Abstract
Sodium-glucose cotransporter-2 inhibitors (SGLT2is) and glucagon-like peptide-1 receptor agonists (GLP1-RAs) reduce cardiovascular events and mortality in patients with type 2 diabetes mellitus (T2DM). We sought to describe trends in prescribing for SGLT2is and GLP1-RAs in diverse care settings, including (1) the outpatient clinics of a midwestern integrated health system and (2) small- and medium-sized community-based primary care practices and health centers in 3 midwestern states. We included adults with T2DM and ≥1 outpatient clinic visit. The outcomes of interest were annual active prescription rates for SGLT2is and GLP1-RAs (separately). In the integrated health system, 22,672 patients met the case definition of T2DM. From 2013 to 2019, the overall prescription rate for SGLT2is increased from 1% to 15% (absolute difference [AD] 14%, 95% confidence interval [CI] 13% to 15%, p <0.01). The GLP1-RA prescription rate was stable at 10% (AD 0%, 95% CI −1% to 1%, p = 0.9). In community-based primary care practices, 43,340 patients met the case definition of T2DM. From 2013 to 2017, the SGLT2i prescription rate increased from 3% to 7% (AD 4%, 95% CI 3% to 6%, p <0.01), whereas the GLP1-RA prescription rate was stable at 2% to 3% (AD 1%, 95% CI −1 to 1%, p = 0.40). In a fully adjusted regression model, non-Hispanic Black patients had lower odds of SGLT2i or GLP1-RA prescription (odds ratio 0.56, 95% CI 0.34 to 0.89, p = 0.016). In conclusion, the increase in prescription rates was greater for SGLT2is than for GLP1-RAs in patients with T2DM in a large integrated medical center and community primary care practices. Overall, prescription rates for eligible patients were low, and racial disparities were observed.
Type 2 diabetes mellitus (T2DM) affects >32 million people in the United States and is associated with an increased risk of cardiovascular disease (CVD) morbidity and mortality.1–4 Intensive glucose control improves microvascular disease but not macrovascular outcomes (such as CVD). In emerging clinical trial evidence, however, newer therapies—namely, sodium-glucose cotransporter-2 inhibitors (SGLT2is) and glucagon-like peptide-1 receptor agonists (GLP1-RAs)—have demonstrated significant reductions in major adverse cardiovascular events and mortality in patients with T2DM.5–11 Despite consensus recommendations from clinical organizations, delays in the uptake of newer therapies are often observed in the clinical setting.12,13 In an effort to inform strategies to enhance implementation practices, we sought to describe trends in prescribing patterns for SGLT2i and GLP1-RA medication classes in an integrated health system and in 226 small- to medium-sized primary care practices in the Midwest. We also aimed to identify racial/ethnic differences in prescription rates for these therapies and to determine whether such disparities may be explained by demographic, clinical, or socioeconomic factors.
Methods
We identified patients with T2DM from 2 settings. The first group of patients received ambulatory care from Northwestern Medicine (NM)—a regional, integrated health system located in Chicago, Illinois and its surrounding suburbs. The second group included patients who received care from any of the 226 small- or medium-sized primary care practices and community health centers in Illinois, Indiana, and Wisconsin that participated in the federally funded Healthy Hearts in the Heartland (H3) research study.14,15
We queried the NM Enterprise Data Warehouse, using a previously validated computable phenotype, to identify patients with T2DM who presented for ≥1 outpatient clinic visit to primary care (general internal or family medicine), cardiology, endocrinology, or nephrology from January 1, 2013 to December 31, 2019.16,17 To be included in the study, patients had to have (1) ≥1 clinic visit with a code (International Classification of Diseases [ICD]-9 or ICD-10) for T2DM and a prescription for an antihyperglycemic agent (Supplementary Table 1) listed within 90 days before or after the visit, (2) a coded T2DM diagnosis with an outpatient glycosylated hemoglobin A1c (HbA1c) value ≥6.5% within 90 days before or after the diagnosis date, and/or (3) any antihyperglycemic medication prescribed within 90 days of an outpatient HbA1c value ≥6.5%. Given our focused patient population, we excluded patients aged <18 years or with a diagnos of type 1 diabetes, gestational diabetes, medication-induced diabetes or hyperglycemia, chronic kidney disease (CKD) stage 5, and end-stage renal disease.17
We further defined a high-risk subgroup with an expanded inclusion profile to identify patients who met specific criteria listed in the American Diabetes Association (ADA) and American College of Cardiology (ACC) consensus recommendations for initiation of SGLT2i or GLP1-RA treatment. Criteria for patient inclusion in this subgroup were (1) pre-existing CVD, documented before or within 6 months of a qualifying clinic visit, (2) HbA1c ≥7.0% within 6 months of a qualifying clinic visit, and (3) a concurrent prescription for metformin. CVD was defined by ICD-9 and ICD-10 codes (based on previous studies), and included a history of (1) ischemic heart disease, (2) coronary revascularization, (3) stroke, (4) peripheral artery disease, or (5) heart failure (HF).18–20 The ICD codes used for each condition are listed in Supplementary Table 2.
Using the DeGAUSS (Decentralized Geomarker Assessment for Multi-Site Studies),21 we linked patient-level street addresses to census block geocodes. We then linked census block geocodes to 2018 national percentile rankings in the Area Deprivation Index, a measure of neighborhood disadvantage based on 17 education, employment, housing-quality, and poverty measures from the 2014 to 2018 American Community Survey.22,23 Each census block was binned in the 1% range according to level of disadvantage relative to other blocks across the United States, with 0 representing the lowest level of disadvantage and 100 representing the highest level of disadvantage.
The H3 study (registered at ClinicalTrials.gov [NCT02598284]) is part of EvidenceNOW, a research network that is funded by the Agency for Healthcare Research and Quality and aims to improve care quality in small- and medium-sized primary care practices.14,15 The study design and trial results have been published; briefly, H3 was a practice-level, randomized, comparative effectiveness study in 226 primary care practices to evaluate the effects of different quality improvement strategies to enhance care.14 A practice was eligible if it (1) was located in Wisconsin, Illinois, or Indiana, (2) was an adult primary care-focused facility, and (3) had ≤20 active primary care clinicians.14 For this study, we retrospectively reviewed individual patient data collected from practices that submitted electronic health records data consistently each year from January 1, 2013 to December 31, 2017 (2017 was the final full year of the H3 study). Inclusion was limited to adults who presented for a clinic visit with a coded (ICD-9 or ICD-10) diagnosis for T2DM. Exclusion criteria were the same as for the integrated health system patients.
