Abstract
Monkeypox virus (MPXV) is a double-stranded DNA virus belonging to the Poxviridae family of the genus Orthopoxvirus with two different clades known as West African and Congo Basin. Monkeypox (MPX) is a zoonosis that arises from the MPXV and causes a smallpox-like disease. The endemic disease status of MPX was updated to an outbreak worldwide in 2022. Thus, the condition was declared a global health emergency independent of travel issues, accounting for the primary reason for its prevalence outside Africa. In addition to identified transmission mediators through animal-to-human and human-to-human, especially sexual transmission among men who have sex with men came to prominence in the 2022 global outbreak. Although the severity and prevalence of the disease differ depending on age and gender, some symptoms are commonly observed. Clinical signs such as fever, muscle and headache pain, swollen lymph nodes, and skin rashes in defined body regions are standard and an indicator for the first step of diagnosis. By following the clinical signs, laboratory diagnostic tests like conventional polymerase chain reaction (PCR) or real-time PCR (RT-PCR) are the most common and accurate diagnostic methods. Antiviral drugs such as tecovirimat, cidofovir, and brincidofovir are used for symptomatic treatment. There is no MPXV-specific vaccine; however, currently available vaccines against smallpox enhance the immunization rate. This comprehensive review covers the MPX disease history and the current state of knowledge by assessing broad topics and views related to disease origin, transmission, epidemiology, severity, genome organization and evolution, diagnosis, treatment, and prevention.
Keywords: Monkeypox virus, Mpox, Genome, Transmission, Diagnosis, Susceptibility
Introduction
Monkeypox (MPX) is a DNA virus that belongs to the Poxviridae family of the genus Orthopoxvirus based on the last update of the International Committee on Taxonomy of Viruses (ICTV) in August 2022 [1] and causes smallpox-like disease. MPX is a zoonotic disease with an unidentified primary host and is pathogenic in animals and humans [2], [3]. It was first discovered in 1958 in a monkey during vaccine research and has been reported in several reservoirs, especially in rodents and other small mammal species [4], [5]. However, the first human case—a pediatric patient from Congo—was not reported until 1970, and the disease was named MPX [6], [7]. The first reported cases were all endemic registered in African countries, but in 1996 –1997, human-to-human transmission became severe [2], [8]. Long-term close contact, respiratory droplets, contaminated personal items, and direct contact with rash regions are some of the mediators for transmission [9].
Approximately thirty years later, the first MPX case outside Africa—due to virus exposure via zoonotic transmission from an infected animal—was reported in the U.S. The main factors in the disease outbreak were travel from African countries and animal importation. From this point on, MPX cases were reported occasionally worldwide. However, in 2022, the MPX outbreak became international and was thus declared a global health emergency independent of travel issues [10] . On November 28, 2022, the World Health Organization (WHO) suggested using the word "mpox" as a replacement for "monkeypox". Both terms will be used for one year, and "monkeypox" is being phased out [8].
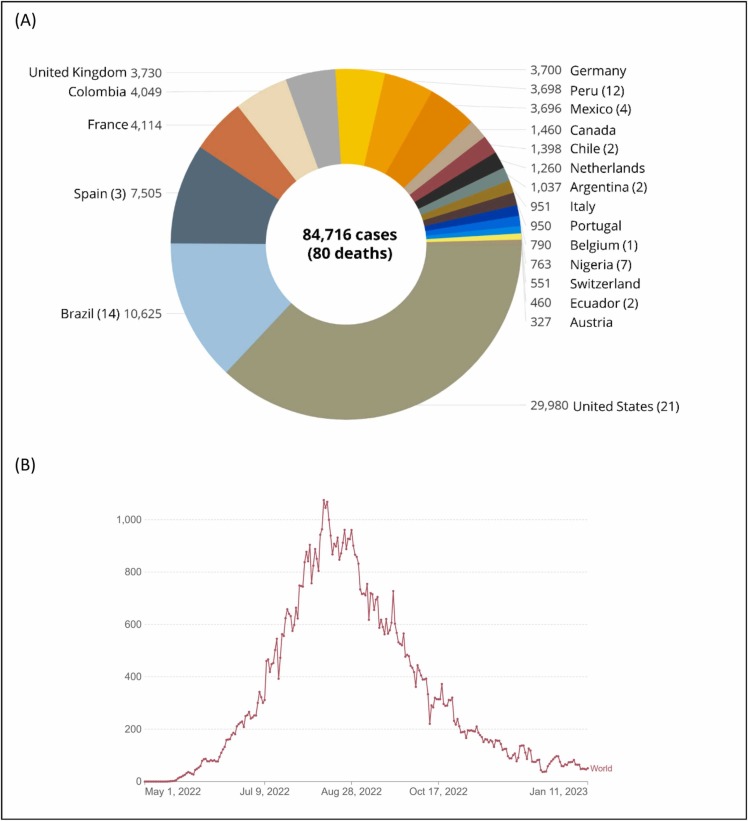
According to the ‘2022 Mpox Outbreak’ presented by the WHO on January 16, 2023, 110 countries, areas, or territories reported MPX cases. Among them, 103 said it for the first time [8], [11]. The total number of confirmed cases reached 84,716 as of January 16, 2023; the U.S. has the highest number, with 29,980 cases. So far, 80 deaths have been reported globally; the top countries are the U.S. (21), Brazil (14), Peru (12), Nigeria (7), Mexico (4), Ghana (4), Spain (3), and Cameroon (3) ( Fig. 1a). According to recent updates by the WHO, the worldwide risk is moderate. Regarding geographical distribution, the risk is high in the Americas and moderate in Africa, the Eastern Mediterranean, Europe, and South-East Asia. The western Pacific region is considered low-risk [8]. Global cases since the outbreak`s beginning reached their highest value in August 2022; then, they gradually decreased and became stable for now (Fig. 1b). However, the virus's potential for mutation or evolution and the relaxation of safety measures could result in more waves of the outbreak.
Fig. 1.
The number of confirmed MPX or mpox cases globally. (a) The top 20 countries have the highest cases as of 16 January 2023. The death tolls are indicated in parenthesis (the World Health Organization, 2022 Mpox Outbreak [8]) (b) Weekly distribution of the global cases from the beginning of the outbreak (OurWorldInData [12]). Note: For the WHO European region, confirmed and probable cases are included within confirmed case counts.
Previously, the MPX was reported to be like a smallpox infection with less fatality. However, over time, the MPX virus became more pathogenic and caused an outbreak with lots of unanswered questions. MPX was previously considered a common fact for African countries, and now it has become more attractive for authorities, organizations, and researchers globally. In this review, MPX (mpox) disease history and the current state of knowledge by assessing broad topics and views related to disease origin and transmission, diagnosis, and treatment, as well as viral genome organization, were comprehensively elucidated.
The origin and classification of MPXV
The family Poxviridae consists of 22 genera and 83 species of two subfamilies: Chordopoxvirinae (18 genera and 52 species) and Entomopoxvarinae (4 genera and 31 species) [1]. The genus Orthopoxvirus affects humans and animals, with 12 identified members. The most well-known member is the variola virus, which causes smallpox; others are MPXV, vaccinia virus (smallpox vaccine virus), Abatino macacapox virus, Akhmeta virus, Camelpox virus, Cowpox virus, Ectromelia virus, Raccoonpox virus, Skunkpox virus, Taterapox virus, and Volepox virus [1]. Two viral clades, the West African and Central African (Congo Basin) clades, have been identified [13]. The central African viruses are more virulent than the West African [14], [15]. During the 2003 U.S. outbreak, the Central African clade of human MPX disease was associated with higher morbidity, death, human-to-human transmission, and viremia [16]. The central African clade is reported to be more severe and shows a higher fatality rate (10%) than the West African clade (4%) [8], [17]. The differences in virulence stem from variabilities in genome organization caused by deleted gene regions and gene fragmentation in open reading frames [9]. Thus, sample collection from the different areas, individuals, and clades is vital for determining the genetic properties of the MPXV and confirming the cases and research facilities [18].
