Abstract
About half of colorectal cancers harbor mutations in the KRAS gene. The presence of these mutations is associated with worse prognosis and, until now, the absence of matched targeted therapy options. In this review, we discuss clinical efforts to target KRAS in colorectal cancer from studies of downstream inhibitors to recent direct inhibitors of KRASG12C and other KRAS mutants. Early clinical trial data, however, suggest more limited activity for these novel inhibitors in colorectal cancer compared to other cancer types, and we discuss the role of receptor tyrosine kinase signaling and parallel signaling pathways in modulating response to these inhibitors. We also review the effect of KRAS mutations on the tumor-immune microenvironment and efforts to induce an immune response against these tumors.
Introduction
The Kirsten rat sarcoma virus (KRAS) gene is the most commonly mutated oncogene in cancer, and its activation promotes tumor proliferation and survival. KRAS mutations occur in 45% of colorectal cancer (CRC) and is a key driver in CRC oncogenesis.[1] Mutant KRAS is frequent in microsatellite stable right-sided CRC and is associated with poor prognosis (Figure 1).[2–4] KRAS mutations preclude response to epidermal growth factor receptor (EGFR) inhibitors and testing for KRAS mutations is recommended for all patients with metastatic CRC.[5,6] Successful targeting of KRAS has been elusive until the recent development of KRASG12C inhibitors. In this review, we will discuss progress in direct KRAS inhibition, highlight other therapeutic approaches for KRAS mutant CRC, and summarize recently completed and ongoing clinical trials (Tables 1 and 2).
Figure 1.
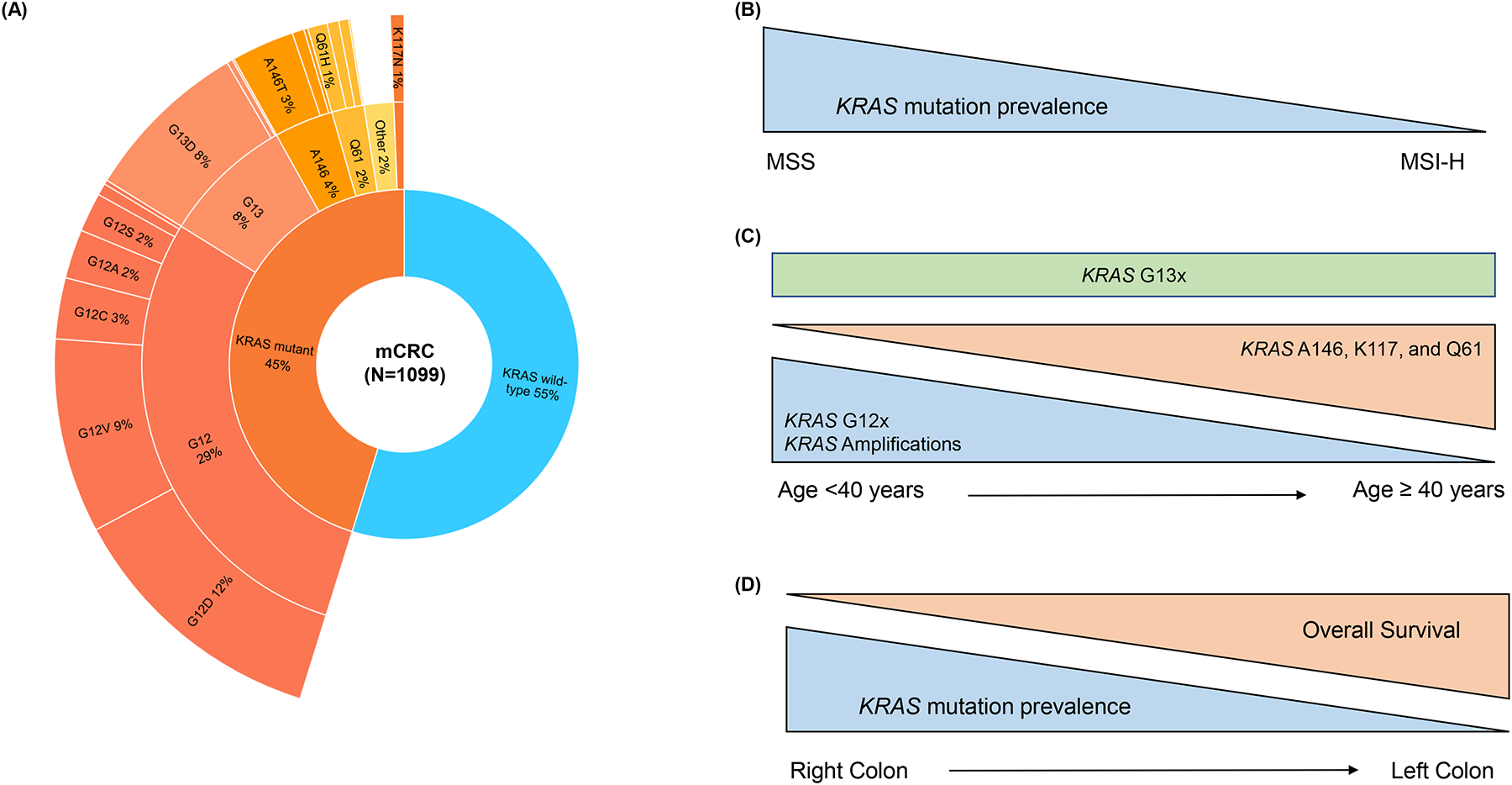
Frequency and clinical associations reported for KRAS alterations in metastatic colorectal cancer (mCRC)
A) Frequencies of KRAS alterations in mCRC at Memorial Sloan Kettering Cancer Center.1 Dataset was obtained through the cBioPortal for Cancer Genomics.
