Abstract
The precise manipulation of immune tolerance is the holy grail of immunotherapies for both autoimmunity and cancer immunity. Thymomas are well known to be associated with autoimmune diseases. The exact mechanism by which autoreactivity is induced after thymectomy remains to be elucidated. We herein present the case of a 50-year-old lady with concurrent de novo type 1 autoimmune hepatitis (AIH) and pure red cell aplasia (PRCA), 1 month after undergoing a successful total thymectomy for combined squamous cell carcinoma and thymoma (Masaoka stage II). Corticosteroids yielded short-term effects for both AIH and PRCA. Literature on thymoma-associated AIH, an extremely rare immune-related comorbidity, was also reviewed.
Keywords: autoimmunity, thymic epithelial tumors, immune tolerance, immunosuppression
Introduction
Autoimmune hepatitis (AIH) is an infrequent immune-mediated inflammatory liver disease that affects all ages, both sexes, and all ethnicities (1). The exact mechanisms underlying the susceptibility, precipitation, escalation, and perpetuation of AIH are not fully understood (2); however, it is generally considered that autoreactive T cells break self-tolerance to hepatic autoantigens as a result of environmental triggers and the inability of autoantigen-specific T regulatory cells to prevent autoreactivity (1-3). Although patients with AIH often demonstrated chronic progressive clinical courses, approximately 25% of them present with acute onset and atypical clinical features, such as negativity for autoantibodies without hypergammaglobulinemia and less responsiveness to corticosteroids (4). Hence, severe acute-onset AIH, a possible cause of acute liver failure, poses great challenges in both diagnosis and management.
The thymus, a primary lymphoid organ responsible for the production of mature, functional T cells and their education through positive and negative selections, plays an important role in central tolerance (5,6). Thymomas or thymic carcinomas, neoplasia derived from the thymic epithelia, are well known to be associated with paraneoplastic autoimmune diseases, with myasthenia gravis (MG) being the mostly common (7,8). Thymomas are also associated with other autoimmune diseases that are mostly described in case reports. Systemic lupus erythematosus (SLE), pure red cell aplasia (PRCA), autoimmune thyroid diseases, polymyositis, bullous pemphigoid, hypogammaglobulinemia, and Good's syndrome are among those with higher frequencies (7,8).
Although thymectomy frequently leads to the remission of MG (9,10), the impact of thymectomy on the outcome of autoimmune diseases other than MG is inconsistent. Reports of de novo onset of thymoma-associated PRCA provide solid evidence that a relapse of PRCA frequently occurs after thymectomy, and that PRCA may develop after thymectomy even in those previously without a history of PRCA (11,12). We herein present a rare case of the de novo onset of thymoma-associated type 1 AIH, accompanying PRCA after thymectomy. We also review the published literature on thymoma-associated paraneoplastic AIH.
Case Report
This 50-year-old Japanese woman was referred to our Department of Thoracic Surgery due to a suspected mediastinal mass during a regular physical examination 3 months prior to this admission. She underwent a successful and uneventful transsternal thymectomy 2 months later. She was discharged 6 days after thymectomy. Pathologically, combined squamous cell carcinoma and thymoma (type B2 to B3) was diagnosed (Masaoka stage II; pT1aN0M0; Fig. 1). The resection margins were tumor free. No vascular or lymphatic invasion was observed.
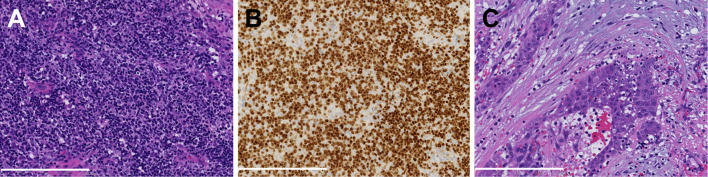
Figure 1.
