the CC group was 69.0 ± 3.7%, which was significantly higher than that in the NC group (61.8 ± 4.6%) (Fig. 3G and fig. S2B), which may be caused by up-regulation of Wnt signaling pathway (fig. S2C) (29). Gene expression levels of DCX and MAP2 were significantly down-regulated by the use of the Wnt signaling pathway inhibitor IWR (fig. S2D). The percentage of glial fibrillary acidic protein–positive (GFAP+) cells in the CC group was 31.0 ± 3.8%, slightly higher than that in the NC group (26.9 ± 4.9%) (Fig. 3G and fig. S2B). In addition, after 14 days of differentiation, genes related to neuroactive ligand–receptor interaction and synapse formation were up-regulated (fig. S2E).
Because the different elastic modulus between LACF and DBCO-LACF may influence cell fate, the NPCs without N3 modification were seeded to LACF and DBCO-LACF, respectively, to analyze the possible effect of changed mechanical modulus on cell adhesion and differentiation. We found that the enhancement of modulus may have a minus effect on the long-term adhesion and growth of cells on scaffolds (fig. S3A), but it did not affect neuronal differentiation (fig. S3, B and C). These results indicated that CC might promote the differentiation of NPCs.
To further evaluate the biological effect of CC between NPCs and LACF, N3-modified NPCs were seeded to LACF and DBCO-LACF (Fig. 3H). To determine the optimal dose of DBCO-Sulfo-NHS, N3-modified NPCs were seeded onto LACFs treated with different concentrations of DBCO-Sulfo-NHS. The results showed that NPCs on DBCO-LACFs treated by NHS-Sulfo-DBCO (0.625 mg/ml) showed the highest number of cells and the best effect on oriented axon growth (fig. S3, D and E). After being cultured for 1, 5, and 10 days, cell morphology was evaluated by fluorescein diacetate (FDA) staining. The results showed that N3-modified NPCs attached and spread on LACF and DBCO-LACF on the first day. Nevertheless, the number of NPCs in the NC group decreased gradually, while the number of NPCs in the CC group did not within 10 days. These findings indicated that CC maintained cell retention on the scaffold surface (Fig. 3I).
To explore the mechanism of azide-labeled NPCs binding to DBCO-modified collagen scaffold, we compared gene expression in cells that adhered to collagen by NC and CC. By analyzing the RNA-seq data, we found that the expression of integrin and focal adhesion–related genes was down-regulated in the CC group compared with the NC group. However, CC between biomaterials and NPCs might promote the expression of some neural-related adhesion molecules, such as NCAM1, NCAM2, CHL, NFASC, CADM2, CADM3, and CNTN2 (fig. S4A). It suggests that CC promotes NPC adhesion by neural-specific adhesion molecules but not integrin-mediated focal adhesion. EDTA can be used to block the integrin and Arg-Gly-Asp (RGD) binding, which is divalent cation dependent (30). To distinguish integrin-based NC adhesion and N3-DBCO–based covalent adhesion, the NPCs attached onto collagen by NC and CC were treated by EDTA. The presence of EDTA led to substantially diminished cell spreading area and cell density in the NC group but not in the CC group (fig. S4B). It suggests that the enhanced cell adhesion is mainly attributed to CC but not integrin-mediated NC interaction. In addition, we also analyzed the stability of azide-DBCO–based covalent bond. We found that 10 days after N3-modification, no positive signal was detected by DBCO-Cy3 staining, indicating that N3-modification of glycoproteins or glycolipids had been metabolically eliminated within 10 days when azide was not conjugated with DBCO at the early stage, but strong fluorescence signal was found after 10 days in N3-modified cells that conjugated with DBCO-Cy3 at the early stage (fig. S4C). These results suggest that CC mediated the interaction between scaffolds and cells, is integrin independent, and promoted the long-term retention of cells on scaffolds by forming stable covalent bonds.
In addition, given the longitudinally aligned topological structure of LACF, two-dimensional (2D) fast Fourier transform (FFT) image analysis was used to evaluate the oriented axon growth of NPCs. Two obvious peaks at 90° and 270° indicated that the outgrowth of neurites of NPCs on DBCO-LACF was linearly distributed (Fig. 3J). Furthermore, SEM images showed that NPCs in the CC group spread along the direction of fibers on the scaffold surface (Fig. 3K). These results indicate that CC could promote retention and differentiation of NPCs and induce the oriented axon growth of NPCs by enhancing the sensing ability of cells for the topological structure of scaffolds.
Eda-loaded DBCO-liposomes attenuate the level of ROS
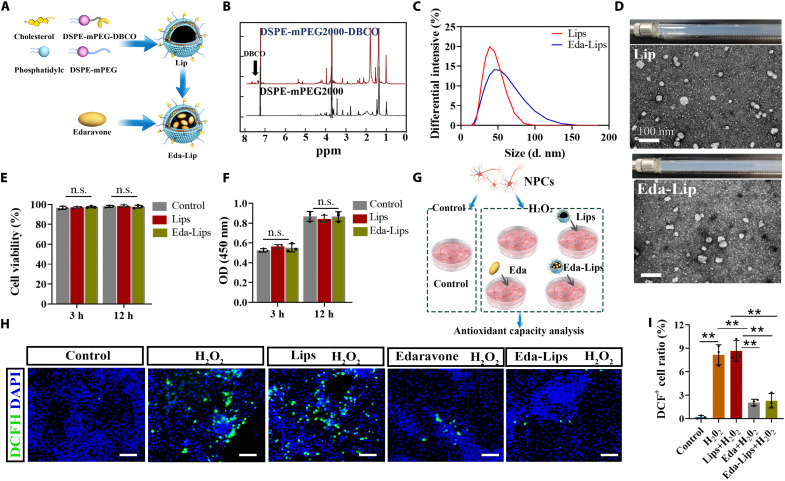
To achieve CC-mediated targeting delivery of Eda to the injury site, cholesterol and soybean phosphatidylcholine (PC), DSPE-mPEG2000 and DSPE-mPEG2000-DBCO, were used to synthesize liposomes (Lips). Subsequently, Eda was loaded into the particles using a calcium acetate gradient method (Fig. 4A). In the 1H-NMR spectrum of DSPE-mPEG2000-DBCO, the peak at 7.0 to 8.0 ppm was identified as the characteristic peak of DBCO (Fig. 4B). The size distribution of Lips was measured by the dynamic light scattering (DLS), which indicated that the average diameters of Lips and Eda-Lips were 41.9 and 52.2 nm, with polydiseperse indexes (PDIs) of 0.156 and 0.20, respectively (Fig. 4C). Through high-performance liquid chromatography (HPLC) analysis, the entrapment rate was determined to be 42.7 ± 4.2%. Lips and Eda-Lips were observed to be similar small unilamellar vesicles by transmission electron microscopy (TEM), suggesting that Eda loading had no obvious influence on the particle morphology (Fig. 4D). To detect the biocompatibility of Lips, Lips and Eda-Lips were added into the NPC culture medium, and live/dead staining and CCK-8 assay were performed after 3 and 12 hours, respectively. The results showed that cell viability was not affected by Lips or Eda-Lips (Fig. 4, E and F, and fig. S5A), suggesting the safety of these particles. The antioxidant capacity of Eda-Lips was assessed using 2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA) kits (Fig. 4G). DCFH-DA is deacetylated by cellular esterases to produce a nonfluorescent compound, which is later oxidized by ROS to produce a fluorescent compound, 2,7-dichlorofluorescein diacetate (DCF), which can be detected at 495 nm. The results showed that there were significantly fewer DCF-positive cells in the Eda and Eda-Lip groups than in the H2O2 group. These findings indicate that Eda can be effectively released from Eda-Lips and strongly attenuate the ROS level of NPCs before or after the encapsulation in Lips (Fig. 4, H and I).
Fig. 4. Preparation and characterization of Eda-Lips.
(A) Schematic illustration of preparation of Eda-Lips. (B) 1H-NMR spectrum of DSPE-mPEG200 and DSPE-mPEG200-DBCO. (C) Size distribution of Eda-Lips and Lips determined by DLS. (D) TEM images of Eda-Lips and Lips. Scale bar, 100 nm. (E) Quantitative analysis of NPC viability (n = 3 biologically independent samples, one-way ANOVA and Tukey’s test). (F) Optical density (OD) values at 450 nm of NPCs treated with Eda-Lips and Lips in CCK-8 analysis (n = 3 biologically independent samples, one-way ANOVA and Tukey’s test). (G) Schematic illustration of antioxidant capacity test. (H) Images of green fluorescent signals of DCF for detecting ROS levels in NPCs with various treatments. Scale bar, 50 μm. (I) DCF+ cell ratio in (H) (n = 3 biologically independent samples, one-way ANOVA and Tukey’s test). Error bars, means ± SD. **P < 0.01. n.s., not significant.
