Abstract
Purpose: This research project aimed to discuss the effect of early enteral nutrition (EEN) plus probiotics on intestinal function of senile patients with sepsis. Methods: 108 senile sepsis patients admitted to our hospital from January 2019 to January 2022 were selected in this retrospective study. These patients including 50 cases in a control group (CG) and 58 cases in a research group (RG). Both groups received EEN, but the research group was given EEN plus probiotics. The two cohorts of patients were compared with respect to treatment efficacy, intestinal mucosal barrier, nutritional status and 28-day mortality. Cox regression was performed to analyze the prognostic factors of elderly patients with sepsis. Results: Compared to the CG, the RG had evidently higher overall response rate and post-treatment albumin (Alb) and prealbumin (PA) levels, as well as statistically lower intestinal fatty acid binding protein, diamine oxidase, D-lactate and 28-day mortality. Furthermore, Alb and PA were identified as independent predictors of prognosis in elderly patients with sepsis. Conclusions: EEN supplemented with probiotics is superior to EEN alone in the treatment of senile patients with sepsis. This combined regimen can significantly improve intestinal function, nutritional status and prognosis of patients. Moreover, Alb and PA are independently related to the prognosis of elderly patients with sepsis.
Keywords: Sepsis in the elderly, probiotics, early enteral nutrition, intestinal function
Introduction
Sepsis is an infection-induced systemic inflammatory disease with its pathological exacerbation being associated with acute kidney injury in most cases [1]. In addition, this disease has the highest morbidity in the intensive care unit and may cause cardiac and autonomic dysfunction in patients [2]. Although the overall mortality of sepsis shows a gradual decline, the 28-day and one-year mortality rates remain up to 25% and 41%, respectively, due to the significant increase in morbidity [3]. Moreover, disease exacerbation may lead to multiple organ dysfunction syndrome or septic shock, which may be life-threatening and induce long-term sequelae [4,5]. Intestinal function is significantly associated with the development of sepsis, and repairing the body’s intestinal mucosal barrier (IMB) is conducive to alleviating intestinal injury and inflammatory response, and curbing disease progression [6]. Evidence has indicated that blood infection in senile patients with sepsis is related to significantly higher mortality and adverse prognosis, more comorbidities and less clinical response, which brings great challenges to clinical treatment [7]. Hence, it is important to seek better treatment strategies for sepsis in the elderly population to improve patients’ intestinal function and prognosis.
Long-term intensive care unit stay in elderly sepsis patients is often accompanied by malnutrition, the risk of which can be effectively reduced by early enteral nutrition (EEN), contributing to improved outcomes of such patients [8]. Compared with parenteral nutrition, enteral nutrition intervention is more conducive to improving intestinal integrity and inhibiting systemic inflammation [9]. There were also studies reporting that giving patients EEN intervention could greatly reduce the risk of serious gastrointestinal complications and accelerate patients’ recovery [10]. In the study of Jiang et al. [11], EEN within 24 hours of admission effectively reduced 60-day mortality in sepsis patients. Besides, the benefits provided by probiotics in various infectious diseases have been gradually revealed, which can improve the flora ecology and restore the lost non-pathogenic digestive flora [12]. Probiotics are also widely used in malnutrition caused by various diseases, providing nutrients for patients while ensuring their safety [13]. Chen et al. [14] reported that while significantly improving the nutritional status of stroke patients, probiotics-containing EEN therapy enhanced patients’ intestinal flora and IMB function.
Considering that there are currently few analyses on the impacts of probiotics plus EEN on intestinal function and clinical effects of elderly sepsis patients, this study conducted relevant research with the aim of providing optimization ideas and new insights into the treatment of sepsis in the elderly.