We identified all SGLT2i (canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin) and GLP1-RA (exenatide, lixisenatide, albiglutide, dulaglutide, liraglutide, and semaglutide) prescriptions, and their combinations, for patients who met eligibility criteria. We also collected data on antihypertensives, antihyperglycemics, diuretics, antiplatelets, and statins. To be defined as having a prescription, a patient was required to have a medication prescription within 90 days before or after the date of any clinic visit. In the integrated health system, a prescription was considered first-time if it was the initial prescription for an SGLT2i or a GLP1-RA in the NM Enterprise Data Warehouse.
Co-morbidities were listed if documented ICD-9 or ICD-10 codes were recorded within 6 months before or after a qualifying clinic visit.17 To increase CKD specificity, we used a previously reported method that defined a patient with CKD as having (1) an ICD-9 or ICD-10 code for CKD and (2) ≥2 estimated glomerular filtration rate measures (using the Modification of Diet in Renal Disease equation) of <60 ml/min/1.73 m2 >90 days apart during the study period.24
For each data source, patient demographics and characteristics were summarized as mean ± SD and number (percentage) of total patients. The primary analysis involved calculation of annual active prescription rates and trends for SGLT2i and GLP1-RA medications (separately) in the integrated health system and community primary care practices. Absolute differences in yearly prescription rates were calculated across the study period; a z test for proportions or Fisher’s Exact test, as appropriate, was used to determine whether the absolute differences were statistically significant. The Cochran-Armitage test for trend was used to assess stagnant versus increasing prescriptions over the analyzed period for each setting. To better ascertain usage rates among groups with varying levels of guideline indications, subgroup analyses were performed for patients in the integrated health system to assess yearly active medication rates by patient demographics, clinical characteristics, and co-morbidities. Race and ethnicity data were reported for non-Hispanic (NH) White, NH Black, and Hispanic patients. Additional investigation included a proportional breakdown, by year, of the prescribing practitioner specialty for all first-time SGLT2i and GLP1-RA medication prescriptions. Given limited data, subgroup and first-time prescriber analyses could not be completed with data from the community clinics. All statistical tests were 2-sided; statistical significance was assessed at p <0.05 and was clinically contextualized by the magnitude of the point estimates.
To further investigate racial disparities in the high-risk cohort among patients with ≥1 eligible encounter in 2019, we used multivariable logistic regression with logit link function to test the association of race/ethnicity with the prescription of SGLT2i and GLP1-RA medications. Models were sequentially adjusted for potential confounders, including (1) demographics (age, gender), (2) co-morbidities (history of CKD, history of atherosclerotic CVD, history of HF) and care team composition (seen by primary care only, seen by a specialist only, seen by both primary and specialist), and (3) markers of socioeconomic status (insurance type, Area Deprivation Index). For sensitivity analyses, we separated the prescription of SGLT2i and GLP1-RA medications into separate models and performed the same sequential adjustment. Missing values for the Area Deprivation Index (6% of the sample) were imputed using predictive mean matching. Analyses were performed using R 3.5.4 and Python 3.8.5 software.
Results
We identified 22,672 patients in the integrated health system from January 1, 2013 to December 31, 2019 who met our case definition. Aggregate data for the group is listed in Table 1. Overall, the mean age was 65 ± 13 years; 47% were female, 62% were NH White, 12% were NH Black, and 11% were Hispanic. Co-morbidities included CKD (29%), ischemic heart disease (24%), HF (13%), ischemic stroke (6%), and peripheral arterial disease (9%). The mean HbA1c level was 8 ± 1%, the mean body mass index was 33 ± 7 kg/m2, and 9% were current smokers. Prevalence of other medications included statins (82%), aspirin (69%), insulin (77%), metformin (83%), thiazolidinediones (18%), sulfonylureas (47%), dipeptidyl peptidase-4 inhibitors (16%), and any class of antihypertensives (74%).
Table 1.
Characteristics of diabetes patients from both an integrated health system (2013–2019) and community primary care clinics (2013–2017).