Transmission of MPXV
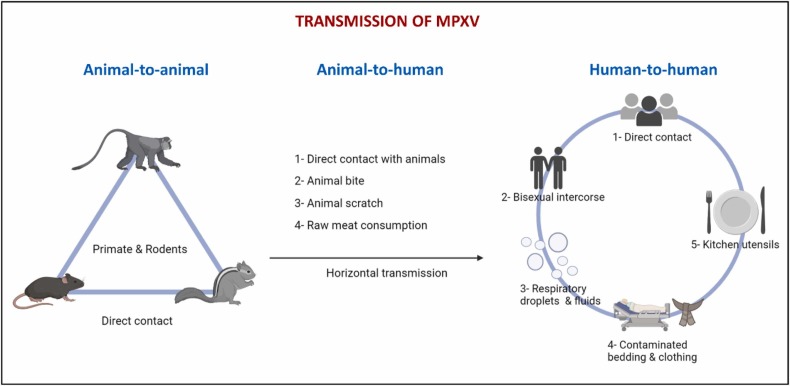
MPX has been primarily observed in West and Central Africa. MPXV transmission can occur in several modes, such as animal-to-animal, animal-to-human, or human-to-human ( Fig. 2). The most common animal-to-human transmission is direct contact with an infected animal or bodily fluids [9]. Human infections have been associated with animal contact. However, it is challenging to determine how exactly a human case was exposed in locations where animal contact is frequent due to rodent infestations in homes and the hunting or preparing of bushmeat from various species [16]. Human-to-human transmission has been found in Nigeria and West Africa [19]. Living in the same home or using the same dishes that an infected person has used are examples of contaminated objects or surfaces that are thought to increase the risk of viral transmission among members of the same household.
Fig. 2.
The transmission of the MPXV is animal-to-animal, animal-to-human, and human-to-human.
The first case of human MPX in the U.S. was discovered in 2003. Although the outbreak was linked to contact with diseased prairie dogs, there was still a risk of transmission from person to person [20]. In the U.K., the MPXV was transmitted from a patient to a health employee related to contaminated bedding in September 2018 [21]. Transmission can occur through close contact, fomites, or exposure to large respiratory droplets during direct contact with the skin lesions of an infected person [16]. Vivancos et al. [22] reported an ongoing outbreak of MPXV infections in the U.K. since May 2022. It was indicated that all previous cases reported were either imported or household or healthcare contacts of imported cases [21]. Besides, the MPX cases detected in Israel and Singapore were related to traveling to Nigeria [23]. Given the initial lack of epidemiological connections to locations in West and Central Africa, the surprising presence of MPX in multiple regions raises the possibility of long-term undiscovered transmission [8].
In addition, the CDC revised new outbreaks for possible transmissions, such as hugging, kissing, oral, anal, and vaginal sexual intercourse, possibly related to genetic changes that ease the human-to-human transmission of the MPXV [17]. On the other hand, most of the diagnosed cases in the 2022 outbreak in Europe originated from the West African clade, indicating a limit in inter-human transmission [24], [25].
Epidemiology of MPXV
Between 1980 and 1985, 282 cases were reported in Zaire at the time of initial identification in humans. Their ages ranged from 1 month to 69 years, and 90% were under 15. No mortality was reported in vaccinated patients, while the average fatality in unvaccinated cases was 11%, with higher rates in kids (15%) [26] ( Table 1).
Table 1.
Distribution of the gender and age factors of MPX.
| Gender/Age (year) | Number/Ratio of cases | Year | Country | Reference |
| Gender: Male | 2.5 male:1 female 99% About 7500 cases, male is 99% of cases. 579/582 (99.5%) confirmed cases are male. |
2017 2022 2022 |
Nigeria U.S. U.S. Ontario, Canada |
[90], [92], [93], [94] |
| Gender: Female | Rare 3/582 (0.5%) are female. |
2022 2022 |
U.S. Ontario, Canada |
[90], [94] |
| Age: Kids younger than 15 | Rare | 2022 | U.S. | [90] |
| Age: 21–40 | Male is 2.5 times than female. | 2017 | Nigeria | [93] |
| Age: 26–40 Age: < 20 – 74 years) with an average is 36.4 years |
Make up the highest proportion of cases. 582 |
2022 2022 |
U.S. Ontario, Canada |
[90], [94] |
| Age: younger than 40–50 | Recently this age became more susceptible to monkeypox because of smallpox’s cessation vaccination in the world after the disease’s elimination | 2022 | [91] | |
| Age: older than 60 | Rare | 2022 | U.S. | [90] |
| Age & Gender are not reported | 3–5 | 2022 | Saudi Arabia | [91], [95] |
Immunity against the MPXV was previously obtained by vaccinia, but the eradication of smallpox and subsequent decrease in the vaccination campaign blocked the gaining of immunity against MPX. The shortage of reports from rural Africa contributed to the undervaluation of this infectious virus’ threatening potential [27]. After a non-reported period of 39 years in Nigeria and Bayelsa state, MPXV re-emerged in 2017, and it was estimated that travellers exported it to Israel and other countries. Then, the issue was raised in 2018 and 2019. Many factors contributed to the MPXV outbreak outside Africa, such as importation, shipping, travelling, contact with infected monkeys, or suspected or associated populations with a risk of acquiring MPXV. This may be attributed to the cessation of the smallpox vaccine, as explained above, that revealed cross-immunity against MPXV and probably resulted in arising of human-to-human transmission.
The outbreaks of MPXV outside Africa demonstrate the global connection of the infection. Therefore, programs to promote surveillance and diagnosis of MPXV are crucial for knowing the fluctuating epidemiology of this infectious disease [28]. MPXV did not stop in Africa, but it reached developed countries. Two MPX cases have been reported in the U.S. in people who came back from Nigeria to Texas in July 2021. A case of a British man was reported on May 6, 2022, following his visit to Nigeria. As of June 2022, about 1500 cases have been reported in more than 43 countries, including North America and Europe. The MPXV is common in central and western Africa, but it has also been reported in the developed world that signs of MPX are spreading worldwide. All infected cases were attributed to travel to Africa or via animal shipping. The U.S. is the new outbreak center, with 29,980 cases and 80 fatalities. This represents a global threat, which insists on designing a strategic plan to prevent MPXV from becoming a pandemic disease [29].
MPXV, gender, and hormones-as immunomodulatory action
One of the critical sex differences in infectious diseases is variations in an endocrine-immune relationship, particularly in males, who are more susceptible to contagion than females because of the gonad’s steroid hormones. Male androgens and female estrogens modify the host`s immune system. In addition, gonad steroids change genes and behaviors, which affect infection and its impedance. Therefore, males are more susceptible to contagion than females; this is not only because of the androgenic suppressing effect on immunity but also because of sex steroid hormones that influence infection-resistance genes. This concept may act significantly in managing infectious diseases and therapy [30]. Males generally have a ≥ 50% infection in most African MPX outbreaks. In addition, outside of Africa, it happens more frequently in males and mainly in the adult population. In the 2022 outbreak, men accounted for most cases (98%). Perfect surveillance of MPX cases is the critical factor for knowing the dynamic epidemiological fluctuations of this infectious disease [21], [23], [28], [31], [32], [33], [34].
