Abstract
Background
Orthostatic intolerance markedly affects the day-to-day activities of patients with myalgic encephalomyelitis (ME) or chronic fatigue syndrome. Chronotropic incompetence (CI), defined as an impaired chronotropic response or reduced increases in heart rate during exercise and resulting in lower exercise capacity, may also be observed during orthostasis in patients with ME.
Methods and Results
In this study, the recordings of 101 adult patients with ME (36 men, 65 women; mean [±SD] age 37±12 years) who underwent conventional active 10-min standing tests at least 3 times to determine the presence of CI were analyzed. Recordings were selected for 13 patients who experienced tests both with and without exhibiting postural orthostatic tachycardia syndrome (POTS; an increase in heart rate of ≥30 beats/min or an actual heart rate of ≥120 beats/min) while also both successfully completing and failing to complete 10-min standing on different occasions. Subjects in whom failure without POTS was observed in any test(s) while success was associated with POTS on other occasions were considered positive for CI during orthostasis. Of the 13 patients, 12 (92%) were CI positive, 5 (38%) of whom exclusively failed the tests without experiencing POTS.
Conclusions
Some patients with ME were CI positive during standing tests, suggesting impaired sympathetic activation. The presence of POTS appears to be essential for maintaining orthostasis in these patients.
Key Words: Chronic fatigue syndrome, Chronotropic incompetence, Myalgic encephalomyelitis, Orthostatic intolerance, Postural orthostatic tachycardia syndrome
Chronic fatigue syndrome (CFS) is characterized by severe and disabling fatigue, prolonged postexertional malaise, and unrefreshing sleep.1 Central nervous system dysfunction associated with myalgic encephalomyelitis (ME) has been postulated to be the main cause of CFS.2 Most patients with ME also experience symptoms related to orthostasis, with orthostatic intolerance (OI), which restricts their daily functional capacity.3,4 OI is characterized by the inability to stand upright without exhibiting severe signs and symptoms, such as palpitations, light-headedness, pallor, fatigue, weakness, dizziness, and nausea.3 Patients with aggravated OI may be unable to even sit, and thus become bedridden.
Most symptoms of OI are believed to be related to reductions in cardiovascular and cerebral blood flow, along with activation of the sympathetic nervous system.5 Postural orthostatic tachycardia syndrome (POTS), orthostatic hypotension, and neurally mediated hypotension have been observed during OI in many patients.3,4–7 Of these conditions, POTS is the most common phenomenon associated with OI, although it remains unclear whether this results from inappropriately exaggerated sympathetic nervous system excitation or from simple compensatory mechanisms working in an upright position in patients with reduced preload.7,8 Although the pathophysiology underlying POTS remains only partially understood, several researchers have reported that many such patients also have a small left ventricle and associated low cardiac output.9–11 Moreover, studies have shown downregulation of both the renin-aldosterone and antidiuretic hormone systems that regulate circulatory blood volume.12–14
It is widely recognized that heart rate is a useful prognostic tool for clinical outcomes and a great therapeutic target in patients with cardiovascular disease.15,16 A normal heart rate response is vital for matching a subject’s cardiac output to the metabolic demand of working muscles during exertion, and for maintaining the required oxygen supply through cerebral blood flow while in an upright position. Chronotropic incompetence or intolerance (CI) is broadly defined as the inability of the heart rate to increase to a degree commensurate with increased activity or demand, and is also defined as a restricted heart rate reserve, which may occur in some patients during orthostasis. However, the importance of acknowledging and understanding the presence of CI is often overlooked in clinical practice.
Patients with cardiovascular diseases have frequently been found to develop CI, promoting exercise intolerance that impairs their quality of life and serves as an independent predictor of major adverse cardiovascular events and mortality.17,18 Several studies have reported CI during exercise in patients with ME/CFS.19–21 In 2000, De Becker et al22 evaluated the ability of female patients with CFS to exercise using a bicycle ergometer. In that study, that most patients were not able to achieve a maximum heart rate of at least 85% of the age-related target heart rate and had lower exercise capacity.22 In addition, the maximum heart rate at exhaustion was lower in female patients with CFS than in control subjects.22
It has been difficult to elucidate the role of POTS during standing tests in patients with ME given that their conditions and characteristics, including age, sex, body mass index, hydration status, leg muscle volume, resting heart rate, and blood pressure, all vary greatly. To overcome this limitation and elucidate whether POTS is causative of OI or not, the present study analyzed the recordings of conventional active 10-min standing tests, which led to the identification of a peculiar phenomenon wherein a normal heart rate response was associated with test failure but, within the same subject, POTS was associated with successful test completion on other occasions.