| Variable | Integrated health system clinics (n = 22,672) | Community primary care practices* (n = 43,340) |
|---|---|---|
|
| ||
| Mean ± SD age at beginning of study period (years) | 65 ± 13 | 63 ± 15 |
| Women | 10,575 (47%) | 23,051 (53%) |
| Race/ethnicity | ||
| Non-Hispanic White | 14,004 (62%) | 10,013 (69%) |
| Non-Hispanic Black | 2,730 (12%) | 1,733 (12%) |
| Hispanic | 2,494 (11%) | 386 (3%) |
| Non-Hispanic Asian | 1,332 (6%) | 1,011 (7%) |
| Other | 1,019 (4%) | 558 (4%) |
| Missing | 1,093 (5%) | 28,802 (66%) |
| Insurance | ||
| Private | 9,522 (42%) | NA |
| Medicare | 9,320 (41%) | NA |
| Medicaid | 1,331 (6%) | NA |
| Self-Pay | 1,176 (5%) | NA |
| Other | 1,322 (6%) | NA |
| Area Deprivation Index National Ranking (0–100) | ||
| Median | 38.84 | NA |
| 25th percentile | 23 | NA |
| 50th percentile | 36 | NA |
| 75th percentile | 53 | NA |
| Smoking status† | ||
| Current | 1,988 (9%) | 5,625 (13%) |
| Former | 7,462 (33%) | NA |
| Never | 12,777 (56%) | |
| Unknown | 355 (2%) | 37,715 (87%) |
| Blood Pressure (mm Hg)‡ | ||
| Systolic | 130 ± 15 | 131 ± 15 |
| Diastolic | 75 ± 9 | 78 ± 9 |
| BMI (kg/m2)‡ | 33 ± 7 | NA |
| Ejection Fraction (%)‡,§ | 58 ± 12 | NA |
| Glycated hemoglobin (%)‡ | 7.5 ± 1.4 | 10.2 ± 2.0 |
| Cholesterol (mg/dl)‡ | ||
| Total (mg/dl) | 163 ± 39 | 183 ± 42 |
| Low-density lipoprotein (mg/dl) | 86 ± 31 | 100 ± 34 |
| High-density lipoprotein (mg/dl) | 45 ± 13 | 47 ±14 |
| Mean ± SD Triglyceride (mg/dl)‡ | 161 ± 103 | 177 ± 107 |
| Cardiovascular Disease | ||
| Ischemic heart disease | 5,469 (24%) | 12,824 (30%) |
| Coronary revascularization† | 1,291 (6%) | NA |
| Ischemic stroke | 1,323 (6%) | 3,358 (8%) |
| Peripheral artery disease | 1,959 (9%) | 4,020 (9%) |
| Heart failure | 2,949 (13%) | 5,068 (12%) |
| Chronic kidney disease | 6,553 (29%) | 17,617 (41%) |
| Medications∥ | ||
| Statin | 18,547 (82%) | 16,703 (39%) |
| Aspirin | 15,534 (69%) | 9,281 (21%) |
| P2Y12 Inhibitor | 3,361 (15%) | 1,038 (2%) |
| Insulin | 17,531 (77%) | 6,871 (16%) |
| Metformin | 18,791 (83%) | 19,008 (44%) |
| Thiazolidinediones | 4,035 (18%) | 2,307 (5%) |
| Sulfonylureas | 10,648 (47%) | 8,416 (19%) |
| Dipeptidyl peptidase 4 inhibitors | 3,711 (16%) | 4,743 (11%) |
| Antihypertensives | 16,872 (74%) | 19,746 (46%) |
Values are n (%) or mean ± standard deviation (SD).
Missing values were high in the community clinics, and ranged from 55% to 75%, when these values were known, they were left out of % calculations.
Smoking status was categorized as ever-smoker or non-smoker in the community clinic setting, current = ever-smoker here.
For patients with more than one value over the study, the average of all values collected was used.
Ejection fraction and revascularization data was not available for patients from the community clinics.
Usage at any time during the study period.
Annual SGLT2i and GLP1-RA prescription rates are shown in Figure 1, Table 2. For all patients with T2DM, the prescription rate for SGLT2is was 1% in 2013 and increased to 15% in 2019 (6-year absolute difference 14%, 95% confidence interval [CI] 13% to 15%, p <0.01, ptrend <0.01). In the overall group, rates of GLP1-RA use varied from 10% to 15% during the study period (6-year absolute difference 0%, 95% CI −1% to 1%, p = 0.99, ptrend = 0.99). Trends in prescribing were largely similar between men and women; NH White patients consistently had the highest prescription rates, and NH Black patients tended to have the lowest rates (Table 3). Higher rates were seen in patients with HbA1c levels of 10.0% to 12.9%. Patients with ejection fraction (EF) <40% had lower prescription rates than patients with EF >50%, and prescription rates were higher in patients with glomerular filtration rates ≥60 ml/min/1.73 m2.
Figure 1.
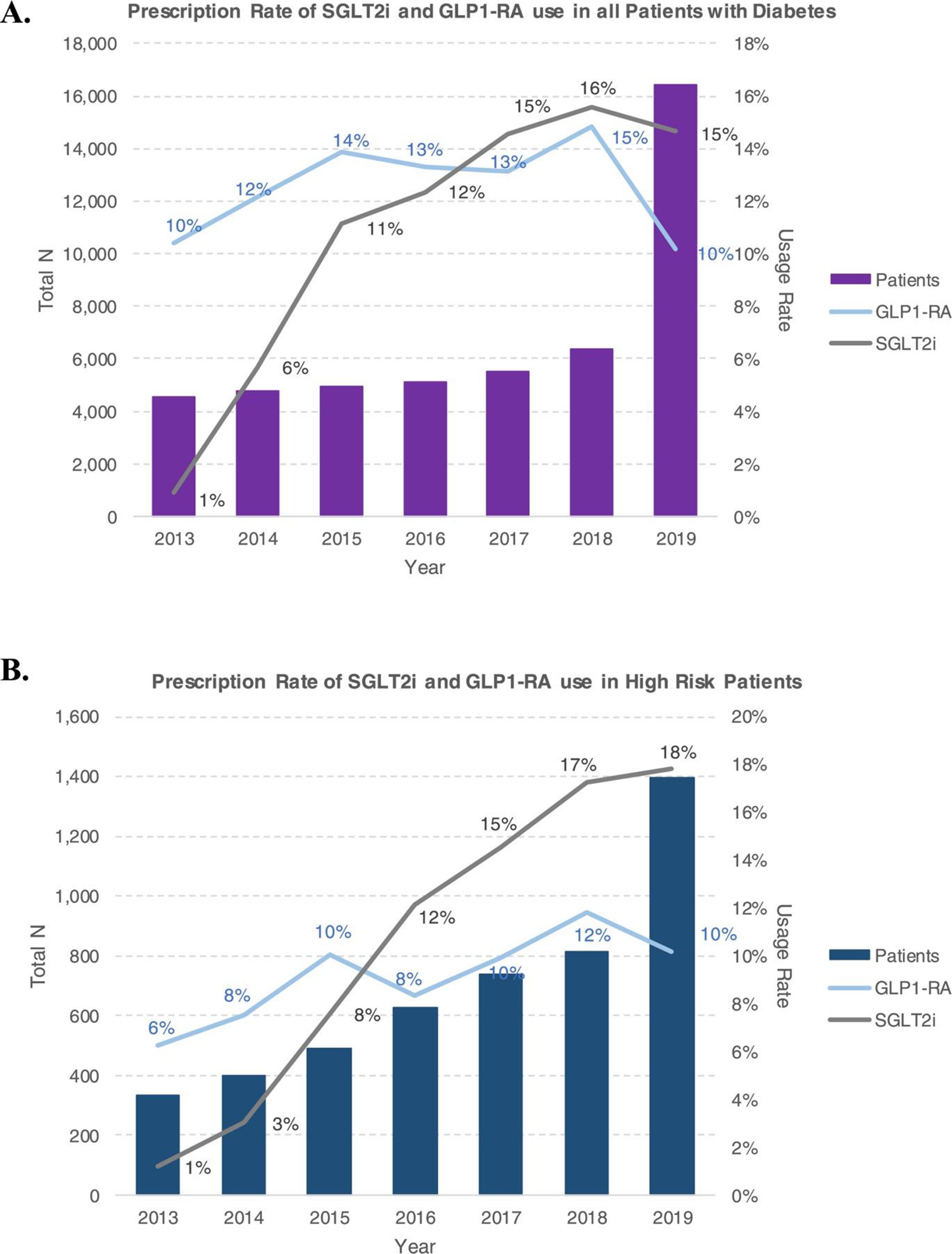
Trends in annual prescription rates for SGLT2 inhibitors and GLP-1 receptor agonists in integrated health system clinics, showing (A) an increase in prescription rates for SGLT2is and no significant change in prescription rates for GLP1-RAs in the overall cohort, and (B) a significant increase in SGLT2i prescription rates and a slight increase in GLP1-RA prescription rates in the high-risk patient cohort. Rates remain low overall in both cohorts.