MPXV has an ortholog to COP-A44L encoding a part of 346 aa. The COP-A44L encodes a 3-β-hydroxysteroid dehydrogenase required to form steroid hormones -either sex hormones or glucocorticoids- to convert pregnenolone to progesterone as dehydroepiandrosterone to androstenedione. It is well known that glucocorticoids have immuno-inhibitory and anti-inflammatory effects, which affect the immune system against the virus. However, A44L is not required for viral replication. Therefore, A44L affects virulence by promoting steroid release and suppresses the immune system’s response [35], [36], [37].
Genome organization and viral entry mechanism of the MPXV
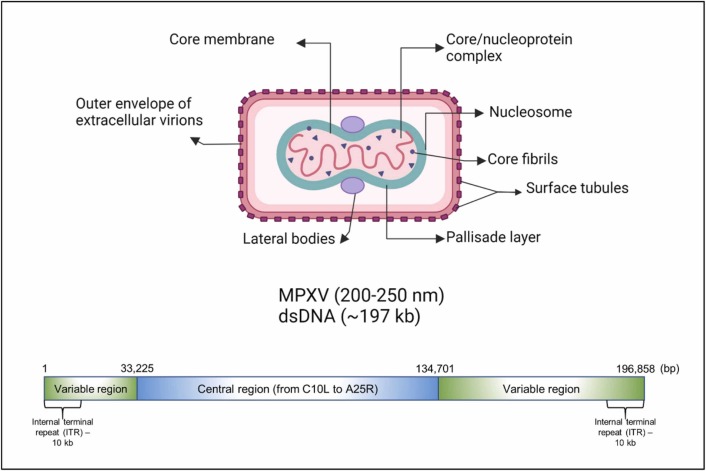
The double-stranded DNA (dsDNA) genome of the MPXV is about 197.2 kb in size and encodes 181 proteins. The linear genome has covalently closed hairpin ends (no free 3′ or 5′ ends). The 10 kb inverted terminal repeats (ITR) locate at both ends of the genome ( Fig. 3). Genes are tightly packed; intergenic areas longer than 100 bp are uncommon. The "housekeeping" genes involved in transcription, replication, and virion assembly are encoded in the conserved central area. The genes encoded in the terminal domains vary between different poxviruses and produce proteins implicated in disease and host range. The first complete MPXV genome of the current MPXV outbreak (isolate name MPXV_U.S._2022_MA001) is released in the GenBank database with an accession ID of ON563414, as dated May 30, 2022 [38]<https://www.ncbi.nlm.nih.gov/nuccore/ON563414 > . The Oxford nanopore technology revealed that the genome size of MPXV is 197,205 bp as linear dsDNA.
Fig. 3.
The structure of the MPXV and its genome.
The average size of the MPXV ranges between 200 and 250 nm. The MPXV replicates in the cytoplasm of the infected host cell and has a core area with lateral bodies, double-stranded deoxyribonucleic acid (dsDNA), and a lipoprotein envelope (Fig. 3). Micropinocytosis, viral endocytosis, and cell membrane fusion facilitate viral entry via the nasopharyngeal, oropharyngeal, subcutaneous, intradermal, and intramuscular pathways. Inflammatory immune-mediated phagocytosis is triggered by MPXV replication at the inoculation, which causes MPXV to spread to the blood, lymph nodes, tonsils, bone marrow, spleen, and other organs. The MPXV genome and proteins are released into host cells under the control of MPXV mature virions (MV) and enveloped virions (EV). Following MPXV mRNA transcription and translation, intracellular mature virions (IMV) with viral DNA encoding the virus are produced. IMVs wrapped in Golgi apparatus-derived membranes create intracellular enveloped virions (IEVs), which fusion with the host inner cell membrane to make cell-associated virions (CEVs) before being released into extracellular areas to form extracellular enveloped virions (EEV) [39].
Evolution of the MPXV genome
Poxviruses are thought to mutate at a rate of between 10−5 and 10−6 mutations per replication site. Sequence analyses of the 2022 outbreak of MPXV in the U.S. revealed many mutations compared to previous MPXV sequences. Those mutations mostly appear as 5′ GA-to-AA within the Apolipoprotein B mRNA Editing Catalytic Polypeptide-like3 (APOBEC3) motif, which indicates APOBEC3 cytosine deaminase activity [38], [40]. However, G-to-A mutations were enriched in the more recently sampled West African MPXV sequences between 2017 and 2022 rather than in West African MPXV clades before 2017 or Congo Basin MPXV clades [38]. The role of APOBEC3 proteins, which primarily work on single-stranded DNA, has been thoroughly investigated in RNA viruses, including HIV [41]. Its role in DNA viruses is also shown [42], [43], [44]. Through cytosine-to-uracil deaminase activity, APOBEC3 proteins are a crucial part of the vertebrate innate immune system that blocks the replication of foreign viruses [41], [45]. However, the activity of APOBEC3 protein in different MPXV lineages should be determined to verify the effect of G-to-A mutations [38].
Jones et al. [46] recently analyzed 47 MPXV genome sequences from Berlin, Germany, sampled between May 20 and July 4, 2022. Compared to the previous MPXV outbreak, non-synonymous amino acid changes were observed. Interestingly, an initial 5′ gene was duplicated in sequences from two lesions of the same patient. Four genes near the 3′ genome end were disrupted or entirely deleted because of an 856-nucleotide translocation between genome termini. Such genome rearrangements in orthopoxviruses are known to confer fitness advantages and thus may provide virus adaptation in the unprecedented human-to-human transmission of the current MPX outbreak [46].
The genome's plasticity of orthopoxviruses is most visible in two inverted terminal repeats (ITR) regions at the ends of the genome, where immunomodulatory host range factors that also affect virulence are primarily present [47], [48]. Modifying these areas is thought to be the primary mechanism underlying the quick adaptability of the orthopoxviruses after changing hosts [49], [50]. The Western and Central African MPXV clade sequences differ significantly in deletions and insertions in the ITR regions, which was also observed in sequences from the 2003 U.S. outbreak [46], [51]. The mutation rate, adaptability, and genetic evolution of MPXV are among the factors that increase transmissibility, virulence, and immune evasion. This could lead to an increase in MPX cases worldwide in the near or long term.
Clinical symptoms
It has been reported that cases between 1970 and 2019 are between four and 21, and males are more vulnerable than women. On top of it, most cases after the 2022 outbreak are also men (98%) who had sexual intercourse with other men—gay or bisexual men—and are in their thirties. 96% of the cases have rashes, while 69% also show flu-like symptoms [17]. Respiratory distress/bronchopneumonia, sepsis, gastrointestinal/mouth, and throat ulcers, fever, superinfection skin, inflammation/lymphadenopathy, corneal infection, and skin scarring/cellulitis/skin lesions are amongst the most common symptoms and complications [9]. Clinical signs that last two to four weeks can appear suddenly and proceed mildly [52]. Nevertheless, the severity and progression of the MPX disease differ between individuals.
Following the entrance of the MPX virus into the cell throughout respiratory mucosa, examining the clinical signs of MPX takes approximately 7–21 days of the incubation period. Non-specific and common symptoms were reported in all MPX cases: fever, headache, myalgia, backache, lymphadenopathy, chills, exhaustion, and rashes. Following infection, some probable complications were estimated as bacterial superinfection in lesion regions, corneal infection, sepsis, dehydration, bronchopneumonia, and respiratory distress [9].
During the 2003 outbreak, a bedside volunteer care team described a hospitalized child in the U.S. who had contact with an infected prairie dog. She has admitted to the hospital with severe symptoms such as fever, headaches, muscle aches, general fatigue, chills, swollen lymph nodes, dysphagia, accompanying vomiting, breathing, and eating problems that worsened within five days: as the progression of flat red macules over the trunk, extremities, palms and soles with the following coverage of remaining part of the body including the mouth, face and oral pharynx. A small number of lesions in the external genital region with protected vulval mucosa were observed, which resulted in difficulty in the usual acts of the human body. On the fourth day of hospitalization, patients’ swallow cervical lymphadenopathy decreased, which resulted in comfort in eating and drinking habits. Afterward, no new lesions were observed, and the previous ones started to crust. Eventually, symptoms almost faded from the seventh day of hospitalization, and the patient was discharged [53]. The same year, a six-year-old child with severe encephalitis accompanying common symptoms was admitted to the hospital [54].
