Abstract
Since December 2020, coronavirus disease 2019 (COVID-19) vaccines have been distributed in most countries to prevent the onset and aggravation of COVID-19. There is little information regarding the long-term safety of the vaccines. We report three cases and a literature review of new-onset adult-onset Still's disease (AOSD) that occurred following COVID-19 vaccination. Our cases include moderate to severe AOSD, and two were complicated with macrophage activation syndrome. Seventeen cases of new-onset or relapse of AOSD following COVID-19 vaccination, including 14 identified in the literature review and our 3 patients, were all treated successfully with glucocorticoid therapy, immunosuppressive drugs, or biologic agents.
Keywords: adult-onset Still's disease, COVID-19 vaccine, innate immune response
Introduction
Coronavirus disease 2019 (COVID-19) has continued to spread worldwide after it was first declared a pandemic by the World Health Organization on March 11, 2020 (1). Since December 2020, the COVID-19 vaccine has been distributed widely in most countries to prevent the onset and aggravation of COVID-19. The results of randomized clinical trials have shown that the BNT162b2 mRNA COVID-19 vaccine and the mRNA-1273 COVID-19 vaccine are 95% and 94% effective, respectively, in preventing the onset of COVID-19 (2,3). The efficacy of these COVID-19 vaccines exceeds the rate of 59% for the influenza inactivated vaccine (4). After confirmation of the high efficacy of COVID-19 vaccines in numerous clinical trials, they became available in Japan from February 2021.
Patients with rheumatic diseases receive immunosuppressive therapy and are therefore considered to be at increased risk of becoming infected with or suffering aggravated COVID-19. In addition, viral infection may trigger the onset and flare of rheumatic diseases. In such patients, COVID-19 vaccination can prevent the onset and high disease activity of COVID-19. The European Alliance of Association for Rheumatology and the American College of Rheumatology generally recommend vaccination for patients with rheumatic diseases and collagen diseases (5,6).
However, there is little information concerning rare adverse events or the long-term safety of the COVID-19 vaccine, as in contrast to existing vaccines, these vaccines are either mRNA or viral vector vaccines. Even conventional vaccines, such as the influenza vaccine, are known to cause new-onset or flare of rheumatic diseases, including adult-onset Still's disease (AOSD) (7-10). There have also been case reports of new-onset or relapsed rheumatic diseases following COVID-19 vaccination, such as rheumatoid arthritis, Behcet's disease, systemic lupus erythematosus, dermatomyositis, vasculitis, and polymyalgia rheumatica (11).
We herein report three cases of new-onset AOSD following COVID-19 vaccination.
Case Reports
Case 1
A 59-year-old woman developed a sore throat and high-grade fever (39.0°C) 7 days after receiving the first dose of the BNT162b2 mRNA COVID-19 vaccine. The symptoms did not improve with acetaminophen or antibiotics. She was admitted to our hospital 29 days after vaccination with a sustained spiking fever, sore throat, salmon-pink eruption on the forearms during the high fever, polyarthralgia, and tenderness of the left cervical lymph nodes.
Laboratory test results were as follows: white blood cell count 13,320 /μL (neutrophils 88.4%, lymphocytes 7.7%), hemoglobin (Hb) 12.9 g/dL, platelets 23.8×104/μL, erythrocyte sedimentation rate 120 mm/h, aspartate aminotransferase (AST) 30 U/L, alanine aminotransferase (ALT) 16 U/L, lactate dehydrogenase (LDH) 326 U/L, γ-GTP 103 U/L, C-reactive protein (CRP) 12.8 mg/dL, serum ferritin 1,004 ng/mL, serum interleukin (IL)-6 19.4 pg/mL, and IL-18 ≥5,000 pg/mL. Rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) antibody were both negative, and antinuclear antibody (ANA) was weakly positive (1:40, homogenous and speckled patterns). Reverse transcription polymerase chain reaction (PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from nasopharyngeal swabs was negative. Real-time PCR for Epstein-Barr virus (EBV) was negative. Other infectious diseases were also excluded, such as by blood, sputum, and urine culture tests.
Computed tomography (CT) of the neck, chest, abdomen, and pelvis revealed multiple lymphadenopathy at the bilateral axillae, dorsal inferior vena cava, right common iliac to external iliac region; and splenomegaly. AOSD was diagnosed according to the standard classification criteria, based on the spiking fever of 39.0°C, sore throat, salmon-pink rash that appeared with the fever, polyarthritis, multiple lymphadenopathy, splenomegaly, leukocytosis, increased neutrophil ratio (>80%), increased serum ferritin level, increased serum liver enzyme level, and increased serum interleukin (IL)-18 level (12).