Table 2.
Annual SGLT2 inhibitor and GLP1 receptor agonist use in the integrated health system clinics (2013–2019), overall and in high-risk patients.
| Variable | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Absolute difference 2013–2019 | 95% confidence interval | p Value for trend |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Integrated health system patients, total population | ||||||||||
|
| ||||||||||
| Eligible patients (n) | 4522 | 4747 | 4915 | 5103 | 5496 | 6342 | 16422 | – | – | – |
| Medication users (n) | ||||||||||
| SGLT2i | 40 | 270 | 547 | 631 | 801 | 987 | 2405 | – | – | P < 0.01 |
| GLP1-RA | 471 | 578 | 682 | 679 | 722 | 940 | 1671 | – | – | P = 0.99 |
| Total | 495 | 742 | 1059 | 1135 | 1302 | 1639 | 3539 | – | – | P < 0.01 |
| Prescription Rate (%) | ||||||||||
| SGLT2i | 1% | 6% | 11% | 12% | 15% | 16% | 15% | 14% | 13%–15% | – |
| GLP1-RA | 10% | 12% | 14% | 13% | 13% | 15% | 10% | 0% | −1%–1% | – |
| Total | 11% | 16% | 22% | 22% | 24% | 26% | 22% | 11% | 10%–12% | – |
|
| ||||||||||
| High-risk patient subgroup * | ||||||||||
|
| ||||||||||
| Eligible patients (n) | 335 | 398 | 489 | 626 | 737 | 812 | 1395 | – | – | – |
| Medication users (n) | ||||||||||
| SGLT2i | 4 | 12 | 37 | 76 | 107 | 140 | 249 | – | – | P < 0.01 |
| GLP1-RA | 21 | 30 | 49 | 52 | 73 | 96 | 142 | – | – | P = 0.01 |
| Total | 24 | 38 | 77 | 114 | 158 | 203 | 340 | – | – | P < 0.01 |
| Prescription Rate (%) | ||||||||||
| SGLT2i | 1% | 3% | 8% | 12% | 15% | 17% | 18% | 17% | 13%–21% | – |
| GLP1-RA | 6% | 8% | 10% | 8% | 10% | 12% | 10% | 4% | 1%–7% | – |
| Total | 7% | 10% | 16% | 18% | 21% | 25% | 24% | 17% | 12%–22% | – |
Usage rates equal the number of medication users divided by the total eligible per year.
This includes patients with diabetes, an A1c >7.0%, an ICD-9 or ICD-10 diagnosis of HF or atherosclerotic CVD and with an active prescription for metformin.
Table 3.
SGLT2 inhibitor or GLP1 receptor agonist use by demographic and clinical subgroups from the integrated health system clinics
| Variable | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Absolute difference 2013–2019 | 95% confidence interval |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Eligible T2DM Patients (n) | 4522 | 4747 | 4915 | 5103 | 5496 | 6342 | 16422 | – | – |
| Total on SGLT2i or GLP1 RA (n) | 495 | 742 | 1059 | 1135 | 1302 | 1639 | 3539 | – | – |
|
| |||||||||
| Prescription of SGLT2 inhibitor or GLP1 receptor agonist by select patient demographics and clinical characteristics (%) * | |||||||||
|
| |||||||||
| Sex | |||||||||
| Female | 11% | 16% | 22% | 22% | 23% | 26% | 20% | 9% | 7%–11% |
| Male | 11% | 15% | 22% | 22% | 24% | 26% | 23% | 12% | 10%–14% |
| Race/ethnicity | |||||||||
| Non-Hispanic White | 13% | 19% | 23% | 24% | 25% | 27% | 22% | 9% | 7%–11% |
| Non-Hispanic Black | 6% | 9% | 18% | 16% | 15% | 20% | 18% | 12% | 9%–15% |
| Hispanic | 9% | 13% | 19% | 21% | 22% | 25% | 21% | 12% | 8%–16% |
| Non-Hispanic Asian | 5% | 8% | 17% | 21% | 21% | 22% | 19% | 14% | 9%–19% |
| Other | 9% | 13% | 17% | 20% | 23% | 25% | 19% | 10% | 5%–16% |
| Hemoglobin A1c Level | |||||||||
| 7.0–9.9% | 16% | 22% | 29% | 29% | 31% | 33% | 30% | 14% | 12%–16% |
| 10.0–12.9% | 14% | 25% | 33% | 35% | 36% | 38% | 34% | 20% | 14%–26% |
| >/=13.0% | 4% | 23% | 23% | 21% | 21% | 28% | 29% | 25% | 14%–36% |
| Heart Failure (EF%) | |||||||||
| > 50% | 4% | 6% | 7% | 8% | 12% | 15% | 13% | 9% | 4%–14% |
| 40%–49% | 4% | 8% | 13% | 6% | 14% | 13% | 8% | 4% | −7%–15% |
| < 40% | 2% | 4% | 5% | 5% | 7% | 10% | 15% | 13% | 6%–20% |
| Missing EF | 8% | 9% | 10% | 10% | 9% | 11% | 15% | 7% | 2%–12% |
| Chronic kidney disease† | |||||||||
| GFR < 60 mL/min/1.73m2 | 4% | 7% | 11% | 12% | 15% | 17% | 17% | 13% | 10%–16% |
| GFR >/= 60 mL/min/1.73m2 | 11% | 16% | 24% | 25% | 25% | 27% | 23% | 12% | 10%–14% |
Usage rates equal the number of medication users divided by the total eligible in each category per year.
