Post-traumatic epilepsy (PTE) is a serious consequence of traumatic brain injury (TBI). PTE develops following a latent postinjury period during which alterations in neuronal network excitability are thought to occur, leading to the development of recurrent and unprovoked seizures. In particular, pathophysiological changes in the dentate gyrus (DG) have been suggested as key mediators of epileptogenesis following TBI.
In order to understand the development of PTE, researchers have focused on aspects of the condition that are shared with other forms of epilepsy, such as temporal lobe epilepsy. One of the most striking observations in patients with either type of epilepsy is the sprouting of granule cell (GC) axons (known as mossy fibers) in the inner molecular layer of the DG, where GC dendrites are located (Swartz et al., 2006). Normally, mossy fibers form excitatory synapses onto CA3 pyramidal cells, hilar mossy cells, and GABAergic interneurons, but rarely synapse onto other GCs (for review, see Amaral et al., 2007). This lack of recurrent synapses is thought to inherently limit the spread of excitation in the DG. Modeling studies predict that mossy fiber sprouting would compromise this, allowing activity to spread and even become self-sustaining: conditions that are favorable for seizures (Santhakumar et al., 2005). In addition to synapsing with GCs, sprouted mossy fibers have also been shown to target inhibitory interneurons (Puhahn-Schmeiser et al., 2021). However, the contribution of such sprouting to the development of epilepsy is currently unclear.
While the relevance of mossy fiber sprouting in epilepsy is still debated (Buckmaster, 2014), it highlights an important concept in epilepsy research, namely, the long-term reorganization of the local DG circuit. This reorganization may also involve abnormal integration of adult-born GCs. Under normal physiological conditions, new neurons are continuously produced throughout adulthood in the DG of rodents. These adult-born GCs integrate into preexisting circuits (for review, see Kempermann et al., 2015). As adult-born GCs mature, they begin to shape the DG circuit in several ways, such as by recruiting feedback inhibition onto mature GCs (Temprana et al., 2015; Drew et al., 2016). However, if these adult-born GCs do not integrate properly into the circuit, they may become a source of aberrant circuit reorganization. Indeed, previous studies in models of temporal lobe epilepsy have shown that adult-born GCs display abnormal plasticity, which is dependent on their maturational stage at the time of the epileptogenic insult (Kron et al., 2010). The functional consequences of abnormal plasticity for the reorganization of the DG circuit were recently identified by the characterization of newly generated synapses arising from GCs born at different times with respect to epileptogenic insult (Hendricks et al., 2017, 2019). However, it remains to be determined whether a GC maturational stage-dependent reorganization of excitatory and inhibitory neuronal circuits occurs in the DG after TBI.
In a recent study, Kang et al. (2022) investigated whether mouse GCs born at different times differentially contribute to shaping the DG local circuit after controlled cortical impact (CCI), a model of TBI. Circuit reorganization was assessed by studying excitation and inhibition of GCs by other GCs: specifically, recurrent excitation in which a GC excites another GC; and feedback inhibition, in which a GC inhibits another GC via an interneuron. Cohorts of GCs born at three different times relative to CCI were selectively labeled with channelrhodopsin2 (ChR2) and EYFP in different mice. Specifically, GCs that were mature at the time of injury (early-born), GCs that were born just before injury (adult-born pre-CCI), or GCs that were born just after injury (adult-born post-CCI) were labeled. Synaptic reorganization was assessed in hippocampal slices by stimulating ChR2-expressing GCs and recording evoked responses in ChR2-negative cells, including parvalbumin-expressing (PV+) interneurons and unlabeled mature GCs. To assess long-term effects, experiments were performed 8-10 weeks after TBI, a time point when adult-born GCs have integrated into the circuit and have mature properties (Zhao et al., 2006).
Kang et al. (2022) first examined whether distinct cohorts of GCs shape feedback inhibition after CCI. Optogenetic stimulation of GCs in all cohorts evoked IPSCs in at least some ChR2-negative mature GCs. However, stimulation of adult-born pre-CCI GCs evoked IPSCs in >70% of recorded mature GCs, substantially more than that evoked by similarly aged GCs from sham-injured mice. This large upregulation of evoked IPSCs compared with respective sham controls was not seen for adult-born post-CCI GCs or early-born GCs, although the latter showed a nonsignificant trend toward an increase. The evoked IPSCs were confirmed to be mediated by polysynaptic feedback inhibition through their pharmacological and electrophysiological properties. These results suggest that GCs that are immature at the time of injury undergo abnormal plasticity that leads to an increased inhibition of surrounding mature GCs.
