Abstract
Calcium controls numerous events within the vessel wall. Permeability of the endothelium is calcium dependent, as are platelet activation and adhesion, vascular smooth muscle proliferation and migration, and synthesis of fibrous connective tissue. Double-helix computerized tomography is a noninvasive technique that can detect, measure, and compare coronary calcification in the coronary arteries. Despite some convincing evidence about the prognostic value and usefulness of coronary artery calcium score (CACS) in the stratification of cardiovascular risk in the high risk general population and also in hypertensive patients, current guidelines for the management of hypertension, do not include such evaluation among the recommended procedures to be performed in the majority of patients even with the intent to detect hypertension-mediated organ damage (HMOD) in an early phase. On the contrary, the European Society of Cardiology guidelines for the diagnosis and management of chronic coronary syndromes, the 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease, and the 2018 Cholesterol Clinical Practice Guidelines indicate that the evaluation of CACS may be of some usefulness in specific subpopulations, although this view is not accepted in the US Preventive Services Task Force document. Very recently, the European Society of Cardiology Guidelines on cardiovascular disease prevention in clinical practice stated that CACS estimation may be considered to improve risk classification around treatment decision thresholds. In conclusion, the use of CACS as a diagnostic tool is still controversial. While some evidence exists about is ability to improve stratification of cardiovascular risk in primary prevention, in particular in selected patients who are at intermediate or borderline risk of atherosclerotic cardiovascular disease, there is insufficient evidence to use it as a standard means to assess HMOD.
Keywords: Atherosclerotic cardiovascular disease (ASCVD), Hypertension-mediated organ damage (HMOD), Hypertension, Atherosclerosis, Calcium score, Coronary
Introduction
Clinical, experimental and pathologic studies strongly indicate that hypertension is a major factor in coronary heart disease, sudden death, stroke congestive heart failure and renal insufficiency. The deleterious effect of the elevated blood pressure on the cardiovascular system appears to be due mainly to the mechanical stress imposed to the heart and blood vessels [1]. Humoral factors and vasoactive hormones such as angiotensin, catecholamines and prostaglandins may play a role in the pathogenesis of hypertensive cardiovascular disease [1–3]. Hypertension and the resulting increase in tangential tension on the myocardial and arterial walls, leads to the development of hypertensive heart disease and congestive heart failure as well as hypertensive vascular disease that affects not only the kidneys but also the heart and brain [1–3]. Hypertensive vascular disease involves both large and small arteries as well as arterioles and is characterized by fibromuscular thickening of intima/media with luminal narrowing of the small arteries and arterioles [4]. The physical stress of hypertension on the arterial wall also results in the aggravation and acceleration of atherosclerosis, particularly of the coronary and cerebral vessels, leading to atherosclerotic cardiovascular disease (ASCVD). Moreover, hypertension appears to increase per se the susceptibility of large arteries to atherosclerosis [1]. Thus, the patient with hypertension is a candidate for both hypertensive and atherosclerotic vascular damage, leading to stenotic/occlusive disease of both large and small coronary and cerebral arteries, resulting in myocardial infarction and stroke [1–3]. Other major complications of hypertensive vascular disease include rupture and thrombotic occlusion of blood vessels, especially in the brain [1–3]. Disease of the arterial media, which begins in childhood with the deposition of calcium in the vessels, may be an important cause of arterial hypertension [1]. This form of hypertension may manifest itself in adults as arteriosclerotic hypertension and lead to cardiovascular complications very similar to those of essential hypertension [1]. An early detection of hypertension-mediated organ damage (HMOD), including that mediated by atherosclerotic mechanisms might provide a better stratification of risk and a more appropriate and timing therapeutic intervention [2, 3]. In this Review the possible role of an estimation of coronary artery calcium score (CACS) in this regard will be addressed.
Atherosclerosis and Hypertension
The molecular mechanism of atherosclerosis is a complex web of cellular events that is only gradually becoming clear. These mechanisms include lipid metabolism, inflammatory signaling, and interaction with the complex vascular system involved in thrombosis [5].
Atherogenesis can be divided into five key steps, which are (1) endothelial dysfunction, (2) formation of lipid layer or fatty streak within the intima, (3) migration of leukocytes and smooth muscle cells into the vessel wall, (4) foam cell formation and (5) degradation of extracellular matrix [5].
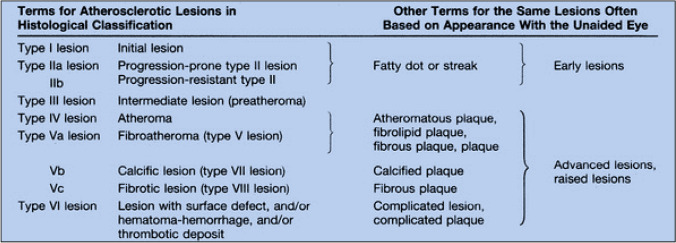
The initial (type I) lesion contains enough atherogenic lipoprotein to elicit an increase in macrophages and formation of scattered macrophage foam cells. As in subsequent lesion types, the changes are more marked in locations of arteries with adaptive intimal thickening. Type II lesions consist primarily of layers of macrophage foam cells and lipid-laden smooth muscle cells and include lesions grossly designated as fatty streaks. Type III is the intermediate stage between type II and type IV (atheroma, a lesion that is potentially symptom-producing). In addition to the lipid-laden cells of type II, type III lesions contain scattered collections of extracellular lipid droplets and particles that disrupt the coherence of some intimal smooth muscle cells. This extracellular lipid is the immediate precursor of the larger, confluent, and more disruptive core of extracellular lipid that characterizes type IV lesions. Beginning around the fourth decade of life, lesions that usually have a lipid core may also contain thick layers of fibrous connective tissue (type V lesion) and/or fissure, hematoma, and thrombus (type VI lesion). Some type V lesions are largely calcified (type Vb), and some consist mainly of fibrous connective tissue and little or no accumulated lipid or calcium (type Vc) [7] (Fig. 1).
