Abstract
Diabetes mellitus (DM) is associated with many health complications and is potentially a morbid condition. As prevalence increases at an alarming rate around the world, research into new antidiabetic compounds with different mechanisms is the top priority. Therefore, the preclinical experimental induction of DM is imperative for advancing knowledge, understanding pathogenesis, and developing new drugs. Efforts have been made to examine recent literature on the various induction methods of Type I and Type II DM. The review summarizes the different in vivo models of DM induced by chemical, surgical, and genetic (immunological) manipulations and the use of pathogens such as viruses. For good preclinical assessment, the animal model must exhibit face, predictive, and construct validity. Among all reported models, chemically induced DM with streptozotocin was found to be the most preferred model. However, the purpose of the research and the outcomes to be achieved should be taken into account. This review was aimed at bringing together models, benefits, limitations, species, and strains. It will help the researcher to understand the pathophysiology of DM and to choose appropriate animal models.
Keywords: Diabetes mellitus, Hyperglycemia, Insulin resistance, Streptozotocin
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by hyperglycemia with less or no insulin secretion. As per the International Diabetes Federation (IDF) 2021, about 537 million adults (20–79 years) have diabetes, which is estimated to rise to 643 million by 2030 and 783 million by 2045 globally (Sun et al. 2022). DM is classified as Type 1 DM (T1DM) or insulin-dependent DM (IDDM) and Type 2 DM (T2DM) or non-insulin-dependent DM (NIDDM) (King 2012). Miscellaneous classes such as diabetes associated with contributing clinical states, diseases, drugs and/or chemicals, gestational diabetes, and malnutrition-associated diabetes are included in T2DM (Jwad et al. 2022). Comorbidity of diabetes mellitus with other chronic diseases exacerbates the condition of patients (Surguchov 2020). Oral hypoglycemic agents are used clinically to control elevated blood glucose (BG) levels in T2DM. Recently many new drugs, like SGLT-2 inhibitors, dipeptidyl peptidase four inhibitors, etc., have been added to the existing regime for the treatment of T2DM, and many others are under research (Dowarah and Singh 2020).
Considering the increasing prevalence of diabetes and the thirst for novel antidiabetic drugs, animal models have become indispensable. The face, predictive, and construct validity is vital to validating and optimizing innovative treatments for safe human usage. Failure to successfully translate human disease into animal models leads to substantial financial losses and wastes valuable time. Therefore, the objective of the current review is to summarize the data about different animal models which have been reported. The review also highlights their shortcomings, emphasizes their advantages, and explains the precautions required with each technique. It will assist the researcher in understanding the pathophysiology of DM and selecting appropriate animal models (Fig. 1).
Fig. 1.
Animal models for Type 1 and Type 2 diabetes mellitus. Streptozotocin (STZ), nicotinamide (NA), non-obese diabetic (NOD), Bio-breeding rat (BB), Kilham rat virus (KRV), high-fat diet (HFD), Goto-Kakizaki (GK), New Zealand obese (NZO) mouse, Zucker Diabetic Sprague–Dawley rat (ZDSD), Gold thioglucose (GTG)
Animal models for T1DM
TIDM in laboratory animals can be mimicked by different mechanisms such as chemical ablation of the β cells, breeding of spontaneous autoimmune animals, and genetic and virally induction of diabetes. The foremost consideration for selecting a suitable animal model for T1DM is the background strain and gender since they markedly alter the phenotype of a model. A more severe phenotype in male mice is observed in high-fat diets and genetic models; an exception is a non-obese diabetic (NOD) mouse, which is more prone to develop diabetes in female mice (Tiano and Mauvais-Jarvis 2012). Moreover, different strains develop diabetes at different ages (Azushima et al. 2017). For example, Akita mice develop diabetes at 3–4 weeks, while Lepdb/db mice take 4–8 weeks (King 2012).
Chemically induced T1DM
Chemically induced T1DM is linked to a rapid rate of endogenous β cell breakdown, resulting in low-insulin production, hyperglycemia, and weight loss. In rodents and higher animals, it provides a simple and cost-effective model. The induction of diabetes takes 5–7 days for stable hyperglycemia. Chemically induced diabetes is preferred when the rise in BG is desired by selective loss of only pancreatic β cells, leaving other alpha and delta cells intact. STZ and alloxan are the most frequently used chemicals administered parentally [intravenous (i.v), intraperitoneal (i.p), or subcutaneous (s.c)] to induce diabetes.
STZ-induced diabetes
STZ [2-deoxy-2-(3-(methyl-3-nitrosoureido)-d-glucopyranose] is a lipophilic nitrosourea analogue synthesized from soil micro-organism Streptomycetes achromogenes (Reusser 1971). It induces diabetes in animals similar to human diabetes (loss of β cells), hence the most preferred model. It is a selective pancreatic cytotoxic agent that causes β cell necrosis through the inflammatory process and lymphocytic infiltration of the pancreatic islets. The dose must be optimized depending on the source of STZ, animal species, and strain used, and body weight for a low dose of STZ does not show the desired effects, and higher doses exhibit mortality. Thus, it is suggested to administer STZ as a single high dose or multiple low doses. For T1DM, attempts to mimic the autoimmune insulitis, multiple low doses are preferred (Rossini et al. 1977). The STZ solution should be freshly prepared and used. Studies suggest that a single high dose of STZ ranging from 100 to 200 mg/kg exhibits high toxicity to pancreatic β cells, resulting in BG rise to > 500 mg/dl within 48 h. Previous research has indicated the regeneration of pancreatic islets in STZ rats; therefore, there should be enough controls to prevent spontaneous β cell regeneration. Multiple low doses of STZ (20–40 mg/kg per day) for over 5 days can result in insults, in mice and rats (Wang and Gleichmann 1998). However, chronic use of STZ is associated with the development of insulinoma, kidney, and liver tumors (Iwase et al. 1991; Steiner et al. 1970; Yamagami et al. 1985). The reported ranges of doses of STZ, animal species, and strains used to induce T1DM are presented in detail in Table 1.
Table 1.
Summary of chemical induced T1DM and T2DM
| Sr. no. | Chemical | Animal species | Dose Range (i.p.) | Latency to develop diabetes | References |
|---|---|---|---|---|---|
| T1DM | |||||
| 1 | STZ |
RAT Wistar rat (8–10 weeks) Neonates (2 days old) |
Single high dose 60 mg/kg |
Fasting BG (FBG) > 250 mg/dL (post 72 h) | Gao et al. (2019) |
| 40 mg/kg | FBG > 20 mmol/l or 360 mg/dl (post 24 h) | Mihailović et al. (2015) | |||
| 90 mg/kg | FBG > 160 mg/dL (post 6 weeks) | Sole and Srinivasan (2012) | |||
|
SD Pups SD rats |
55 mg/kg | FBG > 250 mg/dL (post 72 h) | Naderi et al. (2019), Oguntibeju et al. (2020) | ||
| 65 mg/kg | FBG > 120 mg/ dL (post 72 h) | Hanchang et al. (2019) | |||
| 50 mg/kg | FBG > 120 mg/ dL (post 72 h) | Kocaman and Kuloğlu (2020), Nazir et al. (2021) | |||
|
MICE ICR mice Swiss Albino mice |
200 mg/kg | FBG > 16.7 mmol/l (post 5 days) | Sun et al. (2012) | ||
| 45 mg/kg | FBG > 300 mg/dl (post 72 h) | Tabatabaie and Yazdanparast (2017), Park et al. (2012) | |||
| 40 mg/kg for 5 days | FBG > 300 mg/dl (post 72 h) | Yao et al. (2012) | |||
| 150 mg/kg | FBG > 200 mg/dl (post 72 h) | Hammeso et al. (2019) | |||
|
Multiple doses 180,100,40 mg/kg for 5 consecutive days |
FBG > 300 mg/dl (post 72 h) | Aurora et.al (2009) | |||
| Swiss Albino mice |
Single high dose STZ (200 mg/kg) + NA (110 mg/kg) |
FBG > 300 mg/dl (post 72 h) | Yadav et al. (2013) | ||
|
C57BL/6 or CD-1 mice (8–12 weeks) |
80 mg/kg | FBG > 250 mg/dl (Post 48 h) | Park et al. (2012) | ||
| STZ (40 mg/kg) (5 consecutive days i.p injection) | FBG > 200 mg/dl (12 mmol/l) | Domingues et al. (2011) | |||
| (3–5 weeks) | 50 mg/kg | FBG > 250 mg/dl (post 6 days | Sergeys et al. (2019) | ||
|
Balb/c mice (8–12 weeks) |
60 mg/kg | FBG > 250 mg/dl (post 72 h) | Zhang et al. (2019) | ||
| 200 mg/kg | FBG > 250 mg/dl post 30 h | Himpe et al. (2016) | |||
|
180 mg/kg HFD (4 weeks) + STZ (55 mg/kg) for 5 days |
FBG > 250 mg/dl | Saliu et al. (2022) | |||
| 140, 160, 200, 300, 400 mg/kg | FBG > 300 mg/dl | Wszola et al. (2021) | |||
| 2 | Alloxan | Wistar Rats | 60 mg/kg | FBG BG > 200 mg/dl (post 72 h) | Onyibe et al. (2021) |
| 120 mg/kg | BG > 250 mg/dl | Obasi and Ogugua (2021) | |||
| High carbohydrate/high-fat + Alloxan (105 mg/kg) i.p | FBG > 300 mg/dl (post 72 h) | Xu et al. (2017) | |||
| 150 mg/kg | BG > 200 mg/dl (post 72 h) | Amare (2021), Solikhah and Solikhah (2021), Alkandahri et al. (2021) | |||
| Mice | 180 mg/kg (every 48 h for three times) | Fasting BG > 200 mg/dl (post 72 h) | Raafat et al. (2021) | ||
| 70 mg/kg i.v | Fasting BG > 200 mg/dl (post 48 h) | Badole et al. (2006) | |||
| T2DM | |||||
| 1 | STZ + NA |
Rat SD Rats |
STZ (60 mg/kg)–NA (120 mg/kg) STZ (45 mg/kg)-NA (120 mg/kg) i.p |
Fasting BG > 200 mg/dl (post 72 h), FBG > 16.7 mmol/l (post 72 h) |
Arya et al. (2015) |
|
HFD (4 weeks) + STZ (50 mg/kg) HFD (4 weeks) + STZ 40 mg/kg) HFD + STZ (45, 65, 85 mg/kg) |
FBG > 20 mmol/l FBG > 8.3 mmol/l (determined every week) |
Wickramasinghe et al. (2021) Zhuge et al. (2021) Wang et al. (2014) |
|||
| 2 | STZ + HFD |
Rats Wistar rats |
High-Fat diet (4 weeks) + STZ (120 mg/kg) | FBG ≥ 300 mg/dl (post 7 days) | Srinivasan et al. (2005) |
|
Mice Male Kunming mice (6–8 weeks) |
HFD (4 weeks) + STZ (40 mg/kg) 5 days |
FBG ≥ 11.1 mmol/l (post 7 days) | Li et al. (2019) | ||
|
HFD (4 weeks) + STZ (35 mg/kg) 5 days |
FBG > 11.1 mmol/l (post 7 days) | Hu et al. (2019) | |||
STZ Streptozotocin, NA nicotinamide, FBG fasting blood glucose, BG blood glucose, SD Sprague–Dawley, HFD high fat diet
Alloxan-induced diabetes
Alloxan (2,4,5,6-tetraoxypyrimidine; 5,6-dioxyuracil) is a hydrophilic unstable compound that has a similar structure to glucose and is synthesized by uric acid oxidation. Similar to STZ, alloxan is also chemically unstable; therefore, the parenteral route of dosing is recommended. In addition, the dose of alloxan (150–180 mg/kg) depends mainly on animal species, route of administration, and nutritional status (Federiuk et al. 2004; Gorus et al. 1982; Lenzen and Munday 1991; Weaver et al. 1978). Before commencing the experiment, the animals should be fasted for 24 h to avoid competitive inhibition between alloxan and glucose (Radenković et al. 2016). The uptaken alloxan by the β cell is converted to dialuric acid, a highly reactive oxygen species (ROS), resulting in the fragmentation of β cell DNA (Lenzen 2008; Lenzen and Munday 1991; Munday 1988; Toda and Diano 2014).