Methods
Study Population
Recordings of 101 adult patients with ME who underwent conventional active 10-min standing tests at least 3 times at Miwa Naika Clinic between July 2017 and December 2020 were analyzed. All patients were able to stand and walk, and provided informed consent to undergo conventional active 10-min standing tests for the precise diagnosis of OI.
ME had been diagnosed according to the International Consensus Criteria.2 Briefly, symptoms related to neuroimmune exhaustion, such as marked, rapid physical and/or cognitive fatigability in response to exertion, a prolonged recovery period, and a low threshold of physical and mental fatigability, were compulsory for the diagnosis of ME. In addition, at least 1 symptom from 3 of the 4 symptom categories related to neurological impairments (i.e., neurocognitive impairments; pain; sleep disturbance; and neurosensory, perceptual, and motor disturbances) and at least 1 symptom from 3 of the 5 symptom categories related to immune, gastrointestinal, and genitourinary impairments (i.e., recurrent or chronic flu-like symptoms; susceptibility to viral infections; gastrointestinal tract symptoms; genitourinary symptoms; and sensitivities to food, medications, odors, or chemicals) were required. In addition at least 1 symptom of the symptoms related to energy metabolism/ion transportation impairments (i.e., cardiovascular symptoms such as OI, respiratory symptoms, loss of thermostatic stability, and intolerance of extremes of temperature) was required. Patients with significant comorbidities unrelated to ME, as well as pregnant or lactating women, were excluded.
This study on the conventional active 10-min standing test was approved by the Toyama Prefectural Medical Association Ethics Committee (Approval no. 2016-010) and was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Active 10-min Standing Test
The conventional active 10-min standing test was performed according to the method described by Miwa.8 Patients were required to discontinue adrenergic β-receptor blocking agents and vasopressors (if consuming) before the test, but were not asked to discontinue nutritional supplements or multi-enzyme tablets. After 5 min rest in the recumbent position (and another 5 min if needed for stable hemodynamics), patients were asked to stand still with their feet approximately shoulder-width apart and remain standing without changing their foot positioning. If a patient was unable to maintain this position for various reasons (e.g., palpitation, light-headedness, pallor, fatigue, weakness, dizziness, tremulousness, or nausea) during the test and abandoned the standing position, this was considered a failure to complete the 10-min standing test. POTS was diagnosed based on an increase in heart rate of ≥30 beats/min,23 with symptoms of OI. In addition, in the present study, an actual heart rate of ≥120 beats/min, indicating accentuated sympathetic activity, with symptoms of OI during the test was also considered to occur due to POTS.12 Classic or delayed (after >3 min of upright posture) orthostatic hypotension was diagnosed as a decrease in systolic or diastolic blood pressure of ≥20 or ≥10 mmHg, respectively,23 and/or actual systolic blood pressure of ≤90 mmHg during the test. Recordings of the test with orthostatic hypotension were excluded from those without POTS. Neurally mediated hypotension was diagnosed as orthostatic hypotension with a decrease in heart rate of ≥20 beats/min during the test. Each patient repeated the test every 3 months.
CI During the Active 10-min Standing Test
In this study, recordings of 10-min standing tests performed by patients who had tests both with and without POTS, as well as both success and failure in completing the 10-min standing test were selected. When a patient failed during the test(s) without POTS but succeeded in the test(s) with POTS on other occasion(s), CI during orthostasis was considered positive.
Statistical Analysis
Continuous variables (age) are presented as the mean±SD and were compared using Student’s t-tests. Proportional data were analyzed using Fisher’s exact test with Yates’ correction. Statistical significance was set at P<0.05.
Results
Patient Selection
Of the 101 patients (36 men, 65 women; mean age 37±12 years) in the study who underwent 10-min standing tests, 40 (12 men, 28 women; mean age 32±8 years) exhibited POTS during at least 1 of the standing tests; these 40 patients were significantly (P<0.001) younger than the remaining 61 patients (24 men, 37 women; mean age 40±12 years) without POTS during all standing tests.
Thirteen patients were finally selected; each of these patients showed contradictory test results on different occasions in terms of test success and the presence of POTS during the test. That is, each patient selected underwent at least 1 successful and 1 failed standing test and underwent at least 1 test with and 1 test without POTS (Figure 1). These 13 patients (2 men, 11 women; mean age 29±8 years) were significantly (P<0.01) younger than the other 88 patients (34 men, 54 women; mean age 38±11 years).
Figure 1.