Data and methods
General information
A retrospective analysis was performed on 108 senile sepsis patients admitted between January 2019 and January 2022. Patients were grouped according to different intervention methods, with 50 cases treated with EEN being included in a control group (CG) and 58 cases treated with EEN plus probiotics being assigned to a research group (RG). The ratio of male to female, mean age, and body mass index (BMI) in the CG were 27:23, (71.36±7.71) years, and (22.26±3.05) kg/m2, respectively, while the data in the RG were 34:24, (72.36±7.74) years and (23.19±2.96) kg/m2, respectively. The RG and CG showed comparability in sex, age, BMI and other general data (P>0.05). Inclusion criteria: age ≥60; confirmed diagnosis of sepsis [15]; in accordance with the indications of enteral nutrition therapy; complete clinical records. Exclusion criteria: death within 7 days of admission; malignant tumors or serious organ diseases; nutritional contraindications or inability to receive EEN intervention; recent history of gastrointestinal surgery or abdominal infection; gastrointestinal diseases. This study was approved by the General Hospital of Southern Theatre Command Ethics Committee.
Treatment methods
The CG received EEN therapy. Patients were given water and electrolyte maintenance, perfusion improvement, anti-inflammation and anti-infection treatment, as well as nasogastric tube indwelling. EEN therapy was given within 1-2 days after admission, with the enteral nutrition suspension (NUTRICIA Pharmaceuticals (Wuxi), H20010285) pumped into the patient at a constant speed through the nasal catheter. The dosage was a quarter of the required amount on the first day, and increased by 1/4 until the full amount according to patient tolerance. The enteral nutrient suspension was maintained at 37-42°C, and the feeding rate was set to 20-25 ml per hour, with a daily limit of 500 mL. In cases with gastrointestinal reactions during treatment, the hourly feeding amount was increased to 60-85 mL with a daily limit of 1500-2000 mL.
On this basis, the RG was treated with additional probiotics (Bifid-triple Viable Enteric-coated Capsules; Jincheng Health Pharmaceutical Co., Ltd., S19993065). After grinding and hydration, the probiotics preparations were slowly injected into the patients through the nasal feeding tube, three times a day, three tablets each time, for a two-week course of treatment.
Evaluation of clinical response
Marked response referred to complete resolution of the patient’s clinical symptoms, with the infection focus under control and the laboratory indexes completely restored to normal.
Response was indicated if the patient’s clinical symptoms were improved, the infection focus were not completely controlled, but the laboratory indexes were within the reference range.
If the patient’s clinical symptoms were not improved or even aggravated, and the laboratory indexes were abnormal, it was regarded as non-response.
Endpoints
(1) Efficacy. The number of cases corresponding to marked response, response and non-response was recorded according to the above-mentioned efficacy evaluation criteria, and the overall response rate (ORR) of treatment was calculated. (2) IMB indexes. Serum was isolated via centrifugation after collecting 3 mL venous blood from each patient before and 14 days after enteral nutrition treatment, to detect intestinal fatty acid binding protein (IFABP), diamine oxidase (DAO) and D-lactate (D-Lac) by enzyme-linked immunosorbent assay (ELISA). The experimental process strictly followed the instructions of human IFABP, DAO, and D-Lac ELISA kits (Beijing Bowalls Biotech, EK-H10481, EK-H10657, EK-H12394). (3) Nutritional indicators. Albumin (Alb) and prealbumin (PA) were measured before and after treatment. (4) Prognosis. The 28-day mortality of patients was recorded.
Statistical processing
GraphPad Prism 6 (GraphPad Software, San Diego, USA) was used to analyze the data and plot image rendering in this study. Enumeration data (sex, hypertension, 28-day mortality, etc.) and quantitative data (age, IFABP, DAO, D-Lac) were denoted by the number of cases/percentage [n (%)] and the mean ± standard deviation (mean ± SD), respectively. A Chi-square test was used for comparison of enumeration data between groups. The t test of independent samples was used for comparison of quantitative data between groups, and paired t test for comparison before and after treatment within groups. Cox regression analysis was performed to analyze the prognostic factors of elderly patients with sepsis. A significance level was set at P<0.05.
Results
General information of two cohorts of senile sepsis patients
As shown in Table 1, the general data such as sex, age, hypertension, coronary heart disease, diabetes, chronic obstructive pulmonary disease, smoking history and alcoholism history of the two cohorts of senile sepsis patients were comparable (P>0.05).
Table 1.
