Abstract
A 48-year-old man with poorly controlled HIV presented with severe human monkeypox virus (hMPXV) infection, having completed 2 weeks of tecovirimat at another hospital. He had painful, ulcerating skin lesions on most of his body and oropharyngeal cavity, with subsequent Ludwig's angina requiring repeated surgical interventions. Despite commencing a second, prolonged course of tecovirimat, he did not objectively improve, and new lesions were still noted at day 24. Discussion at the UK National Health Service England High Consequence Infectious Diseases Network recommended the use of 3% topical and then intravenous cidofovir, which was given at 5 mg/kg; the patient made a noticeable improvement after the first intravenous dose. He received further intravenous doses at 7 days and 21 days after the dose and was discharged at day 52. Cidofovir is not licensed for use in treatment of hMPXV infection. Data for cidofovir use in hMPXV are restricted to studies in animals. Four other documented cases of cidofovir use against hMPXV have been reported in the USA in 2022, but we present its first use in the UK. The scarcity of studies into the use of cidofovir in this condition clearly shows the need for robust studies to assess efficacy, optimum dosage, timing, and route of administration.
Introduction
Mpox (formerly known as monkeypox) is caused by an orthopoxvirus that was first identified in 1958 after outbreaks in macaques being used for research in Copenhagen, Denmark.1 The disease was named monkeypox until Nov 28, 2022, when WHO recommended a change to mpox with a year-long transition period because most current and historical literature terms it as monkeypox; this change was to reduce stigma associated with the term monkeypox.2 The first human case was recorded in the 1970s in DR, and subsequently has been reported in several endemic areas in central and western Africa.3 From May, 2022, cases of mpox were reported from several countries worldwide in which domestic acquisition had not previously been reported, and on July 23, 2022 was declared a public health emergency of international concern by WHO.4 As of Jan 20, 2023, 84 916 cases of mpox have been reported worldwide, of which 81 836 are in locations that have not historically reported cases.5 Its presence beyond sub-Saharan Africa has catalysed discussions around optimal treatments, with trials launched in the UK, the USA, DR Congo and elsewhere to gather evidence on potential therapeutics.
Case report
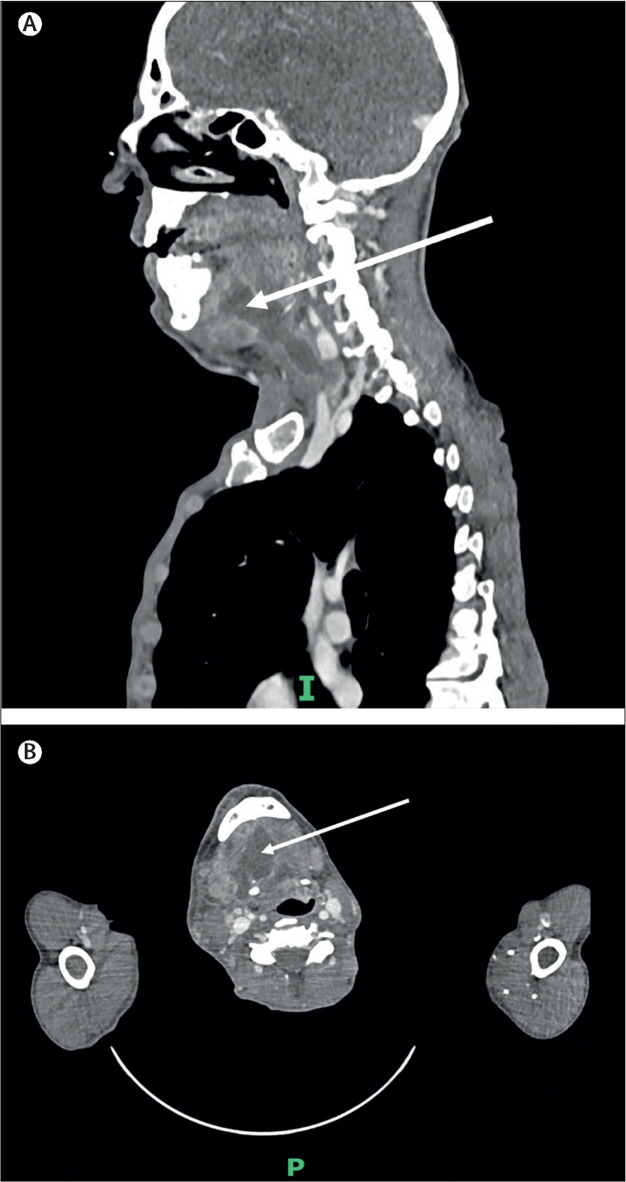
A 48-year-old man with HIV infection was admitted to hospital with an ulcerating and painful rash affecting his limbs and oral cavity, precluding oral intake (figure 1 ). He had a similar presentation 1 month earlier at another hospital. His CD4+ T-lymphocyte count at the time of initial presentation was 27 cells per mm3 but no HIV viral load was available. The patient had been diagnosed with HIV in late 2020 but had been lost to follow-up and off therapy for 12 months. A CT scan of his head showed an oedematous, hypervascular tongue with an ulcer on the lateral margin, but no drainable collections (figure 2 ) Tongue biopsy showed necrotic tissue heavily colonised with Pseudomonas aeruginosa, Leuconostoc spp, and Enterococcus durans, with no evidence of malignancy. Fungal stains were negative and there was no evidence of cytopathic viral change. He was diagnosed with human monkeypox virus (hMPXV) infection and commenced a 2-week course of 600 mg of oral tecovirimat twice a day. He was started on antiretrovirals (bictegravir 50 mg once daily, emtricitabine 200 mg once daily, and tenofovir alafenamide 25 mg once daily) and prophylactic co-trimoxazole at 480 mg once daily. A nasogastric tube was used for medication and nutrition; this was removed once the patient was able to eat and drink, and he was discharged home. The patient received teicoplanin (intravenous for 48 h with a loading dose, then a once daily maintenance dose) and co-amoxiclav (1·2 g three times a day intravenously for 7 days) to treat possible superadded bacterial infection; this was stopped on discharge.