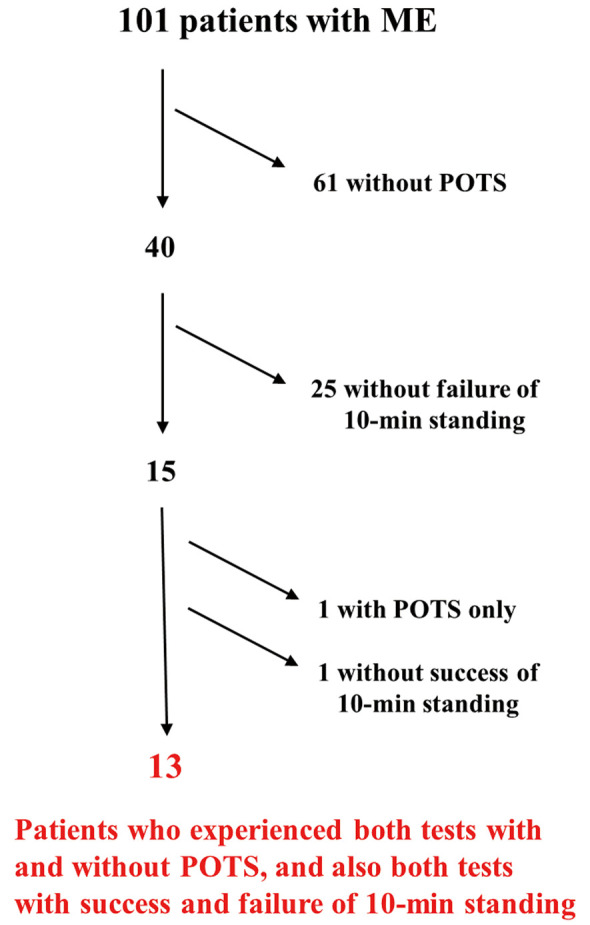
Flowchart of patient selection. ME, myalgic encephalomyelitis; POTS, postural orthostatic tachycardia syndrome.
CI
Of the 13 adult patients selected, 5 (38%; Patients 1–5) failed exclusively during the standing tests without experiencing POTS, 4 (31%; Patients 6–9) had a higher failure rate during tests without POTS than during tests with POTS, and 3 (23%; Patients 10–12) had a higher failure rate during tests with POTS than during tests without POTS. Only 1 (8%) patient (Patient 13) failed exclusively during any of the tests while experiencing POTS (Table A). Thus, CI was positive in 12 (92%) patients (Patients 1–12) among the 13 adult patients who had tests both with and without POTS and both success and failure in completing the 10-min standing test. Summing data for Patients 1–5 (Table B), the total rate of failure in completing the 10-min standing test was significantly higher in tests without than with POTS. For Patients 6–9, the total rate of failure did not differ significantly between tests with and without POTS (Table B). However, among Patients 10–13, the total rate of failure was higher in tests with than without POTS (Table B).
Table.
(A) Results of Conventional Active 10-min Standing Tests With and Without POTS in the 13 Selected Patients With Myalgic Encephalomyelitis, (B) Rate of Failure in Completing 10-min Standing Tests With and Without POTS
| (A) Patient no. | Age/sex | With POTS | Without POTS | ||
|---|---|---|---|---|---|
| No. completed | No. failed | No. completed | No. failed (CI) | ||
| 1 | 40/M | 5 | 0 | 1 | 2 |
| Resting → maximum HR (standing duration) |
79 → 113* | 76 → 105 | 83 → 109 (2’)* | ||
| 81 → 115* | 73 → 100 (8.3’)* | ||||
| 72 → 102* | |||||
| 63 → 104* | |||||
| 76 → 114* | |||||
| 2 | 21/F | 1 | 0 | 5 | 4 |
| Resting → maximum HR (standing duration) |
73 → 110* | 67 → 86 | 64 → 77 (1’)* | ||
| 58 → 76 | 78 → 98 (7’)* | ||||
| 59 → 83 | 62 → 89 (7.5’)* | ||||
| 60 → 82 | 69 → 98 (3’)* | ||||
| 58 → 85 | |||||
| 3 | 46/F | 1 | 0 | 3 | 2 |
| Resting → maximum HR (standing duration) |
71 → 105* | 71 → 99 | 65 → 90 (7.5’)* | ||
| 62 → 87 | 67 → 83 (5’)* | ||||
| 62 → 81 | |||||
| 4 | 28/F | 3 | 0 | 2 | 2 |
| Resting → maximum HR (standing duration) |
83 → 116* | 73 → 100 | 68 → 91 (8’)* | ||
| 69 → 102* | 73 → 99 | ||||
| 91 → 121* | |||||
| 5 | 32/F | 2 | 0 | 3 | 1 |
| Resting → maximum HR (standing duration) |
62 → 92* | 71 → 98 | 58 → 80 (5’)* | ||
| 69 → 104* | 66 → 90 | ||||
| 63 → 89 | |||||
| 6 | 33/F | 5 | 1 | 3 | 2 |
| Resting → maximum HR (standing duration) |
45 → 93* | 53 → 83 (8’) | 40 → 62 | 42 → 67 (9’)* | |
| 45 → 84* | 46 → 73 | 53 → 79 (8.7’)* | |||