Spontaneous autoimmune models of T1DM
The spontaneous animal species is developed from selective breeding over many generations of animals with poor glucose tolerance. The most commonly used animals are non-obese diabetic (NOD) and bio-breeding (BB) in the Wistar colony, Komeda Diabetes-Prone (KDP) rats, and LEW-1AR1/-IDDM rats. BB and NOD spontaneously develop diabetes similar to human TIDM, with identical genotypes and phenotypes. The advantage of the spontaneous animal model also demonstrates the development of obesity and high cholesterol, which makes it useful for metabolic and diabetic research.
NOD mouse
NOD is an inbred albino strain derived from Jcl: ICR mice by Makino in Japan, an excellent model of autoimmune disease that develops spontaneous autoimmune diabetes similar to clinical T1DM in human subjects. The mouse is significantly deficient in insulin mainly due to a dominant negative mutation in the insulin 2 gene (Ins2Akita). Selective breeding of offspring results in insulite in 3–4 weeks, followed by the destruction of β cells by CD4+ and CD8+ lymphocytes and other results in decreased circulating insulin concentration (Jun and Yoon 2001; Herrath and Nepom 2009). The advantages of NOD mice are that they develop spontaneous autoimmunity by generating autoantibodies, increased circulating autoreactive T cells, and inhibit cytokines, namely interleukins, tumor necrosis factor, interferon-γ (Gregori et al. 2003; Ingalls et al. 1950; Kawasaki et al. 2004; Melanitou et al. 2004). Slow disease progression with several months of the prodromal phase of benign islet infiltrations makes the model unique. However, the accelerated onset of diabetes can also be achieved by injecting NOD mice with cyclophosphamide (alkylating agent) that acts by depletion of regulatory (suppressor) T cells. They resemble human diabetes with identical symptoms such as hyperglycemia, glycosuria, polydipsia, and polyuria but very mild ketoacidosis. When the animal becomes over-diabetic, they lose weight and require insulin treatment. The disadvantage of this model is that many drugs showing positive effects in NOD mice were ineffective in humans ( Herrath and Nepom 2009). Moreover, the prime requirement of pathogen-free conditions makes the model expensive. In rat species, the female rat is more likely to develop diabetes than the male rat (Pozzilli et al. 1993). Vitamin D3 and certain probiotics have been studied using NOD mice (Yu et al. 2022; Kim et al. 2020). Single spontaneous mutation results in development of three spontaneous rat models which are Bio-Breeding diabetes-prone rats (BBdp), Komeda diabetes-prone (KDP) rat in a Long-Evans colony, IDDM (LEW.1AR1-iddm) rat in a colony (LEW.1AR1) with a Lewis background. The LEW.1AR1-iddm (IDDM) trait is closely related to the characteristics and pathophysiology of human T1DM.
Bio-breeding rat (BB)
The BB rat is one of the best models of spontaneous autoimmune T1DM, the polygenic type, dependent upon mutations at several loci. BB rats develop diabetes just after puberty in both males and females. There are 2 T1D susceptibility genes which have been identified in the BB rat. They are basically susceptible MHC class II RT1u haplotype on chromosome 20 and a null mutation in the GIMAP5 gene on chromosome 4. Ninety percent of rats develop diabetes between 8 and 16 weeks and require insulin therapy for survival (Roep and Atkinson 2004). The diabetes-prone BB (BBDP) rat develops autoimmune T1DM between 50 and 90 days of age. High T cells, B cells, macrophages, and NK cells result in insulitis. The advantage of the BB rat is that it elucidates the genetics of T1DM and is helpful in the islet transplantation tolerance induction model and diabetic neuropathy (Holmberg et al. 2011; Mordes et al. 2007; Wallis et al. 2009). The model is accompanied with a disadvantage of occurrence of a lymphopenia which is absent in NOD mice, and human Type I diabetes and insulitis are not headed by peri-insulitis (Mordes et al. 2007).
KDP rat and LEW-1AR1 rat model
The KDP rat, formerly called the LETL rat, is a nonlymphopenic model with spontaneous auto-immune diabetes. Mutation in two major susceptibility genes, the major histocompatibility complex (MHC) RT1u haplotype and Cblb (Casitas B-lineage lymphoma b), is responsible for developing diabetes in KDP rats (Yokoi 2007). Disease manifestation occurs between 90 and 100 days of life (Yokoi et al. 2003). Lewis rats with a specified MHC haplotype (LEW-1AR1) develop insulitis and diabetes by 8–9 weeks. The infiltration of islets starts a week before the animal becomes hyperglycemic, which facilitates the examination of the different stages of the infiltration of immune cells. T1DM is established on the 59th day of life (Jörns et al. 2005).
Genetically induced TIDM
In IDDM, the destruction of pancreatic β cells generally occurs by the combined action of several factors, such as autoimmunity, environmental factors, and genetic changes. The Akita mouse was developed from a C57BL/6NSlc mouse in Akita, Japan. Mice have two genes for insulin (Ins1 and Ins 2). Spontaneous mutation in the Ins 2 gene results in the abnormal formation of insulin protein. It consequently results in insult to pancreatic β cells and, therefore, a decreased capacity to secrete insulin, thus triggering the activation of the endoplasmic reticulum stress pathway and β cell apoptosis (Kong et al. 2013; Oyadomari et al. 2002). Akita mice (Ins 2) are hyperglycemic and hypoinsulinemic and display polydipsia and polyuria at an early age of 3–4 weeks. Transplantation studies use them as an alternative to mice treated with STZ due to lack of cell mass β. They are also a preferred model of T1DM macrovascular disease and neuropathy (Drel et al. 2011; Zhou et al. 2011). The disadvantage of the model is its shorter life span of fewer than 12 weeks. Female mice have a higher risk of developing hyperglycemia.
Virus-induced models of TIDM
Viral infection has been involved in the pathophysiology of type 1 diabetes. The destruction of virus-induced β cells occurs by two different mechanisms, firstly by specific auto-immunity of β cells, where the destruction of β cells with or without infection occurs, e.g., Kilham rat virus (KRV). KRV acts through indirect cytolytic infection of pancreatic beta cells (Ellerman 1996). The second mechanism comprises cytolytic conditions leading to the destruction of virus β cell replication leading to hypoinsulinemia and hyperglycemia. Fourteen different viruses have been linked to T1DM development in both human and animal models. Some of the examples of viruses to induce diabetes in the animal model are explained in Table 2.
Table 2.