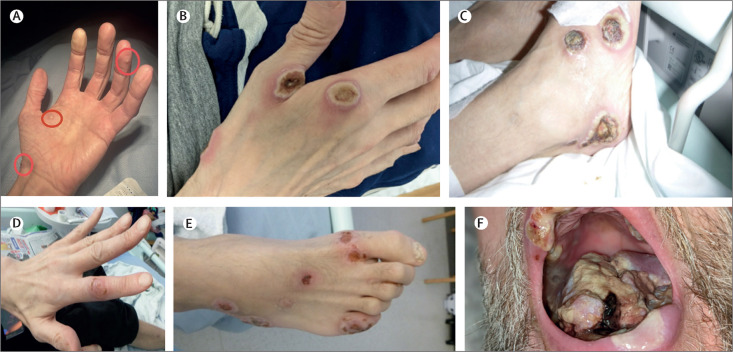
Figure 1.
Photos of the lesions
(A) Early lesions over that patient's hand. (B) Active lesions to hand. (C) Active lesions to foot. (D) Healing lesion to hand. (E) Healing lesions to foot. (F) Active lesions to floor of mouth and lips.
Figure 2.
CT images on day 3 of this admission
Sagittal view (A) and cross-sectional view with the arrows pointing to the floor of mouth areas (B).
2 weeks after discharge, the patient presented to our hospital with worsening of the rash and inability to eat and drink. Examination revealed approximately 20–30 lesions to his hands and feet (including palms and soles), face, scalp, and buttocks with firm, well circumscribed lesions of up to 2·5 cm in diameter, with central umbilication, and some visible slough (figure 1), consistent with worsening hMPXV infection. His oral cavity showed severe ulceration on the tongue and soft palate with areas of visible necrosis and sloughy material. He had a fullness in both submandibular regions. Blood tests showed neutrophilia (white cell count 12·5 × 109 per L, neutrophils 9·8 × 109 per L, lymphocytes 1·6 × 109 per L); a raised C-reactive protein (103 mg/L); normal renal and hepatic function; a CD4+ T-lymphocyte count of 57 cells per mm3 and HIV RNA viral load of 52 800 copies per mL. Serum cryptococcal antigen, galactomannan, and beta-D-glucan were negative. Blood and urine cultures were sterile. Sputum grew P aeruginosa (>100 000 colony-forming unit [CFU] counts per mL) and yeasts (10 000–100 000 CFU counts per L) but no acid-fast bacilli were seen. Syphilis serology was consistent with a previously treated infection. hMPXV DNA was once again detected on skin swabs, with a cycle threshold (Ct) value of 16. Screening swabs taken on admission showed methicillin-resistant Staphylococcus aureus (MRSA) colonisation. The patient had been self-isolating at home before the second admission and presentation. On admission, he was isolated according to UK national guidance with strict infection prevention and control measures with no onward nosocomial transmission.24
A repeat CT scan showed a 6 cm × 4 cm × 2 cm peripherally enhancing collection in the sublingual space of the floor of mouth, tracking down the right side of the neck, causing minor narrowing of the oropharynx and shift of the glottis to the left. Surgical exploration on day 3 of admission revealed no pus but inflamed fibrotic tissue with extensive lymphocyte and neutrophil infiltration; he was diagnosed with Ludwig's angina. He remained intubated after the procedure with a high-risk airway for 7 days before a surgical tracheostomy was performed. Histopathology showed extensive ulceration and inflamed squamous mucosa with no evidence of dysplasia. Pichia kudriavzevii was isolated from tongue biopsy.
He received intravenous piperacillin–tazobactam 4·5 g three times a day, metronidazole 500 mg three times a day, and fluconazole 800 mg loading and 400 mg once daily. On day 6, because of his clinical condition, severe immunocompromised state, and the low Ct value on skin swabs for hMPXV DNA of 16, a second course of enteral tecovirimat (600 mg twice a day) was started after discussion with the National Health Service England High Consequence Infectious Diseases (airborne; HCID) multidisciplinary network. This network includes infection specialists across the UK who discuss patients at high risk who have mpox. Intravenous vancomycin as an infusion at 167 mg/h was added on day 9 in light of the known MRSA colonisation and further clinical deterioration. Despite this addition, the oral and skin lesions did not improve. His antibiotics were broadened to intravenous meropenem 1 g three times daily, linezolid 600 mg twice daily, and anidulafungin 200 mg on day 1, then 100 mg once daily. After rediscussion at the HCID meeting, a trial of 3% topical cidofovir application was commenced for the oral and perinasal lesions as an adjuvant mpox therapy. On day 20, he required further aggressive debridement of necrotic tissue of the floor of the mouth and anterior oral tongue up to bleeding tissue. His HIV viral load was slow to improve, raising concerns about absorption; his antiretrovirals were therefore changed to emtricitabine and tenofovir disoproxil fumarate with crushed dolutegravir and his HIV viral load subsequently fell to 437 copies per mL.