Oral glucocorticoid therapy (prednisolone 35 mg/day; 0.5 mg/kg/day) was administered initially. Her sore throat and polyarthralgia improved, but as the low-grade fever (approximately 37°C) persisted, the daily dose of prednisolone was increased to 50 mg (0.8 mg/kg/day). The serum CRP level was increased despite high-dose glucocorticoid therapy. Following fortnightly treatment with intravenous tocilizumab (8 mg/kg), her symptoms improved, and the inflammatory findings disappeared; the patient was therefore discharged.
At the outpatient examination after the third treatment of intravenous tocilizumab, her laboratory data showed liver dysfunction (AST 2,632 U/L, ALT 4,091 U/L, LDH 2,131 U/L), an increased serum ferritin level (38,101 ng/mL), and thrombocytopenia (platelets 78,000 /μL). Histological findings of a liver biopsy specimen revealed phagocytosis of erythrocytes, platelets, and small lymphocytes by macrophages, and the patient was diagnosed with hemophagocytic syndrome (HPS).
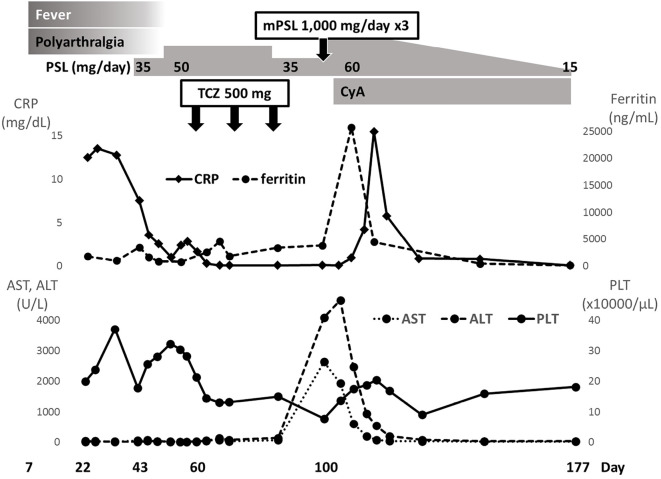
She was treated with 60 mg of oral glucocorticoid (1 mg/kg/day of prednisolone) concomitant with cyclosporine after intravenous methylprednisolone pulse therapy (1,000 mg for 3 days). The dose of methylprednisolone was then gradually reduced to 12 mg, and no relapse of AOSD was observed (Fig. 1).
Figure 1.
Clinical course of patient 1, including symptoms, laboratory findings, and treatment. ‘Day’ means the number of days after the first vaccination date, set as day 0. ALT: aspartate aminotransferase, AST: alanine aminotransferase, CRP: C-reactive protein, CyA: cyclosporine, LDH: lactate dehydrogenase, mPSL: methylprednisolone, PLT: platelets, PSL: prednisolone, TCZ: tocilizumab
Case 2
A 77-year-old woman developed a high-grade fever (39.0°C) and polyarthritis 42 days after receiving the second dose of the BNT162b2 mRNA COVID-19 vaccine. She was admitted to a nearby medical facility 61 days after receiving the second dose, having developed stiffness in both hands and bilateral knee joint pain.
Laboratory tests showed persistent high CRP levels (20-25 mg/dL). Oral glucocorticoid (prednisolone 5 mg/day) was administered initially at 64 days after receiving the second dose. However, a fever of 37-38°C and elevated CRP levels persisted. She was transferred to our hospital at 69 days after receiving the second dose with a sustained spiking fever and polyarthritis involving the right hand, fingers, and knee.
Laboratory tests showed a white blood cell count of 10,090 /μL (neutrophils 86.2%, lymphocytes 9.2%), Hb 8.1 g/dL, platelets 8.7×104/μL, erythrocyte sedimentation rate 93 mm/h, AST 98 U/L, ALT 53 U/L, LDH 553 U/L, γ-glutamyl transpeptidase(GTP) 101 U/L, CRP 14.6 mg/dL, serum ferritin 18,002 ng/mL, serum IL-6 43.6 pg/mL, and IL-18 ≥5,000 pg/mL. RF and anti-CCP antibody were negative, and ANA was positive (1:80; homogenous and speckled patterns). Anti-dsDNA antibody, anti-Sm antibody, anti-U-1RNP antibody, anti-SS-A antibody, proteinase 3 (PR3)-anti-neutrophil cytoplasmic antibodies (ANCA), and myeloperoxidase (MPO)-ANCA were all negative. Blood and urine cultures and PCR for SARS-CoV-2 were all negative. There were no CT findings indicating hepatosplenomegaly or lymphadenopathy in the neck, chest, abdomen, or pelvis.