General data of two cohorts of senile sepsis patients [n (%), mean ± SEM]
| Factors | Control group (n=50) | Research group (n=58) | χ2/t | P |
|---|---|---|---|---|
| Sex (Male/Female) | 27/23 | 34/24 | 0.233 | 0.629 |
| Age (years old) | 71.36±7.71 | 72.36±7.74 | 0.671 | 0.504 |
| Hypertension (Yes/No) | 15/35 | 13/45 | 0.805 | 0.370 |
| Coronary Heart Disease (Yes/No) | 10/40 | 14/44 | 0.266 | 0.606 |
| Diabetes (Yes/No) | 9/41 | 10/48 | 0.011 | 0.918 |
| Chronic Obstructive Pulmonary Disease (Yes/No) | 5/45 | 8/50 | 0.365 | 0.546 |
| History of smoking (Yes/No) | 8/42 | 12/46 | 0.391 | 0.532 |
| History of alcoholism (Yes/No) | 18/32 | 22/36 | 0.043 | 0.836 |
Efficacy of two cohorts of senile sepsis patients
The statistics (Table 2) showed 37 cases of marked response in the CG and 52 cases of that in the RG. The ORR was statistically higher in the RG compared with that in the CG (89.66% vs. 74.00%, P<0.05).
Table 2.
Comparison of curative effects between two cohorts of senile sepsis patients [n (%)]
| Groups | n | Marked response | Response | Non-response | Overall response rate (%) |
|---|---|---|---|---|---|
| Control group | 50 | 12 (24.00) | 25 (50.00) | 13 (26.00) | 37 (74.00) |
| Research group | 58 | 29 (50.00) | 23 (39.66) | 6 (10.34) | 52 (89.66) |
| χ2 value | - | - | - | - | 4.539 |
| P value | - | - | - | - | 0.033 |
IMB indexes of two cohorts of senile sepsis patients
IFABP, DAO and D-Lac were non-significantly different between groups prior to treatment (P>0.05), while the three indexes all decreased statistically in different degrees post treatment (P<0.01), with more obvious reductions in the RG than in the CG (P<0.01, Figure 1).
Figure 1.
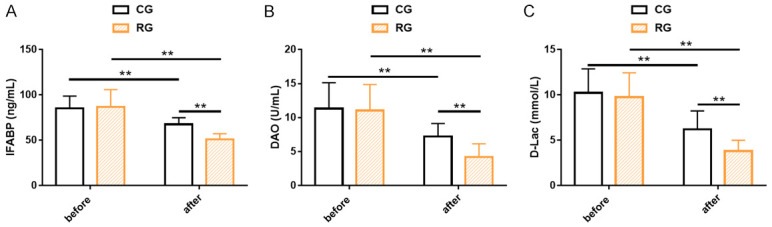
Indicators of intestinal mucosal barrier in two cohorts of senile sepsis patients. A. The IFABP of both groups decreased significantly after treatment, especially in the research group. B. The DAO of both groups decreased significantly after treatment, especially in the research group. C. The D-Lac of both groups decreased significantly after treatment, especially in the research group. Note: **P<0.01. IFABP, Intestinal Fatty Acid Binding Protein; DAO, Diamine Oxidase; D-Lac, D-Lactate.
Nutritional indexes of two cohorts of senile sepsis patients
Alb and PA of both groups were recorded to evaluate the impacts of the two treatments on patients’ nutritional status. The results revealed no significant differences in Alb and PA between the two groups prior to treatment (P>0.05). After treatment, Alb and PA in the RG were markedly higher than those in the CG and those before treatment (P<0.05, Figure 2).
Figure 2.
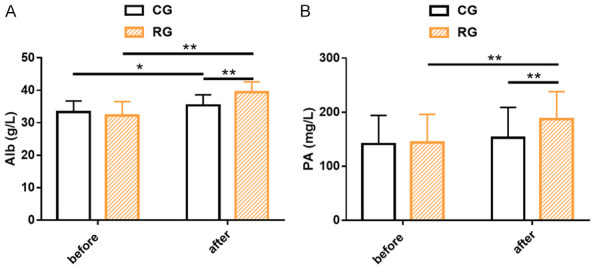
Nutritional indicators of two cohorts of senile sepsis patients. A. The research group had significantly higher Alb levels than the control group after treatment. B. The research group had significantly higher PA levels than the control group after treatment. Note: *P<0.05; **P<0.01. Alb, Albumin; PA, Prealbumin.
