Abstract
A 76-year-old woman with advanced pancreatic cancer developed recurrent cholecystitis after covered self-expandable metal stent (CSEMS) placement. The cholecystitis was refractory to repeated percutaneous transhepatic gallbladder drainage (PTGBD). Cholecystography showed a patent cystic duct with right and cranial side bifurcation, which is indicative of an increased likelihood of success of endoscopic transpapillary gallbladder drainage (ETGBD). We were able to manage the cholecystitis by ETGBD without further recurrence. ETGBD is considered an effective internal drainage method for the management of acute cholecystitis after CSEMS placement, and its indication may be decided on the basis of the findings of cholecystography through the PTGBD route.
Keywords: palliative, gallbladder drainage, endoscopic gallbladder stenting, endoscopic ultrasound, endoscopic retrograde cholangiopancreatography
Introduction
Placement of a covered self-expandable metal stent (CSEMS) is the standard palliative therapy for unresectable malignant biliary obstruction (MBO); however, acute cholecystitis commonly occurs as an adverse event after CSEMS placement, with a reported incidence rate of 5% to 10% (1-3). Cholecystitis after CSEMS placement is occasionally refractory to conservative management and requires percutaneous transhepatic gallbladder drainage (PTGBD), which can lead to prolonged discontinuation of chemotherapy and deterioration of the patient's quality of life (QOL).
We herein report a case of successful endoscopic transpapillary gallbladder drainage (ETGBD) for recurrent cholecystitis after CSEMS placement.
Case Report
The patient was a 76-year-old woman with MBO due to an unresectable locally advanced pancreatic cancer. She underwent palliative endoscopic transpapillary biliary drainage with a CSEMS (diameter, 10 mm; length, 8 cm, fully covered Hanaro stent; Boston Scientific, Natick, USA) for the MBO caused by pancreatic cancer. The cystic duct bifurcation was considered involved by the tumor and covered with the CSEMS based on the findings of the cholangiogram.
Ten days after CSEMS placement, she developed a fever and right upper abdominal pain with elevated levels of inflammatory markers on a blood examination (white blood cell count, 11,800/mm3; C-reactive protein level, 11.8 mg/dL). Computed tomography revealed an enlarged gallbladder with a thickened wall (Fig. 1). These findings indicated acute cholecystitis after CSEMS placement. Percutaneous transhepatic gallbladder aspiration (PTGBA) was performed, but it failed to manage the cholecystitis. PTGBD was performed four days after PTGBA, which successfully managed the cholecystitis. After the improvement of cholecystitis, the drainage tube was removed.
Figure 1.
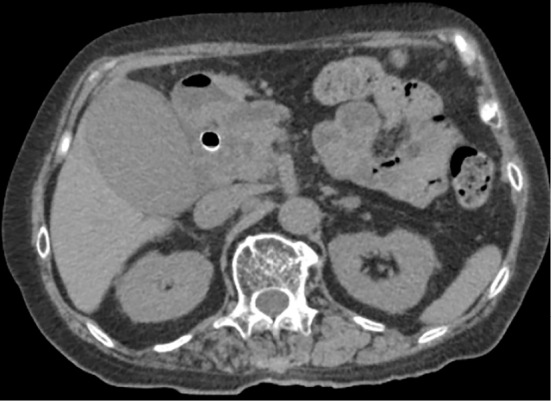
Abdominal computed tomography image showing swelling of the gallbladder and a previously placed covered metal stent.