Animal models for virus induced diabetes, obesity induced diabetes, , and maternal diabetes
| Sr. no. | Virus-induced models | Main feature | Uses | Advantages and limitations | References |
|---|---|---|---|---|---|
| 1 | CVB | Six serotypes CVB (1–6) result in T1DM | Development of T cell-mediated autoimmune T1DM | Disease development within 2 weeks of the viral challenge in NOD mice | Rewers and Ludvigsson (2016) |
| 2 | KRV | The presence of KRV protein in β cells is observed on day 5 post-infection, and islet destruction and diabetes occur post-insulitis around 2–4 weeks following infection. Replication of KRV occurs in the nucleus of infected cells of lymphoid organs such as the spleen, thymus, and lymph nodes | T1DM | About 60% rats successfully develop DM | Tirabassi et al. (2010), Christen et al. (2012) |
| 3 | CMV |
Infection of diabetes-prone (DP)-BB rats, Bio-breeding diabetic prone (BBDP) which develop autoimmune diabetes at 10–14 weeks. Immediately after infection, macrophages predominate, followed by CD41T lymphocytes, and CD81T lymphocytes in intermediate and late stages. Macrophages destroy pancreatic β cells DBA2 mice, EMC-D virus-infected mice, showed a rise in inflammatory mediators, plasma glucose levels, β cells destruction |
T1DM | Clinically induction of T1DM is more common in children. Develops slowly (takes years to diagnose) | Hirasawa et al. (1997), Pak et al. (1988), Baek and Yoon (1990), Yoon et al. (1988) |
| 4 | EMCV |
EMCV is Picornavirus, a rodent virus that selectively infects pancreatic β cells. In the mice model, EMCV causes lesions in the islets of Langerhans EMC-D virus causes cytolytic destruction of β cells in strains of mice such as SJL/J, SWR/J, DBA/1 J, and DBA/2 J resulting in diabetes |
DM, hypoinsulinemia, hyperglycemia, glycosuria, polydipsia, and polyphagia | Craighead and McLane (1968) | |
|
Obesity-induced diabetes model Monogenic model | |||||
| 1 | Ob/Ob (C57BL/6 J-Lepob) mouse |
Autosomal recessive mutation on leptin gene in C57BL/6 J strain. Obesity develops by 4 weeks and hyperinsulinemia by 2–4 weeks Hyperglycemia is developed by 4 weeks rise by 3–5 months) Impaired glucose tolerance is developed by 12 weeks and Blood pressure is reduced |
Obesity, IR, T2DM | Testing products for antiobesity, and antihyperglycemic increases insulin sensitivity effect | Dubuc (1976); Ingalls et al. (1950) |
| 2 | Lep db/db mouse | Single autosomal recessive leptin receptor mutation on chromosome 4 in C57BL/KsJ strain.Mice gets obese by 6 weeks and increase in BG by 8 weeks.Mouse gets hyperglycemic, hyperphagic, obese, hyperinsulinemic by 12 weeks, hyperlipidemic by 13 weeks, ketogenesis, and survives upto 10 months of age. No change in BP observed | Obesity, Hyperphagia, Hyperinsulinemia, Ketogenesis |
Investigation of agents for T2DM, mainly diabetic dyslipidemia Fails to develop T2DM phenotype |
Ingalls et al. (1950), Leiter et al. (1981), Lutz and Woods (2012) |
| 3 | ZDF rats | Substrain of Zucker fatty rat with deficient Leptin receptor. Missense mutation in leptin receptor gene with homozygous fa allele (fa) gene on chromosome 5.Leads in morbid obesity within 3–5 weeks. Diabetes develops due to autosomal recessive. Hyperinsulinemia-euglycemia develops after weaning ~ 4 weeks of age, hyperglycemic insulin deficient state till ~ 12 weeks | Development of diabetes in age-dependent fashion | Test drugs against obesity, IR, hypertension and atherosclerosis | Slieker et al. (1992), Etgen and Oldham (2000), Kava et al. (1990a) |
| 4 | WDF rats | Genetically obese Zucker rats' fa-gene was crossed with Wistar Kyoto (WKY) rats | Obesity, hyperglycemia, hyperinsulinemia, IR, glucose tolerance & peripheral neuropathy | Kava et al. (1990b) | |
| Polygenic model | |||||
| 1 |
KK KK-AY MICE |
Develops obesity at a mild level in adult life Developed by the addition of the diabetic susceptible lethal yellow obese (Ay) gene |
T2DM with complications associated with the renal, retinal, and neurological system. IR, islet cell hyperplasia, compensatory hyperinsulinemia Becomes obese, hyperglycemic, hyperinsulinemic and highly glucose intolerant due to IR |
Nakamura and Yamada (1967) | |
| 2 | NSY mouse |
Developed by selective inbreeding of ancestral laboratory strain of Jc1:ICR mouse Age-dependent spontaneous diabetes development, impaired insulin secretion and mild IR |
Diabetes and IR |
Studying age-related decline in β cell function Gender difference Male mice are more prone to getting hyperglycemic than female mice |
Ueda et al. (1995) |
| 3 | OLETF Rats | Selective outbreeding of the colony of long-Evans rats, defect in the proliferation of beta cells leading to mildly obese, hyperinsulinemic, hypercholesteremic, exhibiting, polyphagia, hypertriglyceridemia and impaired glucose intolerance. Hyperglycemic and islets degeneration occurs at18 weeks, Islet hyperplasia and fibrosis followed by apoptosis and depletion of beta cells by 40 weeks. Absence of cholecystokinin-A receptor, thus blocks stimulation of fat digestion and reduces the number of GLUT4 transporters in muscle | Obesity, Diabetes and IR | Genetic enrichment by inbreeding of known ancestry animals is possible | Kawano et al. (1994) |
| 4 | NZO mice | Early diabetes progression- reduced glycogen synthesis activity in the liver. Hyperleptinemia- Dysfunction of leptin transport across blood–brain barrier. Obese, hyperphagic, hyperinsulinemic, insulin resistant. Impaired β cell function, glucose intolerance is observed by 20 weeks | Diabetes and obesity study | Analyzing possible agents showing a relationship between autoimmunity, obesity and diabetes | Leiter and Reifsnyder (2004) |
| 5 | TALLY HO/JNG mice | Selective breeding of outbred colony of Theiler original mice. Obesity and diabetes develops at 10–14 weeks with enhanced plasma triglycerides, cholesterol and free fatty acid | Obesity, hyperinsulinemia, hyperglycemia, diabetic wound healing | Male mice is more prone to diabetes as compared to female | Kim et al. (2005) |
| Maternal Diabetes models | |||||
| 1 | Maternal low-protein model |
Low protein and nutrition affect the control of energy balance, growth abnormalities, and development of the fetus, which later develops T2DM in adulthood Pregnant dams fed with a diet containing 8% energy-rich protein delivered offspring with low birth weight. Post-birth offspring develop high insulin sensitivity progressing to IR by the age of 15 months and T2DM by the age of 17 months |
Development of T2DM | – | Muhlhausler and Smith (2009), Hales et al. (1996) |
| 2 | Prenatal diet manipulations | Excess supply of nutrients or lack of nutrition in the utero while pregnant alters glucose homeostasis | Lack of nutrition during gestation leads to abnormal pancreas development followed by dysfunction of β cells and insulin deficiency | – | Hegde et al. (2016) |
| 3 | Maternal overnutrition in rats | Pregnant rats fed with HFD increase the chances of offspring with obesity and altered glucose homeostasis lifelong | T2DM in offspring due to leptin resistance, hyperphagia, and IR | - | Schwartz and Niswender (2004) |
| Other models | |||||
| 1 | Drug-induced diabetes | Chronic drug therapy with glucocorticoids, thiazide diuretics, diazoxide, and growth hormone (GH) results in diabetes. Elevated glucose production, loss of pancreatic islets tissues and β cells | T2DM, ketonuria and ketonemia, IR, hyperglycemia, and hyperlipidemia | – | Campbell et al. (1954) |
| 2 |
Iron therapy induced diabetes Phenotypes: hereditary hemochromatosis (HH) Thalassemia and transfusion iron overload |
Iron acts as a pro-oxidant for ROS production, which induces oxidative stress causing β cell tissue damage and IR, thus increasing T2DM HH is transmitted autosomal recession trait by mutation of homeostatic iron (HFe gene), resulting in C282Y substitution in the protein, resulting in Insulin deficiency and IR. Moreover, Iron interferes with the trafficking of transitional metals like (Mn + 2), which decreases the activity of superoxide dismutase (SOD2) and increases oxidative damage Iron overload occurred due to numerous blood transfusions to maintain erythrocytes level. High ferritin increases inflammation which is a symptom of T2DM and vice versa |
Mouse model of hemochromatosis, excess iron results in oxidative stress and increases the susceptibility of pancreatic cells to apoptosis, and decrease insulin secretion Mouse model of HH, HFe gene is deleted or replaced with C282Y mutant, results in decreased insulin secretory capacity, decreased glucose secreted insulin secretion, and increased β cell apoptosis. Iron overload in male C57BL/6 J mice fed a high-iron diet showed decreased insulin secretion | – |
Cooksey et al. (2004), Dandona et al. (1983), |
CVB Coxsackie B Virus, KRV Kilham Rat Virus, CMV Cytomegalovirus, EMCV Encephalomyocarditis Virus, SJL Swiss Jim Lambert, SWR Swiss Webster mouse, IR Insulin resistance, ZDF Rat Zucker Diabetic Fatty rat, WDF Wistar Diabetic Fatty, NSY Nagoda Shibata Yasuda, OLETF Rat Otsuka Long Evans Tokushima Fatty Rat, NZO New Zealand obese
Animal models for T2DM
Down-regulation of insulin receptors and reduced sensitivity of peripheral tissues to utilize insulin resulting in IR combined with the failure of β cells to compensate, leading to insulin deficiency, are the crucial characteristics of T2DM. Pathogenetic factors such as age, genes, IR, glucotoxicity, and incretin are implicated in the progressive impairment of insulin secretion and are targets for developing animal models.