Prognostic indicators of two cohorts of senile sepsis patients
The influences of the two treatments on patient prognosis were evaluated in terms of the 28-day mortality. A lower 28-day mortality was determined in the RG as compared with that in the CG (1.72% vs. 8.00%), but with no significant difference between groups (P>0.05, Figure 3).
Figure 3.
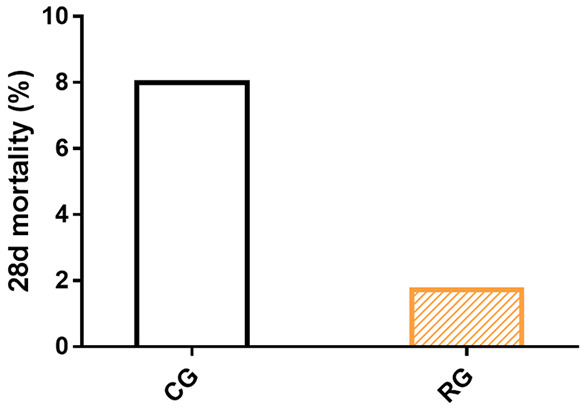
Prognostic indicators of two cohorts of senile sepsis patients.
Analysis of prognostic factors in elderly patients with sepsis
We performed univariate and multivariate analyses on the prognosis of elderly patients with sepsis by Cox regression analysis (Table 3). Univariate analysis showed that IFABP, D-LAC, Alb and PA were significantly associated with the prognosis of senile sepsis patients (P<0.05). Moreover, according to the multivariate analysis, Alb (P=0.003) and PA (P=0.045) were independent prognostic factors in elderly patients with sepsis.
Table 3.
Analysis of prognostic factors in elderly patients with sepsis
| Variate | B | SE | Wald | P | Exp (B) | 95% CI |
|---|---|---|---|---|---|---|
| IFABP | -0.007 | 0.026 | 0.076 | 0.783 | 0.993 | 0.943-1.045 |
| DAO | 0.084 | 0.108 | 0.597 | 0.44 | 1.087 | 0.880-1.344 |
| D-Lac | 0.148 | 0.109 | 1.845 | 0.174 | 1.159 | 0.937-1.434 |
| Alb | -0.127 | 0.043 | 8.856 | 0.003 | 0.881 | 0.810-0.958 |
| PA | 0.008 | 0.004 | 4.002 | 0.045 | 1.008 | 1.000-1.016 |
IFABP, Intestinal Fatty Acid Binding Protein; DAO, Diamine Oxidase; D-Lac, D-Lactate; Alb, Albumin; PA, Prealbumin.
Discussion
Sepsis is a fatal clinical syndrome characterized by organ dysfunction secondary to infection, which can lead to renal failure or even death in severe cases [16]. Senile sepsis patients are associated with elevated risk of disability and death as well as increased treatment difficulty than young patients because of their low immunity, decreased organ reserve function, having basic diseases and atypical clinical symptoms after infection [17,18]. EEN intervention is a safe and reliable nutritional therapy that delivers nutritional fluid to the gastrointestinal tract through a nasal catheter or stoma for patients who are unable or insufficient to achieve nutritional levels orally [19]. This study primarily discusses the intestinal function and the clinical effects of probiotics plus EEN in senile sepsis patients, aiming to contribute to treatment optimization of the disease.