Eda-Lips target into N3-modified SCs
We hypothesized that injured SC could be modified with azide groups by injecting Ac4ManNAz into spared tissues. To test this hypothesis, a complete SC transection was performed in rats. Then, Ac4ManNAz was injected into the spinal parenchyma on both sides of the injured gap using stereotaxic apparatus. To study the labeling kinetics of Ac4ManNAz in SC, the N3-modified SC tissue was lysed for Western blot analysis at different time points after injection. By incubating them with biotin-PEG4-alkyne, N3-modified glycoproteins were biotinylated and thus could be easily detected. The results showed that N3 modification efficiency in SC peaked 4 days post-injury (dpi) and existed at least 10 days before complete metabolic depletion (Fig. 5A). This indicates that endogenous SC resident cells could be N3-modified by Ac4ManNAz. Next, after tail vein injection of DBCO-Cy3, a large number of Cy3-positive cells were found in the parenchyma of N3-modified SC, while little in unmodified SC. These findings indicate that N3 modification of endogenous SC resident cells could provide at least a 10-day targeting window for DBCO-modified drugs (Fig. 5, B and C). We also examined which kinds of cells can be labeled by this method. Immunostaining revealed that 35.1 ± 9.1% of Cy3+ cells were costained with Iba1 (a marker of microglia), 25.7 ± 6.2% with APC+ (a marker of oligodendrocytes), and 17.8 ± 3.9% with GFAP (a marker of astrocytes). Almost no cells positive for both Cy3 and NeuN were found (Fig. 5, D and E). Furthermore, to test the ability of N3-modified SC to enrich DBCO-modified Lips, the DBCO-modified Lips that encapsulated 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide [DiR (DiR-Lips)] were injected into the tail vein at different time points. At 12 hours after injection, the distribution of DiR-Lips was assessed using Living Imaging System (IVIS), and the radiant efficiency of DiR in major organs was detected (Fig. 5F). We found that N3-modified SC showed substantially higher near-infrared fluorescence (NIRF) intensity than unmodified SC in the injury site at 1, 4, 7, and 10 dpi. The radiant efficiencies of N3-modified SC were approximately 2.6- and 2.1-fold higher than those of unmodified SC at 4 and 7 dpi (Fig. 5G and fig. S5, B and C). DiR signals were also detected in the brain in addition to the SC, liver, and spleen. It might be attributed to in situ injected Ac4ManNAz that diffuses into the cerebrospinal fluid and accumulates in brain, which finally led to the dye to be covalently recognized by the N3-modified brain cells.
Fig. 5. Eda-Lips targeted N3-modified SC.
(A) Western blotting analysis of N3-modified SC at various time points after Ac4ManNAz was injected into surrounding area of SCI sites. N3-modified glycoproteins were biotinylated by incubating tissue lysates with biotin-PEG4-alkyne. (B and C) DBCO-Cy3 staining images of N3-modified and unmodified SC. N3 modification and SCI were performed simultaneously, and then DBCO-Cy3 was injected into the tail vein at 1, 4, 7, and 10 dpi. Twelve hours after injection, the SC tissues were harvested to be sectioned for analyzing the intensity of Cy3. Scale bar, 500 μm. (D) Confocal images of GFAP (for astrocytes), Iba1 (for microglia), APC (for oligodendrocytes), and NeuN (for neurons) staining. Four days after N3 modification, DBCO-Cy3 was injected into the tail vein, and the SC tissues were sectioned for immunofluorescence staining. Scale bar, 50 μm. (E) Proportion of various cell types in Cy3+ cell (n = 4 biologically independent samples). (F) IVIS images of the DiR signal of major organs to show the biodistribution of DBCO-Lips. Scale bar, 2 cm. (G) Quantitative analysis of DiR radiant efficiency in SC (n = 3 biologically independent samples, two-tailed t test). (H and I) Eda-Lips were injected into tail vein of SCI rats with N3-modified SC and unmodified SC for evaluation of anti-apoptotic effect. (H) Western blotting analyses of proapoptotic protein. (I) The content of lipid peroxidation marker MDA in SC (n = 4 biologically independent samples, one-way ANOVA and Tukey’s test). Error bars, means ± SD. *P < 0.05, **P < 0.01.
To evaluate whether targeted delivery could enhance the antioxidant effect of Eda in vivo, the rats with N3-modified or unmodified SC received injection of Eda-Lips. The expression levels of proapoptotic proteins in the injured SC were analyzed by Western blot. We demonstrated that cellular apoptosis–related protein Bax and cleaved caspase-3 in N3-modified SC were significantly down-regulated after administration of Eda-Lips compared with the control group and unmodified SC group (Fig. 5H). Meanwhile, a lipid peroxidation marker malondialdehyde (MDA) in the N3-modified SC group decreased to 0.16 nmol/mg compared with 0.21 nmol/mg in the unmodified SC group and 0.35 nmol/mg in the SCI group (Fig. 5I). In conclusion, on the basis of the Ac4ManNAz-mediated endogenous cell labeling in SC, DBCO-modified Eda-Lips could be targeted to SCI sites to attenuate ROS-induced oxidative stress.
Transplantation of NPCs covalently conjugated on LACFs and targeting delivery of Eda-Lips to SC promote neural regeneration after SCI
To investigate the biological effects of CC-based exogenous NPC delivery and Eda-Lips targeting endogenous SC resident cells, green fluorescent protein (GFP)–positive NPCs or GFP-positive N3-modified NPCs were seeded on LACFs and DBCO-LACFs, respectively. NPCs and scaffolds were implanted into rat SCI sites accompanied by Ac4ManNAz injection into the surrounding area of SCI. Eda-Lips were injected into the tail vein at 1, 3, 5, and 7 dpi (Fig. 6A). At 10 dpi, the immunostaining results showed that the number of GFP+ cells in the NCCL (NPCs covalently conjugated on LACFs) group was 1.7-fold higher than that in the NNCAL (NPCs noncovalently attached to the LACF) group (Fig. 6, B and C). The magnified images showed flat morphology of cells in the NCCL group and round cell morphology in the NNCAL group, which could contribute to different adhesion ability (Fig. 6, D and E). In addition, 56.9 ± 4.7% GFP+ cells in the NCCL group were caspase-3+, which was significantly lower compared with the NNCAL group (79.1 ± 6.3%) (Fig. 6, D and F). This is probably because the tighter attachment based on CC prevents NPC anoikis. The combined application of NCCL with Eda-Lips further increased the number of GFP+ cells in the injury area (Fig. 6, B and C). Among them, more GFP+ cells were observed in the tENCCL (targeting Eda-Lips + NCCL) group, which was 1.4-fold of that in ntENCCL (nontargeting Eda-Lips + NCCL) (Fig. 6C). In addition, 24.3 ± 4.8% GFP+ cells in the tENCCL group were caspase-3+, which was lower compared with the ntENCCL groups (35.5 ± 4.6%) (Fig. 6, D and F). This suggests that targeted delivery effectively increases the concentration of Eda in the injury area, improving donor NPC survival. There was no significant difference in the proportion of Tuj-1+GFP+ cells among the four groups at 10 days (Fig. 6, E and G).
Fig. 6. Transplantation of covalently conjugated NPCs on LACF (NCCL) and targeting delivery of Eda-Lips to SC promoted neural regeneration after SCI.
(A) Schematic illustration of entire strategy of cell delivery and targeting modulation to SC. (B) Confocal images of GFP staining (for donor NPCs) at 10 dpi. Scale bar, 500 μm. (C) Quantification of GFP+ cell number per section in (B) (n = 5 biologically independent samples, one-way ANOVA and Tukey’s test). (D and E) Confocal images of Tuj-1 and caspase-3 staining in the lesion core at 10 dpi. Scale bar, 50 μm. (F and G) Proportions of Tuj-1+GFP+/GFP+ and caspase-3+GFP+/GFP+ (n = 5 biologically independent samples, one-way ANOVA and Tukey’s test). (H) Confocal images of GFP staining (for donor NPCs) at 60 dpi. Scale bar, 500 μm. (I) Quantification of GFP+ cell numbers per section in (H) (n = 5 biologically independent samples, one-way ANOVA and Tukey’s test). (J and K) Confocal images of NF and Map2 staining in the lesion core at 60 dpi. Scale bar, 50 μm. (L and M) Proportions of NF+GFP+/ GFP+ and Map2+GFP+/ GFP+ (n = 5 biologically independent samples, one-way ANOVA and Tukey’s test). Error bars, means ± SD. *P < 0.05, **P < 0.01.
At 60 dpi, the number of GFP+ NPCs decreased in all groups, but the trend remained unchanged. The number of GFP cells in the tENCCL group was 3.7-fold higher than that in the NNCAL group (Fig. 6, H and I). To measure the neurons derived from the donor NPCs, immunofluorescence staining and quantification for Map2 (a marker of mature neurons) and NF (a marker of the neuronal axon) were respectively performed. Quantitative analyses showed that 40.9 ± 4.9% and 31.2 ± 3.5% GFP+ cells in the tENCCL group were NF+ and Map2+, respectively, which exceed all groups (Fig. 6, J to M). It suggests that CC promoted the differentiation of donor NPCs in vivo, while targeted delivery of Eda improved the microenvironment in the injury area, which was conducive to the survival of newborn neurons. To assess locomotor functional recovery, an inclined plane assay and BBB (Basso, Beattie, and Bresnahan) test were performed. The results showed that the tENCCL group achieved the highest BBB scores (6 ± 0.75), while the angle inclined plate in the tENCCL group (38.3° ± 1.28°) was larger than that in the ntENCCL group (37.4° ± 1.22°) (fig. S6).
Eda-Lips target N3-modified exogenous astrocytes
Astrocytes provide nutrients to neurons while maintaining ion homeostasis, which is essential for maintaining the physiological function of neurons. In addition, astrocytes have been reported to promote vascular development and axon regeneration (31, 32). Therefore, cotransplantation of astrocytes and NPCs may be a more promising strategy for SCI therapy (33). We hypothesized that astrocytes cultured in vitro could also be N3-modified by Ac4ManNAz and could specifically recognize and enrich Eda-Lips after transplantation in vivo to achieve targeting guiding for DBCO-modified drugs. This approach might further avoid the potential secondary damage of tissues from direct injection of Ac4ManNAz into normal SC. Astrocytes derived from the human fetal SC, highly expressing GFAP and S100β, were used to test this hypothesis (Fig. 7, A and B). To determine the optimal dosage of Ac4ManNAz, astrocytes cultured in vitro were treated with different concentrations of Ac4ManNAz for 48 hours, and the azide group amounts on the cell surface and cell viability were measured to determine the optimal concentration (Fig. 7, C and D, and fig. S7, A and B). The results showed that even the dose of 200 mM had no effect on cell viability, suggesting that the tolerance of astrocytes to Ac4ManNAz was much higher than that of NPCs. However, the content of the azide group on the cell surface reached a plateau at a dose of 50 μM, which was selected for subsequent experiments (Fig. 7D).
Fig. 7. DBCO-Lips targeted N3-modified exogenous astrocyte.