Next, the authors sought to identify the targets of the adult-born pre-CCI GCs that underlie increased feedback inhibition. The DG is composed of several types of inhibitory interneurons that mediate different forms of inhibition (for review, see Houser, 2007). In the DG, a dominant form of feedback inhibition is lateral inhibition among GCs via fast-spiking GABAergic PV+ interneurons (Espinoza et al., 2018). Kang et al. (2022) recorded from basket cells, a subtype of PV+ interneurons, and found reduced intrinsic excitability after injury. Despite this, optogenetic stimulation of adult-born pre-CCI GCs evoked larger EPSCs and triggered action potential firing in a greater proportion of PV+ basket cells than in sham-injured controls. GCs born before CCI thus appear to form strong excitatory synaptic inputs onto PV+ basket cells, driving the increased inhibition of mature GCs. These findings are in contrast to the reduced cortical activation of PV+ interneurons previously found to occur early after TBI, predicted to compromise PV+-interneuron-mediated feedforward inhibition of GCs (Folweiler et al., 2020). These temporally distinct changes in the synaptic reorganization of inputs to PV+ interneurons may underlie the differences in DG excitability seen during these periods (Santhakumar et al., 2001), with early DG hyperexcitability potentially mediated by reduced feedforward inhibition of GCs followed by later increased feedback inhibition of GCs, contributing to the return to baseline excitability observed at 1 month after injury.
The increased synaptic strength between adult-born pre-CCI GCs and PV+ basket cells could potentially have resulted from a change in presynaptic release probability, change in postsynaptic receptors, and/or sprouting of mossy fibers. Although Kang et al. (2022, their Fig. 2) found mossy fiber sprouting in the DG of CCI mice, the synaptic data cannot be solely attributed to mossy fiber sprouting since GCs synapse with PV+ basket cells under physiological conditions. The authors thus concluded that the increased synaptic excitation of PV+ basket cells may be because of increased synaptic contacts and/or changes in the excitatory network.
Last, Kang et al. (2022) examined the contribution of each cohort of GCs to recurrent excitation following CCI. Surprisingly, optogenetic stimulation of ChR2-expressing GCs in all cohorts rarely evoked EPSCs in ChR2-negative mature GCs. Further, external conditions used to evoke hyperexcitability also failed to reveal any recurrent excitation. Overall, these data indicate that, in the CCI model of TBI, early-born and adult-born GCs do not contribute to recurrent excitation. Instead, changes in local circuits appear to be limited to increased input from adult-born pre-CCI GCs to PV+ basket cells, which results in more extensive feedback inhibition of mature GCs.
The findings by Kang et al. (2022) highlight a possible critical period during which adult-born GCs can contribute to circuit reorganization after TBI. The maturation and integration of adult-born GCs into the DG circuit follow a sequential process, in which GABAergic and glutamatergic signaling is important throughout (for review, see Jahn and Bergami, 2018). TBI markedly impacts the DG circuit, with loss of both GABAergic and glutamatergic neurons, as well as changes in the excitability of surviving cells (for review, see Hunt et al., 2013). The GCs born before CCI are <2 weeks postmitosis at the time of injury, a period when GABA-mediated depolarization is involved in GC maturation (Ge et al., 2006; Chancey et al., 2013). GCs that were immature at the time of injury may not be exposed to the necessary GABAergic signaling, perhaps driving their abnormal integration into local feedback inhibitory circuits. In accordance with this, circuit reorganization by adult-born GCs in a model of temporal lobe epilepsy was recently suggested to be driven by increased GABAergic activity following epileptogenic insult (Lybrand et al., 2021). As GC maturation progresses, the impact of the circuit on their development evolves (Schmidt-Hieber et al., 2004; Tashiro et al., 2006). This later maturational stage was not investigated by Kang et al. (2022); thus, it would be worthwhile to determine whether immature GCs that are older at the time of injury differentially contribute to circuit reorganization.
Remarkably, GCs born after injury did not show any enhanced contribution to feedback inhibition or recurrent excitation after CCI, suggesting limited involvement in circuit reorganization. This is consistent with previous work showing that GCs born after injury exhibit normal synaptic innervation and excitability, despite increased dendritic branching and migration in the GC layer (Villasana et al., 2015). Whether neurogenesis after TBI is beneficial or pathologic remains unclear. While neurogenesis may be required for cognitive recovery after TBI (Blaiss et al., 2011), limiting TBI-induced neurogenesis reduces seizure susceptibility (Neuberger et al., 2017). If adult-born post-CCI GCs do contribute to PTE, the findings by Kang et al. (2022) would suggest that this might be via a mechanism other than reorganization of feedback inhibition and recurrent excitation. Further work will be required to understand the underlying mechanism.
Could increased feedback inhibition lead to the development of PTE? The view that seizures occur as a result of an imbalance between inhibition and excitation risks oversimplification. For instance, evidence is emerging for roles of PV+ interneurons in both generating and maintaining seizures (for review, see Magloire et al., 2019). The increased feedback inhibition observed by Kang et al. (2022) suggests that adult-born GCs may influence the activity of a larger proportion of mature GCs after CCI. In this case, PV+ interneurons could paradoxically promote seizure generation by enhancing coordinated spiking in a group of principal cells, a mechanism previously shown in temporal cortex slices (Sessolo et al., 2015).