EF = ejection fraction; GFR = glomerular filtration rate; T2DM = type 2 diabetes mellitus.
There were a variable number of patients missing data for certain categories ranging from 8% to 30%; missing patients were excluded from the analysis.
GFR was calculated using the Modification of Diet in Renal Disease equation.
Among various clinician specialties (Table 4), endocrinology clinicians accounted for the highest number of first-time prescriptions for SGLT2is and GLP1-RAs, although this proportion decreased over time from 80% in 2013 to 29% in 2019 (6-year absolute difference −51%, 95% CI −46% to −56%, p <0.01). Primary care clinicians had an increase in prescriptions over the study period (8% to 57%, 6-year absolute difference 49%, 95% CI 44% to 54%, p <0.01), and ultimately prescribed >50% of first-time prescriptions by 2019. The number of first-time prescriptions was lowest among nephrology and cardiology clinicians, remaining at <1% and 15%, respectively. Cardiology clinician first-time prescriptions increased slightly, from 9% in 2013 to 14% in 2019 (95% CI 3% to 7%, p <0.01).
Table 4.
First-time prescriber rates for SGLT2 inhibitors or GLP1 receptor agonists by clinical specialty in integrated health system clinics
| Variable | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Absolute difference 2013–2019 | 95% confidence interval |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Total Prescriptions (n) | 524 | 388 | 535 | 465 | 549 | 952 | 2031 | – | – |
| Clinician Specialty (%) | |||||||||
| Endocrinology | 80% | 75% | 78% | 67% | 60% | 49% | 29% | 51% | 46%–56% |
| Primary Care | 8% | 6% | 2% | 3% | 4% | 24% | 57% | 49% | 44%–54% |
| Cardiology | 9% | 13% | 14% | 13% | 14% | 12% | 14% | 5% | 3%–7% |
| Nephrology | 0% | 1% | 0% | 1% | 0% | 0% | 0% | 0% | 0% |
Prescriptions were defined as first-time if it was the initial prescription for an SGLT2 inhibitor or GLP1 receptor agonist identified in the Northwestern Medicine Electronic Data Warehouse.
We identified 43,340 patients with T2DM from 20 community clinics from January 1, 2013 to December 31, 2017. Aggregate data for this group are listed in Table 1. Body mass index and EF were not included in the data. A large number of values were missing for race/ethnicity (66%) and laboratory (55% to 75%) data. Overall, the mean age was 63 ± 15 years; 53% were female, 69% were NH White, 12% were NH Black, and 3% were Hispanic. Co-morbidities included CKD (41%), ischemic heart disease (30%), HF (12%), ischemic stroke (8%), and peripheral arterial disease (9%). The mean HbA1c level was 10 ± 1%. Prevalence of other medications included statins (39%), aspirin (21%), insulin (16%), metformin (44%), thiazolidinediones (5%), sulfonylureas (19%), dipeptidyl peptidase-4 inhibitors (11%), and any class of antihypertensives (46%).
The results of the primary analysis in the H3 setting are shown in Table 5. SGLT2i prescriptions increased over time from 2% in 2013 to 7% in 2017 (4-year absolute difference 5%, 95% CI 4% to 6%, p <0.01, ptrend <0.01), whereas GLP1-RA use remained stable at 2% to 3% (4-year absolute difference 1%, 95% CI 0% to 1%, p = 0.22, ptrend = 0.02). The most common antihyperglycemic for comparison was metformin, with prescription rates of 50% to 70%.
Table 5.
SGLT2 inhibitor and GLP1 receptor agonist use per year for all patients with type 2 diabetes mellitus in a community primary care clinic setting (2013–2017)
| Variable | 2013 | 2014 | 2015 | 2016 | 2017 | Absolute difference 2013–2019 | Confidence interval | p Value for trend |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Eligible patients (n) | 3590 | 4843 | 5764 | 6634 | 6035 | – | – | – |
| Medication users (n) | ||||||||
| SGLT2i | 78 | 395 | 583 | 618 | 411 | – | – | P < 0.01 |
| GLP1-RA | 79 | 109 | 139 | 200 | 156 | – | – | P = 0.02 |
| Prescription rate (%) | ||||||||
| SGLT2i | 3% | 8% | 10% | 9% | 7% | 4% | 3%–6% | – |
| GLP1-RA | 2% | 2% | 2% | 3% | 3% | 1% | −1%–1% | – |
Usage rates equal the number of medication users divided by the total eligible per year.
The number of patients who had ≥1 eligible encounter and met specific high-risk criteria listed in the ADA and ACC guideline recommendations increased each year, from 335 in 2013 to 1,395 in 2019 (Table 2). As shown in Figure 1, SGLT2i prescriptions increased from 1% to 18% (6-year absolute difference 17%, 95% CI 13% to 21%, p <0.01, ptrend <0.01); GLP1-RA prescription rates in patients with HF increased from 4% to 17% (6-year absolute difference 13%, 95% CI 10% to 16%, p <0.01). High-risk patients also had an increase in GLP1-RA prescriptions, from 6% to 10% (6-year absolute difference 4%, 95% CI 1% to 7%, p = 0.02, ptrend = 0.01).
