Abstract
Background
The success of elective colorectal surgery is mainly influenced by the surgical procedure and postoperative complications. The most serious complications include anastomotic leakages and surgical site infections (SSI)s, which can lead to prolonged recovery with impaired long‐term health. Compared with other abdominal procedures, colorectal resections have an increased risk of adverse events due to the physiological bacterial colonisation of the large bowel. Preoperative bowel preparation is used to remove faeces from the bowel lumen and reduce bacterial colonisation. This bowel preparation can be performed mechanically and/or with oral antibiotics. While mechanical bowel preparation alone is not beneficial, the benefits and harms of combined mechanical and oral antibiotic bowel preparation is still unclear.
Objectives
To assess the evidence for the use of combined mechanical and oral antibiotic bowel preparation for preventing complications in elective colorectal surgery.
Search methods
We searched MEDLINE, Embase, CENTRAL and trial registries on 15 December 2021. In addition, we searched reference lists and contacted colorectal surgery organisations.
Selection criteria
We included randomised controlled trials (RCTs) of adult participants undergoing elective colorectal surgery comparing combined mechanical and oral antibiotic bowel preparation (MBP+oAB) with either MBP alone, oAB alone, or no bowel preparation (nBP). We excluded studies in which no perioperative intravenous antibiotic prophylaxis was given.
Data collection and analysis
We used standard methodological procedures as recommended by Cochrane. Pooled results were reported as mean difference (MD) or risk ratio (RR) and 95 % confidence intervals (CIs) using the Mantel‐Haenszel method. The certainty of the evidence was assessed with GRADE.
Main results
We included 21 RCTs analysing 5264 participants who underwent elective colorectal surgery.
None of the included studies had a high risk of bias, but two‐thirds of the included studies raised some concerns. This was mainly due to the lack of a predefined analysis plan or missing information about the randomisation process.
Most included studies investigated both colon and rectal resections due to malignant and benign surgical indications. For MBP as well as oAB, the included studies used different regimens in terms of agent(s), dosage and timing. Data for all predefined outcomes could be extracted from the included studies. However, only four studies reported on side effects of bowel preparation, and none recorded the occurrence of adverse effects such as dehydration, electrolyte imbalances or the need to discontinue the intervention due to side effects.
Seventeen trials compared MBP+oAB with sole MBP. The incidence of SSI could be reduced through MBP+oAB by 44% (RR 0.56, 95% CI 0.42 to 0.74; 3917 participants from 16 studies; moderate‐certainty evidence) and the risk of anastomotic leakage could be reduced by 40% (RR 0.60, 95% CI 0.36 to 0.99; 2356 participants from 10 studies; moderate‐certainty evidence). No difference between the two comparison groups was found with regard to mortality (RR 0.87, 95% CI 0.27 to 2.82; 639 participants from 3 studies; moderate‐certainty evidence), the incidence of postoperative ileus (RR 0.89, 95% CI 0.59 to 1.32; 2013 participants from 6 studies, low‐certainty of evidence) and length of hospital stay (MD ‐0.19, 95% CI ‐1.81 to 1.44; 621 participants from 3 studies; moderate‐certainty evidence).
Three trials compared MBP+oAB with sole oAB. No difference was demonstrated between the two treatment alternatives in terms of SSI (RR 0.87, 95% CI 0.34 to 2.21; 960 participants from 3 studies; very low‐certainty evidence), anastomotic leakage (RR 0.84, 95% CI 0.21 to 3.45; 960 participants from 3 studies; low‐certainty evidence), mortality (RR 1.02, 95% CI 0.30 to 3.50; 709 participants from 2 studies; low‐certainty evidence), incidence of postoperative ileus (RR 1.25, 95% CI 0.68 to 2.33; 709 participants from 2 studies; low‐certainty evidence) or length of hospital stay (MD 0.1 respectively 0.2, 95% CI ‐0.68 to 1.08; data from 2 studies; moderate‐certainty evidence).
One trial (396 participants) compared MBP+oAB versus nBP. The evidence is uncertain about the effect of MBP+oAB on the incidence of SSI as well as mortality (RR 0.63, 95% CI 0.33 to 1.23 respectively RR 0.20, 95% CI 0.01 to 4.22; low‐certainty evidence), while no effect on the risk of anastomotic leakages (RR 0.89, 95% CI 0.33 to 2.42; low‐certainty evidence), the incidence of postoperative ileus (RR 1.18, 95% CI 0.77 to 1.81; low‐certainty evidence) or the length of hospital stay (MD 0.1, 95% CI ‐0.8 to 1; low‐certainty evidence) could be demonstrated.
Authors' conclusions
Based on moderate‐certainty evidence, our results suggest that MBP+oAB is probably more effective than MBP alone in preventing postoperative complications. In particular, with respect to our primary outcomes, SSI and anastomotic leakage, a lower incidence was demonstrated using MBP+oAB. Whether oAB alone is actually equivalent to MBP+oAB, or leads to a reduction or increase in the risk of postoperative complications, cannot be clarified in light of the low‐ to very low‐certainty evidence. Similarly, it remains unclear whether omitting preoperative bowel preparation leads to an increase in the risk of postoperative complications due to limited evidence.
Additional RCTs, particularly on the comparisons of MBP+oAB versus oAB alone or nBP, are needed to assess the impact of oAB alone or nBP compared with MBP+oAB on postoperative complications and to improve confidence in the estimated effect. In addition, RCTs focusing on subgroups (e.g. in relation to type and location of colon resections) or reporting side effects of the intervention are needed to determine the most effective approach of preoperative bowel preparation.
Keywords: Adult, Humans, Anastomotic Leak, Anastomotic Leak/drug therapy, Anastomotic Leak/prevention & control, Anti-Bacterial Agents, Anti-Bacterial Agents/administration & dosage, Anti-Bacterial Agents/therapeutic use, Colorectal Surgery, Colorectal Surgery/adverse effects, Ileus, Ileus/drug therapy, Ileus/prevention & control, Preoperative Care, Surgical Wound Infection, Surgical Wound Infection/drug therapy, Surgical Wound Infection/prevention & control
Plain language summary
Can combined mechanical and oral antibiotic bowel preparation reduce the risk of complications after scheduled colon or rectal resections compared with purely mechanical, purely oral antibiotic or no bowel preparation?
Key messages
‐ A combined mechanical (using laxatives) and oral antibiotic bowel preparation probably reduces the occurrence of infections of the surgical site (wound infections and infections in the abdominal cavity) as well as the likelihood of anastomotic leakage (leakage of the suture connection of the bowel) compared with mechanical bowel preparation alone.
‐ Oral antibiotics alone might be as effective as a combined mechanical and oral antibiotic bowel preparation, but this cannot be clearly determined based on the available data.
‐ Whether no bowel preparation compared with a combined mechanical and oral antibiotic bowel preparation has an influence on the occurrence of postoperative complications could not be determined on the basis of the available data.
What is the purpose of preoperative bowel preparation? Due to the naturally bacterial colonisation of the large bowel, infections of the surgical site are more frequent after operations in which the large bowel is opened. To prevent these infections, bowel preparation before surgery is intended to reduce faecal contamination of the bowel and minimise bacterial colonisation.
How is the bowel preparation done? Preoperative bowel preparation can be done mechanically, using laxatives to rinse the bowel, or by taking oral antibiotics that lead to local decontamination. These two methods can be performed either alone or in combination.
What did we want to find out? We wanted to find out whether combined mechanical and oral antibiotic bowel preparation compared with mechanical or oral antibiotic preparation alone or no bowel preparation has an effect on:
‐ the occurrence of surgical site infections
‐ the occurrence of anastomotic leakages
In addition, we wanted to find out whether combined bowel preparation had an effect on mortality, the occurrence of mild or severe postoperative complications, the likelihood of postoperative ileus (bowel motility disorder) or the length of hospital stay. Furthermore, we wanted to investigate whether side effects of the bowel preparation interventions differ between combination therapy and sole mechanical, sole oral antibiotic, or no bowel preparation.
What did we do? We searched for studies comparing combined mechanical and oral antibiotic bowel preparation with sole mechanical, sole oral antibiotic, or no bowel preparation in patients scheduled for colon or rectal resection. We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find? We included 21 studies in which patients scheduled for colon or rectal resection were assigned either to a group receiving combined mechanical and oral antibiotic bowel preparation or to a comparison group. The comparison group received mechanical bowel preparation alone in 17 studies, oral antibiotics alone in three studies, and no bowel preparation at all in one study. All participants received intravenous antibiotic prophylaxis during surgery. The studies included a total of 5968 participants, of whom 5264 were analysed. Most of the studies were conducted in industrialised countries in Europe or Asia. Bowel preparation was conducted over one to three days before surgery and the follow‐up period was 30 days in most of the studies. No industrial funding was reported by any of the studies, but only five of the 21 studies provided information on their funding. Overall, slightly more men (58%) than women (42%) were included. The average age of the study participants varied between 42 and 69 years.
We found moderate‐certainty evidence that combined mechanical and oral antibiotic bowel preparation probably reduces the risk of surgical site infections and leakages without affecting mortality, the occurrence of postoperative ileus or length of hospital stay. When comparing combined bowel preparation with oral antibiotics alone or with no bowel preparation, we found low‐certainty evidence that there is little to no difference between the compared approaches.
What are the limitations of the evidence? There are different reasons why our confidence in the evidence is limited. We are moderately confident in the evidence regarding the reduction of surgical site infections through combined mechanical and oral antibiotic bowel preparation, because different surgical strategies (in terms of surgical access and type and location of bowel resection) and also different methods of bowel preparation (in terms of agent, dose and timing) were used. We are also only moderately confident in the reduction of anastomotic leakage through combined mechanical and oral antibiotic bowel preparation, because just a few cases occurred across the included studies. Regarding the comparison of combination therapy with oral antibiotics alone, we have little confidence in the evidence because not enough studies examined this issue to be certain about the results of our outcomes. In addition, there are some concerns about the methods used in the included studies. As there is only one study, we also have little confidence in the evidence comparing combined bowel preparation with no bowel preparation.
How up to date is this evidence? This evidence is up‐to‐date as of December 2021.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Combined mechanical and oral antibiotic bowel preparation versus mechanical bowel preparation alone.
| Combined mechanical and oral antibiotic bowel preparation versus mechanical bowel preparation alone | ||||||
| Patient or population: Patients undergoing elective colorectal surgery Setting: Any type of hospital offering elective colorectal resections. Both single and multicentre studies are included Intervention: MBP+oAB Comparison: MBP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with MBP | Risk with MBP+oAB | |||||
| SSI follow‐up: 30 days | 137 per 1000 | 77 per 1000 (58 to 101) | RR 0.56 (0.42 to 0.74) | 3917 (16 RCTs) | ⊕⊕⊕⊝ Moderatea | Combined mechanical and oral antibiotic bowel preparation probably results in a reduction in surgical site infections. |
| Anastomotic leakage follow‐up: 30 days | 44 per 1000 | 26 per 1000 (16 to 43) | RR 0.60 (0.36 to 0.99) | 2356 (10 RCTs) | ⊕⊕⊕⊝ Moderateb | Combined mechanical and oral antibiotic bowel preparation may result in a reduction in anastomotic leakage. |
| Mortality follow‐up: 30 days | 18 per 1000 | 16 per 1000 (5 to 51) | RR 0.87 (0.27 to 2.82) | 639 (3 RCTs) | ⊕⊕⊕⊝ Moderatec | Combined mechanical and oral antibiotic bowel preparation may result in no difference in mortality. |
| Incidence of postoperative ileus follow‐up: 30 days | 49 per 1000 | 43 per 1000 (29 to 64) | RR 0.89 (0.59 to 1.32) | 2013 (6 RCTs) | ⊕⊕⊝⊝ Lowd,e | Combined mechanical and oral antibiotic bowel preparation may result in no difference in incidence of postoperative ileus. |
| Length of hospital stay follow‐up: 30 days | MD 0.19 lower (1.81 lower to 1.44 higher) | ‐ | 621 (3 RCTs) | ⊕⊕⊕⊝ Moderated | Combined mechanical and oral antibiotic bowel preparation may result in no difference in length of hospital stay. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_431253836665717559. | ||||||
a The rating was downgraded by one level due to moderate heterogeneity between studies that could not be explained by the subgroup analyses; I2 =44%. b The rating was downgraded by one level for imprecision. Few events occurred in the included trials (28 in the intervention group and 52 in the control group) and the confidence intervals include both benefits and no effect. c The rating was downgraded by one level for imprecision. Few events occurred in the included studies (5 in the intervention group and 6 in the control group) and the confidence intervals include considerable benefit and harm. d The rating was downgraded by one level due to imprecision, as the confidence interval includes considerable benefit and harm. e The rating was downgraded by one level due to possible puplication bias, as small studies reported statistically significant benefits while larger studies showed a much smaller and statistically non‐significant effect.
Summary of findings 2. Summary of findings table ‐ Combined mechanical and oral antibiotic bowel preparation versus oral antibiotics alone.
| Combined mechanical and oral antibiotic bowel preparation versus oral antibiotics alone | ||||||
| Patient or population: Patients undergoing elective colorectal surgery Setting: Any type of hospital offering elective colorectal resections. Both single and multicentre studies are included Intervention: MBP+oAB Comparison: oAB | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with oAB | Risk with MBP+oAB | |||||
| Surgical site infections follow‐up: 30 days | 68 per 1000 | 59 per 1000 (23 to 151) | RR 0.87 (0.34 to 2.21) | 960 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Combined mechanical and oral antibiotic bowel preparation may have no effect on surgical site infections, and the evidence is very uncertain. |
| Anastomotic leakage follow‐up: 30 days | 25 per 1000 | 21 per 1000 (5 to 86) | RR 0.84 (0.21 to 3.45) | 960 (3 RCTs) | ⊕⊕⊝⊝ Lowa,c | Combined mechanical and oral antibiotic bowel preparation may result in no difference in anastomotic leakage. |
| Mortality follow‐up: 30 days | 14 per 1000 | 14 per 1000 (4 to 49) | RR 1.02 (0.30 to 3.50) | 709 (2 RCTs) | ⊕⊕⊝⊝ Lowa,c | Combined mechanical and oral antibiotic bowel preparation may result in no difference in mortality. |
| Incidence of postoperative ileus follow‐up: 30 days | 47 per 1000 | 59 per 1000 (32 to 111) | RR 1.25 (0.68 to 2.33) | 709 (2 RCTs) | ⊕⊕⊝⊝ Lowa,c | Combined mechanical and oral antibiotic bowel preparation may result in no difference in the incidence of postoperative ileus. |
| Length of hospital stay follow‐up: 30 days | In two studies, the reported mean difference between groups was 0.1 and 0.2 (95% CI ‐0.68 to 1.08) days, respectively. | (2 RCTs) | ⊕⊕⊕⊝ Moderatea | Combined mechanical and oral antibiotic bowel preparation probably results in little to no difference in length of hospital stay. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_431253847765418810. | ||||||
a The rating was downgraded by one level because of some concerns about risk of bias, as information on a predefined analysis plan could not be identified for any of the included studies. b The rating was downgraded by one level due to moderate heterogeneity between studies; I2 =69%. c The rating was downgraded by one level due to imprecision, as the confidence interval includes considerable benefit and harm.
Summary of findings 3. Summary of findings table ‐ Combined mechanical and oral antibiotic bowel preparation versus no bowel preparation.
| Combined mechanical and oral antibiotic bowel preparation versus no bowel preparation | ||||||
| Patient or population: Patients undergoing elective colorectal surgery Setting: Any type of hospital offering elective colorectal resections. Both single and multicentre studies are included Intervention: MBP+oAB Comparison: nBP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with nBP | Risk with MBP+oAB | |||||
| Surgical site infections follow‐up: 30 days | 105 per 1000 | 66 per 1000 (35 to 129) | RR 0.63 (0.33 to 1.23) | 396 (1 RCT) | ⊕⊕⊝⊝ Lowa | Combined mechanical and oral antibiotic bowel preparation may result in little to no difference in surgical site infections. |
| Anastomotic leakage follow‐up: 30 days | 40 per 1000 | 36 per 1000 (13 to 97) | RR 0.89 (0.33 to 2.42) | 396 (1 RCT) | ⊕⊕⊝⊝ Lowa | Combined mechanical and oral antibiotic bowel preparation may result in no difference in anastomotic leakage. |
| Mortality follow‐up: 30 days | 10 per 1000 | 2 per 1000 (0 to 42) | RR 0.20 (0.01 to 4.22) | 396 (1 RCT) | ⊕⊕⊝⊝ Lowa | Combined mechanical and oral antibiotic bowel preparation may result in little to no difference in mortlaity. |
| Incidence of postoperative ileus follow‐up: 30 days | 160 per 1000 | 189 per 1000 (123 to 290) | RR 1.18 (0.77 to 1.81) | 396 (1 RCT) | ⊕⊕⊝⊝ Lowa | Combined mechanical and oral antibiotic bowel preparation may result in no difference in incidence of postoperative ileus. |
| Length of hospital stay follow‐up: 30 days | MD 0.1 higher (0.8 lower to 1 higher) | ‐ | 396 (1 RCT) | ⊕⊕⊝⊝ Lowa | Combined mechanical and oral antibiotic bowel preparation may result in no difference in length of hospital stay. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_431253860479140670. | ||||||
a The rating was downgraded by two levels due to imprecision because of the small sample size and the wide confidence intervals, which include considerable benefit and harm.
Background
Description of the condition
Colorectal operations are amongst the most frequently performed surgical procedures worldwide. In addition to emergency surgery (e.g. bowel perforations, diverticulitis, lower gastrointestinal bleeding), colon resections are performed for treating inflammatory diseases (e.g. ulcerative colitis or Crohn's disease) and colorectal cancer (Kuhry 2008; Spanjersberg 2011).
The treatment outcome is significantly influenced by the surgical procedure itself along with the occurrence of postoperative complications. Amongst the most serious complications are anastomotic leaks and surgical site infections (SSIs), which can lead to a prolonged and severe clinical course with impaired long‐term outcomes. In oncological cases, prolonged postoperative recovery can lead to a delayed start of adjuvant oncological treatment and thus to an increased risk of metastasis or local recurrence (Beck 2020; Kulu 2015; Nachiappan 2016; Young 2012). The incidence of complications is, amongst other factors, related to the type of surgery performed. Whilst anastomotic leaks are infrequent for small bowel and elective colon resections (1% to 3%), they occur far more frequently after rectal resections (10% to 23%) (ISOS 2016; Kulu 2015; Toh 2018; Walker 2004; Weidenhagen 2007). Compared with other abdominal operations, colorectal resections present an eight‐fold higher risk for the occurrence of adverse events. For instance, the SSI rate in colorectal surgery lies between 9% and 24 %, while in non‐colorectal surgery it is only 2% to 9 % (Anjum 2017; Migaly 2019). One reason for the increased complication rate in colorectal surgery is the high bacterial colonisation by the physiological intestinal flora, the so‐called microbiome.
Overall, there are numerous recommendations to reduce the risk of surgical complications. These range from specified preoperative preparation of the surgical field (e.g. preoperative whole‐body bathing or showering, hair removal, and disinfection methods) to intravenous antibiotic prophylaxis (Ling 2019; Nelson 2014; NICE 2019; WHO 2018). However, there is disagreement about what might be the best strategy for bowel preparation before elective colon and rectal surgery.
Description of the intervention
The underlying idea of preoperative bowel preparation is to clean the bowel from faeces, thereby reducing the bacterial load, which could lead to a lower rate of postoperative complications, especially SSIs and anastomotic leakage. Furthermore, removing the faeces makes it easier to manipulate the bowel in laparoscopic surgeries and lowers the risk of unwanted faecal spillage into the abdominal cavity.
In summary, there are four widespread interventions for bowel preparation before colorectal surgery in everyday clinical practice:
no bowel preparation;
mechanical bowel preparation (MBP);
oral antibiotics (oAB);
combination of oral antibiotics and mechanical bowel preparation (MBP+oAB).
For MBP, osmotically active solutions are mainly used nowadays (Kumar 2013; WHO 2018). By translocating fluid into the intestine, these lead to the development of diarrhoea and as a result the emptying of the bowel. Given the constant development of the applied solutions in the last decade, complications such as dehydration, electrolyte imbalance, or cardiovascular dysfunction rarely occur (Kumar 2013).
Regarding the antibiotic regimen, a combination of active substances that are effective against both aerobic and anaerobic bacteria are used (Migaly 2019; WHO 2018). Numerous different protocols exist regarding the active substance, dosage, and duration of treatment (WHO 2018). Two meta‐analyses from 2018 recommend a combination of an aminoglycoside (kanamycin or neomycin) and metronidazole or erythromycin administered one to three days before surgery (McSorley 2018; Toh 2018).
There is some evidence that luminal faeces may lead to an inactivation of the topically acting antibiotics. Consequently, oAB should be administered after, or at least in combination with MBP (Schardey 2017). However, the evidence for this hypothesis is ambiguous. Recent meta‐analyses failed to demonstrate a significant difference in SSI or anastomotic leak rates after combined or sole oral antibiotic bowel preparation, calling into question the need for combination therapy (Nelson 2020; Rollins 2019).
How the intervention might work
It is well known that the intestinal microbiome is important for myriads of physiological processes such as metabolism of drugs and nutrition degradation, biosynthesis of neurotransmitters and hormones, and influences immune maturation, host cell proliferation, and neurological signalling – to name but a few examples. However, the microbiome also plays an important role in disease development, for example autoimmune and gastrointestinal disease, but also neuropsychiatric illnesses (Lynch 2016). There is growing evidence that the intestinal microbiome is also involved in wound‐healing processes, especially in the healing of bowel anastomosis or the development of an anastomotic leakage (Schardey 2017). In addition to the surgical technique, bacterial colonisation of the intestinal mucosa of the anastomotic region also influences the occurrence of an insufficiency. Due to the surgical trauma and resulting ischaemia, mucosal bacteria such as Enterococcus faecalis or Pseudomonas aeruginosa develop the ability to express collagenases and activate matrix metalloproteinase 9 in the patient's intestinal tissue. This mechanism promotes the degradation of synthesised tissue leading to vulnerability of the newly created anastomosis (Anjum 2017; Schardey 2017; Shogan 2015).
In order to prevent these wound‐healing disturbances, preoperative preparation of the bowel is intended to create a clean working environment by reducing bacterial contamination of the intestine and respectively of the surgical field.
Why it is important to do this review
In several publications, as well as a Cochrane Review published in 2011, the use of MBP compared with no preparation or rectal enemas did not demonstrate an improved outcome for patients, which led to the recommendation to refrain from preoperative bowel preparation (Güenaga 2011).
In contrast, a current large registry study with more than 8000 patients demonstrated a significantly lower rate of postoperative SSIs as well as a shorter length of hospital stay with combined therapy of oAB and MBP compared with no bowel preparation or monotherapy with MBP or oAB (Klinger 2019). In addition, the combination therapy group also had the lowest readmission rate. Based on these data, the American Society of Colon and Rectal Surgeons recommended a combined mechanical and oral antibiotic bowel preparation prior to elective colon and rectal resections (Migaly 2019).
Despite the evidence for a beneficial effect of supplementing oAB to MBP, a current survey amongst members of the German Society of General and Visceral Surgery revealed that MBP alone is performed in over 50% of colon and over 75% of rectal operations. Additional oAB was only performed in about 10% of these operations (Buia 2019). A comparable survey amongst members of the European Society of Coloproctology revealed similar results (Devane 2017). Whilst the majority of respondents reported to regularly use MBP, less than 10% prescribe oral antibiotic therapy. In the USA, the rate of usage of combination therapies is much higher, although it has decreased over the last few years. In a survey conducted by the American Society of Colon and Rectal Surgeons in 1990, 88% of the participants reported using a combined bowel preparation (Solla 1990). In a more recent survey from 2010, only 36% of the colorectal surgeons still prescribe oral antibiotic therapy (Markell 2010).
The results of these surveys reveal that there is currently no uniform approach. Although there are already several meta‐analyses on this topic, they differ considerably in their conclusions: whilst some meta‐analyses report a benefit of combined oral and intravenous antibiotic therapy, with an unclear effect of concurrent mechanical bowel preparation (Nelson 2020; Rollins 2019), other meta‐analyses have shown superiority of the combination of oral antibiotic prophylaxis and mechanical bowel preparation (Toh 2018). However, these differences are not due to more recent findings, but rather to differences in the literature search, study selection criteria, and data extraction management. In order to establish an evidence‐based therapy, a structured and high‐quality meta‐analysis of the available evidence is necessary to provide optimal guidance on preoperative bowel preparation aiming to reduce the postoperative complication rate as well as overall mortality.
Objectives
The aim of this review is to assess the evidence for the use of combined mechanical and oral antibiotic bowel preparation for preventing complications in elective colorectal surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all published, unpublished, and ongoing randomised controlled trials (RCTs) or quasi‐RCTs (trials in which randomisation is attempted but potentially predictable, such as allocating participants by day of the week or sequence of entry into trial) comparing preoperative bowel preparation using MBP and oAB prior to elective colon or rectal surgery. We considered all identified studies for inclusion regardless of date, location, or language of publication.
Types of participants
Adult participants (18 years of age and older) undergoing elective colorectal surgery.
There were no exclusion criteria regarding the indication for surgery (both benign and malignant diseases were eligible); the type of colorectal surgery performed ((extended) right/left hemicolectomy, transverse colectomy, sigmoid resection, rectal resections, proctocolectomies or reanastomoses (provided a colorectal anastomosis was performed)); any previous treatment of the patient (e.g. neoadjuvant therapy); patient co‐morbidities; or the timing and location of the surgery.
For studies that include both eligible participants and others (e.g. children), we extracted data only from the eligible participants. We requested this information from the study authors if necessary. For one study, it was not possible to extract eligible data separately, and we could not obtain further information from the study author, so this study was classified as awaiting classification.
Types of interventions
We included any combination therapy of preoperative oAB and MBP.
We anticipated that there were different protocols in terms of the timing, duration, and frequency of administration, as well as the dosage of substances used (both in terms of antibiotics and mechanical bowel preparation). Any type of preoperative oral antibiotic prophylaxis, as well as any method of mechanical bowel cleansing, was eligible regardless of the mode of delivery, dose, duration, intensity, and co‐interventions. However, combination therapy and parenteral antibiotic prophylaxis (in both the control and intervention group) was mandatory. Regarding the effect of MBP or oAB alone, analyses already exist in further Cochrane Reviews (Güenaga 2011; Nelson 2014). We included studies using standard of care regarding parenteral antibiotic prophylaxis in both participant groups (intervention and control).
