Abstract
Objective: To systematically assess the efficacy of hematopoietic stem cell transplantation (HSCT) and bone marrow transplantation (BMT) in treating acute myeloid leukemia (AML). Methods: PubMed, EMBASE, ScienceDirect, Cochrane Library, China National Knowledge Infrastructure (CNKI), China VIP database, Wanfang database and China Biomedical Literature Database (CBM) were searched for case-control trials of bone marrow HSCT and peripheral HSCT (PHSCT) for treating AML. Two independent researchers extracted the data between January 2000 and May 2022. Each retrieved article was assessed according to the bias risk defined in Cochrane Handbook 5.3, and data were analyzed by meta-analysis using RevMan5.3. Results: Through computer database retrieval, 7 clinical controlled studies were included, with 1280 samples. A meta-analysis was conducted on the survival rates. The PHSCT and the BMT groups showed no noticeable difference in overall survival (OS) and disease-free survival (DFS) rates (P>0.05). The incidence of acute graft-versus-host disease (GVHD) and chronic GVHD in the BMT group was noticeably lower (P<0.05). The disease recurrence rate in tthe BMT group was lower (P<0.05), but no noticeable differences were found in recurrence-related mortality (P>0.05). Furthermore, there was also no noticeable difference in non-relapse-related mortality (P>0.05). Funnel charts were drawn on the basis of OS rate, DFS rate, incidences of acute GVHD and chronic GVHD, and recurrence. Afterwards publication bias analysis was carried out. Symmetry presented in the majority of the funnel charts and asymmetry was seen in a few, suggesting possible publication bias in the selected literature because of the small sample and the heterogeneity. Conclusion: BMT can be used as an effective treatment for patients with AML, because it can reduce the recurrence rate and the incidence of complications while ensuring a curative effect, suggesting that BMT is worth popularizing in the clinic. Longer follow-up studies are needed to provide more support for the clinical application of BMT in AML patients.
Keywords: Bone marrow transplantation, leukemia, curative effect, hematopoietic stem cell transplantation
Introduction
Leukemia is known as an abnormal clonal growth produced by hematopoietic stem cells [1]. According to the main cell types involved in leukemia, acute leukemia can be divided into two categories, acute myeloid leukemia (AML) and acute lymphoblastic leukemia. AML is a group of heterogeneous diseases originating from hematopoietic stem cells [2]. Uncontrolled proliferation of bone marrow cells can seriously damage hematopoietic function and invade other tissues and organs, resulting in severe anemia, bleeding, infection and central invasion. Today, the treatment for AML is still facing great challenges even with the vigorous development of medical technology. How to reduce the occurrence of adverse reactions and improve the patient’s tolerance while achieving a good therapeutic effect is still an urgent problem to be solved in the clinic.
Standard chemotherapy and hematopoietic stem cell transplantation (HSCT) are the main treatments for AML patients under 60 years old. The “3-7 regimen” (3-d daunorubicin + 7-d cytarabine) proposed in 1970s has long been regarded as a standard treatment, which can cure 30% to 40% of young AML patients [3-5]. An AML study [6] mainly focused on patients aged 50 to 55 years and reported a 5-year survival rate of 40% to 45%. Researchers reported a 30-35% 5-year survival rate in patients with AML aged 60 and older, and the 5-year survival rate was less than 10-15% for elderly patients over 60 years old receiving intensive chemotherapy regimens [6,7]. This may be because elderly patients have difficulty tolerating conventional chemotherapy. In addition, conventional chemotherapy does not seem to be effective in recurrent refractory patients and in patients with high cytogenetic risk. The biological factors leading to these adverse therapeutic effects may be the combination of multiple aspects, such as weak physical function, drug resistance, immune impairment and multiple organ dysfunction. Moreover, the cardiotoxicity related to anthracyclines limits the wide application of anthracyclines to some extent. Therefore, for this large category of patients, it is of great significance to seek a chemotherapy regimen with better efficacy and safety.
The pathogenesis of AML is abnormal proliferation of bone marrow hematopoietic precursor cells. The main clinical manifestations of patients are asthma, fatigue, weak motor ability etc [8]. Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is the only known method to completely cure AML. However, this therapeutic approach is bounded by limited Human leukocyte antigen (HLA)-matched donors, and some patients, especially older patients, are not eligible for the transplantation [9]. Due to the lack of tolerable treatments that can improve survival and life quality in this group of patients, disease management is challenging. Historical data indicates that the overall survival (OS) of patients who do not meet the standard intensive chemotherapy conditions is only about 5 months [10]. In addition, considering the heterogeneity of patients’ disease biology, age-related complications, potential toxicity, and choice of patients, doctors’ preferences and other factors, there is no general standard approach to treat AMI.
Some studies [11] have pointed out that bone marrow transplantation (BMT) can not only enhance the clinical symptoms of patients, but also delay the recurrence time and prolong the survival period. In patients with AML, BMT can reduce the need for repeated chemotherapy, which greatly increases their acceptance of the treatment. Recent studies have shown that BMT is mostly used in patients who are sensitive to chemotherapy or radiotherapy but cannot be cured by ordinary doses [11]. The treatment technology is widely used in clinical practice due to the relatively wide age limit of eligible patients and low transplantation-related mortality. Several case-control studies [11-14] have investigated the clinical efficacy of BMT in treating AMI patients. However, the conclusions of the studies are inconsistent, and there were great differences in study design and evaluation indicators. The conclusions of a single study cannot be used to prove the value of BMT in patients with AMI, and the findings still need to be supported by high quality research evidence. Therefore, more authoritative scientific studies are needed to demonstrate the feasibility and safety of BMT for AMI, in order to offer a theoretical basis for the promotion and application of this treatment. Through Meta-analysis of similar independent studies, this study evaluated the efficacy of BMT for AML in a systematic, quantitative, and comprehensive manner.