Fig. 1.
A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the committee on vascular lesions of the council on arteriosclerosis, American Heart Association.
From reference [7]
As mentioned, hypertension is a risk factor for the development of atherosclerosis through various mechanisms that may include increased shear stress, low grade inflammation, oxidative stress, a procoagulant state and even activation of immune mechanisms [5–7]. According to the 2018 ESC/ESH Guidelines for the management of arterial hypertension [3], an early detection of atherosclerotic vascular damage is mandatory, and the standard workout include a measurement of carotid intima-media thickness quantified by carotid ultrasound, and/or the presence of plaques, the estimation of the ankle-brachial index (ABI) (usually indicative of advanced atherosclerosis) and the measurement of the pulse wave velocity, hallmark of a possibly increased large artery stiffness and partially a proxy of atherosclerotic damage. Being the major part of the risk associated to hypertension related to atherosclerotic damage to the heart and the brain, the estimation of related cardiovascular risk, according to the previously mentioned Guidelines [3], should be made by the calculation of the Systematic COronary Risk Evaluation (SCORE), a validated scoring system that predicts the 10-year risk of a first fatal atherosclerotic cardiovascular event, in relation to age, gender, smoking, total cholesterol, and systolic blood pressure. Similar advices were present in the previous version of the European guidelines [2], as well as in the American and International guidelines [8, 9]. Therefore, the absolute risk associated to hypertension and HMOD that involve heart, brain and vessels is mainly to be ascribed to atherosclerotic damages and its estimation in the early phase of the disease is to be considered mandatory, in order to provide a better stratification of risk and as a guide to treatment [3].
Calcium Score and Atherosclerosis
As previously mentioned, calcium deposition represents an important mechanism in the development of the complicated atherosclerotic plaque (Vb lesions) [7] (Fig. 1). However, calcium particles are often found within the lipid cores even in young adults [7]. Lesions containing a large amount of calcium generally also have increased fibrous connective tissue, and often have the underlying morphology of fibroatheroma. Lesions in which mineralization is the dominant feature may be called type Vb (calcific) lesions. Mineral deposits may replace the accumulated remnants of dead cells and extracellular lipid, including entire lipid cores. Elsewhere, the calcific lesion has been labeled the type VII lesion [7].
Ultrafast computed tomography may allow an early detection of coronary lesions by means of highly sensitive noninvasive demonstration of coronary artery calcium (CAC) [10].
It was suggested that the presence of CAC detected by ultrafast computed tomography, may be a sensitive early noninvasive marker for the presence and progression of atherosclerosis before development of ischemia-producing stenosis or complication [10, 11].
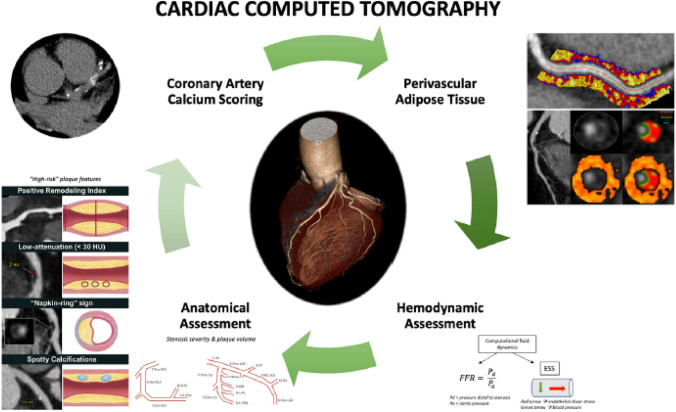
Cardiac computed tomography (CCT) (Fig. 2) has emerged in the last decade as an important non-invasive modality for the evaluation of coronary artery disease (CAD) with actively expanding indications [12]. Initial research led to the establishment of a CACS for improved risk stratification of asymptomatic patients with intermittent probability for adverse atherosclerotic events; a CACS of zero is associated with low rates of future adverse events [13], although also in those patients polygenic risk scores remain associated with ASCVD events [14].
Fig. 2.
Current and emerging roles of cardiac computed tomography in predicting adverse coronary events. Comprehensive assessment of coronary artery disease with cardiac computed tomography (CCT) includes coronary artery calcium score, anatomic assessment to identify stenosis, plaque volume, and high-risk plaque features, hemodynamic assessment using computational fluid dynamics to compute fractional flow reserve (FFR) and endothelial shear stress (ESS), as well as derivation of perivascular fat attenuation index. Perivascular fat attenuation index figure is owned by: Oxford Academic Cardiovascular CT Core Lab and Lab of Inflammation and Cardiometabolic Diseases at NHLBI, published under Attribution-NonCommercial 2.0 Generic (CC BY-NC 2.0). Link to license: https://creativecommons.org/licenses/by-nc/2.0/.