Despite treatment with antibiotics (sulbactam/ampicillin), the elevated serum CRP levels persisted, and anemia progressed. A salmon-pink rash appeared from the neck to the back 76 days after vaccination. AOSD was diagnosed based on a spiking fever of 39.0°C, salmon-pink rash that appeared with the fever, polyarthritis, leukocytosis, increased neutrophil ratio (>80%), increased serum liver enzyme level, and increased serum ferritin level. Furthermore, HPS was suspected due to the progression of anemia, thrombocytopenia, and marked increase in serum ferritin levels (48,377 ng/mL).
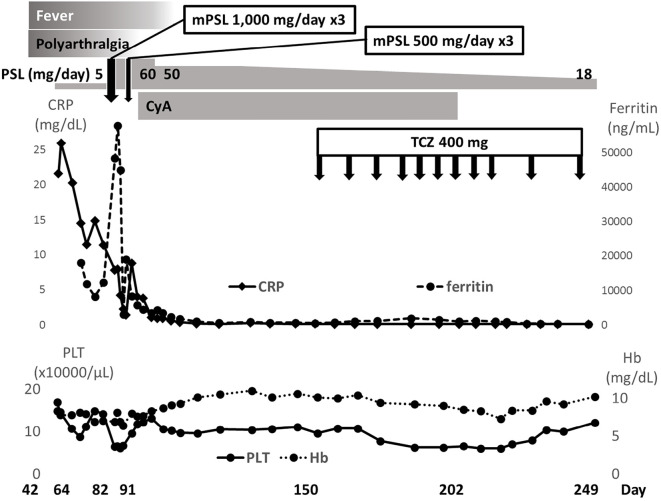
High-dose intravenous methylprednisolone pulse therapy (1,000 mg/day) was performed for 3 days. High-dose glucocorticoid therapy was continued at 60 mg of oral prednisolone daily. Her liver function recovered, and the serum levels of ferritin decreased, but cytopenia progressed. A bone marrow examination showed hemophagocytosis. A second round of methylprednisolone pulse therapy (500 mg/day) was performed for 3 days, she was then treated with high-dose glucocorticoid (prednisolone 60 mg/day) and cyclosporin. The fever resolved, but the recovery of blood cell depletion was poor, and a high serum ferritin level of approximately 600 ng/mL persisted. Tocilizumab was administered intravenously for steroid-resistant hemophagocytosis, but anemia and thrombocytopenia persisted.
Considering the possibility of drug-induced thrombotic microangiopathy, cyclosporine was discontinued. The cytopenia and high serum ferritin levels gradually improved under treatment with glucocorticoid and tocilizumab. While continuing tocilizumab administration, the dose of prednisolone was reduced to 18 mg/day, and no relapse was observed (Fig. 2).
Figure 2.
Clinical course of patient 2, including symptoms, laboratory findings, and treatment. ‘Day’ means the number of days after receiving the second vaccination date, set as day 0. Hb: hemoglobin
Case 3
A 35-year-old man developed a high-grade fever (39.0°C) and sore throat 24 days after receiving the first dose of the mRNA-1273 COVID-19 vaccine. Following initial treatment with antibiotics and antipyretics, he was transferred to our hospital with a persistent spiking fever, high CRP level, leukocytosis, and salmon pink rash on the lower legs.
Laboratory tests showed white blood cell count 15,350 /μL (neutrophils 80.2%, lymphocytes 13.0%), Hb 15.4 g/dL, platelets 50.7×104/μL, erythrocyte sedimentation rate 120 mm/h, AST 25 U/L, ALT 46 U/L, LDH 170 /L, alkaline phosphatase (ALP) 81 U/L, γ-GTP 177 U/L, CRP 10.52 mg/dL, and serum ferritin 1,263 ng/mL. RF was weakly positive (29 IU/mL), and anti-CCP antibody and ANA were negative. Blood and urine cultures, PCR for SARS-CoV-2 and EBV, and anti-parvovirus B19 immunoglobulin M antibody were all negative. AOSD was diagnosed based on the persistent spiking fever, sore throat, polyarthritis, typical eruption, leukocytosis (neutrophils of ≥80%), elevated serum liver enzyme level, and hyperferritinemia.