Alternatively, increased feedback inhibition could reflect a homeostatic mechanism. The DG circuit is hyperexcitable early after injury when GCs born before injury are beginning to integrate into the circuit (Lowenstein et al., 1992). A bias toward synapsing with PV+ interneurons could compensate for this excitability, potentially limiting seizure duration (Hosford et al., 2016). It will be important to determine how selective ablation of GCs born pre-CCI impacts the development of PTE.
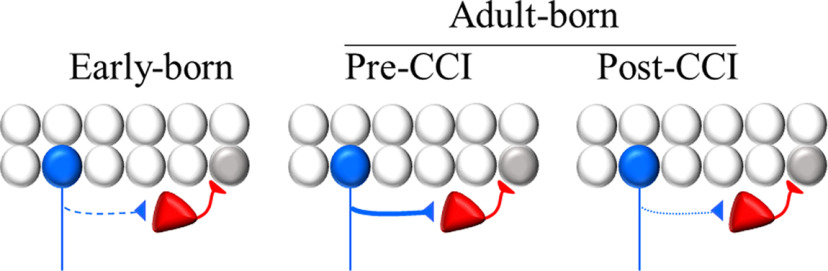
Overall, the study by Kang et al. (2022) provides new evidence for a maturational-stage-dependent contribution of adult-born GCs to network reorganization after TBI (schematically depicted in Fig. 1). The restriction to feedback inhibition brings into the question the hypothesis that recurrent excitation via mossy fiber sprouting is responsible for driving synaptic reorganization that leads to PTE. Although the implications for the development of PTE remain unclear, the current study highlights a role for a specific subpopulation of adult-born GCs in TBI-induced plasticity.
Figure 1.
Schematic of the main conclusions from Kang et al. (2022) illustrating a maturational stage-dependent contribution of GCs to feedback inhibition after CCI. Mature GCs that are born during the early postnatal period (P8-P12; early-born) show a moderate increase in their contribution to feedback inhibition (indicated with a dashed line) compared with similarly aged GCs in sham-injured mice. Whereas GCs that are immature at the time of injury (adult-born pre-CCI) show a substantial increase in feedback inhibition (thick line), GCs born after injury (adult-born post-CCI) have a limited contribution to feedback inhibition that is not different from what would be expected from this population under normal conditions (dotted line). Blue represents ChR2-expressing GC from each cohort. Red represents PV+ basket cell. Gray represents a ChR2-negative mature GC used for electrophysiological recordings.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/jneurosci-journal-club.
The work was supported by grants from the Canadian Institutes of Health Research (#10677), the Koerner Foundation, and the Canada Research Chair in Biotechnology and Genomics-Neurobiology (to Terrance P. Snutch). I thank Dr. Terrance P. Snutch and Dr. Esperanza Garcia for helpful discussions and comments on this paper.
The authors declare no competing financial interests.
References
- Amaral DG, Scharfman HE, Lavenex P (2007) The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res 163:3–22. 10.1016/S0079-6123(07)63001-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaiss CA, Yu TS, Zhang G, Chen J, Dimchev G, Parada LF, Powell CM, Kernie SG (2011) Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J Neurosci 31:4906–4916. 10.1523/JNEUROSCI.5265-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS (2014) Does mossy fiber sprouting give rise to the epileptic state? Adv Exp Med Biol 813:161–168. [DOI] [PubMed] [Google Scholar]
- Chancey JH, Adlaf EW, Sapp MC, Pugh PC, Wadiche JI, Overstreet-Wadiche LS (2013) GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J Neurosci 33:6614–6622. 10.1523/JNEUROSCI.0781-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Kheirbek MA, Luna VM, Denny CA, Cloidt MA, Wu MV, Jain S, Scharfman HE, Hen R (2016) Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons. Hippocampus 26:763–778. 10.1002/hipo.22557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza C, Guzman SJ, Zhang X, Jonas P (2018) Parvalbumin+ interneurons obey unique connectivity rules and establish a powerful lateral-inhibition microcircuit in dentate gyrus. Nat Commun 9:4605. 10.1038/s41467-018-06899-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folweiler KA, Xiong G, Best KM, Metheny HE, Nah G, Cohen AS (2020) Traumatic brain injury diminishes feedforward activation of parvalbumin-expressing interneurons in the dentate gyrus. eNeuro 7:ENEURO.0195-19.2020. 10.1523/ENEURO.0195-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H (2006) GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439:589–593. 10.1038/nature04404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks WD, Chen Y, Bensen AL, Westbrook GL, Schnell E (2017) Short-term depression of sprouted mossy fiber synapses from adult-born granule cells. J Neurosci 37:5722–5735. 10.1523/JNEUROSCI.0761-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks WD, Westbrook GL, Schnell E (2019) Early detonation by sprouted mossy fibers enables aberrant dentate network activity. Proc Natl Acad Sci USA 116:10994–10999. 10.1073/pnas.1821227116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford BE, Liska JP, Danzer SC (2016) Ablation of newly generated hippocampal granule cells has disease-modifying effects in epilepsy. J Neurosci 36:11013–11023. 10.1523/JNEUROSCI.1371-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR (2007) Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog Brain Res 163:217–232. 10.1016/S0079-6123(07)63013-1 [DOI] [PubMed] [Google Scholar]