We included the following comparisons:
combination of MBP and oAB versus MBP alone;
combination of MBP and oAB versus oAB alone;
combination of MBP and oAB versus no preoperative preparation or placebo control (nBP).
Types of outcome measures
We analysed the occurrence of the predefined primary and secondary outcome parameters listed below within 30 days postoperatively. We assessed only the incidence of complications, and not the time of their occurrence. The outcome parameters listed below can be differentiated into efficacy and safety outcomes, whereby all listed outcome parameters, with the exception of length of hospital stay, refer to patient safety.
Reporting one or more of the listed outcomes was not a study inclusion criterion of the review.
Primary outcomes
If an outcome occurred in a participant at multiple sites (e.g. wound infection) or at different time points during the 30‐day postoperative observation interval, we counted such an outcome measure only once.
We considered the following primary outcomes.
Number of participants with SSIs (infection of the incision site involving the skin and subcutaneous tissue (superficial), deep soft tissue (deep), or a part of the body deeper than the fascia/muscle layers that was opened or manipulated during the surgical procedure (organ/space) and occurs within 30 days after the surgical procedure);
Number of participants with anastomotic leakage.
For the above outcome parameters, an absolute risk reduction (RRR) of 5 % was considered clinically relevant. From this RRR value, a risk ratio (RR) of 0.95 can be derived. This means that the RR and the 95% confidence interval (CI) for SSI and anastomotic leakages should be equal to or smaller than 0.95 to be considered a clinically relevant difference.
Secondary outcomes
We recorded the number of participants with the following adverse events (as defined by the primary study author) within 30 days postoperatively.
Mortality.
Mild postoperative complications (Clavien‐Dindo grade I and II).
Severe postoperative complications (Clavien‐Dindo grade III and IV).
Incidence of postoperative ileus.
Length of hospital stay (LOS) [days].
-
Side effect of intervention:
incidence of adverse effects of MBP such as dehydration, electrolyte imbalance, renal failure, or cardiac dysfunction (as defined by the primary study author);
incidence of adverse effects of oAB such as diarrhoea or pseudomembranous enterocolitis (as defined by the primary study author);
number of participants for whom the intervention was discontinued due to side effects, as well as the number of participants for whom therapy was initiated to treat the complications.
C. difficile‐related diarrhoea
Our prespecified outcome measures were not independent of each other. For example, people with SSIs, anastomotic leakages, or other adverse events were additionally classified according to the severity of the complications that occurred using the Clavien‐Dindo classification (Clavien 2009).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases from inception to December 15, 2021:
Cochrane Central Register of Controlled Trials (CENTRAL via the Cochrane Library; 1998 to 2021);
MEDLINE (via PubMed; 1946 to 2021);
Embase (via Ovid; 1988 to 2021).
The search strategies are given in Appendix 1.
Searching other resources
We also searched the trial registries ClinicalTrials.gov (www.clinicaltrials.gov, see Appendix 1) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (trialsearch.who.in, see Appendix 1). Furthermore, we screened the reference lists of all included publications and the included studies of related meta‐analyses for additional studies. We contacted organisations (e.g. regional colorectal surgery societies) to ask if they have knowledge of ongoing or completed studies to complement our database searches. We sought both published and unpublished trials. We did not limit the search by language or date.
Data collection and analysis
Selection of studies
Two review authors (MW and TV) screened the title and abstract of each study identified in the electronic search for potential relevance. Each review author independently decided on trial inclusion using the following predetermined eligibility criteria.
Study design: RCT or quasi‐RCT.
Population: adults undergoing elective colorectal surgery.
Intervention: preoperative bowel preparation using oAB and MBP.
Comparison: no preoperative bowel preparation (nBP) or sole treatment with either MBP or oAB.
Any disagreements regarding study eligibility during title and abstract screening were resolved by a third review author or by contacting the study authors for clarification.
Afterwards, two review authors (MW and TV) independently carried out the full‐text screening using the same inclusion criteria.
Data extraction and management
Two review authors (MW and SS) independently abstracted the following information from the included studies using a standardised form. The data extraction form was previously piloted on two studies and adjusted where necessary.
-
General information
Study ID, study title, corresponding author and contact details, publication date, country where the study was conducted, duration of trial and duration of follow‐up, aim of study (short description), inclusion and exclusion criteria, baseline imbalances, any conflicts of interest stated by the investigators, source of funding, ethics approval, trial registration, and sample size calculation.
-
Number of participants
Number randomised, number analysed, number of participants in the intervention/control group.
-
Population
Age, sex, co‐morbidities, surgery indication, type of operation, subgroups reported, and subgroups measured.
-
Intervention
Description of the intervention, agent and dose used for MBP, agent and dose used for oAB, timing of preparation (MBP), timing of preparation (oAB), modification of intervention, and concomitant medications.
-
Control
Description of control, agent and dose used, and timing of preparation.
-
Outcomes
Incidence of SSI, incidence of incisional (superficial or deep) and organ/space SSI, incidence of anastomotic leakage, spectrum of organisms detected, postoperative complications subdivided according to Clavien‐Dindo in mild (I/II) and severe (III/IV) complications as reported by the authors, incidence of postoperative ileus according to the definition of the primary study author, LOS (days), mortality, and side effects of the intervention (e.g. electrolyte imbalances, renal failure, incidence of Clostridium difficile infection, or termination of the intervention due to side effects).
Outcomes reported in trial but not used in review.
In cases where the majority of the required data was not reported in an identified publication, we searched for the associated study report or attempted to obtain the required data through correspondence with the study author. Trials whose results were published in more than one publication were included as one study. Data extraction was based on the main primary publication, but secondary publications were also considered for additional information. All publications are listed in the references of included studies.
Any disagreements were resolved by discussion or by consultation with a third review author if required.
Assessment of risk of bias in included studies
We assessed risk of bias in the included studies using Cochrane's RoB 2 tool (Sterne 2019; Higgins 2021). Two review authors (MW and SS) independently assessed the risk of bias of the results, with any disagreements resolved through discussion with a third review author.
We were interested in the effect of allocation at baseline, regardless of whether the intervention was delivered as intended (i.e. the 'intention‐to‐treat' effect). Consistent with the RoB 2 tool (Sterne 2019; Higgins 2021), we considered the following five domains in our assessment:
risk of bias arising from the randomisation process;
risk of bias due to deviations from the intended interventions (effect of assignment as well as adhering to intervention);
missing outcome data;
risk of bias in measurement of the outcome;
risk of bias in selection of the reported result.
We assessed the risk of bias for the following five outcomes that also contribute to the summary of findings tables of the review, measured at a time point closest to the 30‐day postoperative window (see 'Summary of findings and assessment of the certainty of the evidence' section below):
SSIs;
anastomotic leakage;
mortality;
incidence of postoperative ileus;
LOS.
We assessed these domains using the 'Excel tool to implement RoB 2' (available at www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2), and followed the recommended algorithm of signalling questions and response options to reach one of the following risk of bias judgements for each of the five domains, and consequently for each of our prespecified outcome measures:
low risk;
some concerns;
high risk.
To assess the overall risk of bias of an outcome, we considered judgements of the five individual domains. To be judged as 'low risk', all domains had to be rated as at low risk of bias. We assessed an outcome as 'some concerns' if risk of bias had been rated as some concerns in at least one domain, and high risk of bias in no domains. We assessed an outcome as 'high risk' if high risk of bias was identified in even one domain. We also classified an outcome as 'high risk' if we judged several domains as 'some concerns', as we consider confidence in such an outcome to be considerably reduced (Higgins 2021).
Measures of treatment effect
For continuous outcomes (e.g. LOS), we extracted the final mean value and standard deviation (SD) of each outcome of interest as well as the number of participants evaluated at the final assessment in each treatment arm. With these data, we calculated the mean difference (MD) and the 95% CI and, if appropriate, a pooled estimate of treatment effects. In cases were MDs and SDs were reported by the primary study authors, those data were used.
For dichotomous outcomes (SSIs, anastomotic leakage, mortality, postoperative complications, incidence of postoperative ileus, treatment‐related adverse effects), we extracted the RR including the 95% CI. If the RR was not reported, the number of affected participants were extracted to estimate an RR and its 95% CI.
Unit of analysis issues
We expected neither cross‐over studies nor cluster‐randomised studies in this clinical area, therefore the protocol did not specify how such studies should have been handled if we had identified one.
Our review includes one intervention group (MBP+oAB) and three comparison groups (comparison 1: MBP alone; comparison 2: oAB alone; comparison 3: no preoperative bowel preparation (nBP)). As there were no restrictions on the methodology of the intervention, no further subdivision of the intervention or comparison groups was intended.
We identified one study with two intervention groups (Espin‐Basany 2005), which we combined for statistical analysis in order to assign it to our predefined intervention group.
Dealing with missing data
For relevant data missing from a trial report, we attempted to contact the corresponding author to obtain the missing information. We did not impute missing information.
Assessment of heterogeneity
Clinical heterogeneity may be caused by several factors, such as patient age and co‐morbidities, indication, and treatment prior to surgery, as well as the procedures used to decontaminate the bowel. In addition, different surgical procedures, as well as the type of access route (minimally invasive versus open surgery), also contribute to heterogeneity. Methodological heterogeneity may be caused by the different risks of bias between studies. To assess the impact of clinical and methodological heterogeneity, we performed subgroup and sensitivity analyses on these topics. We identified statistical heterogeneity through visualisation of the forest plots as well as the Chi² test. We used the I² statistic for the quantification of heterogeneity.
We interpreted the I² value as follows (Deeks 2021).
< 30% to 40%: little or no heterogeneity.
41% to 74%: moderate heterogeneity.
75% to 100%: considerable heterogeneity.
Possible reasons for considerable heterogeneity were investigated and reported.
Assessment of reporting biases
To assess whether selective reporting of outcomes might affect the review findings, we matched the study protocols or registry entries, if available, with the published information on outcomes.
As we identified more than 10 studies reporting on our primary outcomes for the comparison MBP+oAB versus MBP, we created a funnel plots to assess the potential for small‐study effects where possible.
Data synthesis
Before conducting the meta‐analysis, we checked whether the participants, interventions, comparisons, and outcomes were sufficiently similar to ensure that the result of the analysis were clinically meaningful.
To calculate an overall treatment effect, we combined the data using a random‐effects model. As different agents of MBP and a variety of oAB with different administration intervals and combination options were included, a variance between the included trials has been assumed.
We included all eligible studies in the meta‐analysis regardless of their risk of bias rating. We performed the statistical analysis with Review Manager 5 software (Review Manager 2020) and RevMan Web (RevMan Web 2020). To combine dichotomous outcome data, we used the method proposed by Mantel‐Haenszel (Deeks 2021). If meta‐analysis was not appropriate due to an insufficient number of eligible studies or substantial heterogeneity between studies, we provided a narrative description of study characteristics and results.
Subgroup analysis and investigation of heterogeneity
Provided that sufficient studies were identified to justify subgroup analyses or meta‐regressions (at least 10 studies per outcome), we investigated the possible causes of heterogeneity by means of subgroup analyses for the primary outcome parameters.
We conducted subgroup analyses for the first comparison (MBP+oAB versus MBP) with regard to the outcome SSI for the following aspects:
surgery indication;
type of surgery;
surgical approach;
duration of mechanical bowel preparation;
agent combination of oral antibiotics;
duration of intravenous prophylaxis.
We assessed differences between subgroups by performing a test for heterogeneity across subgroups (i.e. Cochran's Q) and analysing I².
Sensitivity analysis
We conducted sensitivity analysis to examine the robustness of our findings, investigating the influence of studies judged at 'some concerns' on the effect size by removing these studies for each outcome and re‐analysing the remaining studies to see if the results were influenced by these factors.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings tables using GRADEpro GDT software (GRADEpro GDT) for each comparison (MBP+oAB versus MBP/oAB/nBP) for the following outcomes, as measured during the 30‐day postoperative duration (Schünemann 2021):
SSIs;
anastomotic leakage;
mortality;
incidence of postoperative ileus;
LOS.
Two review authors independently assessed the certainty of the evidence based on the five GRADE domains (overall RoB 2 judgement, imprecision, inconsistency, indirectness, and publication bias) (Schünemann 2013). Any disagreements were resolved through discussion or by involving a third review author. The GRADE assessment resulted in one of four degrees of certainty (high, moderate, low, or very low certainty), expressing our confidence in the estimate of impact.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification and Characteristics of ongoing studies tables.
We contacted the authors of six studies included in the full‐text screening and asked for more information to better assess whether these studies could be included in our review (Abis 2019; Arezzo 2021; Ikeda 2016; Mulder 2020; Tagliaferri 2020; Uchino 2019). Based on this additional information we received, we included Arezzo 2021; Ikeda 2016 and Uchino 2019 in our review and excluded Mulder 2020. As Abis 2019 includes both eligible and ineligible patients which can not be separated based on the published version of the study, we classified this study as awaiting classification. Tagliaferri 2020 was classified as an ongoing study as only an abstract with primary results was found and no published report of the final study results.
Results of the search
The process of our literature search is shown in a PRISMA flowchart in Figure 1. We identified 1954 records through the electronic searches of the Cochrane Central Register of Controlled Trials (459 records), MEDLINE (922 records) and Embase (573 records). Additionally, 40 records were identified by searching trial registries (clinicaltrials.gov: 17 records; ICTRP: 23 records). Further, 25 records were identified by contacting colorectal surgical societies, screening reference lists and reviewing included studies in related meta‐analyses. Of the 1488 records after removing 531 duplicates, we excluded 1396 clearly irrelevant records by screening their titles and abstracts. We retrieved the full text of 92 records for further assessment. We excluded 30 studies for the reasons listed in the Characteristics of excluded studies tables.
1.
Figure 1: PRISMA flowchart
We also identified 15 ongoing studies (see Characteristics of ongoing studies), and found one study investigating the effect of selective bowel decontamination with oral antibiotics(OABs) and oral antimycotics (Abis 2019). Mechanical bowel preparation (MBP) was performed only in left‐sided colon, sigmoid and low‐anterior resections. To determine if any of the study participants might be eligible for our review, the authors were asked to provide primary data of the study. As we have not received a response yet, the study was rated as awaiting classification (see Studies awaiting classification).
Included studies
We included 21 studies. Seventeen studies compared MBP+oAB with sole MBP (Anjum 2017; Arezzo 2021; Espin‐Basany 2005; Hata 2016; Horie 2007; Ikeda 2016; Ishida 2001; Kobayashi 2007; Lau 1988; Lazorthes 1982; Lewis 2002; Oshima 2013; Papp 2021; Rybakov 2021; Sadahiro 2014; Takesue 2000; Uchino 2019). In three studies, MBP+oAB was compared with oAB alone (Ram 2005; Suzuki 2020; Zmora 2003). Only one study compared MBP+oAB with nBP (Koskenvuo 2019).
Participants
In total, 5968 patients were randomised and 5264 participants analysed in the 21 included studies. Whereby only a subgroup of participants from four studies (Arezzo 2021; Ikeda 2016;Lazorthes 1982; Uchino 2019) were eligible for our meta‐analysis and the corresponding primary data were provided by the authors if necessary. All study participants underwent elective colorectal surgery.
While most studies had a mixed indication profile, 10 studies only included patients who had surgery for colorectal cancer (Arezzo 2021; Hata 2016; Horie 2007; Ikeda 2016; Kobayashi 2007; Lau 1988; Rybakov 2021; Sadahiro 2014; Suzuki 2020; Takesue 2000). Two other studies were limited to resections for inflammatory bowel disease (Oshima 2013: ulcerative colitis; Uchino 2019: Crohn's disease). Regarding the surgical approach, 10 studies included both open and minimally invasive procedures. Seven studies included only open surgery (Horie 2007; Lau 1988; Lazorthes 1982; Oshima 2013; Ram 2005; Takesue 2000; Uchino 2019), and four included only minimally invasive surgery (Arezzo 2021; Hata 2016; Ikeda 2016; Koskenvuo 2019).
Interventions
In 16 studies, MBP was performed on the day before surgery (Anjum 2017; Espin‐Basany 2005; Hata 2016; Horie 2007; Ikeda 2016; Ishida 2001; Kobayashi 2007; Koskenvuo 2019; Lewis 2002; Oshima 2013; Papp 2021; Ram 2005; Rybakov 2021; Takesue 2000; Uchino 2019; Zmora 2003). However, two studies each reported on a two‐day or three‐day laxative programme (Lau 1988; Lazorthes 1982; Sadahiro 2014; Suzuki 2020). The substance most commonly used for MBP was polyethylene glycol (PEG) in 10 studies (Arezzo 2021; Horie 2007; Ishida 2001; Kobayashi 2007; Koskenvuo 2019; Rybakov 2021; Sadahiro 2014; Suzuki 2020; Takesue 2000; Zmora 2003). The second most commonly used substance in nine studies was sodium picosulphate (Anjum 2017; Espin‐Basany 2005; Hata 2016; Ikeda 2016; Lewis 2002; Ram 2005; Sadahiro 2014; Suzuki 2020; Uchino 2019), and the third most commonly used were magnesium preparations in six studies (Hata 2016; Ikeda 2016; Lau 1988; Lazorthes 1982; Oshima 2013; Uchino 2019). A total of eight studies used a combination of several agents fo rMBP, while 13 studies used monotherapy (mostly with PEG solutions). The additional use of enemas was reported in four studies (Lau 1988; Lazorthes 1982; Papp 2021; Zmora 2003).
Regarding oral antibiotic therapy, there were multiple combinations of different substances and doses. In almost all studies, a combination of two active substances was used (exception: Horie 2007: monotherapy with kanamycin; Ram 2005: no information on agent, dosage and time of intake). The most common agents used were metronidazole in 14 studies (Anjum 2017; Espin‐Basany 2005; Hata 2016; Ikeda 2016; Koskenvuo 2019; Lazorthes 1982; Lewis 2002; Oshima 2013; Papp 2021; Rybakov 2021; Sadahiro 2014; Suzuki 2020; Takesue 2000; Uchino 2019), followed by kanamycin in 11 studies (Hata 2016; Horie 2007; Ikeda 2016; Ishida 2001; Kobayashi 2007; Lazorthes 1982; Oshima 2013; Sadahiro 2014; Suzuki 2020; Takesue 2000; Uchino 2019). The combination of these two agents was also the combination therapy most frequently used. Other agents used were neomycin in seven studies (Arezzo 2021; Espin‐Basany 2005; Koskenvuo 2019; Lau 1988; Lewis 2002; Papp 2021; Zmora 2003), erythromycin in five studies (Ishida 2001; Kobayashi 2007; Lau 1988; Rybakov 2021; Zmora 2003) ,and levofloxacin and bacitracin in one study each (Anjum 2017, respectively Arezzo 2021). As for dosage, metronidazole and kanamycin were mostly prescribed at a daily dose of 1500 mg each (in eight and six studies, respectively). However, the daily dose of metronidazole varied between 750 mg and 4000 mg in the included studies. Such differences in daily antibiotic dosage were observed throughout the studies. In terms of timing of administration, in 15 studies oAB was administered after the mechanical preparation of the bowel in two or three single doses on the day before surgery (Anjum 2017; Arezzo 2021; Hata 2016; Ikeda 2016; Kobayashi 2007; Koskenvuo 2019; Lau 1988; Lewis 2002; Oshima 2013; Papp 2021; Rybakov 2021; Sadahiro 2014; Suzuki 2020; Takesue 2000; Uchino 2019). Three studies report a two‐ or three‐day oral antibiotic preparation (Horie 2007; Ishida 2001; Lazorthes 1982). In addition, one three‐armed study compared a three‐day and a one‐day oAB‐regimen in addition to MBP with MBP alone (Espin‐Basany 2005). More detailed information on the agent, dosages used and time of intake for the individual studies can be found in the Characteristics of included studies tables.
Outcomes
Regarding the primary outcomes, all but one of the included studies reported the number of participants with surgical site infections (SSIs) (Lazorthes 1982 subdivided wound infections into major and minor, but only data from major wound infections were reported). Twelve studies also reported a subdivision into incisional and organ/space SSIs. Information on the number of participants with anastomotic leakage was reported in 13 studies. All predefined secondary outcomes were reported in individual included studies. Table 4 provides an overview of which study reported on which outcome.
1. Summary of outcomes reported per study.
| StudyID | SSI | Subdivision in incisional and organ/space SSI | Anastomotic leakage | Mortality | Mild and severe postoperative complications according to Clavien‐Dindo | Incidence of postoperative ileus | LOS | Side effect of Intervention | C. difficile‐related diarrhoea |
| MBP+oAB vs. MBP | |||||||||
| Anjum 2017 | yes | yes | no | no | no | no | no | no | no |
| Arezzo 2021 | yes | no | yes | yes | yes | yes | yes | yes | yes |
| Espin‐Basany 2005 | yes | no | no | no | no | yes | no | yes | no |
| Hata 2016 | yes | yes | yes | no | no | yes | no | no | yes |
| Horie 2007 | yes | no | yes | no | no | no | no | no | no |
| Ikeda 2016 | yes | yes | yes | no | no | yes | yes | no | yes |
| Ishida 2001 | yes | yes | yes | no | no | no | no | no | no |
| Kobayashi 2007 | yes | yes | no | no | no | no | no | no | no |
| Lau 1988 | yes | no | yes | no | no | no | yes | no | no |
| Lazorthes 1982 | incorrect* | no | no | yes | no | no | incorrect** | no | no |
| Lewis 2002 | yes | no | no | no | no | no | no | no | no |
| Oshima 2013 | yes | yes | no | no | no | no | no | yes | no |
| Papp 2021 | yes | yes | yes | yes | yes | yes | no | no | yes |
| Rybakov 2021 | yes | yes | yes | no | yes | yes | no | no | incorrect*** |
| Sadahiro 2014 | yes | yes | yes | no | no | no | no | no | incorrect**** |
| Takesue 2000 | yes | no | yes | no | no | no | no | no | no |
| Uchino 2019 | yes | yes | no | no | no | no | no | no | no |
| MBP+oAB vs. oAB | |||||||||
| Ram 2005 | yes | no | yes | yes | no | yes | yes | no | no |
| Suzuki 2020 | yes | yes | yes | no | no | no | no | no | yes |
| Zmora 2003 | yes | no | yes | yes | no | yes | incorrect** | no | no |
| MBP+oAB vs. nBP | |||||||||
| Koskenvuo 2019 | yes | yes | yes | yes | yes | yes | yes | yes | yes |
* Wound infections were classified into major and minor, but only data from major wound infections are reported **Only the numerical value given with no unit of measurement specified, no information about range or standard deviation *** Detection of Clostridia in general, not specific for C. difficile **** Detection rate of C. difficile toxin pre‐ and postoperatively, regardless of whether diarrhoea was present
Regarding the side effects of the intervention, only four of the 21 included studies reported the occurrence of adverse events related to preoperative bowel preparation (Arezzo 2021; Espin‐Basany 2005; Oshima 2013; Papp 2021). The reported side effects included nausea/vomiting or abdominal pain, but none of the included studies reported adverse effects of MBP such as dehydration, electrolyte imbalances, renal failure or cardiac dysfunction. The number of participants who discontinued the intervention due to side effects was also not reported in any study.
Excluded studies
A total of 30 of the identified studies were excluded. Fifteen because they investigated the wrong intervention, seven due to the lack of perioperative intravenous antibiotic prophylaxis, and five for an inappropriate study design. Two other articles were excluded because of the wrong publication type (congress poster and invited commentary, respectively), and one study was excluded for an inappropriate patient population (patients with anastomotic leakages were excluded from the analysis); see Characteristics of excluded studies.
Risk of bias in included studies
The risk of bias assessments, including all domain evaluations and support for the assessment, are reported in the Risk of bias (tables) section and displayed along the forest plots of each analysis.
None of the included studies was assessed to be of high risk of bias. However, two‐thirds of the studies raised some concerns about the risk of bias. In most cases, these concerns were due to the lack of a predefined analysis plan or the lack of information about the randomisation process.
Detailed data on the risk of bias assessment, such as the Excel file with the consensus responses to the signalling questions, are available on request.
Surgical site infections (SSIs) Twelve of the 20 studies that reported this outcome raised some concerns about the risk of bias (Anjum 2017; Espin‐Basany 2005; Horie 2007; Ishida 2001; Kobayashi 2007; Lau 1988; Lewis 2002; Oshima 2013; Ram 2005; Suzuki 2020; Takesue 2000; Zmora 2003). The concerns were due to the lack of a predefined analysis plan, and in three of these studies, additional inadequate information about the randomisation process (Espin‐Basany 2005; Oshima 2013; Takesue 2000).
Anastomotic leakage Eight of the 14 studies reporting this outcome were at low risk of bias (Arezzo 2021; Hata 2016; Ikeda 2016; Koskenvuo 2019; Papp 2021; Rybakov 2021; Sadahiro 2014; Zmora 2003), six were considered to have some concerns about the risk of bias because no predefined analysis plan could be identified for these studies (Horie 2007; Ishida 2001; Lau 1988; Ram 2005; Suzuki 2020; Takesue 2000). One of the studies also inadequately described the randomisation process, which also raises concerns about risk of bias (Takesue 2000).
Mortality Half of the six studies had a low risk of bias (Arezzo 2021; Koskenvuo 2019; Papp 2021). The other three studies were rated as having some concerns regarding the selection of the reported outcomes of the individual studies (Lazorthes 1982; Ram 2005; Zmora 2003); additionally, in one of the three studies, the randomisation process was not adequately described, leading to some concerns regarding the risk of bias (Lazorthes 1982).
Incidence of postoperative ileus. Only three of the nine studies reporting this outcome raised some concerns about the risk of bias (Espin‐Basany 2005; Ram 2005; Zmora 2003). Again, these concerns were due to the lack of a predefined analysis plan and additional an unclear randomisation method in one case (Espin‐Basany 2005).
Length of hospital stay Only one of the four studies reporting this outcome raised some concerns about the risk of bias (Lau 1988). The reason for this is again the lack of a predefined analysis plan.
Assessment of publication bias The assessment of a small study effect using funnel plots was only possible for the comparison MBP+oAB versus MBP for the outcomes SSI and anastomotic leakage, as no other comparison included ten or more studies. The funnel plots show a nearly symmetrical distribution, indicating that there was no evidence of publication bias (Figure 2; Figure 3). In the funnel plot for the outcome SSI (Figure 2), one study stands out with a much smaller patient population that does not fit the otherwise symmetrical distribution. This study is Arezzo 2021, of which only a subpopulation was included, which explains the smaller sample size resulting in an outlier from symmetry in the funnel plot.
2.