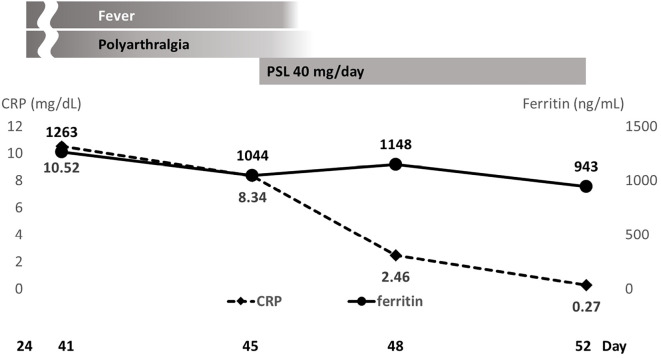
The patient improved following the administration of 40 mg of oral prednisolone (0.5 mg/kg) daily and was discharged from our hospital (Fig. 3). At another hospital, the dose of prednisolone was reduced to 10 mg/day, and no relapse was observed.
Figure 3.
Clinical course of patient 3, including symptoms, laboratory findings, and treatment during hospitalization. ‘Day’ means the number of days after the first vaccination date, set as day 0.
Discussion
We herein report three cases of AOSD that developed following vaccination with mRNA COVID-19 vaccines (two with BNT162b2 and one with mRNA-1273). To our knowledge, 10 other cases of new-onset (cases 1-10 in the Table) and relapse of AOSD (cases 14-17 in Table) have been reported following COVID-19 vaccination (13-23). Seven of those 10 patients developed symptoms 5-21 days after the first or second dose of the COVID-19 vaccine (Table). Among these, the earliest development of symptoms occurred on the day after the first vaccination, and much later development occurred in 2 cases (56 and 90 days after the first vaccination).
Table.
Previous and Present Cases of New-onset or Relapse of AOSD Following COVID-19 Vaccination.
| Case no. | Age (y)/sex | Vaccine | Days from vaccination to new-onset or relapse of AOSD | With (+) or without (-) MAS |
Treatment | Response | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Type | Dose number |
||||||||
| New-onset | |||||||||
| 1 | 36/M | Vector | 1st | 1 | - | mPSL pulse, anakinra 100 mg/day | Improved | 13 | |
| 2 | 45/F | mRNA | 2nd | 5 | - | PSL 60 mg/day | Improved | 14 | |
| 3 | 43/M | mRNA | 2nd | 10 | - | mPSL pulse, PSL 60 mg/day | Improved | 15 | |
| 4 | 56/F | mRNA | 2nd | 7 | - | PSL 60 mg/day | Improved | 15 | |
| 5 | 36/F | mRNA | 1st | 10 | - | mPSL pulse, tocilizumab 8 mg/kg, iv | Improved | 16 | |
| 6 | 22/M | mRNA | 1st | 13 | + | mPSL pulse, IVIg 2 g/kg, PSL 100 mg/ | Improved | 17 | |
| day, anakinra 100 mg/day | |||||||||
| 7 | 53/M | Vector | 1st | 56 | - | PSL 60 mg/day | Improved | 18 | |
| 8 | 20/F | Vector | 1st | 10 | - | Naproxen | Improved | 19 | |
| 9 | 47/F | Vector | 1st | 21 | - | PSL 0.5 mg/kg, MTX, tocilizumab 162 | Improved | 19 | |
| mg, sc | |||||||||
| 10 | 35/F | Vector | 1st | 90 | + | mPSL pulse, IVIg 2 g/kg, tocilizumab 8 | Improved | 19 | |
| mg/kg, iv | |||||||||
| 11 | 59/F | mRNA | 1st | 7 | + | PSL 50 mg/day, tocilizumab 8 mg/kg, iv, | Improved | Our case 1 | |
| mPSL pulse, cyclosporin | |||||||||
| 12 | 77/F | mRNA | 2nd | 42 | + | mPSL pulse, PSL 60 mg/day, mPSL pulse, cyclosporin, tocilizumab 8 mg/kg, iv | Improved | Our case 2 | |
| 13 | 35/M | mRNA | 1st | 24 | - | PSL 40 mg/day | Improved | Our case 3 | |
| Relapse | |||||||||
| 14 | 34/F | Vector | 1st | 7 | - | PSL 50 mg/day, mPSL pulse, etanercept | Improved | 20 | |
| 50 mg, MTX 10 mg/week, PSL 75 mg/day, | |||||||||
| tocilizumab 8 mg/kg, iv | |||||||||
| 15 | 20/F | mRNA | 1st | 6 | + | mPSL pulse, IVIg, anakinra 300 mg/day, | Improved | 21 | |
| ciclosporin | |||||||||
| 16 | 37/F | mRNA | 2nd | A few days | - | PSL 15 mg/day, tocilizumab 162 mg, sc | Improved | 22 | |
| 17 | 49/F | mRNA | 1st | 4 | + | Tocilizumab 400 mg, iv, mPSL pulse, | Improved | 23 | |
| MTX | |||||||||
iv: intravenous, IVIg: intravenous immunoglobulin, MAS: macrophage activation syndrome, mPSL: methylprednisolone, mRNA: messenger ribonucleic acid, MTX: methotrexate, PSL: prednisolone, sc: subcutaneous