On day 25 of admission, the patient showed the first signs of possible improvement to his oral and perinasal lesions; however, the peripheral skin lesions were not improving, and fresh lesions were evident on his face and feet. After rediscussing with the HCID network, tecovirimat was stopped (19 days into the second course) and, because of an observed response with topical cidofovir, an intravenous cidofovir dose (5 mg/kg) was given at day 25 of admission; adjuvant probenecid and hydration were given before and after the dose. The patient showed objective and subjective clinical response to this treatment, with evidence of healing of his skin lesions and improvement in his inflammatory markers; however, his blood, urine, and skin lesion hMPXV Ct values did not shift noticeably. He received two further doses; one at day seven and another at day 21 after the first dose. He showed continued improvement with no adverse drug reactions and stable renal function. He was discharged 52 days after his second admission and is well on follow-up as of Jan 23, 2023.
HIV and hMPXV co-infection
The data on HIV and hMPXV co-infection are conflicting, with two large case series5, 6 concluding that the clinical presentation of hMPXV infection in people living with and without HIV is similar, whereas smaller studies looking specifically at people with severe hMPXV disease have found a larger proportion of HIV-positive individuals. This apparent difference is probably because of the low prevalence of cases of mpox in patients with severe disease and advanced HIV.
A case series by Thornhill and colleagues6 presents 528 cases across 16 countries in a 2-month period during the 2022 outbreak outside of endemic countries. 218 (41%) of 528 individuals in their case series were living with HIV, with 95% in this cohort having well controlled disease with an undetectable viral load. Overall, the clinical presentation was similar, with three cases of serious complications, of which two had co-existing HIV infection: a case of epiglottitis in a person with a CD4+ cell count of less than 200 cells per mm3 that resolved with tecovirimat therapy and a case of self-limiting myocarditis in an individual with a CD4+ cell count of 780 cells per mm3.6
Tarín-Vicente and colleagues7 presented a case series of 181 patients across multiple centres in Spain, of whom 72 (40%) were living with HIV, with 8 of these having a CD4+ cell count less than 500 cells per mm3. There appeared to be no difference in the clinical features, incubation period, and number of lesions between people who were co-infected with HIV or not. The median time from lesion onset to crusting over was 11 days (IQR 8–14 days) in people living with HIV and 10 days (7–12 days) in people without HIV. No major complications of hMPXV infection were noted. However, because of the relatively high CD4+ cell counts of the included patients, the authors were unable to conclude whether more immunosuppressed individuals might be susceptible to more severe disease.7
Of note is a study published by the US Centers for Disease Control and Prevention (CDC), in which findings from 57 cases of severe hMPXV infection in hospitalised adults between August and October, 2022, were reported.8 Of the 57 individuals, 47 (82%) had HIV infection, of whom 43 (72%) had a CD4+ cell count less than 50 cells per mm3. A further 8 (14%) were immunocompromised for other reasons (haematological malignancy, solid organ transplant, and pregnancy.) In this cohort, 17 (30%) received intensive care unit support and 12 (21%) died (although not all deaths have been attributed to mpox).8
Yinka-Ogunleye and colleagues9 present a case series from Nigeria (2017–18) of 118 confirmed cases, among which there were 7 deaths; 4 of the deaths occurred in patients living with HIV who had clinical features of advanced HIV. In another case series from Nigeria of 40 patients, the authors found that the nine HIV-positive individuals were significantly more likely to have lesions larger than 2 cm, prolonged illness, and the presence of genital ulcers and superadded bacterial infection.10 At least a third of the individuals were severely immunosuppressed with a CD4+ cell count of less than 101 cells per mm3, a third had a cell count more than 300 cells per mm3, and there are no data on CD4+ for the final third.10
In terms of the immunological response in patients living with HIV who have low CD4+ counts, there are no data published from the 2022 outbreak. However, a recent paper presents results from an investigation of the immunological signature of hMPXV. In an Italian study of 17 patients (of whom 7 were living with HIV but all with CD4+ counts of >500 cells per mm3), an early expansion of CD4+ and CD8+ T cells which persisted with time was noted; this activation profile reduced during the course of the illness and with recovery.11 In almost all patients, whether HIV-positive or not, a poxvirus-specific Th1 inflammatory cytokine response developed; this persisted until and sometimes after clinical recovery. The signature in patients with and without HIV infection did not differ.11 Further investigations are required on immune responses to hMPXV infection in people with severe immunosuppression are required.
Antiviral therapy
Despite several thousand cases of human hMPXV infection reported annually in west and central Africa, where mortality of up to 10% is reported, there was little sustained investment in therapeutics and vaccinations when cases were localised to these settings.12 There has, however recently been promising progress with the initiation of randomised controlled trials (RCTs). Two are sponsored by the National Institute of Allergy and Infectious Diseases, one primarily based in DR Congo (NCT05559099) and the other in the USA (NCT05534984), looking at the safety and efficacy of tecovirimat in treating mpox.13 Oxford University, Oxford, UK, launched the PLATINUM trial in August, 2022. This UK-wide, community-based RCT randomly allocates patients to receive either oral tecovirimat or placebo.14 Endpoints of interest are symptom resolution and viral clearance. The main antiviral recommended for hMPXV has been tecovirimat, although other therapeutics have been used on compassionate grounds.