In our study, the probiotic preparations used were Bifid-triple Viable Enteric-coated Capsules that mainly include Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus faecalis. Probiotics can stimulate the anti-sepsis protective mechanism in the pathogenesis of sepsis, and play a therapeutic role by inhibiting the secretion of inflammatory mediators and alleviating the conditions relating to irritable bowel disorder [20]. In addition, the probiotics in Bifid-triple Viable Enteric-coated Capsules, as active microorganisms, are also of great help in improving the ecological balance and prognosis of the host’s intestinal flora [21]. Our efficacy assessment data showed that the RG had an obviously higher ORR than the CG, which suggested that EEN plus probiotics were effective in treating senile patients with sepsis. We then assessed the IMB by examining IFABP, DAO and D-LAC in the patients. Increased IFABP is known to indicate intestinal cell injury and intestinal toxicity, while DAO and D-Lac are significantly related to the intestinal flora composition of sepsis patients [22,23]. Our research identified statistically lower IFABP, DAO and D-Lac after treatment than those pre-treatment, and the post-treatment levels were lower in the RG than those in the CG, which indicated that EEN plus probiotics intervention had a prominent improvement effect on IMB. It has also been reported that the IFABP level of rats with nonalcoholic fatty liver disease decreased markedly after the intervention of Saccharomyces boulardii, which is in line with our research results [24]. Similarly, Xin et al. [25] reported that DAO and D-Lac, as intestinal permeability markers, showed significantly reduced serum levels under the intervention of probiotics. Furthermore, patients’ nutritional status was assessed by measuring the patients’ Alb and PA levels. We found that the RG had markedly higher Alb and PA levels than the CG after treatment, demonstrating that the combined intervention can significantly promote the nutritional recovery of senile patients with sepsis. It has been reported that Alb and PA can be used as nutritional indicators of the body, and low levels of Alb and PA are helpful for predicting malnutrition in elderly patients with sepsis [26,27]. In the report of Chen et al. [14], probiotics combined with EEN improved the levels of Alb and PA in stroke patients, and effectively enhanced patients’ nutritional status, which is similar to our research results. In terms of patients’ prognosis, a lower 28-day mortality was observed in the RG (1.72%) as compared with that in the CG (8.00%), suggesting that the combined intervention can effectively improve the outcome of senile sepsis patients. EEN support is shown to exert a significant positive effect on shortening hospital stays and reducing mortality, suggesting that this therapy is helpful for promoting rehabilitation and improving prognosis [28]. Finally, we confirmed through Cox multivariate regression analysis that Alb and PA were independent predictors of the prognosis (28-day mortality) of senile sepsis patients, while IFABP, DAO and D-LAC were not, which was similar to the findings of Zhang et al. [29]. Similarly, Cha et al. [30] also pointed out in their study that Alb was helpful to predict the 28-day mortality of elderly patients with sepsis.
This research has some limitations that need further consideration. To begin with, there may be some information bias as this is a single-center study with limited cases included. Besides, basic experiments should be supplemented to discuss relevant therapeutic mechanisms, so as to help further improve the cognition of therapeutic and pathological mechanisms of sepsis. Moreover, this research would have more important guiding significance for sepsis management optimization if we can further explore the influencing factors that affect the prognosis of sepsis in the elderly. In the future, our research will be gradually improved from the above angles.
In a word, EEN plus probiotics can effectively improve the intestinal function of senile patients with sepsis, mainly by improving the IMB. In addition, this combined intervention mode can validly enhance the curative effect and nutritional level of such patients, contributing to improved prognosis.
Acknowledgements
This work was supported by Science and Technology Program of Guangzhou (No. 202102080528).
Disclosure of conflict of interest
None.