The pathologic reason for the difference in the timing of the onset of AOSD following COVID-19 vaccination is still unknown. Aside from cytokine storm, deficiency of or failure in regulatory or anti-inflammatory mechanisms might be involved in the pathogenesis of autoinflammatory diseases (24). The pathogenesis of AOSD is thought to be due to an imbalance between the activation or amplification of inflammation, referred to as cytokine storm, and the resolution of inflammation. We speculate that there are individual differences in the timing of the development of a hyper-inflammatory state due to the imbalance between the activation and resolution of inflammation after COVID-19 vaccination.
Nine of the 10 new-onset cases had received high-dose prednisolone and/or intravenous pulse methylprednisolone and/or immunosuppressive therapy. One case improved with naproxen, without the use of glucocorticoid or immunosuppressive agents. Two of the three present cases were considered to involve AOSD complicated with macrophage activation syndrome (MAS) because of the extremely high serum ferritin level, elevated serum IL-6 and IL-18 levels, and histological findings of HPS. Four previously reported cases were considered to involve AOSD complicated with MAS (cases 6, 10, 15 and 17).
The period from COVID-19 vaccination to the onset of AOSD and the severity of the disease varied. All 14 previously reported cases and our 3 present cases of AOSD following COVID-19 vaccination were successfully treated with glucocorticoid therapy, immunosuppressive drugs, or a biologic agent. Viral and bacterial infections, including SARS-CoV-2, were excluded in our three patients. According to the clinical course, COVID-19 vaccination was the most likely trigger for AOSD.
AOSD is a rare autoinflammatory disorder that commonly affects young adults and is characterized by a spiking fever, rash, arthralgia or arthritis, and lymphadenopathy. The pathogenesis of AOSD is thought to involve the abnormal activation of the innate immune system with macrophage activation and marked production of inflammatory cytokines, including IL-1, IL-2, IL-6, IL-18, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ, leading to the activation of autoreactive T cells. Systemic hyperinflammation and cytokine storm play a central role in the pathogenesis of both AOSD and severe COVID-19 (25-28). In addition, treatment targeting IL-6 was effective for not only AOSD but also COVID-19 (29-31). Although the cause of AOSD remains unknown, the findings of previous studies have suggested that several micro-organisms, especially viruses, might contribute to its onset or relapse (24,32-34).
Previous studies have reported AOSD that developed after influenza vaccination; since 2021, cases of new-onset AOSD and relapsed immune-mediated disease have also occurred after COVID-19 vaccination (7,8,10,13-23). There are two main types of COVID-19 vaccine formulations: mRNA encoding the SARS-CoV-2 spike protein (S) encapsulated in lipid nanoparticles or adenovirus (AdV) vectors encoding the S protein (35). The mRNA or AdV particle gain entry into dendritic cells, resulting in the production of S protein. Innate sensors are triggered by the adjuvant activity of the vaccines, resulting in the production of type I interferon and multiple pro-inflammatory mediators, which then activates T cells to elicit immunity to SARS-CoV-2. RNA sensors, such as Toll-like receptor 7 (TLR7) and melanoma differentiation-associated gene 5, are triggered by mRNA vaccines, and TLR9 is the major double-stranded DNA sensor for the AdV vaccine. Although mRNA vaccines do not engage TLR9 like AdV vectors, both vaccine formulations cause the production of type I interferon.