From reference [12]
CCT allows quantification of plaque calcium burden by measuring the Agatston score [15]. CACS is based on a low radiation dose, non-contrast CCT [16] and represents a simple, quick, inexpensive, and reproducible test. CACS has shown to correlate well with long-term risk of cardiac events when used as a binary or categorical number [13, 17–20]. For example, the 10-year risk for adverse atherosclerotic events of a 65-year-old male with hyperlipidemia and medically treated hypertension is over 10%. If the same individual has a CACS of zero, then the risk becomes 3.5%. Importantly, the predictive value of CACS is incremental to that of traditional risk factors and risk calculators that have included CACS have outperformed established risk scores, such as the Framingham score and the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) risk estimator [21]. Today, CACS is an established way to better assess the ASCVD risk of asymptomatic individuals that otherwise fall into intermediate risk and start appropriate risk factor modification therapy [class IIb recommendation in European Society of Cardiology guidelines for the diagnosis and management of chronic coronary syndromes [22] and IIa in the 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease [23].
In particular, the former guideline report the following statement: “assessment of coronary artery calcium score with computed tomography may be considered as a risk modifier in the cardiovascular risk assessment of asymptomatic subjects” [22], while in the latter the following recommendation is present “in adults at intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk) or selected adults at borderline risk (5% to < 7.5% 10-year ASCVD risk), if risk-based decisions for preventive interventions (e.g., statin therapy) remain uncertain, it is reasonable to measure a coronary artery calcium score to guide clinician–patient risk discussion” (Table 1).
Table 1.
Different indications in the evaluation of CACS for the assessment of cardiovascular risk between different guidelines
| Guideline | Indication | Class of recommendation | Level of evidence |
|---|---|---|---|
| US Preventive Services Task Force (reference #26) | No indication | NA | NA |
| 2018 Cholesterol Clinical Practice Guidelines (reference #24) |
In intermediate-risk (≥7.5% to <20% 10-year ASCVD risk) adults or selected borderline-risk (5% to <7.5% 10-year ASCVD risk) adults in whom a coronary artery calcium score is measured for the purpose of making a treatment decision, AND If the coronary artery calcium score is zero, it is reasonable to withhold statin therapy and reassess in 5–10 years, as long as higher-risk conditions are absent (e.g., diabetes, family history of premature CHD, cigarette smoking); If coronary artery calcium score is 1 to 99, it is reasonable to initiate statin therapy for patients ≥55 years of age; If coronary artery calcium score is 100 or higher or in the 75th percentile or higher, it is reasonable to initiate statin therapy |
IIa | B |
| 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease (reference # [23]) | In adults at intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk) or selected adults at borderline risk (5% to < 7.5% 10-year ASCVD risk), if risk-based decisions for preventive interventions (e.g., statin therapy) remain uncertain, it is reasonable to measure a coronary artery calcium score to guide clinician–patient risk discussion | IIa | B |
| 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes (reference # [22]) | Assessment of coronary artery calcium score with computed tomography may be considered as a risk modifier in the cardiovascular risk assessment of asymptomatic subjects | IIb | B |
| Kaiser Permanente Atherosclerotic Cardiovascular Disease (ASCVD) Primary Prevention Guideline 2020 (reference # [25]) |
CACS: consider for patients at indeterminate risk or at intermediate risk and undecided about statins CACS: although not routinely recommended, may be helpful for patients at intermediate ASCVD risk who are uncertain about taking a statin, and/or patients whose calculated risk is higher or lower than expected |
NA | NA |
| 2021 European Society of Cardiology (ESC) Guidelines on cardiovascular disease prevention in clinical practice (reference # [39]) | CAC scoring may be considered to improve risk classification around treatment decision thresholds | IIb | B |
ASCVD Atherosclerotic Cardiovascular Disease, CACS coronary artery calcium score, CAC coronary artery calcium, N.A. not available
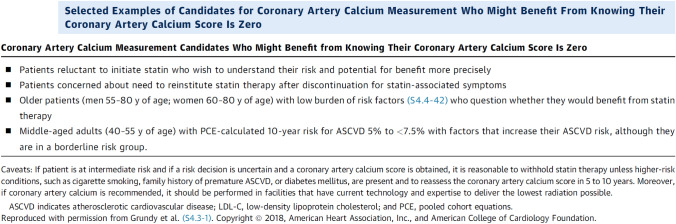
In Table 1 recommendations from the 2018 Cholesterol Clinical Practice Guidelines [24], which are included in the previously mentioned ACC/AHA guidelines [23] are reported; at point 7 the use of CACS is suggested. In Fig. 3 selected examples of candidates for CAC measurement who might benefit from knowing their CACS is zero is reported [23].
Fig. 3.
Selected examples of candidates who might benefit from knowing their coronary artery calcium score is zero.
From reference [23]
In the Kaiser Permanente Atherosclerotic Cardiovascular Disease (ASCVD) Primary Prevention Guideline 2020 [25] the suggested indications to the measurement of CACS were summarized as follows:
CACS: consider for patients at indeterminate risk or at intermediate risk and undecided about statins.
