Abstract
The timing and amplitude of plant signaling are frequently regulated through posttranslational modification of key signaling sectors, which facilitates rapid and flexible responses. Protein ubiquitination can serve as a degradation marker, influence subcellular localization, alter protein-protein interactions, and affect protein activity. Identification of polyubiquitinated proteins has been challenging due to their rapid degradation by the proteasome or removal of modifications by deubiquitination enzymes (DUBs). Tandem ubiquitin binding entities (TUBEs) are based on ubiquitin-associated domains and protect against both proteasomal degradation and DUBs. Here, we provide a protocol for purification of ubiquitinated plant proteins using TUBEs after transient expression in Nicotiana benthamiana. This protocol can also be applied to other plants to purify multiple ubiquitinated proteins or track ubiquitination of a target protein. This methodology provides an effective method for identification of ubiquitin ligase substrates and can be coupled with TUBEs targeting specific ubiquitination linkages.
Keywords: Tandem ubiquitin binding entities, TUBE, Ubiquitination, Plant ubiquitination
1. Introduction
Posttranslational modification of proteins can rapidly regulate their localization, activity, and stability. Attachment of the small protein ubiquitin affects protein function and fate [1]. Polyubiquitination is critical for the degradation of target proteins by the proteasome or lysosome/vacuole [2, 3]. Therefore, the detection and purification of polyubiquitinated proteins help understand protein regulation, abundance, and activity. E1, E2, and E3 enzymes catalyze ubiquitination, with specificity for protein targets provided by the E3 ubiquitin ligase [4, 5]. The majority of E3 ubiquitin ligases present in plant genomes remain uncharacterized.
Identification of ubiquitinated proteins has been mainly dependent on immunoprecipitation after overexpressing epitope-tagged ubiquitin and the use of ubiquitin antibodies. However, ubiquitous expression of ubiquitin and isolation of ubiquitinated proteins remain difficult by conventional immunoprecipitation. Trypsin digestion of cellular lysates also reveals a ubiquitin remnant motif (diGly (Lys-ε-Gly-Gly) that can be detected by mass spectrometry and has been used for large-scale proteomic analyses [6, 7]. In addition, purification of polyubiquitinated proteins can be performed by using ubiquitin-associated domains (UBAs), but their low affinity for ubiquitin is problematic. Physical interactions with an E3 ligase mutant and plant target in yeast have also identified candidates for future investigation.
The identification of plant substrates for ubiquitin ligases is challenging, primarily due to the removal of ubiquitin by deubi-quitination enzymes (DUBs) and rapid degradation of polyubiquitinated proteins [8]. Tandem ubiquitin binding entities (TUBEs) have been developed to overcome this problem and are tandem polymerized UBAs with very strong binding affinity with dissociation constants for tetra-ubiquitin in the nanomolar range (Fig. 1a) [9, 10]. Importantly, TUBEs protect substrate proteins from both DUBs and proteasome-mediated degradation, even in the absence of proteasome inhibitors, which can minimize inhibitor-induced physiological interruption [9]. TUBEs can be pan-selective, binding all polyubiquitin linkages, or chain-selective, binding specific linkages. Furthermore, TUBEs can be conjugated to different moieties enabling enrichment, detection, and imaging of polyubiquitinated proteins (Fig. 1b) [11, 12].
Fig. 1.