Chemically induced T2DM model
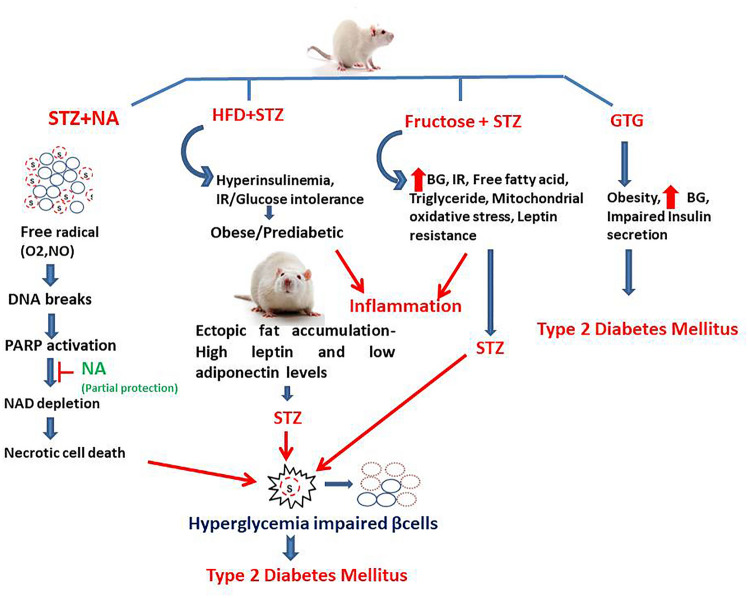
The various agents and mechanisms involved in the induction of chemical-induced T2DM are briefly explained in Fig. 2.
Fig. 2.
Chemicals inducing Type 2 diabetes mellitus and their mechanism of action. (i) NA + STZ model where STZ enters the β cell and damages DNA and increases the activity of the DNA repair enzyme poly(ADP-ribose) polymerase (PARP-1). Excess activity of enzyme results in depletion of intracellular NAD (+) and ATP, and the insulin-secreting cells undergo necrosis. NA shows partial protection due to the inhibition of PARP-1, preventing depletion of NAD (+) and ATP in the cells exposed to STZ activity. (ii) HFD + STZ model-Animals introduced to obesogenic diet, which leads to development of obesity, followed by introduction of STZ, leading to hyperglycemia, IR, hyperinsulinemia, and inflammation. (iii) Fructose + STZ-It leads to increased BG, IR, free fatty acids, oxidative stress, and leptin resistance. (iv) GTG-induced T2DM results in development of obesity, increased BG, and impaired insulin secretion. Streptozotocin (STZ), Nicotinamide (NA), deoxyribonucleic acid (DNA), poly(ADP-ribose) polymerases (PARPs), nicotinamide adenine dinucleotide (NAD), high-fat diet (HFD), insulin resistance (IR), gold thioglucose (GTG), blood glucose (BG)
High-fat diet (HFD) and STZ-induced T2DM
This is the most common T2DM model for reproducing the progression of diabetes in humans. It involves the amalgamation of an HFD (60% calories), which includes approximately (58% fat, 25% protein, and 17% carbohydrate) and STZ injection (~ 30–40 mg/kg i.p.) (Reed et al. 2000). It results in a severe reduction in functional β cell mass, leading to hyperinsulinemia, IR, and/or glucose intolerance (Skovsø 2014; Wang et al. 2014). The key advantages are a shorter timescale achieved by combining the above two interventions and can be customized for slow pathogenesis of T2DM, which mimics the human condition.
NA and STZ-induced T2DM
NA chemically displays its antioxidant property by free radical scavenging effect and thus protects β cells against the cytotoxic effect of STZ (Masiello et al. 1998). NA (110 mg/kg, i.p) is typically administered 15 min before STZ (65 mg/kg, i.p) (Nayak et al. 2014). STZ with NA is one of the popular models, and this combination produces T2DM with a 60% decrease in β cell activity (Ghasemi et al. 2014). Animals with BG greater than 250 mg/dl are considered to have diabetes (Furman 2015). The T2DM, thus induced, also exhibits dyslipidemia, glycosuria, and ketonuria. STZ and alloxan are briefly described under the chemically induced T1DM model. There are various procedures for developing T2DM that mimic the clinical conditions and are described in detail in Table 1.
Fructose and STZ-induced T2DM
Inducing diabetes with a fructose-based solution is a very cost-effective model. Rats (200–250 g) receive variable fructose concentrations (10–40% fructose in normal drinking water) for 2–4 weeks. Before the induction of diabetes, the animals are fasted during the night, followed by a STZ injection (30–40 mg/kg, i.p.). The BG level estimation is carried out after 72 (Oraby et al. 2019). BG levels typically reach more than 300 mg/dL and are considered diabetic (Udumula et al. 2021; Wilson and Islam 2012).
Gold thioglucose (GTG)-induced T2DM
GTG (3,4,5-trihydroxy-6-(hydroxymethyl) oxane-2-thiolate), when administered in three inbred mouse strains (B6, DBA, and BKs) and BDF mice, produces hyperphagia and obesity. The development of obesity-induced diabetes is compared to the insulin secretory capacity of pancreatic cells. GTG-obese mice display significantly reduced islet insulin levels compared to their corresponding control mice. Furthermore, these animals develop the alteration of mRNA expression of hunger and metabolism-related genes in the hypothalamus (Karasawa et al. 2011).
Planned strain development or impaired β cell function model
Planned strain development involves selective breeding techniques or genetic engineering resulting in insulin deficiency.
Goto-Kakizaki Rats GK
GK originated from the repetitive breeding of Wistar rats with an intolerance to glucose, which develops hyperglycemia by adulthood (Goto et al. 1976; Östenson and Efendic 2007). Rats develop IR and impaired insulin secretion along with diabetic complications resulting in renal lesions and alteration in the structure of peripheral nerves and retina of the eyes (Murakawa et al. 2002; Sone et al. 1997). GK rat is further divided into three colonies named Stockholm colony, Dallas colony, and Paris colony. Paris colony showed more than a 50% reduction in the density of β cells at the fetal stage, which is maintained further. GK rats experience hyperglycemia from the third week of birth (Portha et al. 2009).
NONcNZO10/LtJ (NON/NZ)
Non-obese mouse strain was developed by combining two different strains named NON/LtJ strain (88%) and New Zealand obese (NZO/HlJ) (12%), which exhibit maturity onset obesity, hyperglycemia, and insulin resistance (Cho 2007). NON/LtJ males are more susceptible to obesity induced by a high-fat diet (Leiter et al. 2013). An elevated level of IR in the skeletal muscle and liver is observed from 8 weeks of age and gets hyperglycemia by the 12th week (Cho et al. 2007).
UC Davis T2DM rat
UC Davis rats were developed by crossing Sprague–Dawley (SD) and lean-type Zucker diabetic fatty (ZDF) rats. Obese-type SD rats with high fatty acids, triglyceride content, and sustained IR are selected and cross-bred with lean-type ZDF rats with defective β cell production (Cummings et al. 2008). The resulting animals closely resemble human T2DM since they are polygenic, obese, high IR, and glucose intolerant, with deficits in the pancreatic β cell function that ultimately results in altered islet morphology and loss of β cells while retaining the normal leptin signaling.
Zucker diabetic Sprague–Dawley (ZDSD) rats
ZDSD rats are developed by breeding ZDF rats with SD rats which are prone to obesity when supplied with a high-fat diet. SD rats are obese, and ZDF rats have defects in β cell functions. ZDSD maintains a prediabetic condition for more than eight weeks, which mimics the characteristics of T2DM in humans. The inception of diabetes is reflected when glucose is above 250 mg/dl for consequently two weeks, following which the BG level increases steadily (Peterson et al. 2015).
Neonate model of diabetes
Two-day-old neonatal rats are administered STZ leading to hyperglycemic conditions within two days (Portha et al. 1974). Neonatal diabetic glucose maximum level goes up to 300 mg/dl compared to adults who are 500 mg/dl (Barragán-Iglesias et al. 2018). The normal glycemic level is returned due to the regeneration of β cells within ten days. The insulin regeneration process's insufficiency slows down with age, proving inadequate to meet metabolic demands (Bonner-Weir et al. 1981). The model resembles the slow progression of diabetes as in humans and is useful in studying antidiabetic drugs (Portha et al. 2009). Administration of STZ (70 mg/kg, i.p.) in neonate Wistar rats develops DM after 3–5 days and is placed in individual cages till the post-weaning period (Arulmozhi et al. 2004). Hyperglycemia, glucose intolerance, polyphagia, polydipsia, polyuria, and weight loss can be observed.
Pancreas injury models
The pancreas injury model is good for studying normal glucose level compensatory mechanism by β cell regeneration without hyperglycemia (Wang et al. 1995). Pancreatectomy is the ablation of the complete or partial pancreas. At the same time, pancreatic duct ligation involves the ligation of the tail portion of the pancreas, and 90% results in a rise in glucose. The disadvantage of this model is that the small mice size makes the surgery difficult.
Obesity-induced T2DM
Obesity-induced T2DM results from genetic background or unhealthy food habits leading to IR. Genetically induced obesity is further classified as a monogenic and polygenic model.