In another reported case, a four-year-old boy from the Democratic Republic of Congo was admitted to a hospital in December 2016. The child was admitted to the hospital on the third day of symptomatic illness for fever, rhinitis, conjunctivitis, cough, one-sided cervical lymphadenitis, and vesiculopapular rashes in his whole trunk area face. These symptoms were regarded as measles or chickenpox; however, due to a lack of diagnostic tools, no test could be run on the patient. Supportive treatments started immediately but did not prevent the symptoms` progression. On admission day 12, the child passed away, and a blood test for measles also came negative [55].
In the 2017–2018 Nigeria outbreak, 122 confirmed MPX cases aged between 2 days and 50 years were analyzed. All patients had vesiculopustular rashes on their whole body, especially intensely distributed in their face, and with other common symptoms such as fever, pruritus, headache, and lymphadenopathy [56].
A current report from London HCID Center during the 2022 outbreak displayed significant differences between current and previous outbreaks. In previous outbreaks, most of the community was vaccinated against smallpox, and a higher proportion of cases were children. Recent MPX outbreaks of West African and Basin clades have influenced children and adults, specifically males. In the 2022 London outbreak, 197 reported cases were all male − 196 being either gay, bisexual, or having sexual intercourse with another man-. All cases showed mucocutaneous lesions, especially in genital regions and perianal areas. Non-specific symptoms reported previously were also reported in the latest outbreak. On the other side, rectal pain and penile edema (swelling) were newly and commonly reported in this outbreak. While the distribution of lesions in previous cases focused on the trunk, face, leg, head, and arms, in the current outbreaks, lesions were intensively localized in the genitals and perianal regions [52].
Although no study refers to sexual transmission of MPX, the 2022 outbreak describes new symptoms and community in that route. Thus, clinicians and researchers should consider all MPX symptoms as if they are associated with each other, even if they seem irrelevant, especially in diagnosing, treating, and future prevention strategies.
Therapeutic options and prevention
Treatment of the disease is not based on MPXV-specific medications; generally, supportive symptomatic treatments and drugs that act as an inhibitor for viral DNA synthesis are used [9], [24]. Almost all patients with mild symptoms get well without medical treatment. Supportive treatments for reducing pain, preventing dehydration, etc., are sufficient for patient care and comfort, according to the CDC guide`s recommendations for controlling MPX outbreaks. Typically, vaccination against MPX with either smallpox vaccines, antivirals, or vaccinia immune globulin (VIG) is considered effective [57].
Vaccination against smallpox is known to effectively cross-protect humans from MPXV and other poxvirus members. However, the number of people vaccinated lowered after 1980, when the WHO announced that the smallpox virus had been eradicated. Early in 2021, authorities certified the production of smallpox vaccines due to concerns about biological weapon attacks and the MPX outbreak. Currently, two vaccines are approved in the U.S., JYNNEOS (live-attenuated, non-replicating vaccine)—known as Imvamune/Imvanex in Europe— and ACAM2000 (live-attenuated, replicating vaccine). The protection of these vaccines against the MPXV was examined in well-defined animal models, cynomolgus macaques, which have been previously used in smallpox vaccine research. The infection course of the aerosolized MPXV in macaques is similar to that of natural smallpox infection in humans. Animals immunized with two doses of the JYNNEOS vaccine and a single dose of the ACAM2000 vaccine were fully protected against the severe and lethal effects of the disease. In contrast, immunization with a single dose of JYNNEOS did not abolish disease severity and fatal impact [58]. JYNNEOS vaccine was licensed in Europe in 2013, is produced in Denmark for adults over 18 susceptible to disease and was approved by the FDA (U.S. Food and Drug Administration) in 2019 [59], [60], [61]. JYNNEOS is as protective against smallpox as MPX, but vaccination after observing MPX symptoms is not recommended by the CDC [62]. The second smallpox vaccine, ACAM2000, has been FDA-approved since 2007, but it allows the virus to replicate inside cells. Thus, it causes severe side effects [63]. Only individuals at risk, laboratory researchers, and military personnel in Europe and the U.S. can be vaccinated. Although non-replicating viral-based vaccines are suitable, they require multiple times of immunization in higher doses, thus as an alternative and safer method with a higher protection rate, gene and protein subunit vaccines target key protective elements of Orthopoxvirus species seems a good choice [64].
As part of the investigational new drug (IND) protocol, the Aventis Pasteur Smallpox Vaccine (APSV) is available against MPX. APSV is a replication-competent vaccinia vaccine and is considered non-significant against MPX. Yet it is suitable under IND or Emergency Use Authorization (EUA) in case of unavailable licensed vaccines [57]. However, the WHO and the FDA are planning future vaccination programs, vaccine supply, suitable doses, and vaccination intervals; this data will also help detect vaccine effectiveness and all other related topics [65].
Although antiviral efficacy against MPX is unknown, they were approved on animals for smallpox and have been used in humans. Antivirals are only used in severe cases, such as immunocompromised patients, pediatrics, pregnant and breastfeeding women, and patients suffering from lesions near the mouth, eyes, and genitals. Tecovirimat (TPOXX or ST-246), brincidofovir, and cidofovir are approved antivirals against smallpox [57]. The therapeutic action of anti-Orthopoxvirus compound ST-246 (viral envelope VP37 inhibitor) was assessed on animal models, prairie dogs as transmission agents. ST-246 was applied to intranasally infected dogs for 14 days, starting from intranasal infection days zero to three and after post-rash development. Dogs treated before symptom onset were all asymptomatic, while the post-rash group was ill but recovered [66]. In another study, applied to non-human primate models infected with smallpox (variola) and MPXV, ST-246 was established to be safe, effective, and preventive in pre- and post-exposures to the viral agent. ST-246 is protective in disease severity and death and could be used for prophylactic or therapeutic functions [67]. These studies showed that human therapeutic doses of ST-246 are effective in different stages of the illness [68]. Brincidofovir and cidofovir work as viral DNA polymerase inhibitors and are analogs of one another. Due to possible toxicity to several internal organs, their usage protocol is also under EUA or IND. While MPX efficacy of cidofovir in animal tests has been established, brincidofovir activity has only been shown for infections caused by the genus Orthopoxvirus [57].
VIG (vaccinia immunoglobulin) is hyperimmune globulin approved by the FDA for reducing the adverse effects of live-vaccinia virus vaccination (e.g., ACAM2000) [57]. VIG’s efficacy against smallpox and MPX hasn’t been proven. Thus, it should be used under the IND protocol. The hyperimmune plasma collected from individuals vaccinated with live-vaccinia virus contains protective antibodies. Also, non-essential vaccinia virus antibodies with unknown functions in protective immunity are found in plasma [64]. Preparation of well-known monoclonal and polyclonal antibody mixtures that specifically recognize vaccinia virus epitopes may be considered safer alternatives.
Susceptibility to MPX disease
The world faces an outbreak with lots of unknowns. Factors that indicate population immunity to the genus Orthopoxvirus need to be analyzed and reported to prevent diseases involving MPX, smallpox, and the vaccinia virus. Age, sex, medical history, ethnicity, vaccination state, and possible exposure to orthopoxvirus infections indicate MPX susceptibility.
