Abstract
HIV pre-exposure prophylaxis (PrEP) has been associated with incident hepatitis C virus (HCV) infection in men who have sex with men (MSM) due to decreased condom use. We examined rates of HCV among MSM and transgender women at high-risk of HIV on PrEP in southern California using data from two trials (NCT01761643 and NCT01781806). Five of 599 participants (0.84%, 95% CI 0.27–1.93) had HCV antibodies detected at entry. Factors associated with HCV seropositivity included being older (P=0.002) and lower education level (P<0.001). HCV-positive participants had no reported cases of sexually transmitted infection (rectal, urethral or pharyngeal gonorrhea and/or chlamydia) at entry while HCV-negative participants had a prevalence of 18% (95% CI, 15–21%). There were no significant differences in substance use and sexual risk behavior between HCV-positive and HCV-negative participants 1–3 months prior to entry. Among early PrEP adopters, incident HCV did not occur despite ongoing condomless intercourse. Screening intervals for HCV in MSM on PrEP should be led by a risk behavior assessment.
Keywords: PrEP, MSM (men who have sex with men), Hepatitis C
Introduction
HIV pre-exposure prophylaxis (PrEP) uptake, adherence, and persistence is critical to ending the HIV epidemic in the US.1,2 However, there is concern that HIV PrEP use may also promote elevated risk for incident hepatitis C (HCV) via increase in condomless intercourse or similar behaviors.3 HCV infection in North America, Europe, Australia and Asia has been on an upward trend beginning in 1995 and since 2000 has been associated with sexually transmitted infection (STI) among HIV-positive men who have sex with men (MSM).4,5
HIV and HCV are spread through injecting and sexual networks6 commonly associated with condomless anal sex, higher number of sexual partners, recent STIs, and recreational drug use prior to or during sex.7–9 Most data on HCV-HIV co-infection has been among HIV-positive MSM8,10 who have a higher prevalence of co-infection than HIV-negative MSM.11–13 However, sexual transmission of HCV has been reported in HIV-negative MSM14–15 and HIV-negative MSM on PrEP may be at increased risk of HCV infection due to shared transmission networks between HIV-negative and HIV-positive MSM3,13,16 and condomless anogenital contact.19 This overlap in sexual networks between HIV-positive and HIV-negative MSM3,17 can facilitate the introduction of HCV into the HIV-negative population of MSM. In this brief report, we specifically examine the prevalence and incidence of HCV in cohorts of sexually active HIV-negative MSM and transgender women (TGW) in PrEP trials.
Methods
Population
Data from two parallel 48-week prospective trials in Southern California were combined to include adult MSM and TGW at increased risk of acquiring HIV infection who received daily PrEP with tenofovir disoproxil fumarate-emtricitabine (TDF-FTC) and had available paired specimens. These were a randomized clinical trial of text messaging for PrEP adherence (CCTG 595 - NCT01761643)18 with 398 participants enrolled from February 2013 to February 2016, and a strategy study of real-time plasma tenofovir concentration measurement and feedback to support PrEP adherence (PATH-PrEP - NCT01781806)19 with 301 participants enrolled from April 2014 to July 2016. A total of 599 participants with at least one follow-up visit with paired HCV testing samples were included in this analysis (Figure 1), 86 participants were excluded from the analysis due to missing baseline HCV samples (Supplementary Table 3).
Figure 1.