(A) Schematic illustration of N3 modification on astrocytes using Ac4ManNAz. (B) Immunofluorescence images of GFAP and S100β of astrocytes. Scale bar, 50 μm. (C) DBCO-Cy3 staining of astrocytes treated with various concentrations of Ac4ManNAz. Scale bar, 50 μm. (D) Quantitative analysis of the Cy3 fluorescence intensity in (C) (n = 5 biologically independent samples, one-way ANOVA and Tukey’s test). (E and F) DBCO-Cy3 staining images of N3-modified and unmodified implants. N3-modified and unmodified astrocytes labeled by PKH67 were seeded on collagen scaffold. After the implantation of collagen scaffold with astrocytes in SCI sites, DBCO-Cy3 was injected into the tail vein at 1, 4, 7, and 10 dpi. Twelve hours after injection, the SC tissues of SCI rats were harvested to be sectioned to analyze the intensity of Cy3. Scale bar, 50 μm. (G) IVIS images of the DiR signal of major organs to show the biodistribution of DBCO-Lips. Scale bar, 2 cm. (H) Quantitative analysis of DiR radiant efficiency of SC (n = 3 biologically independent samples, two-tailed t test). Error bars, means ± SD. *P < 0.05, **P < 0.01.
Next, to test the effect of targeted delivery based on the covalent recognition of exogenous cells and Eda-Lips, N3-modified astrocytes labeled with PKH67 (a green fluorescent dye used for cell membrane staining) were seeded in collagen sponge scaffolds and transplanted into the injury sites. At different time points after transplantation, we detected the biological distribution of DBCO-Cy3 or DiR-Lips injected into the tail vein (Fig. 7E). The results showed that compared with the unmodified astrocytes, more N3-modified astrocytes were stained with Cy3, especially at 4 and 7 dpi (Fig. 7F). N3-modified astrocytes showed a significantly higher NIRF intensity than unmodified astrocytes in the injury site at 1, 4, 7, and 10 dpi (Fig. 7G). The radiant efficiencies of N3-modified astrocytes were 2.4- and 2.6-fold higher than those of unmodified astrocytes at 4 and 7 dpi, which means that the targeted delivery strategy based on covalent recognition of N3-modified exogenous cells is feasible (Fig. 7H and fig. S7, C and D).
Transplantation of Eda-Lips–targeted spinal cord–like tissues boosts neural regeneration after SCI
The schematic diagram for constructing Eda-Lips–targeted SC-like tissues (Et-SCT) for SCI repair is shown in Fig. 8A. Briefly, N3-modified NPCs were seeded on DBCO-LACF, while N3-modified astrocytes were seeded on collagen conduits. Then, LACFs with NPCs were assembled into the conduits and were together transplanted to the injured gap. SEM images showed that the outer diameter of the collagen conduit was 2.0 mm and the inner diameter was 1.8 mm, which was designed according to the size of the rat SC. The interconnected porous structures with a size of 40 to 200 μm could provide sufficient space for astrocyte adherence and growth and were conducive to the diffusion of neurotrophic factors secreted by astrocytes (Fig. 8B). By labeling NPCs and astrocytes with different fluorescent dyes, we confirmed the structure of the entire implants after assembly (Fig. 8C). For the in vivo transplantation experiment, only NPCs were labeled with GFP, while astrocytes were not. After the transplantation of Et-SCT (NCCL + N3-modified astrocytes on a collagen conduit) or nEt-SCT (NCCL + unmodified astrocytes on collagen conduit), Eda-Lips were intravenously injected into the SCI rats at 1, 3, 5, and 7 dpi. The immunofluorescence staining results at 10 dpi showed that the structures of conduits surrounding LACFs were observed in both groups. GFP+ NPCs were mainly distributed in the center of the injury area, while GFAP+ (a marker of astrocyte) cells distributed around NPCs (Fig. 8D). In addition, the number of GFP+ cells in the Et-SCT group was significantly higher than that in the nEt-SCT group (Fig. 8E). Moreover, only 34.5 ± 6.0% GFP+ cells were caspase-3+ in the Et-SCT group, which was significantly lower compared with the nEt-SCT group (Fig. 8, F and G). These results suggested that the targeted delivery based on exogenous N3-modified cells significantly improved drug enrichment in the injury area and thus promoted NPC survival. There was no significant difference in the proportion of Tuj-1+GFP+ cells between the two groups at 10 days (Fig. 8, H and I). At 60 dpi, the number of GFP+ cells in the Et-SCT group was 1.6-fold of that in the nEt-SCT group (Fig. 8, J and K). Furthermore, the staining for Map2 and NF was performed to evaluate neuronal regeneration. Quantitative analysis revealed that the proportions of GFP+NF+ and GFP+MAP2+ cells in the Et-SCT group were higher than those in the nEt-SCT group (Fig. 8, L to O). To assess locomotor functional recovery, an inclined plane assay and BBB test were performed. The results showed that the Et-SCT group (7.7 ± 0.95) achieved significantly higher BBB scores than the nEt-SCT group (6.3 ± 0.91). In addition, the angle inclined plate in the Et-SCT group (40.0° ± 1.62°) was slightly higher than that in the nEt-SCT group (38.5° ± 1.29°) (fig. S8).
Fig. 8. Transplantation of Eda-Lips–targeted spinal cord–like tissues (Et-SCT) boosted neural regeneration after SCI.
(A) Schematic of cell delivery and targeting modulation by exogenous astrocytes. (B) SEM image of collagen conduit. Scale bars, 1 and 100 μm. (C) Confocal images of Et-SCT. NPCs and astrocytes were labeled by Dil (red) and PKH67 (green), respectively. Scale bar, 1 mm. (D) Confocal images of GFP staining (for donor NPCs) at 10 dpi. Scale bar, 1 and 50 μm. (E) Quantification of GFP+ cell number per section in (D) (n = 5 biologically independent samples, two-tailed t test). (F) Image of caspase-3 staining in the lesion core at 10 dpi. Scale bar, 50 μm. (G) Proportions of caspase-3+GFP+/GFP+ (n = 5 biologically independent samples, two-tailed t test). (H) Image of Tuj-1 staining in the lesion core at 10 dpi. Scale bar, 50 μm. (I) Proportions of Tuj-1+GFP+/GFP+ (n = 5 biologically independent samples, two-tailed t test). (J) Confocal images of GFP staining at 60 dpi. Scale bar, 1 mm. (K) Quantification of GFP+ cell numbers per section in (J) (n = 5 biologically independent samples, two-tailed t test). (L) Images of Map2 staining in the lesion core at 60 dpi. Scale bar, 50 μm. (M) Proportions of Map2+GFP+/GFP+ (n = 5 biologically independent samples, two-tailed t test). (N) Images of NF staining in the lesion core at 60 dpi. Scale bar, 50 μm. (O) Proportions of NF+GFP+/GFP+ (n = 5 biologically independent samples, two-tailed t test). Error bars, means ± SD. *P < 0.05, **P < 0.01.
Next, we compared the effects of tENCCL and Et-SCT to distinguish the difference between endogenous SC labeling and exogenous astrocyte labeling. Although both N3-modified SC and N3-modified astrocytes effectively improved the survival of the donor NPCs and promoted neuronal regeneration, the two methods showed a difference in the regeneration of endogenous nerve fibers and angiogenesis. Furthermore, NF staining demonstrated that the number of NF+GFP+ cells showed no significant difference between the tENCCL and Et-SCT groups, but the number of GFP−NF+ cells in the Et-SCT group was significantly higher than that in the tENCCL group, indicating that the cotransplantation was able to more effectively promote the regeneration of endogenous axons in the injury area (fig. S9, A and B). In addition, stronger rat endothelial cell antigen staining in the Et-SCT group suggested that the cotransplantation might promote angiogenesis in the injury area (fig. S9, C and D). In conclusion, the targeted delivery strategy based on exogenous N3-modified astrocytes not only improves donor NPC viability and newborn neuron number but also promotes the regeneration of endogenous axons and blood vessels. BBB scores and angle of the inclined plate of rats in the tENCCL and Et-SCT groups were used to evaluate the effect of different strategies on motor function recovery. The results showed that the BBB score in the Et-SCT group (7.7 ± 0.95) was significantly higher than that in the tENCCL group (6 ± 0.75) and the control group (2.5 ± 0.92) at 8 weeks after injury (fig. S9E). The angle inclined plate in the Et-SCT group (40.0° ± 1.62°) was slightly higher than that in the tENCCL group (38.3° ± 1.28°) (fig. S9F).
DISCUSSION
Interactions between biomaterials and cells have opened a promising avenue for improving the efficiency of cell transplantation (1–4). In the present study, we designed a strategy based on the click chemical reaction between the azide group and DBCO group to enhance the retention and survival of donor cells on scaffolds to promote neural regeneration after SCI. First, we prepared DBCO-modified scaffolds and N3-modified donor cells and revealed that CC between cells and scaffolds based on azide and DBCO significantly improved neural stem cell (NSC) adhesion, differentiation, and oriented axon growth on scaffolds compared to NC interactions. Subsequently, following the transplantation of stem cell–conjugated biomaterials in SCI rats, the ROS-scavenging agent Eda was loaded into DBCO-modified Lips to target N3-modified SC tissues or N3-modified exogenous implanted astrocytes to promote the accumulation of Eda-Lips in the injured sites. These covalent interaction strategies between biomaterials and cells strongly improved the retention and survival of donor cells and promoted neural regeneration in SCI.
Metabolic labeling techniques based on synthetic azido sugars, such as Ac4ManNAz, have shown a strong application prospect in living cell imaging (15), cell recognition (19), and drug targeting (27). Ac4ManNAz is a convenient and safe labeling reagent for cells. It could be added to the cell culture medium to achieve N3 modification of cell surface glycoproteins and glycolipids. It has been reported that a dose from 50 to 200 μM can be used to label most cells. Considering that the metabolic labeling might affect the adenosine triphosphate (ATP) generation, migration, and channel activity of the neural cells, we analyzed the safe dose for human NSCs and astrocytes. We found that doses of 12.5 and 50 μM were safe for NPCs and astrocytes, respectively. Astrocytes were more tolerant than NPCs, which might be attributed to the differences in metabolic activity between these two types of cells. Two separate research groups have also reported that 10 μM provided a sufficient labeling efficiency for cell labeling (34, 35), which is close to the concentration that we used in this study. In addition, we found that injection of Ac4ManNAz was able to label glial cells but not neurons in the SC. This finding may be because neurons are not as metabolically active as glial cells to be used for endogenous azide metabolic labeling, although they might contain high amounts of glycoproteins.