The cross-protective potential of available vaccines against the Orthopoxvirus genus members is known. Since the eradication of smallpox in the 1980 s, both the vaccination status of the population and studies have slowly dropped. In 2001 a local report from Santé Publique France (the French Institute of Public Health) indicated that the smallpox vaccination rate of people born after 1979 was almost 0%, while it was 90% in those born before 1966 [69]. Seroprevalence investigation of different populations from Europe, Africa, and South America shows that all groups are vulnerable to orthopoxvirus infection; unlike uncontrolled ordinary spreading of orthopoxvirus in African countries, public immunity is also low [59], [69]. In addition, the co-infection of an HIV-positive immunosuppressed individual with MPX causes severe symptoms and a higher mortality rate [55].
Age and vaccination status are considered susceptibility determinants. In the 2003 outbreak, an MPX history of three family members was assessed. A woman aged 30, her 33 years old husband, and her six-year-old daughter had several MPX symptoms. The man who previously received the smallpox vaccine showed mild symptoms with rashes, while the woman who was not vaccinated against MPX showed similar signs. Their child, who received all childhood vaccines except the varicella vaccine, had more severe symptoms and was hospitalized for severe encephalitis [70]. Thus, probable protective effects of vaccination against Orthopoxvirus species and age-related immunity should be considered when considering susceptibility.
In the 2022 outbreak, males, especially those having intercourse with other men, were more susceptible to transmission and showed severe symptoms [52]. It has to be noted that lack of knowledge about how sexual transmission occurs in those people may also be the reason for the worsened outcome and thus makes these people susceptible to spreading the disease.
Connection with possible animal reservoirs and suitable vectors of human transmission make individuals susceptible to MPX [71]. Nevertheless, other potential factors of susceptibility need investigation. In addition, scientific approaches and techniques should be utilized to clarify the relationship between population genetics, viral genomics, public immunity, and disease susceptibility.
Breaking down social distances and physical barriers via social events (i.e., religious meetings and sports events) or natural and man-made disasters (i.e., natural disasters and wars) expose people to infectious agents. Meanwhile, the world refugee crisis also increases spreading rate of infectious agents. At the end of 2021, 89.3 million people fled from their own country, started a dangerous journey, and sought safety in other places [72], which also caused an increase in the spreading rate of viruses such as MPXV [73]. The desperate journey is, by itself, unhealthy and unprotective; men hiding sexual orientation, men having sex with men (MSM), and avoiding safe sex practices at the beginning make this group more vulnerable to diseases. Even after entering a safe country, refugees confront economic problems, and individuals of this vulnerable group may have sex to continue their life [74]. All in all, authorities should promulgate refugee regulations to prevent MPXV spread by improving food aid, health, sanitation, security, camping and movement route, and housing conditions.
Recently, a primary concern in FIFA World Cup 2022 was the risk of increasing the spreading rate of COVID-19 and MPX. Managing crowds is hard on its own; meanwhile, dealing with such a big hosting for the first time and the climate increases the spreading rate of infectious diseases. Dealing with all of this needs professionalism. Otherwise, both Qatar locals and people from around the globe would have been at serious risk for both MPX and other zoonotic diseases [75]. The grouping strategy recommended by the WHO director [76] and using expertise from countries like Saudi Arabia that can manage and overcome huge crowds due to religious meetings every year could be helpful preventive activities.
Diagnostic assays
Diagnosing the MPXV by looking solely at clinical symptoms is challenging; thus, molecular assays and tests of patient specimens are helpful and strongly required for case confirmation. Diagnostic tests are crucial for determining the presence of an orthopoxvirus infection. MPX or orthopoxvirus can now be identified using various diagnostic techniques in clinical specimens from patients [77]. Collection and analysis of samples should be practiced by recommendations for standard precautions.
Viral culture, visualization via electron microscopy, immunohistochemistry, anti-orthopoxvirus IgG and IgM, and real-time PCR techniques are suitable methods for MPXV diagnosis [16]. It is possible to utilize PCR alone or in conjunction with sequencing [78]. Combining these tests with clinical and epidemiological data, such as a patient's immunization history, yields the best results [16]. The best and least intrusive specimens for laboratory confirmation of MPXV is skin lesion material, including swabs of lesion surface and/or exudate, roofs from more than one lesion, or lesion crusts [78]. Besides, the other type of specimens and collection procedures have been described by the CDC [79]. The following is a list of the diagnostic procedures that can be used to distinguish clinical specimens from MPXV or orthopoxvirus:
-
•
Viral culture/isolation, where a patient specimen is used to cultivate and describe a live virus. It can produce a pure, live virus culture allowing accurate species categorization. It takes many days to finish the assay. Well-trained technicians must carry it out to minimize bacterial contamination and recognize further characterization of the viral effect on cells [16].
-
•
Electron microscopy can be used to locate viral particles in viral cultures, scab material, vesicular fluid, or biopsy specimens where a brick-shaped particle is visible after negative staining, allowing for the visual identification of a poxvirus. The assay must be carried out in a significant laboratory with qualified personnel and an electron microscope [16].
-
•
Immunohistochemistry test enables the detection of orthopoxvirus-specific antigens and rules out or becomes aware of other suspect agents in biopsy specimens. The test is not specific to the MPXV and must be performed at a significant laboratory with skilled technicians [16].
-
•
Serology test (Anti-orthopoxvirus IgG, IgM) where immunofluorescences or neutralization assays can be used to measure antibodies against the orthopoxviruses [80]. However, WHO does not recommend the use of antibody testing alone in the diagnosis of MPXV, as vaccination may interfere with serologic testing [78].
-
•
Conventional PCR and real-time (RT)-PCR tests specifically targeted the MPXV’s DNA utilizing lesion material from active cases. It is a highly sensitive method where contamination worries are justified. However, these tests need pricey supplies and equipment and must be carried out by qualified technicians [16], [80], [81]. The RT-PCR protocol for West African-specific MPXV was described by Li et al. [82]. In addition, orthopoxvirus OPX3 real-time PCR assay can be applied to diagnose MPX DNA [38], [71]. Both assays produced similar Ct values in samples originating from USA_2022_FL001, USA_2022_VA001, or 44 USA_2021_TX samples. However, a point mutation (SNP) that is conserved in 2022 MPXV sequences in Europe and partly in the USA, but not in the West African MXPV sequences, decreased the sensitivity of OPX3 RT-PCR assay. The sequence analyses revealed that the SNP (DNA polymerase gene, VACV-Cop E9L, 322 C to T), which corresponds to the binding site of the reverse primer, causes an increase in Ct value ∼6.8 compared to West African specific assay [38]. Possible mutations should be closely screened and assessed for an update on the primer sequences. The updated RT-PCR primer and probe sequences has been released by the CDC [83].
-
•
Tetracore Orthopox BioThreat Alert is a point-of-care diagnostic tool that can quickly detect orthopoxvirus antigens in an active case utilizing lesion material from a patient. It can be done with little expertise at room temperature, but tests must be conducted in endemic areas as the MPXV cannot be detected with this technique, which is less accurate than PCR [16], [80], [81], [84], [85].
Traditional procedures, including virus isolation from clinical specimens, electron microscopy, and immunohistochemistry, are still valid methods. But they need highly developed technical expertise, specialized training, and a high-end laboratory, as mentioned above [16], [80], [81], [84], [85].
To determine the underlying cause of occurrences that are detected after the fact, antibody-based diagnostics are necessary. Orthopoxvirus cross-reactivity makes anti-orthopoxvirus immunological assays effective when the virus-causing sickness has already been identified [16], [80], [81], [84], [85], [86]. For retrospective individuals who have ever been exposed to an orthopoxvirus, including by vaccination, anti-orthopoxvirus immunoglobulin G (IgG) alone will not offer a conclusive diagnosis. Contrarily, serological methods that assess anti-orthopoxvirus immunoglobulin M (IgM) are better suited for detecting recent infections, particularly in those who have already undergone immunization [16], [80], [81], [84], [85], [86].