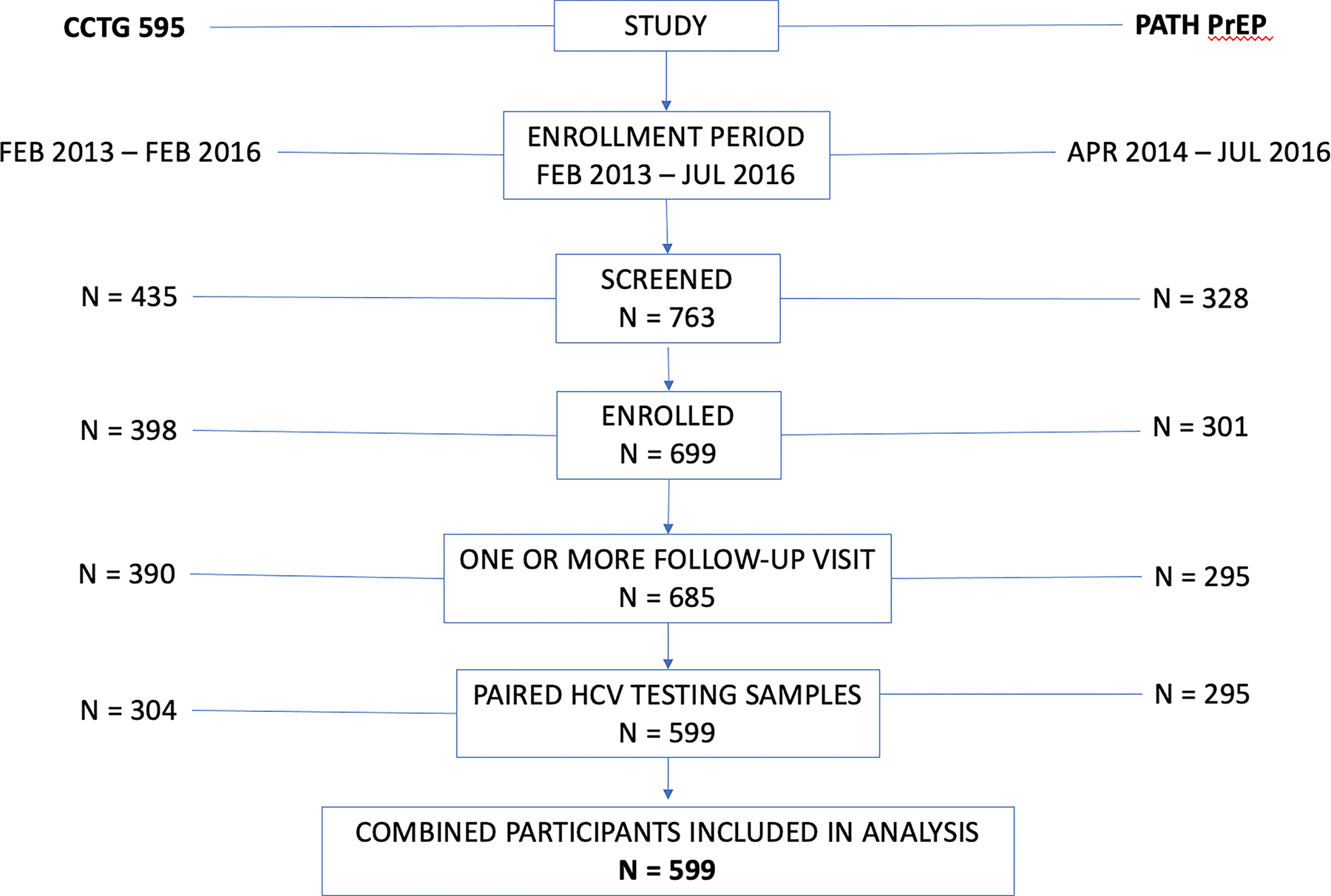
Flow chart of study participants.
Data
The primary outcome for this analysis was HCV status at last study visit. HCV serology was routinely collected in PATH-PrEP study, while paired banked specimens were obtained at entry and last study visit in CCTG 595. CCTG 595 participants with reactive HCV antibodies at last study visit had their entry specimen assayed to determine if seroconversion had occurred since study inception. Participants were also routinely screened for syphilis ( and rectal, urethral, and pharyngeal gonorrhea and chlamydia at regular intervals by NAAT (Hologic Aptima, Long Beach Public Health Lab). Participants with new STI diagnoses were notified and referred for treatment. Screening assessments for syphilis were conducted using serum rapid plasma regain (RPR) and confirmatory treponemal test, but only results for gonorrhea and chlamydia were used for STI measures in this study as incident syphilis could not be differentiated from pre-existing syphilis. PrEP adherence was measured using dried blood spot concentrations for intraerythrocytic TFV-DP at week 12 and 48 using a liquid chromatography–tandem mass spectrometry assay and included as the mean adherence level measured at week 12 and last study visit.20 Concentrations of >719 fmol/punch was considered adequate adherence, and >1246 fmol/punch was near-perfect adherence for participants in CCTG 595. While concentrations of >700 fmol/punch were considered protective in PATH-PrEP, we used the cut off of >719 fmol/punch for this analysis. Sexual risk behavior, and substance use for the past 1–3 months prior to enrollment were collected using a computer assisted self-report survey (Supplementary Table 1).