Chemotherapy with gemcitabine and nab-paclitaxel for pancreatic cancer was subsequently initiated. However, cholecystitis recurred 50 days after the removal of the PTGBD tube. PTGBD was again required to manage the cholecystitis and successfully control the inflammation. We decided to perform permanent drainage of the gallbladder to prevent further recurrence of cholecystitis, as it might interfere with the chemotherapy for pancreatic cancer. Although permanent PTGBD was an option, it risked causing QOL deterioration owing to the nature of the external drainage. Other possible alternative included ETGBD and endoscopic ultrasound-guided gallbladder drainage (EUS-GBD). EUS-GBD was expected to be difficult because the gallbladder cavity could not be enlarged by injecting a contrast agent through the PTGBD route, probably because the gallbladder wall was stiff from repeated inflammation. However, cholecystography through the PTGBD route showed that the cystic duct was patent with right and cranial side bifurcation, although it was obstructed by the CSEMS (Fig. 2). Therefore, we decided to perform ETGBD.
Figure 2.
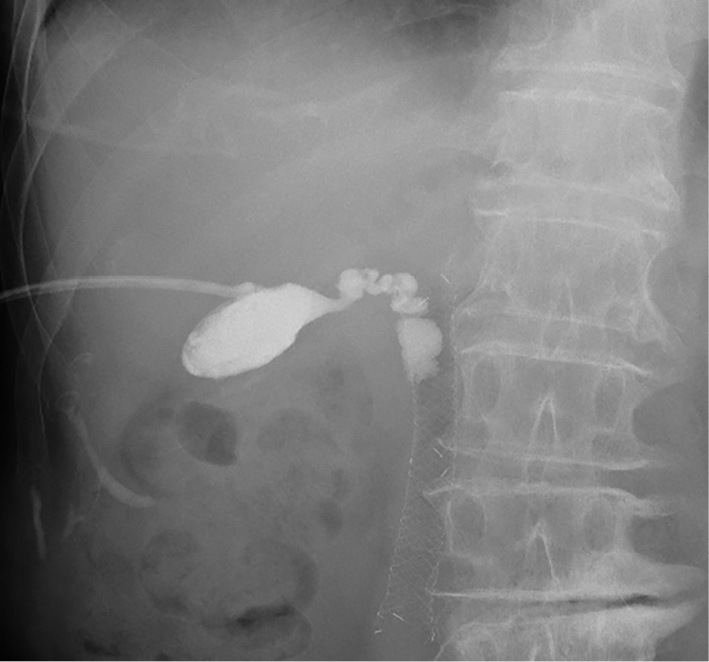
A cholecystogram obtained through the PTGBD route showing that the cystic duct was patent and merged with the metallic stent from the right and cranial sides. PTGBD: percutaneous transhepatic gallbladder drainage
First, a duodenoscope (TJF290V; Olympus Medical System, Tokyo, Japan) was inserted into the duodenum, and the CSEMS was removed with a snare through the scope. After bile duct cannulation through the papilla, a 0.025-inch guidewire (M-Through; MEDICO'S Hirata, Osaka, Japan) was passed through the cystic duct and inserted into the gallbladder. A plastic stent (diameter, 7-Fr; length, 15 cm; Through & Pass double-pigtail stent; Gadelius Medical, Tokyo, Japan) was placed between the gallbladder and the duodenum after dilating the cystic duct with a balloon catheter (REN, 4 mm; Kaneka Medical Products, Osaka, Japan) due to the stricture at the orifice of the cystic duct (Fig. 3). A new CSEMS (diameter, 10 mm; length, 8 cm, fully covered Hanaro stent; Boston Scientific, Natick, USA) was deployed at the biliary obstruction beside the stent for ETGBD (Fig. 4). Thereafter, the PTGBD tube was removed (Fig. 5). After ETGBD, the patient underwent chemotherapy for pancreatic cancer on schedule without cholecystitis recurrence.
Figure 3.
