To the Editor:
Obsessive-compulsive disorder (OCD) is a severe mental condition neuroanatomically characterized by cortico-striato-thalamo-cortical (CSTC) loop dysfunction [1], but also involves alterations in other networks [1, 2] as well as molecular and genetic changes [3–5]. Strategic lesions can result in distinct CSTC loop dysfunctions [6, 7]. This work presents two well-studied OCD patients with strategic basal ganglia pathologies which have been classified in vivo.
The diagnostic work-up in both patients was performed according to an established diagnostic protocol [8, 9]. The magnetic resonance imaging (MRI) analysis included diffusion tensor imaging (DTI) tractography along the brain lesions [10]. The electroencephalography (EEG) was analyzed using independent component analysis (ICA) [11]. Both patients gave written informed consent for publication within a cumulative case study.
Cases 1 and 2 were hospitalized for exacerbations of obsessive-compulsive symptoms (OCS).
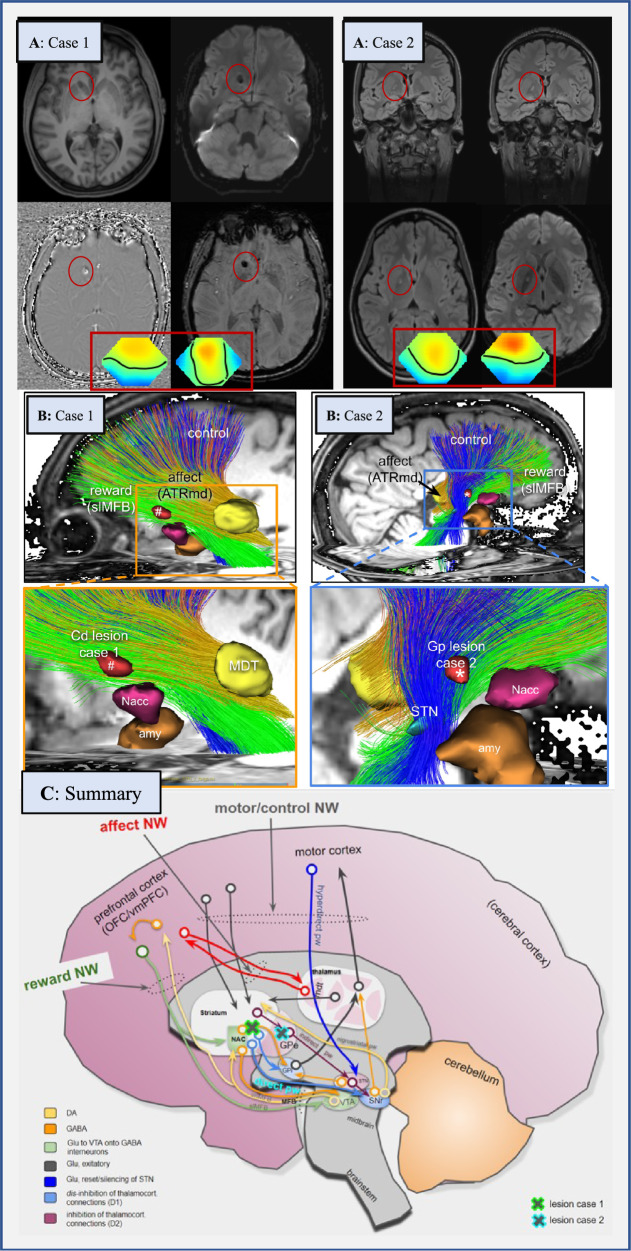
Patient 1 was a 30-year-old female patient who had been suffering from obsessive thoughts and compulsive actions for approximately five years. Her symptoms had exacerbated one year prior to her hospitalization with washing compulsions and concerns about contamination (Y-BOCS score: 27; OCI-R score: 33). The diagnostic work-up identified an MRI lesion in the right caudate with a size of 8.9 mm × 5 mm, most probably a previous microbleeding (Fig. 1). The DTI tractographic rendition of streamlines involved in the strategic unilateral lesion in the right nucleus caudate showed that the crossing fibers connected the superolateral branch of the medial forebrain bundle to the mediodorsal thalamus and potentially to the bed nucleus of the stria terminalis. ICA detected frontocentral theta activity. The CSF analysis showed slightly blood-CSF barrier dysfunction with elevated albumin quotients; no antibodies against extra- or intracellular neuronal antigens could be detected [12]. The long-term blood pressure measurement was normal. Inpatient cognitive behavioral therapy (CBT) with exposure and response prevention (ERP) over approximately 10 weeks and psychopharmacological treatment with sertraline (200 mg/day; blood levels of 54 ng/ml; reference: 10–50 ng/ml) resulted in considerable improvement in OCS, with Y-BOCS decreasing from 27 to 10 (−63%) and OCI-R from 33 to 11 (−67%).