| 64 → 102* | 52 → 78 | ||||
| 49 → 91* | |||||
| 50 → 81* | |||||
| 7 | 25/F | 3 | 1 | 1 | 1 |
| Resting → maximum HR (standing duration) |
65 → 97* | 79 → 120 (7’) | 83 → 107 | 86 → 108 (6’)* | |
| 75 → 108* | |||||
| 92 → 120* | |||||
| 8 | 21/F | 2 | 1 | 1 | 1 |
| Resting → maximum HR (standing duration) |
98 → 121* | 101 → 126 (5.2’) | 93 → 112 | 81 → 96 (7.3’)* | |
| 78 → 112* | |||||
| 9 | 22/M | 1 | 1 | 1 | 3 |
| Resting → maximum HR (standing duration) |
58 → 92* | 69 → 106 (4’, OH) |
69 → 90 | 74 → 102 (6.2’)* | |
| 63 → 88 (8.2’)* | |||||
| 65 → 92 (8.3’)* | |||||
| 10 | 27/F | 5 | 3 | 6 | 1 |
| Resting → maximum HR (standing duration) |
75 → 114* | 81 → 118 (6’) | 74 → 93 | 69 → 88 (3’)* | |
| 96 → 125* | 79 → 116 (5’) | 73 → 102 | |||
| 78 → 114* | 75 → 107 (5’) | 88 → 114 | |||
| 84 → 114* | 78 → 107 | ||||
| 80 → 118* | 77 → 95 | ||||
| 76 → 90 | |||||
| 11 | 23/F | 1 | 3 | 1 | 1 |
| Resting → maximum HR (standing duration) |
68 → 105* | 64 → 96 (8’) | 69 → 88 | 65 → 92 (3’)* | |
| 78 → 117 (5’) | |||||
| 70 → 100 (5’) | |||||
| 12 | 37/F | 2 | 3 | 2 | 1 |
| Resting → maximum HR (standing duration) |
61 → 91* | 66 → 104 (7.2’) | 71 → 100 | 64 → 91 (3.7’)* | |
| 62 → 98* | 61 → 98 (4.5’) | 60 → 85 | |||
| 67 → 100 (3.5’) | |||||
| 13 | 26/F | 0 | 1 | 3 | 0 |
| Resting → maximum HR (standing duration) |
68 → 107 (7.5’, NMH) |
77 → 96 | |||
| 59 → 76 | |||||
| 63 → 86 | |||||
| (B) Patient no. | Rate of failure** | Total rate of failure** | |||
| With POTS | Without POTS | With POTS | Without POTS | P value | |
| 1 | 0 (0/5) | 67 (2/3) | 0 (0/12) | 44 (11/25) | <0.05 |
| 2 | 0 (0/1) | 44 (4/9) | |||
| 3 | 0 (0/1) | 40 (2/5) | |||
| 4 | 0 (0/3) | 50 (2/4) | |||
| 5 | 0 (0/2) | 25 (1/4) | |||
| 6 | 17 (1/6) | 40 (2/5) | 31 (5/16) | 54 (7/13) | 0.27 |
| 7 | 25 (1/4) | 50 (1/2) | |||
| 8 | 33 (1/3) | 50 (1/2) | |||
| 9 | 67 (2/3) | 75 (3/4) | |||
| 10 | 38 (3/8) | 14 (1/7) | 56 (10/18) | 20 (3/15) | 0.07 |
| 11 | 75 (3/4) | 50 (1/2) | |||
| 12 | 60 (3/5) | 33 (1/3) | |||
| 13 | 100 (1/1) | 0 (0/3) | |||
(A) *Values indicate CI. HR is given in beats/min; standing duration is given in minutes. See text for details. (B) Patients 1–5 failed exclusively during standing tests without experiencing POTS, Patients 6–9 had a higher failure rate during tests without than with POTS, and Patients 10–12 had a higher failure rate during tests with than without POTS. **The rate of failure is shown as a percentage, with the number of failures/number of 10-min standing tests given in parentheses. CI, chronotropic incompetence; HR, heart rate; NMH, neurally mediated hypotension; OH, orthostatic hypotension; POTS, postural orthostatic tachycardia syndrome.