PCR analysis of specimens can be used to determine whether a lesion sample contains MPX or orthopoxvirus. These tests are susceptible and practical for the detection of viral DNA. But its application as a diagnostic tool in rural, resource-limited settings still needs to be improved [81], [84]. The point-of-care test is an ideal field deployable. However, there have been few advancements in this area. A recent Tetracore Orthopox BioThreat Alert trial using lesion samples from acute orthopoxvirus infections generated positive findings. This method successfully identified the vaccinia and monkeypox viruses in preparations with 107 plaque-forming units/mL, and 5 out of 6 assessed clinical specimens were accurately identified [85].
Out from these laboratory diagnostic tests, studies are ongoing to find out fast, easy, and accurate molecular methods for the diagnosis of MPXV. For instance, Chen et al. [87] developed a portable CRISPR–Cas-based system for naked-eye detection of MPXV. The system combines the high sensitivity advantage of CRISPR-Cas12 and the isothermal nucleic acid amplification techniques. The assay yielded a limit of detection (LOD) of 13.5 copies/µl and 15 copies/µl by using a microtiter plate reader and one-pot system, respectively. Also, RT-PCR assay was developed by Paniz-Mondolfi et al. [88], which targets F3L-gene of MPXV with a LOD of 7.2 genome copies/reaction. In a recent preprint, Singh et al. [89] described a method to distinguish MPXV from other related orthopoxviruses by targeting a single nucleotide polymorphism (SNP) on the polA gene. The diagnosis is based on the RPA amplification followed by CRISPR-Cas12a detection. In this assay, the LOD was 60 synthetic viral copies per reaction. Because of their high sensitivity, these techniques are promising for use in POC testing of MPXV, which help control the re-emergence and transmission of the MPX disease in populations.
Future perspective
Analysing current and future cases is fundamental to unravelling MPX. For instance, information on how region, animal population, and human interaction effects MPX is limited. On top of it, identifying potential reservoir(s), effects of season on disease incidence, travelling status, and age distribution at MPX infection are also open questions that must be answered. Authorized organizations, research institutes, and governments should agree on early diagnosis signs to reduce the transmission rate and provide medical support. More importantly, developing different approaches for vaccination programs. Thus, a broad range of investigations and further research is needed to prevent other possible public health threats and global concerns.
Conclusion
MPX is not new: African countries have faced MPX for a long time, with ordinary outbreaks in particular regions. Although its worldwide spreading potential is low, some regions have limitations in propagation control, prevention, and vaccination supply. However, it must be noted that there is always a possibility of an unexpected outbreak in a globalized world. MPX’s spreading rate is directly correlated with the pathogenicity-dependent genetic varieties, transmission mechanism, population’s lifestyle, increase in the viable host population, and changes in their habitats and vaccination profile of people. Moreover, other unknown variables also affect the spreading rate, which must be clarified further. Hence, outputs from health centers and scientific research are vital for investigating the efficacy of available vaccines on MPX. Developing a new generation of safe vaccines is essential for public and global health security. Border restrictions, developing rapid and accurate diagnostic tests, and routine screening of risk populations may prevent MPXV rotation. Determining signs and symptoms of the disease is also helpful for discriminating symbols from overlapped co-infection. Diagnostic data accumulated in easy-to-reach applications can be used for early detection and prevention of disease. Yet the best protection method is paying attention to personal hygiene, avoiding touching used objects, and avoiding animal waste. Novel drugs and vaccines lowered the rate of diseases and thus allowed social gatherings. However, we should be aware that these diseases are still in our lives, and our decisions will shape the future and show if we made the right choice against outbreaks.
Funding
Imam Abdulrahman bin Faisal University supported this project under project number 2020-IRMC-S-4.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.International Committee on Taxonomy of Viruses, https://ictv.global/taxonomy; 2022 [accessed 8 August 2022].
- 2.Alakunle E., Moens U., Nchinda G., Okeke M.I. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12:1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heymann D.L., Szczeniowski M., Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54:693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 4.Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 5.Magnus P.V., Andersen E.K., Petersen K.B., et al. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46:156–176. [Google Scholar]
- 6.Farahat R.A., Sah R., El-Sakka A.A., Benmelouka A.Y., Kundu M., Labieb F., et al. Human monkeypox disease (MPX) Infez Med. 2022;30(3):372–391. doi: 10.53854/liim-3003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breman J.G., Kalisa R., Steniowski M.V., Zanotto E., Gromyko A.I., Arita I. Human monkeypox, 1970–79. Bull World Health Organ. 1980;58:165–182. [PMC free article] [PubMed] [Google Scholar]
- 8.The World Health Organization (WHO). 2022 Mpox Outbreak: Global Trends. 2023. https://worldhealthorg.shinyapps.io/mpx_global/ [accessed 19 January 2023].
- 9.Kaler J., Hussain A., Flores G., Kheiri S., Desrosiers D. Monkeypox: a comprehensive review of transmission, pathogenesis, and manifestation. Cureus. 2022;14(7) doi: 10.7759/cureus.26531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farasani Abdullah. Monkeypox virus: future role in Human population. J Infect Public Health. 2022;15 doi: 10.1016/j.jiph.2022.10.002. 1270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel A., Bilinska J., Tam J.C.H., Fontoura D.D.S., Mason C.Y., Daunt A., et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378 doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathieu E., Spooner F., Dattani S., Ritchie H., Roser M. Mpox (monkeypox). OurWorldInData. 2023. https://ourworldindata.org/monkeypox [accessed 16 January 2023].