Comparison 1 (MBP+oAB vs. MBP): Funnel plot regarding the incidence of SSI
3.

Comparison 1 (MBP+oAB vs. MBP): Funnel plot regarding the incidence of anastomotic leakage
Effects of interventions
See: Table 1; Table 2; Table 3
See: Table 1 for combined mechanical and oral antibiotic bowel preparation versus mechanical bowel preparation alone, Table 2 for combined mechanical and oral antibiotic bowel preparation versus oral antibiotics alone and Table 3 for combined mechanical and oral antibiotic bowel preparation versus no bowel preparation.
Comparison 1: Combined mechanical and oral antibiotic bowel preparation versus mechanical bowel preparation alone (MBP+oAB versus MBP)
We identified 17 relevant studies for this comparison (Anjum 2017; Arezzo 2021; Espin‐Basany 2005; Hata 2016; Horie 2007; Ikeda 2016; Ishida 2001; Kobayashi 2007; Lau 1988; Lazorthes 1982; Lewis 2002; Oshima 2013; Papp 2021; Rybakov 2021; Sadahiro 2014; Takesue 2000; Uchino 2019). As only a subpopulation of participants from Arezzo 2021, Ikeda 2016 and Uchino 2019 were eligible for our meta‐analysis, primary data from these studies were requested and received, ensuring that only eligible patients were included in our analysis.
All pre‐defined outcomes were addressed in at least three studies, so that meta‐analyses could be calculated for all outcomes.
Surgical site infections (SSIs)
A total of 3917 participants from 16 studies were included for this outcome and in corresponding meta‐analysis. The results suggested that the intervention (MBP+oAB) reduced the risk of SSI from 137 per 1000 with MBP alone to 77 per 1000 (RR 0.56, 95% CI 0.42 to 0.74; P < 0.0001, I² = 44%; Analysis 1.1). This risk reduction is clinically relevant, as we defined an RR and 95% CI of 0.95 or less as a minimally important difference (MID).
1.1. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 1: SSI
The overall certainty of evidence was moderate, downgraded one level for inconsistency due to moderate heterogeneity amongst these studies (Chi² = 27.01, df = 15 (P = 0.03); I² = 44%). The subgroup analyses performed regarding the indication for surgery (Analysis 1.13), the type of surgery performed (Analysis 1.14) or the surgical approach (Analysis 1.15) as well as concerning the duration of mechanical bowel preparation (Analysis 1.16) or the substances used for oral antibiotic bowel preparation (Analysis 1.17) and with regard to the duration of intravenous antibiotic prophylaxis (Analysis 1.18) could not explain the heterogeneity.
1.13. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 13: SSI_Subgroup analysis regarding surgery indication
1.14. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 14: SSI_Subgroup analysis regarding the type of surgery
1.15. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 15: SSI_Subgroup analysis regarding the surgical approach
1.16. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 16: SSI_Subgroup analysis regarding the duration of mechanical bowel preparation
1.17. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 17: SSI_Subgroup analysis regarding the agent combination of oral antibiotics
1.18. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 18: SSI_Subgroup analysis regarding the duration of intravenous antibiotic prophylaxis
Regarding incisional and organ/space SSIs MBP+oAB also reduced the risk compared with MBP alone (incisional: RR 0.47, 95% CI 0.33 to 0.66; P < 0.0001, I² = 37%; 10 studies, 3054 patients; Analysis 1.2; organ/space: RR 0.65, 95% CI 0.44 to 0.98; P = 0.04, I² = 0%; 10 studies, 3054 patients; Analysis 1.3).
1.2. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 2: Incisional SSI
1.3. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 3: Organ/space SSI
Anastomotic leakage
A total of 2356 participants from 10 studies were included for this outcome and the respective meta‐analysis. The results indicated that the risk of anastomotic leakage could be reduced from 44 per 1000 patients receiving MBP alone to 26 per 1000 patients when MBP+oAB was used (RR 0.60, 95% CI 0.36 to 0.99; P = 0.05, I² = 9%; Analysis 1.4). Whether the risk reduction that can be achieved by MBP+oAB is clinically relevant cannot be ascertained, as the upper limit of the 95% CI slightly exceeds our MID of 0.95. The overall certainty of the evidence was moderate, downgraded by one level due to the limited number of events in the included studies and a rather wide confidence interval, suggesting imprecision of the results.
1.4. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 4: Anastomotic leakage
Mortality
A total of 639 participants from 3 studies were included in this meta‐analysis. The results suggested that there was no difference in mortality between MBP+oAB and MBP alone (RR 0.87, 95% CI 0.27 to 2.82; P = 0.81, I² = 0%; Analysis 1.5). The overall certainty of the evidence was moderate, downgraded by one level due to the limited number of events in the included studies and wide confidence intervals including both, benefit and harm, suggesting imprecision of the results.
1.5. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 5: Mortality
Postoperative complications subdivided according to Clavien‐Dindo in mild (I/II) and severe (III/IV) complications
A total of 695 participants from 3 studies were included in this meta‐analysis. The risk of mild or severe postoperative complications did not differ between MBP+oAB and MBP alone (mild complications: RR 0.76, 95% CI 0.29 to 2.00; P = 0.58, I² = 65%; Analysis 1.6. Severe complications RR 1.00, 95% CI 0.59 to 1.70; P = 0.99, I² = 0%; Analysis 1.7).
1.6. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 6: Mild postoperative complications according to Clavien‐Dindo(I + II)
1.7. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 7: Severe postoperative complications according to Clavien‐Dindo (III + IV)
Incidence of postoperative ileus
A total of 2013 participants from 6 studies were included in this meta‐analysis. The results indicated that the incidence of postoperative ileus was similar between the MBP+oAB and MBP alone groups (RR 0.89, 95% CI 0.59 to 1.32; P = 0.56, I² = 0%; Analysis 1.8). The overall certainty of the evidence was low, downgraded one level due to suspected publication bias as small studies report a statistically significant benefit while larger studies show a much smaller effect, and downgraded another level due to imprecision because of the limited number of events in the included studies with wide confidence intervals that included both benefits and harms.
1.8. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 8: Incidence of postoperative ileus
Length of hospital stay (LOS)
A total of 621 participants from 3 studies were included in this meta‐analysis. LOS seemed to be similar between the MBP+oAB and MBP alone groups as the mean discharge time for patients in the intervention group was only 4.6 hours earlier than for patients in the control group (MD ‐0.19, 95% CI ‐1.81 to 1.44; P = 0.82, I² = 0%; Analysis 1.9). The overall certainty of the evidence was moderate, downgraded by one level due to a small sample size and rather wide confidence intervals, suggesting some imprecision of the effect estimate.
1.9. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 9: Length of hospital stay
Side effect of intervention
A total of 545 participants from 3 studies were included for this outcome. The pooled effect estimate implied that side effects of the intervention, both nausea/vomiting and abdominal pain, occurred more frequently in the MBP+oAB group than in the MBP group (nausea/vomiting: RR 2.22, 95% CI 1.33 to 3.72; P = 0.002, I² = 0%; Analysis 1.10). Abdominal pain: RR 1.79, 95% CI 0.67 to 4.82; P = 0.25, I² = 0%; Analysis 1.11).
1.10. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 10: Side effects of Intervention ‐ Nausea/Vometing
1.11. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 11: Side effects of Intervention ‐ Abdominal pain
C. difficile‐related diarrhoea
Four studies reported on this outcome, but only three were included in the pooled effect estimate. In Arezzo 2021 no patient in this study had C. difficile‐related diarrhoea (n = 50). The results indicated that with regard to the occurrence of C. difficile‐related diarrhoea, there seemed to be no difference between the MBP+oAB and MBP groups (RR 0.89, 95% CI 0.24 to 3.34; P = 0.86, I² = 9%; 3 studies, 1547 patients; Analysis 1.12).
1.12. Analysis.

Comparison 1: MBP+oAB vs. MBP, Outcome 12: C. difficile‐related diarrhoea
Sensitivity analysis
A sensitivity analysis including only studies with a low risk of bias revealed that the studies labelled as "some concern" did not have a considerable impact on the overall effect with regard to SSI, mortality, incidence of postoperative ileus and LOS. For the risk of anastomotic leakage on the other hand, excluding the studies with some concern resulted in a stronger effect (RR 0.48, 95% CI 0.25 to 0.95 instead of RR 0.60, 95% CI 0.36 to 0.99; see Appendix 2).
Comparison 2: Combined mechanical and oral antibiotic bowel preparation versus oral antibiotics alone (MBP+oAB versus oAB)
We identified 3 studies for this comparison (Ram 2005; Suzuki 2020; Zmora 2003). The predefined outcomes SSI, anastomotic leakage, mortality and incidence of postoperative ileus were each reported in more than one study so that the data could be pooled in a meta‐analysis.
Surgical site infections (SSIs)
A total of 960 participants from 3 studies were included in the meta‐analysis for this outcome. The results indicated that there was no difference in the risk of postoperative SSI between the MBP+oAB and the oAB alone group (RR 0.87, 95% CI 0.34 to 2.21; P = 0.76, I² = 69%; Analysis 2.1). The overall certainty of the evidence was very low and was downgraded by three levels in total due to some concerns about the risk of bias, moderate heterogeneity between included studies suggesting inconsistency of results (Chi² = 6.41, df = 2 (P = 0.04); I² = 69%), and a small sample size with wide confidence intervals, indicating imprecision.
2.1. Analysis.

Comparison 2: MBP+oAB vs. oAB, Outcome 1: SSI
Anastomotic leakage
A total of 960 participants from 3 studies were included in this meta‐analysis. The result implicated that the incidence of anastomotic leakage was equal in the MBP+oAB and oAB alone groups (RR 0.84, 95% CI 0.21 to 3.45; P = 0.81, I² = 39%; Analysis 2.2). The overall certainty of the evidence was low, downgraded by two levels due to some concerns the risk of bias in the included studies and a small sample size, limited number of events, and wide confidence intervals, indicating imprecision.
2.2. Analysis.

Comparison 2: MBP+oAB vs. oAB, Outcome 2: Anastomotic leakage
Mortality
A total of 709 participants from 2 studies were included in this meta‐analysis. The results indicated that mortality was equal in the MBP+oAB and oAB alone group (RR 1.02, 95% CI 0.30 to 3.50; P = 0.97, I² = 0%; Analysis 2.3). The overall certainty of the evidence was low, downgraded by two levels due to some concerns about the risk of bias in the included studies and a small sample size, limited number of events, and wide confidence intervals, indicating imprecision.
2.3. Analysis.

Comparison 2: MBP+oAB vs. oAB, Outcome 3: Mortality
Postoperative complications subdivided according to Clavien‐Dindo in mild (I/II) and severe (III/IV) complications
None of the three identified studies collected data on the occurrence of postoperative complications according to the Clavien‐Dindo classification.
Incidence of postoperative ileus
A total of 709 participants from 2 studies were included in this meta‐analysis. The results indicated that the incidence of postoperative ileus was equal in the MBP+oAB and the oAB alone group (RR 1.25, 95% CI 0.68 to 2.33; P = 0.47, I² = 0%; Analysis 2.4). The overall certainty of the evidence was low, downgraded by two levels due to some concerns about the risk of bias in the included studies and a small sample size, limited number of events, and rather wide confidence intervals, indicating possible imprecision.
2.4. Analysis.