Histopathology of the surgically resected thymic tumor. (A, B) A thymoma component including TdT+ immature T cells and (C) a component of squamous cell carcinoma are observed. A and C: Hematoxylin and Eosin staining, B: Immunohistochemistry for TdT. A scale bar equals to 200 μm. TdT: terminal deoxynucleotidyl transferase
One month after thymectomy, she presented to the hospital with progressively generalized malaise. Normocytic normochromic anemia (hemoglobin 5.9 g/dL) with a reticulocyte index <0.2 was observed, and she was therefore admitted on an emergency basis. At admission (33 days after thymectomy), non-icteric elevation of alanine transaminase (ALT) (173 IU/L, reference ranges, 7- 23 for females) was also noticed. Physical manifestations and contrast-enhanced computed tomography excluded the possibility of active bleeding. Due to symptomatic anemia, she underwent a red blood cell transfusion at admission. A bone marrow examination was performed 42 days after thymectomy (Fig. 2), which showed a hypocellular marrow with a cellularity of 5-10%. Neither dysplasia nor significant fibrosis was observed. Only a few glycophorin A-stained, erythroblastic nucleated cells were noticed. Bone marrow aspirate smears showed that normocellular marrow with a significantly decreased number of erythroid cells (0.4%). Human parvovirus B19 DNA assay in serum was negative. Therefore, the patient was diagnosed with thymoma-associated secondary PRCA. Her hepatic dysfunction with hyperbilirubinemia worsened gradually, which prompted consultations with hepatologists.
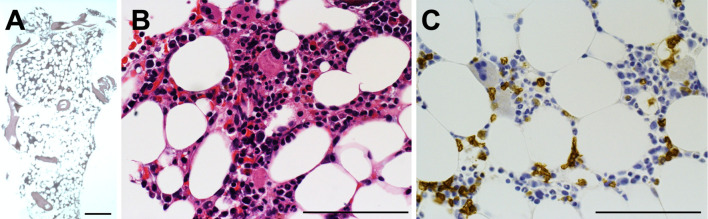
Figure 2.
Histopathology of a bone marrow biopsy. (A, B) A hypocellular marrow with the cellularity of 5-10%. (C) Only a few glycophorin A-stained erythroblastic nucleated cells are observed. A, B: Hematoxylin and Eosin staining, C: Immunohistochemistry for glycophorin A. A scale bar equals to 200 μm.
She was diagnosed as subclinical hypothyroidism in her 30s. She was in a euthyroid state and was followed up without medication thereafter. Five years before admission, the patient had breast adenocarcinoma (pathological stage I) and was successfully treated surgically without any signs of recurrence during the follow-up. Two years before admission, she developed a retroperitoneal liposarcoma (pathological stage I) and was again successfully treated surgically without any signs of recurrence during follow-up. Menstruation had gradually become irregular over the previous 2 years. She is a nonsmoker and had been drinking 2 drinks of alcohol (40 g of ethanol) per day by average for the past 10 years; she quit drinking at the time of the mediastinal tumor diagnosis.
Biochemical and serological manifestations at the peak of liver injury are shown in Table 1. Hepatotropic viral infections were excluded serologically. Her euthyroid state persisted. Wilson disease was serologically excluded. Contrast-enhanced computed tomography excluded obstructive jaundice and metastatic or infiltrating malignancies. Drug-induced liver injury (DILI) was excluded because no medication fulfilled the criteria of the Diagnostic Scale of Digestive Disease Week-Japan 2004 (DDW-J 2004) (13), similar to the Roussel Uclaf Causality Assessment Method of the Council (RUCAM) for International Organizations of Medical Sciences (14). Positive (40-fold) anti-smooth muscle (F-actin) antibody (ASMA), assessed by a fluorescent-labeled antibody method, was observed. Anti-nuclear antibody (ANA) showed a 40-fold homogenous pattern. The serum IgG level was 1,220 mg/dL. With worsening tendencies of both hyperbilirubinemia (total bilirubin, 4.2 mg/dL) and hypertransaminasemia (ALT, 1,032 IU/L), a percutaneous liver biopsy was performed at 44 days after thymectomy (Fig. 3). Histologically, predominant lymphocytic infiltration with a few plasma cells was observed around the portal areas, across liver parenchyma and within sinusoids. These pathological findings (lobular/portal inflammation and perivenular necroinflammatory activity) were congruent to some of the most prevalent features of acute-onset AIH that were reported by Nguyen Canh et al. (15). However, no marked interface activity was observed. Most infiltrated CD3+ T cells were CD8+. Cytomegalovirus (CMV) staining was negative. According to the International Autoimmune Hepatitis Group (IAIHG) scoring system, her pretreatment score was 12, which was compatible with the diagnosis of suspected type 1 AIH (positive ASMA). Owing to the chronological features of an acute de novo onset and the co-occurrence of post-thymectomy thymoma-associated PRCA, thymoma-associated paraneoplastic AIH was thus diagnosed in this case.