Figure 2 shows the heart rate and blood pressure across a 10-min standing test for a patient with CI or reduced increases in heart rate during orthostasis. Figure 3 shows the heart rate and blood pressure across a 10-min standing test for a patient without CI.
Figure 2.
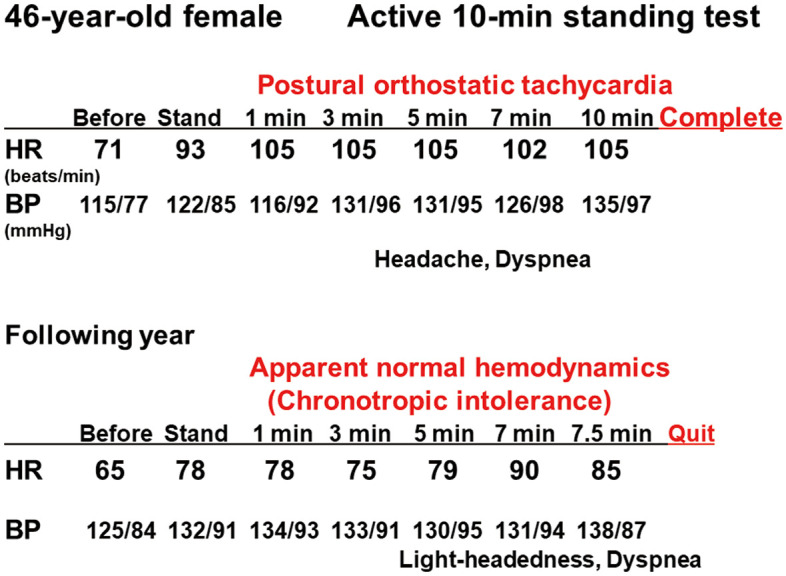
Conventional active 10-min standing test recordings in a 46-year-old female patient (Patient 3) with myalgic encephalomyelitis. This patient completed the 10-min standing test with postural orthostatic tachycardia syndrome (Upper). However, in a test the following year, she failed the test due to light-headedness and dyspnea after 7.5 min, despite having apparent normal hemodynamics, thus demonstrating chronotropic incompetence (Lower). BP, blood pressure; HR, heart rate.
Figure 3.
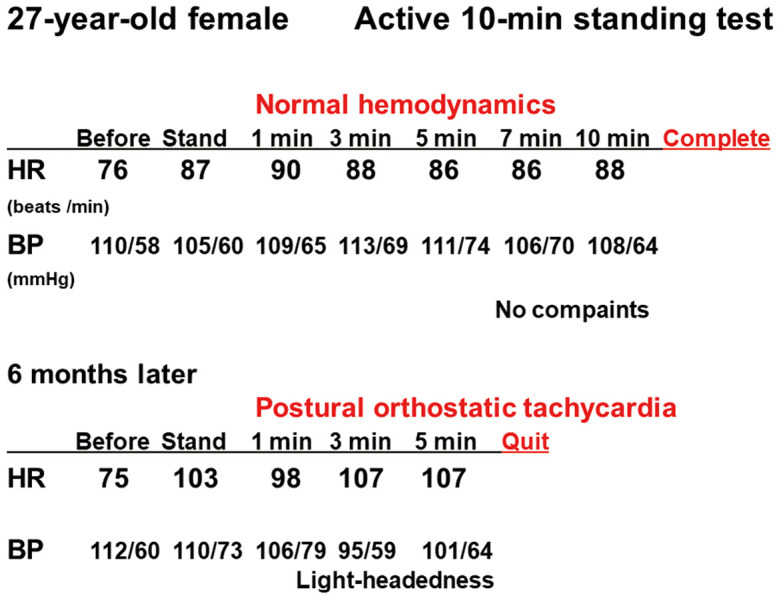
Conventional active 10-min standing test recordings in a 27-year-old female patient (Patient 10) with myalgic encephalomyelitis. The patient completed the 10-min standing test under normal hemodynamics with fatigue (Upper). However, in a test 6 months later, she failed to keep standing during the test due to dyspnea after 5 min with postural orthostatic tachycardia syndrome (Lower). BP, blood pressure; HR, heart rate.