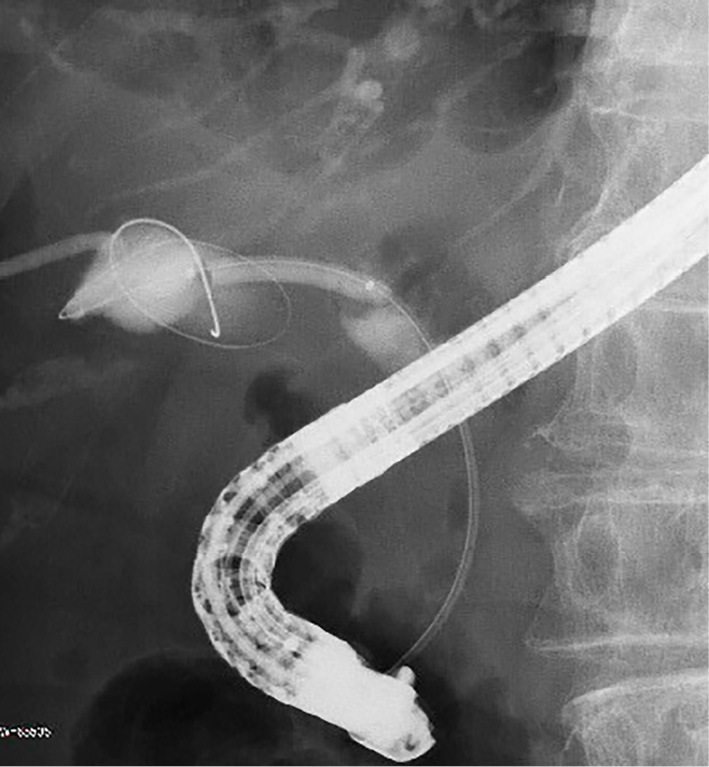
Fluoroscopic image showing cystic duct dilation with a balloon catheter.
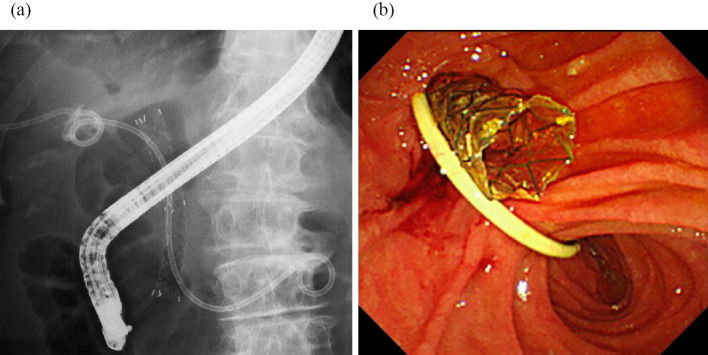
Figure 4.
Transpapillary gallbladder stent and covered metal stent. (a) Fluoroscopic image. (b) Endoscopic image.
Figure 5.
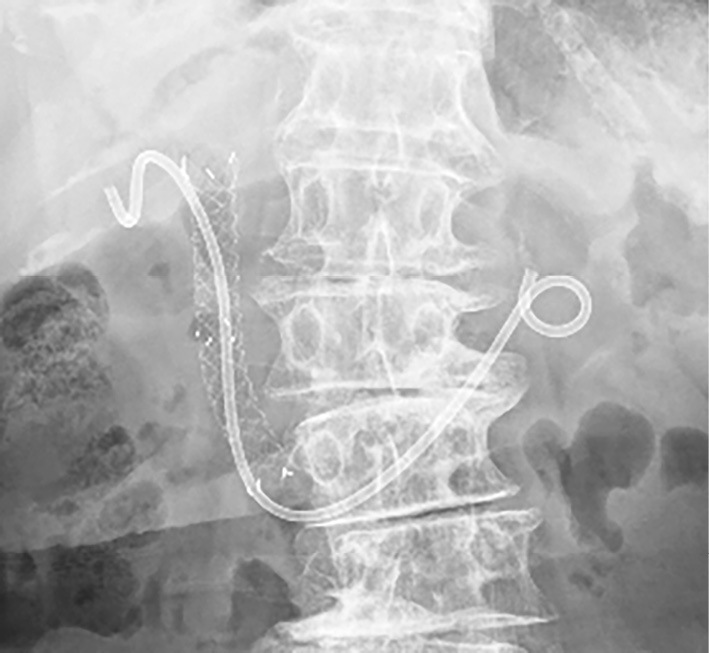
Transpapillary gallbladder stent and covered metal stent after removal of the PTGBD tube. PTGBD: percutaneous transhepatic gallbladder drainage
Discussion
PTGBD is a reliable and well-established procedure that is used to manage cholecystitis even after CSEMS placement in cases that are refractory to conservative methods (1,4). Although PTGBD is an effective management for cholecystitis, it is associated with a reduced QOL because of the nature of external drainage through the skin. Ainley et al. (4) evaluated six patients who underwent PTGBD for the management of cholecystitis after SEMS placement for biliary obstruction. They reported that all cases were successfully managed with PTGBD; however, the time to the removal of the external drainage ranged from two weeks to six months. Furthermore, repeated recurrence of cholecystitis after the removal of the PTGBD tube can affect the clinical course and chemotherapy schedule. Therefore, permanent placement of a PTGBD may be required to manage recurrent cholecystitis, which can further deteriorate the patient's QOL. Nakahara et al. (5) reported that the PTGBD tube could not be removed until death in 3 of 6 (50%) patients who underwent PTGBD for cholecystitis after SEMS placement owing to the recurrence of cholecystitis after tube clamping (n=2) and the appearance of ascites (n=1). Several studies have investigated chemical ablation of the gallbladder mucosa through the injection of ethanol or Aethoxysklerol as an alternative to permanent PTGBD (6-8); however, this technique has not been well evaluated because only case series studies have been performed. In addition, PTGBD has a general disadvantage in that it is contraindicated in the presence of a bleeding tendency due to antiplatelet/anticoagulant use or thrombocytopenia, massive ascites, or an anatomically inaccessible location of the gallbladder (9).