CACS, although not routinely recommended, may be helpful for patients at intermediate ASCVD risk who are uncertain about taking a statin, and/or patients whose calculated risk is higher or lower than expected.
Who should consider getting CACS testing?
Individuals at intermediate ASCVD risk (aged 40–75 years without diabetes and with LDL-C levels ≥ 70 mg/dL, at a 10-year ASCVD risk of ≥ 7.5% and < 20%), if risk status or decision about statin therapy is uncertain (for example, due to patient reluctance to start pharmacotherapy). For these patients, treatment with statin therapy may be withheld or delayed if CACS = 0, except in cigarette smokers and those with a strong family history of premature ASCVD. A CACS of 1–99 favors statin therapy, especially in those aged ≥ 55 years. For any patient, if the CACS is ≥ 100 or ≥ 75th percentile, statin therapy is indicated.
Measurement of CAC may be considered in select adults with borderline elevated ASCVD risk (5–7.4% 10-year ASCVD risk) for further risk stratification, in whom the presence of CAC may change decision-making with regard to statin treatment and intensity of ASCVD risk factor modification.
If patients get CAC testing but remain untreated, repeating CAC measurement in 5–10 years may have some value in reassessing for CAC progression, but data are limited.
Who should not get CAC score testing?
Routine CAC measurement is not recommended in patients at low (< 5% 10-year risk) or high (≥ 20% 10-year risk) ASCVD risk, as the results are generally unlikely to change management.
Patients who are averse to treatment and unlikely to initiate treatment even if CAC is identified should not undergo CAC testing.
However, the US Preventive Services Task Force (USPSTF 2018) examined whether the addition of CAC to the traditional risk factors improves risk classification [26]. The report concluded that—while CACS statistically improves risk stratification—there was insufficient evidence to determine either the benefits and harms of using CACS testing for risk assessment, or whether adding it to the tools currently used would reduce the incidence of cardiac heart disease or mortality following statin therapy [26]. The USPSTF found therefore inadequate evidence to assess whether treatment decisions guided by ABI, high sensitivity C-reactive protein (hsCRP) level, or CAC score, in addition to risk factors in existing cardiovascular disease (CVD) risk assessment models, leads to reduced incidence of CVD events or mortality [26]. The USPSTF found adequate evidence to conceptually bound the harms of early detection and interventions as small [26], however the current evidence is insufficient to assess the balance of benefits and harms of using ABI, hsCRP level, or CAC score in risk assessment for CVD in asymptomatic adults to prevent CVD events [26].
Concerns about the clinical applications of CACS include the followings: the serial use of CACS is less clear, particularly in patients who are on statin therapy [27], while it may be of some help in patients taking aspirin: higher CAC is associated with both ASCVD and bleeding events, with a stronger association with ASCVD [28]. A high CAC score identifies individuals estimated to obtain net benefit from primary prevention with aspirin therapy from those who would not, but only in the setting of lower bleeding risk and estimated ASCVD risk that is not low [28].
Nevertheless, the clinical application of CACS must take into account the pre-test probability of CAD, even when CACS is zero, as plaque can be non-calcified, particularly in younger, high-risk patients. Along these lines, clinical decisions for symptomatic patients should not be based solely on CACS, and in fact, CACS is not recommended in that scenario, as shown in the CORE64 trial where > 10% of symptomatic patients with CACS of zero had obstructive CAD [29], although this was not confirmed in the PROMISE Study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) Study [30], In fact, most patients experiencing clinical events among stable outpatients presenting with suspected CAD have measurable CAC at baseline [30].
In summary, the evaluation of CACS has a good clinical values, since it has good correlation with long-term risk of cardiac events and incremental predictive value on top of traditional risk factors, it has some pros (low radiation, no contrast, quick, inexpensive, reproducible) but also some cons (unclear value of serial CCT assessments, must consider pre-test probability of CAD) [12].
Absolute CACS and CAC percentiles can identify different patient groups, which could be confusing in clinical practice; a relatively simple "rule of thumb" for identifying the American College of Cardiology/American Heart Association [23] endorsed 75th CAC percentile based on age, gender, and the absolute CACS was proposed [31]. Using the Multi-Ethnic Study of Atherosclerosis, the authors calculated the age and gender-specific percent likelihood that a guideline-based absolute CACS group (1 to 100, 100 to 300, > 300) will place a patient above the 75th percentile. Also, gender-specific age cutoffs by which 95% of participants with any (> 0), moderate (≥ 100), or severe (≥ 300) CACS would be over the 75th percentile were derived. The sensitivity analysis was repeated using the 90th percentile threshold. Any CACS > 0 places 95% of women younger than 60 years and over 90% of men younger than 50 years over the 75th percentile. Moderate absolute CACS (> 100) place nearly all men < 60 years and all women < 70 years over the 75th percentile. Confirmatory analysis for age cutoffs was consistent with primary analysis, with cutoffs of 48 years for men and 59 years for women indicating a 95% likelihood that any CACS would place patients over the 75th percentile. In conclusion, the study [31] provides a simple rule of thumb (men < 50 years and women < 60 years with any CACS, men < 60 years and women < 70 years with CAC S > 100) for identifying CACS > 75th percentile that might be readily adopted in clinical practice. CACS could predict coronary artery disease reporting and data system (CAD-RADS) with an accuracy of 80% [32]. Several personalized risk prediction tools, including CACS, polygenic risk scores, and metabolic risk scores may be able to improve risk assessment [33].