The occurrence of immune-mediated disease following vaccination may be due to the interaction between the susceptibility of the vaccinated subjects and vaccine components, such as adjuvants and pathogen-derived peptides (36). Adjuvants, the substances added to a vaccine to enhance the immune response and effectiveness of the vaccine, activate the innate immune system through pattern recognition receptors. The immune-activating component contained in the adjuvant may induce immune-mediated disease. Vaccination is also expected to produce an immune response similar to viral infection by exposure to foreign peptides homologous to human peptides. Molecular mimicry and bystander activation are considered the main mechanisms underlying the association between vaccination and the development of immune-mediated disease (36). Molecular mimicry represents a shared immunologic epitope with a microbe and the host. In a viral system, viruses have been shown to have cross-reactive epitopes with host self-proteins (37,38). Proteins expressed after mRNA translation by vaccination may be structurally similar to self-antigens, which may also cause immune responses targeting these antigens. Bystander activation is caused by viral infections, leading to the significant activation of antigen-presenting cells, which can potentially activate pre-primed autoreactive T cells and then induce autoimmune diseases (37,38). Khan et al. showed that the SARS-CoV-2 spike protein is a potent viral pathogen-associated molecular pattern that leads to the expression of inflammatory mediators in the innate immune response (39). Based on the above findings, viral proteins from vaccines could also theoretically activate the same immune response as viral infections and might cause AOSD.
Other possible immune responses caused by the vaccine itself have also been proposed. mRNA vaccines exhibit a self-adjuvanting property, with the mRNA acting as both antigen and adjuvant (35). Lipid nanoparticles in the mRNA COVID-19 vaccines may trigger inflammatory reactions via several mechanisms (40). In addition, prior to the translation, mRNA may bind pattern recognition receptors in endosomes or cytosol, activating an inflammatory cascade that may trigger a strong innate immune response (41).
New-onset AOSD following COVID-19 vaccination is rare, and the mechanism has not yet been clarified. The continued accumulation of cases of new-onset rheumatic disease or immune-mediated disease after COVID-19 vaccination may provide an opportunity to elucidate the pathogenic mechanism underlying autoimmune and autoinflammatory diseases.
Ethical approval for this study was obtained from INSTITUTIONAL REVIEW BOARD of Saitama Medical University Hospital (APPROVAL NUMBER: 2021-142).
The authors state that they have no Conflict of Interest (COI).
References
- 1.World Health Organization. WHO director-general's opening remarks at the media briefing on COVID-19 [Internet]. [cited 2020 Mar 11]. Available from: http://www.who.int
- 2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 383: 2603-2615, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: 403-416, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12: 36-44, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Bugatti S, Balduzzi S, De Stefano L, et al. Correspondence on ‘EULAR December 2020 viewpoints on SARS-CoV-2 vaccination in patients with RMDs’. Ann Rheum Dis 80: e156, 2021. [DOI] [PubMed] [Google Scholar]
- 6. Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases - version 3. Arthritis Rheumatol 71: 1093-1107, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jatuworapruk K. Adult-onset Still's disease preceded by influenza vaccination: coincidence or true association? Asian Pac J Allergy Immunol. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 8. Shechtman L, Lahad K, Ben-Zvi I. Adult Still's disease triggered by influenza vaccine. Isr Med Assoc J 23: 196-197, 2021. [PubMed] [Google Scholar]
- 9. Olivieri B, Betterle C, Zanoni G. Vaccines and autoimmune diseases of the adult. Vaccines (Basel) 9: 815, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshioka K, Fujimoto S, Oba H, Minami M, Aoki T. Onset of adult-onset Still's disease following influenza vaccination. Mod Rheumatol 21: 432-435, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Watad A, Marco GD, Mahajna H, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines 9: 435, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still's disease. J Rheumatol 19: 424-430, 1992. [PubMed] [Google Scholar]