ETGBD, which involves internal drainage through the papilla, is effective for the management of cholecystitis and can be an alternative therapy to PTGBD (10,11); however, limited reports are available on the application of ETGBD for cholecystitis after SEMS placement (5,12,13). In a retrospective study, Nakahara et al. evaluated 12 patients who underwent ETGBD for cholecystitis after SEMS placement. They performed ETGBD after CSEMS removal if the CSEMS was covering the cystic duct bifurcation; however, the SEMS was kept in place when the cystic duct bifurcation was not covered by a CSEMS or when an uncovered SEMS was in place. The technical and clinical success rates were 83.3% (10/12) and 90.0% (9/10), respectively, suggesting that ETGBD can also provide a relatively good outcome for patients with cholecystitis after SEMS placement (5). However, ETGBD is generally known to have a lower technical success rate than PTGBD because it requires the drainage tube to be placed through the papilla and the cystic duct. In particular, the presence of cystic duct stones dilated common bile duct and a cystic duct direction with proximal or caudal branches can make ETGBD difficult (14). ETGBD can be more challenging to perform for cholecystitis occurring after SEMS placement for MBO than for general cholecystitis, owing to the influence of tumor invasion and the SEMS itself. Therefore, we believe that ETGBD cannot be implemented for the initial management of cholecystitis after SEMS placement because of its uncertain technical feasibility.
In our case, ETGBD was considered feasible after the CSEMS was removed because the cystic duct was determined to be patent and directed toward the cranial side based on the findings of cholecystography through the PTGBD route. Therefore, deciding the indication of ETGBD according to the findings of cholecystography through the PTGBD route may be a useful strategy. If cholecystography shows a proximal or caudal cystic duct direction, which are factors associated with failure of ETGBD, it might be necessary to prepare for more advanced techniques, such as PTGBD-RV. In some cases, EUS-GBD or permanent placement of PTGBD should be considered.