- 13.Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 14.Chen N., Li G., Liszewski M.K., Atkinson J.P., Jahrling P.B., Feng Z., et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo Basin. Virology. 2005;340:46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutson C.L., Abel J.A., Carroll D.S., Olson V.A., Braden Z.H., et al. Comparison of West African and Congo Basin Monkeypox Viruses in BALB/c and C57BL/6 Mice. PLOS ONE. 2010;5(1) doi: 10.1371/journal.pone.0008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58(2):260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 17.Sah R., Abdelaal A., Reda A., Katamesh B.E., Manirambona E., Abdelmonem H., et al. Monkeypox and its possible sexual transmission: where are we now with its evidence? Pathogens. 2022;11(8):924. doi: 10.3390/pathogens11080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornhill J.P., Barkati S., Walmsley S., Rockstroh J., Antinori A., Harrison L.B., et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. New Eng. J Med. 2022;387(8):679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 19.Sklenovská N., Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front Public Health. 2018;6:241. doi: 10.3389/fpubh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischauer A.T., Kile J.C., Davidson M., Fischer M., Karem K.L., Teclaw R., et al. Evaluation of human-to-human transmission of monkeypox from infected patients to health care workers. Clin Infect Dis. 2005;40(5):689–694. doi: 10.1086/427805. [DOI] [PubMed] [Google Scholar]
- 21.Vaughan A., Aarons E., Astbury J., et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26:782–785. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vivancos R., Anderson C., Blomquist P., Balasegaram S., Bell A., Bishop L., et al. Monkeypox incident management team. community transmission of monkeypox in the United Kingdom. April May 2022 Eur Surveill. 2022;27:2200422. doi: 10.2807/1560-7917.ES.2022.27.22.2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erez N., Achdout H., Milrot E., Schwartz Y., Wiener-Well Y., Paran N., et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25:980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alakunle E.F., Okeke M.I. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20(9):507–508. doi: 10.1038/s41579-022-00776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dye C., Kraemer M.U.G. Investigating the monkeypox outbreak. BMJ. 2022;377:o1314. doi: 10.1136/bmj.o1314. [DOI] [PubMed] [Google Scholar]
- 26.Jezek Z., Szczeniowski M., Paluku K.M., Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156(2):293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 27.Moore M.J., Rathish B., Zahra F. Monkeypox. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. PMID: 34662033. [PubMed]
- 28.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., Steffen R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2) doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar N., Acharya A., Gendelman H.E., Byrareddy S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131 doi: 10.1016/j.jaut.2022.102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein S.L. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24(6):627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 31.Yong S.E.F., Ng O.T., Ho Z.J.M., Mak T.M., Marimuthu K., Vasoo S., et al. Imported monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826–1830. doi: 10.3201/eid2608.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huhn G.D., Bauer A.M., Yorita K., Graham M.B., Sejvar J., Likos A., et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41(12):1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 33.Vaughan A., Aarons E., Astbury J., Balasegaram S., Beadsworth M., Beck C.R., et al. Two cases of monkeypox imported to the United Kingdom. Sept 2018 Eur Surveill. 2018;23(38) doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaughan A., Aarons E., Astbury J., Brooks T., Chand M., Flegg P., et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26(4):782–785. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore J.B., Smith G.L. Steroid hormone synthesis by a vaccinia enzyme - a new type of virus virulence factor. EMBO J. 1992;11:1973–1980. doi: 10.1002/j.1460-2075.1992.tb05251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith G.L., Symons J.A., Khanna A., Vanderplasschen A., Alcami A. Vaccinia virus immune evasion. Immunol Rev. 1997;159:137–154. doi: 10.1111/j.1600-065x.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 37.Weaver J.R., Isaacs S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008;225:96–113. doi: 10.1111/j.1600-065X.2008.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gigante C.M., Korber B., Seabolt M.H., Wilkins K., Davidson W., Rao A.K., et al. Multiple lineages of Monkeypox virus detected in the United States, 2021-2022. Science. 2022;378(6619):560–565. doi: 10.1126/science.add4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paharia T., Paharia P.T. Insights into the biology of the monkeypox virus. News-Medical. 2022. https://www.news-medical.net/news/20220823/Insights-into-the-biology-of-the-monkeypox-virus.aspx. [accessed 19 January 2023].
- 40.O’Toole A., Rambaut A. Initial observations about putative APOBEC3 deaminase editing driving short-term evolution of MPXV since 2017. Artic Network 2022. https://virological.org/t/initial-observations-about-putative-apobec3-deaminase-editing-driving-short-term-evolution-of-mpxv-since-2017/830 [accessed 20 December 2022].
- 41.Liddament M.T., Brown W.L., Schumacher A.J., Harris R.S. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr Biol. 2004;14:1385–1391. doi: 10.1016/j.cub.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 42.Vartanian J.P., Guetard D., Henry M., Wain-Hobson S. Evidence for editing of human papillomavirus DNA by APOBEC3 in benign and precancerous lesions. Science. 2008;320(5873):230–233. doi: 10.1126/science.1153201. [DOI] [PubMed] [Google Scholar]
- 43.Vartanian J.P., Henry M., Marchio A., Suspène R., Aynaud M.M., Guétard D., et al. Massive APOBEC3 editing of hepatitis B viral DNA in cirrhosis. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris R.S., Dudley J.P. APOBECs and virus restriction. Virology. 2015;479:131–145. doi: 10.1016/j.virol.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salter J.D., Bennett R.P., Smith H.C. The APOBEC protein family: united by structure, divergent in function. Trends Biochem Sci. 2016;41:578–594. doi: 10.1016/j.tibs.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones T.C., Schneider J., Muehlemann B., Veith T., Beheim-Schwarzbach J., Tesch J., et al. Genetic variability, including gene duplication and deletion, in early sequences from the 2022 European monkeypox outbreak. bioRxiv. 2022 doi: 10.1101/2022.07.23.501239. [DOI] [Google Scholar]
- 47.Hendrickson R.C., Wang C., Hatcher E.L., Lefkowitz E.J. Orthopoxvirus genome evolution: the role of gene loss. Viruses. 2010;2:1933–1967. doi: 10.3390/v2091933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haller S.L., Peng C., McFadden G., Rothenburg S. Poxviruses and the evolution of host range and virulence. Infect Genet Evol. 2014;21:15–40. doi: 10.1016/j.meegid.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elde N.C., Child S.J., Eickbush M.T., Kitzman J.O., Rogers K.S., Shendure J., et al. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell. 2012;150:831–841. doi: 10.1016/j.cell.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brennan G., Kitzman J.O., Rothenburg S., Shendure J., Geballe A.P. Adaptive gene amplification as an intermediate step in the expansion of virus host range. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen N., Li G., Liszewski M.K., Atkinson J.P., Jahrling P.B., Feng Z., et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340:46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel A., Bilinska J., Tam J.C.H., Fontoura D.D.S., Mason C.Y., Daunt A., et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378 doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michael G., Anderson Jd, Lawrence D., Frenkel Scott Homann, Jennifer Guffey. A case of severe monkeypox virus disease in an American child: emerging infections and changing professional values. Pedia Infect Dis J. 2003;22:1093–1096. doi: 10.1097/01.inf.0000101821.61387.a5. [DOI] [PubMed] [Google Scholar]
- 54.Sejvar J.J., Chowdary Y., Schomogyi M., Stevens J., Patel J., Karem K., et al. Human monkeypox infection: a family cluster in the midwestern United States. 2004;190:1833–1840. doi: 10.1086/425039. [DOI] [PubMed] [Google Scholar]
- 55.Eltvedt A.K., Christiansen M., Poulsen A. A case report of monkeypox in a 4-year-old boy from the DR Congo: challenges of diagnosis and management. Case Rep Pediatr. 2020:8572596. doi: 10.1155/2020/8572596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yinka-Ogunleye A., Aruna O., Dalhat M., Ogoina D., McCollum A., Disu Y., et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872–879. doi: 10.1016/S1473-3099(19)30294-4. http://dx.doi.org/10.1016/ S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rizk John G., Lippi Giuseppe, Henry Brandon M., Forthal Donald N., Rizk Youssef. Prevention and treatment of monkeypox. Drugs. 2022;82:957–963. doi: 10.1007/s40265-022-01742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatch G.J., Graham V.A., Bewley K.R., Tree J.A., Dennis M., Taylor I., et al. Assessment of the protective effect of imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J Virol. 2013;87(14):7805–7815. doi: 10.1128/JVI.03481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.U.S. Food and Drug Administration (FDA). Biologics License Application (BLA) for Lynneos vaccine. 2019. https://www.fda.gov/media/131079/download. [accessed 16 September 2022].
- 60.U.S. Food and Drug Administration. Vaccines Licensed for Use in the United States, https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states. [accessed 16 September 2022].
- 61.Rao A.K., Petersen B.W., Whitehill F., Razeq J.H., Isaacs S.N., et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices—United States, 2022. Morb Mortal Wkly Rep. 2022;71(22):734. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention. Jynneos Vaccine. https://www.cdc.gov/poxvirus/monkeypox/interim-considerations/jynneos-vaccine.html. [accessed 16 September 2022].
- 63.Gruber M.F. Current status of monkeypox vaccines. Vaccines. 2022;7:94. doi: 10.1038/s41541-022-00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Golden J.W., Zaitseva M., Kapnick S., Fisher R.W., Mikolajczyk M.G., Ballantyne J., et al. Polyclonal antibody cocktails generated using DNA vaccine technology protect in murine models of orthopoxvirus disease. Virol J. 2011;8:441. doi: 10.1186/1743-422X-8-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.The World Health Organization (WHO). WHO Mpox (monkeypox) Research: What are the knowledge gaps and priority research questions? https://www.who.int/news-room/events/detail/2022/06/02/default-calendar/who-monkeypox-research--what-are-the-knowledge-gaps-and-priority-research-questions. [accessed 19 January 2023].