Research content and methods
The sources and retrieval methods of documents
PubMed, EMBASE, ScienceDirect, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP full-text Database, Wanfang Database and Chinese Biomedical Literature data (CBM), as well as relevant conference papers, degree papers, supplemented by literature review were searched, and we collected data of case-control trials that applied BMT and peripheral HSCT (PHSCT) for treating AML. The literature from January 2000 to May 2022 was searched by free words + subject words, with the search terms of BMT, AML, curative effect analysis, systematic review; leukemia; efficacy analysis; system evaluation.
Literature inclusion and exclusion criteria
Literature inclusion criteria
(1) Study design: case-control trials using BMT and PHSCT to treat leukemia. (2) Subjects: patients with AML who had been diagnosed according to WHO diagnostic criteria [12], and there were no restrictions on clinical classification, sex, age and other biological characteristics. (3) Intervention: BMT or PHSCT was used. (4) Outcome measures: more than one of the following were measured, OS rate, disease-free survival (DFS) rate, recurrence rate, incidence of acute graft-versus-host disease (GVHD) and incidence of chronic GVHD.
Literature exclusion criteria
(1) Case-control studies were not conducted. (2) It is impossible to use the data because the report was incomplete. (3) The research content was repeated. (4) The evaluation of the curative effect of the study was not noticeable. (5) Review of related literature. (6) Case reports.
Quality evaluation and data extraction
1) As recommended in Chapter 5.3 of the Cochrane System Review Manual, bias risk assessment was performed in this study.
2) Literature retrieving and data extraction: an independent review of the literature was carried out by two researchers (Ying Li and Fang Zhou). Data were extracted, and qualities were assessed. Disagreements were discussed and resolved, and a third researcher was included to assist in the decision-making process if needed. To manage and extract research data, Note Express document management software was used, as well as Excel. Whenever incomplete data were found in the literature, this article’s authors were contacted to complete it. The extracted data contained: (1) basic information of the articles: author, publication time, number of cases; (2) intervention: scheme, course of treatment; (3) outcome indicators: OS rate, DFS rate, recurrence rate, incidence of acute GVHD and chronic GVHD.
Statistical process
Meta analysis was performed using RevMan5.3 software. The mean difference was adopted to index counting data, and the relative risk (OR) was adopted to index measurement data. Point estimates and 95% confidence intervals (CIs) were provided for each effect. Heterogeneity was assessed by using I2 and the χ2 test. If P>0.05 and I2<50%, it is considered that the included study is homogeneous, and the modified impact model can be collected for Meta analysis. If P<0.05 and I2≥50%, the combined effect is needed to judge the homogeneity of the included study, then the random effect model was chosen. If P<0.05 and the source of heterogeneity could not be judged, Meta-analysis was not performed, and descriptive analysis was used.
Results and analysis
Literature retrieval and inclusion
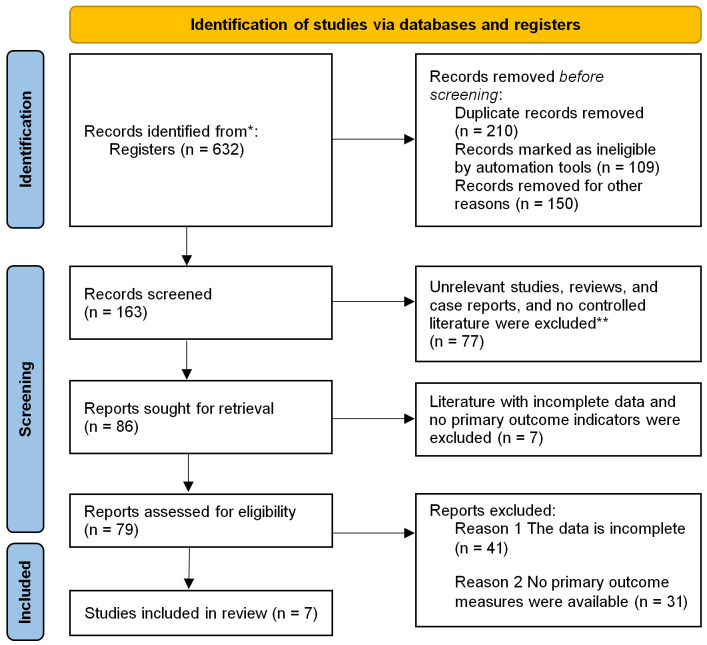
Through computer database retrieval, a total of 632 articles were found, of which 387 repeated studies were removed. Following the exclusion criteria, we obtained 86 articles, but 79 of them had incomplete data and failed to provide main outcomes, and finally 7 controlled trails were included [13-19], with 1280 samples. See Figure 1 for literature screening diagram and Table 1 for basic characteristics of the included articles.
Figure 1.
Illustration of literature screening. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/.
Table 1.