EUS-GBD has recently emerged as an effective management for cholecystitis in poor surgical candidates and can also be an effective drainage approach for cholecystitis after CSEMS placement (15-17). Suzuki et al. reported a case of successful EUS-GBD for cholecystitis after SEMS placement (18). In their case, cholecystoduodenostomy was performed for internal drainage with a CSEMS without any adverse events, and the patient was able to continue palliative chemotherapy with no further episodes of cholecystitis until death from the progression of the primary disease 17 months later. In another retrospective study, Kozakai et al. investigated 10 patients who underwent EUS-GBD for acute cholecystitis after SEMS placement. A double-pigtail plastic stent or CSEMS was deployed as an EUS-GBD stent to bridge the gallbladder and gastrointestinal tract. The technical and clinical success rates were 90% (9/10) and 89% (8/9), respectively. However, bile peritonitis occurred in 4 patients (40%) after EUS-GBD. Among them, three patients recovered with conservative treatment, but the remaining patient required emergency surgery. Furthermore, acute cholecystitis recurred in 3 patients (3/8, 38%) within 1 month after EUS-GBD. In all patients with recurrent cholecystitis, stent dysfunction was endoscopically managed by adding a stent through the previously created fistula (19). Although EUS-GBD is currently a feasible option for the management of acute cholecystitis after SEMS placement, it may be considered only as an alternative to established interventions such as PTGBD or ETGBD because of the dearth of available dedicated devices and scarce evidence supporting the utility of the technique and its safety. More recently, the usefulness of EUS-GBD with a lumen-apposing metal stent (LAMS) in the management of cholecystitis in high-risk surgical patients has also been reported (20-22). Although the application of EUS-GBD with LAMS for the management of cholecystitis after SEMS placement requires further evaluation, this technique may be a viable option, considering the high technical success rate and the nature of internal drainage.
In conclusion, we encountered a case in which recurrent cholecystitis after CSEMS placement was successfully managed with PTGBD followed by ETGBD. The management of cholecystitis after SEMS placement is challenging once it recurs after PTGBD. In such cases, the indication for ETGBD may be decided based on the findings of cholecystography through the PTGBD route, as ETGBD can prevent cholecystitis recurrence through internal drainage.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Isayama H, Kawabe T, Nakai Y, et al. Cholecystitis after metallic stent placement in patients with malignant distal biliary obstruction. Clin Gastroenterol Hepatol 4: 1148-1153, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Suk KT, Kim HS, Kim JW, et al. Risk factors for cholecystitis after metal stent placement in malignant biliary obstruction. Gastrointest Endosc 64: 522-529, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Takinami M, Murohisa G, Yoshizawa Y, Shimizu E, Nagasawa M. Risk factors for cholecystitis after stent placement in patients with distal malignant biliary obstruction. J Hepatobiliary Pancreat Sci 27: 470-476, 2020. [DOI] [PubMed] [Google Scholar]
- 4. Ainley CC, Williams SJ, Smith AC, Hatfield AR, Russell RC, Lees WR. Gallbladder sepsis after stent insertion for bile duct obstruction: management by percutaneous cholecystostomy. Br J Surg 78: 961-963, 1991. [DOI] [PubMed] [Google Scholar]
- 5. Nakahara K, Morita R, Michikawa Y, et al. Endoscopic transpapillary gallbladder drainage for acute cholecystitis after biliary self-expandable metal stent placement. Surg Laparosc Endosc Percutan Tech 30: 416-423, 2020. [DOI] [PubMed] [Google Scholar]