Insights from the CARDIA and MESA Study suggest that elevated remnant cholesterol levels were associated with an increased risk of CAC progression independent of traditional cardiovascular risk factors, even in individuals with optimal LDL-C levels [34].
However, some methodological concerns were recently risen about the evaluation of coronary calcium density, on the basis that the Agatston score may be upweighted based on the assumption that peak calcium density and ASCVD risk are positively correlated [35]. Recent evidence demonstrates that calcium density may be inversely associated with lesion vulnerability and ASCVD risk in population-based cohorts when accounting for age and plaque area [35]. Recently, evaluation of calcium density through CACS with non-contrast computed tomography was reviewed by focusing on 3 main areas: (1) CAC scan acquisition parameters; (2) pathophysiology of calcified plaques; and (3) epidemiologic evidence relating calcium density to ASCVD outcomes [35]. Future and present development in this area include automated cardiovascular risk categorization through artificial intelligence-driven coronary calcium quantification in cardiac PET acquired attenuation correction CCT [36] or machine learning approach for cardiovascular risk and CACS [37] or even new in vitro tools [38].
Finally and most recently, in September 2021, the European Society of Cardiology (ESC) Guidelines on cardiovascular disease prevention in clinical practice were published [39]. CACS was considered as a risk modifier, and its estimation “may be considered to improve risk classification around treatment decision thresholds” (class of recommendation IIb, level of evidence B) (Table 1).
Calcium Score in Hypertension
In 2699 Framingham Heart Study participants who were part of a multidetector computed tomography substudy from 2008 to 2011 it was demonstrated that renal artery calcium (RAC) is common and independently associated with microalbuminuria and hypertension after adjustment for nonrenal vascular calcium [40], therefore RAC may be uniquely associated with these markers of renal end-organ damage [40]. An association was also observed between the presence of aortic valve calcium and coronary calcium score on spiral computed tomography in high-risk hypertensive patients, supporting a high association between aortic valve calcium and increased risk of death from cardiovascular causes [41]. Mild renal dysfunction accelerated coronary artery calcifications, above and beyond conventional risk factors in 547 high-risk Israeli hypertensive patients [42].
The presence of CAC as assessed by dual slice spiral computed tomography predicted cardiovascular events also in high-risk asymptomatic hypertensive patients enrolled in the INSIGHT (International Nifedipine Study Intervention as Goal for Hypertension Therapy) [43], similarly to what previously demonstrated in subjects prone to atherosclerosis. This was confirmed also in an extended follow up (15 years) in the calcification side arm of the INSIGHT [44, 45], as well as in the subgroup of patients with combined diabetes mellitus and systemic hypertension [46] (Table 2).
Table 2.
Evidences concerning CACS estimation in hypertension
| Study | Conclusion | References |
|---|---|---|
| INSIGHT | CACS predicted cardiovascular events also in high-risk asymptomatic hypertensive patients | [43] |
| INSIGHT | In hypertensive patients, progression of CAC is associated with long-term cardiovascular events | [44, 45] |
| INSIGHT | Patients with hypertension and diabetes mellitus can be stratified into a lower cardiovascular risk in the absence of CAC. | [46] |
| Heinz Nixdorf Recall | Risk of myocardial infarction and stroke in hypertension but also in prehypertension depended on the degree of CAC | [47] |
| INSIGHT | Slower progression of coronary calcification in hypertensive patients on nifedipine once daily versus co-amilozide | [48] |
| ACTION | Nifedipine GITS was not effective in slowing down the progression of calcium in advanced atherosclerotic plaques in patients with stable angina pectoris, half of them hypertensives | [49] |
CACS coronary artery calcium score, CAC coronary artery calcium
Prehypertension is a frequent condition and has been demonstrated to increase cardiovascular risk. However, the association with coronary atherosclerosis as part of target organ damage is not well understood. Cross-sectional relationship and longitudinal outcome between blood pressure categories and CAC, quantified by electron beam computed tomography was investigated in 4181 participants from the population-based Heinz Nixdorf Recall Study cohort [47]. Risk of myocardial infarction and stroke in hypertension but also in prehypertension depended on the degree of CAC. According to the Authors, this marker of target-organ damage might be included, when lifestyle modification and pharmacotherapeutic effects in prehypertensive individuals are tested to avoid exposure to risk and increase benefit [47].
Measurement of CACS may be of some help also in treated hypertensive patients. Using double-helix computerized tomography, a side arm of the INSIGHT study (International Nifedipine Study: Intervention as Goal for Hypertension Therapy) was aimed to show the efficacy of nifedipine once daily versus co-amilozide (hydrochlorothiazide plus amiloride) in high-risk hypertensive patients in arresting or slowing-down the progression of coronary artery calcification [48]. Inhibition of coronary calcium progression was significant in the nifedipine versus the co-amilozide group during the first year, not significant during the second year, and significant during the third year [48]. The results point therefore to a slower progression of coronary calcification in hypertensive patients on nifedipine once daily versus co-amilozide [48] (Table 2).