Basic characteristics of the included literature
| Include the literature | Year of publication | Sample size | Age (years) | Gender | Outcome index | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| BMT | PHSCT | BMT | PHSCT | BMT (Male/female) | PHSCT (Male/female) | |||
| Heldal [13] | 2000 | 39 | 31 | 21.8 | 23 | 12/18 | 11/20 | ① ③④⑤ |
| Bensigner [14] | 2001 | 91 | 81 | 42 | 42 | 62/29 | 56/25 | ① ③④⑤ |
| Vigorito [15] | 2001 | 29 | 27 | 29.9 | 36 | 17/12 | 21/6 | ① ③④ |
| Oehler [16] | 2005 | 40 | 32 | 43 | 43 | Unknown | Unknown | ① ③④⑤ |
| Friedrichs [17] | 2010 | 166 | 163 | 37 | 37 | 52/114 | 44/119 | ① ④⑤ |
| Anasetti [18] | 2012 | 278 | 273 | Unknown | Unknown | 168/110 | 146/127 | |
| Zhong Yinghong [19] | 2015 | 11 | 19 | 31.2±3.8 | 29.6±3.2 | 3/8 | 6/13 | ① ②⑤ |
Note: BMT: bone marrow transplantation; PHSCT: peripheral blood stem cell transplantation. ① Overall survival (OS) rate; ② Disease-free survival (DFS) rate; ③ Acute graft-versus-host disease (GVHD); ④ Chronic GVHD; ⑤ Relapse rate.
Quality assessment of the literature’s methodology
All patients in the 7 case-control studies in this meta-analysis were reported to have baseline health status. Random methods and detailed interventions were described in detail in each of the articles. Some of the literature implemented distribution hiding, so the selective bias was small. There was a small implementation bias because only 7 articles described in detail the numbers and reasons for blindness, loss of follow-up, or withdrawal. All the literature obtained their trial plans, so they were less biased. Some reports found other risks, so the bias was larger. The risk bias analysis is shown in Figures 2 and 3.
Figure 2.
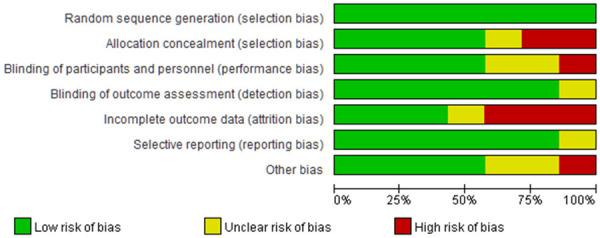
Risk bias chart.
Figure 3.
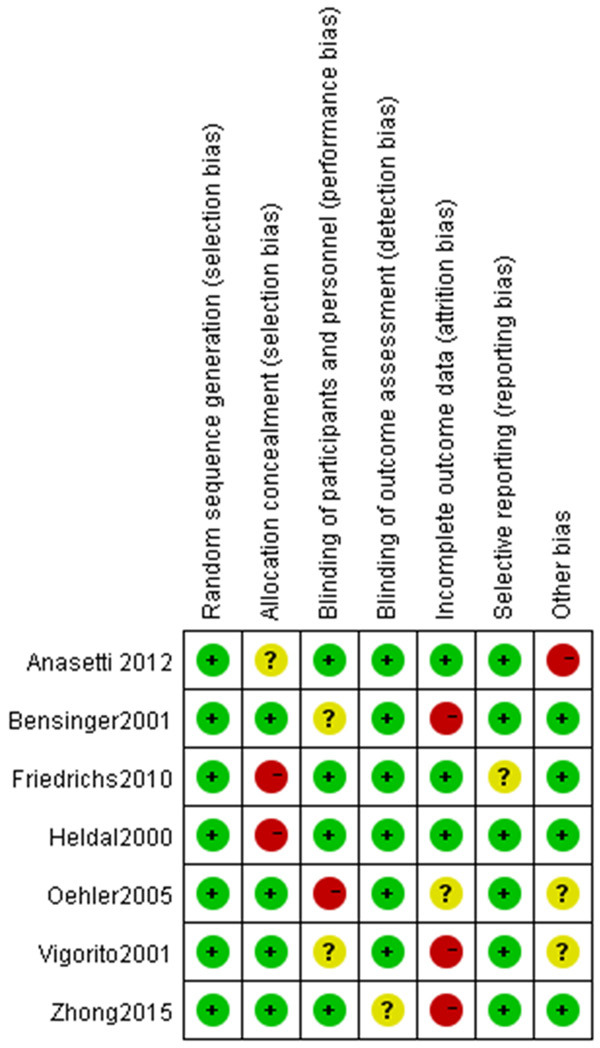
Summary chart of risk bias.
Results of meta analysis
OS rate
Meta-analysis was performed on the OS rates. Tests of heterogeneity revealed the following results: Chi2=9.14, df=6, P=0.17, I2=34%, indicating that the data contained in the study did not appear to be heterogeneous. The fixed-effect model analysis indicated that there was no noticeable difference in the OS rate (P>0.05). See Figure 4.
Figure 4.
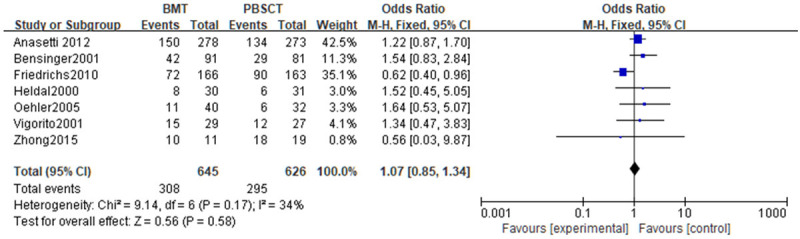
Forest analysis of overall survival in patients who received bone marrow transplantation or peripheral hematopoietic stem cell transplantation.