The evidence base for tecovirimat in hMPXV
Tecovirimat is a novel antiviral drug that works via inhibition of the highly conserved viral envelope protein p37—found in all orthopoxviruses, and essential for replicative formation of new virions. Tecovirimat (or ST-246) was first identified in 2005 during high-throughput screening of 356 240 compounds on their ability to inhibit the cytopathic effect of poxviruses on in-vitro cell cultures.15 Of the 759 compounds identified, tecovirimat was evaluated further—whether others were evaluated further in the study is unclear. It was shown to be a potent but specific inhibitor of poxviruses (including vaccinia, cowpox, mpox, and two strains of variola viruses) in in-vitro models, with low concentrations required to inhibit the cytopathic effects of poxviruses by 50% (median effective dose [ED50]=0·01 uM) but very high concentrations required to inhibit other RNA and DNA viruses (ED50>40 uM). Tecovirimat was also shown to have good oral bioavailability in mice models; however, absolute bioavailability was 31% when comparing intravenous versus oral administration.15
Further in-vitro studies in monkey models have shown efficacy of tecovirimat against mpox infection. In one study, 8 monkeys were infected with the Zaire 1979 strain of hMPXV.16 The monkeys were divided into two cohorts, with three monkeys in each cohort receiving tecovirimat 300 mg/kg for 14 days and the other monkey receiving a placebo. In one cohort, the monkeys received tecovirimat on day 1 after infection, and the other on day 3. Administration of tecovirimat was shown to be protective against disease, with 100% fatality in both monkeys who received placebo (day 9 and 13) but no deaths in the ones who received tecovirimat. In addition, the monkeys in the placebo group developed approximately 1500 skin lesions, whereas none were observed in the drug-treated group. This study also showed an almost 5 log reduction in hMPXV DNA upon administration of tecovirimat.16 There were no differences in side-effects between the two groups.
The effectiveness of tecovirimat in mpox infection was further shown in other monkey models, with reducing efficacy if tecovirimat was commenced from 4 days after infection,17, 18 with one study showing survival rates of 67% when tecovirimat was commenced at day 6 and 50% when commenced at day 8 (6 monkeys in each cohort).18 In addition, a Canadian study examined the hMPXV strain responsible for the 2022 outbreak, and found that tecovirimat reduced viral titres after 1 week and 2 weeks in their mouse model.19
A phase 3 trial20 has shown tecovirimat appears to be well tolerated with only one adverse event recorded (pulmonary embolus) in the 359 people who received the drug.20 The most common recorded adverse events were headache (16·99% affected), nausea (5·57%), diarrhoea (3·06%), vomiting (2·51%) and dizziness (2·51%).
There have been two case reports of the use of tecovirimat in human cases of hMPXV in the UK, one in an adult patient (presented as part of a case series of 7),21 and the other in a neonate.22 The first patient acquired hMPXV while caring for her daughter; she developed malaise, headache, pharyngitis, and lesions to her chest.21, 22 She had been isolating either at home or in hospital for 35 days but still tested positive for hMPXV DNA. As such, a decision was made to commence a 14-day course of 600 mg tecovirimat twice daily to shorten the duration of viral shedding and expedite the end of her isolation. Compared with the other six patients in the case series reported by Adler and colleagues21 (three of whom received oral brincidofovir and the remainder supportive treatment only), she had a shorter duration of both viral shedding and hospitalisation (10 days vs 13–39 days). 24 h after starting treatment, she developed no new lesions, and her blood and pharyngeal hMPXV PCR became negative after 48 h. She remained clinically well throughout and was discharged from hospital at day 10. By contrast, the other patients in the case series had a median hospital stay of 24 days (range 13–39). She had no adverse effects from her tecovirimat, whereas all three patients who received brincidofovir had their treatment course stopped after developing a drug-related transaminitis.21
The second case report of tecovirimat use was in a neonate who developed a vesicular rash on day 9 of life.22 The father had a widespread rash and associated fever 9 days before the birth and the mother presented with a similar rash 4 days after birth. On day 15, the infant developed respiratory failure and was subsequently transferred to intensive care, where blood, urine, vesicular fluid, and throat swabs from both mother and child were PCR-positive for hMPXV. The patient also tested positive for adenovirus. After an initial clinical deterioration, the patient was treated with a 14-day course of tecovirimat alongside cidofovir; cidofovir was used for adenovirus pneumonia rather than for mpox. The patient was discharged home after a 4-week intensive care stay and remains well.22
Tecovirimat was approved for use by the US Food and Drug Administration (FDA) in July, 2018, for the treatment of smallpox after concerns about the virus being used as a bioweapon.23 It was granted authorisation for use in hMPXV infections under exceptional circumstances by the European Medicines Agency on Jan 6, 2022, and subsequently was authorised in the UK by the Medicines and Healthcare products Regulatory Agency on June 30, 2022, although it currently remains unlicensed. Oral tecovirimat is available for severe cases of mpox within the UK. A national commissioning policy outlining criteria for use is available (panel ).25
Panel. NHS England eligibility criteria for the use of tecovirimat in the treatment of hospitalised patients with human monkeypox virus infection in the UK Clinical Commissioning Rapid Policy Statement.
Eligibility criteria published on Sept 20, 2022,15 on the use of tecovirimat as treatment for patients hospitalised due to monkeypox virus infection.
