Abstract
Background:
Data on the humoral vaccine response in patients on anti-interleukin-6 (IL-6) receptor therapy remain scarce.
Objective:
The main objective of our study was to investigate the humoral response after vaccination against SARS-CoV-2 in neuromyelitis optica spectrum disorder (NMOSD)/myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) patients treated with anti-IL-6 receptor therapy. Secondarily, we analyzed relapse activity timely associated with vaccination.
Methods:
In this retrospective cross-sectional multicenter study, we included 15 healthy controls and 48 adult NMOSD/MOGAD patients without previous COVID-19 infection. SARS-CoV-2 spike protein antibody titers during anti-IL-6 receptor therapy were compared to anti-CD20 antibody therapy, oral immunosuppressants, and to nonimmunosuppressed individuals.
Results:
We observed 100% seroconversion in the anti-IL-6 receptor treatment group. Titers of SARS-CoV-2 spike protein antibodies were lower compared to healthy controls (720 vs 2500 binding antibody units (BAU)/mL, p = 0.004), but higher than in the anti-CD20 (720 vs 0.4 BAU/mL, p < 0.001) and comparable to the oral immunosuppressant group (720 vs 795 BAU/mL, p = 1.0). We found no association between mRNA-based vaccines and relapse activity in patients with or without immunotherapy.
Conclusions:
Despite being lower than in healthy controls, the humoral vaccine response during anti-IL-6 receptor therapy was evident in all patients and substantially stronger compared to anti-CD20 treatment. No relevant disease activity occurred after mRNA vaccination against SARS-CoV-2.
Keywords: Vaccination, vaccine response, COVID-19, SARS-CoV-2, MOGAD, tocilizumab, satralizumab
Introduction
Interleukin-6 (IL-6) plays a central role in humoral immunity by regulating B-cell maturation and stimulating immunoglobulin G (IgG) secretion. 1 Confirming its role in antibody-mediated diseases, high efficacy of IL-6 receptor inhibiting (anti-IL6R) monoclonal antibodies satralizumab (SAT) and tocilizumab (TCZ) has been demonstrated in neuromyelitis optica spectrum disorder (NMOSD). 2 Despite the potentially increased risk of infection, data on the humoral vaccine response in patients treated with these therapies remain scarce. Few studies demonstrated a sufficient vaccine immune response to influenza or pneumococcal vaccines during TCZ therapy, but no data on the recently approved SAT are available. 3
The primary objective of our study was to investigate the effects of IL-6R inhibition on the humoral response after vaccination against SARS-CoV-2 in comparison to anti-CD20 therapy and oral immunosuppressants in NMOSD/myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD). Secondarily, we analyzed the incidence of relapses timely associated with vaccination.
Material and methods
We conducted a retrospective cross-sectional multicenter study in 10 university hospitals. Healthy controls (HC) and patients (⩾18 years) with a diagnosis of NMOSD according to IPND-criteria 2015 4 and MOGAD 5 were included. We investigated SARS-CoV-2 spike protein antibody (spike-ab) titers collected between May 2021 and February 2022 in patients on anti-IL-6R therapy, anti-CD20 antibodies (anti-CD20 monoclonal antibody (mAbs)), oral immunosuppressants (azathioprine (AZA)/mycophenolat mofetil (MMF)) or nonimmunosuppressed individuals. All participants denied a laboratory-confirmed or symptomatic COVID-19 infection before serum sampling. There was no change in therapy regimens prior to vaccination.
Immunoglobulin G antibodies against SARS-CoV-2 spike protein (Spike-abs) were analyzed with the Elecsys anti–SARS-CoV-2S enzyme immunoassay and SARS-CoV-2 total antibody assay (Siemens) 4 to 26 weeks after the second vaccine dose. Titers are indicated in binding antibody units (BAU)/mL according to the WHO International Standard (NIBSC Code 20/136) for transferability of results. The cutoff value for seropositivity was set at 0.8 BAU/mL. Demographic, disease-specific, vaccination, and laboratory data were collected for further analysis. Mild lymphopenia was defined as lymphocyte counts <1500/µL 6 and IgG deficiency below 700 mg/dL. Relapses were classified as temporally associated if they occurred up to 4 weeks after vaccination.
Statistical analysis
Statistical analyses were conducted using SPSS software version 26 (IBM Corp., Armonk, USA). Regression analysis was performed to predict spike-ab titers based on age, time interval between the second vaccine and spike-ab investigation, previous anti-CD20 therapy, last available prevaccination lymphocyte count, and IgG serum level. Continuous variables were compared by nonparametric statistical tests (Mann–Whitney U/Kruskal–Wallis test, significance threshold p < 0.05).