Copper-free click chemistry strategies have been used for tissue engineering previously. Hydrogels composed of hyaluronic acid (HA)–DBCO, heparin-DBCO, RGD-azide, and polyethylene glycol (PEG)–diazide were used to encapsulate dopaminergic neurons for neuronal implantation to treat Parkinson’s disease (24). Chondrocytes embedded in an Strain-promoted azide-alkyne cycloaddition (SPAAC)-based hydrogel composed of HA-DBCO and PEG-azide were injected into mice to regenerate cartilaginous tissue (36). Click chemical reactions have shown a strong application prospect in cell surface CC due to the high selectivity and fast reaction efficiency in a complex physiological environment. This kind of CC has been reported for cell-cell adhesion (19), cell-drug targeting (27), and cell-dye recognition (21), but there has been no application for cell-scaffold adhesion until now. The anchorage-dependent signals mediated by focal adhesion kinase, phosphoinositide 3-kinase, protein kinase B, and mitogen-activated protein promote cell survival. Many studies have investigated the influence of various characteristics of biomaterials on cell behavior. Taking NSCs as an example, their adhesion was significantly enhanced at a collagen-coated substrate with a stiffness of 7 kPa compared with a lower stiffness substrate (9). Biomaterials of nano- to microtopographies with a grating ridge width from 500 to 2000 nm illustrated the relationship between surface structures and PC12 cell adhesion (10). Li et al. (11) reported that gelatin sponge with sufficient porosity (100 to 500 μm) and silk fibroin coating facilitated NSCs adhesion. In previous studies, we have confirmed that epidermal growth factor receptor (EGFR) antibody (13) and N-cadherin modification (12) on the surface of collagen scaffold could effectively improve the adhesion of the scaffold to NSCs. Unlike these strategies of improving NC adhesion, in the present study, we attached the cells to the scaffold through CC. CC helped the scaffold to capture more cells in a short time compared with NC, which may be partially due to the higher density of the azide group than integrin (17), which can yield stronger, multivalent adhesion between cell and scaffold. Moreover, the binding efficiency of DBCO and azide ligation was higher than that of integrin-mediated adhesion. During the long-term culture in vitro, the number of NPCs in the CC group remained basically unchanged, but some cells in the NC group lost adhesion signal and detached from the scaffold. This was probably because of the limited tensile strength and stability of NC methods, which could be influenced by secreted proteases or varied microenvironment (14). In contrast, covalent chemical bonds are irreversible and therefore more stable and robust than NC bonds. CC effectively promoted cell growth along the fibers, possibly because enhanced adhesion between cells and scaffolds increased the sensitivity of cells to respond to the linear ordered topology. CC is a controllable strategy for regulating the adhesion between cells and scaffolds, because the metabolic label can be easily regulated by changing the amount of Ac4ManNAz addition and the number of DBCO groups on the surface of scaffolds.
Stem cells sense mechanical stimuli, such as viscosity, elasticity, and nanotopography, and translate them into biochemical signals to regulate cell differentiation (37). CC might have an impact on stem cell differentiation as a result of the different mechanotransduction from NC. Using RNA-seq and immunofluorescence staining, we confirmed that CC accelerated NPC differentiation. Although this differentiation did not have a specific tendency toward neurons or astrocytes, it would lead to fast maturation of the implanted cells and promote the integration of the implants into the host tissues. Previous studies have also reported that the cell differentiation can be influenced by the characteristics of biomaterials. Benoit et al. (38) demonstrated that the tether of different chemical functional groups could directly regulate the differentiation of mesenchymal stem cells encapsulated in hydrogels. Matrices with hydrophobic tertiary butyl groups caused adipogenesis, while phosphate groups led to osteogenesis. Osteogenic lineage was favored on rigid micropost arrays, whereas adipogenic differentiation was enhanced on soft ones (39). It has been reported that stem cell differentiation was regulated by fiber diameters (40). The deep molecular mechanism of CC-mediated NSC differentiation should be investigated in the future.
Over the recent few decades, mounting evidence has suggested that click chemistry could be used for nanoparticle delivery. Cheng and colleagues (26) introduced azido sugar–conjugated glycopolyester nanoparticle, which underwent controlled release of azido sugars in the presence of cellular esterase for metabolic cancer cell labeling. This azido modification can be used for targeted delivery of DBCO-Dox. In another study, selective metabolic glycoengineering of cancer cells was achieved by modifying Ac4ManNAz with a group that could be cleaved by highly expressed enzymes in tumors to specifically recruit DBCO-Dox (27). A more general approach is to use DBCO-modified Lips to encapsulate drugs to target azide-modified tissues. Koo et al. (25) directly injected Ac4ManNAz into tumor tissues of mice to label tumor cells, and Lips modified with DBCO groups were injected into mice intravenously. They showed that the accumulation of DBCO-modified Lips was significantly increased by ligation of azido and DBCO. In our study, Ac4ManNAz was injected into spared SC to label tissues and DBCO-modified Lips were injected intravenously to target the N3-modified SC. Although the accumulation of DBCO-modified Lips significantly increased and the survival rate of donor cells was significantly improved, direct injections might cause physical damage to the spared SC tissue, including neural circuit damage, blood-SC barrier destruction, and activation of astrocytes and microglia. Second, metabolic labeling in vivo usually requires high concentrations of azido sugars. In the Cheng’s and Koo’s groups, the effective concentration for tumor labeling was 50 μM, the same concentration that we used in this work, which is fourfold higher than in vitro NPC labeling. High concentrations of azido sugars may cause safety concerns especially for tissue regeneration studies. Hence, we cotransplanted metabolically labeled astrocytes with NPCs to avoid endogenous injection of azido sugars. Compared with NPCs, astrocytes showed higher tolerance of Ac4ManNAz, and metabolic labeling of astrocytes cultured in vitro with dose-controlled Ac4ManNAz might be safer than endogenous labeling. In addition, astrocytes have the biological function of promoting angiogenesis and endogenous axon regeneration (41, 42). We demonstrated that N3 modification of donor cells can be used for chemical recognition and enrichment of DBCO-modified Lips in vivo. The survival of NPCs in the N3-modified implantation group was significantly improved, compared with the normal implantation group without a metabolic label, while the endogenous axonal regeneration and angiogenesis were significantly promoted compared with the N3-modified SC group.
In the past few decades, tissue engineering–based SCI repair strategies have been shown to be effective in the recovery of motor function after SCI. Tuszynski and colleagues (5) used biomimetic 3D-printed scaffolds to load rat NPCs into a 2-mm complete cross-sectional model. They evaluated motor function for 20 weeks, and at week 8, the rats from NPC-scaffold group achieved around six points. Lai et al. (43) used collagen-sponge scaffolds with rat NPC cells to build bioengineered transplantable SC-like tissue. These rats achieved more than nine points at 8 weeks after being transplanted into 2-mm gaps. In the present study, we used a 4-mm complete cross-sectional model of rats to evaluate the effect of our combined strategy on recovery of motor function. After 8 weeks, the BBB score improved to 7.7 ± 0.95. Although it may not be impressive for motor function recovery, it is important to note that the injury model in our study is more severe because of the longer gap than previous studies. In addition, it needs to emphasize that comparing motor function recovery results from different reports should take into account systematic errors related with injury model, surgical procedure, and postoperative care.
The present study also has limitations. The targeted delivery of Eda significantly improved the viability of the transplanted cells, but compared with 10 dpi, the number and proportion of surviving cells decreased at 60 dpi. This may be related to lymphocyte-mediated immune reaction, poor blood supply, non–ROS-dependent secondary inflammation, cytotoxic neural excitement, and other factors. Therefore, it may be difficult to achieve satisfactory efficacy by relying on the targeted delivery of single drug. In addition, the methods we currently report are complex in operation, and in particular, metabolic labeling strategies for endogenous cells may encounter difficulties in clinical translation. The therapeutic window for drug targeting based on metabolic labeling can only last 10 days. It needs to be optimized for long-term treatment of patients with SCI in the future.
MATERIALS AND METHODS
Ethics statement
The use of human fetal tissue was approved by the Reproductive Study Ethics Committee of Nanjing Drum Tower Hospital, Nanjing University (2018-223-01), and informed consent was provided by the donors. The aborted fetal tissues were obtained after legal termination of pregnancy. Animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals, formulated by the National Institutes of Health (USA) and approved by the Institute of Genetics and Developmental Biology, the Chinese Academy of Sciences.
Preparations and characterization of DBCO-LACFs
Bovine aponeurosis tissue was used to prepare LACFs according to a previously described method (44). DBCO-Sulfo-NHS ester (Sigma-Aldrich, USA) was dissolved in phosphate-buffered saline (PBS; pH 7.2). Then, 100-μl volume DBCO-Sulfo-NHS solution at different concentrations (5, 2.5, 1.25, 0.625, and 0 mg ml−1) was added to 20 mg of LACFs at 4°C for 2 hours to prepare DBCO-LACFs. After that, DBCO-LACFs were washed with PBS five times. Then, DBCO-LACFs were dissolved in 1 M acetic acid for 48 hours to obtain UV-vis absorption spectra in a U-4100 UV-Vis/NIR spectrophotometer (Hitachi, Japan) at a range of 250 to 400 nm. The standard curve of DBCO-Sulfo-NHS was used to calculate the content of the DBCO group on DBCO-LACFs. DBCO-Sulfo-NHS (0.625 mg ml−1) was used for subsequent experiments. DBCO-LACFs were dissolved in C2H3DO2 and DO2 for 1H NMR spectroscopy (Beckman, 700 MHz). To detect the DBCO group by fluorescence microscopy, Cy3-azide (1.25 mg ml−1; Sigma-Aldrich, USA) was used. To measure mechanical properties, DBCO-LACFs and LACFs were placed in a mechanical testing machine (Shimadzu AGS-X, Japan) at room temperature (RT). The load cell was set at 1 kN, and the lifting speed of the upper plate was 200 mm min−1.