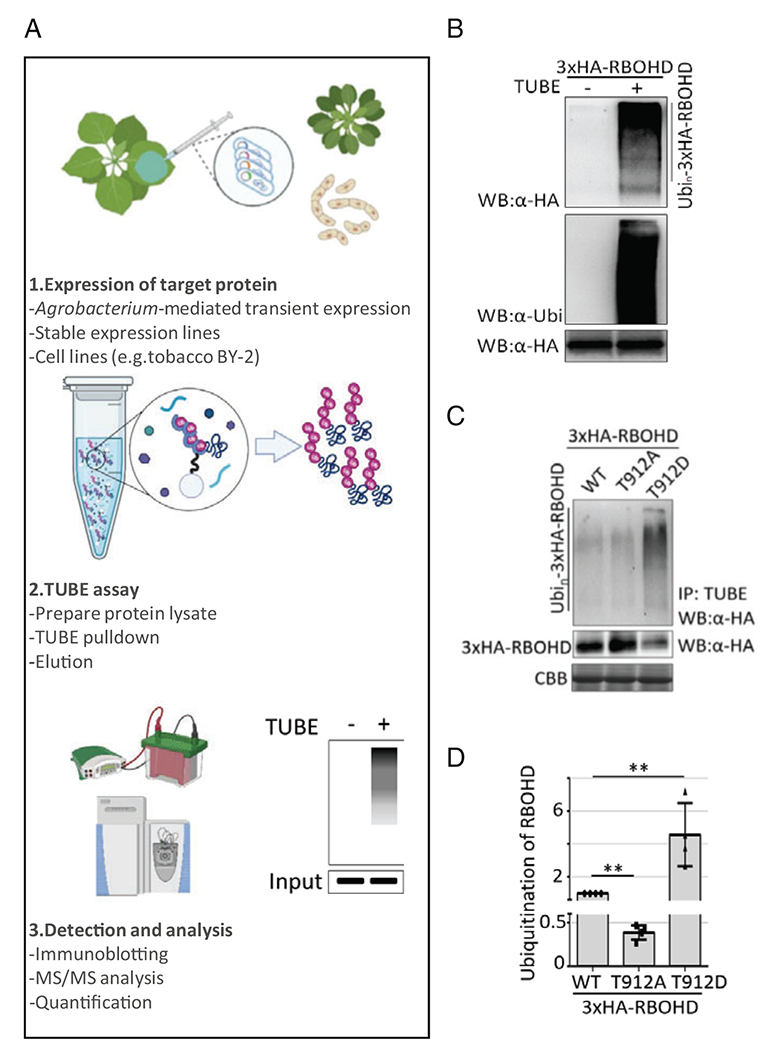
Detection and quantification of polyubiquitination by TUBE assay. (a) Schematic protocol of the TUBE assay to identify ubiquitination in vivo. (b) Ubiquitination of RBOHD is detected by pull-down using the TUBE assay described in this paper. Polyubiquitinated proteins were purified using agarose resin conjugated with TUBE (+) from lysate of leaf tissue expressing 3xHA-RBOHD in N. benthamiana after Agrobacterium-mediated transient expression. Canonical smearing band pattern induced by addition of ubiquitin proteins was detected on the immunoblotting after TUBE pull-down. Agarose resin (−) was used as a control to verify unspecific binding on the resin matrices. Ubiquitinated RBOHD (top panel) and input of RBOHD (bottom panel) were detected by immunoblotting with anti-HA. Immunoblotting using anti-ubiquitin (middle panel) shows the specificity of the TUBE assay to purify ubiquitinated proteins. (c) Detection of the level of ubiquitination of RBOHD variants in planta. Phosphomimetic RBOHDT912D exhibited enhanced polyubiquitination of RBOHD, and phospho-null RBOHDT912A showed reduced polyubiquitination in vivo (C, top panel). Bottom panels indicated input. RBOHD’s ubiquitination was detected by TUBE assay after expressing 3xHA-RBOHD variants (WT, T912A, and T912D) in N. benthamiana. (d) Quantification of ubiquitination of RBOHD phosphomutants. The level of ubiquitination of each variant was calculated by the ratio of polyubiquitination intensity compared to input signal intensity (intensity of top panel/intensity of middle panel). Relative intensity of ubiquitination in samples was normalized to the intensity of wild-type (WT) RBOHD ubiquitination. (Images in B–D are reprinted from Ref. [13] with permission from the Nature Publishing Group)
While TUBEs have been broadly used to purify and characterize polyubiquitinated proteins in mammalian cell lines and tissues, the use of TUBEs in plant systems is less common. Here, we report a TUBE assay combined with Agrobacterium-mediated transient expression in Nicotiana benthamiana as a simple and highly sensitive method to detect polyubiquitination in planta. This straightforward method represents a powerful tool for investigating protein ubiquitination and can be easily modified to detect and isolate various ubiquitinated proteins in planta.