B) KRAS mutations are more frequent in microsatellite stable (MSS) CRC as compared to microsatellite instability-high (MSI-H) CRC.3
C) In a CRC dataset from Foundation Medicine (N = 13,336), there was higher prevalence of KRAS G12 mutations and KRAS amplifications in younger patients; more frequent KRAS A146, K117, and Q61 mutations in older patients; and no difference in KRAS G13 alterations by age. Analysis used age as a continuous variable as well as used age 40 as a stratification cut-off.3
D) Prevalence of KRAS mutations decreases sharply from right to left-sided colon cancer but starts rising again in rectal cancer.2 Right-sided CRC is independently associated with worse overall survival as compared to left-sided CRC.2 Specific KRAS mutations are also associated with poor prognosis.4
Table 1.
Ongoing and recent clinical trials of KRAS inhibitors for KRAS mutant colorectal cancer..
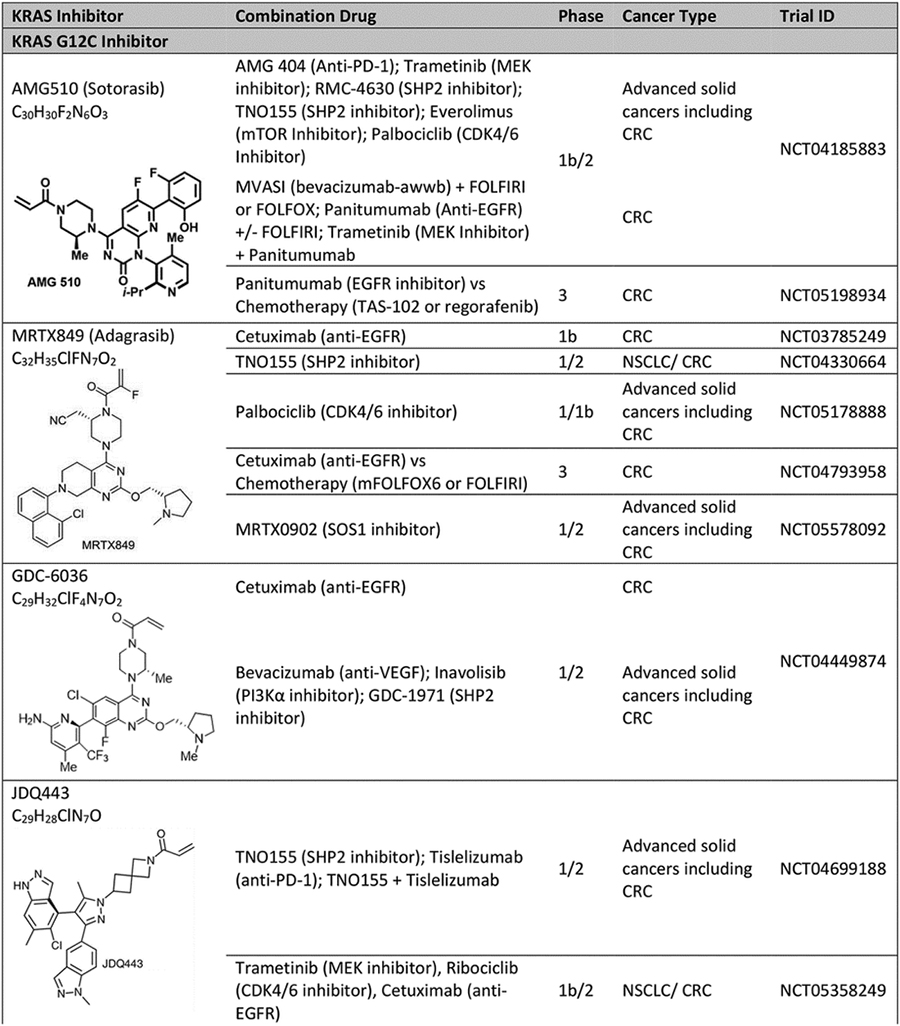
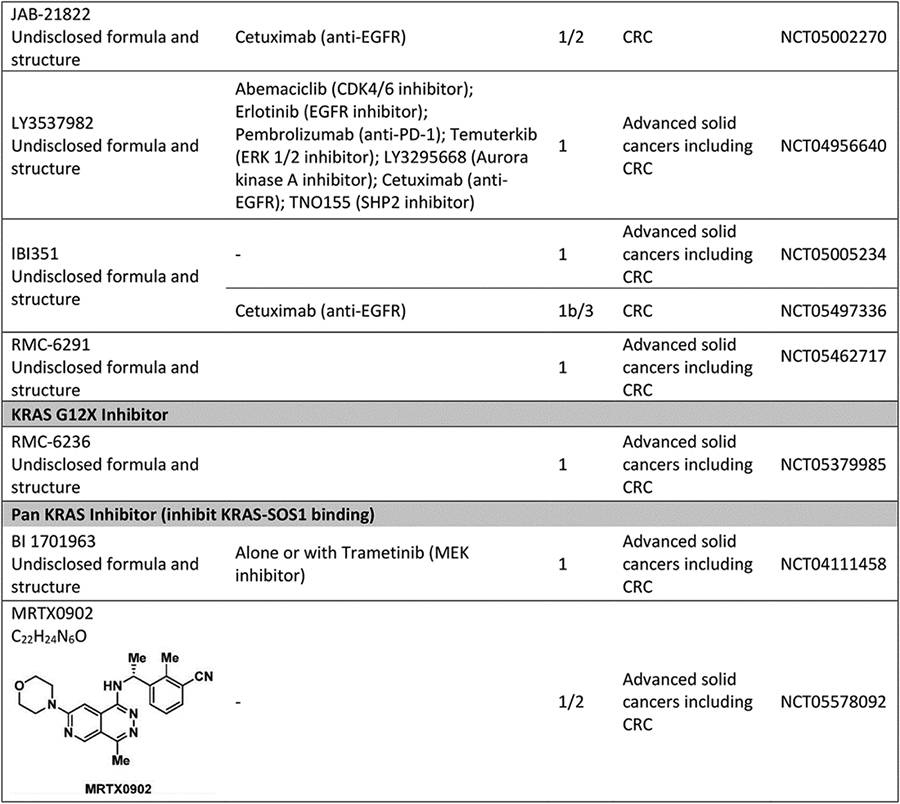
|
Abbreviation: CRC: colorectal cancer.