Preparation collagen scaffold for astrocytes
The collagen from bovine skin was immersed in a 0.5 M acetic acid solution for 8 hours at 4°C, and collagen solution was neutralized with NaOH and dialyzed in deionized water for 5 days and subsequently lyophilized in a custom mold to form a collagen conduit (outer diameter 2.0 mm and inner diameter 1.8 mm) or collagen cylinders (3 mm in diameter and 3 mm in height). The porous collagen scaffolds were cross-linked in 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (1 mg/ml) and NHS (0.5 mg/ml) at 37°C for 4 hours. After washing, the scaffolds were lyophilized again and sterilized using Co60.
Cell culture
Primary NPCs were obtained from two 2-cm-long human fetal SC tissues at gestational 8 and 14 weeks, respectively. The single-cell suspension of SC was prepared at 37°C with accutase (Sigma-Aldrich, USA). Next, an appropriate volume of Dulbecco’s modified Eagle’s medium (DMEM)/F-12 medium was added to end the digestion, and the suspension was centrifuged at 500g for 5 min. Cells were cultured in 10-cm wide Petri dishes (Corning, USA) precoated with 125 μg of laminin (Sigma-Aldrich, USA) in the proliferation medium (2:1 mixture of DMEM and DMEM/F-12, Gibco, USA) containing 1% nonessential amino acid (Gibco, USA), 1% sodium pyruvate (Gibco, USA), 1% penicillin-streptomycin (Gibco, USA), 15 mM Hepes (Sigma-Aldrich, USA), 2% B27 (Gibco, USA), recombinant human insulin (25 mg liter−1; Yeasen, Shanghai, China), human plasma apo-transferrin (100 mg liter−1; Millipore, USA), glucose (15 g liter−1; Sigma-Aldrich, USA), 20 nM progesterone (Sigma-Aldrich, USA), 30 nM sodium selenite (Sigma-Aldrich, USA), 100 μM putrescine (Sigma-Aldrich, USA), EGF (40 ng ml−1; PeproTech, USA), basic fibroblast growth factors (bFGFs; 40 ng ml−1; PeproTech, USA), leukemia inhibitory factor (LIF; 20 ng ml−1; PeproTech, USA), neurotrophins-3 (10 ng ml−1; PeproTech, USA), and heparin (1.83 μg liter−1;Sigma-Aldrich, USA). NPCs were passaged upon reaching about 80% confluence and were stored in liquid nitrogen or passaged for subsequent experiments. For cell differentiation experiments, EGF, bFGF, and LIF were replaced by brain-derived neurotrophic factor (10 ng ml−1; PeproTech, USA) and glial cell line–derived neurotrophic factor (20 ng ml−1; PeproTech, USA).
Primary astrocytes were extracted from 2-cm-long gestational 22-week-old human fetal SC tissues that were dissociated into a single-cell suspension with TrypLE (Invitrogen, USA). Next, appropriate volume of DMEM/F-12 medium was added to end the digestion and then centrifuged at 500g for 5 min. Subsequently, the cells were resuspended in DMEM containing 10% fetal bovine serum (FBS; Gibco, USA) and 1% penicillin-streptomycin (Gibco, USA), which is an astrocyte proliferation culture medium, and seeded in culture flasks. After the confluence of primary cells reached 50 and 80%, the cells were purified twice. The flasks were shaken for 8 hours at a speed of 200 rpm using an orbital shaker. Following shaking, the cells in the culture flasks were cultured in new medium. The purified astrocytes were then cultured in DMEM containing 10% FBS (Gibco, USA) and 1% penicillin-streptomycin (Gibco, USA). The cells were passaged until confluence reached 80%.
Preparation and characterization of N3-modified cells
NPCs were treated with different concentrations of Ac4ManNAz (100, 50, 25, 12.5, 6.25, and 0 μM) in cell culture medium for 48 hours to prepare N3-modified NPCs. After washing with PBS for three times, NPCs were incubated with 50 μM DBCO-Cy3 for 40 min at 37°C. Subsequently, NPCs were fixed with 4% paraformaldehyde (PFA) and blocked with 5% bovine serum albumin (BSA) containing 0.3% Triton X-100 for 1 hour at RT. Hoechst 33342 (1 mg ml−1) was used to stain cell nuclei for 10 min at RT. Images were taken using an inverted fluorescence microscope (Axio Vert.A1, Zeiss, Germany), and the fluorescence intensity of Cy3 was analyzed by microscopic imaging software ZEN. Astrocytes were treated with different concentrations of Ac4ManNAz (200, 100, 50, 25, 12.5, and 0 μM) for 96 hours to prepare N3-modified astrocytes. The method of characterization was the same as that of N3-modified NPCs.
Cytotoxicity assay
Cell viability was assessed using a Live/Dead cell viability kit (US Everbright, China). Cells were double-stained with calcein-AM (2 μM) and ethidium homodimer-1 (4 μM) for 15 min at 37°C. Images were taken using an inverted fluorescence microscope (Axio Vert.A1, Zeiss, Germany). CCK-8 (YEASEN, CHINA) was added into the medium at a ratio of 10%, and the cells were incubated at 37°C for 1 hour. A plate reader (SpectraMax Plus384, USA) was used to measure the absorbance of the medium at 450 nm.
Conjugation of N3-modified NPCs onto DBCO-LACFs
For the CC group, NPCs were treated with 12.5 μM Ac4ManNAz for 48 hours, and then the cells were harvested and washed with PBS three times. After that, N3-modified NPC suspension (5 × 107/ml) was added to DBCO-LACFs at 37°C for 4 hours. We added a cell culture medium and gently dispersed the cell suspension using a pipette to avoid clumping every half hour. For the NC group, unmodified NPCs were harvested and soaked in LACFs using the same method described above. After being cultured for 1, 5, and 10 days, NPCs on DBCO-LACFs and LACFs after different time points were stained by FDA (5 μg ml−1) for 10 min to analyze the cell adhesion. All images were collected on a confocal microscope (Leica STELLARIS 5) under the same parameters.
Quantification of oriented axon growth of NPCs on scaffold
A 2D FFT image analysis method was adopted to quantitatively evaluate NPC alignment on LACFs and DBCO-LACFs (45, 46). A 1024 × 1024 pixel image was overlaid with a compatible-sized black square mask with a transparent concentric circle (1024 pixels in diameter) to avoid the edge effect, and we then proceeded with the FFT function in ImageJ. Subsequently, pixel intensity along each angle (from 0° to 359° with 1° increment) was summed through the plugin “Oval Profile” in ImageJ. The obtained pixel intensity was normalized by subtracting the minimum intensity value and dividing the difference between the maximum and the minimum.
SEM analysis
To observe the surface morphology of collagen scaffold, DBCO-LACFs and LACFs were characterized by a SEM (EVO LS 10, Germany) after being dried in a lyophilizer for 24 hours and sputter-coated with gold. To observe cell morphology of NPCs attached on LACF by CC and NC, the samples cultured for 5 days were washed with PBS and fixed with 2.5% glutaraldehyde overnight at 4°C. Then, the samples were dehydrated gradually using a series of ethanol solutions (30, 50, 70, 80, 90, 95, 98, and 100%) in water. Last, the samples were imaged with SEM after being coated with gold using a sputterer.
Inhibition assay of EDTA
A 200-μl volume of collagen solution (1 mg/ml) was added to each well of a 48-well plate, incubated overnight at 4°C, and washed with PBS until neutral. The collagen was DBCO-modified by treatment with NHS-Sulfo-DBCO (0.01 mg/ml). NPCs at the density of 2 × 104 per well were seeded onto collagen by NC or CC. After 4 hours or 3 days, NPCs were treated with 15 mM EDTA at 37°C for 20 min before FDA staining. Images were taken using an inverted fluorescence microscope (Axio Vert.A1, Zeiss, Germany).
Stability assay of azide-DBCO conjugation
Collagen-coated 48-well plates were prepared using the above method without DBCO modification. NPCs were seeded into 48-well plates at a density of 2 × 104 per well and subsequently treated with 12.5 μM Ac4ManNAz for 48 hours. Immediately after that, the NPCs were incubated with 50 μM DBCO-Cy3 for 40 min at 37°C. After 10 days of culture, the NPCs were fixed with 4% PFA and stained with Hoechst 33342. For the control group, after N3 modification, it was cultured for 10 days first and then incubated with 50 μM DBCO-Cy3. Images were taken using an inverted fluorescence microscope (Axio Vert.A1, Zeiss, Germany).
RNA-seq of NPCs
A 1-ml volume of collagen solution (1 mg/ml) was added to each well of a six-well plate, incubated overnight at 4°C, and washed with PBS until neutral. The collagen was DBCO-modified by treatment with NHS-Sulfo-DBCO (0.01 mg/ml). NPCs attached to collagen by CC and NC were cultured for 1, 7, and 14 days in the differentiation medium. Total RNA from NPCs was extracted with TRIzol (CWBio, China). RNA samples were qualified and quantified with the Agilent 2100 RNA Nano 6000 Assay Kit (Thermo Fisher Scientific, USA), and the resulting mRNA was purified with oligo(dT) magnetic beads. Purified double-stranded cDNA was then subjected to terminal repair with base A additions and joint sequence processing. The template was enriched by PCR, and the PCR product was purified to obtain the final library. The constructed libraries were sequenced using an Illumina HiSeq 2500 with the SE50 sequencing strategy. Reads were mapped to the human genome GRCh38 using StringTie with default parameters.