DFS rate
A meta-analysis was performed on the DFS rates. Tests of heterogeneity revealed the following results: Chi2=8.43, df=4, P=0.08, I2=53%, indicating that the data contained in the study did not appear to be heterogeneous. Analyses were conducted using a random effect model. The DFS rates did not differ noticeably (P>0.05). See Figure 5.
Figure 5.
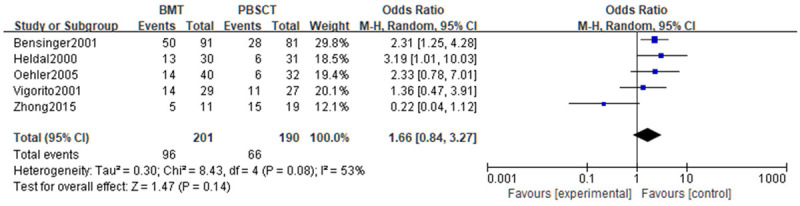
Forest analysis of disease-free survival in patients who received bone marrow transplantation or peripheral hematopoietic stem cell transplantation.
Acute GVHD
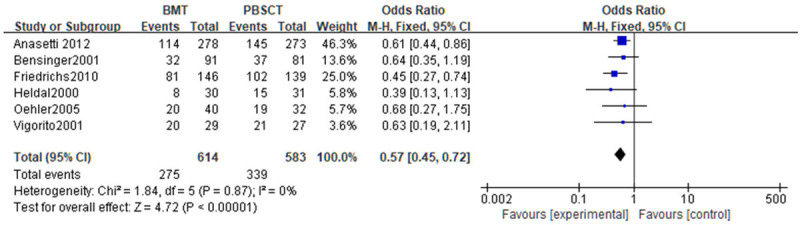
A meta-analysis was performed on the acute GVHD. Tests of heterogeneity revealed the following results: Chi2=1.00, df=4, P=0.91, I2=0%, indicating that the data contained in the study did not appear to be heterogeneous. BMT patients had a noticeably lower incidence of acute GVHD, according to the fixed-effect model analysis (P<0.05). See Figure 6.
Figure 6.
Forest analysis map of incidence of acute graft-versus-host disease in patients who received bone marrow transplantation or peripheral hematopoietic stem cell transplantation.
Chronic GVHD
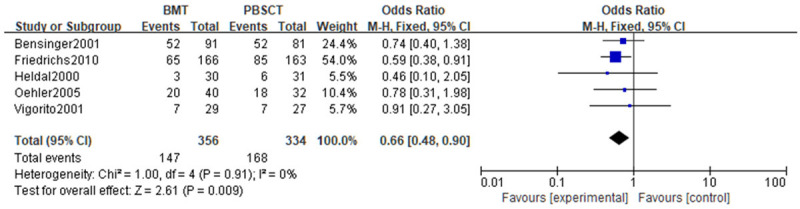
Chronic GVHD was analyzed in a meta-analysis. Tests of heterogeneity revealed the following results: Chi2=1.84, df=5, P=0.87, I2=0%, indicating that the data contained in the study did not appear to be heterogeneous, and the fixed-effect model analysis indicated that the incidence of chronic GVHD in the BMT group was noticeably lower (P<0.05). See Figure 7.
Figure 7.
Forest analysis map of incidence of chronic graft-versus-host disease in patients who received bone marrow transplantation or peripheral hematopoietic stem cell transplantation.
Relapse rate
A meta-analysis was performed on the recurrence rate after treatment. Tests of heterogeneity revealed the following results: Chi2=7.06, df=4, P=0.13, I2=43%, indicating that the data contained in the study did not appear to be heterogeneous. According to the fixed effect model analysis, the BMT group experienced a lower recurrence rate (P<0.05). See Figure 8.
Figure 8.
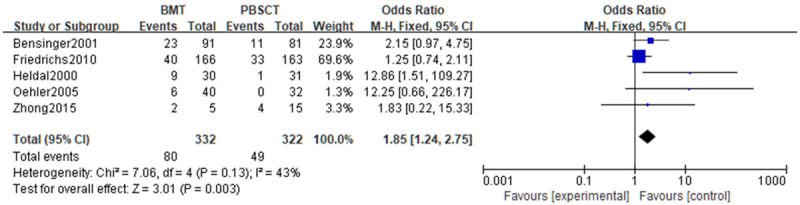
Forest analysis map of recurrence rate in patients who received bone marrow transplantation or peripheral hematopoietic stem cell transplantation.
Recurrence-related mortality
A meta-analysis of recurrence-related mortality was carried out. Tests of heterogeneity revealed the following results: Chi2=4.09, df=4, P=0.39, I2=2%, indicating that the data contained in the study did not appear to be heterogeneous. The fixed-effect model analysis indicated that there was no statistical difference in the recurrence-related mortality (P>0.05). See Figure 9.
Figure 9.
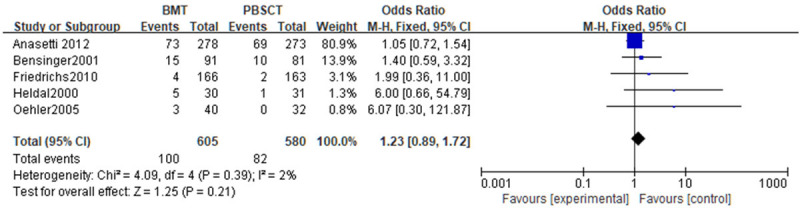
Forest analysis map of relapse-related mortality in patients that received bone marrow transplantation or peripheral hematopoietic stem cell transplantation.