Monogenic and polygenic models of obesity
The monogenic pattern of obesity is the combination of conditions affecting energy homeostasis throughout the body by increasing dietary intake and reducing energy expenditure. Dietary intake and appetite in a healthy person are controlled by circulating leptin which signals hypothalamus leading to reduced food intake and increased energy consumption. Abnormalities in leptin signaling pathways translate into overeating, uncontrolled appetite, and reduced energy expenditure. Leptin is produced in adipocytes in response to the quantity of triglycerides accumulated in the body (Farooqi and Rahilly 2009). A genetically engineered animal model where a lack of leptin production or leptin receptor mutations with extreme leptin resistance is designed. Ob-gene mutation results in LepOb/Ob and Lepdb/DB mice. Polygenic models are very similar to human obesity, which is why they are used to investigate T2DM and glucose tolerance. Four animal models have been developed: KK-AY mice, New Zealand Obese (NZO) mice, TallHo/Jng mice, and Otsuka Long Evans Tokushima Fat rat (OLETF). A detailed explanation of the above animals is given in Table 2.
Diet-Induced models of diabetes and obesity
Changes in lifestyle and eating habits in the modernized world lead to obesity, the leading cause of diabetes in people. T2DM is induced in rodents through a high-fat, high-energy diet. The diet includes 54% basic feed, 15% lard, 15% sucrose, 4% milk powder, 3% peanut, 5% egg yolk powder, 1% sesame oil, 2% salt, 0.6% dicalcium phosphate, and 0.4% mountain flour. Obesity accompanied by IR develops at eight weeks. Along with a high-fat diet, a cafeteria diet, high sucrose, high fructose diet can contribute to obesity leading to IR and T2DM (Aswar et al. 2019; Nakajima et al. 2015). The C57BL/6 J mouse strain is prone to diet-induced extreme obesity, increased adiposity, glucose intolerance, and mild IR (Hayashi et al. 1989). C57BL/6N strain is the ideal choice for developing obesity and diabetes, as when fed with HFD, they develop hepatosteatosis, hyperglycemia, and hyperinsulinemia. Since it displays significant aspects of T2DM and IR, it is suitable for testing new therapy options. The C57BL/6 mouse when fed with HFD (60% lipids), resulted into fatty lever and fatty pancreas along with marked hyperinsulinemia and corresponding IR with reduced adiponectin. The hyperlipidemia along with IR results in abnormal adipose-insular signaling leading to metabolic syndrome, thus making C57BL/6 an appropriate model to study the antidiabetic drugs that not only reduce BG and upsurge blood insulin but also improve β cell and hepatocyte function as well reduces obesity (Souza-Mello 2010).
Sand RAT (Psammomys obesus)
The Sand Rat (Psammomys obesus) is a polygenic model of a diurnal gerbil. It is lean in physique with average BG and body weight in its natural habitat and survives on a low-calorie plant diet. Nevertheless, when they receive HFD, they become obese and hyperglycemic (Ziv et al. 1999). It is a polygenic model of early-onset obesity with diabetic complications and helps study the effects of diet and exercise on T2DM.
Animal models for maternal diabetes
Due to intra-uterine changes, maternal diabetes and its impact on offspring are vital in diabetes research. The chances of offspring getting diabetic are related to the dam's pre-pregnancy or post-pregnancy diabetic condition. Pups from the diabetic dam are more prone to getting diabetes in later life (Minokoshi et al. 2003). The various models of maternal diabetes are described in Table 2.
Other miscellaneous models for T2DM include drug-induced and iron therapy-induced diabetes models, including hereditary hemochromatosis (HH), thalassemia, and transfusion iron overload, as described in Table 2.
Conclusion
With the alarming rise in diabetes cases worldwide, preclinical models play a vital role in understanding the pathogenesis of human diabetes. They also play a crucial role in the development and study of new drug molecules to treat DM. These animal models can be incorporated into the preclinical evaluation of pharmacotherapies, pathogenicity, and genetic studies and to improve the understanding of the various pathways involved. Each model varies depending on the animal strain, gender, and pathogenesis, as well as benefits and limitations. For selecting an animal model for T1DM, consideration must be given to models of autoimmunity and predictability of onset. In developing the T2DM, selecting an appropriate model depends upon the researcher's objective, such as whether the drug decreases IR or improves β cell function, whether the test molecule has to be tested for obesity-induced diabetes, etc. The selection of strain, species, and sex of the animal also becomes crucial here, as in T1DM, and should also be given strong consideration to improve susceptibility to diabetes induction and treatment. Thus, the selection of the appropriate animal model should be made based on face construct and predictive validity, along with it being reproducible, reliable, and affordable.
Acknowledgements
The authors would like to acknowledge Dr A.P. Pawar, Principal, PCP, BVDU, for providing the necessary facilities to prepare the manuscript. Graphical abstract was prepared by Biorender.The authors would like to acknowledge BANRF, India for supporting Ms Rashmi Patil.
Funding
Not applicable
Data availability
It is a review article; hence, no laboratory data was generated.
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical statement
Not applicable (review article).
References
- Alkandahri MY, Sujana D, Hasyim DM, Shafirany MZ, Sulastri L, et al. Antidiabetic activity of extract and fractions of castanopsis costata leaves on alloxan-induced diabetic mice. Pharmacogn J. 2021;13(6):1589–1593. doi: 10.5530/pj.2021.13.204. [DOI] [Google Scholar]
- Amare YE. Methanolic Extract of Myrsine africana leaf ameliorates hyperglycemia and dyslipidemia in alloxan-induced diabetic albino mice. eCAM. 2021;398:765263. doi: 10.1155/2021/3987656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arulmozhi DK, Veeranjaneyulu A, Bodhankar SL. Neonatal streptozotocin-induced rat model of type 2 diabetes mellitus: a glance. Indian J Pharmacol. 2004;36(4):217–221. [Google Scholar]
- Arya A, Jamil Al-Obaidi MM, Karim RB, Taha H, Khan AK, et al. Extract of Woodfordia fruticosa flowers ameliorates hyperglycemia, oxidative stress and improves β-cell function in streptozotocin-nicotinamide induced diabetic rats. J Ethnopharmacol. 2015;175:229–240. doi: 10.1016/j.jep.2015.08.057. [DOI] [PubMed] [Google Scholar]
- Aswar U, Patil R, Bodhankar S. Short review on the induction of obesity in laboratory animals. Diabesity. 2019;5(4):25–31. doi: 10.15562/diabesity.2019.58. [DOI] [Google Scholar]
- Aurora S, Shreesh KO, Divya V. Characterization of Streptozotocin Induced Diabetes Mellitus in Swiss Albino Mice. Glob J Pharmacol. 2009;3(2):81–84. [Google Scholar]
- Azushima K, Gurley SB, Coffman TM. Modelling diabetic nephropathy in mice. Nat Rev Nephrol. 2017;14(1):48–56. doi: 10.1038/nrneph.2017.142. [DOI] [PubMed] [Google Scholar]
- Badole S, Patel N, Bodhankar S, Jain B, Bhardwaj S. Antihyperglycemic activity of aqueous extract of leaves of Cocculus hirsutus (L.) Diels in alloxan-induced diabetic mice. Indian J Pharmacol. 2006;38(1):49–53. doi: 10.4103/0253-7613.19853. [DOI] [Google Scholar]
- Baek HS, Yoon JW. Role of macrophages in the pathogenesis of encephalomyocarditis virus-induced diabetes in mice. J Virol. 1990;64(12):5708–5715. doi: 10.1128/jvi.64.12.5708-5715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragán-Iglesias P, Oidor-Chan VH, Loeza-Alcocer E, Pineda-Farias JB, Velazquez-Lagunas I, et al. Evaluation of the neonatal streptozotocin model of diabetes in rats: evidence for a model of neuropathic pain. Pharmacol Rep. 2018;70(2):294–303. doi: 10.1016/j.pharep.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, Trent DF, Honey RN, Weir GC. Responses of neonatal rat islets to streptozotocin. Limited B cell regeneration and hyperglycemia. Diabetes. 1981;30(1):64–69. doi: 10.2337/diab.30.1.64. [DOI] [PubMed] [Google Scholar]
- Campbell J, Chaikof L, Davidson IW. Metahypophyseal diabetes produced by growth hormone. Endocrinology. 1954;54(1):48–58. doi: 10.1210/endo-54-1-48. [DOI] [PubMed] [Google Scholar]
- Cho YR, Kim HJ, Park SY, Ko HJ, Hong EG, et al. Hyperglycemia, maturity-onset obesity, and insulin resistance in NONcNZO10/LtJ males, a new mouse model of type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;293(1):E327–E336. doi: 10.1152/ajpendo.00376.2006. [DOI] [PubMed] [Google Scholar]
- Christen U, Bender C, Von Herrath MG. Infection as a cause of type 1 diabetes? Curr Opin Rheumatol. 2012;24(4):417–423. doi: 10.1097/BOR.0b013e3283533719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey RC, Jouihan HA, Ajioka RS, Hazel MW, Jones DL, et al. Oxidative stress, β-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology. 2004;145(11):5305–5312. doi: 10.2119/2007-00114.Jouihan. [DOI] [PubMed] [Google Scholar]