In 2019, of the 1,395 patients who were considered high-risk by the ADA/ACC guidelines, 340 (24%) were prescribed an SGLT2i or a GLP1-RA (Table 2); 26% of NH White patients, but only 16% of NH Black patients, had prescriptions. In the unadjusted model, NH Black patients had 47% lower odds (odds ratio 0.53, CI 0.35 to 0.79, p = 0.002) than NH White patients of being prescribed an SGLT2i or GLP1-RA (Table 6). In a model fully adjusted for age, gender, co-morbidities, care team composition, and markers of socioeconomic status, NH Black patients remained at similarly low odds (odds ratio 0.56, CI 0.34 to 0.89, p = 0.016), compared with NH White patients, of being prescribed an SGLT2i or GLP1-RA (Table 3). Findings were similar in models that tested prescription rates for SGLT2is only but were different in models that tested prescription rates for GLP1-RAs only; in the latter, NH Black race was not associated with prescription rates in the adjusted model.
Table 6.
Association of race/ethnicity with prescription of SGLTi or GLP1-RA in the high risk cohort of patients from the Integrated System Clinics, year 2019
| Variable | Model 1 | Model 2* | Model 3† | Model 4‡ |
|---|---|---|---|---|
|
| ||||
| Outcome: Prescription of SGLTi or GLP1-RA | ||||
| Race / Ethnicity | ||||
| Non-Hispanic White | Ref. | Ref. | Ref. | Ref. |
| Non-Hispanic Black | 0.53 (0.35–0.79)§ | 0.41 (0.26–0.62)§ | 0.54 (0.33–0.84)§ | 0.56 (0.34–0.89)§ |
| Hispanic | 1.10 (0.73–1.63) | 0.83 (0.53–1.27) | 0.89 (0.56–1.40) | 0.91 (0.57–1.43) |
| Other∥ | 1.03 (0.62–1.66) | 1.01 (0.59–1.68) | 1.04 (0.59–1.68) | 1.18 (0.68–1.99) |
| Outcome: Prescription of SGLTi | ||||
| Race / Ethnicity | ||||
| Non-Hispanic White | Ref. | Ref. | Ref. | Ref. |
| Non-Hispanic Black | 0.39 (0.22–0.64)§ | 0.51 (0.26–0.86)§ | 0.66 (0.35–1.19)† | 0.39 (0.22–0.64)† |
| Hispanic | 0.93 (0.57–1.48) | 1.04 (0.62–1.69) | 0.77 (0.41–1.36) | 0.93 (0.57–1.48) |
| Other∥ | 0.98 (0.54–1.71) | 1.02 (0.54–1.84) | 1.46 (0.73–2.74) | 0.98 (0.54–1.71) |
| Outcome: Prescription of GLP1-RA | ||||
| Race / Ethnicity | ||||
| Non-Hispanic White | Ref. | Ref. | Ref. | Ref. |
| Non-Hispanic Black | 0.50 (0.27–0.86)§ | 0.68 (0.37–1.19) | 0.66 (0.35–1.19) | 0.50 (0.27–0.86)§ |
| Hispanic | 0.74 (0.40–1.30) | 0.77 (0.41–1.37) | 0.77 (0.41–1.36) | 0.74 (0.40–1.30) |
| Other∥ | 1.17 (0.58–2.18) | 1.25 (0.61–2.38) | 1.46 (0.73–2.74) | 1.17 (0.58–2.18) |
High risk defined as pre-existing CVD documented prior to or within 6 months of a qualifying clinic visit, HbA1c ≥ 7.0% within 6 months of a qualifying clinic visit, and a concurrent prescription for metformin based on ADA and ACC consensus recommendations for initiation of an SGLT2i or GLP1-RA.
Adjusted for age and sex.
Adjusted for Model 2 covariates plus history of chronic kidney disease, history of atherosclerotic cardiovascular disease, history of heart failure, and team composition (primary care clinician only vs specialist only vs primary care clinician and specialist).
Adjusted for Model 3 covariates plus insurance type and national area deprivation rank.
Represents a statistically significant adjusted odds ratio at p value <0.05.
Other includes unknown.
Discussion
In this analysis of SGLT2i and GLP1-RA medication prescription rates for patients with T2DM in an integrated health system and community primary care practices in the Midwest, we identified very low prescription rates for both classes of medication. Although prescription rates increased over time, the prevalence of both medication classes remained <25%. Similar rates and trends were found for a more narrowly defined, high-risk group of patients targeted in both ADA guidelines and ACC consensus recommendations. Rates for other medication classes with cardiovascular benefits (statins, aspirin, and antihypertensives) were notably higher. After metformin, the most common noninsulin antihyperglycemic class used was sulfonylureas, which have not been shown to reduce CVD mortality or morbidity.25
The highest prescription rates were seen in NH White patients with normal EF, with moderately elevated HbA1c levels, and without significant CKD. Black patients and those of Hispanic ethnicity had consistently lower prescription rates, despite their elevated CV risks at the population level.26,27 No differences based on patient gender, co-morbidities (with the exception of higher prescription rates among patients without CKD), or insurance status were observed. During the study period, randomized controlled trials demonstrated that patients with HF (with and without T2DM) derived a significant benefit from SGLT2is, but the presence of HF (regardless of EF) was not associated with increased prescription rates in this study.
Endocrinology clinicians prescribed the majority (initially >50%) of first-time SGLT2is and GLP1-RAs. Primary care clinicians increased prescription rates over time, possibly because of a growth in the overall and relative numbers of primary care clinicians in the integrated health system. Notably, cardiology and nephrology clinicians initiated <15% and approximately 1%, respectively, of all prescriptions each year, although both specialties see a large number of patients with both T2DM and CVD.