Table 1.
Biochemical and Serological Findings (Biochemical Findings were Sampled on Day 47 after Thymectomy).
| Variables (Unit) | Variables (Unit) | Variables (Unit) | Variables (Unit) | ||||
|---|---|---|---|---|---|---|---|
| Biochemistry | CBC | Coombs tests | +- | ||||
| TP (g/dL) | 6.4 | LDH (U/L) | 655 | WBC (/µL) | 4,500 | Direct | - |
| ALB (g/dL) | 3.2 | AST (U/L) | 1,056 | RBC (104/µL) | 262 | Indirect | 1+ |
| T-Bil (mg/dL) | 5.3 | ALT (U/L) | 1,022 | Hb (g/dL) | 8.5 | Urinalysis | |
| D-Bil (mg/dL) | 4.2 | ALP (U/L) | 356 | HCT (%) | 24.7 | Protein | +- |
| UN (mg/dL) | 8.2 | Ch-E (U/L) | 193 | MCV (fL) | 94.3 | Occult blood | - |
| Cr (mg/dL) | 0.66 | γ-GTP (U/L) | 437 | MCHC (g/dL) | 34.4 | Bil | 1+ |
| UA (mg/dL) | 2.3 | Fe (μg/dL) | 285 | PLT (103/µL) | 5.9 | ||
| Na (mEq/L) | 139.1 | TIBC (μg/dL) | 295 | Reticulocyte (%) | 0.3 | ||
| K (mEq/L) | 3.5 | UIBC (μg/dL) | <28 | Ret # (104/µL) | 8.6 | ||
| Cl (mEq/L) | 101 | Cu (μg/dL) | 182 | Coagulation | |||
| IP (mg/dL) | 3.5 | Zn (μg/dL) | 50 | APTT (s) | 35 | ||
| GLU (mg/dL) | 103 | Vit B12 (pg/mL) | 1,280 | PT-% (%) | 85 | ||
| HbA1c (%) | 5.4 | FA (ng/mL) | 8.5 | PT-INR | 1.11 | ||
| T-Cho (mg/dL) | 144 | EPO (IU/mL) | 1,120 | FNG (µg/mL) | 247 | ||
| TG (mg/dL) | 79 | FDP-P (μg/mL) | <2.0 | ||||
| Variables (Unit) | Variables (Unit) | ||||||
| Serology | IgM-HAV | (-) | |||||
| ANA | 40X | HBs-Ag | (-) | ||||
| ASMA | 40X | IgM-HBc | (-) | ||||
| Anti-mitochondria M2 | (-) | HBV-DNA | (-) | ||||
| Anti-LKM1 | (-) | HCV-Ab | (-) | ||||
| pANCA | (-) | HCV-RNA | (-) | ||||
| IgG (mg/dL) | 1,220 | IgA-HEV | (-) | ||||
| IgM (mg/dL) | 103 | IgM-EBV VCA | (-) | ||||
| IgA (mg/dL) | 184 | IgG-EBV VCA | (+) | ||||
| F-T3 (pg/mL) | 3.2 | IgM-CMV | (±) | ||||
| F-T4 (ng/dL) | 1.4 | IgG-CMV | (+) | ||||
| TSH (μIU/mL) | 1.05 | CMV antigenemia | (0, 0) | ||||
| Haptoglobin (mg/dL) | 170 | Measles IgM | (+) | ||||
| Ceruloplasmin (mg/dL) | 44 | Measles IgG | (+) | ||||
| CRP (mg/dL) | 1.14 | Human parvovirus DNA | (-) | ||||
| Ferritin (ng/dL) | 5,202 |