- 13. Leone F, Cerasuolo PG, Bosello SL, et al. Adult-onset Still's disease following COVID-19 vaccination. Lancet Rheumatol 3: e678-e680, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Magliulo D, Narayan S, Ue F, Boulougoura A, Badlissi F. Adult-onset Still's disease after mRNA COVID-19 vaccine. Lancet Rheumatol 3: e678-e680, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharabi A, Shiber S, Molad Y. Adult-onset Still's disease following mRNA COVID-19 vaccination. Clin Immunol 233: 108878, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park SY, Lee KH. Adult-onset Still's disease after BNT162b2 mRNA COVID-19 vaccine. J Korean Med Sci 36: e344, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baicus C, Delcea C, Pinte L, Dan GA. Hyper-inflammation after COVID-19 mARN vaccination: at the crossroads of multisystem inflammatory disease and adult-onset Still's disease. Does terminology matter? Rom J Intern Med 60: 3-5, 2022. [DOI] [PubMed] [Google Scholar]
- 18. Sweeney A, Tracey G, Garnham K. Adult-onset Still disease post-adenovirus vector COVID-19 vaccine. Intern Med J 51: 2144-2145, 2021. [DOI] [PubMed] [Google Scholar]
- 19. Padiyar S, Kamath N, Mathew J, et al. New-onset adult-onset Still's disease-like syndrome after ChAdOx1 nCoV-19 vaccination-a case series with review of literature. Clin Rheumatol 41: 1569-1575, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeon YH, Lim DH, Choi SW, Choi SJ. A flare of Still's disease following COVID-19 vaccination in a 34-year-old patient. Rheumatol Int 42: 743-748, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muench F, Krusche M, Sander LE, Rose T, Burmester GR, Schneider U. Macrophage activation syndrome in a patient with adult-onset Still's disease following first COVID-19 vaccination with BNT162b2. BMC Rheumatol 5: 60, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamamoto S, Nishimura K, Yo K, Waki D, Murabe H, Yokota T. Flare-up of adult-onset Still's disease after receiving a second dose of BNT162b2 COVID-19 mRNA vaccine. Clin Exp Rheumatol 39 Suppl 132: 139-140, 2021. [DOI] [PubMed] [Google Scholar]
- 23. Kim JW, Jung JY, Suh CH, Kim HA. Flare of adult-onset Still's disease following mRNA COVID-19 vaccination: a case report and review of literature. Clin Rheumatol 19: 1-7, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still's disease. Nature Rev Rheumatol 14: 603-618, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahmed-Hassan H, Sisson B, Shukla RK, et al. Innate immune responses to highly pathogenic coronaviruses and other significant respiratory viral infections. Front Immunol 11: 1979, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beng J, Ma Y, Jia J, et al. Cytokine storm in coronavirus disease 2019 and adult-onset Still's disease: similarities and differences. Front Immunol 11: 603389, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roy RK, Sharma U, Wasson MK, Jain A, Hassan MI, Prakash H. Macrophage activation syndrome and COVID-19: impact of MAPK driven immune-epigenetic programming by SARS-Cov-2. Front Immnol 12: 763313, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bamidis AD, Koehler P, Di Cristanziano V, et al. First manifestation of adult-onset Still's disease after COVID-19. Lancet Rheumatol 3: e319-e321, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev 19: 102568, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aziz M, Haghbin H, Abu Sitta E, et al. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J Med Virol 93: 1620-1630, 2021. [DOI] [PubMed] [Google Scholar]
- 31. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Internal Med 181: 41-51, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jia J, Shi H, Liu M, et al. Cytomegalovirus infection may trigger adult-onset Still's disease onset or relapses. Front Immunol 10: 898, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tomaras S, Goetzke CC, Kallinich T, Feist E. Adult-onset Still's disease: clinical aspects and therapeutic approach. J Clin Med 10: 733, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jamilloux Y, Gerfaud-Valentin M, Martinon F, Belot A, Henry T, Seve P. Pathogenesis of adult-onset Still's disease: new insights from the juvenile counterpart. Immunol Res 61: 53-62, 2015. [DOI] [PubMed] [Google Scholar]
- 35. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 21: 195-197, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol 15: 586-594, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev 19: 80-94, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Munz C, Lunemann JD, Getts MT, Miller SD. Antiviral immune responses: trigger of or triggered by autoimmunity? Nat Rev Immunol 9: 246-258, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khan S, Shafiei MS, Longoria C, Schoggins J, Savani RC, Zaki H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-kB pathway. bioRxiv. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ndeupen S, Qin Z, Jacobsen S, Bouteau A, Estanbouli H, Igyarto BZ. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 24: 103479, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin Immunol 224: 108665, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]