Results
Patient characteristics
Thirty patients with NMOSD (25 AQP4-IgG+, 5 AQP4-IgG−/MOG-IgG−), 18 with MOGAD, and 15 HC were included (Table 1). They were mostly vaccinated with BNT162b2/Comirnaty® (n = 50, 79%), followed by a combination of Comirnaty® and ChAdOx1/Vaxzevria® (n = 10, 16%) or mRNA-1273/Spikevax® (n = 3, 5%). Thirty-nine of 48 patients (81%) received immunotherapy, including rituximab (n = 17, 44%), anti-IL6R therapy (n = 13, 33%, 4 SAT/9 TCZ), and oral immunosuppressants (n = 9, 23%, 6 AZA/3 MMF).
Table 1.
Clinical characteristics, seropositivity rate, and humoral vaccine response in patients and healthy controls.
| NMOSD/MOGAD | HC (n = 15) |
||||
|---|---|---|---|---|---|
| Anti-IL6R (n = 13) a | Anti-CD20 (n = 17) b | Oral IT (n = 9) b | No IT (n = 9) | ||
| Female sex, n (%) | 12 (93) | 16 (94) | 5 (56) | 7 (78) | 11 (73) |
| Age, years, median (range) | 48 (23–62) | 49 (29–68) | 42 (32–72) | 34 (20–63) | 33 (22–67) |
| AQP4-IgG+/-IgG-/MOG-IgG+ | 8/0/5 | 12/3/2 | 2/2/5 | 3/0/6 | – |
| Disease duration, years, median (range) | 9 (3–42) | 10 (2–40) | 19 (15–33) | 10 (1–22) | – |
| Duration of treatment, months, median (range) | 29 (10–139) | 56 (10–172) | 40 (21–181) | – | – |
| Vaccination type | |||||
| Comirnaty, n (%) | 12 (92) | 15 (88) | 7 (78) | 6 (67) | 10 (67) |
| Comirnaty/Vaxzevria, n (%) | 0 | 1 (6) | 1 (11) | 3 (33) | 5 (33) |
| Spikevax, n (%) | 1 (8) | 1 (6) | 1 (11) | 0 | 0 |
| Spike-ab seropositivity, n (%) | 13/13 (100) | 7/17 (41) | 9/9 (100) | 9/9 (100) | 15/15 (100) |
| Spike-ab titer, BAU/mL, median (range) | 720 (102–2500) | 0.4 (0–213) | 795 (250–2500) | 2500 (181–2500) | 2500 (809–2500) |
| Time btw. 2nd vaccination and spike-ab, days, median (range) | 72 (22–154) | 67 (28–174) | 77 (48–138) | 91 (39–169) | 76 (34–165) |
| Lymphopenia, n (%) | 7/13 (54) | 7/16 (44) | 2/9 (22) | 1/6 (17) | – |
| IgG deficiency, n (%) | 6/12 (50) | 3/15 (20) | 0/6 | 0/4 | – |
| Pretreatment with RTX, n (%) | 7/13 (54) | – | 0/8 | 1/7 (14) | – |
| Time between last anti-CD20 and first vaccination, days (range) | 833 (317–4357) | 136 (76–289) | – | 508 | – |
anti-IL6R: IL-6 receptor inhibiting monoclonal antibodies; HC: healthy controls; IT: immunotherapy; MOGAD: Myelin oligodendrocyte glycoprotein antibody-associated disease; NMOSD: Neuromyelitis optica spectrum disorder; RTX: rituximab; spike-ab: Immunoglobulin G antibody against SARS-CoV-2 spike protein.
Three of 13 patients are treated with concomitant steroid therapy.
One patient from the group is treated with concomitant steroid therapy.
Effect of different immunotherapies on spike-ab titers
Seropositivity rate was 100% in patients on anti-IL-6R therapy, oral immunosuppressants, and those without immunotherapy. Only 41% of patients treated with anti-CD20 mAbs were seropositive (Table 1).