Comparison 2: MBP+oAB vs. oAB, Outcome 4: Incidence of postoperative ileus
Length of hospital stay (LOS)
Both Zmora 2003 and Ram 2005 found no significant differences in the length of hospital stay between the MBP+oAB and the oAB groups. The mean difference between the groups was 0.1 respectively 0.2 (95% CI ‐0.68 to 1.08) days.
Side effects of the Intervention
None of the three identified studies provided data on the occurrence of side effects of the intervention.
C. difficile‐related diarrhoea
The detection rate of C. difficile toxin was collected both pre‐ and postoperatively by Suzuki 2020. It was demonstrated that the number of C. difficile detections tended to increase after surgery in all groups, although the absolute number was quite low. C. difficile‐related diarrhoea was not observed in any group.
Sensitivity analysis
No sensitivity analyses were performed for this comparison as all studies were judged as "some concerns".
Comparison 3: Combined mechanical and oral antibiotic bowel preparation versus no bowel preparation (MBP+oAB versus nBP)
For this comparison, we identified a single relevant study (Koskenvuo 2019) that addressed all of our pre‐defined outcomes.
Surgical site infections (SSIs)
The study results suggested that the intervention (MBP+oAB) may result in little to no difference in surgical site infections. (RR 0.63, 95% CI 0.33 to 1.23; P = 0.17; 396 participants). While SSI occurred in 105 per 1000 patients with MBP alone, it only occurred in 66 per 1000 patients in the MBP+oAB group. However, as only one study with a limited number of participants was identified for this comparison and the 95% CI includes both, benefit and harm, the confidence in the evidence was downgraded by two levels due to imprecision. The possible positive effect of MBP+oAB was confirmed for incisional wound infections (RR 0.45, 95% CI 0.14 to 1.45; P = 0.18; 396 participants), but no important difference was demonstrated for organ/space infections (RR 0.77, 95% CI 0.33 to 1.78; P = 0.53; 396 participants).
Anastomotic leakage
The risk of an anastomotic leakage was equal in the MBP+oAB and nBP group (RR 0.89, 95% CI 0.33 to 2.42; P = 0.82; 396 participants). The certainty of evidence was however low, as only one study with a limited number of participants was identified, which led to a downgrading of the certainty of evidence by two levels due to imprecision.
Mortality
The study results suggested that MBP+oAB may result in little to no difference in mortality (RR 0.20, 95% CI 0.01 to 4.22; P = 0.30; 396 participants). However, due to the small number of events (MPB+oAB 0/196; nBP 2/200), the validity of the data was limited. The overall certainty of the evidence was low, downgraded by two levels due to imprecision.
Postoperative complications subdivided according to Clavien‐Dindo in mild (I/II) and severe (III/IV)
The risk of mild postoperative complications was equal in the MBP+oAB and nBP group (RR 1.15, 95% CI 0.93 to 1.41; P = 0.20; 396 participants). The risk of severe postoperative complications on the other hand seemed to be increased by the Intervention (RR 1.47, 95% CI 0.80 to 2.69; P = 0.22; 396 participants).
Incidence of postoperative ileus
The risk of postoperative ileus was equal in the MBP+oAB and nBP group (RR 1.18, 95% CI 0.77 to 1.81; P = 0.45; 396 participants). The certainty of evidence was however low, as only one study with a limited number of participants was identified, which led to a downgrading of the certainty of evidence by two levels due to imprecision.
Length of hospital stay (LOS)
The length of the hospital stay did not differ between the MBP+oAB and nBP group (MD 0.10, 95% CI ‐0.80 to 1.00; P = 0.83; 396 participants). The certainty of evidence was however low, as only one study with a limited number of participants was identified, which led to a downgrading of the certainty of evidence by two levels due to imprecision.
Side effect of Intervention
The incidence of side effects of the intervention was the same in the MBP+oAB and nBP group (RR 0.94, 95% CI 0.44 to 2.01; P = 0.88; 396 participants)
C. difficile‐related diarrhoea
The study results suggested that MBP+oAB reduce the risk of C. difficile‐related diarrhoea compared with nBP (RR 0.34, 95% CI 0.01 to 8.30; P = 0.51; 396 participants). However, due to the small number of events (MPB+oAB 0/196; nBP 1/200), the validity of the data is limited.
Sensitivity analysis
No sensitivity analyses were performed for this comparison as only one study with a low risk of bias was identified.
Discussion
Summary of main results
We identified 21 studies (with 5264 participants analysed) that were eligible for this review.
For the first comparison (mechanical bowel preparation plus oral antibiotics (MBP+oAB versus MBP)), 17 studies (with 3908 participants) were included in our meta‐analyses. Based on moderate certainty of evidence, our review shows that MBP+oAB probably reduces the risk of postoperative wound infections and anastomotic leakage, while having no effect on mortality, incidence of postoperative ileus or length of hospital stay compared with MBP alone. More specifically, combined bowel preparation can reduce the risk of SSI from 137 per 1000 patients with MBP alone to 77 per 1000 patients. The risk of anastomotic leakage also decreases from 44 per 1000 with MBP alone to 26 per 1000 with combination therapy (see Table 1). However, nausea/vomiting as a side effect of the intervention occurred more frequently in the MBP+oAB group than in the MBP alone group. It should be noted that there was moderate statistical heterogeneity between studies regarding the incidence of SSI, which could not be explained by any subgroup analyses. Although the risk of bias for the SSI outcome was predominantly rated as "some concerns", while the risk of bias for the other outcomes was evenly split between "low risk of bias" and "some concerns", the sensitivity analyses conducted showed no substantial impact of the risk of bias rating on the overall effect of all outcomes.
For the second comparison (MBP+oAB versus oAB), 3 studies (with 960 participants) were identified. Due to some concerns about the risk of bias in the included studies and the small sample size, we have only a low to very low certainty of the evidence. As far as we can conclude, MBP+oAB may result in little to no difference of SSI rates, anastomotic leakage, mortality or postoperative ileus compared with oAB alone.
For the third comparison (MBP+oAB versus nBP), a single study (with 396 participants) was identified. This suggests that MBP+oAB may reduce SSIs and mortality, while resulting in little to no difference for anastomotic leakage, postoperative ileus, length of hospital stay or the occurrence of side effects of the intervention compared with nBP. However, certainty of evidence is low.
Overall completeness and applicability of evidence
The question of superiority of combined mechanical and oral antibiotic bowel preparation to prevent postoperative complications can only be answered incompletely by this review.
A major limitation is the lack of evidence for the second (MBP+oAB versus oAB) and third (MBP+oAB versus nBP) comparison. While 17 studies were identified for our first comparison (MBP+oAB versus MBP), data for the second and third comparison come from three and one studies, respectively.
However, a further indication of the possible superiority of combination therapy over nBP (third comparison) can be derived from a comparison with the Cochrane review on mechanical bowel preparation by Güenaga 2011. In this meta‐analysis, MBP alone was found to be equivalent to nBP, particularly in terms of wound infections and anastomotic leakage. Considering that our review found superiority of combination therapy over MBP alone with respect to these outcomes, one might conclude that combination therapy may be advantageous over nBP. Unfortunately, there is no evidence to support this conclusion.
Another limitation regarding the applicability of our results is that most studies included both colon and rectal resections. There was only one study that included only colon respectively only rectal resections. Furthermore, a differentiation between resection of the right colon versus resection of the left colon was not carried out in the trials. A review of the effect depending on the type and location of the surgery performed, in particular a further subdivision of colon resections, was not possible based on the available data. The same applies to the other performed subgroup analyses, so that further investigation of the effect depending on the indication for surgery, the surgical approach or the agents used for the intervention as well as the duration of the intervention was only conclusive to a limited extent.
In addition, insufficient information about the side effects of the intervention, especially regarding dreaded effects of MBP such as dehydration, electrolyte imbalances, and even cardiac problems, limit the applicability of our results.
We included both small and large studies published over a long period from 1992 to the present. We also identified 15 ongoing studies, indicating that interest in this area remains high. Especially for the second and third comparisons, it is very likely that further research will have an important impact on the findings and our confidence in the estimated effect.
Quality of the evidence
There is moderate to low certainty‐evidence comparing MBP+oAB versus MBP for the outcomes assessed in Table 1. For SSIs, there was moderate heterogeneity between the included studies that could not be explained by the subgroup analyses conducted, so confidence in the evidence was downgraded by one level. However, the subgroup analyses are not reliable since they could not be carried out as planned on the basis of the available data. For example, our subgroup analysis on the type of surgery performed is not very conclusive, as only one study reported results for rectal resections only. The other studies all reported on a mixed population, and based on the reported data it was not possible to subdivide the results according to the type and location of surgery performed. The same applies to the subgroup analysis regarding the indication for surgery (benign versus malignant) and the surgical approach (open versus laparoscopic). Similarly, the subgroup analysis regarding the agents used for oral antibiotic bowel preparation is of limited value, as different dosages were used within the subgroups, which in turn may contribute to heterogeneity. Due to the limited number of events and wide confidence intervals of the included studies, the certainty of the estimated effect of anastomotic leakage, mortality, incidence of postoperative ileus and LOS was also downgraded by one level. Additionally, certainty of the estimated effect on postoperative ileus was downgraded another level because small studies reported statistically significant benefits, while larger studies showed a much smaller effect, indicating possible publication bias.
Only low to very low certainty of the effect estimate was found for the MBP+oAB versus oAB comparison (Table 2). This is primarily due to the small overall sample size of only three identified studies and "some concerns" about the risk of bias in all included studies, as no predefined analysis plan could be identified for any of these studies.
There is also only low confidence in the evidence for the MBP+oAB versus nBP comparison (Table 3). Due to the very small sample size with wide confidence intervals in only one identified study, the confidence in the effect estimate was downgraded by two levels due to imprecision.
Potential biases in the review process
A broad search strategy was used to identify studies in this area, searching both electronic databases and study registries without making any restrictions, e.g. based on language. Unfortunately, first order problems in the conceptualisation of the search strategy may have resulted in missed eligible studies, which is a considerable limitation of this review. Several national and international colorectal surgery organisations were contacted and asked whether they were aware of any ongoing or completed studies on this topic. In addition, all included studies of similar meta‐analyses were screened for eligibility. We believe that, even though our review is limited by conceptualisation issues of the search strategies, it is unlikely that previously conducted and published studies were overlooked; however, unpublished studies or ongoing studies not registered in study registries may be missing. If such studies are identified, we will include them in future updates of the review. Furthermore, our search strategies will be redesigned for future updates.
We attempted to reduce bias as much as possible by having at least two authors work independently on study selection, data extraction, and risk of bias as well as GRADE assessment.
We were only able to examine the potential for publication bias using funnel plots for the two primary outcomes of the first comparison, as there were no other outcomes of interest with 10 or more studies included in any meta‐analyses (Figure 2; Figure 3).
Agreements and disagreements with other studies or reviews
There are already numerous reviews and meta‐analyses on various aspects of the topic of preoperative bowel preparation, but they all come to different conclusions. Comparing three high‐quality meta‐analyses published in the last four years, we find that Toh 2018 associated MBP+oAB with the lowest risk of SSI in a network meta‐analysis. Oral antibiotics alone were ranked as second best, but available data on this approach were limited. In contrast, Rollins 2019 found that MBP+oAB was largely equivalent to oAB alone, and Nelson 2020 proclaimed the superiority of oAB alone over MBP+oAB. While our results overlap with those of Toh 2018 and Rollins 2019, we were unable to show superiority of oAB alone over combination therapy, as Nelson 2020 did. An examination of the included studies shows that the different conclusions are mainly not due to more recent publications that were not yet included in the older reviews, but to different inclusion and exclusion criteria or variations in the literature search. A major difference between our review and the earlier ones is that only studies in which all patients received perioperative intravenous antibiotic prophylaxis were included. Perioperative intravenous antibiotic prophylaxis has a significant impact especially on the likelihood of the occurrence of SSI and should be standard of care (Nelson 2014; WHO 2018). Therefore, studies without perioperative intravenous antibiotic prophylaxis would confound the results of the meta‐analysis and were excluded from our review.
Authors' conclusions
Implications for practice.
The lack of benefit from MBP alone has already been demonstrated in the past leading to the recommendation in several guidelines to avoid MBP (Gustafsson 2019; Güenaga 2011; NICE 2019; WHO 2018). Based on the data from study registries, the "American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Use of Bowel Preparation in Elective Colon and Rectal Surgery" (Migaly 2019) recommends combined mechanical and oral antibiotic bowel preparation.
Our results support the hypothesis that combination therapy is likely superior to MBP alone for reducing the incidence of SSI and anastomotic leakage. Our evidence indicates that MBP only has the desired effect on postoperative complications in combination with oAB. The efficacy of oAB alone compared with combination therapy has not yet been clearly demonstrated and needs to be investigated in further RCTs before valid conclusions can be drawn. The same applies to the comparison of combination therapy and nBP.
Implications for research.
Based on the available evidence, conclusions can only be drawn for one of the three comparisons in our review. The comparisons of MBP+oAB with oAB alone or nBP are insufficiently studied. Of the ongoing studies identified, the majority continues to investigate the comparison of MBP+oAB versus MBP. Only four trials focus on the comparison MBP+oAB versus oAB (NCT03042091; NCT04931173; ORALEV2; Tagliaferri 2020) and only two investigate the comparison of MBP+oAB versus nBP (MECCLANT –C and –R Trials; Panaiotti 2020). In order to be able to make an evidence‐based assessment of the most effective preoperative bowel preparation, further RCTs, especially on the last two comparisons are required.
In addition, future studies should also examine the different options of bowel preparation in terms of timing, active ingredient, and dosage in more detail. Although our review was able to support the assumption that MBP+oAB has an advantage over MBP alone, there is no clear recommendation regarding the antibiotic regimen to be used. Combination therapies of metronidazole and an aminoglycoside (neomycin or kanamycin) or erythromycin and an aminoglycoside (neomycin or kanamycin) appear to be the most commonly used therapies and do not differ in efficacy (Analysis 1.17). But even within these combination therapies, the dosage used varies across all included studies, necessitating further investigation. The same applies to the substance and duration of mechanical bowel preparation.
Another aspect that should be investigated in future studies are the side effects of the intervention, so that in addition to the benefits, the potential harms of the intervention can also be considered in treatment decisions.
Furthermore, the differences in the effect of MBP+oAB depending on the type of surgery performed, not only colon versus rectum, but also regarding the site of colon surgery, should be further investigated. Since the right colon is physiologically filled with fewer faeces than the left, there are surgeons who prescribe MBP only before resections of the left colon or rectum, but not before resections of the right colon. Studies analysing the different effect of preoperative bowel preparation depending on the localisation of the colon resection have not been identified, so that a corresponding subgroup analysis was not possible in our review. The benefit of oAB alone might also differ due to the physiologically lower contamination of the right colon compared with the left colon and rectum, so that depending on the location of the planned resection, a different type of bowel preparation might be most effective.
Another aspect that should be further investigated would be a separate evaluation of the benefits and harms of preoperative bowel preparation in minimally invasive and open procedures. Pooled data from studies including both laparoscopic and open procedures demonstrate a clear advantage of combination therapy over MBP alone in terms of SSI rates. However, separate analysis of studies that included only laparoscopic or only open approaches shows a much smaller effect with wide 95% CIs that include both benefit and harm (Analysis 1.15). Therefore, further studies are needed here to better assess the differential effects depending on the surgical approach.
History
Protocol first published: Issue 1, 2022
Risk of bias
Risk of bias for analysis 1.1 SSI.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lau 1988 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as SSIs are clearly defined clinically | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Takesue 2000 | Some concerns | There was no information on method of randomisation, but there was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 83 % of the 100 randomised patients were analysed. The reasons for the exclusion of 17 patients are given and comprehensible. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as SSIs are clearly defined clinically. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about the randomisation method and about a predefined analysis plan. |
| Ishida 2001 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the diagnosis was made using well‐defined criteria. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Lewis 2002 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | All participants were included in the analysis | Low risk of bias | The asessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the diagnosis was made using well‐defined criteria. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Espin‐Basany 2005 | Some concerns | There was no information on method of randomisation, there was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, SSIs are clearly defined clinically | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns about the lack of information about the randomisation method and about a predefined analysis plan. |
| Kobayashi 2007 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The asessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the rating, as the diagnosis was made using well‐defined criteria. | Some concerns | The existence of a study protocol was mentioned in the publication but was not available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Horie 2007 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | All participants were included in the analysis | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the diagnosis was made using well‐defined criteria. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Oshima 2013 | Some concerns | There was no information on the method of randomisation, but there was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | 91 % of the randomised patients were analysed. 13 participants were excluded due to protocol violations (6 patients in group A and 7 patients in group B). | Low risk of bias | The asessors were blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the diagnosis was made using well‐defined criteria. | Some concerns | The existence of a study protocol was mentioned in the publication but was not available. | Some concerns | Some concerns due to the lack of information about the randomisation method and about a predefined analysis plan. |
| Sadahiro 2014 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 95% of the randomised patients were analysed. 11 patients were excluded after randomisation due to the following reasons:
|
Low risk of bias | The assessors were blinded to the allocation of patients. | Low risk of bias | The trial was registered as University Hospital Medical Information Network (UMIN) Clinical Trials Registry 000003435. | Low risk of bias | Low risk of bias in all domains. |
| Ikeda 2016 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | >95% of randomised patients were analysed. For our meta‐analysis, however, only patients who had MBP and oAB as intervention were eligible. The relevant data was provided to us by the study authors. | Low risk of bias | The infection control doctors and nurses who determined SSIs were blinded to the assignment. | Low risk of bias | This trial was registered with the UMIN Clinical Trials Registry (UMIN000019339). | Low risk of bias | Low risk of bias in all domains. |
| Hata 2016 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention could have influenced the assessment, as the CDC criteria for diagnosing SSI were used in both groups. | Low risk of bias | This trial was registered with ClinicalTrials.gov, Number NCT00508690. | Low risk of bias | Low risk of bias in all domains. |
| Anjum 2017 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were blinded, but even without blinding it is unlikely that knowledge of the intervention would have influenced the rating, as the diagnosis is made using well‐defined criteria | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Uchino 2019 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were blinded to the allocation of patients. | Low risk of bias | The trail was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR 000013369). | Low risk of bias | Low risk of bias in all domains. |
| Rybakov 2021 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 77 % of the 150 randomised patients were analysed. 34 participants were excluded due to unexpected changes in surgical procedure (e.g. inoperability or no restoration of bowel continuity via anastomosis; 18 in the oral + IV AP group and 16 in the IV AP group). None of the patients were lost to follow‐up. | Low risk of bias | The assessors were blinded to the allocation of patients. | Low risk of bias | The study protocol was registered on ClinicalTrials.gov (registrationnumber NCT03436719). | Low risk of bias | Low risk of bias in all domains. |
| Papp 2021 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 89 % of the 597 randomised patients were analysed. The reasons for exclusion were mostly protocol violations (n=71) or that no anastomosis was created during surgery (n= 47). 7 patients were excluded due to adverse events. | Low risk of bias | The assessors were blinded to the allocation of patients. | Low risk of bias | This trial was registered as EudraCT 2015‐005614‐27 | Low risk of bias | Low risk of bias in all domains. |
| Arezzo 2021 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | All participants were included in the analysis. For our meta‐analysis, however, only patients who had MBP and oAB as intervention were eligible. The relevant data was provided to us by the study authors. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention has influenced the assessment, as the CDC criteria for diagnosing SSI were used in both groups. | Low risk of bias | The trial was registered with ClinicalTrials.gov (NCT: 04438655). | Low risk of bias | Low risk of bias in all domains. |
Risk of bias for analysis 1.4 Anastomotic leakage.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lau 1988 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as anastomotic leakages are clearly defined clinically. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Takesue 2000 | Some concerns | There was no information on method of randomisation, but there was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 83 % of the 100 randomised patients were analysed. The reasons for the exclusion of 17 patients are given and comprehensible. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as anastomotic leakages are clearly defined clinically. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about the randomisation method and about a predefined analysis plan. |
| Ishida 2001 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as anastomotic leakages are clearly defined clinically. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Horie 2007 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | All participants were included in the analysis. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as anastomotic leakages are clearly defined clinically | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Sadahiro 2014 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 95% of the randomised patients were analysed. 11 patients were excluded after randomisation due to the following reasons:
|
Low risk of bias | The assessors were blinded to the allocation of patients. | Low risk of bias | The trial was registered as University Hospital Medical Information Network (UMIN) Clinical Trials Registry 000003435. | Low risk of bias | Low risk of bias in all domains. |
| Hata 2016 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as anastomotic leakages are clearly defined clinically | Low risk of bias | This trial was registered with ClinicalTrials.gov, Number NCT00508690. | Low risk of bias | Low risk of bias in all domains. |
| Ikeda 2016 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | >95% of randomised patients were analysed. For our meta‐analysis, however, only patients who had MBP and oAB as intervention were eligible. The relevant data was provided to us by the study authors. | Low risk of bias | The infection control doctors and nurses who determined SSIs were blinded to the assignment. The ward doctors and nurses who probably diagnosed all other outcomes were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as anastomotic leakages are clearly defined clinically. | Low risk of bias | This trial was registered with the UMIN Clinical Trials Registry (UMIN000019339). | Low risk of bias | Low risk of bias in all domains. |
| Rybakov 2021 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 77 % of the 150 randomised patients were analysed. 34 participants were excluded due to unexpected changes in surgical procedure (e.g. inoperability or no restoration of bowel continuity via anastomosis; 18 in the oral + IV AP group and 16 in the IV AP group). None of the patients were lost to follow‐up. | Low risk of bias | The assessors were blinded to the allocation of patients. | Low risk of bias | The study protocol was registered on ClinicalTrials.gov (registration number NCT03436719). | Low risk of bias | Low risk of bias in all domains. |