Although the dynamics of clinician prescribing behaviors are not as well-studied as patient adherence factors, there is some evidence to support the possible impacts of specific influences.28–31 The substantial utilization gap may be related largely to novelty, as a majority of SGLT2is and GLP1-RAs have been introduced only in the last decade. The angiotensin receptor-neprilysin inhibitor sacubitril/valsartan, another recent medication shown to have a significant impact on HF morbidity and mortality, was approved for use by the Food and Drug Administration in 2015; it has reported prescription rates as low as 13%.32,33 Furthermore, patent exclusivity and high medication launch prices can make novel therapies unaffordable for many patients.34 Patients frequently report medication cost as a barrier to adherence; this has an impact on prescription choices by their physicians.35–38 Similarly, route of administration may affect patient preferences; the first oral GLP1-RA was not approved until 2019, and this may have impacted the prescription rates observed in this study. New medications are often utilized within a narrow scope of practice by select specialty clinicians before they are adopted more broadly among primary care clinicians and clinicians within other specialties.29,30 It can similarly take time for evidence to become incorporated into society guidelines; despite the first positive cardiovascular outcome trial release in 2016, the ADA and ACC did not highlight these medications in their recommendations until 2018.2,8 Ultimately, the slow uptake of novel medical therapies represents a critical challenge that contributes to adverse patient outcomes and increased health care costs. It represents an important opportunity for process improvements and implementation practices that are positioned to have a large impact on the quality and cost-effectiveness of health care in the United States.39,40
We observed persistent disparities between NH Black and NH White patients in the prescription of these therapies for the highest-risk patients in 2019, even after adjustment for demographics, co-morbidities, and markers of socioeconomic status. This is consistent with other studies in cardiovascular quality of care and is likely related to upstream social determinants of health—including structural racism and systemic barriers to optimal care—that we could not measure in this study. This disparity has been cited previously for both medical and interventional therapies for CVD and T2DM.41–44 These disparities have been exacerbated further by the current pandemic and public health crisis.45 Although the underpinnings for this are complex and interconnected, they must be addressed as part of any comprehensive plan that aims to improve outcomes.46,47 In addition to the discussion presented here, several further strengths and limitations are noted in the Supplementary Material.
In conclusion, we found that SGLT2i and GLP1-RA prescription rates for patients with T2DM were very low—<25% for both primary and secondary prevention of CVD—despite existing guidelines and recommendations from expert professional societies. Rates were especially low among nonendocrinology specialists (e.g., cardiology and nephrology), who can have a large impact on the care of patients with T2DM in the future. Important differences in current prescribing among racial/ethnic groups were also observed. Effective interventions to increase the use of proven medical therapies for patients with T2DM are urgently needed, given the persistently elevated rates of CVD morbidity and mortality among these patients.
Supplementary Material
Disclosures
Dr. Ahmad has received consulting fees, unrelated to this manuscript, from Amgen, Teladoc Livongo, and Pfizer. Dr. Persell receives research support from Omron Healthcare Co. and consulting fees from the RAND Corporation and Quio Technologies and previously received research support from Pfizer; all support was unrelated to this manuscript. Dr. Petito receives research support from Omron Healthcare Co. Dr. Shah has received research grants from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer and consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Axon Therapies, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Cardiora, CVRx, Cytokinetics, Edwards, Eidos, Eisai, Ionis, Ironwood, Eli Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Sanofi, Shifamed, Tenax, and United Therapeutics. In the past 3 years, Dr. Huffman has received support from the American Heart Association, Verily, and AstraZeneca for work unrelated to this research. Dr. Huffman has received salary support from the American Medical Association for his role as an associate editor for JAMA Cardiology. The George Institute for Global Health has a patent and license and has received investment funding with intent to commercialize fixed-dose combination therapy through its social enterprise business, George Medicines. Dr. Huffman plans to submit patents for heart failure polypills. The remaining authors have no conflicts of interest to declare.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2022.10.041.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report 2020. Available at: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed on XXX.
- 2.Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL Jr, Kalyani RR, Kosiborod M, Magwire ML, Morris PB, Sperling LS. 2018 ACC Expert Consensus Decision Pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert consensus Decision Pathways. J Am Coll Cardiol 2018;72:3200–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregg EW, Hora I, Benoit SR. Resurgence in diabetes-related complications. JAMA 2019;321:1867–1868. [DOI] [PubMed] [Google Scholar]
- 4.Rawshani A, Rawshani A, Franzén S, Eliasson B, Svensson AM, Miftaraj M, McGuire DK, Sattar N, Rosengren A, Gudbjörnsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017;376:1407–1418. [DOI] [PubMed] [Google Scholar]
- 5.Cardiovascular benefits of SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes. JAMA 2019;321:1720–1721. [DOI] [PubMed] [Google Scholar]
- 6.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 7.Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, Lam CSP, Lopes RD, McMurray JJV, Pratley RE, Rosenstock J, Gerstein HC. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021;9:653–662. [DOI] [PubMed] [Google Scholar]
- 8.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 10.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS. DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347– 357. [DOI] [PubMed] [Google Scholar]
- 11.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 12.Brownson RC, Kreuter MW, Arrington BA, True WR. Translating scientific discoveries into public health action: how can schools of public health move us forward? Public Health Rep 2006;121:97– 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colaco NA, Kazi DS. From molecules to markets: broadening the focus of cardiovascular innovation. Circ Heart Fail 2018;11:e004815. [DOI] [PubMed] [Google Scholar]
- 14.Ciolino JD, Jackson KL, Liss DT, Brown T, Walunas TL, Murakami L, Chung I, Persell SD, Kho AN. Design of Healthy Hearts in the Heartland (H3): a practice-randomized, comparative effectiveness study. Contemp Clin Trials 2018;71:47–54. [DOI] [PubMed] [Google Scholar]
- 15.Persell SD, Liss DT, Walunas TL, Ciolino JD, Ahmad FS, Brown T, French DD, Hountz R, Iversen K, Lindau ST, Lipiszko D, Makelarski JA, Mazurek K, Murakami L, Peprah Y, Potempa J, Rasmussen LV, Wang A, Wang J, Yeh C, Kho AN. Effects of 2 forms of practice facilitation on cardiovascular prevention in primary care: a practice-randomized, comparative effectiveness trial. Med Care 2020;58:344–351. [DOI] [PubMed] [Google Scholar]
- 16.Starren JB, Winter AQ, Lloyd-Jones DM. Enabling a learning health system through a unified enterprise data warehouse: the experience of the Northwestern University Clinical and Translational Sciences (NUCATS) Institute. Clin Transl Sci 2015;8:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiese AD, Roumie CL, Buse JB, Guzman H, Bradford R, Zalimeni E, Knoepp P, Morris HL, Donahoo WT, Fanous N, Epstein BF, Katalenich BL, Ayala SG, Cook MM, Worley KJ, Bachmann KN, Grijalva CG, Rothman RL, Chakkalakal RJ. Performance of a computable phenotype for identification of patients with diabetes within PCORnet: the Patient-Centered Clinical Research Network. Pharmacoepidemiol Drug Saf 2019;28:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad FS, Ricket IM, Hammill BG, Eskenazi L, Robertson HR, Curtis LH, Dobi CD, Girotra S, Haynes K, Kizer JR, Kripalani S, Roe MT, Roumie CL, Waitman R, Jones WS, Weiner MG. Computable phenotype implementation for a national, multicenter pragmatic clinical trial: lessons learned From ADAPTABLE. Circ Cardiovasc Qual Outcomes 2020;13:e006292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roumie CL, Patel NJ, Muñoz D, Bachmann J, Stahl A, Case R, Leak C, Rothman R, Kripalani S. Design and outcomes of the Patient Centered Outcomes Research Institute coronary heart disease cohort study. Contemp Clin Trials Commun 2018;10:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Medicare and Medicaid Services. Chronic Conditions Data Warehouse. Available at: https://www2.ccwdata.org/web/guest/home. Accessed on XXX.