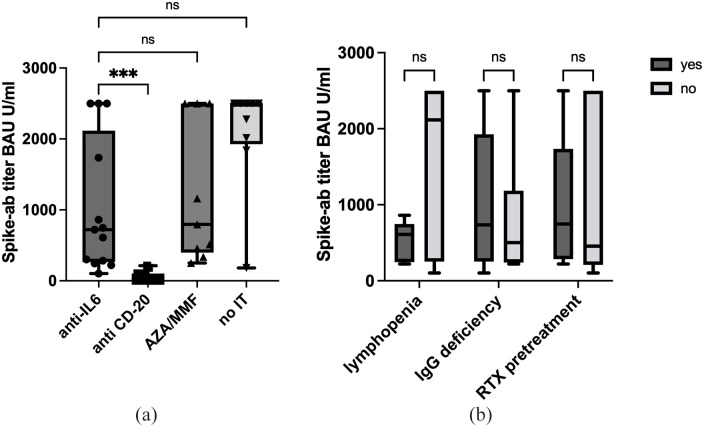
Patients on anti-IL-6R inhibitors had significantly lower spike-ab titers than HC (720 vs 2500 BAU/mL, p = 0.004). Vaccine response in this group was significantly stronger compared to anti-CD20 mAbs (720 vs 0.4 BAU/mL, p < 0.001), and similar to oral immunosuppressants (720 vs 795 BAU/mL, p = 1.0) (Figure 1(a)).
Figure 1.
Boxplots showing SARS-CoV2 spike protein-specific antibodies (BAU/mL) in NMOSD/MOGAD patients separated for all immunosuppressants, anti-IL-6 n = 13, anti-CD20 n = 17, AZA/MMF n = 9, no IT n = 9 (a) and for anti-IL6 therapy alone (b), according to possible influencing factors, lymphopenia n = 7, IgG deficiency n = 6, RTX pretreatment n = 7. Data were analyzed with a Kruskal–Wallis test.
AZA: azathioprin; IT: immunotherapy; MMF: mycophenolat-mofetil; ns: not significant; RTX: rituximab.
***p < 0.001.
Univariate regression analysis revealed age (F(1,61) = 11.9, p = 0.001, R 2 = 0.15) and time interval between the second vaccine and spike-ab investigation (F(1,61) = 4.03, p = 0.049, R 2 = 0.047) as two potential predictors of humoral response. Only age remained significant in a multivariate analysis. Both factors were comparable among all treatment groups (Table 1).
Factors influencing vaccine response on anti-IL6R therapy
To explore the broad spike-ab range during anti-IL-6R therapy, subgroup analysis was performed (Figure 1(b)). Mild lymphopenia (7/13 patients, 3 SAT/4 TCZ) was associated with lower antibody titers (609 vs 2117 BAU/mL, p = 0.058), without reaching the significance level. Neither previous treatment with Rituximab (RTX) (7/13, median 833 days before vaccination, 746 vs 456 BAU/mL, p = 0.667), nor IgG deficiency (735 vs 502 BAU/mL, p = 0.575) influenced the humoral immune response.
Relapse activity after vaccination
Only one MOGAD patient with recurrent optic neuritis (13 in 4 years, current treatment TCZ/prednisolone) experienced another attack 1 week after the first Comirnaty® vaccination. One week prior to vaccination prednisolone dose was tapered (from 12.5 to 10 mg). No further relapses were observed after both vaccinations in the other 47 patients, including 11 without immunotherapy (6 MOGAD, 5 AQP4-IgG+-NMOSD).
Discussion
We observed seroconversion in all patients on anti-IL6R therapy; however, spike-ab titers were significantly lower than in HC. These findings are in line with rheumatological studies, demonstrating sufficient antibody response rate to influenza/pneumococcal and SARS-CoV-2 vaccinations during TCZ treatment.3,7
Anti-IL-6R-treated patients had a significantly better humoral immune response than those on RTX, the most frequent long-term therapy for NMOSD/MOGAD in Germany. For anti-CD20 mAbs, lower spike-ab titers were already documented in multiple sclerosis (MS). 8 Even though IL-6 is an important driver of B-cell maturation and antibody production and TCZ/SAT can result in significant functional changes in these cells, no B-cell depletion itself occurs in contrast to RTX. 1
We observed a broad range of spike-ab titers in the anti-IL-6R therapy group. Previous anti-CD20 treatment seems to have no significant influence on the spike-ab titers. As previously reported lymphocyte count influences the humoral COVID-19 vaccine response in MS 9 ; similarly, we observed a trend to lower absolute spike-ab titers in patients with mild lymphopenia not reaching the significance level probably due to a small sample size.
Vaccination led to no relevant disease exacerbation, besides one patient with a very actively relapsing MOGAD and simultaneous prednisolone tapering. Previously relapse activity has been described mostly after Vaxzevria® vector-vaccination in MS and MOGAD 10 ; however, most patients in our study received mRNA-based vaccines.