TP: total protein, ALB: albumin, T-Bil: total bilirubin, D-Bil: direct bilirubin, UN: urea nitrogen, Cr: creatinine, UA: uric acid, Na: sodium, K: potassium, Cl: chloride, IP: ionic phosphate, GLU: glucose, HbA1c: hemoglobn A1c, T-Cho: total cholesterol, TG: triacylglycerol, LDH: lactate dehydrogenase, AST: aspartate transaminase, ALT: alanine transaminase, ALP: alkaline phosphatase, γ-GTP: gamma-glutamine transpeptidase, Ch-E: choline esterase, Fe: serum iron, TIBC: total iron binding capacity, UIBC: unsaturated iron binding capacity, vit B12: vitamin B12, FA: folic acid, WBC: white blood cells, RBC: red blood cells, Hb: hemoglobin, HCT: hematocrit, MCV: mean corpuscular volume, PLT: platelet, Ret #: reticulocyte number, APTT: activated partial thromboplastin time, PT: prothrombin time, INR: international ratio, FNG: fibrinogen, FDP: fibrin degradation product, ANA: anti-nuclear antibody, ASMA: anti-smooth muscle antibody, LKM: liver/kidney microsomal, pANCA: perinuclear anti-neutrophil cytoplasmic antibody, F-T3: free T3, F-T4: free T3, TSH: thyroid-stimulating hormone, CRP: C-reactive protein, Ig: immunoglobulin, HAV: hepatitis A virus, HBV: hepatitis B virus, HCV: hepatitis C virus, HEV: hepatitis E virus, Ag: antigen, EBV Epstein-Barr virus, VCA: virus capsid antigen, CMV: cytomegalovirus
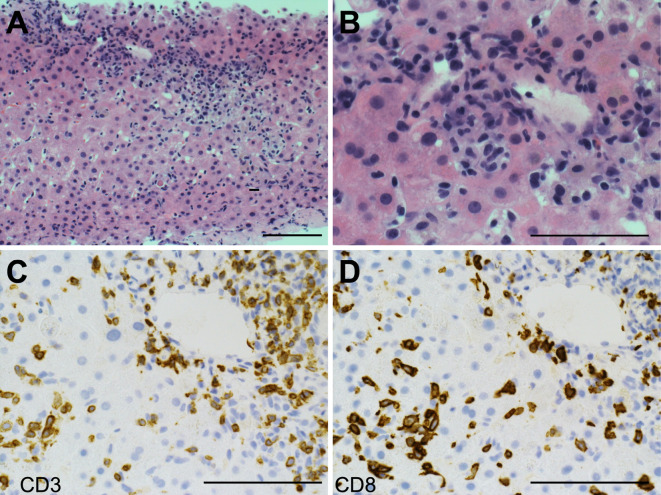
Figure 3.
Histopathology of a liver biopsy. (A, B) Liver tissue shows the characteristic of mixed lymphoplasmacytic infiltrate across the portal area and parenchyma. (C, D) Most of the infiltrated CD3+ T lymphocytes are CD8 positive. A, B: Hematoxylin and Eosin staining. C, D: Immunohistochemistry for CD3 and CD8, respectively. A scale bar equals to 200 μm.
Seropositivity of IgM type antibodies to CMV and measles was also noticed. However, clinical manifestation and liver histology precluded them from being possible etiologies of liver injury.
Owing to the worsening tendency of hyperbilirubinemia (total bilirubin 5.3 mg/dL), which suggested impeding liver failure, after a detailed discussion with the attending hematologist, prednisolone 1 mg/kg body weight was started on day 47 after thymectomy. Her serum transaminases decreased immediately, and hyperbilirubinemia improved accordingly. Approximately 8 days after the initiation of prednisolone, the peripheral reticulocyte count started to increase. Clinical improvement persisted after tapering of prednisolone, and she was discharged on day 57 after thymectomy. The clinical course is summarized in Fig. 4.