2. Materials
2.1. Plant Growth Conditions
N. benthamiana plants were grown in a growth chamber under the following conditions: 25 °C, 50% relative humidity, a 14 h light/10 h dark photoperiod, and a light intensity of 180 μE/m2/s.
2.2. Expression of Target Protein: Agrobacterium- Mediated Transient Expression
Expression constructs for target proteins: Gateway binary (pGWB) 13 vector harboring wild-type (WT) RBOHD or T912 mutants (RBOHD T912A or RBOHD T912D). The vector, tag, and tag orientation for a target protein need to be determined experimentally.
Agrobacterium tumefaciens GV101 Strain
LB medium: 10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl. Autoclave and store at room temperature.
Antibiotics: Kanamycin (50 ug/ml), rifampicin (50 ug/ml), and chloramphenicol (25 ug/ml, see Note 1).
Agrobacterium infiltration buffer: 10 mM MgCl2, 10 mM 2-(N-morpholino) ethanesulfonic acid (MES), and 150 μM acetosyringone.
1 ml needleless syringe.
2.3. TUBE Assay
TUBE-conjugated agarose resin and control agarose (TUBE 1 cat# UM401, control agarose cat# UM400).
Lysis buffer: 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM dithiothreitol (DTT), 1 mM ethylenediaminetetraacetic acid (EDTA), 10% glycerol, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1× protease inhibitor, 2% octylphenoxypo-lyethoxyethanol (IGEPAL), 50 μM PR-619, and 5mM 1–10-phenanthoroline (see Notes 2 and 3).
1X Phosphate-buffered saline (PBS): 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4, pH 7.4
1X Tris-buffered saline with 0.1% tween (TBST): 20 mM Tris, 150 mM NaCl, 0.1% tween-20, and pH 8.0
6x Laemmli buffer: 0.375 M Tris pH 6.8, 12% SDS, 60% glycerol, 0.6 M DTT, and 0.06% bromophenol blue.
2.4. Detection and Analysis
SDS-PAGE gel: 30% Bis/acrylamide, 1.5 M Tris-HCl pH 8.8 (for resolving gel), 0.5 M Tris-HCl pH 6.8 (for stacking gel), 10% sodium dodecyl sulfate (SDS), 10% ammonium persulfate, and N,N,N′,N′-tetramethylenediamine (TEMED) (see Note 4).
SDS-PAGE running buffer: 25 mM Tris, 192 mM glycine, and 0.1% SDS.
Transfer buffer: 25 mM Tris, 192 mM glycine, and 10% methanol.
Five percent skim milk in TBST.
Antibodies: anti-HA antibody conjugated with horseradish peroxidase (HRP) (a clone 3F10, cat# 12013819001), anti-ubiquitin antibody (clone P4D1-A11, cat# 05–994), goat anti-mouse IgG (Biorad,1,706,516).
Enhanced chemiluminescence (ECL) substrate.
2.5. Equipment
Temperature-controlled centrifuge.
Rotator (earthquake shaker).
Shaker platform (for immunoblotting).
Temperature-controlled shaking incubator.
Controlled growth chamber or room.
Heat block.
SDS-PAGE gel caster, gel running, and wet transfer system.
Immunoblotting imaging system (e.g., Biorad Chemidoc Imaging System).
3. Methods
3.1. Plant Growth Conditions
N. benthamiana plants are grown vegetatively in a controlled growth room (see Note 5). Four–five-week-old N. benthamiana were used for most of our experiments (see Note 6). For most of our experiments, 0.5–1 g of leaf tissue sample is used.