However, in the ACTION (A Coronary Disease Trial Investigating Outcome with Nifedipine GITS) trial, nifedipine GITS was not effective in slowing down the progression of calcium in advanced atherosclerotic plaques in patients with stable angina pectoris, half of them hypertensives [49] (Table 2).
According to the 2013 Guidelines for the management of arterial hypertension of the European Society of Hypertension and of the European Society of Cardiology, coronary calcium score is one of the recommended strategies for the search for HMOD, with cardiovascular predictive value rated ++, availability +, reproducibility +++ and cost-effectiveness + [2], while the 2018 version of the European Guidelines [3], as well as in the current American [8] and International [9] guidelines do not consider coronary calcium score among the suggested procedures to evaluate HMOD.
Conclusions
Calcium controls numerous events within the vessel wall. Permeability of the endothelium is calcium dependent, as are platelet activation and adhesion, vascular smooth muscle proliferation and migration, and synthesis of fibrous connective tissue. Double-helix computerized tomography is a noninvasive technique that can detect, measure, and compare coronary calcification in the coronary arteries [48]. Despite some convincing evidence about the prognostic value and usefulness of CACS in the stratification of cardiovascular risk in the high risk general population and also in hypertensive patients, current guidelines for the management of hypertension, do not include such evaluation among the recommended procedure to be performed in the majority of patients even with the intent to detect HMOD in an early phase. On the contrary, the European Society of Cardiology guidelines for the diagnosis and management of chronic coronary syndromes [22], the 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease [23], the 2018 Cholesterol Clinical Practice Guidelines [24] and the 2021 European Society of Cardiology Guidelines on cardiovascular disease prevention in clinical practice [39] indicate that the evaluation of CACS may be of some usefulness in specific subpopulations (Table 1), although this view is not accepted in the US Preventive Services Task Force document [26].
Therefore, the use of CAC as a diagnostic tool is still controversial. While some evidence exists about is ability to improve stratification of cardiovascular risk in primary prevention, in particular in selected patients who are at intermediate or borderline risk of ASCVD [50, 51], there is insufficient evidence to use it as a standard means to assess HMOD.
Funding
Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement. The authors did not receive support from any organization for the submitted work.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
N.A.
Data availability
Being a Review there is no data availability. The manuscript has no original data.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
References
- 1.Hollander W. Role of hypertension in atherosclerosis and cardiovascular disease. Am J Cardiol. 1976;38(6):786–800. doi: 10.1016/0002-9149(76)90357-x. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Members TF. ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;2013(31):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 3.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, Authors/Task Force Members 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Agabiti-Rosei C, Bruno RM, Rizzoni D. Microcirculation and macrocirculation in hypertension: a dangerous cross-link? Hypertension. 2022;79(3):479–490. doi: 10.1161/HYPERTENSIONAHA.121.17962. [DOI] [PubMed] [Google Scholar]
- 5.Ross R, Faggiotto A, Bowen-Pope D, Raines E. The role of endothelial injury and platelet and macrophage interactions in atherosclerosis. Circulation. 1984;70(5 Pt 2):III77–III82. [PubMed] [Google Scholar]
- 6.Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25(2):155–161. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- 7.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92(5):1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 8.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Sr, Williamson JD, Wright JT., Jr 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 9.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, Wainford RD, Williams B, Schutte AE. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 10.Janowitz WR, Agatston AS, Kaplan G, Viamonte M., Jr Differences in prevalence and extent of coronary artery calcium detected by ultrafast computed tomography in asymptomatic men and women. Am J Cardiol. 1993;72:247–254. doi: 10.1016/0002-9149(93)90668-3. [DOI] [PubMed] [Google Scholar]
- 11.Wong ND, Kouwabunpat D, Vo AN, Detrano RC, Eisenberg H, Goel M, Tobis JM. Coronary calcium and atherosclerosis by ultrafast computed tomography in asymptomatic men and women: relation to age and risk factors. Am Heart J. 1994;127:422–430. doi: 10.1016/0002-8703(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 12.Emfietzoglou M, Mavrogiannis MC, Samaras A, Rampidis GP, Giannakoulas G, Kampaktsis PN. The role of cardiac computed tomography in predicting adverse coronary events. Front Cardiovasc Med. 2022;9:920119. doi: 10.3389/fcvm.2022.920119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of coronary calcium and incident coronary heart disease events: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2013;61:1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Rifai M, Yao J, Guo X, Post WS, Malik S, Blumenthal RS, Ballantyne CM, Budoff M, Taylor KD, Lin HJ, Rich SS, Hajek C, Greenland P, Rotter JI, Virani SS. Association of polygenic risk scores with incident atherosclerotic cardiovascular disease events among individuals with coronary artery calcium score of zero: the multi-ethnic study of atherosclerosis. Prog Cardiovasc Dis. 2022;S0033-0620(22)00085-8. 10.1016/j.pcad.2022.08.003 [DOI] [PMC free article] [PubMed]