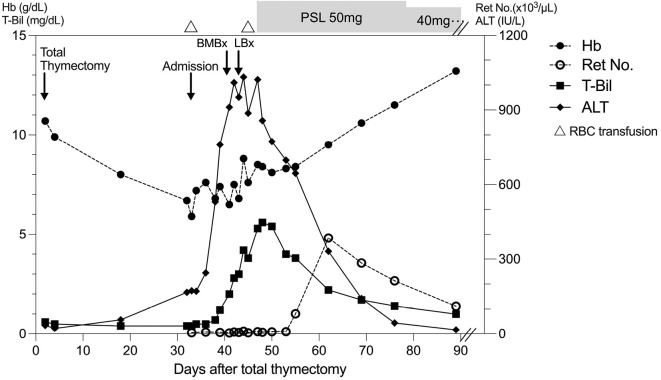
Figure 4.
Summary of the clinical course after thymectomy. Hb: hemoglobin, T-Bil: total bilirubin, ALT: alanine transaminase, Ret No.: reticulocyte number, RBC: red blood cell, BMBx: bone-marrow biopsy, LBx: liver biopsy, PSL: prednisolone
No recurrence of thymoma and thymic carcinoma was observed 1.5 year after thymectomy. The dose of prednisolone was gradually tapered to 5 mg/day. However, at 8 months after the initiation of prednisolone, a relapse of PRCA was observed, whereas the course of AIH was uneventful. After a dose increase of prednisolone, PRCA subsided again. No onset of any other paraneoplastic autoimmune disease, such as MG, was observed. She is now closely followed-up, and the use of cyclosporine A (CsA) will be considered if another relapse of PRCA is observed.
Discussion
The association between AIH and thymoma is extremely rare (8). To the best of our knowledge, our case is the first report of concurrent de novo onset of thymoma-associated type 1 AIH and PRCA after thymectomy. We conducted a database (PubMed, National Library of Medicine) searching for publications up until December 2021, with keywords “thymoma” and “autoimmune hepatitis.” Including the current case, only 13 cases of thymoma-associated AIH were identified (Table 2) (7,16-26). The median age of onset is 35 years (range 25-77 years), with a female preponderance (61.5%, 8/13). There is no obvious ethnical tendency. Eleven out of 13 patients (84.6%) with thymoma-associated AIH had at least one autoimmune disease other than AIH. Among them, MG (72.7%, 8/11) is the most common comorbid paraneoplastic autoimmune disease, followed by autoimmune thyroiditis (27.3%, 3/11). Another case of PRCA that occurred before thymectomy has also been reported (24). Most patients suffer from AIH before thymectomy; only three patients (23.1%) suffered from an acute de novo onset of AIH after thymectomy, including the current case. It is noteworthy that it took a relatively long time from thymectomy to onset of AIH in the other two cases [23 months and 4 years, respectively (20,22)], compared to the current case (Table 2). Interestingly, DILI of specific medication or nutritional supplement has been suspected before the diagnosis of AIH in the four cases described in previous reports (20,24-26), thus indicating the need for awareness of the possibility of AIH. A short-term survival outcome is generally good (92.3%); however, AIH-related acute liver failure was reportedly the cause of mortality in only one patient (18), thus suggesting that immune-mediated liver injury should not be overlooked during the management of patients with thymoma.
Table 2.
Reported Cases of Thymoma-associated Paraneoplastic Autoimmune Hepatitis.
| No | Age | Sex | Country | Onset of AIH before/ after Thx |
Other paraneoplastic autoimmune disease | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 25 | F | Hong Kong | Before | MG/PM | PSL 1 mg/kg; AZA | Survived | (16) |
| 2 | 30 | M | Korea | Before | MG | PSL 0.5 mg/kg | Survived | (17) |
| 3 | 77 | F | Japan | Before | MG/Thyroiditis/T1DM | PE | Died#1 | (18) |
| 4 | 57 | F | Austria | Before | Polyneuropathy | PSL 1 mg/kg | Survived | (19) |
| 5 | 31 | M | Turkey | After#2 | MG/Thyroiditis/SjS | PSL 0.6 mg/kg | Survived | (20) |
| 6 | 42 | F | US | Before | MG/Myocarditis | PE; CTx | Survived | (21) |
| 7 | 29 | M | Tunisia | After#3 | T1DM | CTx | Survived | (22) |
| 8 | 47 | F | France | Before | MG/Thyroiditis | N/A | Survived | (7) |
| 9 | 31 | M | Italy | Before | MG | PSL 0.3 mg/kg | Survived | (23) |
| 10 | 34 | F | US | Before | PRCA/MG | Thx; CRT | Survived | (24) |
| 11 | 63 | F | Japan | Before | None | PSL 0.5 mg/kg | Survived | (25) |
| 12 | 35 | M | US | Before | None | Thx; CTx | Survived | (26) |
| This Case | 50 | F | Japan | After | PRCA | PSL 1 mg/kg | Survived | - |
#1. Due to acute liver failure. #2. Interval until the onset of AIH: 23 months after thymectomy. #3. Interval until the onset of AIH: 4 years after thymectomy.