3.2. Expression of the Target Protein: Agrobacterium-Mediated Transient Expression
This section describes the expression of your protein of interest to test polyubiquitination in planta by Agrobacterium-mediated transient expression in N. benthamiana. The samples harvested after transient expression will subsequently be used for the TUBE assay. The protocol can be modified to detect proteins stably expressed in planta by skipping this section.
Inoculate 5 ml LB media with Agrobacterium containing the target gene in a binary vector, add appropriate antibiotics, and culture overnight at 28 °C in a shaking incubator.
Transfer 1 ml of culture into a fresh 1.5 ml microtube and pellet cells at 10,000 × g for 3 min at 16 °C and discard supernatant.
Resuspend pellet in 1 ml of infiltration buffer and repeat centrifugation. Discard supernatant to completely remove residual culture media.
Measure the absorbance at 600 nm after tenfold dilution of cells in the infiltration buffer and calculate the OD of the resuspension.
Adjust OD600 of the bacterial suspension to 0.2 using the infiltration buffer (see Note 7).
Incubate for 2–3 h at room temperature under rotation in the infiltration buffer to induce Agrobacteria.
Choose fully expanded healthy leaves from 3 to 4-week-old N. benthamiana and infiltrate the Agrobacteria suspension in the leaves with a 1 ml needleless syringe and mark the infiltrated leaf or area.
Remove excess suspensionon the leaves by gently tapping with a paper tissue and move the plants back to the growth room.
Harvest samples after 24–48 h post-infiltration, weigh samples, and store in −80 °C after wrapping in an aluminum foil packet and snap freezing in liquid Nitrogen.
3.3. Sample Preparation and TUBE Assay
The isolation and detection of polyubiquitination by the TUBE assay relies on the expression of the target protein and quality of protein extracts. Thus, much care should be taken during buffer preparation and tissue processing. This section describes the preparation of samples and step-by-step procedures for the TUBE assay. After pull-down using TUBE-conjugated agarose resin, the proteins eluted from the resin will subsequently be resolved on an SDS-PAGE gel, and the status of polyubiquitination of target protein will be determined by immunoblotting (see Note 8).
3.3.1 Protein Extraction
Thaw 40 μl of a 50% slurry of TUBE-conjugated resin slurry per sample, resuspend on an earthquake shaker at 4 °C, and keep on ice (see Note 9).
Grind 0.5–1 g of leaf tissue in liquid N2 with mortar and pestle. Resuspend in 2 ml per 1 g of sample in lysis buffer (see Note 10).
Transfer the sample into a 1.5 ml microtube and incubate for 30 min with rotation on an earthquake shaker (see Note 11).
Centrifuge at 10,000 × g for 10 min at 4 °C to remove large tissue debris.
Transfer supernatant to a new microtube and centrifuge at 20,000 × g for 20 min at 4 °C to remove cell debris.
Filter the supernatant using a 0.45 μM sterile membrane with low protein binding affinity to reduce contamination caused by tissue/cell debris (see Note 12).
Measure the protein concentration of the samples using Bradford assay, and save 50 μl of sample to use as an input (see Note 13).
Add Laemmli buffer to a final concentration at 1.5× and incubate for 10 min at 65 °C; store extracted protein at −20 °C.
During centrifugation of the cell lysate, equilibrate TUBE-conjugated resin and control agarose resin in the lysis buffer. Add 1 ml of PBS into 40 μl of TUBE-conjugated resin slurry, centrifuge at 3000 rpm for 2 min, and discard supernatant. Repeat the wash of the TUBE resin; remove supernatant.
Preclear lysate with 40 μl of equilibrated control agarose (50%) slurry per sample in 2 ml for 2 hr. at 4 °C with rotation in order to avoid nonspecific binding caused by the agarose matrices (see Note 14).