- Craighead JE, McLane MF. Diabetes mellitus: Induction in mice by encephalomyocarditis virus. Science. 1968;162(3856):913–914. doi: 10.1126/science.162.3856.913. [DOI] [PubMed] [Google Scholar]
- Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, et al. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R1782–R1793. doi: 10.1152/ajpregu.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P, Hussain MAM, Varghese Z, Politis D, Flynn DM, Hoffbrand AV. Insulin Resistance and Iron Overload. Ann Clin Biochem. 1983;20(2):77–79. doi: 10.1177/000456328302000203. [DOI] [PubMed] [Google Scholar]
- Domingues A, Sartori A, Golim M, Valente L, Rosa L, Ishikawa L, Siani A, Viero R. Prevention of experimental diabetes by Uncaria tomentosa extract: Th2 polarization, regulatory T cell preservation or both? J Ethnopharmacol. 2011;137(1):635–642. doi: 10.1016/j.jep.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Dowarah J, Singh VP. Anti-diabetic drugs recent approaches and advancements. Bioorg Med Chem. 2020;28(5):115263. doi: 10.1016/j.bmc.2019.115263. [DOI] [PubMed] [Google Scholar]
- Drel VR, Pacher P, Stavniichuk R, Xu W, Zhang J, et al. Poly(ADP-ribose)polymerase inhibition counteracts renal hypertrophy and multiple manifestations of peripheral neuropathy in diabetic Akita mice. Int J Mol Med. 2011;28(4):629–635. doi: 10.3892/ijmm.2011.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc PU. The development of obesity, hyperinsulinemia, and hyperglycemia in ob/ob mice. Metabolism. 1976;25(12):1567–1574. doi: 10.1016/0026-0495(76)90109-8. [DOI] [PubMed] [Google Scholar]
- Ellerman KE, Richards CA, Guberski DL, Shek WR, Like AA. Kilham rat triggers T cell-dependent autoimmune diabetes in multiple strains of rat. Diabetes. 1996;45(5):557–562. doi: 10.2337/diab.45.5.557. [DOI] [PubMed] [Google Scholar]
- Etgen GJ, Oldham BA. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metab. 2000;49(5):684–688. doi: 10.1016/S0026-0495(00)80049-9. [DOI] [PubMed] [Google Scholar]
- Sadaf Farooqi I, O’Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr. 2009;89(3):980–984. doi: 10.3945/ajcn.2008.26788C. [DOI] [PubMed] [Google Scholar]
- Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- Federiuk IF, Casey HM, Quinn MJ, Wood MD, Ward WK. Induction of type-1 diabetes mellitus in laboratory rats by use of alloxan: route of administration, pitfalls, and insulin treatment. Comp Med. 2004;54(3):252–257. [PubMed] [Google Scholar]
- Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70(1):5–47. doi: 10.1002/0471141755.ph0547s70. [DOI] [PubMed] [Google Scholar]
- Gao T, Jiao Y, Liu Y, Li T, Wang Z, Wang D. Protective effects of konjac and inulin extracts on type 1 and type 2 diabetes. J Diabetes Res 2019. 2019 doi: 10.1155/2019/3872182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi A, Khalifi S, Jedi S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes. Acta Physiol Hung. 2014;101(4):408–420. doi: 10.1556/APhysiol.101.2014.4.2. [DOI] [PubMed] [Google Scholar]
- Gorus FK, Malaisse WJ, Pipeleers DG. Selective uptake of alloxan by pancreatic B cells. Biochem J. 1982;208(2):513–515. doi: 10.1042/bj2080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med. 1976;119(1):85–90. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171(8):4040–4047. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- Hales CN, Desai M, Ozanne SE, Crowther NJ. Fishing in the stream of diabetes: From measuring insulin to the control of fetal organogenesis. Biochem Soc Trans. 1996;24(2):341–350. doi: 10.1042/bst0240341. [DOI] [PubMed] [Google Scholar]
- Hammeso WW, Emiru YK, Getahun KA, Kahaliw W. Antidiabetic and antihyperlipidemic activities of the leaf latex extract of aloe megalacantha baker (Aloaceae) in streptozotocin-induced diabetic model. eCAM 2019. 2019 doi: 10.1155/2019/8263786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchang W, Khamchan A, Wongmanee N, Seedadee C. Hesperidin ameliorates pancreatic β-cell dysfunction and apoptosis in streptozotocin-induced diabetic rat model. Life Sci. 2019;235:116858. doi: 10.1016/j.lfs.2019.116858. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Utsuyama M, Kurashima C, Hirokawa K. Spontaneous development of organ-specific autoimmune lesions in aged C57BL/6 mice. Clin Exp Immunol. 1989;78(1):120–126. [PMC free article] [PubMed] [Google Scholar]
- Himpe E, Cunha DA, Song I, Bugliani M, Marchetti P. Phenylpropenoic acid glucoside from rooibos protects pancreatic beta cells against cell death induced by acute injury. PLoS ONE. 2016;11(6):1–13. doi: 10.1371/journal.pone.0157604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa K, Jun HS, Maeda K, Kawaguchi Y, Itagaki S, et al. Possible role of macrophage-derived soluble mediators in the pathogenesis of encephalomyocarditis virus-induced diabetes in mice. J Virol. 1997;71(5):4024–4031. doi: 10.1128/jvi.71.5.4024-4031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg R, Refai E, Höög A, Crooke RM, Graham M, et al. Lowering apolipoprotein CIII delays onset of type 1 diabetes. Proc Natl Acad Sci U S A. 2011;108(26):10685–10689. doi: 10.1073/pnas.1019553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hu TG, Wen P, Shen WZ, Liu F, Li Q, et al. Effect of 1-Deoxynojirimycin isolated from mulberry leaves on glucose metabolism and gut microbiota in a streptozotocin-induced diabetic mouse. Model J Nat Prod. 2019;82(8):2189–2200. doi: 10.1021/acs.jnatprod.9b00205. [DOI] [PubMed] [Google Scholar]
- Huang J, Simcox J, Mitchell TC, Jones D, Cox J, et al. Iron regulates glucose homeostasis in liver and muscle via AMP-activated protein kinase in mice. FASEB J. 2013;27(7):2845–2854. doi: 10.1096/fj.12-216929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):315–317. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- Iwase M, Nunoi K, Wakisaka M, Kikuchi M, Maki Y, et al. Spontaneous recovery from non-insulin-dependent diabetes mellitus induced by neonatal streptozotocin treatment in spontaneously hypertensive rats. Metabolism. 1991;40(1):10–14. doi: 10.1016/0026-0495(91)90184-X. [DOI] [PubMed] [Google Scholar]
- Jörns A, Günther A, Hedrich HJ, Wedekind D, Tiedge M, Lenzen S. Immune cell infiltration, cytokine expression, and β-cell apoptosis during the development of type 1 diabetes in the spontaneously diabetic LEW.1AR1/Ztm-iddm rat. Diabetes. 2005;54(7):2041–2052. doi: 10.2337/diabetes.54.7.2041. [DOI] [PubMed] [Google Scholar]
- Jouihan HA, Cobine PA, Cooksey RC, Hoagland EA, Boudina S, et al. Iron-mediated inhibition of mitochondrial manganese uptake mediates mitochondrial dysfunction in a mouse model of hemochromatosis. Mol Med. 2008;14(3–4):98–108. doi: 10.2119/2007-00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun HS, Yoon JW. The role of viruses in Type I diabetes: two distinct cellular and molecular pathogenic mechanisms of virus-induced diabetes in animals. Diabetologia. 2001;44(3):271–285. doi: 10.1007/s001250051614. [DOI] [PubMed] [Google Scholar]
- Jwad M, Yousif Al-Fatlawi H, Student PD. Types of diabetes and their effect on the immune system. J Adv Pharm Pract. 2022;4(1):21–30. [Google Scholar]
- Karasawa H, Takaishi K, Kumagae Y. Obesity-induced diabetes in mouse strains treated with gold thioglucose: a novel animal model for studying β-cell dysfunction. Obesity. 2011;19(3):514–521. doi: 10.1038/oby.2010.171. [DOI] [PubMed] [Google Scholar]
- Kava R, Peterson RG, West DB, Greenwood MRC. Wistar diabetic fatty rat. ILAR J. 1990;32(3):9–13. doi: 10.1093/ilar.32.3.9. [DOI] [Google Scholar]
- Kava R, Greenwood MRC, Johnson PR. Zucker ( fa/fa ) Rat. ILAR J. 1990;32(3):4–8. doi: 10.1093/ilar.32.3.4. [DOI] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract. 1994;24:S317–S320. doi: 10.1016/0168-8227(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Kawasaki E, Abiru N, Eguchi K. Prevention of type 1 diabetes: From the view point of β cell damage. Diabetes Res Clin Pract. 2004;66(66):S27–S32. doi: 10.1016/j.diabres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Kim JH, Stewart TP, Zhang W, Kim HY, Nishina PM, Naggert JK. Type 2 diabetes mouse model TallyHo carries an obesity gene on chromosome 6 that exaggerates dietary obesity. Physiol Genomics. 2005;22(26):171–181. doi: 10.1152/physiolgenomics.00197.2004. [DOI] [PubMed] [Google Scholar]
- Kim TK, Lee JC, Im SH, Lee MS. Amelioration of autoimmune diabetes of NOD mice by immunomodulating probiotics. Front Immunol. 2020;11:1832. doi: 10.3389/fimmu.2020.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012;166(3):877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocaman N, Kuloğlu T. Expression of asprosin in rat hepatic, renal, heart, gastric, testicular and brain tissues and its changes in a streptozotocin-induced diabetes mellitus model. Tissue Cell. 2020;66:101397. doi: 10.1016/j.tice.2020.101397. [DOI] [PubMed] [Google Scholar]