M: male, F: female, AIH: autoimmune hepatitis, Thx: thymectomy, DILI: drug-induced liver injury, MG: myasthenia gravis, PM: polymyositis, T1DM: Type 1 diabetes mellites, SjS: Sjögren's syndrome, PRCA: pure red cell aplasia, PSL: prednisolone, PE: plasma exchange, CTx: chemotherapy, CRT: chemoradiation therapy, N/A: not available
AIH is a multifactorial disease that requires the interplay among genetic, epigenetic, immunologic, and environmental factors. A rare exception is AIH associated with an autosomal recessive mutation in the autoimmune regulator (AIRE) gene (1), a transcriptional modulator that is mainly expressed in major histocompatibility complex (MHC)-II high medullary thymic epithelial cells (27,28), clinically associated with autoimmune polyendocrine syndrome type 1 (APS-1). However, impaired self-tolerance due to immature T cell development [the “immature T cell theory” (6)] or increased autoreactivity due to increased proliferation rates [the “neoplastic-genetic theory” (6)] could not completely explain de novo paraneoplastic diseases post-thymectomy (29). A previous observation of thymoma-associated PRCA revealed that cytotoxic CD8 T cells in bone marrow, especially those with perforin+ effector memory phenotypes, are important for its pathogenesis (28,30). Recently, the accumulation of intrahepatic CD8+ CD103+ perforin+ T cells, the so-called tissue resident memory T cells, have been observed in pediatric patients with AIH, particularly in indeterminate acute liver failure (31). Moreover, a cohort study of 13 patients with hypertransaminasemia and thymoma demonstrated significantly decreased frequencies of CD8 T cells and an elevated CD4/CD8 ratio after thymectomy (32). As observed in the present case, liver histopathology showed predominant infiltration of CD8 T cells (Fig. 3), in contrast to the CD4 T cell predominance in classical chronic AIH (3), the failure of hematopoiesis and liver injury may both originate from CD8 T cell-mediated immune dysregulation after thymectomy. Interestingly, the role of cytotoxic CD8 T cells in hepatitis-associated aplastic anemia (33) also illustrates its common pathological role in both liver and bone marrow. Thus, pre-existing populations of T cells that have escaped positive and negative selection may persist with minimal self-reactivity in the periphery, such as in the liver and the bone marrow. Thymectomy itself disables further maintenance of immune tolerance, and dysregulates the development of T regulatory cells, leading to de novo autoimmunity (29,34). This hypothesis for post-thymectomy autoimmunity is also supported by an observation in a murine model wherein fatal hepatitis resembling the human AIH developed only after neonatal thymectomy that severely reduced the number of T regulatory cells in programmed death (PD)1-ablated mice (35).
Including this current case, the seropositivity of ASMA (anti F-actin) has been reported in two other cases of thymoma-associated AIH (16,26). Immunofluorescent reactivity of human anti-smooth muscle antibodies has been detected in thymic medulla (36). In addition, anti-liver-kidney microsomal (LKM)-3 (UDP glucuronosyltransferase 1 family, polypeptide A cluster, UGT1A) and cytochrome P450 (CYP) 1A2 have been reported in cases with thymoma-associated type 2 AIH and APS-1 associated hepatitis, respectively (19,37). However, the molecular and immunological links to hepatic autoantigens and tissue injury are still unclear, even in classical AIH. Therefore further investigations are needed.