Table 2:
Novel non-direct KRAS inhibition treatment strategies for KRAS mutant colorectal cancer.
| Mechanism | Combination Drug | Phase | Cancer Type | Trial ID | Status |
|---|---|---|---|---|---|
| Immunotherapy | |||||
| Cancer Vaccines | |||||
| mRNA-5671/V941 vaccine | Alone or with Pembrolizumab (anti-PD-1) | 1 | Microsatellite Stable CRC; KRAS G12D, G12V, G13D or G12C; HLA-A11:01 or HLA C08:02 | NCT03948763 | Completed 2022 |
| Pooled Mutant KRAS-Targeted Long Peptide Vaccine | Nivolumab and ipilimumab | 1 | Microsatellite Stable CRC with KRAS mutation | NCT04117087 | Ongoing |
| Adoptive T cell therapies | |||||
| Peripheral blood lymphocytes transduced with HLA-A11:01-restricted anti-KRAS G12D murine T cell receptor | Preparative lymphodepletion (cyclophosphamide, fludarabine), post-infusion high dose aldesleukin (IL-2). | 1/2 | Advanced solid cancers including CRC; KRAS G12D; HLA-A11:01 | NCT03745326 | Ongoing |
| Peripheral blood lymphocytes transduced with HLA-A11:01-restricted anti-KRAS G12V murine T cell receptor | Preparative lymphodepletion (cyclophosphamide, fludarabine), post-infusion high dose aldesleukin (IL-2). | 1/2 | Advanced solid cancers including CRC; KRAS G12V; HLA-A11:01 | NCT03190941 | Ongoing |
| Stimulators of Innate Immunity | |||||
| SX-682 (inhibits chemokine receptors CXCR1/2, thereby decreasing myeloid- derived suppressor cells in tumor microenvironment) | Nivolumab | 1/2 | RAS mutated, MSS advanced CRC | NCT04599140 | Ongoing |
| Magrolimab (anti-CD47, promotes phagocytosis of cancer cells by macrophages) | Cetuximab | 1/2 | Advanced solid cancers, with KRAS mutant CRC cohorts | NCT02953782 | Completed 2020 |
| REOLYSIN® (Reovirus Type 3 Dearing replicates selectively in Ras-transformed cells causing cell lysis, and stimulates antigen presenting cells) | FOLFIRI and Bevacizumab | 1 | KRAS mutant metastatic CRC | NCT01274624 | Completed 2018 |
| Imprime PGG (soluble beta-1,3/1,6 glucan which binds to complement receptor 3 on innate immune cells) | Cetuximab | 2 | KRAS mutant metastatic CRC | NCT00912327 | Completed 2012 |
| Lenalidomide (activates natural killer cells) | Cetuximab | 2 | Advanced KRAS mutant CRC | NCT01032291 | Terminated due to lack of efficacy |
| Autophagy inhibitors | |||||
| DCC-3116 (ULK1/2 inhibitor) | Trametinib (MEK inhibitor) | 1/2 | Advanced solid cancers with RAS/MAPK pathway mutations (KRAS, NRAS, NF1, or BRAF), with a CRC cohort | NCT04892017 | Ongoing |
| Hydroxychloroquine | Ulixertinib (ERK inhibitor) | 2 | Advanced gastrointestinal cancers with MAPK mutations (KRAS, NRAS, HRAS, BRAF non-V600, MAP2K1/2 or ERK1/2), with a CRC cohort | NCT05221320 | Ongoing |
| Apoptosis inducers | |||||
| ABBV-621/Eftozanermin (TRAIL receptor agonist) | FOLFIRI ± bevacizumab (VEGF inhibitor) | 1 | Advanced cancers, with KRAS-mutant CRC cohort | NCT03082209 | Completed 2022 |
| HDM201 (MDM2 inhibitor) | Trametinib (MEK inhibitor) | 1 | RAS/RAF mutant and TP53 wild-type advanced colorectal cancer | NCT03714958 | Ongoing |
| RGX-202–01 (inhibits creatine transporter SLC6a8) | FOLFIRI ± bevacizumab (VEGF inhibitor) | 1 | RAS mutant advanced CRC | NCT03597581 | Ongoing |
| NBF-006 (inhibits Glutathione-S- Transferase P, GST-π, thereby causing oxidative stress) | - | 1 | CRC, NSCLC, Pancreatic Cancer | NCT03819387 | Ongoing |
| Conatumumab (Death Receptor 5 agonist) | FOLFIRI vs FOLFIRI alone | 2 | KRAS mutant metastatic CRC | NCT00813605 | Completed 2011 |
| Metabolism inhibitors | |||||
| Telaglenastat (Glutaminase Inhibitor) | Palbociclib (CDK4/6 inhibitor) | 1b/2 | Advanced solid cancers with expansion cohort in metastatic KRAS mutant CRC | NCT03965845 | Completed 2021 |
| Inhibition of Epithelial to Mesenchymal Transition | |||||
| TP-0903 (AXL kinase inhibitor, reverses mesenchymal phenotype) | - | 1 | BRAF, KRAS, or NRAS Mutant metastatic CRC | NCT02729298 | Ongoing |
Abbreviation: CRC: colorectal cancer.