The main limitations of our study are the observational nature, resulting in heterogeneous patient groups and vaccine types. We studied neither the T-cell immune response nor the COVID-19 infection rate postvaccination.
In conclusion, despite being lower than in HC, humoral vaccine response with anti-IL6R therapy was evident in all patients and substantially stronger compared to anti-CD20 treatment. An mRNA vaccination against SARS-CoV-2 seems not to be associated with increased disease activity in NMOSD/MOGAD.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.S., T.P., S.J., U.C., P.L., E.O. and C.G. report no disclosures relevant to the manuscript. I.K. has received personal fees from Alexion, Biogen, Celgene, Chugai, Novartis, Merck, and Roche and received research support from Chugai and Diamed, none related to this study. M.R. received speaker honoraria from Novartis, Bayer Vital GmbH, Roche, Alexion, and Ipsen and travel reimbursement from Bayer Schering, Biogen Idec, Merz, Genzyme, Teva, Roche, and Merck, none related to this study. O.A. has received personal fees from Alexion, Bayer HealthCare, Biogen, Celgene, Merck Serono, MedImmune, Novartis, Roche, Teva, and Zambon, outside the submitted work. M.K.-K. has received speaker honoraria from BMS, Novartis, Merck Serono as well as travel expenses from Novartis, none related to this study. B.W. received grants from the German Ministry of Education and Research, Deutsche Forschungsgemeinschaft, Dietmar Hopp Foundation, Klaus Tschira Foundation, grants and personal fees from Merck, and personal fees from Alexion, Bayer, Biogen, Roche, none related to this work A.B. has received personal compensation from Merck Serono, Biogen, Novartis, Teva, Roche, Sanofi/Genzyme, Alexion, and Celgene/ Bristol Myers Squibb, Janssen, Sandoz/HEXAL and grants for congress trips and participation from Biogen, Teva, Novartis, Sanofi/Genzyme, Merck Serono, and Celgene, none related to this study. R.P. received honoraria for lectures from Alexion, Bayer Healthcare, Biogen, Celgene, Novartis, Merck, Roche, Sanofi-Aventis, and Teva. He received research grants from HERZ Burgdorf, Novartis, and Merck. S.F. has received speaker’s and/or scientific board honoraria from Biogen, BMS, Celgene, Genesis Pharma, Novartis and Roche, congress support from Biogen and Janssen and grant support from Ruhr-University Bochum, DMSG, Stiftung für therapeutische Forschung, Lead Discovery Center GmbH and Novartis. K.H. received speaker’s, board honoraria, and research support from Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, and Teva, none related to this study. A.B.A.G.R.G. has received travel grants from Roche and Biogen, outside the submitted work. A.-K.P. received speaker honorario and research support from Biogen, none related to this study. T.K. has received speaker honoraria and/or personal fees for advisory boards from Bayer HealthCare, Teva Pharma, Merck, Novartis Pharma, Sanofi Aventis/Genzyme, Roche Pharma, and Biogen and grant support from Novartis and Chugai Pharma in the past, none related to this study. F.T.B. received speaker honoraria from Actelion, Alexion, Bayer, Biogen, Genzyme, Merck-Serono, Novartis, Roche, and Teva; travel reimbursement to attend scientific meetings from Bayer, Biogen, Genzyme, Merck-Serono, Novartis, Roche, and Teva; research support for investigator-initiated studies, through his institution, from the German Research Foundation (DFG), the German Federal Ministry of Education and Research (BMBF), Actelion, Bayer, Merck-Serono, Novartis, and Teva; none of these funds were related to this study. C.T. has received honoraria for consultation and expert testimony from Alexion Pharma Germany GmbH, Chugai Pharma Germany GmbH, and Roche Pharma GmbH. None of this interfered with the current report. R.G. received speaker’s and board honoraria from Baxter, Bayer Schering, Biogen Idec, CLB Behring, Genzyme, Merck Serono, Novartis, Stendhal, Talecris, and Teva. His department received grant support from Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, and Teva. All not related to the content of this manuscript. I.A. received personal fees from Roche, Alexion, Horizon, Sanofi Aventis/Genzyme, and Merck and received research support from Diamed, none related to this study.