Non-relapse-related mortality
A meta-analysis of non-relapse-related mortality was conducted. Tests of heterogeneity revealed the following results: Chi2=3.47, df=4, P=0.48, I2=0%, indicating that the data contained in the study did not appear to be heterogeneous. The fixed-effect model analysis indicated that there was no statistical difference in the non-relapse-related mortality (P>0.05). See Figure 10.
Figure 10.
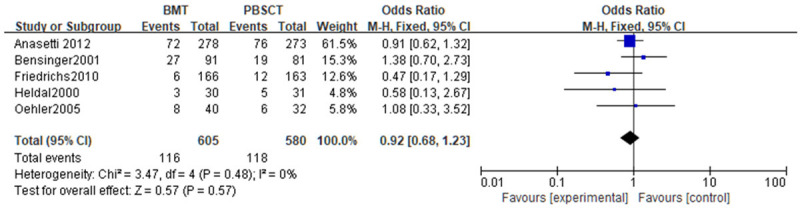
Forest analysis chart of non-relapse-related mortality in patients that received bone marrow transplantation or peripheral hematopoietic stem cell transplantation.
Publication bias analysis
Funnel charts were drawn on the basis of OS rate, DFS rate, incidences of acute and chronic GVHD, and recurrence, and publication bias analysis was carried out. There was a noticeable amount of symmetry and a small amount of asymmetry in the funnel charts, suggesting possible publication bias in the selected literature because of the small sample size and the heterogeneity. See Figures 11, 12, 13, 14, 15, 16 and 17.
Figure 11.
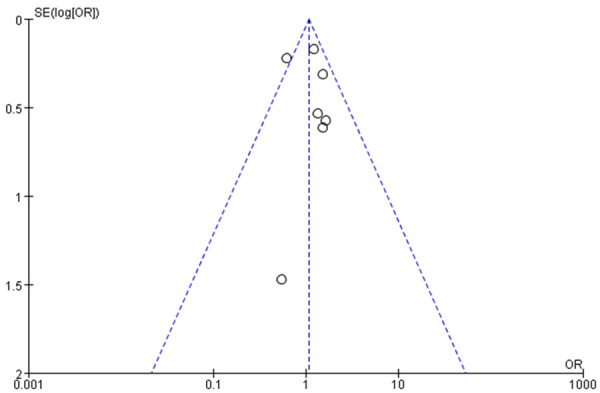
Funnel chart based on overall survival rate.
Figure 12.
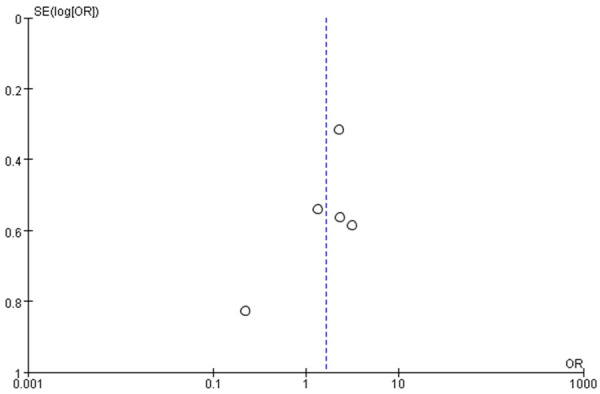
Funnel chart based on disease-free survival rate.
Figure 13.
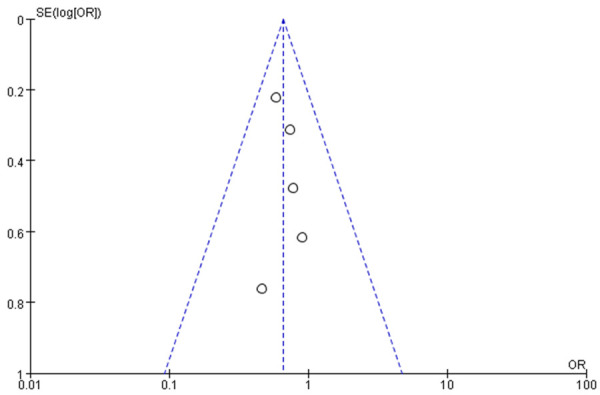
Funnel diagram based on incidence of acute graft-versus-host disease.
Figure 14.
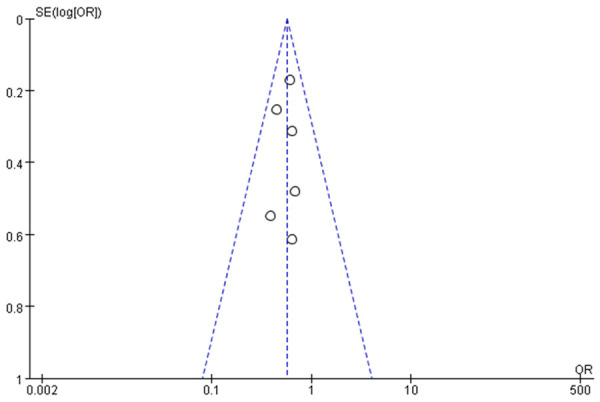
Funnel diagram based on incidence of chronic graft-versus-host disease.
Figure 15.