Eligibility criteria
Hospitalised patients must meet all of the eligibility criteria and none of the exclusion criteria listed below:
-
•
Mpox is confirmed by PCR testing
and
-
•
Symptomatic with a syndrome compatible with ongoing monkeypox virus infection
and
-
•
Meeting any one or more of the criteria24 for severe or complicated disease as outlined below:
-
•Critical illness where monkeypox virus infection is considered to be a key factor driving the critical condition of the patient
-
•Intractable pain
-
•Rectal abscess or fistula formation
-
•Upper respiratory tract mucocutaneous involvement that is affecting swallowing or airways
-
•Patient with primary or acquired immunodeficiency, or on immunosuppressive medication as per Green Book definitions
-
•Ocular or periocular disease
-
•Encephalitis, meningitis, or other neurological manifestation
-
•Extensive cutaneous disease (eg, having more than 100 lesions)
-
•Complex genital disease: difficulty passing urine due to swelling or lesions causing direct urinary obstruction
-
•
Exclusion criteria
Patients are not eligible for treatment if any of the following apply:
-
•
Hospitalised for reasons other than monkeypox virus infection or do not meet any of the criteria for severe and complicated disease
-
•
Known hypersensitivity reaction to the active substances or to any of the excipients of the medications listed in their respective Summary of Product Characteristics
-
•
Adults and children with a bodyweight less than 13 kg
Tecovirimat absorption requires concurrent high fat content meals; however, in the patient described in this Grand Round, his antiretroviral medicines required administration on an empty stomach. This required careful planning as he was also fed enterally through the nasogastric tube. It is recommended that 600 kcal, which includes 25 g of lipid, is provided with tecovirimat. The patient was provided with enteral nutrition via boluses twice daily with the tecovirimat, which included a high-energy feed with additional fat emulsion. An overnight continuous feed was also provided for 8 hours between doses of antiretroviral medicines to allow nutritional requirements to be met. Complexities of delivery included the risk of overfeeding during the acute phase due to additional calories received from sedatives such as propofol and poor tolerance of bolus feed. Tolerance issues included loose stools, abdominal pain, and vomiting.
Although an intravenous preparation of tecovirimat has been developed, it is not available in the UK and we were unable to source it. In addition, we did not have genomic sequencing of the patient's hMPXV at that time so there was a concern as to whether his virus had developed any tecovirimat-resistance mutations. Since then, we have preliminary sequencing results that are not yet ready for dissemination; there is further work underway to systematically investigate potential drug resistance through sequencing this (and other patients') hMPXV DNA, the results of which will be disseminated in due course. This might have explained the apparent treatment failure with slow improvement to existing lesions and newly developing lesions despite his extended course of tecovirimat. Mutations leading to tecovirimat resistance have been described in in-vitro studies of other orthopoxviruses, with single-point mutations in the F13L gene conferring resistance. In addition, the CDC sent a Health Alert Network alert on Nov 17, 2022, confirming two cases of tecovirimat-resistant hMPXV infection.26, 27 Genomic sequencing identified mutations in the F13L gene, and further cell cultures confirmed phenotypic resistance. As in our case, both patients were severely immunocompromised with disseminated hMPXV disease, and showed progression of disease despite an extended (>14 days) course of tecovirimat. In a case report of progressive vaccinia (another orthopoxvirus) in an individual who was immunocompromised secondary to acute myeloid leukaemia, resistance to tecovirimat developed late into the disease, with tecovirimat levels fluctuating below the therapeutic level during his extended oral treatment.28 It was hypothesised in this reported case that the fluctuating levels could have been due to absorption issues.
Anecdotally, in patients with immunosuppression, longer courses of tecovirimat might be needed; however, there is insufficient guidance in the public domain for this indication. Tecovirimat also appears to be relatively well tolerated; however, this patient developed abdominal discomfort, a recognised side-effect with this medication.
Potential for cidofovir use in severe hMPXV infection
Because of concerns about this patient's clinical state, including his severe immunocompromised state and the severity of his hMPXV infection, intravenous cidofovir was used. Cidofovir is a prodrug that is phosphorylated intracellularly to its active form cidofovir diphosphate. It inhibits DNA synthesis by incorporation into the DNA strand during replication.29 Previous studies have shown that cidofovir appears to have good in-vitro activity against many DNA viruses, including adenovirus, herpesviruses, papillomavirus, polyomavirus, and poxviruses, such as hMPXV.30, 31, 32 It is currently licenced in the UK and by the FDA for the treatment of cytomegalovirus retinitis but is not approved by any regulatory bodies in the treatment of hMPXV.33 Cidofovir has poor oral bioavailability at less than 5% and is usually administered intravenously.34 The drug's main limitation is dose-dependent nephrotoxicity and it is therefore often co-administered with oral probenecid alongside pre-hydration fluids.35 Of interest, despite objective clinical improvement with intravenous cidofovir after only a few days, the virological response did not correlate with the clinical progress initially (table ).
Table.
Summary of the Ct values for orthopoxvirus DNA detection
| EDTA | Serum | Skin swab | Throat swab | Urine | |
|---|---|---|---|---|---|
| 34 days before admission | NA | NA | Positive; Ct 26 | NA | NA |
| Day 5 | NA | NA | Positive; Ct 16 | NA | NA |
| Day 9 | Positive; Ct 27 | NA | NA | NA | NA |
| Day 16 | NA | NA | NA | NA | Positive; Ct 36 |
| Day 22 | Positive; Ct 29 | NA | Positive; Ct 22 | Positive; Ct 20 | Positive; Ct 36 |
| Day 23 | Positive; Ct 30 | NA | Positive; Ct 20 | NA | Positive; Ct 36 |
| Day 27 | Positive; Ct 31 | NA | Positive; Ct 25 | NA | Positive; Ct 30 |
| Day 30 | Positive; Ct 32 | NA | Positive; Ct 23 | Positive; Ct 25 | Positive; Ct 36 |
| Day 41 | Positive; Ct 31/32 | Positive; Ct 33/31 | Positive; Ct 25/22 | NA | Negative/Negative |
| Day 42 | Positive; Ct 32 | Positive; Ct 32 | Positive; Ct 18 | Positive; Ct 25 | Positive; Ct 36 |
| Day 44 | Positive; Ct 33 | Positive; Ct 34 | Positive; Ct 19 | NA | Negative |
| Day 50 | NA | NA | Positive; Ct 25 | Positive; Ct 30 | |
| Day 59 | NA | Positive; Ct 35 | Positive for foot and hand; Ct 22/26 | Positive; Ct 36 | Negative |
Found on PCR of blood samples (EDTA and serum), swabs of skin lesions, throat swabs, and urine samples. Quoted as day of hospital admission. Ct=cycle threshold. NA=data not available.