Statistical analysis
Prevalence and incidence of HCV and STI was calculated at baseline and last study visit with binomial exact method to calculate 95% confidence interval. Cumulative incidence is defined as the proportion of participants who were HCV positive over 48 weeks of follow up time between 2013–2016 and calculated as the number of new cases of HCV over number of individuals in the study population at risk. Baseline differences by HCV-status were assessed using Fisher’s exact for categorial data and Wilcoxon rank sum and T-test for continuous data. A P-value less than 0.05 was considered statistically significant. Data management and statistical analysis were conducted using Stata/SE 15.1 (StataCorp, College Station, Texas, USA).
Results
A total of 599 participants with paired HCV serology samples were included in this analysis. Five participants had HCV antibodies detected at entry (prevalence 0.84%, 95% CI 0.27–1.93), there were no new positives by end of study, thus, zero incident HCV cases (upper limit of 1-sided 95% CI, 0.49%) were detected during the study period. Factors associated with HCV seropositivity at entry included being older in age (median 51 years HCV-positive vs. median 38 years HCV-negative, P=0.002) and having a lower education level (60% high-school or less HCV-positive vs. 9% high-school or less HCV-negative, P<0.001). HCV-positive individuals were less likely to maintain PrEP adherence over the course of the study (40% not adequate HCV-positive vs. 13% not adequate HCV-negative, P=0.029) (Table 1).
Table 1:
Characteristic and risk factor by Hepatitis C (HCV) status at baseline.
| HCV Negative | HCV Positive | P-value | HCV Negative | HCV Positive | P-value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| N=594 | N=5 | N=594 | N=5 | ||||
|
|
|
||||||
| Demographic | Substance Use (past 30 days) | ||||||
| Gender | 1.00 | Ecstasy | 1.00 | ||||
| Male to Female | 2 (<1%) | 0 (0%) | No | 450 (76%) | 3 (60%) | ||
| Male | 592 (100%) | 5 (100%) | Yes | 122 (21%) | 0 (0%) | ||
| Race and Ethnicity | 0.38 | Missing | 22 (4%) | 2 (40%) | |||
| White | 306 (52%) | 2 (40%) | Heroin | 1.00 | |||
| African American | 65 (11%) | 2 (40%) | No | 555 (93%) | 3 (60%) | ||
| Hispanic/Latino | 164 (28%) | 1 (20%) | Yes | 15 (3%) | 0 (0%) | ||
| Asian | 23 (4%) | 0 (0%) | Missing | 24 (4%) | 2 (40%) | ||
| Other | 36 (6%) | 0 (0%) | Marijuana | 0.25 | |||
| Education Level | 0.008 | No | 290 (49%) | 3 (60%) | |||
| Highschool or less | 56 (9%) | 3 (60%) | Yes | 282 (47%) | 0 (0%) | ||
| Some college or more | 538 (91%) | 2 (40%) | Missing | 22 (4%) | 2 (40%) | ||
| Age at enrollment, mean (SD) | 38 (10) | 51 (5) | 0.002 | Methamphetamines | 0.071 | ||
| Sexually Transmitted Infections | No | 480 (81%) | 1 (20%) | ||||
| Hepatitis B | 1.00 | Yes | 92 (15%) | 2 (40%) | |||
| Negative | 534 (90%) | 5 (100%) | Missing | 22 (4%) | 2 (40%) | ||
| Positive | 2 (<1%) | 0 (0%) | Hallucinogens | 1.00 | |||
| Missing | 58 (10%) | 0 (0%) | No | 532 (90%) | 3 (60%) | ||
| Any Gonorrhea | 1.00 | Yes | 39 (7%) | 0 (0%) | |||
| Negative | 267 (45%) | 3 (60%) | Missing | 23 (4%) | 2 (40%) | ||
| Positive | 58 (10%) | 0 (0%) | Dissociative | 1.00 | |||
| Missing | 269 (45%) | 2 (40%) | No | 541 (91%) | 3 (60%) | ||
| Any Chlamydia | 1.00 | Yes | 32 (5%) | 0 (0%) | |||
| Negative | 269 (45%) | 3 (60%) | Missing | 21 (4%) | 2 (40%) | ||