Centrifuge at 5000 rpm for 2 min at 4 °C and transfer the resulting supernatant to a new microtube.
Divide precleared lysate into two fresh tubes, and incubate with either the TUBE-conjugated agarose or the control agarose resin at 4 °C for 18 h under rotation (see Note 15).
After incubation, wash the resin with 1 ml of TBST buffer by inverting gently, centrifuge at 3000 rpm for 2 min at 4 °C, and discard supernatant.
Repeat the previous step four times.
Elute bound proteins with 50 μl of 1.5× Laemmli buffer and incubate for 10 min at 65 °C.
Store the eluted samples in −20 °C.
3.4. Analysis of Ubiquitination by Immunoblotting
This procedure explains SDS-PAGE electrophoresis and immunoblotting to detect the accumulation of the target protein and the status of polyubiquitination by immunoblotting:
Prepare the appropriate percentage of SDS-PAGE gel based on your target protein’s molecular weight (see Note 16).
Resolve 20 μl of sample by SDS-PAGE including input and elution.
Transfer proteins to activated PVDF (polyvinylidene) membranes by wet transfer (see Note 17).
After transfer, block the membrane with 5% skim milk and incubate with primary antibody for 18 h at 4 °C with agitation (see Note 18).
Wash the membrane with TBST with agitation for 5 minutes, discard the buffer, and repeat. A 10 mL of TBST is sufficient for an 8 × 10 cm membrane.
Incubate with 10 mL secondary antibody for 1–2 h at RT in 5% skim milk.
Wash the membrane using 10 mL TBST with agitation for 5 minutes, discard TBST, and repeat three times.
Detect your target protein after incubation with ECL using a ChemiDoc. Smeared high molecular weight bands are typically detected in the elution sample after incubation with TUBE due to addition of polyubiquitin on the target protein (Fig. 1b, c) (see Note 19).
Analyze the level of target protein’s ubiquitination using an image quantification software such as ImageJ or Bio-Rad Image lab software. The level of ubiquitination can be quantified by comparing the signal intensity of elution samples after TUBE pull-down to the signal intensity of input samples (Fig. 1c, d).
Acknowledgments
GC and DH were supported by a grant from the National Institutes of Health (NIH 1R35GM136402).
4 Notes
The A. tumefaciens GV101 strain is resistant to chloramphenicol and rifampicin; kanamycin resistance is imparted by the binary vector.
1x Protease inhibitor, DTT (1 M), and PMSF (100 mM) stocks are unstable at room temperature or 4 °C and should be stored at −20 °C. Aliquot small volumes to avoid repeated freezethawing. Add to solutions immediately before use.
IGEPAL is chosen for solubilizing membrane proteins. The buffer and detergent composition will need to be determined based on target protein localization and features.
Adjust the amount of each reagent for appropriate percentage of SDS-PAGE gel according to target protein’s molecular weight.
For consistent protein expression by Agrobacterium-mediated transient expression, controlled growth conditions and tissue treatments are required. The conditions can be adapted to your target protein and should be determined empirically. Transgenic plants or cell lines expressing target proteins (e.g. Arabidopsis transgenic lines or tobacco BY-2 cell lines, respectively) can also be used. Detectable expression of the target protein in the input is required for the detection of polyubiquitination by the TUBE assay (Fig. 1a).
Young N. benthamiana plants exhibit higher transient protein expression. Use plants that are less than five weeks old and have not flowered.
Verify the final density of Agrobacterium by measuring the OD600 after dilution. The titer of the Agrobacterium suspensionshould be determined empirically (typically in the range of OD600 = 0.4–1.0). In order to enhance expression, a silencing inhibitor such as P19 can be co-infiltrated with target genes.
Multiple TUBEs are available, and the protocol can be modified if protein detection by mass spectrometry is required.
TUBE-conjugated agarose resin should be stored in −20 °C. Repeated freeze/thaw cycles should be avoided.