The long-term effect of corticosteroid alone on PRCA is unsatisfactory with a 39% response rate (38). With an overall response rate >75%, CsA, a calcineurin inhibitor, can be considered to be the agent of choice for immunosuppression in PRCA (39). CsA should also be considered as an alternative first line treatment for AIH, especially in pediatric patients (1), and it has also been reported for second line use in corticosteroid non-responders with severe acute-onset AIH in adults (40). Therefore, CsA may be considered if a relapse of PRCA or AIH is observed in the current case. Nevertheless, a possible recurrence of thymic carcinoma should also be carefully monitored.
In the era in which novel immunotherapies flourish, how “immune tolerance” can be precisely manipulated is still considered the Holy Grail in coping with both autoimmunity and cancer immunity. It should be noted that, to date, little is still known about the exact mechanisms by which thymic neoplasia induces autoreactivity after thymectomy in multiple organs. Therefore, this and other related reports provide opportunities to learn more about this rare but insightful disease.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Mack CL, Adams D, Assis DN, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology 72: 671-722, 2020. [DOI] [PubMed] [Google Scholar]
- 2. Czaja AJ. Examining pathogenic concepts of autoimmune hepatitis for cues to future investigations and interventions. World J Gastroenterol 25: 6579-6606, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmmune hepatitis. Cell Mol Immunol 19: 158-176, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol 63: 971-1004, 2015. [DOI] [PubMed] [Google Scholar]
- 5. Bluestone JA, Anderson M. Tolerance in the age of immunotherapy. N Engl J Med 383: 1156-1166, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shelly S, Agmon-Levin N, Altman A, et al. Thymoma and autoimmunity. Cell Mol Immunol 8: 199-202, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernard C, Frih H, Pasquet F, et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev 15: 82-92, 2016. [DOI] [PubMed] [Google Scholar]
- 8. Blum TG, Misch D, Kollmeier J, et al. Autoimmune disorders and paraneoplastic syndromes in thymoma. J Thorac Dis 12: 7571-7590, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emeryk B, Strugalska MH. Evaluation of results of thymectomy in myasthenia gravis. J Neurol 211: 155-168, 1976. [DOI] [PubMed] [Google Scholar]
- 10. Okumura M, Fujii Y, Shiono H, et al. Immunological function of thymoma and pathogenesis of paraneoplastic myasthenia gravis. Gen Thorac Cardiovasc Surg 56: 143-150, 2008. [DOI] [PubMed] [Google Scholar]
- 11. Thompson CA, Steensma DP. Pure red cell aplasia associated with thymoma: clinical insights from a 50-year single-institution experience. Br J Haematol 135: 405-407, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Hirokawa M, Sawada K, Fujishima N, et al. Long-term response and outcome following immunosuppressive therapy in thymoma-associated pure red cell aplasia: a nationwide cohort study in Japan by the PRCA collaborative study group. Haematologica 93: 27-33, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Takikawa H, Onji M. A proposal of the diagnostic scale of drug-induced liver injury. Hepatol Res 32: 250-251, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Danan G, Benichou C. Causality assessment of adverse reactions to drugs - I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 46: 1323-1330, 1993. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen Canh H, Harada K, Ouchi H, et al. ; Intractable Liver and Biliary Diseases Study Group of Japan. Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol 70: 961-969, 2017. [DOI] [PubMed] [Google Scholar]
- 16. Ko KF, Ho T, Chan KW. Autoimmune chronic active hepatitis and polymyositis in a patient with myasthenia gravis and thymoma. J Neurol Neurosurg Psychiatry 59: 558-559, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han YS, Kim BH, Kim TH, et al. Autoimmune hepatitis in a patient with myasthenia gravis and thymoma - a report on the first case in Korea. Korean J Intern Med 15: 151-155, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Asakawa H, Kashihara T, Fukuda H, et al. A patient with thymoma and four different organ-specific autoimmune diseases. Neth J Med 60: 292-295, 2002. [PubMed] [Google Scholar]