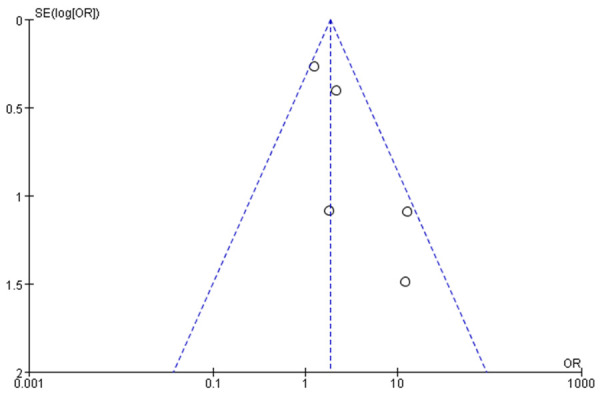
Funnel chart based on recurrence rate.
Figure 16.
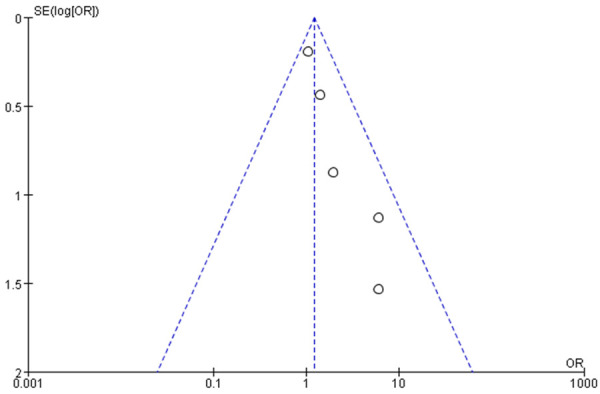
Funnel chart based on recurrence-related death.
Figure 17.
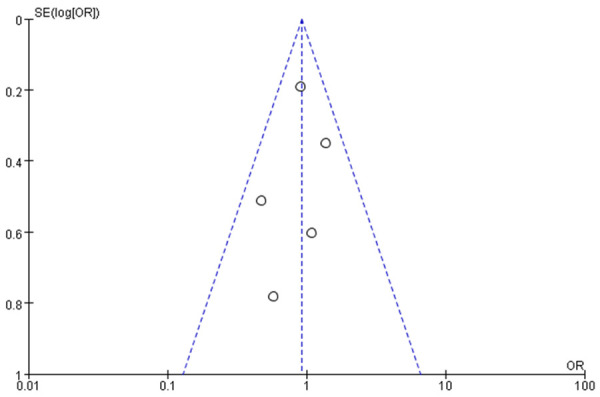
Funnel chart based on non-recurrence-related death.
Analysis and discussion
AML is a hematological malignant tumor with abnormal proliferation of bone marrow hematopoietic germ cells (rather than lymphoid germ cells) [20]. At present, it is generally believed that most leukemias are caused by a combination of environmental factors and genetic material in cells. When it comes to AML in adults, age is a determining factor. Symptoms of AML include fatigue, asthma, decreased motor ability, skin and mucosal bruising and bleeding [21]. HSCT is a difficult and high-tech clinical treatment technology. At present, it is recognized as the most effective treatment for malignant hematological diseases, malignant solid tumors sensitive to radiotherapy and chemotherapy, non-malignant hematological diseases, hereditary diseases, autoimmune diseases and acute radiation sickness [22,23]. HSCT refers to the use of normal hematopoietic stem cells from various sources. The patients who received overdose radiotherapy/chemotherapy were infused intravenously to replace the original pathological hematopoietic stem cells, so as to reconstruct normal hematopoietic and immune function of the recipients. The advantages are that there is no GVHD, no donor is required and it is well tolerated in the elderly. The obvious disadvantage, however, is the re-entry of leukemia cells.
With the improvement of a variety of in vitro purification methods, BMT may become the best scheme for early intensive therapy. BMT replaces diseased bone marrow by intravenous infusion of normal bone marrow into the patient. It is used to treat hematopoietic dysfunction, immune deficiency, hematological malignant tumors and some other malignant tumors. The use of this therapy can improve the curative effect, improve the prognosis, prolong survival and radically cure patients. BMT simply means to transplant normal bone marrow into the patient’s abnormal or lost hematopoietic bone marrow to restore the patient’s hematopoietic function [24]. According to the various sources of bone marrow, BMT can be divided into allogeneic BMT and autologous BMT. Allogeneic BMT is donated by the patient’s siblings or other close relatives, whereas autologous BMT is from an individual’s own body. When the patient is in remission with chemotherapy, the patient’s bone marrow is extracted and frozen, and then BMT is carried out appropriately [25]. Over the past few years, considerable progress has been made in the control of bone marrow rejection and GVHD. Many BMT centers have used unrelated bone marrow donors as long as human white blood cell antigens are the same. Currently, there have been many successful cases. In the past, induction chemotherapy was the first choice for patients with AML to achieve complete remission of bone marrow, followed by multiple courses of consolidation chemotherapy to achieve continuous remission. However, in adult patients, relapse is often inevitable without autologous or allogeneic stem cell transplantation. After the relapse of leukemia, patients often die within six months. In order to reduce drug resistance and relapse of leukemia and achieve long-term survival or cure, autologous BMT is required. This meta-analysis, included 7 clinical controlled studies with 1280 samples, showed that there was no difference in the OS rate and the DFS rate between PHSCT and BMT. This has shown that both PHSCT and BMT have good therapeutic effects on AML patients with the similar curative effects, and both of them can successfully prolong the survival time of patients and protect their physical health. Therefore, it is necessary to include more indictors to judge which treatment scheme has more advantages.