| Papp 2021 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 89 % of the 597 randomised patients were analysed. The reasons for exclusion were mostly protocol violations (n=71) or that no anastomosis was created during surgery (n= 47). 7 patients were excluded due to adverse events. | Low risk of bias | The assessors were blinded to the allocation of patients. | Low risk of bias | This trial was registered as EudraCT 2015‐005614‐27 | Low risk of bias | Low risk of bias in all domains. |
| Arezzo 2021 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | All participants were included in the analysis For our meta‐analysis, however, only patients who had MBP and oAB as intervention were eligible. The relevant data was provided to us by the study authors. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as anastomotic leakages are clearly defined clinically | Low risk of bias | The trial was registered with ClinicalTrials.gov (NCT: 04438655). | Low risk of bias | Low risk of bias in all domains. |
Risk of bias for analysis 1.5 Mortality.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lazorthes 1982 | Some concerns | There was no information on method of randomisation, but there was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | All participants were included in the analysis. | Low risk of bias | The assessors were not blinded, but it is not possible that knowledge of the intervention would have influenced the assessment, as death cannot be misdiagnosed. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about the randomisation method and about a predefined analysis plan. |
| Arezzo 2021 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | All participants were included in the analysis. For our meta‐analysis, however, only patients who had MBP and oAB as intervention were eligible. The relevant data was provided to us by the study authors. | Low risk of bias | The assessors were not blinded, but it is not possible that knowledge of the intervention would have influenced the assessment, as death cannot be misdiagnosed. | Low risk of bias | The trial was registered with ClinicalTrials.gov (NCT: 04438655). | Low risk of bias | Low risk of bias in all domains. |
| Papp 2021 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 89 % of the 597 randomised patients were analysed. The reasons for exclusion were mostly protocol violations (n=71) or that no anastomosis was created during surgery (n= 47). 7 patients were excluded due to adverse events. | Low risk of bias | The assessors were blinded to the allocation of patients. | Low risk of bias | This trial was registered as EudraCT 2015‐005614‐27. | Low risk of bias | Low risk of bias in all domains. |
Risk of bias for analysis 1.8 Incidence of postoperative ileus.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Espin‐Basany 2005 | Some concerns | There was no information on method of randomisation, there was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the occurrence of postoperative ileus is associated with clear clinical diagnostic criteria. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns about the lack of information about the randomisation method and about a predefined analysis plan. |
| Hata 2016 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the occurrence of postoperative ileus is associated with clear clinical diagnostic criteria. | Low risk of bias | This trial was registered with ClinicalTrials.gov, Number NCT00508690. | Low risk of bias | Low risk of bias in all domains. |
| Ikeda 2016 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | >95% of randomised patients were analysed. For our meta‐analysis, however, only patients who had MBP and oAB as intervention were eligible. The relevant data was provided to us by the study authors. | Low risk of bias | The infection control doctors and nurses who determined SSIs were blinded to the assignment. The ward doctors and nurses who probably diagnosed all other outcomes were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the occurrence of postoperative ileus is associated with clear clinical diagnostic criteria. | Low risk of bias | This trial was registered with the UMIN Clinical Trials Registry (UMIN000019339). | Low risk of bias | Low risk of bias in all domains. |
| Rybakov 2021 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 77 % of the 150 randomised patients were analysed. 34 participants were excluded due to unexpected changes in surgical procedure (e.g. inoperability or no restoration of bowel continuity via anastomosis; 18 in the oral + IV AP group and 16 in the IV AP group). None of the patients were lost to follow‐up. | Low risk of bias | The assessors were blinded to the allocation of patients. | Low risk of bias | The study protocol was registered on ClinicalTrials.gov (registrationnumber NCT03436719). | Low risk of bias | Low risk of bias in all domains. |
| Papp 2021 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 89 % of the 597 randomised patients were analysed. The reasons for exclusion were mostly protocol violations (n=71) or that no anastomosis was created during surgery (n= 47). 7 patients were excluded due to adverse events. | Low risk of bias | The assessors were blinded to the allocation of patients. | Low risk of bias | This trial was registered as EudraCT 2015‐005614‐27 | Low risk of bias | Low risk of bias in all domains. |
| Arezzo 2021 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | All participants were included in the analysis For our meta‐analysis, however, only patients who had MBP and oAB as intervention were eligible. The relevant data was provided to us by the study authors. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the occurrence of postoperative ileus is associated with clear clinical diagnostic criteria. | Low risk of bias | The trial was registered with ClinicalTrials.gov (NCT: 04438655). | Low risk of bias | Low risk of bias in all domains. |
Risk of bias for analysis 1.9 Length of hospital stay.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lau 1988 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | Patients are discharged from inpatient therapy after recovery. The timing of discharge is influenced by many factors. Therefore, knowledge about the intervention has little to no influence on the assessment of this outcome. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Ikeda 2016 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | >95% of randomised patients were analysed. For our meta‐analysis, however, only patients who had MBP and oAB as intervention were eligible. The relevant data was provided to us by the study authors. | Low risk of bias | The infection control doctors and nurses who determined SSIs were blinded to the assignment. The ward doctors and nurses who probably diagnosed all other outcomes were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the occurrence of postoperative ileus is associated with clear clinical diagnostic criteria. | Low risk of bias | This trial was registered with the UMIN Clinical Trials Registry (UMIN000019339). | Low risk of bias | Low risk of bias in all domains. |
| Arezzo 2021 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | All participants were included in the analysis. For our meta‐analysis, however, only patients who had MBP and oAB as intervention were eligible. The relevant data was provided to us by the study authors. | Low risk of bias | Patients are discharged from inpatient therapy after recovery. The timing of discharge is influenced by many factors. Therefore, knowledge about the intervention has little to no influence on the assessment of this outcome. | Low risk of bias | The trial was registered with ClinicalTrials.gov (NCT: 04438655). | Low risk of bias | Low risk of bias in all domains. |
Risk of bias for analysis 2.1 SSI.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Zmora 2003 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 92% of the 415 randomised patients were analysed. Twenty‐nine patients were excluded after randomization due to the intraoperative exclusion criteria (18 had abdominoperineal resection and 11 had a proximal stoma). Six patients withdrew their consent before surgery, leaving 380 patients for the data analysis. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the diagnosis was made using well‐defined criteria. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Ram 2005 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | All participants were included in the analysis. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the rating, as the diagnosis was made using well‐defined criteria. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Suzuki 2020 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as SSIs are clearly defined clinically. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
Risk of bias for analysis 2.2 Anastomotic leakage.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Zmora 2003 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 92% of the 415 randomised patients were analysed. Twenty‐nine patients were excluded after randomization due to the intraoperative exclusion criteria (18 had abdominoperineal resection and 11 had a proximal stoma). Six patients withdrew their consent before surgery, leaving 380 patients for the data analysis. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as anastomotic leakages are clearly defined clinically. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Ram 2005 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | All participants were included in the analysis. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as anastomotic leakages are clearly defined clinically. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Suzuki 2020 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | >95% of randomised patients were analysed. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as anastomotic leakages are clearly defined clinically. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
Risk of bias for analysis 2.3 Mortality.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Zmora 2003 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 92% of the 415 randomised patients were analysed. Twenty‐nine patients were excluded after randomization due to the intraoperative exclusion criteria (18 had abdominoperineal resection and 11 had a proximal stoma). Six patients withdrew their consent before surgery, leaving 380 patients for the data analysis. | Low risk of bias | The assessors were not blinded, but it is not possible that knowledge of the intervention would have influenced the assessment, as death cannot be misdiagnosed. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Ram 2005 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | All participants were included in the analysis. | Low risk of bias | The assessors were not blinded, but it is not possible that knowledge of the intervention would have influenced the assessment, as death cannot be misdiagnosed. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
Risk of bias for analysis 2.4 Incidence of postoperative ileus.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Zmora 2003 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 92% of the 415 randomised patients were analysed. Twenty‐nine patients were excluded after randomization due to the intraoperative exclusion criteria (18 had abdominoperineal resection and 11 had a proximal stoma). Six patients withdrew their consent before surgery, leaving 380 patients for the data analysis. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the occurrence of postoperative ileus is associated with clear clinical diagnostic criteria. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
| Ram 2005 | Low risk of bias | The generation of the randomisation sequence and the allocation concealment were appropriate. There was no baseline imbalance that would indicate a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | All participants were included in the analysis. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the occurrence of postoperative ileus is associated with clear clinical diagnostic criteria. | Some concerns | No pre‐registered method (registry or protocol) available. | Some concerns | Some concerns due to the lack of information about a predefined analysis plan. |
Risk of bias for analysis 3.1 SSI.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Koskenvuo 2019 | Low risk of bias | Generation of randomisation sequence and concealment of allocation were adequate. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 95% of randomised patients were analysed in the modified intention‐to‐treat analysis. | Low risk of bias | The raters were blinded, but even without blinding it is unlikely that knowledge of the intervention would have influenced the rating, as the diagnosis is made using well‐defined criteria. | Low risk of bias | The trial was registered with ClinicalTrials.gov (NCT02652637) and EudraCT (2015‐004559‐38). | Low risk of bias | Low risk of bias in all domains. |
Risk of bias for analysis 3.4 Anastomotic leakage.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Koskenvuo 2019 | Low risk of bias | Generation of randomisation sequence and concealment of allocation were adequate. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 95% of randomised patients were analysed in the modified intention‐to‐treat analysis. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as anastomotic leakages are clearly defined clinically | Low risk of bias | The trial was registered with ClinicalTrials.gov (NCT02652637) and EudraCT (2015‐004559‐38). | Low risk of bias | Low risk of bias in all domains. |
Risk of bias for analysis 3.8 Incidence of postoperative ileus.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Koskenvuo 2019 | Low risk of bias | Generation of randomisation sequence and concealment of allocation were adequate. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 95% of randomised patients were analysed in the modified intention‐to‐treat analysis. | Low risk of bias | The assessors were not blinded, but it is unlikely that knowledge of the intervention would have influenced the assessment, as the occurrence of postoperative ileus is associated with clear clinical diagnostic criteria. | Low risk of bias | The trial was registered with ClinicalTrials.gov (NCT02652637) and EudraCT (2015‐004559‐38). | Low risk of bias | Low risk of bias in all domains. |
Risk of bias for analysis 3.9 Length of hospital stay.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Koskenvuo 2019 | Low risk of bias | Generation of randomisation sequence and concealment of allocation were adequate. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. There were no deviations from intervention. | Low risk of bias | 95% of randomised patients were analysed in the modified intention‐to‐treat analysis. | Low risk of bias | Patients are discharged from inpatient therapy after recovery. The timing of discharge is influenced by many factors. Therefore, knowledge about the intervention has little to no influence on the assessment of this outcome. | Low risk of bias | The trial was registered with ClinicalTrials.gov (NCT02652637) and EudraCT (2015‐004559‐38). | Low risk of bias | Low risk of bias in all domains. |
Acknowledgements
The authors would like to acknowledge the strong support of the Cochrane Germany Foundation and the Institute for Evidence in Medicine, Freiburg.
Cochrane Colorectal and the Cochrane Editorial and Methods Department supported the authors in the development of this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Mike Brown, Michigan State University College of Human Medicine, USA;
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Sam Hinsley, Cochrane Central Editorial Service;
Editorial Assistant (conducted editorial policy checks and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service
Copy Editor (copy editing and production): Heather Maxwell;
Peer‐reviewers (provided comments and recommended an editorial decision): Richard Garfinkle, MD, MSc, PhD, Department of Surgery, McGill University, Montreal, QC, Canada (clinical/content review), Dr Kari Clifford, Department of Surgical Sciences, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand (clinical/content review), Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods review), Robin Featherstone, Cochrane Central Editorial Service (search review).
Appendices
Appendix 1. Search strategies
CENTRAL was searched via the Cochrane library using the following search string:
#1 [mh "colorectal surgery"] OR [mh "colectomy"] OR [mh "proctectomy"]
#2 (colorectal OR colon OR rectal OR proctolog* OR proctocolonic):ti,ab,kw
#3 (surger* OR surgical* OR resect* OR incisi* OR excisi* OR invasive* OR restorati* OR operation* OR operative* OR perioperati* OR peri‐operati*):ti,ab,kw OR [mh "elective surgical procedures"]
#4 #2 AND #3
#5 #4 OR #1
#6 [mh "laxatives"] OR [mh "cathartics"] OR [mh "enema"] OR [mh "antibiotic prophylaxis"] OR [mh "Anti‐Bacterial Agents"]
#7 (antibacterial* OR anti bacterial* OR antibiotic* OR neomycin OR metronidazole OR ciprofloxacin OR colistin OR tobramycin OR paromomycin OR erythromycin OR levofloxacin):ti,ab,kw
#8 (oral OR orally):ti,ab,kw
#9 #7 AND #8
#10 (bowel preparat* OR intestine preparat* OR colon preparat* OR gut preparat* OR bowel cleansing OR intestine cleansing OR colon cleansing OR gut cleansing OR laxative* OR purgative OR enema):ti,ab,kw
#11 #9 AND #10
#12 #6 OR #11
#13 #5 AND #12
MEDLINE was searched via PubMed using the following search string:
#1 "Colorectal Surgery"[MeSH Terms] OR "Colectomy"[Mesh]
#2 colorectal [tiab] OR colon [tiab] OR rectal [tiab] OR proctolog* [tiab] OR proctocolonic [tiab]
#3 surger* [tiab] OR surgical* [tiab] OR resect* [tiab] OR incisi* [tiab] OR excisi* [tiab] OR invasive* [tiab] OR restorati* [tiab] OR operation* [tiab] OR operative* [tiab] OR perioperati* [tiab] OR peri‐operati* [tiab] OR "surgery"[Subheading] OR "Surgical Procedures, Operative"[Mesh]
#4 #2 AND #3
#5 #4 OR #1
#6 "Gastrointestinal Agents"[Mesh] OR "Laxatives" [Pharmacological Action] OR "Enema"[Mesh] OR "Cathartics"[Mesh] OR "Laxatives"[Mesh] OR "Antibiotic Prophylaxis"[Mesh] OR "Anti‐Bacterial Agents"[Mesh]
#7 antibacterial* [tiab] OR anti bacterial* [tiab] OR antibiotic* [tiab] OR neomycin [tiab] OR metronidazole [tiab] OR ciprofloxacin [tiab] OR colistin [tiab] OR tobramycin [tiab] OR paromomycin [tiab] OR erythromycin [tiab] OR levofloxacin [tiab]
#8 oral [tiab] OR orally [tiab]
#9 #7 AND #8
#10 bowel preparat* [tiab] OR intestine preparat* [tiab] OR colon preparat* [tiab] OR gut preparat* [tiab] OR bowel cleansing [tiab] OR intestine cleansing [tiab] OR colon cleansing [tiab] OR gut cleansing [tiab] OR laxative* [tiab] OR purgative [tiab] OR enema [tiab]
#11 #9 AND #10
#12 #6 OR #11
#13 #5 AND #12
#14 ("randomized controlled trial"[Publication Type] OR "controlled clinical trial"[Publication Type] OR "randomized"[Title/Abstract] OR "placebo"[Title/Abstract] OR "clinical trials as topic"[MeSH Terms:noexp] OR "randomly"[Title/Abstract] OR "trial"[Title]) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms])
#15 #13 AND #14
Embase was searched via Ovid using the following search string:
#1 exp *colorectal surgery/ or exp *colon resection/ or exp *rectum resection/
#2 (colorectal or colon or rectal or proctolog* or proctocolonic).ti,ab,kw.
#3 (surger* or surgical* or resect* or incisi* or excisi* or invasive* or restorati* or operation* or operative* or perioperati* or peri‐operati*).ti,ab,kw. or exp *elective surgery/
#4 2 and 3
#5 1 or 4
#6 exp *laxative/ or exp *intestine preparation/ or exp *enema/ or exp *antibiotic prophylaxis/ or exp *antiinfective agent/
#7 (antibacterial* or anti bacterial* or antibiotic* or neomycin or metronidazole or ciprofloxacin or colistin or tobramycin or paromomycin or erythromycin or levofloxacin).ti,ab,kw.
#8 (oral or orally).ti,ab,kw.
#9 7 and 8
#10 (bowel preparat* or intestine preparat* or colon preparat* or gut preparat* or bowel cleansing or intestine cleansing or colon cleansing or gut cleansing or laxative* or purgative or enema).ti,ab,kw.
#11 9 and 10
#12 6 or 11
#13 5 and 12
#14 limit 13 to (clinical trial or randomized controlled trial)
ClinicalTrials.gov was searched using the following search strategies:
Advanced Search Condition or disease: colorectal surgery Other terms: bowel preparation AND oral antibiotic Study type: Interventional Studies (Clinical Trails) Study Results: All Studies Eligibility Criteria: Adult (18‐64) , Older Adult (65+) Sex: all
Expert Search ((Colorectal Surgery OR colectomy OR proctectomy OR ((colorectal OR colon OR rectal OR proctolog* OR proctocolonic) AND (surger* OR surgical* OR resect* OR incisi* OR excisi* OR invasive* OR restorati* OR operation* OR operative* OR perioperati* OR peri‐operati))) AND ((bowel preparat* OR intestine preparat* OR colon preparat* OR gut preparat* OR bowel cleansing OR intestine cleansing OR colon cleansing OR gut cleansing OR laxative* OR purgative OR enema) AND ((oral OR orally) AND (antibacterial* OR anti bacterial* OR antibiotic* OR neomycin OR metronidazole OR ciprofloxacin OR colistin OR tobramycin OR paromomycin OR erythromycin OR levofloxacin))))
The World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) was searched using the following search strategy:
mechanical bowel preparation AND oral antibio* AND ((colorectal OR colo* OR rectal*) AND (surgery OR operat*))
Appendix 2. Sensitivity analysis
Investigate sensitivity ‐ 1.1 SSI: Figure 37
4.
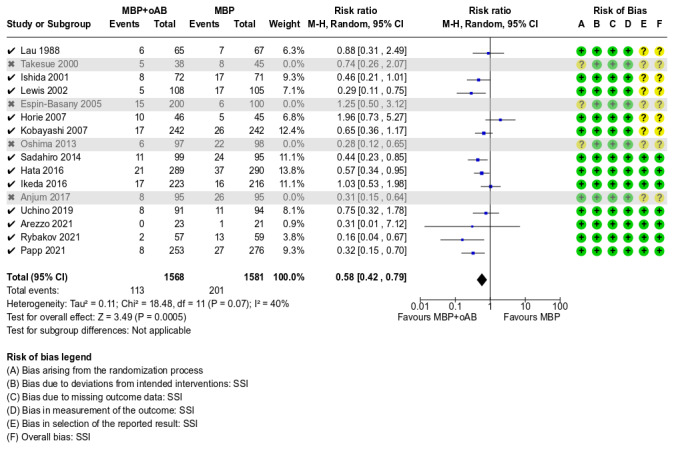
Investigate sensitivity ‐ 1.1 SSI
Investigate sensitivity ‐ 1.4 Anastomotic leakage: Figure 38
5.
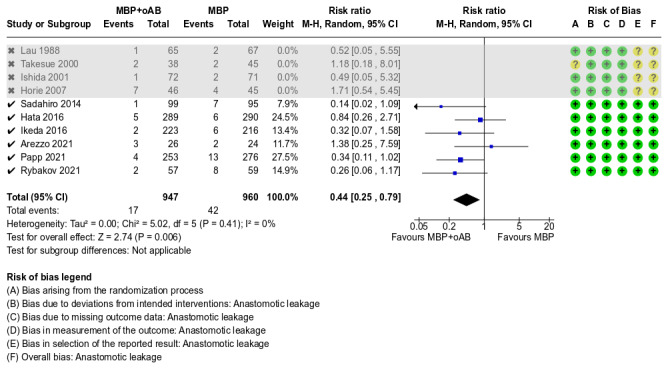
Investigate sensitivity ‐ 1.4 Anastomotic leakage
Investigate sensitivity ‐ 1.5 Mortality: Figure 39
6.
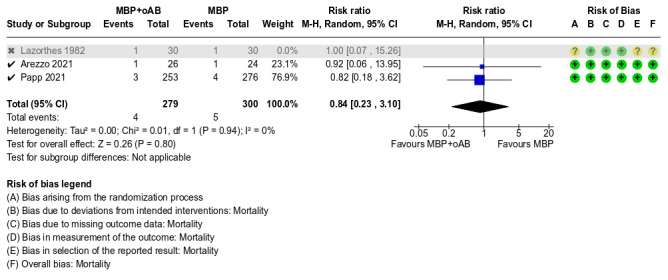
Investigate sensitivity ‐ 1.5 Mortality
Investigate sensitivity ‐ 1.8 Incidence of postoperative ileus: Figure 40
7.
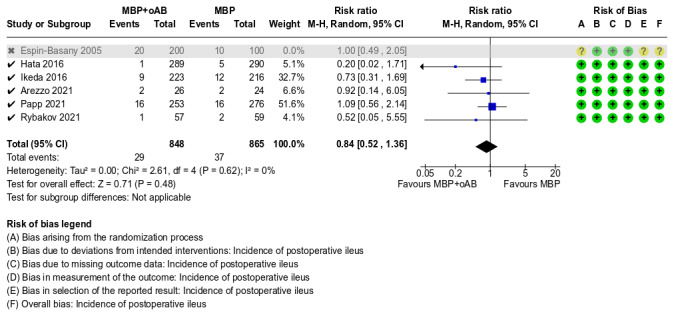
Investigate sensitivity ‐ 1.8 Incidence of postoperative ileus
Investigate sensitivity ‐ 1.9 Length of hospital stay: Figure 41
8.
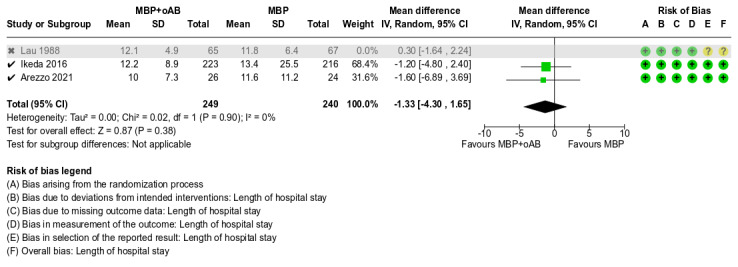
Investigate sensitivity ‐ 1.9 Length of hospital stay
Data and analyses
Comparison 1. MBP+oAB vs. MBP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 SSI | 16 | 3917 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.42, 0.74] |
| 1.2 Incisional SSI | 10 | 3054 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.33, 0.66] |
| 1.3 Organ/space SSI | 10 | 3054 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.98] |
| 1.4 Anastomotic leakage | 10 | 2356 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 0.99] |
| 1.5 Mortality | 3 | 639 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.82] |
| 1.6 Mild postoperative complications according to Clavien‐Dindo(I + II) | 3 | 695 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.29, 2.00] |
| 1.7 Severe postoperative complications according to Clavien‐Dindo (III + IV) | 3 | 695 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.59, 1.70] |
| 1.8 Incidence of postoperative ileus | 6 | 2013 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.32] |
| 1.9 Length of hospital stay | 3 | 621 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.81, 1.44] |
| 1.10 Side effects of Intervention ‐ Nausea/Vometing | 3 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.33, 3.72] |
| 1.11 Side effects of Intervention ‐ Abdominal pain | 3 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.67, 4.82] |
| 1.12 C. difficile‐related diarrhoea | 3 | 1547 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.24, 3.34] |
| 1.13 SSI_Subgroup analysis regarding surgery indication | 16 | 3915 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.42, 0.74] |
| 1.13.1 Only malignant surgical indications | 9 | 2160 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.48, 0.96] |
| 1.13.2 Only benign surgical indications | 2 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.17, 1.22] |
| 1.13.3 Both malignant and benign surgical indications | 5 | 1375 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.26, 0.71] |
| 1.14 SSI_Subgroup analysis regarding the type of surgery | 14 | 3577 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.44, 0.80] |
| 1.14.1 Colon and rectum resections | 13 | 3461 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.46, 0.83] |
| 1.14.3 Rectum resections only | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.04, 0.67] |
| 1.15 SSI_Subgroup analysis regarding the surgical approach | 13 | 3075 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.41, 0.82] |
| 1.15.1 Minimally invasive surgical approach | 3 | 1062 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.46, 1.10] |
| 1.15.2 Open surgical approach | 5 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.39, 1.40] |
| 1.15.3 Both open and minimally invasive surgical approach | 5 | 1329 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.24, 0.72] |
| 1.16 SSI_Subgroup analysis regarding the duration of mechanical bowel preparation | 15 | 3871 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.42, 0.74] |
| 1.16.1 Bowel preparation on the day before surgery | 13 | 3547 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.40, 0.77] |
| 1.16.2 Bowel preparation over several days before the operation | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 0.99] |
| 1.17 SSI_Subgroup analysis regarding the agent combination of oral antibiotics | 16 | 3915 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.42, 0.74] |
| 1.17.1 Combination of metronidazole and an aminoglycoside (neomycin or kanamycin) | 9 | 2717 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.39, 0.78] |
| 1.17.2 Combination of erythromycin and an aminoglycoside (neomycin or kanamycin) | 3 | 757 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.40, 0.94] |
| 1.17.3 Other oral antibiotic combinations | 4 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.13, 1.59] |
| 1.18 SSI_Subgroup analysis regarding the duration of intravenous antibiotic prophylaxis | 16 | 3915 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.42, 0.74] |
| 1.18.1 Only perioperative intravenous antibiotic prophylaxis | 9 | 2290 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.36, 0.72] |
| 1.18.2 Continuation of perioperative intravenous antibiotic prophylaxis for 24 hours | 3 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.19, 1.09] |
| 1.18.3 Continuation of perioperative intravenous antibiotic prophylaxis beyond 24 hours | 4 | 801 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.44, 1.30] |
Comparison 2. MBP+oAB vs. oAB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 SSI | 3 | 960 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.34, 2.21] |
| 2.2 Anastomotic leakage | 3 | 960 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.21, 3.45] |
| 2.3 Mortality | 2 | 709 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.50] |
| 2.4 Incidence of postoperative ileus | 2 | 709 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.68, 2.33] |
Comparison 3. MBP+oAB vs. nBP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 SSI | 1 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.33, 1.23] |
| 3.2 Incisional SSI | 1 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.14, 1.45] |
| 3.3 Organ/space SSI | 1 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.33, 1.78] |
| 3.4 Anastomotic leakage | 1 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.33, 2.42] |
| 3.5 Mortality | 1 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.22] |
| 3.6 Mild postoperative complications according to Clavien‐Dindo(I + II) | 1 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.93, 1.41] |
| 3.7 Severe postoperative complications according to Clavien‐Dindo(III + IV) | 1 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.80, 2.69] |
| 3.8 Incidence of postoperative ileus | 1 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.77, 1.81] |
| 3.9 Length of hospital stay | 1 | 396 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.80, 1.00] |
| 3.10 Side effects of Intervention | 1 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.44, 2.01] |
| 3.11 C. difficile‐related diarrhoea | 1 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.30] |
3.1. Analysis.