| Positive | 58 (10%) | 0 (0%) | Popper | 0.61 | |||
| Missing | 267 (45%) | 2 (40%) | No | 273 (46%) | 2 (40%) | ||
| Syphilis | 0.78 | Yes | 300 (51%) | 1 (20%) | |||
| Negative | 269 (45%) | 3 (60%) | Missing | 21 (4%) | 2 (40%) | ||
| Positive | 43 (97%) | 0 (0%) | Cocaine | 1.00 | |||
| Missing | 282 (48%) | 2 (40%) | No | 464 (78%) | 3 (60%) | ||
| Yes | 109 (18%) | 0 (0%) | |||||
| Missing | 21 (4%) | 2 (40%) | |||||
| Sexual Risk Behavior (past 30 days) | PrEP Adherence | ||||||
| Anal sex in past 30 days | 1.00 | Mean PrEP Adherence | 0.029 | ||||
| No | 11 (2%) | 0 (0%) | Not adequate/below quantification | 80 (13%) | 2 (40%) | ||
| Yes | 563 (95%) | 3 (60%) | Adequate/Protective | 267 (45%) | 0 (0%) | ||
| Missing | 20 (3%) | 2 (40%) | Perfect | 223 (38%) | 2 (40%) | ||
| No. male partners for anal sex, median (IQR) | 5 (3, 10) | 3.5 (3, 4) | 0.39 | Missing | 24 (4%) | 1 (20%) | |
| No. unprotected ARS, median (IQR) | 1 (0, 3.5) | 4.5 (1, 8) | 0.34 | ||||
| No. unprotected AIS, median (IQR) | 2 (0, 4) | 1 (1, 1) | 0.60 | ||||
| No. HIV negative partners, median (IQR) | 1 (0, 3) | 1 (0, 2) | 0.68 | ||||
NOTES:
Percentages are column totals
P-value is for Fisher’s Exact Test and Wilcoxon Rank Sum/T-Test
Any Gonorrhea: any of rectal, urine and/or pharyngeal cases; Any Chlamydia: any of rectal and/or urine cases
ARS/AIS: Anal receptive sex/Anal insertive sex
Mean PrEP adherence is mean value measured at week 12 and last study visit
HCV-positive participants had no reported cases of STIs (positive for rectal, urethral or pharyngeal gonorrhea and/or chlamydia) at entry, thus zero prevalence (upper limit of 1-sided 95% CI, 45%) of STI among HCV-positive participants and no incident cases through week 48. Among HCV-negative participants, there were more chlamydia than gonorrhea cases (Figure 1A) and prevalence of STIs at entry was 18% (106 of 594; 95% CI, 15–21%), increasing to 20% (97 of 485; 95% CI, 17–24%) by week 48 (Figure 1B).
There were no significant differences in substance use and sexual risk behavior between HCV-positive and HCV-negative participants 1–3 months days prior to entry. Among HCV-negative participants, methamphetamines (40%) and poppers (20%) were the only drugs reported by participants. Poppers, an inhalant form of amyl nitrite, was the most common drug among HCV-negative participants of whom 51% reported use in the past 1–3 months prior to entry. Majority of participants (95% of HCV-negative and 60% of HCV-positive) engaged in anal sex in the past 1–3 months prior to entry (Table 1).
Discussion
HCV infection was not common among HIV-negative MSM and TGW on PrEP trials in Southern California. Among early PrEP adopters, the prevalence of HCV antibodies was less than 1% and no incident HCV was detected during the study period despite ongoing risk behavior and high prevalence of STIs. PrEP use has been linked to high rates of STI due to decreased condom use. There is little research available on HCV and HIV co-infection in Southern California, and with the increased benefits of PrEP in prevention of HIV, it was of interest to see how PrEP would be associated with HCV in the HIV-negative population. Our results show participants who were undergoing daily PrEP had low adherence level among HCV positive participants which may be an indication of increased risk of acquiring HIV infection and possible transmission route between HIV-negative to HIV-positive MSM.