References
- 1.Gao Q, Zheng Y, Wang H, Hou L, Hu X. circSTRN3 aggravates sepsis-induced acute kidney injury by regulating miR-578/toll like receptor 4 axis. Bioengineered. 2022;13:11388–11401. doi: 10.1080/21655979.2022.2061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mollura M, Lehman LW, Barbieri R. Assessment of sepsis in the ICU by linear and complex characterization of cardiovascular dynamics. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:862–865. doi: 10.1109/EMBC46164.2021.9630521. [DOI] [PubMed] [Google Scholar]
- 3.Vesteinsdottir E, Sigurdsson MI, Gottfredsson M, Blondal A, Karason S. Temporal trends in the epidemiology, management, and outcome of sepsis-A nationwide observational study. Acta Anaesthesiol Scand. 2022;66:497–506. doi: 10.1111/aas.14026. [DOI] [PubMed] [Google Scholar]
- 4.Gao YL, Yao Y, Zhang X, Chen F, Meng XL, Chen XS, Wang CL, Liu YC, Tian X, Shou ST, Chai YF. Regulatory T cells: angels or demons in the pathophysiology of sepsis? Front Immunol. 2022;13:829210. doi: 10.3389/fimmu.2022.829210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhan L, Pu J, Zheng J, Hang S, Pang L, Dai M, Ji C. Tetrastigma hemsleyanum Diels et Gilg ameliorates lipopolysaccharide induced sepsis via repairing the intestinal mucosal barrier. Biomed Pharmacother. 2022;148:112741. doi: 10.1016/j.biopha.2022.112741. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Quiles R, Merino-Lucas E, Boix V, Fernandez-Gil A, Rodriguez-Diaz JC, Gimeno A, Valero B, Sanchez-Martinez R, Ramos-Rincon JM. Bacteraemia and quick Sepsis Related Organ Failure Assessment (qSOFA) are independent risk factors for long-term mortality in very elderly patients with suspected infection: retrospective cohort study. BMC Infect Dis. 2022;22:248. doi: 10.1186/s12879-022-07242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Huang T, Xu F, Li S, Zheng S, Lyu J, Yin H. Prediction of prognosis in elderly patients with sepsis based on machine learning (random survival forest) BMC Emerg Med. 2022;22:26. doi: 10.1186/s12873-022-00582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojo O, Ojo OO, Feng Q, Boateng J, Wang X, Brooke J, Adegboye ARA. The effects of enteral nutrition in critically ill patients with COVID-19: a systematic review and meta-analysis. Nutrients. 2022;14:1120. doi: 10.3390/nu14051120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farina N, Nordbeck S, Montgomery M, Cordwin L, Blair F, Cherry-Bukowiec J, Kraft MD, Pleva MR, Raymond E. Early enteral nutrition in mechanically ventilated patients with COVID-19 infection. Nutr Clin Pract. 2021;36:440–448. doi: 10.1002/ncp.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song M, Zhao P, Hu W. Application effect of intra-abdominal pressure monitoring system in early enteral nutrition nursing of ICU patients. Contrast Media Mol Imaging. 2022;2022:3545278. doi: 10.1155/2022/3545278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Y, Hu B, Zhang S, Cai M, Chu X, Zheng D, Lou M, Cui K, Zhang M, Sun R, Lin R. Effects of early enteral nutrition on the prognosis of patients with sepsis: secondary analysis of acute gastrointestinal injury study. Ann Palliat Med. 2020;9:3793–3801. doi: 10.21037/apm-20-1650. [DOI] [PubMed] [Google Scholar]
- 12.Wan G, Wang L, Zhang G, Zhang J, Lu Y, Li J, Yi X. Effects of probiotics combined with early enteral nutrition on endothelin-1 and C-reactive protein levels and prognosis in patients with severe traumatic brain injury. J Int Med Res. 2020;48:300060519888112. doi: 10.1177/0300060519888112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Liu X, Zhang Y, Chu J, Zheng J, Cheng X, Li X, Long J. Effect of probiotics on the nutritional status of severe stroke patients with nasal feeding that receive enteral nutrition: a protocol for systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2021;100:e25657. doi: 10.1097/MD.0000000000025657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Hu Y, Yuan X, Yang J, Ka L. Effect of early enteral nutrition combined with probiotics in patients with stroke: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2022;76:592–603. doi: 10.1038/s41430-021-00986-3. [DOI] [PubMed] [Google Scholar]
- 15.Gauer R, Forbes D, Boyer N. Sepsis: diagnosis and management. Am Fam Physician. 2020;101:409–418. [PubMed] [Google Scholar]