Discussion
To the best of the author’s knowledge, this is the first study to report that CI or a restricted heart rate reserve during orthostasis, suggesting possible impaired sympathetic activation, causes OI during standing tests in patients with ME. This is similar to the phenomenon of CI observed during exercise testing among patients with chronic heart failure and ME/CFS.17–19
Possible Mechanisms for OI and Role of the Autonomic Nervous System
Patients with OI have been clinically recognized.3 These patients predictably develop symptoms of disabling fatigue, dizziness, diminished concentration, tremulousness, and nausea while standing upright. Simple activities such as eating, showering, or low-intensity exercise may profoundly exacerbate these symptoms. Reduced cerebral blood flow with impaired cerebral oxygenation during an upright posture is considered the major mechanism for OI, although compensatory sympathetic activation also seems to play an important role in the development of aggravated symptoms in cases in which it is exaggerated.3
Under normal conditions, the upright posture results in a shift of blood from the chest to the lower abdomen and legs, which reduces venous return, causing a transient decrease in cardiac filling, stroke volume, and arterial pressure.24 Unloading of baroreceptors triggers compensatory sympathetic activation, which increases the heart rate and leads to systemic vasoconstriction. This compensation results in the restoration of venous return and cardiac output. These compensatory physiological regulations are considered to be compromised in OI with POTS, and thus venous return remains reduced, upright cardiac output and stroke volume are not normalized, and standing heart rate is elevated. Many factors, such as hypovolemia, an excessive orthostatic shift in plasma volume, autonomic dysfunction, increased sympathetic tone, physical deconditioning, poor venous return, enhanced blood venous pooling, and immunological factors, may contribute to the occurrence of OI with POTS.24 Impaired activation of the renin-angiotensin-aldosterone and the antidiuretic systems, which regulate circulatory blood volume, may also play a role in the pathophysiology of OI with POTS in patients with ME.12–14
The markedly increased heart rate or POTS observed in most adult patients with ME involved in the present study seems to be an essential physiologic compensatory response to a decrease in stroke volume upon standing, preserving cardiac output enough to maintain cerebral circulation. The autonomic nervous system plays an important role when standing upright to overcome the gravitational effects of blood pooling in the lower limbs, causing an imbalance in arterial and venous blood pressures. To re-equilibrate arterial and venous blood pressures by promoting increased venous return, sympathetic activity is required to induce vasoconstriction of large veins in the legs.
Several previous studies showed a clear correlation between levels of fatigue and muscle sympathetic nerve activity during static contraction.25,26 Furthermore, exhaustive incremental exercise provokes a sustained increase in plasma norepinephrine concentrations that outlasts postexercise termination by several hours.27 In these patients, inappropriate sympathetic overactivity at rest may represent a neural functional correlation with fatigue26,27 and may further be linked to restricted sympathetic reserves, resembling the tachyphylaxis phenomenon.
The feeling of fatigue and exhaustion experienced by patients with ME/CFS has been attributed to their difficulty in maintaining an erect posture.28 Symptom severity and hierarchy frequently fluctuate or enter “relapsing-remitting patterns” among patients with ME/CFS, although the precise mechanism remains unknown.2 One study reported that the hydration status or preload may be linked to fluctuations in symptoms.8 Moreover, the condition of the prevailing sympathetic modulation at rest and reduced responsiveness to excitatory stimuli, as shown by the failure to develop POTS upon standing in association with the failure to maintain standing on some occasions, was also seen in some patients with ME.8 Residual sympathetic modulation appeared to be extremely limited on such occasions, similar to what was seen in patients in the present study.
Possible Mechanisms for CI During Orthostasis
The mechanism behind attenuated fluctuations in standing heart rate or postural orthostatic CI remains unexplained. Nevertheless, 4 major abnormalities of cardiac neural regulation may be linked to this mechanism: downregulation of β-adrenergic receptors, resulting in adrenergic insensitivity; sympathetic fiber dysfunction, resulting in decreased norepinephrine output; diminished sympatho-adrenal-medullary activation, resulting in a lesser increase in epinephrine; and the relative dominance of vagus inputs inhibiting the influence of epinephrine and norepinephrine. These abnormalities have been suggested to be associated with the CI during exercise in patients with ME/CFS.21
Among patients with chronic heart failure, CI during exercise has been associated with β-adrenergic receptor downregulation and desensitization in the presence of increased circulating catecholamine concentrations.29,30 Moreover, the reduction in sinus node reserves due to significant sinus node remodeling has been suggested to be responsible, at least in part, for the CI commonly seen during heart failure.31 However, whether these suggested mechanisms for CI among patients with heart failure could be related to the postural orthostatic CI observed during orthostasis among patients with ME remains unknown.
Among patients who had ME with OI, the exaggerated activation of the sympathetic nervous system while standing appeared to cause a switch to impaired sympathetic activation. This occurs after being loaded with additional accentuated stimuli, such as preload reduction, exaggerated effort with sustained exhaustion, and disequilibrium, which has been recently proposed as another cause of OI.32,33 The therapeutic effects of administering β-adrenergic receptor blocking agents34 or the sinus node blocker ivabradine35,36 on relieving symptoms of OI may differ according to patient condition.