Inhibition of signaling downstream from KRAS
The role of KRAS in oncogenic signaling of CRC is shown in Figure 2. Early clinical efforts used selective inhibitors of downstream effectors to target signaling in KRAS mutant CRC but were unable to achieve meaningful clinical activity. In the phase 1 expansion for the MEK inhibitor trametinib, for example, no objective responses were seen in 28 patients with metastatic CRC, including 12 patients with KRAS mutant CRC.[7] Based on preclinical studies suggesting that, in KRAS mutant CRC, ERK signaling depends on KRAS activation, while phosphatidylinositol 3-kinase (PI3K) activation results from receptor tyrosine kinase (RTK) signaling, particularly IGF-IR, or PIK3CA mutation, combination therapy against ERK and PI3K signaling was tested.[8,9] The combination of the MEK inhibitor binimetinib plus the PI3K inhibitor buparlisib was not tolerated at continuous dosing in the phase 1 trial and responses were not seen in the CRC patients enrolled.[10] The narrow therapeutic index of MEK/ERK inhibitors likely limited the ability to sufficiently inhibit ERK signaling in these studies. The complexity of intracellular interactions among different signaling mediators also precluded success by release of negative regulatory holds on ERK signaling and reactivation of other oncogenic pathways.
Figure 2.
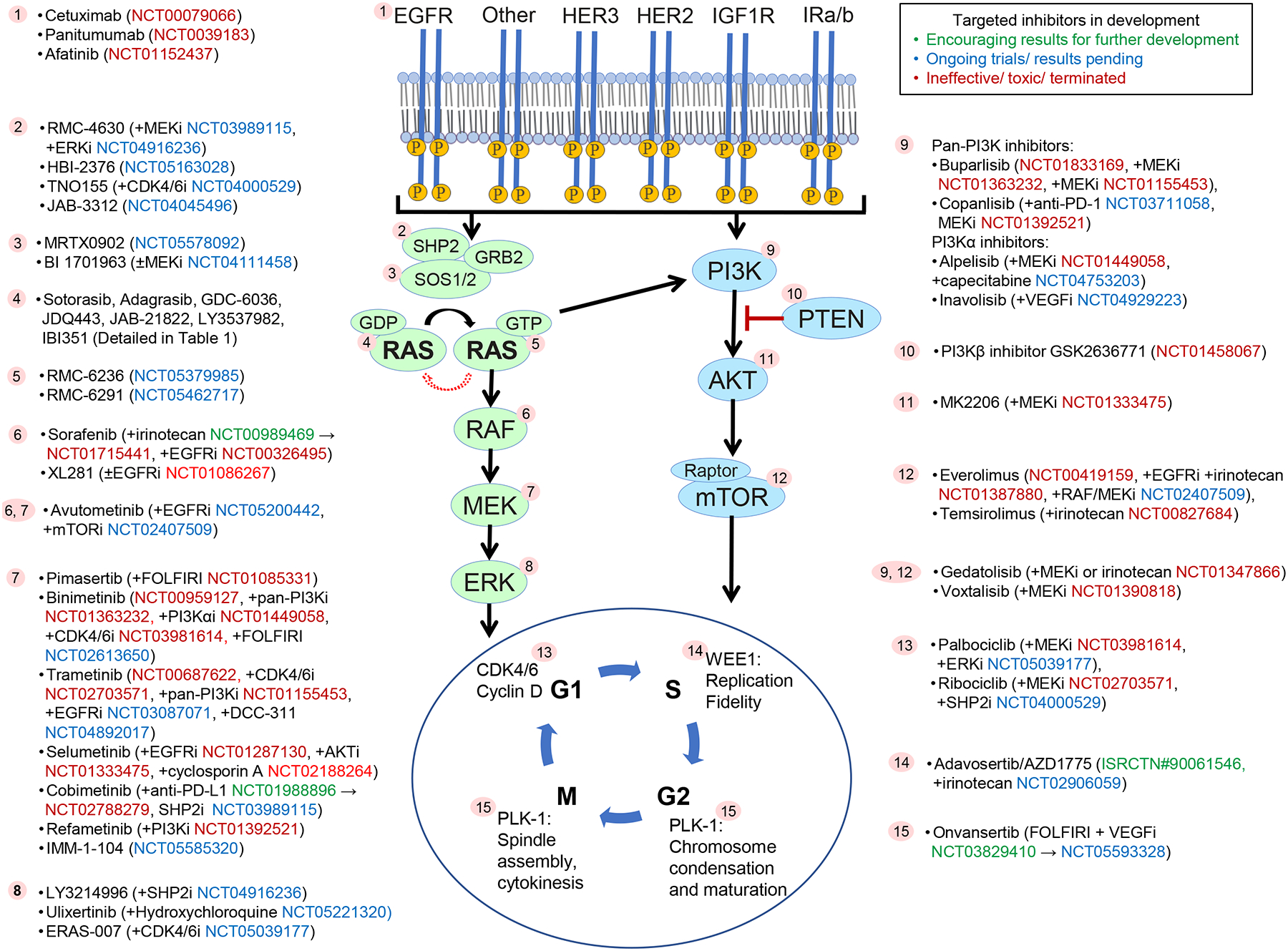
KRAS in oncogenic signaling.
Binding of ligands (e.g., growth factors, cytokines) to receptor tyrosine kinases activates guanine nucleotide exchange factors (e.g., SOS1/2), which mediates conversion of inactive GDP-bound RAS to active GTP-bound RAS. GTP-bound RAS promotes cancer growth mainly via activating ERK signaling and, to a lesser extent, via activating the phosphatidylinositol 3-kinase (PI3K) and other oncogenic pathway. An intrinsic GTPase of RAS inactivates it. Activating mutations of RAS are most frequent in the KRAS isoform. Signaling mediators of RAS against which targeted inhibitors have been / are in clinical development for KRAS mutant metastatic colorectal cancer are marked with numbers. Targeted drugs along with combination drugs/regimens (if applicable) and respective clinical trial numbers are shown next to each number. Clinical trials colors indicate encouraging results for further development (green), ongoing trials (blue) and ineffective/toxic regimens (red).