If your target expression is low, or you need more input for further experiments such as mass spectrometry, the amount of sample can be increased. Additionally, the enrichment of certain organelles prior to the TUBE assay could reduce the background and enhance detection of polyubiquitinated proteins depending on the target protein localization.
This step is required for proteins with transmembrane domains but should be omitted for soluble proteins. Longer incubation could enhance the solubility of your target protein but also decrease its stability; optimal timing of incubation should be checked empirically.
Supernatant can also be filtered using miracloth.
For simple detection of polyubiquitination, protein quantification can be omitted since the same amount of lysate will be incubated with control agarose and TUBE-conjugated agarose. However, protein quantification is necessary to compare the level of polyubiquitination among different treatments (e.g., chemical treatment, gene mutation effect, etc.). In our experiment conditions, more than 5 ug/ul of total proteins from 1 g of leaf tissues was repeatedly obtained.
The timing of preclearing should be adjusted empirically depending on the level of background based on immunoblotting.
The incubation time should be checked and adjusted empirically for your target proteins.
A smear signal from the target protein is typical due to addition of ubiquitins on the target protein.
We prefer to use wet transfer compared to semidry or quick transfer machines to maximize the efficacy of transfer for high molecular weight polyubiquitinated target proteins. We obtained good transfer at 300 mA for 2.5 h at 4 °C.
The incubation time, antibody concentration, and other steps during immunoblotting can be adjusted based on the target protein and antibody combination. The optimal condition should be determined empirically.
Different types of secondary antibody-enzyme conjugates, substrates, and imaging systems can be used according to your secondary antibody (e.g. alkaline phosphatase [AP], fluorescence, etc.). We used HRP-conjugated primary or secondary antibody with an ECL substrate.
References
- 1.Kliza K, Husnjak K (2020) Resolving the Complexity of Ubiquitin Networks. Front Mol Biosci 7(21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clague MJ, Urbé S (2010) Ubiquitin: same molecule, different degradation pathways. Cell 143(5):682–685 [DOI] [PubMed] [Google Scholar]
- 3.Varshavsky A (2017) The ubiquitin system, autophagy, and regulated protein degradation. Annu Rev Biochem 86:123–128 [DOI] [PubMed] [Google Scholar]
- 4.Callis J (2014) The ubiquitination machinery of the ubiquitin system. Arabidopsis Book 12: e0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deol K, Lorenz S, Strieter ER (2019) Enzymatic logic of ubiquitin chain assembly. Front Physiol 10:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grubb LE et al. (2021) Large-scale identification of ubiquitination sites on membrane-associated proteins in Arabidopsis thaliana seedlings. Plant Physiol 185(4):1483–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udeshi ND et al. (2013) Large-scale identification of ubiquitination sites by mass spectrometry. Nat Protoc 8(10):1950–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reyes-Turcu FE, Ventii KH, Wilkinson KD (2009) Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem 78:363–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hjerpe R et al. (2009) Efficient protection and isolation of ubiquitylated proteins using tandem ubiquitin-binding entities. EMBO Rep 10(11):1250–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopitz-Otsoa F, Rodriguez MS, Aillet F (2010) Properties of natural and artificial proteins displaying multiple ubiquitin-binding domains. Biochem Soc Trans 38(1):40–45 [DOI] [PubMed] [Google Scholar]
- 11.Akinjiyan FA et al. (2020) A Novel Luminescence-Based High-Throughput Approach for Cellular Resolution of Protein Ubiquitination Using Tandem Ubiquitin Binding Entities (TUBEs). SLAS DISCOVERY: Advancing the Science of Drug Discovery 25(4):350–360 [DOI] [PubMed] [Google Scholar]
- 12.Mattern M et al. (2019) Using ubiquitin binders to decipher the ubiquitin code. Trends Biochem Sci 44(7):599–615 [DOI] [PubMed] [Google Scholar]
- 13.Lee D et al. (2020) Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat Commun 11(1):1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]