- Hunt RF, Boychuk JA, Smith BN (2013) Neural circuit mechanisms of post-traumatic epilepsy. Front Cell Neurosci 7:89. 10.3389/fncel.2013.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn HM, Bergami M (2018) Critical periods regulating the circuit integration of adult-born hippocampal neurons. Cell Tissue Res 371:23–32. 10.1007/s00441-017-2677-x [DOI] [PubMed] [Google Scholar]
- Kang YJ, Lee SH, Boychuk JA, Butler CR, Juras JA, Cloyd RA, Smith BN (2022) Adult born dentate granule cell mediated upregulation of feedback inhibition in a mouse model of traumatic brain injury. J Neurosci 42:7077–7093. 10.1523/JNEUROSCI.2263-21.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Song H, Gage FH (2015) Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol 7:a018812. 10.1101/cshperspect.a018812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron MM, Zhang H, Parent JM (2010) The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J Neurosci 30:2051–2059. 10.1523/JNEUROSCI.5655-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK (1992) Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci 12:4848–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lybrand ZR, Goswami S, Zhu J, Jarzabek V, Merlock N, Aktar M, Smith C, Zhang L, Varma P, Cho KO, Ge S, Hsieh J (2021) A critical period of neuronal activity results in aberrant neurogenesis rewiring hippocampal circuitry in a mouse model of epilepsy. Nat Commun 12:1423. 10.1038/s41467-021-21649-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magloire V, Mercier MS, Kullmann DM, Pavlov I (2019) GABAergic interneurons in seizures: investigating causality with optogenetics. Neuroscientist 25:344–358. 10.1177/1073858418805002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger EJ, Swietek B, Corrubia L, Prasanna A, Santhakumar V (2017) Enhanced dentate neurogenesis after brain injury undermines long-term neurogenic potential and promotes seizure susceptibility. Stem Cell Rep 9:972–984. 10.1016/j.stemcr.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhahn-Schmeiser B, Leicht K, Gessler F, Freiman TM (2021) Aberrant hippocampal mossy fibers in temporal lobe epilepsy target excitatory and inhibitory neurons. Epilepsia 62:2539–2550. 10.1111/epi.17035 [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Ratzliff AD, Jeng J, Toth Z, Soltesz I (2001) Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann Neurol 50:708–717. 10.1002/ana.1230 [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Aradi I, Soltesz I (2005) Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: a network model of the dentate gyrus incorporating cell types and axonal topography. J Neurophysiol 93:437–453. 10.1152/jn.00777.2004 [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jones P, Bischofberger J (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429:184–187. 10.1038/nature02553 [DOI] [PubMed] [Google Scholar]
- Sessolo M, Marcon I, Bovetti S, Losi G, Cammarota M, Ratto GM, Fellin T, Carmignoto G (2015) Parvalbumin-positive inhibitory interneurons oppose propagation but favor generation of focal epileptiform activity. J Neurosci 35:9544–9557. 10.1523/JNEUROSCI.5117-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz BE, Houser CR, Tomiyasu U, Walsh GO, DeSalles A, Rich JR, Delgado-Escueta A (2006) Hippocampal cell loss in posttraumatic human epilepsy. Epilepsia 47:1373–1382. 10.1111/j.1528-1167.2006.00602.x [DOI] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH (2006) NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442:929–933. 10.1038/nature05028 [DOI] [PubMed] [Google Scholar]
- Temprana SG, Mongiat LA, Yang SM, Trinchero MF, Alvarez DD, Kropff E, Giacomini D, Beltramone N, Lanuza GM, Schinder AF (2015) Delayed coupling to feedback inhibition during a critical period for the integration of adult-born granule cells. Neuron 85:116–130. 10.1016/j.neuron.2014.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasana LE, Kim KN, Westbrook GL, Schnell E (2015) Functional integration of adult-born hippocampal neurons after traumatic brain injury. eNeuro 2:ENEURO.0056-15.2015. 10.1523/ENEURO.0056-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Ming GL, Gage FH (2006) Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26:3–11. 10.1523/JNEUROSCI.3648-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]