- 21.Brokamp C, Wolfe C, Lingren T, Harley J, Ryan P. Decentralized and reproducible geocoding and characterization of community and environmental exposures for multisite studies. J Am Med Inform Assoc 2018;25:309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Center for Health Disparities Research, University of Wisconsin School of Medicine and Public Health. Neighborhood atlas: area deprivation index v3.1. Available at: https://www.neighborhoodatlas.medicine.wisc.edu/. Accessed on July 7 2021.
- 23.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible - the Neighborhood Atlas. N Engl J Med 2018;378:2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frigaard M, Rubinsky A, Lowell L, Malkina A, Karliner L, Kohn M, Peralta CA. Validating laboratory defined chronic kidney disease in the electronic health record for patients in primary care. BMC Nephrol 2019;20:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44(suppl 1):S125–S150. [DOI] [PubMed] [Google Scholar]
- 26.Sidney S, Quesenberry CP Jr, Jaffe MG, Sorel M, Nguyen-Huynh MN, Kushi LH, Go AS, Rana JS. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol 2016;1:594–599. [DOI] [PubMed] [Google Scholar]
- 27.Leigh JA, Alvarez M, Rodriguez CJ. Ethnic minorities and coronary heart disease: an update and future directions. Curr Atheroscler Rep 2016;18:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar-Jacob JM. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. J Am Heart Assoc 2016;5:e002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason A New medicines in primary care: a review of influences on general practitioner prescribing. J Clin Pharm Ther 2008;33:1–10. [DOI] [PubMed] [Google Scholar]
- 30.Lublóy Á Factors affecting the uptake of new medicines: a systematic literature review. BMC Health Serv Res 2014;14:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy AE, Huang C, Huang A, Ho MP. Recent approaches to improve medication adherence in patients with coronary heart disease: progress towards a learning healthcare system. Curr Atheroscler Rep 2018;20:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 33.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 34.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Health Care Services, Committee on Ensuring Patient Access to Affordable Drug Therapies. Making medicines affordable: a national imperative. Washington: National Academies Press (US); 2017. [PubMed] [Google Scholar]
- 35.Piette JD, Wagner TH, Potter MB, Schillinger D. Health insurance status, cost-related medication underuse, and outcomes among diabetes patients in three systems of care. Med Care 2004;42:102–109. [DOI] [PubMed] [Google Scholar]
- 36.Piette JD, Heisler M, Wagner TH. Cost-related medication underuse among chronically ill adults: the treatments people forgo, how often, and who is at risk. Am J Public Health 2004;94: 1782–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persaud N, Bedard M, Boozary AS, Glazier RH, Gomes T, Hwang SW, Juni P, Law MR, Mamdani MM, Manns BJ, Martin D, Morgan SG, Oh PI, Pinto AD, Shah BR, Sullivan F, Umali N, Thorpe KE, Tu K, Laupacis A. Carefully Selected and Easily Accessible at No Charge Medicines (CLEAN Meds) Study Team. Effect on treatment adherence of distributing essential medicines at no charge: the CLEAN Meds randomized clinical trial. JAMA Intern Med 2019;180:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madden JM, Graves AJ, Zhang F, Adams AS, Briesacher BA, Ross-Degnan D, Gurwitz JH, Pierre-Jacques M, Safran DG, Adler GS, Soumerai SB. Cost-related medication nonadherence and spending on basic needs following implementation of Medicare Part D. JAMA 2008;299:1922–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 2005;43:521–530. [DOI] [PubMed] [Google Scholar]
- 40.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166:1836–1841. [DOI] [PubMed] [Google Scholar]
- 41.Youmans QR, Hastings-Spaine L, Princewill O, Shobayo T, Okwuosa IS. Disparities in cardiovascular care: past, present, and solutions. Cleve Clin J Med 2019;86:621–632. [DOI] [PubMed] [Google Scholar]
- 42.Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev 2007;64 (suppl):101S–156S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson PA, Lee TH, Cook EF, Rouan GW, Goldman L. Effect of race on the presentation and management of patients with acute chest pain. Ann Intern Med 1993;118:593–601. [DOI] [PubMed] [Google Scholar]
- 44.Eberly LA, Yang L, Eneanya ND, Essien U, Julien H, Nathan AS, Khatana SAM, Dayoub EJ, Fanaroff AC, Giri J, Groeneveld PW, Adusumalli S. Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open 2021;4:e216139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yancy CW. COVID-19 and African Americans. JAMA 2020;323:1891–1892. [DOI] [PubMed] [Google Scholar]
- 46.Purnell TS, Calhoun EA, Golden SH, Halladay JR, Krok-Schoen JL, Appelhans BM, Cooper LA. Achieving health equity: closing the gaps in health care disparities, interventions, and research. Health Aff (Millwood) 2016;35:1410–1415. [DOI] [PubMed] [Google Scholar]
- 47.Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal treatment: confronting racial and ethnic disparities in health care. Washington: National Academies Press (US); 2003. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