A study by Milam et al. reported an estimated one additional condomless receptive anal intercourse act per month after PrEP initiation in a demonstration study21 and a cohort of PrEP users in San Francisco also had high rates of STI with 41% of participants reporting decreased condom use over study period.22 STI rates among PrEP users in both studies included in this secondary analysis were high19,23, with a prevalence ranging from 4–20% among HCV-negative participants.
HCV prevalence in this study is similar to studies among MSM cohorts in the United States, United Kingdom (UK), Canada and Australia. Among 485 HIV-uninfected MSM receiving PrEP in a medical center in San Francisco from 2011–2014, only two incident HCV infections were reported for patients older than 35 years.24 Similarly to our cohort of HCV positive participants, the only reported risk factor for the two patients for HCV infection was condomless sexual intercourse. A survey of 2,030 MSM in Manchester, UK, had 0.9% of new HCV diagnosis, among which 1.8% were HIV-positive MSM and 0.2% HIV-negative MSM. The authors found HCV positivity was significantly associated with HIV status, with HIV-positive MSM reporting lower rates of sexual partners and insertive unprotected anal intercourse (P<0.05) compared with HIV-negative MSM.25 A study in Canada of 442 MSM from primary care settings reported 1.4% prevalence among HIV-negative MSM without history of injection drug use (IDU)12 and a study in Australia with HCV prevalence of 1.07% among HIV-negative MSM.13 While only the San Francisco study included MSM on PrEP, the findings are consistent with low HCV prevalence among HIV-negative MSM reporting higher numbers sexual partners and condomless anal insertive sex. To get a sense of the prevalence and incidence of HCV infection in HIV-positive MSM in San Diego, CA, Chaillon A et al26 reported a stable incidence of HCV between 2000–2014 (0.83/100 person year; 95% CI 0.41 – 1.48) from two primary HIV clinics, and an increase to 3.01/100 person year (95% CI 1.97 – 4.42) in 2015. Our cohort enrolled patients between 2013 – 2016 out of which only eight patients were enrolled in 2015 from San Diego. It is likely that our cohort enrollment in San Diego was near complete prior to the peak in HCV incidence. Additionally, a study by Chew et al27 shows low HCV prevalence among newly HIV-diagnosed MSM in Los Angeles.
The low HCV prevalence in our study differs from a cohort in Amsterdam3 suggesting high incidence of HCV among HIV-negative MSM on PrEP. Hoornenborg et al had 18 of 375 HIV-negative MSM (4.8%, 95% CI 2.9–7.5) positive for HCV at baseline, with median age of 33 years (IQR 28–42) compared with 40 years of age for HCV-negative MSM. They also reported significant differences in sexual risk behaviors at baseline between HCV-positive and HCV-negative MSM on PrEP including self-reported STI, number of receptive condomless anal sex in past 3 months, injection drug use in past 3 months and use of gamma-hydroxybutyrate/gamma-butyrolactone (GHB/GBL), methamphetamine or mephedrone during sex in past 3 months. There were no significant differences in drug use between HCV-positive and HCV-negative participants at baseline in our study, possibly due to low power to detect a difference. The MSM/TGW in our cohort were older and had lower baseline HCV prevalence compared with the Amsterdam cohort. It is possible that the HCV positive participants in our cohort are cases from other sources as these individuals were comparatively older and did not have any STI, fitting more in line with CDC recommendations for screening individuals born between 1945 and 1965.
Global prevalence of HCV in HIV negative MSM was estimated to be 1.5% by Jin et al6 with notably higher rates in current or past IDU. In MSM taking PrEP, global incidence was found to be 14.8 per 1000 person years. However, global incidence was determined by only four studies, none of which took place in the United States. Wynn et al28 estimated that HCV seroprevalence in San Diego was 2.1% in 2018, with a majority of infections found in IDU and those aged 55–74. Although this study did not examine rates specific to those taking PrEP, HCV seroprevalence was no different in HIV negative MSM aged 18–54 compared to men aged 18–54.
This report is limited by having to combine datasets from two separate studies without exact mirroring of the questionnaires, thus limited data is combined. However, lab results of STI and HCV were not subject to data collection differences. We did not have information on mode of drug administration (i.e. snorting, injection drug use) in the questionnaire which would be informative towards understanding the mechanism of infection. The low prevalence rate of HCV in this group limit the power to conduct further analysis of factors related to HCV. We were unable to determine if the five anti-HCV antibody positive MSM had active HCV infection, conducting a phylogenetic analysis on background sequences of HCV infections in HIV-positive MSM from Southern California to determine potential transmission between HIV-positive and HIV-negative individuals would be very informative. Finally, as only two TGW enrolled in the study, our results are driven by MSM at elevated risk for HIV seeking PrEP shortly after regulatory approvals by the US Food and Drug Administration of TDF-FTC.