- 66.Smith S.S., Self J., Weiss S., Carroll D., Braden Z., Regnery R.L., Davidson W., et al. Effective antiviral treatment of systemic orthopoxvirus disease: ST-246 treatment of prairie dogs infected with monkeypox virus. J Virol. 2011;85(17):9176–9187. doi: 10.1128/JVI.02173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huggins J., Goff A., Hensley L., Mucker E., Shamblin J., Wlazlowski C., et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53(6):2620–2625. doi: 10.1128/AAC.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.(a) Robert Jordan, Arthur Goff, Annie Frimm, Michael L.Corrado, Lisa E.Hensley, Chelsea M.Byrd, Eric Mucker, Josh Shamblin, Tove’ C.Bolken, Carly Wlazlowski, Wendy Johnson, Jennifer Chapman, Nancy Twenhafel, Shanthakumar Tyavanagimatt, Adams Amantana, Jarasvech Chinsangaram, Hruby Dennis E., John Huggins. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrobial agents chemotherapy. 2009;Vol. 53(No. 5):1817–1822. doi: 10.1128/AAC.01596-08. (May) [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jordan O., Goff A., Frimm A., Corrado M.L., Hensley L.E., Byrd C.M., et al. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob Agents Chemother. 2009;53(5):1817–1822. doi: 10.1128/AAC.01596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luciani L., Lapidus N., Amroun A., Falchi A., Souksakhone C., Mayxay M., et al. Susceptibility to monkeypox virus infection: seroprevalence of orthopoxvirus in 4 population samples; France, Bolivia. Laos Mali medRxiv. 2022 doi: 10.1101/2022.07.15.2227766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sejvar J.J., Chowdary Y., Schomogyi M., Stevens J., Patel J., Karem K., et al. Human monkeypox infection: a family cluster in the Midwestern United States. 2004;190:1833–1840. doi: 10.1086/425039. [DOI] [PubMed] [Google Scholar]
- 71.Reynolds M.G., Carroll D.S., Karem K.L. Factors affecting the likelihood of monkeypox’s emergence and spread in the post-smallpox era. Curr Opin Virol. 2012;2:335–343. doi: 10.1016/j.coviro.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.The United Nations Refugee Agency (UNHCR). Refugee’s Global report. 2022. https://www.unhcr.org/uk/figures-at-a-glance.html. [accessed 19 January 2023].
- 73.Pipito L., Carlo P.D., Cascio A. Pustular lesions and itching in a couple of young migrants. Travel Med Infect Dis. 2022;50 doi: 10.1016/j.tmaid.2022.102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manirambona E., Shomuyiwa E.O. Monkeypox among refugees: a call for a global protection. Travel Med Infect Dis. 2022;50 doi: 10.1016/j.tmaid.2022.102458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Subedi D., Pantha S., Chandran D., Bhandari M., Acharya K.P., Dhama K. FIFA World Cup 2022 and the Risk of Emergence of Zoonotic Diseases. J Pure Appl Microbiol. 2022;16(4):2246–2258. doi: 10.22207/JPAM.16.4.47. [DOI] [Google Scholar]
- 76.Farahat R.A., Setti M.O., Benmelouka A.Y., Ali I., Umar T.P., et al. Monkeypox emergence and hosting a safe FIFA World Cup 2022 in Qatar: Challenges and recommendations. Int J Surg. 2022;106 doi: 10.1016/j.ijsu.2022.106935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kulesh D.A., Loveless B.M., Norwood D., et al. Monkeypox virus detection in rodents using real-time 3′-minor groove binder TaqMan assays on the Roche LightCycler. Lab Invest. 2004;84:1200–1208. doi: 10.1038/labinvest.3700143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.The world health organization. Laboratory testing for the monkeypox virus: Interim guidance. 2022. https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1 [accessed 10 January 2023].
- 79.The Centers for Disease Control and Prevention (CDC). Specimen Collection Procedures. 2023. https://www.cdc.gov/smallpox/lab-personnel/specimen-collection/specimen-collection-procedures.html. [accessed 21 January 2023].
- 80.Robert Koch Institut (RKI). Diagnostics of infections with poxviruses. 2023. https://www.rki.de/EN/Content/infections/Diagnostics/NatRefCentresConsultantLab/POX/Diagnostics-Pox.html [accessed 18 January 2023].
- 81.Li Y., Olson V.A., Laue T., Laker M.T., Damon I.K. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36:194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y., Zhao H., Wilkins K., Hughes C., Damon I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Method. 2010;169(1):223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.The Centers for Disease Control and Prevention (CDC). Laboratory Procedures and Testing. 2023. https://www.cdc.gov/poxvirus/monkeypox/lab-personnel/index.html. [accessed 10 January 2023].
- 84.Olson V.A., Laue T., Laker M.T., et al. Real-time PCR system for detection of orthopoxviruses and simultaneous identification of smallpox virus. J Clin Microbiol. 2004;42:1940–1946. doi: 10.1128/JCM.42.5.1940-1946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Townsend M.B., Macneil A., Reynolds M.G., et al. Evaluation of the Tetracore Orthopox BioThreat® antigen detection assay using laboratory grown orthopoxviruses and rash illness clinical specimens. J Virol Methods. 2013;187:37–42. doi: 10.1016/j.jviromet.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sapkal A., Agrawal S. Monkeypox: the re-emerging terror. Cureus. 2022;14:8. doi: 10.7759/cureus.28597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Q., Gul I., Liu C., Lei Z., Li X., Raheem M.A., et al. CRISPR‐Cas12–based field‐deployable system for rapid detection of synthetic DNA sequence of the monkeypox virus genome. J Med Virol. 2022 doi: 10.1002/jmv.28385. [DOI] [PubMed] [Google Scholar]
- 88.Paniz‐Mondolfi A., Guerra S., Muñoz M., Luna N., Hernandez M.M., Patino L.H., et al. Evaluation and validation of an RT‐PCR assay for specific detection of monkeypox virus (MPXV) J Med Virol. 2022 doi: 10.1002/jmv.28247. [DOI] [PubMed] [Google Scholar]
- 89.Singh, M., Misra, C.S., Bindal, G., Rangu, S.S., & Rath, D. (2022). CRISPR/Cas12a assisted specific detection of monkeypox virus. medRxiv. [DOI] [PubMed]
- 90.The Centers for Disease Control and Prevention (CDC). Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/demographics.html#print, [accessed 10 January 2023].
- 91.World Health Organization. Monkeypox. 2022. https://www.who.int. [accessed 10 September 2022].
- 92.Center for Infectious Disease Research and Policy (CIDRAP), University of Minnesota. Monkeypox cases reach 7,500 in US; 99% of cases in males. 2022. https://www.cidrap.umn.edu/news-perspective/2022/08/monkeypox-cases-reach-7500-us-99-cases-males. [accessed 8 August 2022].
- 93.Fowotade A., Fasuyi T.O., Bakare R.A. Re-emergence of monkeypox in Nigeria: a cause for concern and public enlightenment. Afr J Clin Exp Microbiol. 2018;19:307–313. [Google Scholar]
- 94.Public Health Ontario. Monkeypox in Ontario: May 1, 2022 to December 13, 2022. 2022. https://www.publichealthontario.ca/-/media/Documents/M/2022/monkeypox-episummary.pdf?sc_lang=en. [accessed 28 August 2022].
- 95.The Ministry of Health (MoH) of the Kingdom of Saudi Arabia (KSA). 2022. https://www.moh.gov.sa/en/CCC/events/international/Pages/Monkeypox.aspx [accessed 1 September 2022].