The effects of cidofovir have been shown in multiple animal models.36, 37 hMPXV does not cause disease in adult mice and hence mouse models have mainly studied the effects of cidofovir on other orthopoxviruses, such as cowpox and vaccinia.38 Stittelaar and colleagues39 evaluated the use of intraperitoneal cidofovir in hMPXV infection in monkey models. In their study, macaques were infected with a lethal dose of hMPXV. In the untreated control group, all six animals died by day 15. In the other groups, either 5 or 6 doses of cidofovir were given, at the same dose used in humans, between day 1 and day 13 after infection. In these groups, five animals did not suffer any severe morbidity and four did not die from their hMPXV infection. In addition, no monkeys suffered substantial renal dysfunction. Plasma viral load of hMPXV DNA was also statistically lower in the monkeys treated with cidofovir than in the non-treated control group.39 Similar results have been seen in other monkey models when intravenous cidofovir at a dose of 5 mg/kg was either given as prophylaxis or on day 2 after hMPXV infection, with positive findings of reducing viral titres and number of body lesions on the animals and preventing deaths.40, 41 However, these studies are only available as abstracts and do not have peer-reviewed data, with their results simply being referenced in review papers.36, 37
A small number of case studies support the use of cidofovir for the treatment of hMPXV in humans. A case series from the San Raffaele Scientific Institute in Milan, Italy, described four men with severe hMPXV infection who were each given a single dose of intravenous cidofovir between June and August, 2022.42 One of these patients presented with pharyngeal and laryngeal involvement, similar to the case described here. The other cases presented with disease at various sites including cutaneous and genital, rectal, and ocular involvement.43 The authors reported a rapid improvement within days in all cases, evidenced by a decrease in numbers and crusting of mpox lesions and resolution of presenting symptoms. Hence, further administrations of cidofovir were not required. There were no reported adverse events or any new symptoms after cidofovir administration.42 Separately, another study described the treatment of one patient at the Luigi Sacco Hospital in Milan who was given two administrations of intravenous cidofovir at day 13 and day 20 due to tecovirimat unavailability.43 This patient had initially presented with several nasal vesicular lesions associated with high fever and possible superimposed bacterial infection with a large eschar that subsequently developed despite antibiotic treatment. After cidofovir administration and an 8-day period of hospitalisation, he had complete recovery of his symptoms within 6 weeks.43 There has also been a published case report describing the compassionate use of 1% topical cidofovir to treat a nasal lesion with anatomical deformity, co-infected with hMPXV and HSV-1. After 5 days, there was improvement both aesthetically and symptomatically with cessation of pain.44
Brincidofovir is an oral prodrug of cidofovir that is cleaved to form cidofovir after ingestion and cellular uptake. One study in prairie dogs from 2021 reported a 29% survival rate when administering brincidofovir on day one compared with placebo group survival of 14%.45 This survival improved the sooner brincidofovir was administered. Human studies are scarce with one study showing no convincing benefit despite early discontinuation due to deranged liver enzymes.46 However, it was not available in the UK at the time and there was concern about enteral absorption in this patient.
Ludwig's angina as a rare complication of hMPXV infection
This case of hMPXV infection is associated with Ludwig's angina, something that has not been reported in the literature previously. Superficial oropharyngeal lesions are common in mpox;47 Patel and colleagues48 noted they affected 13·7% (95% CI 9·2–19·3) of infected individuals.48 Deeper seated infections (tonsillar abscesses) were noted in 2 of 197 individuals in the same case series, and an earlier report documents a child with retro-pharyngeal phlegmon.49 However, an association between hMPXV infection and Ludwig's angina has not been recorded in the previous literature, which makes this an unusual and particularly severe case. The case also presented challenges to both airway and surgical management requiring careful multidisciplinary management. The Ludwig's angina severely restricted hyolaryngeal movement, which resulted in a substantial oropharyngeal dysphagia characterised by aspiration with all food and drink and restricted transfer through the upper oesophageal sphincter. The patient decided to eat and drink acknowledging aspiration and the nasogastric tube was removed. The long-term prognosis for his dysphagia is dependent on his Ludwig's angina.
In this patient, ascertaining how much of his systemic features were being driven by bacterial rather than hMPXV infection is difficult. Biopsies from the Ludwig's angina grew organisms that were covered by the antimicrobials given. However, despite this, there was little objective evidence of improvement until the intravenous cidofovir was commenced suggesting that hMPXV was an important driver of his symptoms.
Conclusion
We present the case of a patient with severe HIV-related immunosuppression who had prolonged, active lesions due to hMPXV infection despite commencing a second, extended course of tecovirimat on his re-presentation. He had severe Ludwig's angina as a complication that required careful surgical debridement and airway management. He had a persistently positive hMPXV DNA on swabs, blood, and urine with low Ct values. We highlight three main points. Because of objective and subjective improvement in the patient's clinical condition with intravenous cidofovir, we explore the evidence base for this treatment and suggest that it can be considered in severe cases of hMPXV in which there is no improvement on tecovirimat. However, cidofovir is not without side-effects, particularly nephrotoxicity. Nephrotoxicity did not occur in this patient despite three doses, but we would suggest caution with the use of intravenous cidofovir in patients at high risk of this complication. The topically administered cidofovir appeared to provide objective improvement to the nasal lesions. We highlight practical challenges in managing the need for tecovirimat administration with a high-fat meal and the need for antiretrovirals to be administered on an empty stomach. We emphasise the need for trials and data that investigate the role of intravenous cidofovir in severe hMPXV infection as well as optimal dosing and number of doses. Careful consideration of the threshold to commence treatment and balanced against the risk of adverse events is essential. Additionally, the route of administration (eg, topical or oral preparations of tecovirimat and brincidofovir) needs further investigation.