Conclusion
Among early PrEP adopters engaged in Southern California PrEP demonstration projects, the seroprevalence of HCV was low. Incident HCV did not occur during the study period, despite ongoing risk behavior and a high prevalence of bacterial STIs. While routine testing of HCV among sexually active MSM may be most reasonable among those with additional risk factors for HCV acquisition, our findings do not support a need for frequent screening of HCV for all MSM on PrEP and should be guided by risk behavior assessment.
Supplementary Material
Figure 2.
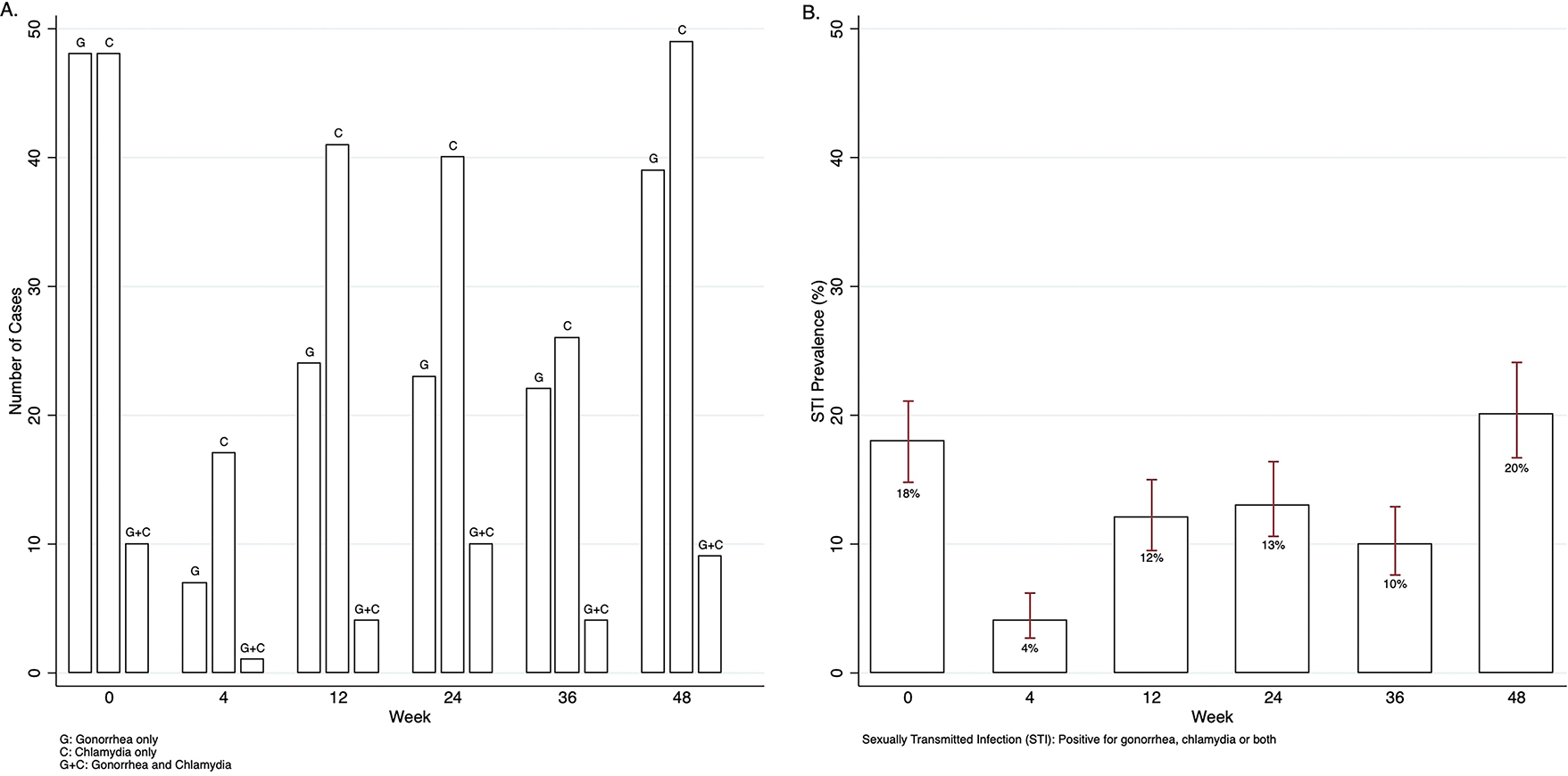
A) Number of cases of gonorrhea and chlamydia among Hepatitis C negative participants by visit week, and B) Prevalence and 95% confidence intervals of sexually transmitted infections (positive for gonorrhea, chlamydia or both) among Hepatitis C negative participants by visit week.