- 15.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 16.Blankstein R, Gupta A, Rana JS, Nasir K. The implication of coronary artery calcium testing for cardiovascular disease prevention and diabetes. Endocrinol Metab. 2017;32:47–57. doi: 10.3803/EnM.2017.32.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obaid DR, Calvert PA, Gopalan D, Parker RA, Hoole SP, West NE, et al. Atherosclerotic plaque composition and classification identified by coronary computed tomography: assessment of computed tomography-generated plaque maps compared with virtual histology intravascular ultrasound and histology. Circ Cardiovasc Imaging. 2013;6:655–664. doi: 10.1161/CIRCIMAGING.112.000250. [DOI] [PubMed] [Google Scholar]
- 18.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 19.Elias-Smale SE, Proenca RV, Koller MT, Kavousi M, van Rooij FJ, Hunink MG, et al. Coronary calciumscore improves classification of coronary heart disease risk in the elderly: the Rotterdam study. J Am Coll Cardiol. 2010;56:1407–1414. doi: 10.1016/j.jacc.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Razavi A, Iftekhar Uddin S, Dardari Z, Berman D, Budoff M, Miedema M, et al. Coronary artery calcium for risk stratification of sudden cardiac death: the coronary artery calcium consortium. JACC Cardiovasc Imaging. 2022 doi: 10.1016/j.jcmg.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (multi-ethnic study of atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study) J Am Coll Cardiol. 2015;66:1643–1653. doi: 10.1016/j.jacc.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, ESC Scientific Document Group 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 23.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Jr, Virani SS, Williams KA, Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines Clinical Practice Guideline. J Am Coll Cardiol. 2019;74(10):e177–e232. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Sparks A & Cohen A et al. Kaiser Permanente Atherosclerotic Cardiovascular Disease (ASCVD) Primary Prevention Guideline 2020. https://wa.kaiserpermanente.org/static/pdf/public/guidelines/ascvd-primary.pdf
- 26.US Preventive Services Task Force. Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW, Jr, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Tseng CW, Wong JB. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272–280. doi: 10.1001/jama.2018.8359. [DOI] [PubMed] [Google Scholar]
- 27.Nakazato R, Gransar H, Berman DS, Cheng VY, Lin FY, Achenbach S, et al. Statins use and coronary artery plaque composition: results from the International Multicenter CONFIRM Registry. Atherosclerosis. 2012;225:148–153. doi: 10.1016/j.atherosclerosis.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ajufo E, Ayers CR, Vigen R, Joshi PH, Rohatgi A, de Lemos JA, Khera A. Value of coronary artery calcium scanning in association with the net benefit of aspirin in primary prevention of atherosclerotic cardiovascular disease. JAMA Cardiol. 2021;6(2):179–187. doi: 10.1001/jamacardio.2020.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottlieb I, Miller JM, Arbab-Zadeh A, Dewey M, Clouse ME, Sara L, et al. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol. 2010;55:627–634. doi: 10.1016/j.jacc.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Budoff MJ, Mayrhofer T, Ferencik M, Bittner D, Lee KL, Lu MT, Coles A, Jang J, Krishnam M, Douglas PS, Hoffmann U, PROMISE Investigators Prognostic value of coronary artery calcium in the PROMISE Study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) Circulation. 2017;136(21):1993–2005. doi: 10.1161/CIRCULATIONAHA.117.030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osei AD, Mirbolouk M, Dardari Z, Shea S, Blankstein R, Dzaye O, Nasir K, Blumenthal RS, Blaha MJ. A simple approach to the identification of guideline-based coronary artery calcium score percentiles (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2022;179:18–21. [DOI] [PMC free article] [PubMed]
- 32.Moradi M, Rafiei E, Rasti S, Haghbin H. Coronary artery calcification-does it predict the CAD-RADS category? Emerg Radiol. 2022 doi: 10.1007/s10140-022-02082-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma KP, Inouye M, Meikle PJ, Nicholls SJ, Carrington MJ, Marwick TH. New cardiovascular risk assessment techniques for primary prevention: JACC Review Topic of the Week. J Am Coll Cardiol. 2022;80(4):373–387. doi: 10.1016/j.jacc.2022.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Hao QY, Gao JW, Yuan ZM, Gao M, Wang JF, Schiele F, Zhang SL, Liu PM. Remnant cholesterol and the risk of coronary artery calcium progression: insights from the CARDIA and MESA Study. Circ Cardiovasc Imaging. 2022;15(7):e014116. doi: 10.1161/CIRCIMAGING.122.014116. [DOI] [PubMed] [Google Scholar]