- Kong LL, Wu H, Cui WP, Zhou WH, Luo P, et al. Advances in murine models of diabetic nephropathy. J Diabetes Res. 2013 doi: 10.1155/2013/797548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter EH, Reifsnyder PC. Differential levels of diabetogenic stress in two new mouse models of obesity and type 2 diabetes. Diabetes. 2004;53(1):S4–11. doi: 10.2337/diabetes.53.2007.s4. [DOI] [PubMed] [Google Scholar]
- Leiter EH, Strobel M, O’Neill A, Schultz D, Schile A, Reifsnyder PC. Comparison of two new mouse models of polygenic type 2 diabetes at the Jackson Laboratory, NONcNZO10Lt/J and TALLYHO/JngJ. J Diabetes Res. 2013 doi: 10.1155/2013/165327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- Lenzen S, Munday R. Thiol-group reactivity, hydrophilicity and stability of alloxan, its reduction products and its N-methyl derivatives and a comparison with ninhydrin. Biochem Pharmacol. 1991;42(7):1385–1391. doi: 10.1016/0006-2952(91)90449-F. [DOI] [PubMed] [Google Scholar]
- Li S, Huang Q, Zhang L, Qiao X, Zhang Y, Tang F, Li Z. Effect of CAPE-pNO2 against type 2 diabetes mellitus via the AMPK/GLUT4/ GSK3β/PPARα pathway in HFD/STZ-induced diabetic mice. Eur J Pharmacol. 2019;853:1–10. doi: 10.1016/j.ejphar.2019.03.027. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol. 2012;58(1):5–61. doi: 10.1002/0471141755.ph0561s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiello P, Broca C, Gross R, Roye M, Manteghetti M, et al. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47(2):224–229. doi: 10.2337/diab.47.2.224. [DOI] [PubMed] [Google Scholar]
- Melanitou E, Devendra D, Liu E, Miao D, Eisenbarth GS. Early and quantal (by litter) expression of insulin autoantibodies in the nonobese diabetic mice predict early diabetes onset. J Immunol. 2004;173(11):6603–6610. doi: 10.4049/jimmunol.173.11.6603. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kahn CR, Kahn BB. Tissue-specific ablation of the GLUT4 glucose transporter or the insulin receptor challenges assumptions about insulin action and glucose homeostasis. J Biol Chem. 2003;278(36):33609–33612. doi: 10.1074/jbc.R300019200. [DOI] [PubMed] [Google Scholar]
- Mordes JP, Poussier P, Rossini AA, Blankenhorn EP, Greiner DL (2007) Rat models of type 1 diabetes: genetics, environment, and autoimmunity. Anim Model Diabetes Second Ed Front Res 10.1093/ilar.45.3.278 [DOI] [PubMed]
- Muhlhausler B, Smith SR. Early-life origins of metabolic dysfunction: role of the adipocyte. Trends Endocrinol Metab. 2009;20(2):51–57. doi: 10.1016/j.tem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Munday R. Dialuric acid autoxidation. Biochem Pharmacol. 1988;37(3):409–413. doi: 10.1016/0006-2952(88)90207-9. [DOI] [PubMed] [Google Scholar]
- Murakawa Y, Zhang W, Pierson CR, Brismar T, Östenson CG, Sima AAF. Impaired glucose tolerance and insulinopenia in the GK-rat causes peripheral neuropathy. Diabetes Metab Res Rev. 2002;18(6):473–483. doi: 10.1002/dmrr.326. [DOI] [PubMed] [Google Scholar]
- Naderi A, Zahed R, Aghajanpour L, Amoli FA, Lashay A. Long term features of diabetic retinopathy in streptozotocin-induced diabetic Wistar rats. Exp Eye Res. 2019;184:213–220. doi: 10.1016/j.exer.2019.04.025. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Hira T, Hara H. Postprandial glucagon-like peptide-1 secretion is increased during the progression of glucose intolerance and obesity in high-fat/high-sucrose diet-fed rats. Br J Nutr. 2015;113(9):1477–1488. doi: 10.1017/S0007114515000550. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Yamada K. Studies on a diabetic (KK) strain of the mouse. Diabetologia. 1967;3:212–221. doi: 10.1007/BF01222198. [DOI] [PubMed] [Google Scholar]
- Nayak Y, Hillemane V, Daroji VK, Ayashree BSJ, Unnikrishnan MK. Antidiabetic activity of benzopyrone analogues in nicotinamide-streptozotocin induced type 2 diabetes in rats. Sci World J. 2014 doi: 10.1155/2014/854267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir N, Muhammad J, Ghaffar R, Nisar M, Zahoor M, Uddin F, Ullah R, Alotaibi A. Phytochemical profiling and antioxidant potential of Daphne mucronata Royle and action against paracetamol-induced hepatotoxicity and nephrotoxicity in rabbits. Saudi J Biol Sci. 2021;28(9):5290–5301. doi: 10.1016/j.sjbs.2021.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obasi DC, Ogugua VN. GC-MS analysis, pH and antioxidant effect of Ruzu herbal bitters on alloxan-induced diabetic rats. Biochem Biophys Reports. 2021;27:101057. doi: 10.1016/j.bbrep.2021.101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguntibeju OO, Aboua GY, Omodanisi EI. Effects of Moringa oleifera on oxidative stress, apoptotic and inflammatory biomarkers in streptozotocin-induced diabetic animal model. South African J Bot. 2020;129:354–365. doi: 10.1016/j.sajb.2019.08.039. [DOI] [Google Scholar]
- Onyibe PN, Edo GI, Nwosu LC, Ozgor E. Effects of vernonia amygdalina fractionate on glutathione reductase and glutathione-S-transferase on alloxan induced diabetes wistar rat. Biocatal Agric Biotechnol. 2021;36:102118. doi: 10.1016/j.bcab.2021.102118. [DOI] [Google Scholar]
- Oraby MA, El-Yamany MF, Safar MM, Assaf N, Ghoneim HA. Dapagliflozin attenuates early markers of diabetic nephropathy in fructose-streptozotocin-induced diabetes in rats. Biomed Pharmacother. 2019;109:910–920. doi: 10.1016/j.biopha.2018.10.100. [DOI] [PubMed] [Google Scholar]
- Östenson CG, Efendic S. Islet gene expression and function in type 2 diabetes; studies in the Goto–Kakizaki rat and humans. Diabetes, Obes Metab. 2007;9(Suppl 2):180–186. doi: 10.1111/j.1463-1326.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic β cells. Apoptosis. 2002;7(4):335–345. doi: 10.1111/j.1463-1326.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- Pak CY, Mcarthur RG, Eun HM, Yoon JW. Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet. 1988;332(8601):1–4. doi: 10.1016/S0140-6736(88)92941-8. [DOI] [PubMed] [Google Scholar]
- Park EY, Kim HJ, Kim YK, Park SU, Choi JE, et al. Increase in insulin secretion induced by panax ginseng berry extracts contributes to the amelioration of hyperglycemia in streptozotocin induced diabetic mice. J Ginseng Res. 2012;36(2):153–160. doi: 10.5142/jgr.2012.36.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RG, Jackson CV, Zimmerman K, De Winter W, Huebert N, Hansen MK. Characterization of the ZDSD rat: a translational model for the study of metabolic syndrome and type 2 diabetes. J Diabetes Res. 2015 doi: 10.1155/2015/487816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portha B, Levacher C, Picon L, Rosselin G. Diabetogenic effect of streptozotocin in the rat during the perinatal period. Diabetes. 1974;23(11):889–895. doi: 10.2337/diab.23.11.889. [DOI] [PubMed] [Google Scholar]
- Portha B, Lacraz G, Kergoat M, Homo-Delarche F, Giroix MH, et al. The GK rat beta-cell: a prototype for the diseased human beta-cell in type 2 diabetes? Mol Cell Endocrinol. 2009;297(1–2):73–85. doi: 10.1016/j.mce.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Pozzilli P, Signore A, Williams AJK, Beales PE. NOD mouse colonies around the world- recent facts and figures. Immunol Today. 1993;14(5):193–196. doi: 10.1016/0167-5699(93)90160-M. [DOI] [PubMed] [Google Scholar]
- Raafat K, Aboul-Ela M, El-Lakany A. Alloxan-induced diabetic thermal hyperalgesia, prophylaxis and phytotherapeutic effects of Rheum ribes L. in mouse model. Arch Pharm Res. 2021;44(8):1–10. doi: 10.1007/s12272-014-0372-y. [DOI] [PubMed] [Google Scholar]
- Radenković M, Stojanović M, Prostran M. Experimental diabetes induced by alloxan and streptozotocin: the current state of the art. J Pharmacol Toxicol Methods. 2016;78:13–31. doi: 10.1016/j.vascn.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, et al. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49(11):1390–1394. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- Reusser F. Mode of action of streptozotocin. J Bacteriol. 1971;105(2):580–588. doi: 10.1128/jb.105.2.580-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387(10035):2340–2348. doi: 10.1016/S0140-6736(16)30507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roep BO, Atkinson M. Animal models have little to teach us about Type 1 diabetes: 1. In support of this proposal. Diabetologia. 2004;47(10):1650–1656. doi: 10.1007/s00125-004-1517-1. [DOI] [PubMed] [Google Scholar]
- Rossini AA, Like AA, Chick WL, Appel MC, Cahill GF. Studies of streptozotocin induced insulitis and diabetes. Proc Natl Acad Sci U S A. 1977;74(6):2485–2489. doi: 10.1073/pnas.74.6.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliu TP, Yazawa N, Hashimoto K, Miyata K, Kudo A. Serum amyloid a3 promoter-driven luciferase activity enables visualization of diabetic kidney disease. Int J Mol Sci. 2022;23(2):899. doi: 10.3390/ijms23020899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Niswender KD. Adiposity signaling and biological defense against weight gain: Absence of protection or central hormone resistance? J Clin Endocrinol Metab. 2004;89(12):5889–5897. doi: 10.1210/jc.2004-0906. [DOI] [PubMed] [Google Scholar]