Comparison 3: MBP+oAB vs. nBP, Outcome 1: SSI
3.2. Analysis.

Comparison 3: MBP+oAB vs. nBP, Outcome 2: Incisional SSI
3.3. Analysis.

Comparison 3: MBP+oAB vs. nBP, Outcome 3: Organ/space SSI
3.4. Analysis.

Comparison 3: MBP+oAB vs. nBP, Outcome 4: Anastomotic leakage
3.5. Analysis.

Comparison 3: MBP+oAB vs. nBP, Outcome 5: Mortality
3.6. Analysis.

Comparison 3: MBP+oAB vs. nBP, Outcome 6: Mild postoperative complications according to Clavien‐Dindo(I + II)
3.7. Analysis.

Comparison 3: MBP+oAB vs. nBP, Outcome 7: Severe postoperative complications according to Clavien‐Dindo(III + IV)
3.8. Analysis.

Comparison 3: MBP+oAB vs. nBP, Outcome 8: Incidence of postoperative ileus
3.9. Analysis.

Comparison 3: MBP+oAB vs. nBP, Outcome 9: Length of hospital stay
3.10. Analysis.

Comparison 3: MBP+oAB vs. nBP, Outcome 10: Side effects of Intervention
3.11. Analysis.

Comparison 3: MBP+oAB vs. nBP, Outcome 11: C. difficile‐related diarrhoea
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anjum 2017.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomised trials Duration of trial: July 2014 to January 2016 Duration of follow‐up: "All outcomes were evaluated daily during the hospital stay, and postoperative follow‐up was conducted on postoperative days 30 and 90" Country of origin: China |
| Participants |
Baseline characteristics
Number randomised: 190 participants
Number analysed: 184 participants
Intervention: MBP+oAB
Control: MBP
Inclusion criteria 18 years of age or older and scheduled for elective colorectal surgery Exclusion criteria Patients were excluded if they had any preoperative infection or bowel obstruction, if they were undergoing emergency laparotomy, if they used antibiotics 2 weeks preoperatively, or were being treated with steroids or immunosuppressants preoperatively Baseline imbalances None |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: Two patients were excluded due protocol violations |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: This work was supported by the National Natural Science Foundation of China (grant number 81270478) Conflicts of interest: ‐ Ethics approval: The study was approved by the ethical committee of the Jinling Hospital Informed consent: All participants gave their informed consent before randomisation Clinical trials registration: ‐ Sample size calculation: "To reach a power of 80%, it was estimated that 90 patients would be required in each group to detect the difference of SSI between 18% and 6% with a type I error of 0.05. A total sample size of 95 patients was established for each arm." |
Arezzo 2021.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomised trials Duration of trial: July 2019 to June 2020 Duration of follow‐up: patients were followed for at least 30 days after surgery Country of origin: Italy |
| Participants |
Baseline characteristics
Number randomised: 204 participants
Number analysed: 204 participants
Number eligible and included in this review: 50 Only study patients who underwent preoperative mechanical bowel preparation were included in the meta‐analysis. Intervention: MBP+oAB
Control: MBP
Inclusion criteria Quote: "Patients who were scheduled for colorectal resection in participating centers for any indication (cancer, chronic diverticulitis, inflammatory bowel disease), > 18 years old and in general health condition permitting general anesthesia (ASA, American Society for Anaesthesiology classification I–III) were eligible for inclusion Open, laparoscopic, laparoscopic‐assisted, or laparoscopic converted to open were all suitable techniques, as well as any mechanical bowel preparation as indicated by each centre." Exclusion criteria Quote: "Emergency surgery; appendectomy; primarily urological/gynaecological or vascular procedure; diagnostic laparotomy/laparoscopy without intestinal resection; surgery involving multi‐visceral surgery (e.g. pelvic exenteration); contraindication for MBP; allergy to used drugs; patients who refuse to participate in the study; patients with intra‐abdominal sepsis before surgery (abscess); patients who received antibiotics for any reason within two weeks prior to surgery; patients who do not comply strictly with the assigned prophylaxis regimen; patients who cannot be followed at least four weeks after surgery." Baseline imbalances ‐ |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial ‐ Outcomes reported in trial but not used in review ‐ |
| Notes |
Source of funding: Quote: "Open access funding provided by Università degli Studi di Torino within the CRUI‐CARE Agreement. Authors have nothing to disclose. This study was supported by Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) under the programme ‘Dipartimenti di Eccellenza ex L.232/2016’ to the Department of Surgical Sciences, University of Torino" Conflicts of interest: quote: "No confict of interest or financial ties to disclose" Ethics approval: The study was approved by the local ethics committee Informed consent: Written informed consent was obtained from all patients Clinical trials registration: NCT04438655 Sample size calculation: "Statistical analysis showed that considering the closest limits of the two CI intervals (13.2 and 8.4%), with a β‐error of 0.20 and a one‐sided α‐error of 0.05, 656 patients were needed per group." |
Espin‐Basany 2005.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: ‐ Duration of follow‐up: 30 days Country of origin: Spain |
| Participants |
Baseline characteristics
Number randomised: 306 participants
Number analysed: 300 participants
Intervention 1: MBP+oAB
Intervention 2: MBP+oAB
Control: nBP
Inclusion criteria Quote: "Patients with elective colorectal resections" Exclusion criteria Quote: Pregnancy, penicillin allergy (cross reactions with cephalosporins) and contra‐in dications for a sodium phosphate (NaP) preparation (renal impairment with serum creatinine over 200 μg/l, massive ascites or severe heart failure)" Baseline imbalances "Treatment groups were similar, regarding age, sex, medical history of diabetes mellitus, hypertension or chronic obstructive pulmonary disease (COPD), preoperative serum albumin level and haematocrit, preoperative final diagnosis and operations performed" |
| Interventions |
Comparison
MBP
oAB (3x)
oAB (1x)
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review ‐ |
| Notes |
Source of funding: ‐ Conflicts of interest: ‐ Ethics approval: ‐ Informed consent: Quote: "Threehundred consecutive patients with elective colorectal resections who consented to participate in the study were included." Clinical trials registration: ‐ Sample size calculation: ‐ |
Hata 2016.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: November 2007 to December 2012 Duration of follow‐up: 30 days Country of origin: Japan |
| Participants |
Baseline characteristics
Number randomised: 584 participants
Number analysed: 579 participants
Intervention: MBP+oAB
Control: MBP
Inclusion criteria Quote:"Patients undergoing elective laparoscopic colorectal surgery for colorectal cancer or adenoma; aged 20 years or older, having good oral intake, and having adequate organ function." Exclusion criteria Quote:"Bowel obstruction, preoperative infections, antibiotic use within 2 weeks before the surgery; preoperative steroid use, neoadjuvant radiation and/or chemo therapy, uncontrolled diabetes mellitus, pregnant or lactating woman, and severe allergy" Baseline imbalances Quote:"The 2 groups were well balanced at the baseline." |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: Quote: "This study was funded by JMTO, a general incorporated association established in 1999 to support clinical trials, especially multicenter or multinational randomised controlled trials, for the diagnosis, treatment, and prevention of the diseases. The sponsor had no involvement in the design or conduct of the study." Conflicts of interest: Quote: "The authors have no conflicts of interest to declare." Ethics approval: "This study was approved by the JMTO Ethics Committee in February 2007 and also by the institutional review boards of all of the participating hospitals." Informed consent: Quote:"All patients provided written informed consent before randomization." Clinical trials registration: NCT00508690 Sample size calculation: Quote:"It was planned to enroll 566 patients during the trial design. This sample size would provide an 80% power with a 2‐sided significance level of 0.05 to demonstrate the superiority of the Oral‐IV group in the reduction of SSI rate." |
Horie 2007.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: April 2002 to December 2006 Duration of follow‐up: ‐ Country of origin: Japan |
| Participants |
Baseline characteristics
Number randomised: 91 participants
Number analysed: 91 participants Intervention: MBP+oAB
Control: MBP
Inclusion criteria Elective surgery for colorectal cancer Exclusion criteria Quote: "Any patient with a colonic obstriction, experience of abdominal operation and resection of other organs synchronously were excluded" Baseline imbalances none |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: ‐ Ethics approval: ‐ Informed consent: "Informed consent was obtained from all patients" Clinical trials registration: ‐ Sample size calculation: ‐ |
Ikeda 2016.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: June 2013 to April 2014 Duration of follow‐up: 30 days Country of origin: Japan |
| Participants |
Baseline characteristics
Number randomised: 515 participants
Number analysed: 511 participants
Number eligible and in this review included participants: 439 Only study patients who underwent preoperative mechanical bowel preparation were included in the meta‐analysis. Intervention: MBP+oAB
Control: MBP
Inclusion criteria Quote: ""All consecutive patients with colorectal cancer undergoing elective laparoscopic colorectal resection" Exclusion criteria Quote: ""Age less than 20 years; patients with bowel obstruction and who could not tolerate liquid intake; pregnancy; history of allergy to the drugs in the protocol; administration of antibiotics in the 2 weeks before surgery; severe dysfunction of liver, kidney, heart or lung; and synchronous resection of other major organs such as the stomach, liver or uterus" Baseline imbalances Quote: ""The patient characteristics of the two groups were well balanced at baseline" |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen:Quote: " "All patients except for two received the planned antimicrobial doses." |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: None Ethics approval: Quote:" "The study was conducted with the approval of the ethics committee of the Cancer InstituteHospital of the Japanese Foundation for Cancer Research, Tokyo, Japan" Informed consent: Written informed consent was obtained from all participants Clinical trials registration: Quote: ""This trial is registered with the UMIN Clinical Trials Registry (UMIN000019339)." Sample size calculation: Quote: ""Assuming a one‐sided α of 0⋅05, a power of 80 per cent and a 5 per cent incidence of overall SSI in both groups, 235 patients per group were needed (Dunnett–Gent test)7. Assuming an 8 per cent drop‐out rate, the planned required sample size was 253 patients." |
Ishida 2001.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: April 1998 to August 2000 Duration of follow‐up: Quote: ""The wounds were inspected daily until the patients were discharged from the hospital." Country of origin: Japan |
| Participants |
Baseline characteristics
Number randomised: 146 participants
Number analysed: 143 participants Intervention: MBP+oAB
Control: MBP
Inclusion criteria Quote: ""Patients with colorectal diseases, surgically treated" Exclusion criteria Quote: ""The patients in both groups were excluded if a full mechanical bowel preparation was not feasible or if they had taken any antibiotics within 14 days before surgery" Baseline imbalances none |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: ‐ Ethics approval: ‐ Informed consent: ‐ Clinical trials registration: ‐ Sample size calculation: ‐ |
Kobayashi 2007.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: May 2001 to December 2004 Duration of follow‐up: 6 weeks Country of origin: Japan |
| Participants |
Baseline characteristics
Number randomised: 491 participants
Number analysed: 484participants Intervention: MBP+oAB
Control: MBP
Inclusion criteria "At least 20 years of age and referred for elective surgery for colorectal cancer" Exclusion criteria Quote: ""Contraindication to mechanical bowel preparation, known allergy to a penicillin or cephalosporin, treatment with any antibiotic within the past 2 weeks, pregnancy, and evidence of an infection at the time of surgery moderate or severe liver disease (alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, or total bilirubin more than five times the upper limit of normal), or severe renal impairment (serum creatinine >220 µmol/l)" Baseline imbalances none |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: ‐ Ethics approval: "The study was approved by the ethics boards of the participating centers." Informed consent: "Written informed consent was required" Clinical trials registration: ‐ Sample size calculation: "On the basis of an SSI rate of 11% at the last evaluable follow‐up assessment for both treatment groups, a power of 0.80, and the requirement to show that intravenous antimicrobial prophylaxis was noninferior to oral and intravenous antimicrobial prophylaxis with a δ of 8%, a total sample size of 482 patients (241 assigned to intravenous anti microbial prophylaxis, and 241 assigned to oral and intravenous antimicrobial prophylaxis) satisfying the criteria for the intention‐to‐treat population was calculated to be required. Taking into account an estimated ineligibility rate before the start of the study of 5%, a total of about 500 patients was thus considered to be needed." |
Koskenvuo 2019.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: March 2016 to August 2018 Duration of follow‐up: Quote: ""During the hospital stay and at 1‐month clinical follow‐up visit at the outpatient clinic" Country of origin: Finland |
| Participants |
Baseline characteristics
Number randomised: 417 participants
Number analysed: 396 participants
Intervention: MBP+oAB
Control: nBP
Inclusion criteria Quote: ""Patients scheduled for colon resection in participating centres. Both benign and malignant indications were eligible, as were both laparoscopic and open procedures." Exclusion criteria Quote: ""Need for emergency surgery; bowel obstruction; colonoscopy planned to be undertaken during surgery; other indications for mechanical preparation or contraindications; allergy to drugs used in the trial (polyethylene glycol, neomycin, metronidazole); and age younger than 18 years or older than 95 years." Baseline imbalances Quote: ""Patient baseline characteristics were similar between the two groups" |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: Quote: " "Vatsatautien Tutkimussäätiö Foundation, Mary and Georg Ehrnrooth’s Foundation, and Helsinki University Hospital research funds" Conflicts of interest: "VS reports grants from Vatsatautien Tutkimussäätiö Foundation, Mary and Georg Ehrnrooth’s Foundation, and Helsinki University Hospital research funds, during the conduct of the study; grants from Finnish Surgical Society, Finska Läkaresällskapet, and Finnish Gastroenterological Society; personal fees from City of Vantaa, Finnish Gastroenterological Society, Novartis, and University of Helsinki; and non‐financial support from Astellas, outside of the submitted work. TS reports personal fees from Johnson & Johnson’s laparoscopic colorectal surgery advisory board, outside of the submitted work. All other authors declare no competing interests" Ethics approval: Quote: ""The research plan was approved by the Finnish National Committee on Medical Research Ethics and Finnish Medicines Agency. The research plan was further approved by the local ethics committee of Helsinki University Hospital and by each participating centre’s institutional review board (Helsinki University Hospital, Oulu University Hospital, Central Finland Central Hospital, and Seinäjoki Central Hospital)" Informed consent: Patients provided written informed consent Clinical trials registration: ClinicalTrials.gov (NCT02652637) and EudraCT (2015–004559–38) Sample size calculation: Quote: " "With a power of 80% and significance at 5%, 396 patients would be needed to show this difference. The sample size was adjusted for a possible 5% loss, yielding a final sample size of 415 patients" |
Lau 1988.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: May 1981 to June 1987 Duration of follow‐up: 3 months Country of origin: Hong Kong |
| Participants |
Baseline characteristics
Number randomised: 140 participants
Number analysed: 132 participants Intervention: MBP+oAB
Control: MBP
Inclusion criteria "Elective colorectal surgery for carcinoma" Exclusion criteria Quote: "Inflammatory bowel disease, an existing colostomy, an active infection, a history of sensitivity to any of the antibiotics used, a history of renal insufficiency or eighth nerve dysfunction, an obstructing colonic lesion, or a history of receiving antibiotics within the 2 weeks before operation." Baseline imbalances Quote:"The randomised groups were well matched with respect to patient characteristics and surgical procedures" |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: Quote:"3 patients were excluded due to violation of the study protocol" |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: ‐ Ethics approval: Quote: "The study was approved by the ethics boards of the participating centers" Informed consent: Quote: "After informed consent was obtained, patients were stratified into three categories" Clinical trials registration: ‐ Sample size calculation: Quote: "The initial study was projected to require 200 patients to show a statistical difference. We analysed our results statistically after inclusion of the first 202 patients" |
Lazorthes 1982.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: January 1979 to March 1980 Duration of follow‐up: 2 months Country of origin: France |
| Participants |
Baseline characteristics
Number randomised: 90 participants
Number analysed: 90 participants
Number eligible and included in this review: 60 Only a subset of the study patients was included in the meta‐analysis. Patients in study group O did not receive perioperative intravenous antibiotic prophylaxis and were therefore excluded from the meta‐analysis. Intervention: MBP+oAB
Control: MBP
Inclusion criteria Elective colorectal surgery Exclusion criteria Quote:"Patients requiring colostomy alone* or having to undergo surgery for hemorrhagic rectocolitis, as well as patients who had received antibiotics for seven days prior to surgery, were excluded from the study." Baseline imbalances None |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: ‐ Ethics approval: ‐ Informed consent: ‐ Clinical trials registration: ‐ Sample size calculation: ‐ |
Lewis 2002.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: 1992 ‐ 1995 Duration of follow‐up: Quote: "The patients were followed up by the infection control nurse on postoperative days 3, 5 to 7, 10 to 14, and at 1 month for diagnosis of surgical site infection, using the modified CDC criteria." Country of origin: Canada |
| Participants |
Baseline characteristics
Number randomised: 215 participants
Number analysed: 213 participants Intervention: MBP+oAB
Control: MBP
Inclusion criteria Quote: "All patients who underwent elective surgery of the colon at the Queen Elizabeth Hospital in Montreal were eligible to enter the study" Exclusion criteria Quote: "Patients who were allergic to the study antibiotics or who had received antibiotics within the 2 weeks before operation, pregnant patients and those who refused informed consent were excluded." Baseline imbalances Quote: "The treatment groups were evenly matched with respect to age, gender, body mass index and preoperative serum albumin level and blood lymphocyte count. There were no significant differences between the groups with respect to the preoperative final diagnoses and operations performed." |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: ‐ Ethics approval: ‐ Informed consent: Quote: "All patients gave informed consent to participate in the study" Clinical trials registration: ‐ Sample size calculation:Quote: "The sample size was calculated assuming an infection rate at the surgical site of 10% to 15%, and a treatment difference of 10% (α risk 0.05, β risk 0.20)" |
Oshima 2013.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: July 2006 to April 2009 Duration of follow‐up:Quote: "during the hospital stay, and at 4‐weeek and 3‐month postoperative" Country of origin: Japan |
| Participants |
Baseline characteristics
Number randomised: 200 participants
Number analysed: 195 participants Intervention: MBP+oAB
Control: MBP
Inclusion criteria Quote: "Patients with ulcerative colitis scheduled to undergo restorative proctocolectomy with IPAA with an open approach" Exclusion criteria Quote:"Patients were excluded if they had received antibiotics within 2 weeks before randomization, were allergic to any of the drugs used, were aged less than 18 years, had abdominal sepsis within 6 months before randomization, were pregnant or breast feeding, were being treated with steroids or had any form of chronic immunosuppression, or had obstructive symptoms. Patients were excluded after randomization if they did not receive the study drugs according to the study protocol or if they did not have ananastomosis created during surgery for any reason." Baseline imbalances none |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: Protocol violations were detected in 6 patients in group A (MBP+oAB) and 7 patients in group B (MBP without oAB) |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: ‐ Ethics approval: Quote: "The protocol was approved by institutional review board" Informed consent:Quote: "Informed consent was required from all participants" Clinical trials registration: ‐ Sample size calculation:Quote: "When the power was 80%, it was assumed that 90 patients would be required in each group to detect a difference between 18% and 6% SSI rate (favoring oral antibiotics) with a probability of a type 1 error less than 0.05. Allowing for a loss of evaluable patients, a total sample size of 100 patients in each arm was chosen." |
Papp 2021.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: November 2016 to June 2018 Duration of follow‐up: 30 days Country of origin: Hungary |
| Participants |
Baseline characteristics
Number randomised: 600 participants
Number analysed: 529 participants Intervention: MBP+oAB
Control: MBP
Inclusion criteria Quote: "All indications for colorectal anastomosis were considered eligible, including Hartmann’s reversal, with the exception of loop colostomy closure." Exclusion criteria Quote: "Patients requiring colostomy alone or having to undergo surgery for hemorrhagic rectocolitis, as well as patients who had received antibiotics for seven days prior to surgery, were excluded from the study." Baseline imbalances none |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: Quote: "The SOAP study non‐commercial" Conflicts of interest:Quote: "The authors declare no conflict of interest" Ethics approval: Quote: "Ethical approval was granted by both the Hungarian National Institute of Pharmacy and Nutrition and the Hungarian Medical Research Council." Informed consent: All patients gave informed consent Clinical trials registration: EudraCT 2015‐005614‐30 Sample size calculation: Quote: "The study power calculation was based on the international literature, with an estimated 11 per cent incidence of SSI in the OABP– group and 5 per cent in the OABPþ group. Postoperative ileus was estimated to occur in 6 percent of patients in the OABP– group and 3 percent in the OABP+ group. Using d ¼ 3 and an adjusted study power of 80 percent with a 95 percent confidence interval, it was calculated that 282 patients were required for the SSI primary endpoint and 374 for the postoperative ileus endpoint. This was rounded up to 400 patients and, after adjusting for a possible 12.5 percent loss, the final sample size was estimated to be 450 patients." |
Ram 2005.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: April 1999 to March 2002 Duration of follow‐up: Quote: "Complications were registered daily after surgery, and patients were re‐examined at the outpatient clinic 1, 3, and 6 weeks following surgery" Country of origin: Israel |
| Participants |
Baseline characteristics
Number randomised: 329 participants
Number analysed: 329 participants Intervention: MBP+oAB
Control: oAB
Inclusion criteria Quote: "Elective colorectal procedures for nonobstructive large bowel pathologic features" Exclusion criteria Quote: "Patients in both groups were excluded if they had taken antibiotics for the last 10 days before surgery or if there was evidence of infection. Patients undergoing emergency operations were not included. Patients randomised to group 2 were excluded if they had bowel preparation for colonoscopy within 6 days prior to surgery. Patients undergoing proctectomy with low rectal anastomosis or surgery for polypoid lesion were also excluded." Baseline imbalances none |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: ‐ Ethics approval: Quote:"The study was approved by the hospital ethics committee." Informed consent: All patients gave informed consent Clinical trials registration: ‐ Sample size calculation: ‐ |
Rybakov 2021.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: November 2017 to October 2018 Duration of follow‐up: 30 days Country of origin: Russia |
| Participants |
Baseline characteristics
Number randomised: 150 participants
Number analysed: 116 participants Intervention: MBP+oAB
Control: MBP
Inclusion criteria Quote: "All the patients with rectal cancer scheduled for elective rectal resection" Exclusion criteria "The presence of inflammatory process at the preoperative stage, intestinal obstruction, which contraindicate preoperative mechanical preparation, antibacterial drugs intake for 30 days before surgery, planned simultaneous operation on liver or other major organs, severe renal and/or liver failure, allergy to used antibacterial drugs" Baseline imbalances Quote: "Both groups were well matched in terms of demography and laboratory parameters, the presence of diabetes mellitus, ASA score, adjuvant chemoradiation, and surgery." |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: 33 patients excluded due to violation of the protocol (18 in MBP+oAB group an 15 in oAB group) |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: None Ethics approval: Quote:"The study protocol was approved by the local Ethics Committee" Informed consent: Informed consent was obtained from all the participants Clinical trials registration: NCT03436719 Sample size calculation: Quote: "To reach a power of 80%, it was estimated, that 176 patients would be required in each group to detect significant differences between them." |
Sadahiro 2014.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: May 2008 to October 2011 Duration of follow‐up: Four weeks Country of origin: Japan |
| Participants |
Baseline characteristics
Number randomised: 206 participants
Number analysed: 194 participants
Intervention: MBP+oAB
Control: nBP
Inclusion criteria Quote: "Patients scheduled to undergo elective colon cancer operations in whom curative resection of tumor(s) was considered feasible. The eligibility criteria were as follows: 20‐80 years of age; preoperative performance status of 0 or 1; and no serious coexisting medical conditions." Exclusion criteria Quote: "Patients with a history of intestinal resection, patients with a stoma, patients with intestinal stenosis or obstruction that would preclude routine preoperative mechanical bowel preparation, and patients with stage IV disease on preoperative diagnosis were excluded from this study." Baseline imbalances None |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: ‐ Ethics approval: Quote:"This study was approved by the Ethics Committee of Tokai University" Informed consent: Quote: "Patients provided written informed consent" Clinical trials registration: Quote:"The trial registration number is University Hospital Medical Information Network (UMIN) Clinical Trials Registry 000003435" Sample size calculation: Quote: "Assuming an SSI rate of 9% each in the oral antibiotics group and the probiotics group and an SSI rate of 30% in the control group, we calculated the number needed to treat that would have 90% power to detect differences between the oral antibiotics group and the control group and between the probiotics group and the control group by the Fisher exact test at an overall level of significance of 0.05 (two‐sided). We then calculated the number needed to treat per group at a two‐sided significance level of 0.0253 for each comparison, adjusting for multiplicity associated with multiple tests by the Dunn‐Sidak method. The number needed to treat was thus calculated to be 92 per group. To allow for possible dropouts, a sample size of 300 patients (100 patients per group) was established for this study." |
Suzuki 2020.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: August 2014 to April 2017 Duration of follow‐up: 30 days Country of origin: Japan |
| Participants |
Baseline characteristics
Number randomised: 254 participants
Number analysed: 251 participants
Intervention: MBP+oAB
Control: nBP
Inclusion criteria Quote: "Diagnosis of primary colon cancer, age of 20 to 85 years, and Eastern Cooperative Oncology Group performance status of 0 or 1." Exclusion criteria Quote: "Patients with a stoma, patients in whom conventional preoperative MBP could not be performed because of stenosis or obstruction, patients with a preoperative diagnosis of stage 4 disease, patients with an American Society of Anesthesi ologists score of ≥4, and patients who were scheduled to simultaneously undergo resection of other organs were excluded from the study" Baseline imbalances Quote: "There was no significant difference between the groups in age, sex, or hemoglobin or albumin levels in peripheral blood before surgery, the presence or absence of diabetes mel litus, American Society of Anesthesiologists score, tumor location, or histological stage. There was also no significant difference between the groups in operation time, bleeding volume, or the presence or absence of blood transfusion." |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: ‐ |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest:Quote: "The authors declare that they have no conflicts of interest to disclose" Ethics approval:Quote: "Ethical approval of Institutional Review Board of Tokai Uni versity was obtained" Informed consent: All patients provided written informed consent Clinical trials registration: ‐ Sample size calculation: Quote: "The required number of patients in each group was estimated to be 115. Given a dropout rate of 10%, the target number of patients per group was set at 127, and the total number of patients in both groups combined was set at 254" |
Takesue 2000.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: April 1997 to November 1997 Duration of follow‐up: Quote: "until discharged from the hospital" Country of origin: Japan |