Quantitative real-time polymerase chain reaction
Total RNAs were extracted with TRIzol reagent (Invitrogen Life Technologies), and cDNAs were obtained through reverse transcription performed using a kit (K1622, Thermo Fisher Scientific, Waltham, MA, USA). The abundance of mRNAs was determined using a kit (Bio-Rad, Hercules, CA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was selected as the internal reference gene. The relative expression levels of the target genes were calculated according to the 2−ΔΔCt method. The primers used are shown in table S2. To detect the effect of inhibition of Wnt signaling pathway on NPC differentiation, IWR-1 (S7086) was added to the medium at a concentration of 5 μM from the first day after inoculation until total RNA was collected.
Immunofluorescence staining of cells
Cells in 48-well plates were fixed with 4% formaldehyde for 20 min at RT. Then, 5% BSA containing 0.3% Triton X-100 was added and incubated at RT for 1 hour. The sections were incubated with primary antibodies overnight at 4°C (table S1). After washing the slides with PBS, we added species-specific secondary antibodies (Sigma-Aldrich or Thermo Fisher Scientific, USA) and kept them at RT for 1 hour. Hoechst 33342 (1 mg ml−1) was used to stain cell nuclei for 10 min at RT. Images were taken using an inverted fluorescence microscope (Axio Vert.A1, Zeiss, Germany).
For proliferation and differentiation analysis, NPCs at the density of 1 × 104 per well were seeded onto collagen by NC or CC, and the medium was changed every 3 days. After 1 and 3 days of culture in the proliferation medium, ki67 staining was performed, and after 14 days of culture in the differentiation medium, Map2 and GFAP staining were performed.
Preparations of Eda-Lips
Eda-Lips were prepared by the calcium acetate gradient method (47, 48). Soybean PC, DSPE-mPEG2000-DBCO, cholesterol, and DSPE-mPEG2000 (Ruixi Biotech; 11:3:1:1 mass ratio) were dissolved in a small amount of chloroform and methanol (2:1 volume ratio) in a round-bottom flask and dried in a rotary evaporator under reduced pressure at 50°C to form a thin lipid film. Subsequently, the lipid film was hydrated at 55°C with calcium acetate solution (120 mM, pH 6.0) by vortexing. The obtained multilamellar vesicles were extruded through a size-controlled polycarbonate membrane (50-nm membrane filter pore size; Whatman Japan). Extrusion was performed 10 times. Then, the calcium acetate of the external Lip medium was replaced with sodium sulfate solution by a dialysis step to create a calcium acetate concentration gradient across the liposomal membrane. Eda (equivalent in mass to DSPE-mPEG2000-DBCO, Selleck, USA) was dissolved in sodium sulfate solution and was mixed with Lips. We incubated the Lips and Eda mixture at 37°C for 30 min to load Eda into Lips. Excess Eda was removed by dialysis.
Characterization of Eda-Lips
The mean diameters of Lips and Eda-Lips were determined by DLS using DelsaNano C (Beckman Coulter, USA). The morphology of Lips and Ede-Lips was observed by TEM (JEM-1400, Japan) after negative staining with 1% uranyl acetate solution. To determine the encapsulation efficiency of Eda, Eda-Lips were separated from free Eda by dialysis using a size-controlled polycarbonate membrane (50-nm membrane filter pore size; Whatman Japan) at 4°C for 24 hours. The Eda concentration in the supernatant was determined by HPLC carried out under the following conditions: symmetry, C18; mobile phase, methanol and 0.05 M ammonium dihydrogen phosphate solution (2:3 volume ratio); wavelength for spectrophotometry, 240 nm.
Evaluation of ROS removal efficiency of EDA-Lips in vitro
NPCs were seeded on a 48-well plate at a density of 5 × 104 cells per well and cultured for 24 hours. First, Eda or Eda-Lips [equivalent to Eda (0.1 mg ml−1)] or Lips (0.1 mg ml−1) were added and incubated for 2 hours. Subsequently, 200 μM H2O2 was added and incubated for half an hour to induce the generation of ROS. Then, the cells were loaded with DCFH-DA (10 μM) (GOYOO, China) to detect intracellular ROS with a fluorescence microscope (Axio Vert.A1, Zeiss, Germany).
SCI surgical procedure
Female SD rats aged 6 to 8 weeks were used in this study. An SD rat T8–9 SC complete transection model was used to evaluate the effects of exogenous NPC delivery and Eda-Lips targeting. Briefly, the rats were intraperitoneally anesthetized with sodium pentobarbital (50 mg/kg). The skin and muscle tissue on the midline of the back were successively incised, and the T7–T9 vertebrae were removed to expose the SC. Subsequent laminectomy resulted in a 4-mm complete cross-sectional injury. Bleeding was ceased using a gelatin sponge in the transection site.
N3 modification of SC
To evaluate the targeting delivery of Eda-Lips to the SC tissue, the SC tissue was modified with Ac4ManNAz. Ac4ManNAz (100 μM) was injected into eight sites (on the rostral and caudal regions 12 mm from the injury edge) at a dose of 5 μl per site through a pulled glass micropipette (outer diameter 100 μm) using a UMP3 microoperated pump (WPI, USA) at a speed of 5 μl/min.
Evaluation of targeting capability of Lips in vivo
To study the labeling kinetics of Ac4ManNAz in SCI rats, the SD rat T8–9 SC complete transection model was made and the SC was N3-modified as described above. The rats were injected with the same amount of dimethyl sulfoxide and H2O (1:3 volume ratio) in the same way as controls. Subsequently, DBCO-Cy3 (2 mg/kg) was intravenously injected into the rats at 1, 3, 5, and 7 dpi. Twelve hours after each injection, the rats were euthanized for tissue section. Fluorescent images of Cy3 were acquired with a confocal microscope (Leica STELLARIS 5, Germany).
To test the ability of N3-modified SC to enrich DBCO-modified Lips, DiR-Lips were intravenously injected into SCI rats with N3-modified SC at 1, 3, 5, and 7 dpi (5.5 mg of PCs per animal). Twelve hours after injection, the distribution of fluorescence in major organs, including the SC, brain, heart, lung, liver, kidney, and spleen, was determined by the IVIS system (PerkinElmer, USA). The radial efficiency was quantified (Living Image Software). To prepare Dir-Lips, DiR, PC, DSPE-mPEG2000-DBCO, cholesterol, and DSPE-mPEG2000 (Ruixi Biotech; 0.15:11:3:1:1 mass ratio) were dissolved in chloroform and methanol (2:1 volume ratio) in a round-bottom flask and dried in a rotary evaporator under reduced pressure at 50°C to form a thin lipid film. Subsequently, the lipid film was hydrated at 55°C with PBS by vortexing. The obtained multilamellar vesicles were extruded through a size-controlled polycarbonate membrane (50-nm membrane filter pore size; Whatman Japan). Extrusion was performed 10 times.
To evaluate the targeting ability of Eda-Lips to N3-modified exogenous astrocytes in SCI rats, astrocytes seeded onto the collagen cylinders were implanted into the SCI sites. Briefly, the scaffolds were immersed in 50 μl of N3-modified or unmodified astrocyte suspension (2 × 107/ ml) for 4 hours and cultured overnight. The scaffolds with N3-modified or unmodified astrocytes were implanted into the injured gap. The methods for injection of DBCO-Cy3 and DiR-Lips were the same as described above. The distribution of fluorescence in major organs, including the SC, brain, heart, lung, liver, kidney, and spleen, was determined by the IVIS system (PerkinElmer, USA).
Western blot assay for SC tissue
SC tissue after injury was harvested and lysed in ice-cold radioimmunoprecipitation assay buffer (Beyotime, China). Next, total protein concentration was determined using a bicinchoninic acid (BCA) kit (Beyotime, China). Then, 40 μg of protein was separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking with 5% nonfat dry milk, the membrane was incubated with primary antibodies overnight at 4°C. Horseradish peroxidase (HRP)–conjugated secondary antibodies were diluted at 1:3000 and incubated for 1 hour at RT. Protein bands were detected using an enhanced chemiluminescence Western blot detection reagent (Thermo Fisher Scientific, USA). The primary antibodies used in this study included caspase-3 (1:1000, 9661, CST) and Bax (1:1000, 60267, Proteintech).
For detection of N3-modified glycoprotein in the SC, the SC lysate was first conjugated with the biotin-PEG4-alkyne (25 μM; Sigma-Aldrich, USA) using the Click-it Cell Reaction Buffer Kit (Invitrogen, USA). After conjugation, the protein was separated by SDS-PAGE and transferred to a PVDF membrane. The blots were probed with HRP-conjugated avidin (Solarbio, China).
Lipid peroxidation assay of SC tissue
The SC tissue lysate was prepared, and total protein concentration was quantified using a BCA kit. MDA content was assayed using an MDA assay kit (Beyotime, China). In brief, the tissue lysate was heated at 100°C for 15 min with twice the volume of the thiobarbituric acid solution. After cooling, the absorbance value at 532 nm was measured with a plate reader (SpectraMax Plus384, USA). MDA standards with different concentrations were tested in the same way to draw the standard curve. The absorbance values of the samples at 532 nm were converted into MDA concentrations according to the standard curve. The concentrations were then divided by the corresponding protein mass to indicate the level of MDA in the SCs.
Construction of Eda-Lips targeted Et-SCT
N3-modified NPCs were seeded onto DBCO-LACFs (NCCL) following the method mentioned above. N3-modified or unmodified astrocytes were seeded on collagen conduits. Each conduit was soaked into 100 μl of cell suspension (1 × 107 /ml). Subsequently, astrocytes loaded on the conduits were cultured overnight. During this process, for N3-modified astrocytes, Ac4ManNAz was used to treat cells. The next day, LACFs with N3-modified NPCs were carefully assembled into the conduits with N3-modified or unmodified astrocytes for subsequent characterization or transplantation. To characterize the distribution of cells in spinal-like tissues in vitro, NPCs and astrocytes were treated with Dil (10 μM; Beijing Fluorescence Biotechnology, China) and PKH67 (5 μM; Beijing Fluorescence Biotechnology, China) for 30 min at 37°C before being seeded. After assembly, spinal-like tissues were cultured in an NPC proliferation medium for 2 days. Then, the spinal-like tissues were fixed in 4% PFA for 12 hours at 4°C. The tissues were treated with 20 and 30% sucrose overnight, embedded into Optimum Cutting Temperature compound (ZLI-9302, Japan), and sliced into 20-μm histologic sections with a cryostat microtome (CM-1950, Leica, Germany). A mounting medium containing 4′-6-diamidino-2-phenylindole (DAPI; ZSGB-Bio, China) was used for nuclear staining. Images were obtained on a confocal microscope (Leica STELLARIS 5, Germany).