Study Limitations
This study has several limitations. First, autonomic nervous functions that may modulate both cerebrovascular and cardiac function during upright posture were not evaluated. Second, cerebral circulation and oxygenation, important indicators of OI, were not measured or estimated. Third, the difference between cardiac output at rest and that during an upright posture was not investigated. Fourth, only 13 patients were selected for analysis from 101 study patients. CI could be identified using some other appropriate methods even in patients without POTS. Further investigations are required to identify OI with reduced heart rate reserve or CI and to clarify the precise mechanisms responsible for CI during orthostasis found in ME patients.
Conclusions
Postural orthostatic CI, comprised of reduced increases in heart rate during orthostasis, causes OI among some adult patients with ME, similar to the phenomenon reportedly observed during exercise.
Sources of Funding
This research did not receive grant funding from any agencies in the public, commercial, or not-for-profit sectors.
Disclosures
None.
IRB Information
This study was approved by the Toyama Prefectural Medical Association Ethics Committee (Approval no. 2016-010).
Acknowledgment
The author thanks Ms. Takako Miwa for her technical help.
References
- 1. Fukuda K, Straus SE, Hickle I, Sharpe MC, Dobbins JG, Komaroff A, et al.. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann Intern Med 1994; 121: 953–959. [DOI] [PubMed] [Google Scholar]
- 2. Carruthers BM, van de Sande MI, DeMeirleir KL, Klimas NG, Broderick G, Mitchell T, et al.. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med 2011; 270: 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schondorf R, Freeman R.. The importance of orthostatic intolerance in the chronic fatigue syndrome. Am J Med Sci 1999; 317: 117–123. [DOI] [PubMed] [Google Scholar]
- 4. Costigan A, Elliott C, McDonald C, Newton JL.. Orthostatic symptoms predict functional capacity in chronic fatigue syndrome: Implications for management. QJM 2010; 103: 589–595. [DOI] [PubMed] [Google Scholar]
- 5. Tanaka H, Matsushima R, Tamai H, Kajimoto Y.. Impaired postural cerebral hemodynamics in young patients with chronic fatigue with and without orthostatic intolerance. J Pediatr 2002; 140: 412–417. [DOI] [PubMed] [Google Scholar]
- 6. Rowe PC, Bou-Holaigah I, Kan JS, Calkins H.. Is neurally mediated hypotension an unrecognised cause of chronic fatigue? Lancet 1995; 345: 623–624. [DOI] [PubMed] [Google Scholar]
- 7. IOM (Institute of Medicine).. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an illness. National Academies (Abstract), Washington DC 2015. [PubMed]
- 8. Miwa K.. Variability of postural orthostatic tachycardia in patients with myalgic encephalomyelitis and orthostatic tachycardia. Heart Vessels 2016; 31: 1522–1528. [DOI] [PubMed] [Google Scholar]
- 9. Peckerman A, LaManca, JJ, Dahl KA, Chemitiganti R, Qureish B, Natelson BH.. Abnormal impedance cardiography predicts symptom severity in chronic fatigue syndrome. Am J Med Sci 2003; 326: 55–60. [DOI] [PubMed] [Google Scholar]
- 10. Miwa K, Fujita M.. “Small heart syndrome” in patients with chronic fatigue syndrome. Clin Cardiol 2008; 31: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu Q, VanGundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, et al.. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2010; 55: 2858–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, et al.. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 2005; 111: 1574–1582. [DOI] [PubMed] [Google Scholar]
- 13. Miwa K, Fujita M.. Renin-aldosterone paradox in patients with myalgic encephalomyelitis and orthostatic intolerance. Int J Cardiol 2014; 172: 514–515. [DOI] [PubMed] [Google Scholar]
- 14. Miwa K.. Down-regulation of renin-aldosterone and antidiuretic hormone systems in patients with myalgic encephalomyelitis/chronic fatigue syndrome. J Cardiol 2017; 69: 684–688. [DOI] [PubMed] [Google Scholar]
- 15. Hagiya K, Ozaki K, Nanasato M, Iguchu N, Takayama M, Shimokawa T, et al.. Relationship between heart rate and long-term outcomes of surgically treated patients with type A acute aortic dissections. Circ J 2021; 85: 2191–2200. [DOI] [PubMed] [Google Scholar]