Fig. 1. Neuroimaging findings in both patients (A, B) and conceptual considerations (C).
A: Case 1 shows a lesion in the right caudate nucleus with a size of 8.9 × 5 mm. The lesion was classified as a probable microbleeding, but calcification as a late consequence of microbleeding could not be definitively ruled out. Case 2 presents with a FLAIR-hyperintense lesion in the globus pallidus on the right (defect with a gliotic rim in the direction of a lenticulostriate vessel). Because Case 2 had febrile seizures during her first decade, and since then chronic obsessive-compulsive disorder (OCD), a post-inflammatory origin could be plausible. Framed below in red are the topographies of the prominent theta electroencephalography (EEG) components for the two patients. In both cases the pronounced frontocentral theta activity is suggestive of sleepiness. Topographies are against common average reference with standard orientation (top = front), positive excursions in red, negative in blue. Zero potential is indicated by a black line. Spontaneous EEG was recorded during a 12-minute series of standard maneuvers (eyes open, eyes closed, and hyperventilation) according to the 10–20 system (21 head channels), leading to 21 independent component analysis (ICA) components. Artifact-free sections across the whole recording length were submitted to ICA training. ICA maps as well as associated EEG time courses were reviewed and components classified by their topography, reaction to the maneuvers, and spectrum. Additionally, intermittent rhythmic delta/theta activity was detected in the artifact-free sections using an automated algorithm. The software used for these analyses is a combination of the EEG data processor “avg_q” (https://github.com/berndf/avg_q) and custom python scripts. B: Case 1 has tractographic rendition of streamlines involved in strategic unilateral lesions in the inferior and anterior caudate nucleus (nucleus accumbens spared) (#, right side). Tractographic analysis of Case 2 shows a pallidal lesion (*) involving the “motor-control network” (blue fibers). C: Interplay of three involved networks (“affect, control, and reward network”) within the whole “OCD framework” (existing of the “affect, control, reward, and default-mode network” [2, 13]) at distinct network hubs (taken and altered from [13]). The locations of lesions in Cases 1 and 2 are shown as green/blue crosses. Abbreviations: Amy amygdala, ATR anterior thalamic radiation, ATRmd anterior thalamic radiation from dorsomedial thalamus, BNST bed nucleus of stria terminalis, Cd caudate nucleus, DA dopamine, EEG electroencephalography, GABA gamma-aminobutyric acid, GPe globus pallidus externus, GPi globus pallidus internus, Glu glutamate, imMFB infero-medial MFB, ICA independent component analysis, MDT mediodorsal thalamus, MFB medial forebrain bundle, NAC/Nacc nucleus accumbens, NW network, OCD obsessive-compulsive disorder, OFC orbitofrontal cortex, pw pathway, slMFB superolateral branch of the medial forebrain bundleMFB, SNr substantia nigra, STN subthalamic nucleus, VTA ventral tegmental area, vmPFC ventromedial prefrontal cortex.
Patient 2 was a 26-year-old female patient who had suffered from mixed obsessive thoughts and compulsive actions since childhood. This patient’s OCS were dominated by washing compulsions (Y-BOCS: 16; OCI-R: 27). Her symptoms had exacerbated two years prior to her hospitalization without any identifiable trigger. The patient experienced febrile seizures during her childhood, and she had subsyndromal ADHD and autism symptomatology (not fulfilling diagnostic ICD-10 criteria) since her first decade. The diagnostic work-up identified a FLAIR-hyperintense lesion in the right globus pallidus (Fig. 1). A tractographic analysis showed the involvement of fibers from the “motor-control network”. The lesion was in her right globus pallidus externus and at the same time encroached on the “indirect pathway”. ICA detected regional slowing in form of frontocentral theta activity. CSF analysis identified isolated increased lactate levels; neuronal antibodies were negative [12]. The results of the stroke screening and lactate ischemia tests were normal (see Supplemental Table 1). Therapy with 50 mg of sertraline (blood levels of 39 ng/ml; reference 10–50 ng/ml; higher doses led to side effects and increased serum levels) and CBT with ERP did not lead to unequivocal improvement in OCS (Y-BOCS increased to 18 [+12.5%]; OCI-R reduced to 17 [−37%]).