Statement of significance.
HIV pre-exposure prophylaxis (PrEP) has been associated with incident hepatitis C virus (HCV) infection in men who have sex with men (MSM) due to decreased condom use. A recent systematic review and meta-analysis by Jin et al. has described the incidence and prevalence of HCV in MSM on PrEP globally, with particular concern of higher incidence for those taking PrEP, and provided recommendations to increase screening. Our findings in a large study population of concern on PrEP are novel, as prevalence of HCV was very low and no incident cases occurred.
Source of Funding:
Data for this analysis were collected as part of the California Collaborative Treatment Group 595 study supported by the California HIV/AIDS Research Program EI-11-SD-005. PATH-PrEP demonstration study was funded by the California HIV Research Program EI11-LA-002 with additional support from the Center for HIV Identification, Prevention, and Treatment (CHIPTS) NIMH grant MH58107; the UCLA Center for AIDS Research (CFAR) grant 5P30AI028697; and the National Center for Advancing Translational Sciences through UCLA CSTI Grant UL1TR000124. Gilead Sciences provided drug supply and additional support for some drug-assay testing. RJL received honoraria and travel support from Gilead Sciences.
We gratefully acknowledge and thank all participants who contributed to the study and the CCTG clinical site staff for implementation and data collection.
Footnotes
Author Disclosure Statement: No competing financial interests exist.
Ethics Statement. The studies were approved by the Institutional Review Boards at UC San Diego and UC Los Angeles.
viii. REFERENCES
- 1.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV Epidemic: A Plan for the United States Ending the HIV Epidemic Editorial. JAMA. 2019;321(9):844–845. [DOI] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoornenborg E, Coyer L, van Laarhoven A, et al. Change in sexual risk behaviour after 6 months of pre-exposure prophylaxis use: results from the Amsterdam pre-exposure prophylaxis demonstration project. Aids. 2018;32(11):1527–1532. [DOI] [PubMed] [Google Scholar]
- 4.van de Laar TJ, Matthews GV, Prins M, Danta M. Acute hepatitis C in HIV-infected men who have sex with men: an emerging sexually transmitted infection. Aids. 2010;24(12):1799–1812. [DOI] [PubMed] [Google Scholar]
- 5.Ghosn J, Pierre-François S, Thibault V, et al. Acute hepatitis C in HIV-infected men who have sex with men. HIV Med. 2004;5(4):303–306. [DOI] [PubMed] [Google Scholar]
- 6.Jin F, Dore GJ, Matthews G, et al. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: a systematic review and meta-analysis, Lancet Gastroenterology and Hepatology. 2021;6:39–56 [DOI] [PubMed] [Google Scholar]
- 7.Vanhommerig JW, Lambers FA, Schinkel J, et al. Risk Factors for Sexual Transmission of Hepatitis C Virus Among Human Immunodeficiency Virus-Infected Men Who Have Sex With Men: A Case-Control Study. Open Forum Infect Dis. 2015;2(3):ofv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS (London, England). 2015;29(17):2335–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Center for Disease Control and Prevention (CDC). Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men--New York City, 2005–2010. MMWR Morb Mortal Wkly Rep. 2011;60(28):945–950. [PubMed] [Google Scholar]
- 10.Jordan AE, Perlman DC, Neurer J, Smith DJ, Des Jarlais DC, Hagan H. Prevalence of hepatitis C virus infection among HIV+ men who have sex with men: a systematic review and meta-analysis. Int J STD AIDS. 2017;28(2):145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houlihan CF, Larke NL, Watson-Jones D, et al. Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. Aids. 2012;26(17):2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remis RS, Liu J, Loutfy MR, et al. Prevalence of Sexually Transmitted Viral and Bacterial Infections in HIV-Positive and HIV-Negative Men Who Have Sex with Men in Toronto. PLoS One. 2016;11(7):e0158090–e0158090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin F, Prestage GP, Matthews G, et al. Prevalence, incidence and risk factors for hepatitis C in homosexual men: data from two cohorts of HIV-negative and HIV-positive men in Sydney, Australia. Sexually Transmitted Infections. 2010;86(1):25–28. [DOI] [PubMed] [Google Scholar]