Our study found that the PHSCT and the BMT groups showed no noticeable difference in OS rates and DFS rates (P>0.05). The incidence of acute and chronic GVHD in the BMT group were noticeably lower (P<0.05). Also, the recurrence rate of the BMT group was lower (P<0.05), but no noticeable difference was found in recurrence-related mortality and non-relapse-related mortality (P>0.05). Funnel charts were drawn on the basis of OS rate, DFS rate, acute GVHD incidence, chronic GVHD incidence and recurrence, and publication bias analysis was carried out. There was symmetry in the majority of funnel charts and asymmetry in a few, suggesting possible publication bias in the selected literature because of the small sample size and the heterogeneity. Xue et al. [26] reported that the main causes of early death after allogeneic HSTC were poor implantation and early complications. Serious complications in the early stage of transplantation include organ failure related to preconditioning toxicity, hepatic vein occlusion syndrome, acute GVHD, transplantation-related infections (such as bacteria, fungi or viruses) and thrombotic microangiopathy. A large number of clinical data indicated that PHSCT could be the first choice for auto-transplantation instead of BMT [27,28]. It has definite advantages over BMT, including rapid implantation, low immediate mortality caused by stem cell extraction, short hospital stays and rapid immune reconstruction. However, it was also reported that PHSCT led to an increased incidence of GVHD [29,30]. We carried out meta-analysis of acute and chronic GVHD, and our analysis suggested that the incidences of acute and chronic GVHD in the BMT group were noticeably lower compared to the PHSCT group, implying that BMT can noticeably reduce the incidence of acute GVHD and risk of chronic GVHD in AML patients, showing higher safety. To some extent, the Th2-regulated immune machinery of GVHD can lead to autoimmune disease. In contrast, BMT contains noticeably fewer Th2 cells than PHSCT mobilized by G-CSF and is therefore has a lower risk of GVHD, showing more application advantages and clinical value.
Meta-analysis was performed on the recurrence rate, recurrence-related mortality and non-relapse-related mortality after treatment. The recurrence rate of the BMT group was lower, suggesting that BMT treatment could noticeably reduce the risk of relapse in AML patients, but no noticeable difference was found in recurrence-related mortality and non-relapse-related mortality (P>0.05). Namely, no noticeable effect on the mortality of patients was discovered, which is consistent with the previous analysis conclusion. Different disease stages and the median number of transplanted CD34+ cells are related. In this analysis, due to incomplete data and small sample size, the different stages of the disease are not explained. This study had a number of limitations. Firstly, because of a relatively strict inclusion and exclusion criteria, even though the search scope of this study included a number of authoritative databases, only 7 articles were included in the final analysis, and the heterogeneity was strong, but there was no detailed subgroup analysis of studies with heterogeneity. Secondly, follow-up times in all studies were not consistent, which restricted the results. In view of the above, we used a random effect model to analyze the heterogeneity of the article. Also, the search scope will be expanded in future research to reduce the article heterogeneity and publication bias.
Conclusions
To sum up, the efficacy of BMT and PHSCT is comparable. There is no noticeable difference in OS, DFS, recurrence-related mortality and non-relapse mortality between patients receiving BMT and PHSCT. However, BMT can noticeably reduce the disease recurrence rate and the occurrence of acute GVHD in AML patients. In order to verify the results, more studies with higher methodological standards and longer intervention time are need.
Disclosure of conflict of interest
None.
References
- 1.Pelcovits A, Niroula R. Acute myeloid leukemia: a review. R I Med J (2013) 2020;103:38–40. [PubMed] [Google Scholar]
- 2.Vago L, Gojo I. Immune escape and immunotherapy of acute myeloid leukemia. J Clin Invest. 2020;130:1552–1564. doi: 10.1172/JCI129204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol. 2018;93:1267–1291. doi: 10.1002/ajh.25214. [DOI] [PubMed] [Google Scholar]
- 4.Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392:593–606. doi: 10.1016/S0140-6736(18)31041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du W, Xu A, Huang Y, Cao J, Zhu H, Yang B, Shao X, He Q, Ying M. The role of autophagy in targeted therapy for acute myeloid leukemia. Autophagy. 2021;17:2665–2679. doi: 10.1080/15548627.2020.1822628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 7.Medinger M, Heim D, Halter JP, Lengerke C, Passweg JR. Diagnosis and therapy of acute myeloid leukemia. Ther Umsch. 2019;76:481–486. doi: 10.1024/0040-5930/a001126. [DOI] [PubMed] [Google Scholar]
- 8.Thol F, Ganser A. Treatment of relapsed acute myeloid leukemia. Curr Treat Options Oncol. 2020;21:66. doi: 10.1007/s11864-020-00765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozdemir ZN, Civriz Bozdağ S. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus Apher Sci. 2018;57:163–167. doi: 10.1016/j.transci.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Falini B, Brunetti L, Sportoletti P, Martelli MP. NPM1-mutated acute myeloid leukemia: from bench to bedside. Blood. 2020;136:1707–1721. doi: 10.1182/blood.2019004226. [DOI] [PubMed] [Google Scholar]