Here, two paradigmatic OCD cases with different strategic basal ganglia lesions and diverse therapy responses are presented. ICA detected frontocentral electrophysiological changes in both patients. The DTI tractography revealed the precise localization of the lesions. In Case 1, the lesion was in the inferior part of the caudate nucleus (ventral striatum adjacent to nucleus accumbens) and the border to the anterior limb of the internal capsule, through which the anterior thalamic radiation out of the medio-dorsal thalamus, the superolateral branch of the medial forebrain bundle, and many frontopontine connections pass. Lesions in the left caudate nucleus have previously been described as associated with OCD [6, 7]. The tractography in Patient 2 showed an involvement of the “motor-control network”. Therefore, the tractographic work-up of both patients showed lesions in two distinct parts of the “OCD framework” [2, 13]. The unilateral lesion in the right caudate of Patient 1 might have disturbed the interconnection of the “reward and affect network,” with reduced dampening of the latter and consecutive exacerbation of OCS. It can be assumed that the right globus pallidus lesion in Patient 2 involved the “motor-control network”. Therefore, the motor consequences of obsessions, including emotional consequences, could have been influenced. The globus pallidus is the exit of the basal ganglia and inherits part of the indirect motor pathway. Motor actions (proceeding or stopping) can be altered via GABA-ergic projection from the nucleus accumbens to the globus pallidus internus; and a lesion in the globus pallidus externus might disturb parts of the indirect pathway as such responsible for stopping non-intended movements and actions. It could be hypothesized that the compensated motor state (no compulsive actions) might have been altered through the lesion (compulsive actions), thus possibly unmasking the OCD.
Interestingly, only Patient 1, who had an involvement of the “reward and affect network”—but not Patient 2, with involvement of the “motor-control network”—benefited clearly from the guideline-based treatment applied in both cases. The lesion in Patient 2 showed involvement of the indirect pathway which is D2 receptor dominated. The D2 receptor, moreover, is involved in decreased reinforcement learning as a side-effect of agonistic medication [14]. Therefore, in similar cases, a pharmacological therapy targeting dopaminergic transmission might be beneficial.
The changes within the “OCD framework” level do not explain the cause of the lesions. In Patient 1, microbleeding, and in Patient 2, a post-inflammatory origin was suspected.
One limitation of both case studies is that (epi)genetic and neurochemical processes were not analyzed in detail [4, 15]. Further, psychological factors [5] may also be involved, and the role of MRI lesions may be overinterpreted.
In summary, these cases show that a broad diagnostic work-up can accurately locate lesions that might lead to “OCD framework” dysfunction [1, 2, 5, 15]. Thus, the investigation of such cases could help to better understand the pathophysiology of OCD and may support biomarker-guided treatment in the future.
Supplementary information
Acknowledgements
We would like to thank both patients for the opportunity to publish this cumulative case report. DE is members of the Immuno-NeuroPsychiatry Network of the European College of Neuropsychopharmacology (ECNP). MAS is a member of the Obsessive Compulsive and Related Disorders (OCRN) network of the ECNP.
Author contributions
DE, KvZ, and VAC wrote the paper. KvZ has collected the clinical data. DE, KvZ, and KR treated the patients. HU was responsible for the neuroradiological interpretation. RD and HP were responsible for the neurological co-assessment. RD was responsible for CSF analysis. HP performed the tissue-based assays on unfixed murine brain sections. BF performed the EEG assessment and ICA analyses. KN was responsible for the ophthalmological measurements. KD and MAS carefully revised the manuscript. MR and VAC performed the DTI analyses. MR and VAC were responsible for the neurostructural interpretation and prepared the conceptional Fig. 1C. All authors read and approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
All necessary data can be found in the paper.
Competing interests
RD: Lecture fees from Roche, Alexion, Bayer, and Sanofi. Travel grant from Biogen. KD: Steering Committee Neurosciences, Janssen. Speaker fees from Janssen. VAC: Consultation work for CereGate (Munich, Germany), Cortec (Freiburg, Germany), and Inbrain (Barcelona, Spain). Honoraria for Talks (Boston Scientific, USA). IITs in DBS with Medtronic (USA) and Boston Scientific (USA). All other authors declare no potential competing interests.