Cell transplantation into SCI rats
To study the biological effects of tENCCL in vivo, NPCs or N3-modified NPCs were infected with a lentivirus expressing GFP to trace exogenous cells after transplantation. Subsequently, 50 μl of N3-modified NPCs and unmodified NPCs (5 × 107 /ml) were incubated with DBCO-LACFs or LACFs at 37°C for 4 hours, respectively. After that, we added a 5-ml medium for overnight culture. The next day, LACF or DBCO-LACF with NPCs was carefully transplanted into the injury gap.
To study the biological effects of Et-SCT in vivo, GFP-labeled N3-modified NPCs were seeded onto DBCO-LACFs (NCCL). N3-modified or unmodified astrocytes were seeded on collagen conduits. Each conduit was soaked into 100 μl of cell suspension (1 × 107 /ml). After that, astrocytes loaded on the conduits were cultured overnight. During this process, for N3-modified astrocytes, Ac4ManNAz was used to treat cells. The next day, LACFs with N3-modified NPCs were carefully assembled into the conduits with N3-modified or unmodified astrocytes for subsequent transplantation.
Cyclosporin A (10 mg kg−1 rat per day; MCE, USA) was injected intraperitoneally into all of the animals daily from 7 days before the surgery to sacrifice. After surgery, all of the animals were given an antibiotic injection once per day for 5 days and subjected to artificial urination twice a day until partially autonomous urination was restored. For rats in the tENCCL, ntENCCL, Et-SCT, and nEt-SCT groups, Eda-Lips (equivalent Eda 3.0 mg/kg) were injected through the tail vein at 1, 3, 5, and 7 dpi. The rats were sacrificed either 10 days or 2 months after surgery.
Behavioral analysis
Two observers blinded to the experimental groups tested the ability of rats to move their hindlimbs based on the BBB open-field 21-point locomotion rating scale (49), every week after the injury. The rats were placed on a special inclined plate with rubber on the test surface, which gradually increased the angle from the horizontal position of 0° until the rats maintained a constant position of just 5 s. This angle was recorded and analyzed 8 weeks after injury.
Histological analyses
Ten and 60 days after surgery, 2-cm-thick SC tissue sections containing the injury site were harvested and fixed in 4% PFA for 24 hours at 4°C. The tissues were treated with 20 and 30% sucrose overnight, embedded into Optimum Cutting Temperature compound (ZLI-9302, Japan), and sliced into 20-μm histologic sections with a cryostat microtome (CM-1950, Leica, Germany). The sections were rinsed with Dulbecco's PBS (DPBS), permeabilized, and blocked with 5% BSA containing 0.3% Triton X-100. Subsequently, the samples were incubated with primary antibodies (table S1) at 4°C overnight, rinsed with DPBS, and incubated with the corresponding secondary antibodies, Alexa Fluor 488 donkey anti-chicken immunoglobulin G (IgG) (1:500), Alexa Fluor 594 donkey anti-rabbit IgG (1:500), or Alexa Fluor 647 donkey anti-mouse IgG (1:500) (Jackson ImmunoResearch), in 5% BSA for 1 hour at RT. A mounting medium containing DAPI (ZSGB-Bio, China) was used for nuclear staining. Images were obtained on a confocal microscope (Leica STELLARIS 5, Germany).
Statistical analysis
All data collection and data analyses were blinded in this study. All experiments were independently repeated for at least three times. Statistical analyses were performed using SPSS software (version 26, IBM, USA) and presented as means ± SD. The Shapiro-Wilk test was used to analyze the normality. The significance between two groups was analyzed using a two-tailed Student’s t test. For multiple comparisons, one-way analysis of variance with Tukey’s test for equal variances was performed. * and ** represent P < 0.05 and P < 0.01, respectively.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (NSFC) (grant nos. 81891002 and 32071338), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDA16040700/XDA16040601), and Youth Innovation Promotion Association CAS (grant no. Y202031). We also thanked the support from CAS Project for Young Scientists in Basic Research (grant no. YSRB073).
Author contributions: Conceptualization and supervision: J.D., Y.Z., and Weiyuan Liu. Writing—original draft: Y.Z. and Weiyuan Liu. Writing—review and editing: J.D. and Y.Z. Visualization: Weiyuan Liu, B.X., S.Z., and S.H. Methodology: Wenbin Liu, C.J., B.C., Z.X., M.Y., and Y.Y. Investigation: S.Z., S.H., and R.Q.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S9
Tables S1 and S2
REFERENCES AND NOTES
- 1.Z. Alvarez, A. N. Kolberg-Edelbrock, I. R. Sasselli, J. A. Ortega, R. Qiu, Z. Syrgiannis, P. A. Mirau, F. Chen, S. M. Chin, S. Weigand, E. Kiskinis, S. I. Stupp, Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury. Science 374, 848–856 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.X. L. Wang, N. Rivera-Bolanos, B. Jiang, G. A. Ameer, Advanced functional biomaterials for stem cell delivery in regenerative engineering and medicine. Adv. Funct. Mater. 29, 1809009 (2019). [Google Scholar]
- 3.B. N. Kharbikar, P. Mohindra, T. A. Desai, Biomaterials to enhance stem cell transplantation. Cell Stem Cell 29, 692–721 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A. P. Liu, E. A. Appel, P. D. Ashby, B. M. Baker, E. Franco, L. Gu, K. Haynes, N. S. Joshi, A. M. Kloxin, P. H. J. Kouwer, J. Mittal, L. Morsut, V. Noireaux, S. Parekh, R. Schulman, S. K. Y. Tang, M. T. Valentine, S. L. Vega, W. Weber, N. Stephanopoulos, O. Chaudhuri, The living interface between synthetic biology and biomaterial design. Nat. Mater. 21, 390–397 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.J. Koffler, W. Zhu, X. Qu, O. Platoshyn, J. N. Dulin, J. Brock, L. Graham, P. Lu, J. Sakamoto, M. Marsala, S. Chen, M. H. Tuszynski, Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 25, 263–269 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.E. S. Rosenzweig, J. H. Brock, P. Lu, H. Kumamaru, E. A. Salegio, K. Kadoya, J. L. Weber, J. J. Liang, R. Moseanko, S. Hawbecker, J. R. Huie, L. A. Havton, Y. S. Nout-Lomas, A. R. Ferguson, M. S. Beattie, J. C. Bresnahan, M. H. Tuszynski, Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 24, 484–490 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A. Trounson, C. McDonald, Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 17, 11–22 (2015). [DOI] [PubMed] [Google Scholar]
- 8.L. M. Marquardt, V. M. Doulames, A. T. Wang, K. Dubbin, R. A. Suhar, M. J. Kratochvil, Z. A. Medress, G. W. Plant, S. C. Heilshorn, Designer, injectable gels to prevent transplanted Schwann cell loss during spinal cord injury therapy. Sci. Adv. 6, eaaz1039 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Y. Han, D. Baltriukiene, E. N. Kozlova, Effect of scaffold properties on adhesion and maintenance of boundary cap neural crest stem cells in vitro. J. Biomed. Mater. Res. A 108, 1274–1280 (2020). [DOI] [PubMed] [Google Scholar]
- 10.A. Ferrari, M. Cecchini, A. Dhawan, S. Micera, I. Tonazzini, R. Stabile, D. Pisignano, F. Beltram, Nanotopographic control of neuronal polarity. Nano Lett. 11, 505–511 (2011). [DOI] [PubMed] [Google Scholar]
- 11.G. Li, B. Zhang, J. H. Sun, L. Y. Shi, M. Y. Huang, L. J. Huang, Z. J. Lin, Q. Y. Lin, B. Q. Lai, Y. H. Ma, B. Jiang, Y. Ding, H. B. Zhang, M. X. Li, P. Zhu, Y. Q. Wang, X. Zeng, Y. S. Zeng, An NT-3-releasing bioscaffold supports the formation of TrkC-modified neural stem cell-derived neural network tissue with efficacy in repairing spinal cord injury. Bioact. Mater. 6, 3766–3781 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.W. Liu, B. Xu, W. Xue, B. Yang, Y. Fan, B. Chen, Z. Xiao, X. Xue, Z. Sun, M. Shu, Q. Zhang, Y. Shi, Y. Zhao, J. Dai, A functional scaffold to promote the migration and neuronal differentiation of neural stem/progenitor cells for spinal cord injury repair. Biomaterials 243, 119941 (2020). [DOI] [PubMed] [Google Scholar]
- 13.B. Xu, Y. Zhao, Z. Xiao, B. Wang, H. Liang, X. Li, Y. Fang, S. Han, X. Li, C. Fan, J. Dai, A dual functional scaffold tethered with EGFR antibody promotes neural stem cell retention and neuronal differentiation for spinal cord injury repair. Adv. Healthc. Mater. 6, 1601279 (2017). [DOI] [PubMed] [Google Scholar]
- 14.X. Xiong, H. Liu, Z. Zhao, M. B. Altman, D. Lopez-Colon, C. J. Yang, L. J. Chang, C. Liu, W. Tan, DNA aptamer-mediated cell targeting. Angew. Chem. Int. Ed. Engl. 52, 1472–1476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli, C. R. Bertozzi, Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. U.S.A. 104, 16793–16797 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.J. Rong, J. Han, L. Dong, Y. Tan, H. Yang, L. Feng, Q. W. Wang, R. Meng, J. Zhao, S. Q. Wang, X. Chen, Glycan imaging in intact rat hearts and glycoproteomic analysis reveal the upregulation of sialylation during cardiac hypertrophy. J. Am. Chem. Soc. 136, 17468–17476 (2014). [DOI] [PubMed] [Google Scholar]