In conclusion, to our knowledge, this is the first use of intravenous cidofovir in the UK for severe hMPXV infection with objective and subjective clinical improvement and no adverse events. This is a single case, however, which we hope will stimulate discussion about its use. At present, similar presentations need careful, expert, multidisciplinary discussion, and informed consent from patients before use.
Search strategy and selection criteria
We searched PubMed for the terms “(monkeypox OR mpox OR hMPX) AND (tecovirimat OR cidofovir OR brincidofovir OR treatment OR Ludwig's angina)”. All literature prevailing to the treatment of mpox (formerly known as monkeypox) up to the date Dec 20, 2022, was included.
Declaration of interests
DA-J has received consultancy fees or honoraria from Gilead and Pulmocide and has stock or stock options in Pulmocide. MG has received consultancy fees from Pfizer. FD receives a Clinical Academic Research Partnerships Fellowship from the Medical Research Council. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We would like to acknowledge and thank the National Health Service (NHS) England High Consequence Infectious Diseases (airborne) Network for their guidance and support over the course of this patient's management and the patient himself for encouraging and agreeing to a write-up of his case. We would also like to thank the UK Health Security Agency (UKHSA) Rare and Imported Pathogens Laboratory team for their support and the UKHSA Mpox Genomics Group. This study was supported in part by the Biomedical Research Centre of Imperial College NHS Healthcare Trust.
Contributors
AS, SR, KS, EPD, MG, and AA contributed to the writing of the first draft and subsequent revisions. MG, CSW, CC, DA-J, TS, FD, CHMG, VK, AEH, ZA, CS, BM-P, DM, PR, JP, MC, CEW, TR, GC, SD, VC, CD, and SO contributed to reviews, discussions, and comments that were incorporated into the text. All authors reviewed the final version of the manuscript.
References
- 1.Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8:129–157. doi: 10.2217/fvl.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO WHO recommends new name for monkeypox disease. 2022. https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease
- 3.WHO Multi-country monkeypox outbreak: situation update 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON390
- 4.WHO Director-General declares the ongoing monkeypox outbreak a public health emergency of international concern. 2022. https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern [PMC free article] [PubMed]
- 5.Centers for Disease Control and Prevention 2022 monkeypox outbreak global map. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- 6.Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 7.Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400:661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller MJ, Cash-Goldwasser S, Marx GE, et al. Severe monkeypox in hospitalized patients—United States, August 10–October 10, 2022. 2022. https://www.cdc.gov/mmwr/volumes/71/wr/pdfs/mm7144e1-H.pdf Centers for Disease Control and Prevention. [DOI] [PMC free article] [PubMed]
- 9.Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogoina D, Iroezindu M, James HI, et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis. 2020;71:e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 11.Agrati C, Cossarizza A, Mazzotta V, et al. Immunological signature in human cases of monkeypox infection in 2022 outbreak: an observational study. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00662-4. published online Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozlov M. Monkeypox in Africa: the science the world ignored. Nature. 2022;607:17–18. doi: 10.1038/d41586-022-01686-z. [DOI] [PubMed] [Google Scholar]
- 13.Sherwat A, Brooks JT, Birnkrant D, Kim P. Tecovirimat and the treatment of monkeypox—past, present, and future considerations. N Engl J Med. 2022;387:579–581. doi: 10.1056/NEJMp2210125. [DOI] [PubMed] [Google Scholar]
- 14.University of Oxford Oxford University launch new clinical trial to test a treatment for monkeypox. 2022. https://www.ox.ac.uk/news/2022-08-24-oxford-university-launch-new-clinical-trial-test-treatment-monkeypox
- 15.Yang G, Pevear DC, Davies MH, et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53:2620–2625. doi: 10.1128/AAC.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berhanu A, Prigge JT, Silvera PM, Honeychurch KM, Hruby DE, Grosenbach DW. Treatment with the smallpox antiviral tecovirimat (ST-246) alone or in combination with ACAM2000 vaccination is effective as a postsymptomatic therapy for monkeypox virus infection. Antimicrob Agents Chemother. 2015;59:4296–4300. doi: 10.1128/AAC.00208-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo AT, Grosenbach DW, Brasel TL, et al. Effects of treatment delay on efficacy of tecovirimat following lethal aerosol monkeypox virus challenge in cynomolgus macaques. J Infect Dis. 2018;218:1490–1499. doi: 10.1093/infdis/jiy326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warner BM, Klassen L, Sloan A, et al. In vitro and in vivo efficacy of tecovirimat against a recently emerged 2022 monkeypox virus isolate. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.ade7646. [DOI] [PubMed] [Google Scholar]
- 20.Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44–53. doi: 10.1056/NEJMoa1705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramnarayan P, Mitting R, Whittaker E, et al. Neonatal monkeypox virus infection. N Engl J Med. 2022;387:1618–1620. doi: 10.1056/NEJMc2210828. [DOI] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration FDA approves the first drug with an indication for treatment of smallpox. 2018. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-indication-treatment-smallpox