Consent for publication
Both patients have given her signed written informed consent for this cumulative case study to be published.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dominique Endres, Katharina von Zedtwitz.
These authors jointly supervised this work: Marco Reisert, Volker A. Coenen.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-022-01853-8.
References
- 1.Shephard E, Stern ER, van den Heuvel OA, Costa DLC, Batistuzzo MC, Godoy PBG, et al. Toward a neurocircuit-based taxonomy to guide treatment of obsessive-compulsive disorder. Mol Psychiatry. 2021;26:4583–4604. doi: 10.1038/s41380-020-01007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coenen VA, Schlaepfer TE, Sajonz B, Döbrössy M, Kaller CP, Urbach H, et al. Tractographic description of major subcortical projection pathways passing the anterior limb of the internal capsule. Corticopetal organization of networks relevant for psychiatric disorders. Neuroimage Clin. 2020;25:102165. doi: 10.1016/j.nicl.2020.102165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endres D, Domschke K, Schiele MA. Neurobiology of obsessive-compulsive disorder. Nervenarzt. 2022;93:670–677. doi: 10.1007/s00115-022-01331-0. [DOI] [PubMed] [Google Scholar]
- 4.Saraiva LC, Sato JR, Cappi C. Probing the genetic and molecular correlates of connectome alterations in obsessive-compulsive disorder. Mol Psychiatry. 2022. 10.1038/s41380-022-01590-y. [DOI] [PubMed]
- 5.Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, et al. Obsessive-compulsive disorder. Nat Rev Dis Prim. 2019;5:52. doi: 10.1038/s41572-019-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz BS, Flemming KD. Obsessive compulsive disorder due to a cavernous malformation hemorrhage in the dominant caudate head. J Clin Neurosci. 2015;22(Feb):398–9. doi: 10.1016/j.jocn.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Thobois S, Jouanneau E, Bouvard M, Sindou M. Obsessive-compulsive disorder after unilateral caudate nucleus bleeding. Acta Neurochir (Wien) 2004;146:1027–31. doi: 10.1007/s00701-004-0312-6. [DOI] [PubMed] [Google Scholar]
- 8.Endres D, Mertens L, Berger B, Reisert M, Runge K, Nickel K, et al. Autoimmune obsessive-compulsive disorder with novel anti-basal ganglia antibodies. Psychother Psychosom. 2022:1-3. 10.1159/000522136.. [DOI] [PubMed]
- 9.Endres D, Pankratz B, Robinson T, Pitsch K, Göbel T, Runge K, et al. Autoimmune obsessive-compulsive disorder with novel anti-CNS autoantibodies in cerebrospinal fluid. Mol Psychiatry. 2022. 10.1038/s41380-022-01688-3. [DOI] [PMC free article] [PubMed]
- 10.Coenen VA, Döbrössy MD, Teo SJ, Wessolleck J, Sajonz BEA, Reinacher PC, et al. Diverging prefrontal cortex fiber connection routes to the subthalamic nucleus and the mesencephalic ventral tegmentum investigated with long range (normative) and short range (ex-vivo high resolution) 7T DTI. Brain Struct Funct. 2022;227:23–47. doi: 10.1007/s00429-021-02373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endres D, Maier S, Feige B, Mokhtar NB, Nickel K, Goll P, et al. Increased rates of intermittent rhythmic delta and theta activity in the electroencephalographies of adult patients with attention-deficit hyperactivity disorder. Epilepsy Behav. 2017;75:60–65. doi: 10.1016/j.yebeh.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 12.Prüss H. Autoantibodies in neurological disease. Nat Rev Immunol. 2021;21:798–813. doi: 10.1038/s41577-021-00543-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coenen VA, Schlaepfer TE, Sajonz BEA, Reinacher PC, Döbrössy MD, Reisert M. “The Heart Asks Pleasure First”-Conceptualizing Psychiatric Diseases as MAINTENANCE Network Dysfunctions through Insights from slMFB DBS in Depression and Obsessive-Compulsive Disorder. Brain Sci. 2022;12:438. doi: 10.3390/brainsci12040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, et al. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacol (Berl) 2008;196:221–32. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pauls DL, Abramovitch A, Rauch SL, Geller DA. Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat Rev Neurosci. 2014;15:410–24. doi: 10.1038/nrn3746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All necessary data can be found in the paper.