| Participants |
Baseline characteristics
Number randomised: 100 participants
Number analysed: 83 participants Intervention: MBP+oAB
Control: MBP
Inclusion criteria Quote: "Elective colorectal surgery" Exclusion criteria Quote:"MBP not possible; antibiotics within least 15 days; emergency colonic obstruction" Baseline imbalances None |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: 17 excluded after randomisation (12 in MBP+oAB group an 5 in oAB group) |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: ‐ Ethics approval: ‐ Informed consent: All patients gave informed consent Clinical trials registration: ‐ Sample size calculation: ‐ |
Uchino 2019.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: March 2014 to February 2017 Duration of follow‐up: Quote: "The wounds were inspected daily by a nurse, once a week by the surveillance member, and an attending physician during the hospital stay, and at the 4‐week and 3‐month postoperative follow‐up visits." Country of origin: Japan |
| Participants |
Baseline characteristics
Number randomised: 325 participants
Number analysed: 321 participants
Number eligible and included in this review: 185 Only a subset of the study patients was included in the meta‐analysis. All patients who underwent colon or rectal surgery with intestinal anastomosis were eligible. Intervention: MBP+oAB
Control: nBP
Inclusion criteria "Patients undergoing surgery for Crohn disease." Exclusion criteria Quote: "Patients with emergent surgery, allergy to antibiotics, and antibiotic use within the 2 weeks before surgery were excluded. Moreover, patients treated with a long‐tube insertion due to bowel obstruction or surgery for an anal lesion alone were also excluded." Baseline imbalances None |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen Quote: "Protocol violations were detected in 2 patients in group A and 3 patients in group B, and the remaining 320 patients were included in the PP analysis." |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review ‐ |
| Notes |
Source of funding: ‐ Conflicts of interest: Quote: "The authors report no conflicts of interest." Ethics approval: Quote:"All study protocols were approved by the Institutional Review Board of the Hyogo College of Medicine (No. 1679)." Informed consent: Quote: "Informed consent was required from all participants" Clinical trials registration:Quote: "The study protocols were registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR 000013369)." Sample size calculation: Quote: "It was previously reported that the incidence of SSI in CD surgery without oral antibiotic prophylaxis was approximately 25%. When the power was set to 80%, it was assumed that 149 patients would be required in each group to detect a difference between an SSI rate of 12.5% and 25% (favoring oral antibiotic prophylaxis) with a probability of a type 1 error <0.05. To allow for the potential loss of evaluable patients, a total sample size of 160 patients in each arm was chosen." |
Zmora 2003.
| Study characteristics | |
| Methods |
Study design: Parallel‐group randomised trials Duration of trial: July 1997 to July 2000 Duration of follow‐up: 30 days Country of origin: Israel |
| Participants |
Baseline characteristics
Number randomised: 415 participants
Number analysed: 380 participants Intervention: MBP+oAB
Control: oAB
Inclusion criteria Quote: "Patients undergoing elective colon and rectal surgery with primary anastomosis" Exclusion criteria Quote: "Patients with tumors smaller than 2 cm were excluded from the study, as palpation of small tumors may be difficult in an unprepared bowel, and these patients may require intraoperative colonoscopy to identify these lesions. Patients who required a diverting stoma proximal to the anastomosis and those who were found to have an abdominal abscess at the time of surgery were also excluded from the data analysis." Baseline imbalances Quote:"Demographic characteristics, indications for surgery, and type of surgery did not significantly differ between the two groups." |
| Interventions |
Comparison
MBP
oAB
Perioperative intravenous antibiotic prophylaxis
Adherence to regimen: 193 Patient excluded since they did not have MBP |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: ‐ Conflicts of interest: ‐ Ethics approval: Quote:"The study was approved by the Institutional Review Board (Helsinki committee)" Informed consent: Quote:Patients gave their informed consent before randomization." Clinical trials registration: ‐ Sample size calculation: ‐ |
D/C: Clavien‐Dindo classification; IV: intravenous; MBP: mechanical bowel preparation; MBP+oAB: combined mechanical and oral antibiotic bowel preparation; nMB: no bowel preparation; oAB: oral antibiotics as bowel preparation; PEG: polyethylene glycol; SD: standard deviation; SSI: surgical site infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alverdy 2019 | Wrong study design |
| Anthony 2011 | Wrong intervention |
| Barker 1971 | No perioperative intravenous antibiotic prophylaxis |
| Champault 1981 | No perioperative intravenous antibiotic prophylaxis |
| Clarke 1977 | No perioperative intravenous antibiotic prophylaxis |
| COLONPREP | Wrong study design |
| Condon 1979 | Wrong intervention |
| Contant 2007 | No perioperative intravenous antibiotic prophylaxis |
| Coppa 1983 | Wrong intervention |
| Eisenberg 1981 | Wrong intervention |
| Emir 2012 | Wrong intervention |
| Flückiger 1980 | Wrong intervention |
| Güenaga 2011 | Wrong study design |
| Hjalmarsson 2015 | Wrong intervention |
| Ishibashi 2009 | Wrong intervention |
| Jagelman 1985 | Wrong intervention |
| Kolovrat 2012 | Wrong intervention |
| Mehdorn 2021 | Wrong study design |
| Mendes Da Costa 1977 | No perioperative intravenous antibiotic prophylaxis |
| Mulder 2020 | Wrong intervention |
| Playforth 1988 | Wrong intervention |
| Reddy 2007 | No perioperative intravenous antibiotic prophylaxis |
| Schardey 2020 | Wrong intervention |
| Tajima 2016 | Wrong intervention |
| Takesue 2009 | Wrong publication type (congress poster) |
| Taylor 1994 | Wrong patient population |
| Thalheimer 2008 | Wrong publication type (invited commentary) |
| Vadhwana 2020 | Wrong study design |
| Wolff 1988 | No perioperative intravenous antibiotic prophylaxis |
| Yabata 1997 | Wrong intervention |
Characteristics of studies awaiting classification [ordered by study ID]
Abis 2019.
| Methods |
Study design: randomised clinical trial Duration of trial: May 2013 to March 2017 Duration of follow‐up: follow‐up was done at least twice a year in the first 2 years after surgery and then yearly according the Dutch guidelines on colorectal cancer Country of origin: the Netherlands |
| Participants |
Baseline characteristics
Number randomised: 485
Number analysed: 565 Number of eligible participants that could be included in our meta‐analysis: 316 Only study patients who underwent preoperative mechanical bowel preparation (left‐sided colonic, sigmoid and low anterior resections) can be included in our meta‐analysis. A request to the study authors to provide us with the primary data for the inclusion of eligible patients from the study has not yet been fulfilled. |
| Interventions |
Comparison
Mechanical bowel preparation Was given for left‐sided colectomies, sigmoid and anterior resections. Oral antibiotics Oral colistin, tobramycin and amphotericin B were administered to patients in the SDD group to decontaminate the digestive tract. Perioperative intravenous antibiotic prophylaxis Both treatment and control group received intravenous cefazolin and metronidazole for perioperative prophylaxis. |
| Outcomes |
Outcomes sought in review and reported in trial
Outcomes sought but not reported in trial
Outcomes reported in trial but not used in review
|
| Notes |
Source of funding: this study was funded by the DutchDigestive Foundation, Spaarne Gasthuis Academy Fund. The funder had no role in study design, data collection, data analysis, data interpretation or writing of the report. Conflicts of interest: the authors declare no conflict of interest Ethics approval: the ethics board at the VU University Medical Centre and the institutional review board at each participating centre approved the study. Informed consent: all patients provided written informed consent. Clinical trials registration: the trial was registered with ClinicalTrials.gov (number NCT01740947). Sample size calculation: considering a 9 per cent anastomotic leakage rate in the control group, based on numbers of the Dutch Surgical Colorectal Audit at onset of the trial, and an estimated 4 percent in the intervention group, 381 patients needed to be included per treatment arm (total of 762 patients). |
SDD: selective decontamination of the digestive tract; SSI: surgical site infections
Characteristics of ongoing studies [ordered by study ID]
COMBINE.
| Study name | Intravenous Versus Combined Oral and Intravenous Antimicrobial Prophylaxis for the Prevention of Surgical Site Infection in Elective Colorectal Surgery |
| Methods |
Study type: interventional Study design
|
| Participants |
Condition or disease: elective colorectal surgery Inclusion criteria
Exclusion criteria
Target sample size: ‐ Actual Enrolment: 920 |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome:
Secondary outcome:
|
| Starting date |
Date of registration: November 5, 2015 Study Start Date: May 25, 2016 Actual Study Completion Date: June 30, 2020 Recruitment Status: Completed |
| Contact information |
Contact person: Professor Emmanuel Futier Affiliation: University Hospital, Clermont‐Ferrand Country of origin: France |
| Notes |
Ethics approval: COMBINE trial has been approved by an independent ethics committee for all study centres Source of funding: COMBINE trial is supported by funding from French Ministry of Health (Programme Hospitalier de Recherché Clinique (PHRC) National 2014) and from the University Hospital of Clermont‐Ferrand |
CTRI/2018/07/014938.
| Study name | Role of combined oral antibiotic and mechanical bowel preparation in reducing incidence of surgical site infections in comparison to only mechanical bowel preparation in patients undergoing elective resection for rectal cancer at a tertiary care centre in India: A Randomized Control Trial |
| Methods |
Study type: interventional Study design
|
| Participants |
Condition or disease: patients with rectal cancer Inclusion criteria
Exclusion criteria Patients with rectal cancer who are not eligible for mechanical bowel preparation/ where bowel preparation is contraindicated such as Crohn’s disease, obstructed bowel and renal or cardiac impairment.Also patients with known drug allergy to the medications used in the trial. Target sample size: 118 |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date |
Date of registration: 18/07/2018 Study Start Date: 25/04/2018 Study Completion Date: ‐ Recruitment Status: open to recruitment |
| Contact information |
Contact person: Mark Ranjan Jesudason Affiliation: Christian Medical College, Vellore Country of origin: India |
| Notes |
Ethics approval: Silver, Research and Ethics Committee, Christian Medical College, Vellore Source of funding: Fluid RFesearch Grant, Christian Medical College, Vellore |
EUCTR2019‐002002‐43‐ES.
| Study name | Phase IV, unicentric, randomized and open study to confirm the decrease of the incidence of the surgical site infection after elective right hemicolectomy with anterographic mechanical preparation associated with oral and intravenous antibiotic therapy versus oral and intravenous antibiotic and versus only intravenous antibiotic |
| Methods |
Study type: interventional clinical trial of medicinal product Study design
|
| Participants |
Condition or disease: patients undergoing right hemicolectomy Inclusion criteria
Exclusion criteria
Target sample size: 108 |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcomes
Secondary outcomes
Time point(s) of evaluation the end point(s): for 30 days after surgery |
| Starting date |
Date of registration: 21/10/2019 Study Start Date: 16/12/2019 Study Completion Date: ‐ Recruitment Status: authorised‐recruitment may be ongoing or finished |
| Contact information |
Contact person: P. Millan (Unidad de Investigación Clínica) Affiliation: Fundación Instituto de Investigación Sanitaria Aragón Country of origin: Spain |
| Notes |
Ethics approval: Favourable Ethics Committee Opinion of the trial application (2019‐12‐12) Source of funding: Fundación Instituto de Investigación Sanitaria Aragón |
KCT0004822.
| Study name | Infectious surgical site Complications after Oral antibiotic Bowel preparation for minimally‐invasive Rectal cAncer surgery (COBRA) – multicenter prospective randomized controlled trial |
| Methods |
Study type: interventional study Study design
|
| Participants |
Condition or disease: neoplasms Inclusion criteria
Exclusion criteria
Target sample size: 438 |
| Interventions |
Treatment arms
Mechanical bowel preparation
Oral antibiotics
Perioperative intravenous antibiotic prophylaxis
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date |
Date of registration: 12.03.2020 Estimated Study Start Date: ‐ Estimated Study Completion Date: ‐ Recruitment status: recruiting |
| Contact information |
Contact person: Ji Woong Bae Affiliation: Korea University Ansan Hospital Country of origin: Korea |
| Notes |
Ethics approval: Approval date: 05/03/2020 Source of funding: Korea University |
MECCLANT –C and –R Trials.
| Study name | Preparation with Mechanical Bowel Cleansing or/and Oral Antibiotics or Nothing for Elective Colorectal Surgery: Two‐Two‐Arm Multicentre Randomised Controlled Studies (MECCLANT –C and –R Trials) |
| Methods |
Study type: interventional Study design
|
| Participants |
Condition or disease
Inclusion criteria
Exclusion criteria
Target sample size: 356 |
| Interventions |
Treatment arms
Mechanical bowel preparation
Oral antibiotic prophylaxis
Perioperative intravenous antibiotic prophylaxis
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date |
Date of registration: ‐ Study Start Date: ‐ Estimated Primary Completion Date: ‐ Recruitment Status: Open/recruiting |
| Contact information |
Contact person: Nikolaos Gouvas Affiliation: Acute Hospitals, Worcester, UK Country of origin: Greece |
| Notes |
Ethics approval: ‐ Source of funding: ‐ |
MOBILE2.
| Study name | Mechanical Bowel Preparation and Oral Antibiotics Versus Mechanical Bowel Preparation Only Prior Rectal Surgery (MOBILE2) |
| Methods |
Study type: interventional (Clinical Trial) Study design
|
| Participants |
Condition or disease: Rectal Adenocarcinoma, Rectum Neoplasm, Rectum Carcinoma, Colorectal Cancer, Colorectal Neoplasms, Colorectal Carcinoma, Surgical Site Infection, Surgery‐‐Complications Inclusion criteria
Exclusion criteria
Target sample size: 604 |
| Interventions |
Treatment arms
Mechanical bowel preparation
Oral antibiotic prophylaxis
Perioperative intravenous antibiotic prophylaxis
|
| Outcomes |
Primary outcome
Secondary outcomes
Tertiary outcomes (long‐term follow‐up)
|
| Starting date |
Date of registration: February 24, 2020 Actual Study Start Date: March 18, 2020 Estimated Primary Completion Date: March 2022 Recruitment Status: Recruiting (Last Update Posted: June 3, 2021) |
| Contact information |
Contact person: Laura Koskenvuo Affiliation: Helsinki University Hospital Country of origin: Finland |
| Notes |
Ethics approval: The research plan has been evaluated by the Finnish National Committee on Medical Research Ethics (TUKIJA) and Finnish Medicines Agency (FIMEA) has been notified. The EUDRA CT number for the clinical drug trials has been applied (No 2018‐004355‐20). The research plan was further approved by the local ethics committee of Helsinki University Hospital and in each participating centres’ institutional review board (Helsinki University Hospital, Tampere University Hospital and Turku University Hospital) Source of funding: Cancer Society of Finland |
NCT03042091.
| Study name | Neomycin and Metronidazole Hydrochloride With or Without Polyethylene Glycol in Reducing Infection in Patients Undergoing Elective Colorectal Surgery |
| Methods |
Study type: interventional Study design
|
| Participants |
Condition or disease: Colorectal Neoplasms, Diverticulitis, Inflammatory Bowel Diseases, Surgical Site Infection Inclusion criteria
Exclusion criteria
Target sample size: 224 |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date |
Date of registration: February 3, 2017 Actual Study Start Date: September 2016 Estimated Primary Completion Date: October 2020 (Last Update Posted: August 22, 2018) Recruitment Status: Unknown |
| Contact information |
Contact person: Benjamin Phillips Affiliation: Thomas Jefferson University Country of origin: USA |
| Notes |
Ethics approval: ‐ Source of funding: ‐ |
NCT03491540.
| Study name | Mechanical Bowel Preparation and Oral Antibiotics Before Rectal Cancer Surgery (PREPACOL2) |
| Methods |
Study type: ‐ Study design:
|
| Participants |
Condition or disease: rectal cancer surgery Inclusion criteria
Exclusion criteria
Target sample size: 400 |
| Interventions |
Treatment arms
Mechanical bowel preparation
Oral antibiotics
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date |
Date of registration: April 9, 2018 ActualStudy Start Date: September 3, 2018 Estimated Primary Completion Date: May 31, 2023 Recruitment Status: Recruiting |
| Contact information |
Contact person: Yves Panis and Massimo Giacca Affiliation: Service de chirurgie Colorectale/Hôpital Beaujon Country of origin: France |
| Notes |
Ethics approval: ‐ Source of funding: Assistance Publique ‐ Hôpitaux de Paris |
NCT03563586.
| Study name | Mechanical Bowel Preparation With or Without Oral Antibiotics for Colorectal Cancer Surgery (MECCA) |
| Methods |
Study type: interventional (Clinical Trial) Study design
|
| Participants |
Condition or disease: Antibiotic, Bowel Cancer, Colorectal Cancer, Surgical Site Infection Inclusion criteria
Exclusion criteria
Target sample size: 105 |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date |
Date of registration: June 9, 2018 Study Start Date: April 1, 2018 Estimated Primary Completion Date: April 1, 2021 Recruitment Status: Recruiting (Last Update Posted: September 11, 2020) |
| Contact information |
Contact person: George Theodoropoulos Affiliation: National and Kapodistrian University of Athens Country of origin: Greece |
| Notes |
Ethics approval: ‐ Source of funding: National and Kapodistrian University of Athens |
NCT03856671.
| Study name | Prophylactic Effect Preoperative Antibiotics With Mechanical Bowel Preparation in SSIs |
| Methods |
Study type: interventional Study design
|
| Participants |
Condition or disease: Surgical Site Infection, Postoperative Complications, Bowel Preparation, Oral Antibiotics, Colorectal Cancer Inclusion criteria
Exclusion criteria
Target sample size: 360 |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date |
Date of registration: February 27, 2019 Actual Study Start Date: January 17, 2019 Estimated Primary Completion Date: June 30, 2021 (Last Update Posted: February 5, 2020) Recruitment Status: Recruiting (Last Update Posted: February 5, 2020) |
| Contact information |
Contact person: Purun Lei Affiliation: The Third Affiliated Hospital of Sun Yat‐Sen university Country of origin: China |
| Notes |
Ethics approval: ‐ Source of funding: ‐ |
NCT04931173.
| Study name | Mechanical Bowel Prep Randomized Study |
| Methods |
Study type: interventional Study design
|
| Participants |
Condition or disease: Colorectal surgery Inclusion criteria
Exclusion criteria
Target sample size: 1062 |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date |
Date of registration: June 18, 2021 EstimatedStudy Start Date: April 2022 Estimated Primary Completion Date: December 2025 Recruitment Status: not yet recruiting |
| Contact information |
Contact person: Erin Kennedy Affiliation: Division of General Surgery, Mount Sinai Hospital, Canada Country of origin: Canada |
| Notes |
Ethics approval: ‐ Source of funding: ‐ |
ORALEV2.
| Study name | Preoperative Oral Antibiotics With vs Without Mechanical Bowel Preparation to Reduce Surgical Site Infections Following Colonic Resection: an International Randomized Controlled Trial. (ORALEV2) |
| Methods |
Study type: Interventional Study design
|
| Participants |
Condition or disease: Wounds and Injuries, Surgery‐‐Complications Inclusion criteria
Exclusion criteria
Target sample size: 968 |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date |
Date of registration: November 13, 2019 EstimatedStudy Start date: September 14, 2021 Estimated Primary Completion Date: May 2023 Recruitment Status: Not yet recruiting (Last Update Posted: August 31, 2021) |
| Contact information |
Contact person: Eloy Espín‐Basany Affiliation: Colorectal Surgery Unit, Hospital Vall d’Hebron, Country of origin: Spain |
| Notes |
Ethics approval: The study has been approved by the Ethics Committee of the Coordinating Centre and by the Spanish Agency of Medicines and Medical Devices (Agencia Española de Medicamentos y Productos Sanitarios, AEMPS) Source of funding: This study is funded by the Instituto de Salud Carlos III of the Spanish Ministry of Science and Innovation through grant PI20/00622 |
Panaiotti 2020.
| Study name | Mechanical Bowel Preparation with Oral Antibiotics Vs No Preparation Before Elective Colon Resection for Colon Cancer |
| Methods |
Study type: Study design: randomised controlled trial |
| Participants |
Condition or disease: elective colon resection Inclusion criteria
Exclusion criteria
Target sample size: 712 |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date |
Date of registration: ‐ EstimatedStudy Start date: ‐ Estimated Primary Completion Date: ‐ Recruitment Status: ‐ |
| Contact information |
Contact person: Aleksandra Olkina Affiliation: Surgical department of abdominal oncology, Saint Petersburg Country of origin: Russia |
| Notes |
Ethics approval: ‐ Source of funding: ‐ |
REaCT‐NSQIP.
| Study name | An RCT Protocol Using the REaCT and NSQIP Platforms to Compare Oral Antibiotics and No Mechanical Bowel Preparation for Surgical Site Infection in Colon Surgery |
| Methods |
Study type: ‐ Study design: multi‐centre randomised controlled trail |
| Participants |
Condition or disease: elective colon surgery Inclusion criteria ‐ Exclusion criteria ‐ Target sample size: ‐ |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date |
Date of registration: ‐ ActualStudy Start Date: ‐ Estimated Primary Completion Date: ‐ Recruitment Status: ‐ |
| Contact information |
Contact person: S.S. Apte Affiliation: The Ottawa Hospital, Ottawa Country of origin: Canada |
| Notes |
Ethics approval: ‐ Source of funding: ‐ |
Tagliaferri 2020.
| Study name | Combined mechanical and oral antibiotic bowel preparation versus oral antibiotics alone for the reduction of surgical site infection following elective colorectal resection: Interim analysis |
| Methods |
Study type: ‐ Study design: randomised controlled trial |
| Participants |
Condition or disease: Elective colon resection Inclusion criteria All patients over 18 years of age undergoing elective colon resections were included in the study Exclusion criteria ‐ Target sample size: ‐ |
| Interventions |
Treatment arms
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date |
Date of registration: ‐ Study Start Date: ‐ Estimated Primary Completion Date: ‐ Recruitment Status: ‐ |
| Contact information |
Contact person: A. R. Tagliaferri Affiliation: ‐ Country of origin: USA |
| Notes |
Ethics approval: ‐ Source of funding: ‐ |
BMI: body mass index; IV: intravenous; ECOG: Eastern Cooperative Oncology Group; IBD: irritable bowel disease; MRI: magnetic resonance imaging; RCT: randomised controlled trial; SSI: surgical site infections
Differences between protocol and review
Ms Sophia Soltau (SS) was included as an additional author and carried out the data extraction and risk of bias assessment together with MW instead of TV. Together with IT, TV took on the role of the third reviewer and was consulted in case of disagreements between MW and SS. The reasons for this were, firstly, to involve SS in the project so that she could learn how to conduct a systematic review and, secondly, to add another person to the project resources to ensure that the schedule was adhered to.
The planned subdivision of SSI into superficial, deep and organ/space infections had to be adjusted because most studies that made a subdivision only distinguished between incisional and organ/space SSI. For those studies that reported superficial SSI and deep SSI separately, these data were combined into incisional SSI for inclusion in the meta‐analysis.
The secondary outcome "treatment‐related adverse events", including its sub‐items, was replaced by the two outcomes "side effect of the intervention" and "C. Difficile‐related diarrhoea". Adverse events of the intervention were mainly reported as nausea/vomiting or abdominal pain. Information on dehydration, electrolyte imbalance, renal failure or cardiac dysfunction due to MBP, or the number of participants who discontinued the intervention due to side effects and the number of participants for whom therapy was initiated to treat the complications, were not reported in any of the included studies. Furthermore, the identified studies did not distinguish between MBP or oAB as a trigger for the side effect of the intervention.
The planned subgroup analyses also had to be adjusted based on the data available to us. For example, the planned subdivision into (extended) right‐sided versus (extended) left‐sided versus rectal resections was not possible. A meaningful subdivision into colon versus rectal resections could also not be made, as most studies reported a mixed collective (Analysis 1.14). The comparison of operations with restoration of bowel continuity (primary anastomosis) with or without creation of a protective stoma, could also not be performed due to lack of data.
For the primary endpoint anastomotic leakage, no subgroup analysis was performed for the comparison MBP+oAB versus MBP as no statistical heterogeneity was found (Chi² = 9.84, df = 9 (P = 0.36); I² = 9%; Analysis 1.4).
The sensitivity analyses were also conducted differently than intended in the protocol. As no study with a high risk of bias was identified, an analysis with only those studies that were found to have a low risk of bias was conducted as a sensitivity analysis for the first comparison. The planned sensitivity analysis with exclusion of all studies that had > 20% missing data was not performed, as only one study (Rybakov 2021) analysed less than 80% of randomised patients, and the reasons for this are well explained. The missing data is not due to loss to follow‐up, but partly due to the fact that no surgery was performed, inoperability was given, or no anastomosis was done, so that the patients no longer fulfilled the inclusion criteria of the study.
The MID for SSI and anastomotic leakage given in the protocol had to be revised because the derivation of the given values led to conflicting estimates of clinical relefance. The premise that an intervention must lead to an absolute risk reduction of 5% to achieve a clinically important difference was retained. From the formula RRR = 100% x (1‐ RR) it can be deduced that we achieve an absolute risk reduction of 5% with a risk reduction of less than or equal to 0.95, so this value was reported as the new MID for both SSIs and anastomotic leaks.
Contributions of authors
Maria A Willis (MW): Conception of the review, search strategy development, acquisition of trial reports, trial selection, data extraction, risk of bias assessment, data analysis, data interpretation, review drafting.
Ingrid Toews (IT): Methodological advice and revision of the review.
Sophia LV Soltau (SS): Data extraction and risk of bias assessment.
Joerg C Kalff (JK): Clinical advice on the conception of the review and revision of the review.
Joerg J Meerpohl (JM): Data interpretation, final revision of the review, in particular with regard to the methodology.
Tim O Vilz (TV): Conception of the review, trial selection, revision of the review.
All authors have read and approved the final manuscript and declare that they are accountable for all aspects of the review.
Sources of support
Internal sources
-
Department of General, Visceral, Thorax and Vascular Surgery, University Hospital Bonn, Bonn, Germany, Germany
Salary (TV); Salary (MW before and after the nine‐month leave to work full time on the review)
-
Institute for Evidence in Medicine, Freiburg, Germany
Salary (IT andJM)
External sources
-
German Cancer Aid, Germany
Scientific grant for the development of a guideline on 'Perioperative Management of Gastrointestinal Tumours (POMGAT)' including salary payment for MW during a nine‐month leave of absence from clinical practice to complete the review in collaboration with Cochrane Germany and funding for a student assistant (SS) to support the guideline project.
Declarations of interest
MW: Surgical resident at the University Hospital Bonn. This review is intended as a partial aspect of the evidence workup for a new guideline on perioperative medicine for gastrointestinal tumours. This guideline is being developed with the AWMF (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V.) and the German Guideline Program in Oncology, and is funded by the German Cancer Aid (see Sources of support).
IT: None.
SS: None.
JK: None.
JM: None.
TV: Scientific grant from the German Cancer Aid to create a guideline for 'Perioperative Management of Gastrointestinal Tumours (POMGAT)' (see Sources of support); publications in the field of perioperative medicine; head of the Colorectal Surgery and Proctology section at the University Hospital Bonn.
New
References
References to studies included in this review
Anjum 2017 {published data only}
- Anjum N, Ren J, Wang G, Li G, Wu X, Dong H, et al. A randomized control trial of preoperative oral antibiotics as adjunct therapy to systemic antibiotics for preventing surgical site infection in clean contaminated, contaminated, and dirty type of colorectal surgeries. Diseases of the Colon and Rectum 2017;60(12):1291–8. [DOI: 10.1097/DCR.0000000000000927] [PMID: ] [DOI] [PubMed] [Google Scholar]
Arezzo 2021 {published and unpublished data}
- Arezzo A, Mistrangelo M, Bonino MA, Salusso P, Forcignano E, Vettoretto N, et al. Oral neomycin and bacitracin are effective in preventing surgical site infections in elective colorectal surgery: a multicentre, randomized, parallel, single-blinded trial (COLORAL-1). Updates in Surgery 2021;73(5):1775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arezzo A. Primary data of the study [personal communication]. Email to: M Morino and A Arezzo 13 January 2022.
- NCT04438655. Prospective randomized clinical trial on oral and intravenous antibiotic prophylaxis in colorectal surgery (COLORAL1). clinicaltrials.gov/ct2/show/NCT04438655 (first received 19 June 2020).
Espin‐Basany 2005 {published data only}
- Espin-Basany E, Sanchez-Garcia JL, Lopez-Cano M, Lozoya-Trujillo R, Medarde-Ferrer M, Armadans-Gil L, et al. Prospective, randomised study on antibiotic prophylaxis in colorectal surgery. Is it really necessary to use oral antibiotics? International Journal of Colorectal Disease 2005;20(6):542–6. [DOI: 10.1007/s00384-004-0736-8] [DOI] [PubMed] [Google Scholar]