- 24.UK Health Security Agency Mpox (monkeypox): background information. 2022. https://www.gov.uk/guidance/monkeypox#infection-prevention-and-control
- 25.NHS England Clinical Commissioning Rapid Policy Statement: tecovirimat as treatment for patients hospitalised due to monkeypox virus infection. 2022. https://www.england.nhs.uk/commissioning/publication/tecovirimat-as-treatment-for-patients-hospitalised-due-to-monkeypox-virus-infection/
- 26.Duraffour S, Lorenzo MM, Zöller G, et al. ST-246 is a key antiviral to inhibit the viral F13L phospholipase, one of the essential proteins for orthopoxvirus wrapping. J Antimicrob Chemother. 2015;70:1367–1380. doi: 10.1093/jac/dku545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Update on managing monkeypox in patients receiving therapeutics. 2022. https://emergency.cdc.gov/han/2022/han00481.asp
- 28.Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:1372–1385. doi: 10.1093/infdis/jis510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magee WC, Hostetler KY, Evans DH. Mechanism of inhibition of vaccinia virus DNA polymerase by cidofovir diphosphate. Antimicrob Agents Chemother. 2005;49:3153–3162. doi: 10.1128/AAC.49.8.3153-3162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu J, Raj SM. Efficacy of three key antiviral drugs used to treat orthopoxvirus infections: a systematic review. Glob Biosecurity. 2019;1:28. [Google Scholar]
- 31.Baker RO, Bray M, Huggins JW. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 2003;57:13–23. doi: 10.1016/S0166-3542(02)00196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Clercq E. Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clin Microbiol Rev. 2003;16:569–596. doi: 10.1128/CMR.16.4.569-596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Food and Drug Administration FDA approves cidofovir for treatment of CMV retinitis. J Int Assoc Physicians AIDS Care. 1996;2:30. [PubMed] [Google Scholar]
- 34.Wachsman M, Petty BG, Cundy KC, et al. Pharmacokinetics, safety and bioavailability of HPMPC (cidofovir) in human immunodeficiency virus-infected subjects. Antiviral Res. 1996;29:153–161. doi: 10.1016/0166-3542(95)00829-2. [DOI] [PubMed] [Google Scholar]
- 35.Cundy KC, Petty BG, Flaherty J, et al. Clinical pharmacokinetics of cidofovir in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1995;39:1247–1252. doi: 10.1128/aac.39.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smee DF. Progress in the discovery of compounds inhibiting orthopoxviruses in animal models. Antivir Chem Chemother. 2008;19:115–124. doi: 10.1177/095632020801900302. [DOI] [PubMed] [Google Scholar]
- 37.Andrei G, Snoeck R. Cidofovir activity against poxvirus infections. Viruses. 2010;2:2803–2830. doi: 10.3390/v2122803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bray M, Martinez M, Smee DF, Kefauver D, Thompson E, Huggins JW. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J Infect Dis. 2000;181:10–19. doi: 10.1086/315190. [DOI] [PubMed] [Google Scholar]
- 39.Stittelaar KJ, Neyts J, Naesens L, et al. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature. 2006;439:745–748. doi: 10.1038/nature04295. [DOI] [PubMed] [Google Scholar]
- 40.Huggins J, Raymond JL, Fisher R, Jahrling P, Hensley L. Sequential determination of virus in blood and tissues of the variola cynomolgus monkey model of classical smallpox reveals that IV cidofovir can effectively treat monkeys with extensive viral burden. 19th International Conference on Antiviral Research. May 7–11, 2006 [Google Scholar]
- 41.Huggins J, Martinez M, Hartmann C, et al., editors. Successful cidofovir treatment of smallpox-like disease in Variola and monkeypox primate models. 17th International Conference on Antiviral Research; May 2–6, 2004. [Google Scholar]
- 42.Raccagni AA-O, Candela C, Bruzzesi E, et al. Real-life use of cidofovir for the treatment of severe monkeypox cases. J Med Virol. 2023;95:e28218. doi: 10.1002/jmv.28218. [DOI] [PubMed] [Google Scholar]
- 43.Moschese D, Giacomelli A, Beltrami M, et al. Hospitalisation for monkeypox in Milan, Italy. Travel Med Infect Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escudero-Tornero RA-O, Sobral-Costas TA-O, De Moraes-Souza RA-O. Monkeypox lesions affecting the nose: a therapeutical challenge. J Eur Acad Dermatol Venereol. 2022 doi: 10.1111/jdv.18540. published online Aug 20. [DOI] [PubMed] [Google Scholar]
- 45.Hutson CL, Kondas AV, Mauldin MR, et al. Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model. MSphere. 2021;6:e00927–e00930. doi: 10.1128/mSphere.00927-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao AK, Schulte J, Chen TH, et al. Monkeypox in a traveler returning from Nigeria—Dallas, Texas, July 2021. MMWR Morb Mortal Wkly Rep. 2022;71:509–516. doi: 10.15585/mmwr.mm7114a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ardila CM, Arrubla-Escobar DE, Vivares-Builes AM. Oral lesions in patients with human monkeypox: a systematic scoping review. J Oral Pathol Med. 2022 doi: 10.1111/jop.13375. jop.13375. [DOI] [PubMed] [Google Scholar]
- 48.Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson MG, Frenkel LD, Homann S, Guffey J. A case of severe monkeypox virus disease in an American child: emerging infections and changing professional values. Pediatr Infect Dis J. 2003;22:1093–1096. doi: 10.1097/01.inf.0000101821.61387.a5. [DOI] [PubMed] [Google Scholar]