- 11.Chopra M, Bohlander SK. The cell of origin and the leukemia stem cell in acute myeloid leukemia. Genes Chromosomes Cancer. 2019;58:850–858. doi: 10.1002/gcc.22805. [DOI] [PubMed] [Google Scholar]
- 12.Ni L, Yu W. The value of cellular immunophenotype detection in the diagnosis of acute myeloid leukemia. Shanghai Journal of Medical Laboratory. 1998;13:136–137. [Google Scholar]
- 13.Heldal D, Tjønnfjord G, Brinch L, Albrechtsen D, Egeland T, Steen R, Solheim BG, Evensen SA. A randomised study of allogeneic transplantation with stem cells from blood or bone marrow. Bone Marrow Transplant. 2000;25:1129–36. doi: 10.1038/sj.bmt.1702422. [DOI] [PubMed] [Google Scholar]
- 14.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, Kashyap A, Flowers ME, Lilleby K, Chauncey TR, Storb R, Appelbaum FR. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–81. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 15.Vigorito AC, Marques Júnior JF, Aranha FJ, Oliveira GB, Miranda EC, De Souza CA. A randomized, prospective comparison of allogeneic bone marrow and peripheral blood progenitor cell transplantation when treating hematologic malignancies: an update. Haematologica. 2001;86:665–6. [PubMed] [Google Scholar]
- 16.Oehler VG, Radich JP, Storer B, Blume KG, Chauncey T, Clift R, Snyder DS, Forman SJ, Flowers ME, Martin P, Guthrie KA, Negrin RS, Appelbaum FR, Bensinger W. Randomized trial of allogeneic related bone marrow transplantation versus peripheral blood stem cell transplantation for chronic myeloid leukemia. Biol Blood Marrow Transplant. 2005;11:85–92. doi: 10.1016/j.bbmt.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Friedrichs B, Tichelli A, Bacigalupo A, Russell NH, Ruutu T, Shapira MY, Beksac M, Hasenclever D, Socié G, Schmitz N. Long-term outcome and late effects in patients transplanted with mobilised blood or bone marrow: a randomised trial. Lancet Oncol. 2010;11:331–8. doi: 10.1016/S1470-2045(09)70352-3. [DOI] [PubMed] [Google Scholar]
- 18.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, Cutler CS, Westervelt P, Woolfrey A, Couban S, Ehninger G, Johnston L, Maziarz RT, Pulsipher MA, Porter DL, Mineishi S, McCarty JM, Khan SP, Anderlini P, Bensinger WI, Leitman SF, Rowley SD, Bredeson C, Carter SL, Horowitz MM, Confer DL Blood and Marrow Transplant Clinical Trials Network. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–96. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong Y, Luo H, Li M, Guo L, Feng S. Clinical study of autologous peripheral blood stem cells and autologous bone marrow transplantation when treating acute myeloid leukemia. Contemporary Medicine. 2015;21:20–21. [Google Scholar]
- 20.Saleh K, Khalifeh-Saleh N, Kourie HR. Acute myeloid leukemia transformed to a targetable disease. Future Oncol. 2020;16:961–972. doi: 10.2217/fon-2019-0670. [DOI] [PubMed] [Google Scholar]
- 21.Kayser S, Levis MJ. Clinical implications of molecular markers in acute myeloid leukemia. Eur J Haematol. 2019;102:20–35. doi: 10.1111/ejh.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai G. Summary of hematopoietic stem cell transplantation. Chinese Medical Innovation. 2022;19:179–184. [Google Scholar]
- 23.Su N, Liu Z, Li Y, Cai D. Analysis of 12 cases of recurrence after allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia. Journal of China Medical University. 2022;51:638–642. [Google Scholar]
- 24.Wojcicki AV, Kasowski MM, Sakamoto KM, Lacayo N. Metabolomics in acute myeloid leukemia. Mol Genet Metab. 2020;130:230–238. doi: 10.1016/j.ymgme.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 25.YiBaDiti·Semedi , Han C, Pan L. Clinical nursing observation of 17 cases of autologous bone marrow transplantation. Smart Health. 2019;5:193–194. [Google Scholar]
- 26.Xue S, Liu F, Zhang Y, Tan Y, Wang J. Clinical study of cord blood mesenchymal stem cells combined with Eltrombopag when treating graft dysfunction after allogeneic hematopoietic stem cell transplantation. Int J Blood Transfus Hematol. 2022;45:132–139. [Google Scholar]
- 27.Luo R, Tian Z, Zhang X, Man Q, Gu W, Wang J. Analysis of the curative effect of salvage hematopoietic stem cell transplantation in advanced childhood acute leukemia. Chinese Journal of Pediatric Blood and Oncology. 2022;27:178–183. [Google Scholar]
- 28.Xie C, Zhang L. Prognosis and related factors after autologous hematopoietic stem cell transplantation in patients with acute myeloid leukemia. Clinical Medical Engineering. 2022;29:731–732. [Google Scholar]
- 29.Gao J, Zhang Y, Su L. Advances in prevention of acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Chinese Journal of Medical Frontiers (Electronic Edition) 2022;14:53–58. [Google Scholar]
- 30.Zhang W, Zhou R, Ma L, Wang Z, Mo W, Zhou M, Quan X. Study on the relationship between muscle mass and acute graft-versus-host disease in patients with allogeneic hematopoietic stem cell transplantation. Chinese Journal of Experimental Hematology. 2021;29:1950–1956. doi: 10.19746/j.cnki.issn.1009-2137.2021.06.045. [DOI] [PubMed] [Google Scholar]