- Sergeys J, Etienne I, Van Hove I, Lefevere E, Stalmans I, et al. Longitudinal in vivo characterization of the streptozotocin-induced diabetic mouse model: Focus on early inner retinal responses. Investig Ophthalmol vis Sci. 2019;60(2):807–822. doi: 10.1167/iovs.18-25372. [DOI] [PubMed] [Google Scholar]
- Skovsø S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig. 2014;5(4):349–358. doi: 10.1111/jdi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slieker LJ, Sundell KL, Heath WF, Osborne HE, Bue J, et al. Glucose transporter levels in tissues of spontaneously diabetic Zucker fa/fa rat (ZDF/drt) and viable yellow mouse (A(vy)/a) Diabetes. 1992;41(2):187–193. doi: 10.2337/diab.41.2.187. [DOI] [PubMed] [Google Scholar]
- Sole SS, Srinivasan BP. Aqueous extract of tamarind seeds selectively increases glucose transporter-2, glucose transporter-4, and islets’ intracellular calcium levels and stimulates β-cell proliferation resulting in improved glucose homeostasis in rats with streptozotocin-induce. Nutr Res. 2012;32(8):626–636. doi: 10.1016/j.nutres.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Solikhah TI, Solikhah GP. Effect of Muntingia calabura L. Leaf Extract on blood glucose levels and body weight of alloxan-induced diabetic mice. Pharmacogn J. 2021;13(6):1450–1455. doi: 10.5530/PJ.2021.13.184. [DOI] [Google Scholar]
- Sone H, Kawakami Y, Okuda Y, Sekine Y, Honmura S, et al. Ocular vascular endothelial growth factor levels in diabetic rats are elevated before observable retinal proliferative changes. Diabetologia. 1997;40(6):726–730. doi: 10.1007/s001250050740. [DOI] [PubMed] [Google Scholar]
- Souza-Mello V, Gregorio BM, Cardoso-de-Lemos FS, de Carvalho L, Aguila MB, Mandarim-de-Lacerda CA. Comparative effects of telmisartan, sitagliptin and metformin alone or in combination on obesity, insulin resistance, and liver and pancreas remodelling in C57BL/6 mice fed on a very high-fat diet. Clin Sci. 2010;119(6):239–250. doi: 10.1042/CS20100061. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat : A model for type 2 diabetes and pharmacological screening. Pharmacological Res. 2005;52:313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Steiner H, Oelz O, Zahnd G, Froesch ER. Studies on islet cell regeneration, hyperplasia and intrainsular cellular interrelations in long lasting streptozotocin diabetes in rats. Diabetologia. 1970;6(6):558–564. doi: 10.1007/BF00418221. [DOI] [PubMed] [Google Scholar]
- De SC, Zhang B, Zhang JK, Xu CJ, Wu YL, et al. Cyanidin-3-glucoside-rich extract from Chinese bayberry fruit protects pancreatic β cells and ameliorates hyperglycemia in streptozotocin-induced diabetic mice. J Med Food. 2012;15(3):288–298. doi: 10.1089/jmf.2011.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguchov A. Caveolin: a new link between diabetes and AD. Cell Mol Neurobiol. 2020;40(7):1059–1066. doi: 10.1007/s10571-020-00796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabaie PS, Yazdanparast R. Teucrium polium extract reverses symptoms of streptozotocin-induced diabetes in rats via rebalancing the Pdx1 and FoxO1 expressions. Biomed Pharmacother. 2017;93:1033–1039. doi: 10.1016/j.biopha.2017.06.082. [DOI] [PubMed] [Google Scholar]
- Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol. 2012;8(6):342–351. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- Toda C, Diano S. Mitochondrial UCP2 in the central regulation of metabolism. Best Pract Res Clin Endocrinol Metab. 2014;28(5):757–764. doi: 10.1016/j.beem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Udumula MP, Mangali S, Kalra J, Dasari D, Goyal S, et al. High fructose and streptozotocin induced diabetic impairments are mitigated by Indirubin-3-hydrazone via downregulation of PKR pathway in Wistar rats. Sci Rep. 2021;11(1):1–11. doi: 10.1038/s41598-021-92345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Ikegami H, Yamato E, Fu J, Fukuda M, et al. The NSY mouse: a new animal model of spontaneous NIDDM with moderate obesity. Diabetologia. 1995;38(5):503–508. doi: 10.1007/BF00400717. [DOI] [PubMed] [Google Scholar]
- Von Herrath M, Nepom GT. Animal models of human type 1 diabetes. Nat Immunol. 2009;10(2):129–132. doi: 10.1038/ni0209-129. [DOI] [PubMed] [Google Scholar]
- Wallis RH, Wang K, Marandi L, Hsieh E, Ning T, et al. Type 1 diabetes in the BB rat: a polygenic disease. Diabetes. 2009;58(4):1007–1017. doi: 10.2337/db08-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gleichmann H. GLUT2 in pancreatic islets: crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes. 1998;47(1):50–56. doi: 10.2337/diab.47.1.50. [DOI] [PubMed] [Google Scholar]
- Wang RN, Kliippel G, Bouwens L, Jette C. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38:1405–1411. doi: 10.1007/BF00400600. [DOI] [PubMed] [Google Scholar]
- Wang ZH, Hsu CC, Lin HH, Chen JH. Antidiabetic effects of carassius auratus complex formula in high fat diet combined streptozotocin-induced diabetic mice. Evid-Based Complement Altern Med. 2014 doi: 10.1155/2014/628473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DC, McDaniel ML, Naber SP, Barry CD, Lacy PE. Alloxan stimulation and inhibition of insulin release from isolated rat islets of Langerhans. Diabetes. 1978;27(12):1205–1214. doi: 10.2337/diab.27.12.1205. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe ASD, Kalansuriya P, Attanayake AP. Herbal medicines targeting the improved β -cell functions and β -cell regeneration for the management of diabetes mellitus. Evidence-Based Complement Altern Med. 2021;2021:1–32. doi: 10.1155/2021/2920530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RD, Islam MS. Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Rep. 2012;64(1):129–139. doi: 10.1016/S1734-1140(12)70739-9. [DOI] [PubMed] [Google Scholar]
- Wszola M, Klak M, Kosowska A, Tymicki G, Berman A, et al. Streptozotocin-induced diabetes in a mouse model (balb/c) is not an effective model for research on transplantation procedures in the treatment of type 1 diabetes. Biomedicines. 2021;9(12):1790. doi: 10.3390/biomedicines9121790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Sun B, Li D, Mao R, Li H, et al. Beneficial effects of small molecule oligopeptides isolated from Panax ginseng meyer on pancreatic beta-cell dysfunction and death in diabetic rats. Nutrients. 2017;9(10):1061. doi: 10.3390/nu9101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami T, Miwa A, Takasawa S, Yamamoto H, Okamoto H. Induction of rat pancreatic B cell tumors by the combined administration of streptozotocin or alloxan and poly(adenosine diphosphate ribose) synthetase inhibitors. Cancer Res. 1985;45(4):1845–1849. [PubMed] [Google Scholar]
- Yadav KS, Yadav NP, Shanker K, Thomas SC, Srivastav S, et al. Assessment of antidiabetic potential of Cissampelos pareira leaf extract in streptozotocin–nicotinamide induced diabetic mice. J Pharm Res. 2013;6(8):874–878. doi: 10.1016/j.jopr.2013.06.027. [DOI] [Google Scholar]
- Yao HW, Ling P, Chen SH, Tung YY, Chen SH. Factors affecting herpes simplex virus reactivation from the explanted mouse brain. Virology. 2012;433(1):116–123. doi: 10.1016/j.virol.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Yokoi N, Namae M, Fuse M, Wang HY, Hirata T, et al. Establishment and characterization of the Komeda diabetes-prone rat as a segregating inbred strain. Exp Anim. 2003;52(4):295–301. doi: 10.1538/expanim.52.295. [DOI] [PubMed] [Google Scholar]
- Yoon JW, Ko W, Bae YS, Pak CY, Amano K, et al. Identification of antigenic differences between the diabetogenic and non-diabetogenic variants of encephalomyocarditis virus using monoclonal antibodies. J Gen Virol. 1988;69(5):1085–1090. doi: 10.1099/0022-1317-69-5-1085. [DOI] [PubMed] [Google Scholar]
- Yu J, Sharma P, Girgis CM, Gunton JE. Vitamin D and beta cells in type 1 diabetes: a systematic review. Int J Mol Sci. 2022;23(22):14434. doi: 10.3390/ijms232214434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Yanni J, Qureshi MA, Logantha S, et al. Electrical conduction system remodeling in streptozotocin-induced diabetes mellitus rat heart. Front Physiol. 2019;10:1–15. doi: 10.3389/fphys.2019.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Pridgen B, King N, Xu J, Breslow JL. Hyperglycemic Ins2AkitaLdlr–/– mice show severely elevated lipid levels and increased atherosclerosis: a model of type 1 diabetic macrovascular disease. J Lipid Res. 2011;52(8):1483–1493. doi: 10.1194/jlr.M014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuge F, Ni Y, Wan C, Liu F, Fu Z. Anti-diabetic effects of astaxanthin on an stz-induced diabetic model in rats. Endocr J. 2021;68(4):451–459. doi: 10.1507/endocrjEJ20-0699. [DOI] [PubMed] [Google Scholar]
- Ziv E, Shafrir E, Kalman R, Galer S, Bar-On H. Changing pattern of prevalence of insulin resistance in Psammomys obesus, a model of nutritionally induced type 2 diabetes. Metab Clin Exp. 1999;48(12):1549–1554. doi: 10.1016/s0026-0495(99)90244-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
It is a review article; hence, no laboratory data was generated.