- 14.van de Laar TJ, Paxton WA, Zorgdrager F, Cornelissen M, de Vries HJ. Sexual transmission of hepatitis C virus in human immunodeficiency virus-negative men who have sex with men: a series of case reports. Sex Transm Dis. 2011;38(2):102–104. [DOI] [PubMed] [Google Scholar]
- 15.Richardson D, Fisher M, Sabin CA. Sexual transmission of hepatitis C in MSM may not be confined to those with HIV infection. J Infect Dis. 2008;197(8):1213–1214, author reply 1214–1215. [DOI] [PubMed] [Google Scholar]
- 16.Nijmeijer BM, Koopsen J, Schinkel J, Prins M, Geijtenbeek TB. Sexually transmitted hepatitis C virus infections: current trends, and recent advances in understanding the spread in men who have sex with men. J Int AIDS Soc. 2019;22 Suppl 6(Suppl Suppl 6):e25348–e25348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoornenborg E, Coyer L, Boyd A, et al. High incidence of HCV in HIV-negative men who have sex with men using pre-exposure prophylaxis. J Hepatol. 2020;72(5):855–864. [DOI] [PubMed] [Google Scholar]
- 18.Moore DJ, Jain S, Dube MP, et al. Randomized Controlled Trial of Daily Text Messages to Support Adherence to Preexposure Prophylaxis in Individuals at Risk for Human Immunodeficiency Virus: The TAPIR Study. Clin Infect Dis. 2018;66(10):1566–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landovitz RJ, Beymer M, Kofron R, et al. Plasma Tenofovir Levels to Support Adherence to TDF/FTC Preexposure Prophylaxis for HIV Prevention in MSM in Los Angeles, California. J Acquir Immune Defic Syndr. 2017;76(5):501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29(2):384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milam J, Jain S, Dube MP, et al. Sexual Risk Compensation in a Pre-exposure Prophylaxis Demonstration Study Among Individuals at Risk of HIV. J Acquir Immune Defic Syndr. 2019;80(1):e9–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volk JE, Marcus JL, Phengrasamy T, et al. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin Infect Dis. 2015;61(10):1601–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassan A, Blumenthal JS, Dube MP, et al. Effect of rectal douching/enema on rectal gonorrhoea and chlamydia among a cohort of men who have sex with men on HIV pre-exposure prophylaxis. Sexually Transmitted Infections. 2018;94(7):508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volk JE, Marcus JL, Phengrasamy T, Hare CB. Incident Hepatitis C Virus Infections Among Users of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(11):1728–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ireland G, Higgins S, Goorney B, et al. Evaluation of hepatitis C testing in men who have sex with men, and associated risk behaviours, in Manchester, UK. Sexually Transmitted Infections. 2017;93(6):404–409. [DOI] [PubMed] [Google Scholar]
- 26.Chaillon A, Sun X, Cachay ER, et al. Primary Incidence of Hepatitis C Virus Infection Among HIV-Infected Men Who Have Sex With Men in San Diego, 2000–2015. Open Forum Infect Dis. 2019;6(4):ofz160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chew KW, Javanbakht M, Clare L, et al. Hepatitis C in Men Who Have Sex with Men With New HIV Diagnoses in Los Angeles. CROI; February 23–26, 2015; Seattle, Washington. [Google Scholar]
- 28.Wynn A, Tweeten S, McDonald E, et al. The estimated hepatitis C seroprevalence and key population sizes in San Diego in 2018. PLOS ONE. 2021;16(6):e0251635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