Cell cycle proteins have also been targeted to inhibit KRAS mutant CRC. Gene expression profiling of CRCs from 55 patients showed that cell cycle and mitosis pathways were significantly upregulated in KRAS mutant tumors.[11] MEK and CDK4/6 inhibitors synergize to downregulate expression of cell cycle proteins and increase tumor regression in KRAS mutant CRC cell lines and patient-derived xenograft models[11,12]. This led to a randomized phase 2 clinical trial (ACCRU-GI-1618) of binimetinib (MEK inhibitor) and palbociclib (CDK4/6 inhibitor) versus TAS-102 in refractory metastatic KRAS/NRAS mutant CRC (NCT03981614). Median progression-free survival (PFS) of 2.1 months was seen in each arm, and median overall survival (OS) was also not improved (7.7 months in experimental arm, 6.6 months in TAS-102 arm).[13] Polo-like kinase 1 (PLK1) is a serine/threonine-protein kinase that promotes cell cycle progression. In KRAS mutant irinotecan resistant patient-derived xenograft models, PLK1 inhibitor (onvansertib) re-sensitized tumors to irinotecan.[14] An objective response rate (ORR) of 36% was seen in phase 1/2 clinical trial of onvansertib with FOLFIRI + bevacizumab in patients with KRAS mutant metastatic CRC, with plans for a follow-up randomized, phase 2 trial of this combination versus FOLFIRI + bevacizumab alone.[15]
Direct KRAS inhibition
KRASG12C inhibitors:
KRASG12C is present in about 3% of metastatic CRC.[16] The potential to target KRAS has been revolutionized with selective KRASG12C inhibitors that bind to a pocket in the switch II region exposed in GDP-bound RAS. These drugs take advantage of the cysteine residue in the mutant protein and the intrinsic GTPase of KRASG12C, which is relatively higher than for other KRAS mutants.[17] The two KRASG12C inhibitors furthest along in clinical development are sotorasib (AMG510) and adagrasib (MRTX849).
Early data suggest response rates of 7–20% for KRASG12C inhibitor monotherapy in patients with metastatic KRASG12C CRC. The phase 1 clinical trial of sotorasib had a 7% response rate (3 of 42 patients), 73.8% disease control rate (DCR) and median PFS of 4.0 months.[18] The subsequent phase 2 trial of sotorasib at 960 mg daily reported objective response in 6 out of 62 (9.7%) patients but did not meet the primary endpoint of ORR of 20%. DCR was 82.3%; median PFS and OS were 4 and 10.6 months, respectively. The phase 2 CRC cohort of the KRYSTAL-1 study of adagrasib had an ORR of 19% (in 8 patients) and DCR of 86% (in 37 patients) among 43 evaluable patients. Median PFS was 5.6 months.[19] This clinical activity of KRASG12C inhibitors in metastatic CRC is lower than that seen in metastatic non-small cell lung cancer, for which sotorasib has been granted accelerated approval by FDA based on results of phase 2 trial.[20]
Several research groups have shown reactivation of RAS signaling through RTKs to be a key mechanism of resistance to KRASG12C inhibition in CRC.[21–23] Amadeo et al showed that in KRASG12C CRC cell lines, KRASG12C inhibition results in transient ERK inhibition, after which there is phospho-ERK rebound. Compared to non-small cell lung cancer cells, the KRASG12C CRC cells have high basal RTK activation and are more responsive to growth factor stimulation, which induces higher level of phospho-ERK. The combination of cetuximab (EGFR inhibitor) and sotorasib resulted in sustained inhibition of phospho-ERK, increased cell death rate, and inhibited growth of patient derived CRC organoids and xenografts.[21] Ryan et al showed that multiple RTKs can drive feedback reactivation of wild-type RAS (including NRAS and HRAS) after KRASG12C inhibition and provided evidence for co-inhibition of convergent nodes such as SHP2 or MEK to overcome adaptive resistance.[22,23] Clinical trials of KRASG12C and SHP2 inhibitors are ongoing.
Consistent with the preclinical studies, clinical outcomes of patients with KRASG12C metastatic CRC improved with co-targeting of KRASG12C and EGFR in ongoing trials. Results reported from the phase 1b (n=40) cohort of sotorasib with panitumumab combination demonstrated ORR of 30% and DCR of 93%.[24] Median PFS was 5.7 months. Similarly, in the KRYSTAL-1 study phase 1b expansion cohort, the addition of cetuximab to adagrasib improved ORR to 46% (13 patients) and DCR to 100% among 28 evaluable patients; median PFS was 6.9 months. Treatment related adverse events of grade 3/4 were seen in 16% of patients.[19] These results have paved the way for registrational phase 3 clinical trials in patients with KRASG12C metastatic CRC. The KRYSTAL-10 trial (NCT04793958) randomizes patients to adagrasib (600 mg twice a day) with cetuximab (500 mg/m2 every 2 weeks) versus chemotherapy (mFOLFOX6 or FOLFIRI +/− anti-VEGF/VEGFR) after progression on first line fluoropyrimidine-based doublet regimen. CodeBreaK300 (NCT05198934) is comparing the combination of sotorasib (960 mg daily in Arm A and 240 mg daily in Arm B) with panitumumab (6mg/kg every 2 weeks) versus chemotherapy (Trifluridine and Tipiracil or Regorafenib) in the third-line setting. In contrast with the higher efficacy of this combination, co-inhibition of KRASG12C and MEK with sotorasib and trametinib combination among 18 patients with KRASG12C metastatic CRC achieved ORR and DCR of 11% (2 patients) and 83% (15 patients), respectively, and is not being further pursued. [25] Further combination regimens that are being investigated in metastatic CRC in the CodeBreaK101 study include triplet regimens, such as, sotorasib + trametinib + panitumumab, sotorasib + panitumumab + chemotherapy, and sotorasib + bevacizumab + chemotherapy.