Hata 2016 {published data only}
- Goto S, Hasegawa S, Hata H, Yamaguchi T, Hida K, Nishitai R, et al. Differences in surgical site infection between laparoscopic colon and rectal surgeries: sub-analysis of a multicenter randomized controlled trial (Japan-multinational trial organization PREV 07-01). International Journal of Colorectal Disease 2016;31(11):1775–84. [DOI: 10.1007/s00384-016-2643-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hata H, Yamaguchi T, Hasegawa S, Nomura A, Hida K, Nishitai R, et al. Oral and parenteral versus parenteral antibiotic prophylaxis in elective laparoscopic colorectal surgery (JMTO PREV 07-01) a phase 3, multicenter, open-label, randomized trial. Annals of Surgery 2016;263(6):1085–91. [DOI] [PubMed] [Google Scholar]
- NCT00508690. Trial of antibiotic prophylaxis in elective laparoscopic colorectal surgery: oral and systemic versus systemic antibiotics. clinicaltrials.gov/ct2/show/NCT00508690 (first received 30 July 2007).
Horie 2007 {published data only}
- Horie T. Randomized controlled trial on the necessity of chemical cleaning as preoperative preparation for colorectal cancer surgery. Dokkyo Journal of Medical Sciences 2007;34(3):205-12. [Google Scholar]
Ikeda 2016 {published and unpublished data}
- Ikeda A, Konishi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, et al. Randomized clinical trial of oral and intravenous versus intravenous antibiotic prophylaxis for laparoscopic colorectal resection. British Journal of Surgery 2016;103(12):1608–15. [DOI] [PubMed] [Google Scholar]
- Konishi T. Primary data of the study [personal communication]. Email to: T Konishi 12 January 2022.
Ishida 2001 {published data only}
- Ishida H, Yokoyama M, Nakada H, Inokuma S, Hashimoto D. Impact of oral antimicrobial prophylaxis on surgical site infection and methicillin-resistantstaphylococcus aureus infection after elective colorectal surgery. Results of a prospective randomized trial. Surgery Today 2001;31(11):979–83. [DOI] [PubMed] [Google Scholar]
Kobayashi 2007 {published data only}
- Kobayashi M, Mohri Y, Tonouchi H, Miki C, Nakai K, Kusunoki M. Randomized clinical trial comparing intravenous antimicrobial prophylaxis alone with oral and intravenous antimicrobial prophylaxis for the prevention of a surgical site infection in colorectal cancer surgery. Surgery Today 2007;37(5):383–8. [DOI] [PubMed] [Google Scholar]
Koskenvuo 2019 {published data only}
- EudraCT 2015–004559–38. Bowel preparation before bowel resection. clinicaltrialsregister.eu/ctr-search/search?query=2015-004559-38 (first received 11 December 2015).
- Koskenvuo L, Lehtonen T, Koskensalo S, Rasilainen S, Klintrup K, Ehrlich A, et al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet 2019;394(10201):840–8. [DOI] [PubMed] [Google Scholar]
- NCT02652637. MOBILE Trial. Mechanical and oral antibiotic bowel preparation versus no bowel preparatIon for elective colectomy - a multicenter, prospective, randomized, controlled trial. clinicaltrials.gov/ct2/show/NCT02652637 (first received 12 January 2016).
Lau 1988 {published data only}
- Lau WY, Chu KW, Poon GP, Ho KK. Prophylactic antibiotics in elective colorectal surgery. British Journal of Surgery 1988;75(8):782–5. [DOI: 10.1002/bjs.1800750819] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lazorthes 1982 {published data only}
- Lazorthes F, Legrand G, Monrozies X, Fretigny E, Pugnet G, Cordova JA, et al. Comparison between oral and systemic antibiotics and their combined use for the prevention of complications in colorectal surgery. Diseases of the Colon & Rectum 1982;25(4):309-11. [DOI] [PubMed] [Google Scholar]
Lewis 2002 {published data only}
- Lewis RT. Oral versus systemic antibiotic prophylaxis in elective colon surgery: a randomized study and meta-analysis send a message from the 1990s. Canadian Journal of Surgery 2002;45(3):173–80. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Oshima 2013 {published data only}
- Oshima T, Takesue Y, Ikeuchi H, Matsuoka H, Nakajima K, Uchino M, et al. Preoperative oral antibiotics and intravenous antimicrobial prophylaxis reduce the incidence of surgical site infections in patients with ulcerative colitis undergoing IPAA. Diseases of the Colon and Rectum 2013;56(10):1149–55. [DOI] [PubMed] [Google Scholar]
Papp 2021 {published data only}
- EudraCT 2015-005614-30. SOAP antibiotic prophylaxis trial systemic versus combined systemic and oral antibiotic prophylaxis in elective colorectal surgery. clinicaltrialsregister.eu/ctr-search/search?query=2015-005614-30 (first received 07 July 2016).
- Papp G, Saftics G, Szabo BE, BaracsJ, Vereczkei A, Kollar D, et al. Systemic versus oral and systemic antibiotic prophylaxis (SOAP) study in colorectal surgery: prospective randomized multicentre trial. British Journal of Surgery 2021;108(3):271–6. [DOI] [PubMed] [Google Scholar]
Ram 2005 {published data only (unpublished sought but not used)}
- Ram E, Sherman Y, Weil R, Vishne T, Kravarusic D, Dreznik Z. Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Archives of Surgery 2005;140(3):285–8. [DOI] [PubMed] [Google Scholar]
Rybakov 2021 {published data only}
- NCT03436719. Antibiotic prophylaxis in rectal cancer surgery: oral with intravenous versus intravenous antibiotics. clinicaltrials.gov/ct2/show/NCT03436719 (first received 19 February 2018).
- Rybakov E, Nagudov M, Sukhina M, Shelygin Y. Impact of oral antibiotic prophylaxis on surgical site infection after rectal surgery: results of randomized trial. International Journal of Colorectal Disease 2021;36(2):323–30. [DOI: 10.1007/s00384-020-03746-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sadahiro 2014 {published data only}
- Sadahiro S, Suzuki T, Tanaka A, Okada K, Kamata H, Ozaki T, et al. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: prospective randomized trial. Surgery (United States) 2014;155(3):493-503. [DOI] [PubMed] [Google Scholar]
Suzuki 2020 {published data only}
- Suzuki T, Sadahiro S, Tanaka A, Okada K, Saito G, Miyakita H, et al. Usefulness of preoperative mechanical bowel preparation in patients with colon cancer who undergo elective surgery: a prospective randomized trial using oral antibiotics. Digestive Surgery 2020;37(3):192-8. [DOI] [PubMed] [Google Scholar]
Takesue 2000 {published data only}
- Takesue Y, Yokoyama T, Akagi S, Ohge H, Murakami Y, Sakashita Y, et al. A brief course of colon preparation with oral antibiotics. Surgery Today 2000;30(2):112-6. [DOI] [PubMed] [Google Scholar]
Uchino 2019 {published and unpublished data}
- Uchino M, Ikeuchi H, Bando T, Chohno T, Sasaki H, Horio Y, et al. Efficacy of pre-operative oral antibiotic prophylaxis for the prevention of wound infections in patients with crohn's disease. Annals of Surgery 2019;269(3):420–6. [DOI: 10.1097/SLA.0000000000002567] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Uchino M. Primary data of the study [personal communication]. Email to: M Uchino 14 January 2022.
Zmora 2003 {published data only}
- Zmora O, Mahajna A, Bar-Zakai B, Rosin D, Hershko D, Shabtai M, et al. Colon and rectal surgery without mechanical bowel preparation: a randomized prospective trial. Annals of Surgery 2003;237(3):363–7. [DOI: 10.1097/01.SLA.0000055222.90581.59] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alverdy 2019 {published data only}
- Alverdy JC, Shogan D. Preparing the bowel for surgery: rethinking the strategy. Nature Reviews Gastroenterology & Hepatology 2019;16(12):708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anthony 2011 {published data only}
- Anthony T, Murray BW, Sum-Ping JT, Lenkovsky F, Vornik VD, Parker BJ, et al. Evaluating an evidence-based bundle for preventing surgical site infection: a randomized trial. Archives of Surgery 2011;146(3):263-9. [DOI] [PubMed] [Google Scholar]
- Dallas VA Medical Center. Minimization of surgical site infections for pts undergoing colorectal surgery. ClinicalTrials.gov. [CLINICALTRIALS.GOV: NCT00953784]
Barker 1971 {published data only}
- Barker K, Graham NG, Mason MC, De Dombal FT, Goligher JC. The relative significance of preoperative oral antibiotics, mechanical bowel preparation, and preoperative peritoneal contamination in the avoidance of sepsis after radical surgery for ulcerative colitis and Crohn's disease of the large bowel. British Journal of Surgery 1971;58(4):270-3. [PubMed] [Google Scholar]
Champault 1981 {published data only}
- Champault G, Adloff M, Alexandre JH, Arnaud JP, Boutelier P, Chevrel JP, et al. Pre-operative preparation of the colon: a controlled prospective multicentre study in 215 patients (author's transl). Journal de Chirurgie 1981;118(11):677-84. [PubMed] [Google Scholar]
Clarke 1977 {published data only}
- Clarke JS, Condon RE, Bartlett JG, Gorbach SL, Nichols RL, Ochi S. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double-blind clinical study. Annals of Surgery 1977;186(3):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
COLONPREP {published data only}
- Assistance Publique - Hôpitaux de Paris. Mechanical bowel preparation and oral antibiotics before colon cancer surgery (COLONPREP). clinicaltrials.gov. [CLINICALTRIALS.GOV: NCT03475680]
Condon 1979 {published data only}
- Condon RE, Bartlett JG, Nichols RL, Schulte WJ, Gorbach SL, Ochi S. Preoperative prophylactic cephalothin fails to control septic complications of colorectal operations: results of controlled clinical trial: a Veterans Administration cooperative study. American Journal of Surgery 1979;137(1):68-74. [DOI] [PubMed] [Google Scholar]
Contant 2007 {published data only}
- Contant CM, Hop WC, van't Sant HP, Oostvogel HJ, Smeets HJ, Stassen LP, et al. Mechanical bowel preparation for elective colorectal surgery: a multicentre randomised trial. Lancet 2007;370(9605):2112-7. [DOI] [PubMed] [Google Scholar]
Coppa 1983 {published data only}
- Coppa GF, Eng K, Gouge TH, Ranson JH, Localio SA. Parenteral and oral antibiotics in elective colon and rectal surgery: a prospective, randomized trial. American Journal of Surgery 1983;145(1):62-5. [DOI] [PubMed] [Google Scholar]
Eisenberg 1981 {published data only}
- Eisenberg Henry W. Cefamandole preparation for colonic surgery. Diseases of the Colon & Rectum 1981;24(8):610-2. [DOI] [PubMed] [Google Scholar]
Emir 2012 {published data only}
- Emir S, Kavlakoglu B, Sozen S, Yazar MF, Ozkan Z. Mechanical bowel preparation before elective colorectal surgery: is it necessary? A prospective, randomized and controlled study. Turkish Journal of Surgery 2012;28(2):84. [Google Scholar]
Flückiger 1980 {published data only}
- Flückiger M, Berger J, Casey P, Aeberhard P. Antibiotic preparation of the colon or preoperative parenteral prophylaxis in colon surgery. A randomized study. Helvetica Chirurgica Acta 1980;47(5):659-62. [PubMed] [Google Scholar]
Güenaga 2011 {published data only}
- Güenaga KF, Matos D, Wille‐Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD001544. [DOI: 10.1002/14651858.CD001544.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hjalmarsson 2015 {published data only}
- Hjalmarsson C, Karlberg J, Törnqvist P, Arbman G, Frisk B, Modin M, et al. Orally administered trimethoprim-sulfamethoxazole and metronidazole as infection prophylaxis in elective colorectal surgery. Surgical Infections 2015;16(5):604-10. [DOI] [PubMed] [Google Scholar]
Ishibashi 2009 {published data only}
- Ishibashi K, Kuwabara K, Ishiguro T, Ohsawa T, Okada N, Miyazaki T, et al. Short-term intravenous antimicrobial prophylaxis in combination with preoperative oral antibiotics on surgical site infection and methicillin-resistant staphylococcus aureus infection in elective colon cancer surgery: results of a prospective randomized trial. Surgery Today 2009;39(12):1032-9. [DOI] [PubMed] [Google Scholar]
Jagelman 1985 {published data only}
- Jagelman DG, Fazio VW, Lavery IC, Weakley FL. A prospective, randomized, double-blind study of 10% mannitol mechanical bowel preparation combined with oral neomycin and short-term, perioperative, intravenous flagyl as prophylaxis in elective colorectal resections.. Surgery 1985;98(5):861-5. [PubMed] [Google Scholar]
Kolovrat 2012 {published data only}
- Kolovrat M, Busic Z, Lovric Z, Amic F, Cavka V, Boras Z, et al. Mechanical bowel preparation in colorectal surgery. Collegium antropologicum 2012;36(4):1343-6. [PubMed] [Google Scholar]
Mehdorn 2021 {published data only}
- Mehdorn M, Lübbert C, Chaberny IF, Gockel I, Jansen-Winkeln B. Mechanical plus oral bowel preparation with paromomycine and metronidazole reduces infectious complications in elective colorectal surgery: a matched case-control study. International Journal of Colorectal Disease 2021;36(9):1839-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendes Da Costa 1977 {published data only}
- Mendes Da Costa P, Klastersky J, Gerard A. Controlled study of oral administration of antibiotics in the preparation of digestive surgery [Etude controlee de l'administration orale d'antibiotiques comme preparation a la chirurgie digestive]. Acta chirurgica Belgica (Ed. bilingue) 1977;76(5):475-80. [PubMed] [Google Scholar]
Mulder 2020 {published and unpublished data}
- Mulder T, Kluytmans-van den Bergh M, Vlaminckx B, Roos D, Smet AM, Vos Tot Nederveen Cappel R, et al. Prevention of severe infectious complications after colorectal surgery using oral non-absorbable antimicrobial prophylaxis: results of a multicenter randomized placebo-controlled clinical trial. Antimicrobial Resistance and Infection Control 2020;9(1):84-95. [DOI: 10.1186/s13756-020-00745-2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Playforth 1988 {published data only}
- Playforth MJ, Smith GM, Evans M, Pollock AV. Antimicrobial bowel preparation. Diseases of the Colon & Rectum 1988;31(2):90-3. [DOI] [PubMed] [Google Scholar]
Reddy 2007 {published data only}
- Reddy BS, Macfie J, Gatt M, Larsen CN, Jensen SS, Leser TD. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Journal of British Surgery 2007;94(5):546-54. [DOI] [PubMed] [Google Scholar]
Schardey 2020 {published data only}
- Schardey HM, Wirth U, Strauss T, Kasparek MS, Schneider D, Jauch KW. Prevention of anastomotic leak in rectal cancer surgery with local antibiotic decontamination: a prospective, randomized, double-blind, placebo-controlled single center trial. International Journal of Colorectal Disease 2020;35(5):847-57. [DOI] [PubMed] [Google Scholar]
Tajima 2016 {published data only}
- Tajima Y, Ishida H, Yamamoto A, Chika N, Onozawa H, Matsuzawa T, et al. Comparison of the risk of surgical site infection and feasibility of surgery between sennoside versus polyethylene glycol as a mechanical bowel preparation of elective colon cancer surgery: a randomized controlled trial. Surgery Today 2016;46(6):735-40. [DOI] [PubMed] [Google Scholar]
Takesue 2009 {published data only}
- Takesue Y, Hirata A, Kobayashi M, Yamagishi D, Matsuoka H, Tanaka K. Bowel preparation with oral antibiotics for the surgery in patients with ulcerative colitis (UC) and colorectal cancer (CRC): prospective, randomized study. Surgical Infections 2009;10:223-4. [Google Scholar]
Taylor 1994 {published data only}
- Taylor TW, Lindsay G. Selective decontamination of the colon before elective colorectal surgery. World Journal of Surgery 1994;18(6):926-31. [DOI] [PubMed] [Google Scholar]
Thalheimer 2008 {published data only}
- Thalheimer A, Germer CT. Mechanical bowel preparation before colorectal surgery? Digestive Surgery 2008;25(5):328. [PubMed] [Google Scholar]
Vadhwana 2020 {published data only}
- Vadhwana B, Pouzi A, Surjus Kaneta G, Reid V, Claxton D, Pyne L, et al. Preoperative oral antibiotic bowel preparation in elective resectional colorectal surgery reduces rates of surgical site infections: a single-centre experience with a cost-effectiveness analysis. Annals of The Royal College of Surgeons of England 2020;102(2):133-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolff 1988 {published data only}
- Wolff BG, Beart RW, Dozois RR, Pemberton JH, Zinsmeister AR, Ready RL, et al. A new bowel preparation for elective colon and rectal surgery: a prospective, randomized clinical trial. Archives of Surgery 1988;123(7):895-900. [DOI] [PubMed] [Google Scholar]
Yabata 1997 {published data only}
- Yabata E, Okabe S, Endo M. A prospective, randomized clinical trial of preoperative bowel preparation for elective colorectal surgery-comparison among oral, systemic, and intraoperative luminal antibacterial preparations. Journal of Medical and Dental Sciences 1997;44(4):75-80. [PubMed] [Google Scholar]
References to studies awaiting assessment
Abis 2019 {published and unpublished data}
- Abis GS, Stockmann HB, Bonjer HJ, Veenendaal N, Doorn-Schepens ML, Budding AE, et al. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). British Journal of Surgery 2019;106(4):355–63. [DOI: 10.1002/bjs.11117] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
COMBINE {unpublished data only}2015‐002559‐84
- Vignaud M, Paugam-Burtz C, Garot M, Jaber S, Slim K, Panis Y, et al. Comparison of intravenous versus combined oral and intravenous antimicrobial prophylaxis (COMBINE) for the prevention of surgical site infection in elective colorectal surgery: study protocol for a multicentre, double-blind, randomised controlled clinical trial. BMJ Open 2018;8(4):e020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTRI/2018/07/014938 {published data only}CTRI/2018/07/014938
- CTRI/2018/07/014938. Role of oral antibiotics in reducing the chance of infection after operations for cancer of the rectum [Role of combined oral antibiotic and mechanical bowel preparation in reducing incidence of surgical site infections in comparison to only mechanical bowel preparation in patients undergoing elective resection for rectal cancer at a tertiary care centre in India: a randomized control trial]. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=25532 (first received 18 July 2018).
EUCTR2019‐002002‐43‐ES {published data only}EUCTR2019‐002002‐43‐ES
- Unidad de Investigación Clínica. Phase IV, unicentric, randomized and open study to confirm the decrease of the incidence of the surgical site infection after elective right hemicolectomy with anterographic mechanical preparation associated with oral and intravenous antibiotic therapy versus oral and intravenous antibiotic and versus only intravenous antibiotic. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2019-002002-43-ES (first received 21 October 2019).
KCT0004822 {published data only}KCT0004822
- KCT0004822. Infectious surgical site complications after oral antibiotic bowel preparation for minimally-invasive rectal cancer surgery [Infectious surgical site Complications after Oral antibiotic Bowel preparation for minimally-invasive Rectal cAncer surgery (COBRA) – multicenter prospective randomized controlled trial]. trialsearch.who.int/?TrialID=KCT0004822 (first received 12 March 2020).
MECCLANT –C and –R Trials {published data only}2016‐000254‐352016‐001404‐32
- Xynos E, Gouvas N, Agalianos C, Balogiannis I, Christodoulakis M, Korkolis D, et al. Preparation with mechanical bowel cleansing or/and oral antibiotics or nothing for elective colorectal surgery: two-two-arm multicentre randomised controlled studies (MECCLANT –C and –R trials). Journal of Clinical Trials 2017;7(2):1233-46. [DOI: ] [Google Scholar]
MOBILE2 {published data only}
- Koskenvuo L, Lunkka P, Varpe P, Hyöty M, Satokari R, Haapamäki C, et al. Mechanical bowel preparation and oral antibiotics versus mechanical bowel preparation only prior rectal surgery (MOBILE2): a multicentre, double-blinded, randomised controlled trial-study protocol. BMJ Open 2021;11(7):e051269. [DOI: 10.1136/bmjopen-2021-051269] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03042091 {published data only}
- NCT03042091. Neomycin and metronidazole hydrochloride with or without polyethylene glycol in reducing infection in patients undergoing elective colorectal surgery. clinicaltrials.gov/ct2/show/record/NCT03042091 (first received 3 February 2017).
NCT03491540 {unpublished data only}
- NCT03491540. Mechanical bowel preparation and oral antibiotics before rectal cancer surgery (PREPACOL2). clinicaltrials.gov/ct2/show/record/NCT03491540 (first received 4 October 2021).
NCT03563586 {unpublished data only}
- NCT03563586. Mechanical bowel preparation with or without oral antibiotics for colorectal cancer surgery (MECCA). clinicaltrials.gov/ct2/show/study/NCT03563586 (first received 20 June 2018).
NCT03856671 {published data only}
- NCT03856671. Prophylatic effect preoperative antibiotics with mechanical bowel preparation in SSIs. clinicaltrials.gov/ct2/show/study/NCT03856671 (first received 27 February 2019).
NCT04931173 {published data only}
- NCT04931173. Mechanical bowel prep randomized study. clinicaltrials.gov/ct2/show/study/NCT04931173 (first received 23 July 2021).
ORALEV2 {published data only}
- Pellino G, Solís-Peña A, Kraft M, Huguet BM, Espín-Basany E. Preoperative oral antibiotics with versus without mechanical bowel preparation to reduce surgical site infections following colonic resection: Protocol for an international randomized controlled trial (ORALEV2). Colorectal Disease: the official Journal of the Association of Coloproctology of Great Britain and Ireland 2021;23(8):2173–81. [DOI: 10.1111/codi.15681] [DOI] [PubMed] [Google Scholar]
Panaiotti 2020 {published data only}
- Panaiotti L, Olkina A, Petrov A, Lankov T, Karachun A. Mechanical bowel preparation with oral antibiotics vs no preparation before elective colon resection for colon cancer. European Journal of Surgical Oncology 2020;46:e92. [DOI: 10.1016/j.ejso.2019.11.223] [DOI] [Google Scholar]
REaCT‐NSQIP {unpublished data only}
- Apte SS, Moloo H, Jeong A, Liu M, Vandemeer L, Suh K, et al. Prospective randomised controlled trial using the rethinking clinical trials (REaCT) platform and national surgical quality improvement program (NSQIP) to compare no preparation versus preoperative oral antibiotics alone for surgical site infection rates in elective colon surgery: a protocol. BMJ Open 2020;10(7):e036866. [DOI: 10.1136/bmjopen-2020-036866] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tagliaferri 2020 {published and unpublished data}
- Tagliaferri AR, Kucejko R, Costanzo CM, Phillips B. Combined mechanical and oral antibiotic bowel preparation versus oral antibiotics alone for the reduction of surgical site infection following elective colorectal resection: Interim analysis. Diseases of the Colon and Rectum 2020;63(6):383. [Google Scholar]
Additional references
Anjum 2017
- Anjum N, Ren J, Wang G, Li G, Wu X, Dong H, et al. A randomized control trial of preoperative oral antibiotics as adjunct therapy to systemic antibiotics for preventing surgical site infection in clean contaminated, contaminated, and dirty type of colorectal surgeries. Diseases of the Colon & Rectum 2017;12:1291–8. [DOI: 10.1097/DCR.0000000000000927] [DOI] [PubMed] [Google Scholar]
Beck 2020
- Beck C, Weber K, Brunner M, Agaimy A, Semrau S, Grützmann R, et al. The influence of postoperative complications on long-term prognosis in patients with colorectal carcinoma. International Journal of Colorectal Disease 2020;6:1055–66. [DOI: 10.1007/s00384-020-03557-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buia 2019
- Buia A, Post S, Buhr HJ, Hanisch E. Bowel preparation for elective colorectal surgery in Germany 2017: results of a survey among members of the German Society of General and Visceral Surgery [Darmvorbereitung bei elektiven kolorektalen Resektionen in Deutschland 2017: Ergebnisse einer Umfrage unter den Mitgliedern der DGAV]. Der Chirurg 2019;90(7):564–9. [DOI: 10.1007/s00104-018-0773-4] [DOI] [PubMed] [Google Scholar]
Clavien 2009
- Clavien PA, Barkun J, Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Annals of Surgery 2009;250(2):187-96. [DOI: 10.1097/SLA.0b013e3181b13ca2] [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Devane 2017
- Devane LA, Proud D, O'Connell PR, Panis Y. A European survey of bowel preparation in colorectal surgery. Colorectal Disease 2017;19(11):402-6. [DOI: 10.1111/codi.13905] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 20 December 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2021. Available at gradepro.org.
Gustafsson 2019
- Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World Journal of Surgery 2019;43(3):659-95. [DOI] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
ISOS 2016
- International Surgical Outcomes Study group. Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. British Journal of Anaesthesia 2016;117(5):601–9. [DOI: 10.1093/bja/aew316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klinger 2019
- Klinger AL, Green H, Monlezun DJ, Beck D, Kann B, Vargas HD, et al. The role of bowel preparation in colorectal surgery: results of the 2012-2015 ACS-NSQIP data. Annals of Surgery 2019;269(4):671–7. [DOI: 10.1097/SLA.0000000000002568] [DOI] [PubMed] [Google Scholar]
Kuhry 2008
- Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD003432. [DOI: 10.1002/14651858.CD003432.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kulu 2015
- Kulu Y, Büchler MW, Ulrich A. Perioperative complications of the lower gastrointestinal tract. Prevention, recognition and treatment [Perioperative Komplikationen des unteren Gastrointestinaltraktes]. Der Chirurg 2015;86(4):311-8. [DOI: 10.1007/s00104-014-2848-1] [DOI] [PubMed] [Google Scholar]
Kumar 2013
- Kumar AS, Kelleher DC, Sigle GW. Bowel preparation before elective surgery. Clinics in Colon and Rectal Surgery 2013;26(3):146–52. [DOI: 10.1055/s-0033-1351129] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ling 2019
- Ling ML, Apisarnthanarak A, Abbas A, Morikane K, Lee KY, Warrier A, et al. APSIC guidelines for the prevention of surgical site infections. Antimicrobial Resistance & Infection Control 2019;8:174. [DOI: 10.1186/s13756-019-0638-8] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lynch 2016
- Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. New England Journal of Medicine 2016;375(24):2369–79. [DOI: 10.1056/NEJMra1600266] [DOI] [PubMed] [Google Scholar]
Markell 2010
- Markell KW, Hunt BM, Charron PD, Kratz RJ, Nelson J, Isler JT, et al. Prophylaxis and management of wound infections after elective colorectal surgery: a survey of the American Society of Colon and Rectal Surgeons membership. Journal of Gastrointestinal Surgery 2010;14(7):1090–8. [DOI: 10.1007/s11605-010-1218-7] [DOI] [PubMed] [Google Scholar]
McSorley 2018
- McSorley ST, Steele CW, McMahon AJ. Meta-analysis of oral antibiotics, in combination with preoperative intravenous antibiotics and mechanical bowel preparation the day before surgery, compared with intravenous antibiotics and mechanical bowel preparation alone to reduce surgical-site infections in elective colorectal surgery. BJS Open 2018;2(4):185–94. [DOI: 10.1002/bjs5.68] [DOI] [PMC free article] [PubMed] [Google Scholar]
Migaly 2019
- Migaly J, Bafford AC, Francone TD, Gaertner WB, Eskicioglu C, Bordeianou L, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Use of Bowel Preparation in Elective Colon and Rectal Surgery. Diseases of the Colon & Rectum 2019;62(1):3-8. [DOI: 10.1097/DCR.0000000000001238] [DOI] [PubMed] [Google Scholar]
Nachiappan 2016
- Nachiappan S, Askari A, Mamidanna R, Munasinghe A, Currie A, Stebbing J, et al. Initiation of adjuvant chemotherapy within 8 weeks of elective colorectal resection improves overall survival regardless of reoperation. Colorectal Disease 2016;18(11):1041–9. [DOI: 10.1111/codi.13308] [DOI] [PubMed] [Google Scholar]
Nelson 2014
- Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD001181. [DOI: 10.1002/14651858.CD001181.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 2020
- Nelson RL, Hassan M, Grant MD. Antibiotic prophylaxis in colorectal surgery: are oral, intravenous or both best and is mechanical bowel preparation necessary? Techniques in Coloproctology 2020;24(12):1233–46. [DOI: 10.1007/s10151-020-02301-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment. www.nice.org.uk/guidance/ng125 (accessed 20 December 2021). [ISBN: 978-1-4731-3394-5]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
RevMan Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Rollins 2019
- Rollins KE, Javanmard-Emamghissi H, Acheson AG, Lobo DN. The role of oral antibiotic preparation in elective colorectal surgery: a meta-analysis. Annals of Surgery 2019;270(1):43–58. [DOI: 10.1097/SLA.0000000000003145] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schardey 2017
- Schardey HM, Rogers S, Schopf SK, Ahnen T, Wirth U. Are gut bacteria associated with the development of anastomotic leaks? [Sind Darmbakterien an der Entstehung der Anastomoseninsuffizienz beteiligt?: Eine Übersicht über experimentelle und klinische Arbeiten]. Coloproctology 2017;39:94–100. [DOI: 10.1007/s00053-016-0136-x] [DOI] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Shogan 2015
- Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel C, et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contributes to intestinal anastomotic leak. Science Translational Medicine 2015;7(286):286ra68. [DOI: 10.1126/scitranslmed.3010658] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solla 1990
- Solla JA, Rothenberger DA. Preoperative bowel preparation. A survey of colon and rectal surgeons. Diseases of the Colon & Rectum 1990;33(2):154–9. [DOI: 10.1007/BF02055549] [PMID: ] [DOI] [PubMed] [Google Scholar]
Spanjersberg 2011
- Spanjersberg WR, Reurings J, Keus F, Laarhoven C. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No: CD007635. [DOI: 10.1002/14651858.CD007635.pub2] [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:4898. [DOI: 10.1136/bmj.l4898] [PMID: ] [DOI] [PubMed] [Google Scholar]
Toh 2018
- Toh JW, Phan K, Hitos K, Pathma-Nathan N, El-Khoury T, Richardson AJ, et al. Association of mechanical bowel preparation and oral antibiotics before elective colorectal surgery with surgical site infection: a network meta-analysis. JAMA Network Open 2018;1(6):e183226. [DOI: 10.1001/jamanetworkopen.2018.3226] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2004
- Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, et al. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Annals of Surgery 2004;240(2):255–9. [DOI: 10.1097/01.sla.0000133186.81222.08] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weidenhagen 2007
- Weidenhagen R, Spelsberg F, Strauss T, Jauch K-W. Anastomotic dehiscence in colorectal surgery [Anastomoseninsuffizienz in der kolorektalen Chirurgie]. Viszeralchirurgie 2007;42:165–72. [DOI: 10.1055/s-2007-960720] [DOI] [Google Scholar]
WHO 2018
- World Health Organization. Global guidelines for the prevention of surgical site infection. www.ncbi.nlm.nih.gov/books/NBK536404/ (accessed 20 December 2021).
Young 2012
- Young H, Knepper B, Moore EE, Johnson JL, Mehler P, Price CS. Surgical site infection after colon surgery: National Healthcare Safety Network risk factors and modeled rates compared with published risk factors and rates. Journal of the American College of Surgeons 2012;214(5):852–9. [DOI: 10.1016/j.jamcollsurg.2012.01.041] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Willis 2022
- Willis MA, Toews I, Meerpohl JJ, Vilz TO. Preoperative combined mechanical and oral antibiotic bowel preparation for preventing complications in elective colorectal surgery. Cochrane Database of Systematic Reviews 2022, Issue 1. Art. No: CD014909. [DOI: 10.1002/14651858.CD014909] [DOI] [PMC free article] [PubMed] [Google Scholar]