- 16.Hei B, Yue C, Sun Y. Long noncoding RNA ZFAS1 protects HK-2 cells against sepsis-induced injury through targeting the miR3723p/PPARalpha axis. J Healthc Eng. 2022;2022:7768963. doi: 10.1155/2022/7768963. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Carbajal-Guerrero J, Cayuela-Dominguez A, Fernandez-Garcia E, Aldabo-Pallas T, Marquez-Vacaro JA, Ortiz-Leyba C, Garnacho-Montero J. Epidemiology and long-term outcome of sepsis in elderly patients. Med Intensiva. 2014;38:21–32. doi: 10.1016/j.medin.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Clifford KM, Dy-Boarman EA, Haase KK, Maxvill K, Pass SE, Alvarez CA. Challenges with diagnosing and managing sepsis in older adults. Expert Rev Anti Infect Ther. 2016;14:231–241. doi: 10.1586/14787210.2016.1135052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Onofrio V, Del Chierico F, Belci P, Vernocchi P, Quagliariello A, Reddel S, Conta G, Mancino MV, Fadda M, Scigliano MC, Morelli R, De Francesco A, Guagnini F, Fassio F, Galletti R, Putignani L. Effects of a synbiotic formula on functional bowel disorders and gut microbiota profile during long-term home enteral nutrition (LTHEN): a pilot study. Nutrients. 2020;13:87. doi: 10.3390/nu13010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar R, Tripathi AS, Sharma N, Singh G, Mohapatra L. Is regular probiotic practice safe for management of sepsis? Chin J Integr Med. 2022;28:185–192. doi: 10.1007/s11655-021-3334-5. [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Zhang H, Huang W. Efficacy of Bifidobacterium triple viable enteric-coated capsules combined with enteral nutrition on patients with chronic critical illness and influence on immune and coagulation function. Evid Based Complement Alternat Med. 2021;2021:3718255. doi: 10.1155/2021/3718255. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Perananthan V, Wijerathna T, Mohamed F, Gawarammana IB, Dawson AH, Buckley NA. Circulating intestinal fatty acid binding protein and intestinal toxicity in Russell’s viper envenomation. Clin Toxicol (Phila) 2022;60:311–318. doi: 10.1080/15563650.2021.1965160. [DOI] [PubMed] [Google Scholar]
- 23.Yang XJ, Liu D, Ren HY, Zhang XY, Zhang J, Yang XJ. Effects of sepsis and its treatment measures on intestinal flora structure in critical care patients. World J Gastroenterol. 2021;27:2376–2393. doi: 10.3748/wjg.v27.i19.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu YT, Li YQ, Wang YZ. Protective effect of Saccharomyces boulardii against intestinal mucosal barrier injury in rats with nonalcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi. 2016;24:921–926. doi: 10.3760/cma.j.issn.1007-3418.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xin J, Wang H, Sun N, Bughio S, Zeng D, Li L, Wang Y, Khalique A, Zeng Y, Pan K, Jing B, Ma H, Bai Y, Ni X. Probiotic alleviate fluoride-induced memory impairment by reconstructing gut microbiota in mice. Ecotoxicol Environ Saf. 2021;215:112108. doi: 10.1016/j.ecoenv.2021.112108. [DOI] [PubMed] [Google Scholar]
- 26.Su X, Li Y, Zhang Y, Han S. Efficacy of alanyl glutamine in nutritional support therapy for patients with sepsis: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e24861. doi: 10.1097/MD.0000000000024861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleming D, Jiang Y, Opoku K, Alhaj Saleh A, Larumbe-Zabala E, Kesey JE, Griswold JA, Dissanaike S. Prophylactic probiotics in burn patients: risk versus reward. J Burn Care Res. 2019;40:953–960. doi: 10.1093/jbcr/irz132. [DOI] [PubMed] [Google Scholar]
- 28.Li PF, Wang YL, Fang YL, Nan L, Zhou J, Zhang D. Effect of early enteral nutrition on outcomes of trauma patients requiring intensive care. Chin J Traumatol. 2020;23:163–167. doi: 10.1016/j.cjtee.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Liu D, Wang Y, Yan J, Yang X. Clinical significance on serum intestinal fatty acid binding protein and D-lactic acid levels in early intestinal injury of patients with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31:545–550. doi: 10.3760/cma.j.issn.2095-4352.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Cha K, Choi SP, Kim SH, Oh SH. Prognostic value of ambulation ability with albumin and C-reactive protein to predict 28-day mortality in elderly sepsis patients: a retrospective multicentre registry-based study. BMC Geriatr. 2022;22:661. doi: 10.1186/s12877-022-03339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