A mechanism of intrinsic resistance to targeted therapy may be co-occurrence of other genomic alterations in the tumor that sustain oncogenic signaling through the same or different pathways when the driver oncogene is inhibited.[26] Over a quarter of patients with KRASG12C CRC have activating alterations in the PI3K/mTOR pathway, and 8% of patients have other likely pathogenic co-alterations in ERK signaling (e.g., mutations in BRAF, RAF1, HRAS, NRAS, MAP2K1, PTPN11, and other mutations in KRAS).[27] Data from the adagrasib monotherapy and combination cohorts showed no association between PIK3CA mutation status and response, but analysis was limited by the small sample size.[19] Furthermore, CRISPR screens in lung and pancreatic cancer models treated with KRASG12C inhibitor have revealed collateral dependencies on genes in cell cycle, RTKs that promote target engagement upstream of KRAS, and parallel PI3K signaling pathway.[28] Hence, combinations of sotorasib with everolimus (mTOR inhibitor), sotorasib with palbociclib (CDK 4/6 inhibitor), and adagrasib with palbociclib are also being investigated in advanced solid tumors. Other KRASG12C inhibitors in development include GDC-6036 (alone and in combination with inhibitors of EGFR, VEGF, PI3Kα, SHP2), JDQ443 (alone and with co-inhibition of SHP2, EGFR, MEK, CDK4/6, anti-PD-1), JAB21822 (alone and with cetuximab), LY3537982 (alone and with co-inhibition of SHP2, EGFR, ERK1/2, AurA, CDK4/6, anti-PD-1).
Early studies of progression samples suggest multiple resistance alterations can emerge with KRASG12C inhibition in CRC and primarily converge to reactivate ERK signaling. Data from the 74 gene circulating tumor DNA assay (Guardant360) from baseline and progression samples collected from 45 CRC patients treated with sotorasib in CodeBreaK100 study [29,30] revealed detectable acquired genomic alterations in 32 patients (71%), involving RTK genes in 27% (including EGFR, ERBB2, KIT, ROS1, FGFR1, FGFR1, MET and PDGFRA), cell cycle genes in 22%, DNA damage repair genes in 22%, and secondary RAS alterations in 16%. Genomic mechanisms of acquired resistance were also evaluated in 10 patients with metastatic CRC treated with adagrasib monotherapy on KRYSTAL-1 study using next generation sequencing of tumor tissue and or circulating tumor DNA.[31] Six patients had at least one putative resistance mechanism and five patients had multiple resistance alterations. Secondary KRAS mutations within the drug binding pocket (H95Q, H95R) were noted in two patients, other activating KRAS alterations in three and MAP2K1 alterations in 4 patients. KRASG12C amplification, NRASQ61K, BRAFV600E, and likely oncogenic PIK3R1S361fs and PTENN48K mutations were noted in one patient each. Three patients developed acquired gene fusions (EML4-ALK rearrangement; CCDC6-RET fusion; and multiple fusions involving FGFR3, BRAF, and RAF1). A functionally distinct KRASG12C inhibitor, RM-018, that forms a tricomplex (RM-018, cyclophilin A, GTP-bound KRASG12C), was able to overcome resistance due to acquired KRASY96D mutation affecting the switch-II binding pocket in cell lines from KRASG12C lung and pancreatic cancers.[32]
KRASG12D inhibitors:
KRASG12D mutation is present in 12% of metastatic CRC and is the most common KRAS mutation in CRC. The switch II pocket of KRASG12D molecule lacks a reactive residue that could form a potent covalent bond with an inhibitor, thereby limiting efforts at targeting it. Another challenge is a lower intrinsic GTP hydrolysis rate with KRASG12D mutation than with KRASG12C.[17] Recently, MRTX 1133 was developed as a noncovalent KRASG12D inhibitor that occupies the switch II pocket and extends three substituents (piperazine, pyrrolidine and naphthyl groups) for binding with a picomolar affinity. Binding of MRTX 1133 prevented the formation of KRASG12D/GTP/RAF1 complex, inhibited signaling in cell lines and also showed in vivo tumor regression in 04.03 xenograft model of pancreatic cancer.[33] Investigational New Drug application for MRTX 1133 is planned in the second half of 2022.
KRASG12x inhibitors:
Targeting of other KRAS G12 mutations, similarly, has been challenging due to the lack of a deep reactive binding pocket. KRASG12A or KRASG12R have about 40–80 fold decrease in GTP hydrolysis rate[17]. RMC-6236 has been developed to prevent interaction of activated RAS with downstream proteins by forming a non-covalent high affinity complex with intracellular chaperone protein, cyclophilin A, and GTP-RAS.[34] A phase I clinical trial of RMC-6236 in patients with advanced refractory solid cancers harboring specific KRAS mutations (G12A, G12D, G12R, G12S, G12V) is ongoing (NCT05379985).
Pan-RAS inhibitors:
BI-3406 and BI 1701963 are small molecule inhibitors that bind the catalytic site of SOS1 and prevent interaction of SOS1 with RAS-GDP, which reduces activation to RAS-GTP. In preclinical models of KRAS mutant tumors, these agents also attenuate MEK inhibitor-induced feedback reactivation of RAS signaling, synergized with KRASG12C inhibitor, and potentiated irinotecan-induced DNA damage.[35,36] BI 1701963 is currently in phase I clinical development as monotherapy and in combination with MEK inhibitor (trametinib) in KRAS mutant advanced solid tumors (NCT04111458).[36]
Immunotherapy
The immune microenvironment of KRAS mutant CRC is immunosuppressive. An analysis of immune signatures using The Cancer Genome Atlas (TCGA) RNA-seq and the Koo Foundation Sun Yat-Sen Cancer Center microarray datasets showed decrease in Th1-centric co-ordinate immune response cluster in KRAS mutant CRC. [37] There was reduced expression of cytotoxic T cells, neutrophils, interferon gamma pathway, STAT1 and CXCL10 in KRAS mutant samples as compared to KRAS wild-type samples. KRAS mutant CRC also have lower expression of immune inhibitory molecules (CTLA4, PDL1, PDL2, LAG3, and TIM3) and CD4.[38] Similarly, another TCGA analysis showed significantly lower levels of IL6/JAK/STAT3, IFN-γ, complement, and IL2/STAT5 in KRASG12C as compared to KRASnonG12C and KRASwild-type CRC patients.[16] Liao et al showed in mouse models of CRC with doxycycline-inducible oncogenic KRAS that mutant KRAS expressing tumors showed de novo resistance to anti-PD-1 therapy.[39] This was mediated by suppression of interferon regulatory factor 2, which results in increased expression of CXCL3, which bind CXCR2 and promote infiltration of myeloid derived suppressor cells in the tumor microenvironment. A phase I/II trial of SX-628 (CXCR1/2 inhibitor) in combination with nivolumab is ongoing in refractory RAS mutant MSS CRC (NCT04599140).