Ethics: The local ethics committees of each participating NEMOS center approved this study. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Carolin Schwake  https://orcid.org/0000-0003-3669-7244
https://orcid.org/0000-0003-3669-7244
Martin W Hümmert  https://orcid.org/0000-0002-5928-2343
https://orcid.org/0000-0002-5928-2343
Contributor Information
Carolin Schwake, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
Thivya Pakeerathan, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
Ingo Kleiter, Marianne-Strauß-Klinik, Behandlungszentrum Kempfenhausen für Multiple Sklerose Kranke, Berg, Germany/Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
Marius Ringelstein, Department of Neurology, Medical Faculty, Heinrich Heine University Düsseldorf, Düsseldorf, Germany/Department of Neurology, Center for Neurology and Neuropsychiatry, LVR-Klinikum, Heinrich Heine University Düsseldorf, Düsseldorf, Germany.
Orhan Aktas, Department of Neurology, Medical Faculty, Heinrich Heine University Düsseldorf, Düsseldorf, Germany.
Mirjam Korporal-Kuhnke, Molecular Neuroimmunology Group, Department of Neurology, University Hospital Heidelberg, Heidelberg, Germany.
Brigitte Wildemann, Molecular Neuroimmunology Group, Department of Neurology, University Hospital Heidelberg, Heidelberg, Germany.
Sven Jarius, Molecular Neuroimmunology Group, Department of Neurology, University Hospital Heidelberg, Heidelberg, Germany.
Antonios Bayas, Department of Neurology and Clinical Neurophysiology, Medical Faculty, University of Augsburg, Augsburg, Germany.
Refik Pul, Department of Neurology, University of Duisburg-Essen, Essen, Germany.
Ulas Ceylan, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
Simon Faissner, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
Kerstin Hellwig, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
Ana Beatriz Ayroza Galvao Ribeiro Gomes, Departments of Neurology, Biomedicine and Clinical Research and Research Center for Neuroimmunology and Neuroscience, University Hospital Basel, University of Basel, Basel, Switzerland.
Philipp Lipps, Departments of Neurology, Biomedicine and Clinical Research and Research Center for Neuroimmunology and Neuroscience, University Hospital Basel, University of Basel, Basel, Switzerland.
Anne-Katrin Pröbstel, Departments of Neurology, Biomedicine and Clinical Research and Research Center for Neuroimmunology and Neuroscience, University Hospital Basel, University of Basel, Basel, Switzerland.
Tania Kümpfel, Institute of Clinical Neuroimmunology, LMU Hospital, Ludwig-Maximilians-Universität München, Munich, Germany.
Eva Oswald, Institute of Clinical Neuroimmunology, LMU Hospital, Ludwig-Maximilians-Universität München, Munich, Germany.
Florian Then Bergh, Department of Neurology, University of Leipzig, Leipzig, Germany.
Clemens Gödel, Department of Neurology, University of Leipzig, Leipzig, Germany.
Martin W Hümmert, Department of Neurology, Hannover Medical School, Hannover, Germany.
Corinna Trebst, Department of Neurology, Hannover Medical School, Hannover, Germany.
Ralf Gold, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
Ilya Ayzenberg, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany.
References
- 1.Tanaka T, Narazaki M, Kishimoto T.IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014; 6: a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamura T, Kleiter I, Fujihara K, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med 2019; 381: 2114–2124. [DOI] [PubMed] [Google Scholar]
- 3.Tsuru T, Terao K, Murakami M, et al. Immune response to influenza vaccine and pneumococcal polysaccharide vaccine under IL-6 signal inhibition therapy with tocilizumab. Mod Rheumatol 2014; 24(3): 511–516. [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation 2018; 15: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naeim F, Nagesh Rao P, Song SX, et al. Lymphocytopenia and lymphocytosis. In: Naeim F, Nagesh Rao P, Song SX, et al. (eds) Atlas of hematopathology. Cambridge, MA: Academic Press, 2013, pp. 627–633. [Google Scholar]
- 7.Farroni C, Aiello A, Picchianti-Diamanti A, et al. Booster dose of SARS-CoV-2 messenger RNA vaccines strengthens the specific immune response of patients with rheumatoid arthritis: a prospective multicenter longitudinal study. Int J Infect Dis 2022; 125: 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021; 27(11): 1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achiron A, Mandel M, Gurevich M, et al. Immune response to the third COVID-19 vaccine dose is related to lymphocyte count in multiple sclerosis patients treated with fingolimod. J Neurol 2022; 269(5): 2286–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fragoso YD, Gomes S, Goncalves MVM, et al. New relapse of multiple sclerosis and neuromyelitis optica as a potential adverse event of AstraZeneca AZD1222 vaccination for COVID-19. Mult Scler Relat Disord 2022; 57: 103321. [DOI] [PMC free article] [PubMed] [Google Scholar]