- 16. Matsumoto S, Nakanichi R, Ishibashi R, Honda M, Hayashida K, Sakurai A, et al.. Heart rate and mortality after resuscitation in patients with out-of-hospital cardiac arrest: Insights from the SOS-KANTO registry. Circ J 2022; 86: 1562–1571. [DOI] [PubMed] [Google Scholar]
- 17. Katritsis D, Camm AJ.. Chronotropic incompetence: A proposal for definition and diagnosis. Br Heart J 1993; 70: 400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brubaker PH, Kitzman DW.. Chronotropic incompetence: Causes, consequences, and management. Circulation 2011; 123: 1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Montague TJ, Marrie TJ, Klassen GA, Bewick DJ, Horacek BM.. Cardiac function at rest and with exercise in the chronic fatigue syndrome. Chest 1989; 95: 779–784. [DOI] [PubMed] [Google Scholar]
- 20. Vanness JM, Snell CR, Strayer DR, Dempsey L.. Subclassifying chronic fatigue syndrome through exercise testing. Med Sci Sports Exerc 2003; 35: 908–913. [DOI] [PubMed] [Google Scholar]
- 21. Davenport TE, Lehnen M, Stevens SR, VanNess JM, Stevens J, Snell CR.. Chronotropic intolerance: An overlooked determinant of symptoms and activity limitation in myalgic encephalomyelitis/chronic fatigue syndrome? Front Pediatr 2019; 7: 82, doi:10.3389/fped.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Becker P, Roeykens J, Reynders M, McGreger N, De Meirleir K.. Exercise capacity in chronic fatigue syndrome. Arch Int Med 2000; 160: 3270–3277. [DOI] [PubMed] [Google Scholar]
- 23. Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, et al.. ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017; 136: e25–e59. [DOI] [PubMed] [Google Scholar]
- 24. Zadourian A, Doherty TA, Swiatkiewicz I, Taub PR.. Postural orthostatic tachycardia syndrome: Prevalence, pathophysiology, and management. Drugs 2018; 78: 983–994. [DOI] [PubMed] [Google Scholar]
- 25. Saito M, Mano T, Iwase S.. Sympathetic nerve activity related to local fatigue sensation during static contraction. J Appl Physiol 1989; 67: 980–984. [DOI] [PubMed] [Google Scholar]
- 26. Pagani M, Lucini D.. Chronic fatigue syndrome: A hypothesis focusing on the autonomic nervous system. Clin Sci (Lond) 1999; 96: 117–125. [PubMed] [Google Scholar]
- 27. Strobel G, Hack V, Kinscherf R, Weicker H.. Sustained noradrenaline sulphate response in long-distance runners and untrained subjects up to 2 h after exhausting exercise. Eur J Appl Physiol Occup Physiol 1993; 66: 421–426. [DOI] [PubMed] [Google Scholar]
- 28. Freeman R, Komaroff AL.. Does the chronic fatigue syndrome involve the autonomic nervous system? Am J Med 1997; 102: 357–364. [DOI] [PubMed] [Google Scholar]
- 29. Bristow MR, Hershberger RE, Port JD.. Beta-adrenergic pathways in non-failing and failing human ventricular myocardium. Circulation 1990; 82: 12–25. [PubMed] [Google Scholar]
- 30. Colucci WS, Ribeiro JP, Rocco MB, Quigg RJ, Creager MA, Marsh JD, et al.. Impaired chronotropic response to exercise in patients with congestive heart failure: Role of postsynaptic beta-adrenergic desensitization. Circulation 1989; 80: 314–323. [DOI] [PubMed] [Google Scholar]
- 31. Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM.. Remodeling of sinus node function in patients congestive heart failure: Reduction in sinus node reserve. Circulation 2004; 110: 897–903. [DOI] [PubMed] [Google Scholar]
- 32. Miwa K, Inoue Y.. The etiologic relation between disequilibrium and orthostatic intolerance in patients with myalgic encephalomyelitis (chronic fatigue syndrome). J Cardiol 2018; 72: 261–264. [DOI] [PubMed] [Google Scholar]
- 33. Miwa K, Inoue Y.. Paradigm shift to disequilibrium in the genesis of orthostatic intolerance in patients with myalgic encephalomyelitis and chronic fatigue syndrome. Int J Cardiol Hypertens 2020; 5: 100032, doi:10.1016/j.ijchy.2020.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raj SR, Black BK, Biaggioni I, Paranjape SY, Ramirez M, Dupont WD, et al.. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: Less is more. Circulation 2009; 120: 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ewan V, Norton M, Newton JL.. Symptom improvement in postural orthostatic tachycardia syndrome with the sinus node blocker ivabradine. Europace 2007; 9: 1202. [DOI] [PubMed] [Google Scholar]
- 36. Kim BH, Cho KI, Kim SM, Kim N, Han J, Kim JY, et al.. Heart rate reduction with ivabradine prevents thyroid hormone-induced cardiac remodeling in rat. Heart Vessels 2013; 28: 524–535. [DOI] [PubMed] [Google Scholar]