Ebert et al showed that MEK inhibition improved intratumoral CD8+ cell infiltration and increased anti-tumor activity in combination with anti-PD-L1 in vivo.[40] Canon et al also showed that in CT26 KRASG12C mutant CRC model, sotorasib increased intratumoral cytotoxic T-cell infiltration and pro-inflammatory cytokines including interferon gamma signaling.[41] Similarly, adagrasib was shown to increase MHC class 1 expression and effector cell infiltration while decreasing immune inhibitory cytokines and myeloid-derived suppressor cells in CT26 mice expressing KRASG12C [42]. Marked in vivo synergy between KRASG12C inhibitor and anti-PD-1 was shown by both groups. Clinically, the combination of sotorasib with anti-PD-1 (pembrolizumab or atezolizumab) in non-small cell lung cancer resulted in grade 3/4 transaminitis in about half of the patients. A low dose sotorasib lead-in strategy before the combination treatment appears to be better tolerated and is being further investigated.[43] The phase 3 clinical trial of atezolizumab and cobimetinib and of atezolizumab monotherapy versus regorafenib in microsatellite stable CRC did not meet the primary end point of improved OS in the combination arm.[44] About half of the patients (99 out of 183) in the combination arm were KRAS mutant.
Cancer vaccines:
Various vaccination strategies, including mRNA and peptide vaccines, are under investigation to potentiate T cell sensitization to mutant KRAS neoantigens. A phase 1 clinical trial is ongoing using mRNA-5671/V941 vaccine alone or combined with anti-PD-1 (pembrolizumab) in patients with advanced or metastatic microsatellite stable CRC with one of the four KRAS mutations (G12D, G12V, G13D or G12C) and HLA types of HLA-A11:01 or HLA C08:02 (NCT03948763). Another phase 1 trial of pooled mutant-KRAS peptide vaccine with nivolumab and ipilimumab in microsatellite stable CRC is ongoing (NCT04117087). TG02 is a neoantigen peptide cancer vaccine developed by Targovax that contains eight synthetic peptides representing fragments of the most frequent RAS mutant peptides seen in rectal cancer. A phase 1b clinical trial of TG02 alone or in combination with pembrolizumab was conducted in locally advanced primary or recurrent KRAS codon 12 or 13 (exon 2) mutant CRC (NCT02933944). TG02 doses were administered along with granulocyte–macrophage colony-stimulating factor before pelvic surgery. The trial was terminated early. Out of the 6 patients enrolled, 4 had TG02 specific immune response assessed by delayed type hypersensitivity and 3 had systemic presence of TG02 specific T cells.
Adoptive T cell therapies:
Adoptive T cell therapy involves ex vivo expansion of patient’s tumor infiltrating T cells that have specificity for neopeptides generated by somatic mutations in the patient’s tumor followed by autologous transfusion of these T cells. Tran et al published a case report of a patient with KRASG12D metastatic CRC who received a polyclonal infusion of HLA-C*08:02–restricted tumor-infiltrating lymphocytes targeting four neopeptides of KRASG12D and achieved objective response in all lung metastases.[45] On progression of one of the lung metastasis after 9 months, loss of chromosome 6 haplotype that encoded the HLA-C*08:02 allele was observed in the tumor. Maoz et al reported that (18.4%) of patients (687 of 3734) with CRC have at least one copy of HLA-C*08:02, and 2.3 % of patients (85 of 3734) have HLA-C*08:02 and KRASG12D.[46] HLA-A*11:01-restricted T-cell receptors specifically targeting KRASG12D and KRASG12V neo-peptides have also been reported.[47] Another strategy to enhance neopeptide recognition by T cells is to genetically modify patient’s tumor infiltrating lymphocytes by transduction of an HLA-restricted murine T-cell receptor that specifically recognizes mutant RAS neopeptides using a retroviral vector, followed by adoptive T cell transfer.[47] This strategy is being investigated in a phase 1/2 clinical trial in patients with HLA-A*11:01 positive metastatic or unresectable RASG12D or RASG12V cancers, including CRC (NCT03745326, NCT03190941).
Additional Strategies to target KRAS mutant colorectal cancer
Novel non-direct KRAS inhibition treatment strategies for KRAS mutant CRC are detailed in Table 2, such as, inhibition of autophagy, induction of apoptosis, inhibition of metabolism and reversal of mesenchymal phenotype.
Conclusions
In conclusions, there has been encouraging progress in development of novel therapeutics for KRAS mutant CRC, which include mutation specific KRAS inhibitors as well as immunotherapeutic approaches. Further research is needed to elucidate mechanisms of resistance to targeted therapy for combination development and to effectively immunomodulate the tumor microenvironment.
Funding
This work was funded by a National Institutes of Health Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center (P30 CA08748).