- 6. Lee TH, Park SH, Kim SP, et al. Chemical ablation of the gallbladder using alcohol in cholecystitis after palliative biliary stenting. World J Gastroenterol 15: 2041-2043, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu Z, Wang L, Zhang N, Ling X, Hou C, Zhou X. Chemical ablation of the gallbladder: clinical application and long-term observations. Surg Endosc 19: 693-696, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Atar E, Khasminsky V, Friehmann T, Choen A, Bachar GN. Cystic duct embolization with chemical gallbladder ablation for the treatment of acute calculous cholecystitis in high-risk patients: a prospective single-center study. J Vasc Interv Radiol 31: 644-648, 2020. [DOI] [PubMed] [Google Scholar]
- 9. Itoi T, Sofuni A, Itokawa F, et al. Endoscopic transpapillary gallbladder drainage in patients with acute cholecystitis in whom percutaneous transhepatic approach is contraindicated or anatomically impossible (with video). Gastrointest Endosc 68: 455-460, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Sagami R, Hayasaka K, Ujihara T, et al. Endoscopic transpapillary gallbladder drainage for acute cholecystitis is feasible for patients receiving antithrombotic therapy. Dig Endosc 32: 1092-1099, 2020. [DOI] [PubMed] [Google Scholar]
- 11. Maruta A, Iwashita T, Iwata K, et al. Permanent endoscopic gallbladder stenting versus removal of gallbladder drainage, long-term outcomes after management of acute cholecystitis in high-risk surgical patients for cholecystectomy: multi-center retrospective cohort study. J Hepatobiliary Pancreat Sci 28: 1138-1146, 2021. [DOI] [PubMed] [Google Scholar]
- 12. Kawakubo K, Isayama H, Sasahira N, et al. Endoscopic transpapillary gallbladder drainage with replacement of a covered self-expandable metal stent. World J Gastrointest Endosc 3: 46-48, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakahara K, Michikawa Y, Morita R, Suetani K, Itoh F. Endoscopic transpapillary gallbladder stent placement in the presence of uncovered biliary metal stents using a through-the-mesh technique. VideoGIE 5: 296-299, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maruta A, Iwata K, Iwashita T, et al. Factors affecting technical success of endoscopic transpapillary gallbladder drainage for acute cholecystitis. J Hepatobiliary Pancreat Sci 27: 429-436, 2020. [DOI] [PubMed] [Google Scholar]
- 15. Park SW, Lee SS. Current status of endoscopic management of cholecystitis. Dig Endosc 34: 439-450, 2022. [DOI] [PubMed] [Google Scholar]
- 16. Atalla H, Shiomi H, Nakano R, et al. EUS-guided cholecystoduodenostomy using novel dumbbell-shaped fully covered metal stent (with video). J Hepatobiliary Pancreat Sci 28: e19-e20, 2021. [DOI] [PubMed] [Google Scholar]
- 17. Ogura T, Ueno S, Okuda A, Nishioka N, Higuchi K. One-step deployment for EUS-guided gallbladder drainage using a novel fully covered metal stent (with video). J Hepatobiliary Pancreat Sci 28: e4-e5, 2021. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki Y, Hashimoto Y, Shibuki T, et al. Endoscopic ultrasound-guided gallbladder drainage for aberrant right posterior duct obstruction developing after placement of a covered self-expandable metallic stent in a patient with distal biliary obstruction. Case Rep Gastroenterol 12: 722-728, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kozakai F, Kanno Y, Ito K, et al. Endoscopic ultrasonography-guided gallbladder drainage as a treatment option for acute cholecystitis after metal stent placement in malignant biliary strictures. Clin Endosc 52: 262-268, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teoh AYB, Kitano M, Itoi T, et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: an international randomised multicentre controlled superiority trial (DRAC 1). Gut 69: 1085-1091, 2020. [DOI] [PubMed] [Google Scholar]
- 21. Teoh AYB, Kongkam P, Bapaye A, et al. Use of a novel lumen apposing metallic stent for drainage of the bile duct and gallbladder: long term outcomes of a prospective international trial. Dig Endosc 33: 1139-1145, 2021. [DOI] [PubMed] [Google Scholar]
- 22. Torres Yuste R, Garcia-Alonso FJ, Sanchez-Ocana R, et al. Safety and efficacy of endoscopic ultrasound-guided gallbladder drainage combined with endoscopic retrograde cholangiopancreatography in the same session. Dig Endosc 32: 608-615, 2020. [DOI] [PubMed] [Google Scholar]