- 19. Aigner E, Strassburg CP, Strasser M, et al. Transient autoimmune hepatitis induced by a thymoma. Am J Gastroenterol 104: 1332-1334, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Yapali S, Oruc N, Ilgun S, et al. Acute presentation of autoimmune hepatitis in a patient with myasthenia gravis, thymoma, Hashimoto thyroiditis and connective tissue disorder. Hepatol Res 42: 835-839, 2012. [DOI] [PubMed] [Google Scholar]
- 21. Rajan A, Kotlyar D, Giaccone G. Acute autoimmune hepatitis, myositis, and myasthenic crisis in a patient with thymoma. J Thorac Oncol 8: e87-e88, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mejri N, Chabchoub I, Gargouri I, et al. Effect of chemotherapy on autoimmune hepatitis in thymoma: a case report and literature review. Cancer Biol Med 10: 169-173, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mendogni P, Rosso L, Tosi D, et al. Autoimmune hepatitis: an uncommon presentation of thymoma. Tumori 102: 2016. [DOI] [PubMed] [Google Scholar]
- 24. Feinsilber D, Mears KA, Pettiford BL. Polyparaneoplastic manifestations of malignant thymoma: a unique case of myasthenia, autoimmune hepatitis, pure red cell aplasia, and keratoconjunctivitis sicca. Cureus 9: e1374, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishimura T, Tsunezuka H, Miyata N, et al. Autoimmune hepatitis during preoperative chemotherapy in a patient with thymoma. Interact Cardiovasc Thorac Surg 29: 635-637, 2019. [DOI] [PubMed] [Google Scholar]
- 26. Stilwell KT, Musick SR, Cebe KM, et al. Thymoma-induced autoimmune hepatitis: a rare paraneoplastic syndrome. Cureus 11: e5637, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rossi SW, Kim MY, Leibbrandt A, et al. RANK signals from CD4+3- inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med 204: 1267-1272, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marx A, Yamada Y, Simon-Keller K, et al. Thymus and autoimmunity. Semin Immunopathol 43: 45-64, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mollaeian A, Haas C. A tale of autoimmunity: thymoma, thymectomy, and systemic lupus erythematosus. Clin Rheumatol 39: 2227-2234, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nitta H, Mihara K, Sakai A, et al. Expansion of CD8+/perforin+ effector memory T cells in the bone marrow of patients with thymoma-associated pure red cell aplasia. Br J Haematol 150: 712-715, 2010. [DOI] [PubMed] [Google Scholar]
- 31. Chapin CA, Burn T, Meijome T, et al. Indeterminate pediatric acute liver failure is uniquely characterized by a CD103+CD8+ T-cell infiltrate. Hepatology 68: 1087-1100, 2018. [DOI] [PubMed] [Google Scholar]
- 32. Yu XT, Yu L, Du X, et al. Clinical and genetic characteristics of thymoma patients with autoimmune hepatitis and myocarditis. Front Oncol 11: 746304, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rauff B, Idrees M, Shah SA, et al. Hepatitis associated aplastic anemia: a review. Virol J 8: 87, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gerli R, Paganelli R, Cossarizza A, et al. Long-term immunologic effects of thymectomy in patients with myasthenia gravis. J Allergy Clin Immunol 103: 865-872, 1999. [DOI] [PubMed] [Google Scholar]
- 35. Kido M, Watanabe N, Okazaki T, et al. Fatal autoimmune hepatitis induced by concurrent loss of naturally arising regulatory T cells and PD-1-mediated signaling. Gastroenterology 135: 1333-1343, 2008. [DOI] [PubMed] [Google Scholar]
- 36. Toh BH. Smooth muscle autoantibodies and autoantigens. Clin Exp Immunol 38: 621-628, 1979. [PMC free article] [PubMed] [Google Scholar]
- 37. Clemente MG, Obermayer-Straub P, Meloni A, et al. Cytochrome P450 1A2 is a hepatic autoantigen in autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab 82: 1353-1361, 1997. [DOI] [PubMed] [Google Scholar]
- 38. Means RT, Jr. Pure red cell aplasia. Hematology Am Soc Hematol Educ Program 2016: 51-56, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sawada K, Fujishima N, Hirokawa M. Acquired pure red cell aplasia: updated review of treatment. Br J Haematol 142: 505-514, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noguchi F, Chu PS, Taniki N, et al. Long-term observation of cyclosporine as second-line therapy in adults for severe acute autoimmune hepatitis. Hepatology 73: 2594-2597, 2021. [DOI] [PubMed] [Google Scholar]