- 35.Razavi AC, Agatston AS, Shaw LJ, De Cecco CN, van Assen M, Sperling LS, Bittencourt MS, Daubert MA, Nasir K, Blumenthal RS, Mortensen MB, Whelton SP, Blaha MJ, Dzaye O. Evolving role of calcium density in coronary artery calcium scoring and atherosclerotic cardiovascular disease risk. JACC Cardiovasc Imaging. 2022;15(9):1648–62. 10.1016/j.jcmg.2022.02.026. [DOI] [PMC free article] [PubMed]
- 36.van Velzen SGM, Dobrolinska MM, Knaapen P, van Herten RLM, Jukema R, Danad I, Slart RHJA, Greuter MJW, Išgum I. Automated cardiovascular risk categorization through AI-driven coronary calcium quantification in cardiac PET acquired attenuation correction CT. J Nucl Cardiol. 2022 doi: 10.1007/s12350-022-03047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aditya CR, Sattaru NC, Gopal K, Rahul R, Chandra Shekara G, Nasif O, Alharbi SA, Raghavan SS, Jayadhas SA. Machine Learning approach for cardiovascular risk and coronary artery calcification score. Biomed Res Int. 2022;2022:2632770. doi: 10.1155/2022/2632770. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Jaminon AMG, Rapp N, Akbulut AC, Dzhanaev R, Reutelingsperger CP, Jahnen-Dechent W, Schurgers LJ. A semi-automated and reproducible biological-based method to quantify calcium deposition in vitro. J Vis Exp. 2022 doi: 10.3791/64029. [DOI] [PubMed] [Google Scholar]
- 39.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B, ESC National Cardiac Societies; ESC Scientific Document Group 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 40.Roseman DA, Hwang SJ, Manders ES, O'Donnell CJ, Upadhyay A, Hoffmann U, Fox CS. Renal artery calcium, cardiovascular risk factors, and indexes of renal function. Am J Cardiol. 2014;113(1):156–161. doi: 10.1016/j.amjcard.2013.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adler Y, Shemesh J, Tenenbaum A, Hovav B, Fisman EZ, Motro M. Aortic valve calcium on spiral computed tomography (dual slice mode) is associated with advanced coronary calcium in hypertensive patients. Coron Artery Dis. 2002;13(4):209–213. doi: 10.1097/00019501-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Bursztyn M, Motro M, Grossman E, Shemesh J. Accelerated coronary artery calcification in mildly reduced renal function of high-risk hypertensives: a 3-year prospective observation. J Hypertens. 2003;21(10):1953–1959. doi: 10.1097/00004872-200310000-00024. [DOI] [PubMed] [Google Scholar]
- 43.Shemesh J, Morag-Koren N, Goldbourt U, Grossman E, Tenenbaum A, Fisman EZ, Apter S, Itzchak Y, Motro M. Coronary calcium by spiral computed tomography predicts cardiovascular events in high-risk hypertensive patients. J Hypertens. 2004;22(3):605–610. doi: 10.1097/00004872-200403000-00024. [DOI] [PubMed] [Google Scholar]
- 44.Shemesh J, Motro M, Grossman C, Morag-Koren N, Apter S, Grossman E. Progression of coronary artery calcification is associated with long-term cardiovascular events in hypertensive adults. J Hypertens. 2013;31(9):1886–1892. doi: 10.1097/HJH.0b013e328362b9f8. [DOI] [PubMed] [Google Scholar]
- 45.Shemesh J, Motro M, Morag-Koren N, Tenenbaum A, Apter S, Weiss A, Grossman E. Coronary artery calcification predicts long-term mortality in hypertensive adults. Am J Hypertens. 2011;24(6):681–686. doi: 10.1038/ajh.2011.28. [DOI] [PubMed] [Google Scholar]
- 46.Shemesh J, Motro M, Morag-Koren N, Konen E, Grossman E. Relation of coronary artery calcium to cardiovascular risk in patients with combined diabetes mellitus and systemic hypertension. Am J Cardiol. 2012;109(6):844–850. doi: 10.1016/j.amjcard.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 47.Erbel R, Lehmann N, Möhlenkamp S, Churzidse S, Bauer M, Kälsch H, Schmermund A, Moebus S, Stang A, Roggenbuck U, Bröcker-Preuss M, Dragano N, Weimar C, Siegrist J, Jöckel KH, Heinz Nixdorf Recall Study Investigators Subclinical coronary atherosclerosis predicts cardiovascular risk in different stages of hypertension: result of the Heinz Nixdorf Recall Study. Hypertension. 2012;59(1):44–53. doi: 10.1161/HYPERTENSIONAHA.111.180489. [DOI] [PubMed] [Google Scholar]
- 48.Motro M, Shemesh J. Calcium channel blocker nifedipine slows down progression of coronary calcification in hypertensive patients compared with diuretics. Hypertension. 2001;37(6):1410–1413. doi: 10.1161/01.hyp.37.6.1410. [DOI] [PubMed] [Google Scholar]
- 49.Motro M, Kirwan BA, de Brouwer S, Poole-Wilson PA, Shemesh J, ACTION CC side-arm study Tracking coronary calcification and atherosclerotic lesions in patients with stable angina pectoris undergoing nifedipine therapy. Cardiology. 2007;107(3):165–171. doi: 10.1159/000095308. [DOI] [PubMed] [Google Scholar]
- 50.Greenland P, Lloyd-Jones DM. Role of coronary artery calcium testing for risk assessment in primary prevention of atherosclerotic cardiovascular disease: a review. JAMA Cardiol. 2022;7(2):219–224. doi: 10.1001/jamacardio.2021.3948. [DOI] [PubMed] [Google Scholar]
- 51.Dzaye O, Dudum R, Reiter-Brennan C, Kianoush S, Tota-Maharaj R, Cainzos-Achirica M, Blaha MJ. Coronary artery calcium scoring for individualized cardiovascular risk estimation in important patient subpopulations after the 2019 AHA/ACC primary prevention guidelines. Prog Cardiovasc Dis. 2019;62(5):423–430. doi: 10.1016/j.pcad.2019.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Being a Review there is no data availability. The manuscript has no original data.