- 17.J. B. Haun, N. K. Devaraj, S. A. Hilderbrand, H. Lee, R. Weissleder, Bioorthogonal chemistry amplifies nanoparticle binding and enhances the sensitivity of cell detection. Nat. Nanotechnol. 5, 660–665 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.E. Kim, H. Koo, Biomedical applications of copper-free click chemistry: In vitro, in vivo, and ex vivo. Chem. Sci. 10, 7835–7851 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.H. Koo, M. Choi, E. Kim, S. K. Hahn, R. Weissleder, S. H. Yun, Bioorthogonal click chemistry-based synthetic cell glue. Small 11, 6458–6466 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Y. Luo, Z. Chen, M. Sun, B. Li, F. Pan, A. Ma, J. Liao, T. Yin, X. Tang, G. Huang, B. Zhang, H. Pan, M. Zheng, L. Cai, IL-12 nanochaperone-engineered CAR T cell for robust tumor-immunotherapy. Biomaterials 281, 121341 (2022). [DOI] [PubMed] [Google Scholar]
- 21.R. Xie, L. Dong, Y. Du, Y. Zhu, R. Hua, C. Zhang, X. Chen, In vivo metabolic labeling of sialoglycans in the mouse brain by using a liposome-assisted bioorthogonal reporter strategy. Proc. Natl. Acad. Sci. U.S.A. 113, 5173–5178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.P. Contessotto, D. Orbanic, M. Da Costa, C. Jin, P. Owens, S. Chantepie, C. Chinello, J. Newell, F. Magni, D. Papy-Garcia, N. G. Karlsson, M. Kilcoyne, P. Dockery, J. C. Rodriguez-Cabello, A. Pandit, Elastin-like recombinamers-based hydrogel modulates post-ischemic remodeling in a non-transmural myocardial infarction in sheep. Sci. Transl. Med. 13, eaaz5380 (2021). [DOI] [PubMed] [Google Scholar]
- 23.S. Y. Wang, H. Kim, G. Kwak, H. Y. Yoon, S. D. Jo, J. E. Lee, D. Cho, I. C. Kwon, S. H. Kim, Development of biocompatible HA hydrogels embedded with a new synthetic peptide promoting cellular migration for advanced wound care management. Adv. Sci. (Weinh) 5, 1800852 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.M. M. Adil, T. Vazin, B. Ananthanarayanan, G. M. C. Rodrigues, A. T. Rao, R. U. Kulkarni, E. W. Miller, S. Kumar, D. V. Schaffer, Engineered hydrogels increase the post-transplantation survival of encapsulated hESC-derived midbrain dopaminergic neurons. Biomaterials 136, 1–11 (2017). [DOI] [PubMed] [Google Scholar]
- 25.H. Koo, S. Lee, J. H. Na, S. H. Kim, S. K. Hahn, K. Choi, I. C. Kwon, S. Y. Jeong, K. Kim, Bioorthogonal copper-free click chemistry in vivo for tumor-targeted delivery of nanoparticles. Angew. Chem. Int. Ed. Engl. 51, 11836–11840 (2012). [DOI] [PubMed] [Google Scholar]
- 26.H. Wang, Y. Bo, Y. Liu, M. Xu, K. Cai, R. Wang, J. Cheng, In vivo cancer targeting via glycopolyester nanoparticle mediated metabolic cell labeling followed by click reaction. Biomaterials 218, 119305 (2019). [DOI] [PubMed] [Google Scholar]
- 27.H. Wang, R. Wang, K. Cai, H. He, Y. Liu, J. Yen, Z. Wang, M. Xu, Y. Sun, X. Zhou, Q. Yin, L. Tang, I. T. Dobrucki, L. W. Dobrucki, E. J. Chaney, S. A. Boppart, T. M. Fan, S. Lezmi, X. Chen, L. Yin, J. Cheng, Selective in vivo metabolic cell-labeling-mediated cancer targeting. Nat. Chem. Biol. 13, 415–424 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.W. Xu, L. Chi, R. Xu, Y. Ke, C. Luo, J. Cai, M. Qiu, D. Gozal, R. Liu, Increased production of reactive oxygen species contributes to motor neuron death in a compression mouse model of spinal cord injury. Spinal Cord 43, 204–213 (2005). [DOI] [PubMed] [Google Scholar]
- 29.P. Vanderhaeghen, Wnts blow on NeuroD1 to promote adult neuron production and diversity. Nat. Neurosci. 12, 1079–1081 (2009). [DOI] [PubMed] [Google Scholar]
- 30.G. Cseke, M. S. Maginnis, R. G. Cox, S. J. Tollefson, A. B. Podsiad, D. W. Wright, T. S. Dermody, J. V. Williams, Integrin αvβ1 promotes infection by human metapneumovirus. Proc. Natl. Acad. Sci. U.S.A. 106, 1566–1571 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.L. Ben Haim, D. H. Rowitch, Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 18, 31–41 (2017). [DOI] [PubMed] [Google Scholar]
- 32.G. Bonvento, J. P. Bolanos, Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 33, 1546–1564 (2021). [DOI] [PubMed] [Google Scholar]
- 33.J. Wang, P. Jiang, W. Deng, Y. Sun, Y. Liu, Grafted human ESC-derived astroglia repair spinal cord injury via activation of host anti-inflammatory microglia in the lesion area. Theranostics 12, 4288–4309 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.S. S. Han, D. E. Lee, H. E. Shim, S. Lee, T. Jung, J. H. Oh, H. A. Lee, S. H. Moon, J. Jeon, S. Yoon, K. Kim, S. W. Kang, Physiological effects of Ac4ManNAz and optimization of metabolic labeling for cell tracking. Theranostics 7, 1164–1176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.P. Kranaster, C. Karreman, J. Dold, A. Krebs, M. Funke, A. K. Holzer, S. Klima, J. Nyffeler, S. Helfrich, V. Wittmann, M. Leist, Time and space-resolved quantification of plasma membrane sialylation for measurements of cell function and neurotoxicity. Arch. Toxicol. 94, 449–467 (2020). [DOI] [PubMed] [Google Scholar]
- 36.S. S. Han, H. Y. Yoon, J. Y. Yhee, M. O. Cho, H. E. Shim, J. E. Jeong, D. E. Lee, K. Kim, H. Guim, J. H. Lee, K. M. Huh, S. W. Kang, In situ cross-linkable hyaluronic acid hydrogels using copper free click chemistry for cartilage tissue engineering. Polym. Chem. 9, 20–27 (2018). [Google Scholar]
- 37.M. G. Tupone, M. d’Angelo, V. Castelli, M. Catanesi, E. Benedetti, A. Cimini, A state-of-the-art of functional scaffolds for 3D nervous tissue regeneration. Front. Bioeng. Biotechnol. 9, 639765 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D. S. Benoit, M. P. Schwartz, A. R. Durney, K. S. Anseth, Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat. Mater. 7, 816–823 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.J. Fu, Y. K. Wang, M. T. Yang, R. A. Desai, X. Yu, Z. Liu, C. S. Chen, Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods 7, 733–736 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.G. T. Christopherson, H. Song, H. Q. Mao, The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 30, 556–564 (2009). [DOI] [PubMed] [Google Scholar]
- 41.S. Stern, B. J. Hilton, E. R. Burnside, S. Dupraz, E. E. Handley, J. M. Gonyer, C. Brakebusch, F. Bradke, RhoA drives actin compaction to restrict axon regeneration and astrocyte reactivity after CNS injury. Neuron 109, 3436–3455.e9 (2021). [DOI] [PubMed] [Google Scholar]
- 42.S. Zhang, B. Kim, X. Zhu, X. Gui, Y. Wang, Z. Lan, P. Prabhu, K. Fond, A. Wang, F. Guo, Glial type specific regulation of CNS angiogenesis by HIFα-activated different signaling pathways. Nat. Commun. 11, 2027 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.B. Q. Lai, B. Feng, M. T. Che, L. J. Wang, S. Cai, M. Y. Huang, H. Y. Gu, B. Jiang, E. A. Ling, M. Li, X. Zeng, Y. S. Zeng, A modular assembly of spinal cord-like tissue allows targeted tissue repair in the transected spinal cord. Adv. Sci. (Weinh) 5, 1800261 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.H. Lin, B. Chen, B. Wang, Y. N. Zhao, W. J. Sun, J. W. Dai, Novel nerve guidance material prepared from bovine aponeurosis. J. Biomed. Mater. Res. A 79a, 591–598 (2006). [DOI] [PubMed] [Google Scholar]
- 45.C. E. Ayres, B. S. Jha, H. Meredith, J. R. Bowman, G. L. Bowlin, S. C. Henderson, D. G. Simpson, Measuring fiber alignment in electrospun scaffolds: A user’s guide to the 2D fast Fourier transform approach. J. Biomater. Sci. Polym. Ed. 19, 603–621 (2008). [DOI] [PubMed] [Google Scholar]
- 46.L. F. Tseng, P. T. Mather, J. H. Henderson, Shape-memory-actuated change in scaffold fiber alignment directs stem cell morphology. Acta Biomater. 9, 8790–8801 (2013). [DOI] [PubMed] [Google Scholar]
- 47.K. Hironaka, Y. Inokuchi, T. Fujisawa, H. Shimazaki, M. Akane, Y. Tozuka, K. Tsuruma, M. Shimazawa, H. Hara, H. Takeuchi, Edaravone-loaded liposomes for retinal protection against oxidative stress-induced retinal damage. Eur. J. Pharm. Biopharm. 79, 119–125 (2011). [DOI] [PubMed] [Google Scholar]
- 48.H. Shimazaki, K. Hironaka, T. Fujisawa, K. Tsuruma, Y. Tozuka, M. Shimazawa, H. Takeuchi, H. Hara, Edaravone-loaded liposome eyedrops protect against light-induced retinal damage in mice. Invest. Ophthalmol. Vis. Sci. 52, 7289–7297 (2011). [DOI] [PubMed] [Google Scholar]
- 49.D. M. Basso, M. S. Beattie, J. C. Bresnahan, Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 139, 244–256 (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S9
Tables S1 and S2