Conflict of Interest Statement
Maliha Nusrat: None
Rona Yaeger: Consulting or Advisory Role: Array BioPharma, Natera, Mirati Therapeutics. Research Funding: Array BioPharma (Inst), Boehringer Ingelheim (Inst), Daiichi Sankyo (inst), Pfizer (Inst), Mirati Therapeutics (Inst)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yaeger R, Chatila WK, Lipsyc MD, Hechtman JF, Cercek A, Sanchez-Vega F, Jayakumaran G, Middha S, Zehir A, Donoghue MTA, et al. : Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018, 33:125–136 e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loree JM, Pereira AAL, Lam M, Willauer AN, Raghav K, Dasari A, Morris VK, Advani S, Menter DG, Eng C, et al. : Classifying Colorectal Cancer by Tumor Location Rather than Sidedness Highlights a Continuum in Mutation Profiles and Consensus Molecular Subtypes. Clinical Cancer Research 2018, 24:1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serebriiskii IG, Connelly C, Frampton G, Newberg J, Cooke M, Miller V, Ali S, Ross JS, Handorf E, Arora S, et al. : Comprehensive characterization of RAS mutations in colon and rectal cancers in old and young patients. Nat Commun 2019, 10:3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RP, Sutton PA, Evans JP, Clifford R, McAvoy A, Lewis J, Rousseau A, Mountford R, McWhirter D, Malik HZ: Specific mutations in KRAS codon 12 are associated with worse overall survival in patients with advanced and recurrent colorectal cancer. Br J Cancer 2017, 116:923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peeters M, Oliner KS, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, Andre T, Chan E, Lordick F, et al. : Analysis of KRAS/NRAS Mutations in a Phase III Study of Panitumumab with FOLFIRI Compared with FOLFIRI Alone as Second-line Treatment for Metastatic Colorectal Cancer. Clin Cancer Res 2015, 21:5469–5479. [DOI] [PubMed] [Google Scholar]
- 6.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, et al. : K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008, 359:1757–1765. [DOI] [PubMed] [Google Scholar]
- 7.Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, DeMarini DJ, Cox DS, Xu Y, Morris SR, et al. : Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol 2012, 13:773–781. [DOI] [PubMed] [Google Scholar]
- 8.Ebi H, Corcoran RB, Singh A, Chen Z, Song Y, Lifshits E, Ryan DP, Meyerhardt JA, Benes C, Settleman J, et al. : Receptor tyrosine kinases exert dominant control over PI3K signaling in human KRAS mutant colorectal cancers. J Clin Invest 2011, 121:4311–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halilovic E, She QB, Ye Q, Pagliarini R, Sellers WR, Solit DB, Rosen N: PIK3CA mutation uncouples tumor growth and cyclin D1 regulation from MEK/ERK and mutant KRAS signaling. Cancer Res 2010, 70:6804–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bardia A, Gounder M, Rodon J, Janku F, Lolkema MP, Stephenson JJ, Bedard PL, Schuler M, Sessa C, LoRusso P, et al. : Phase Ib Study of Combination Therapy with MEK Inhibitor Binimetinib and Phosphatidylinositol 3-Kinase Inhibitor Buparlisib in Patients with Advanced Solid Tumors with RAS/RAF Alterations. Oncologist 2020, 25:e160–e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pek M, Yatim S, Chen Y, Li J, Gong M, Jiang X, Zhang F, Zheng J, Wu X, Yu Q: Oncogenic KRAS-associated gene signature defines co-targeting of CDK4/6 and MEK as a viable therapeutic strategy in colorectal cancer. Oncogene 2017, 36:4975–4986. [DOI] [PubMed] [Google Scholar]
- 12.Lee MS, Helms TL, Feng N, Gay J, Chang QE, Tian F, Wu JY, Toniatti C, Heffernan TP, Powis G, et al. : Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models. Oncotarget 2016, 7:39595–39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MS, Zemla TJ, Ciombor KK, McRee AJ, Akce M, Dakhil SR, Jaszewski BL, Ou F-S, Bekaii-Saab TS, Kopetz S: A randomized phase II trial of MEK and CDK4/6 inhibitors vesus tipiracil/trifluridine (TAS-102) in metastatic KRAS/NRAS mutant (mut) colorectal cancer (CRC). Journal of Clinical Oncology 2022, 40:116–116. [Google Scholar]
- 14.Kopetz S, Ridinger M, Sorokin A, Kanikarla P, Gao F, Liu Z, Samuelsz E, Smeal T, Starr JS, Sharma MR: 366P The PLK1 inhibitor onvansertib overcomes irinotecan resistance in RAS-mutated (mRAS) metastatic colorectal cancer (mCRC) in vivo and in patients (pts). Annals of Oncology 2022, 33:S704. [Google Scholar]
- 15.Lenz H-J, Kasi A, Mendelsohn L, Cannon TL, Starr JS, Hubbard JM, Bekaii-Saab TS, Ridinger M, Samuelsz E, Ruffner KL, et al. : A phase 1b/2 trial of the PLK1 inhibitor onvansertib in combination with FOLFIRI-bev in 2L treatment of KRAS-mutated (mKRAS) metastatic colorectal carcinoma (mCRC). Journal of Clinical Oncology 2022, 40:100–100. [Google Scholar]
- 16.Henry JT, Coker O, Chowdhury S, Shen JP, Morris VK, Dasari A, Raghav K, Nusrat M, Kee B, Parseghian C, et al. : Comprehensive Clinical and Molecular Characterization of KRASG12C-Mutant Colorectal Cancer. JCO Precision Oncology 2021:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter JC, Manandhar A, Carrasco MA, Gurbani D, Gondi S, Westover KD: Biochemical and Structural Analysis of Common Cancer-Associated KRAS Mutations. Molecular Cancer Research 2015, 13:1325–1335. [DOI] [PubMed] [Google Scholar]
- 18.Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS, et al. : KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. New England Journal of Medicine 2020, 383:1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klempner SJ WJ, Pelster M, Spira A, Barve M, Ou SI, Leal TA, Bekaii-Saab T, Christensen JG, Kheoh T, Velastegui K, Torossian HD, Yaeger R: LBA24 - KRYSTAL-1: Updated efficacy and safety of adagrasib (MRTX849) with or without cetuximab in patients with advanced colorectal cancer (CRC) harboring a KRASG12C mutation. Annals of Oncology 2022, 33:S808–S869. [Google Scholar]
- 20.Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, Italiano A, Schuler M, Borghaei H, Barlesi F, et al. : Sotorasib for Lung Cancers with KRAS p.G12C Mutation. New England Journal of Medicine 2021, 384:2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amodio V, Yaeger R, Arcella P, Cancelliere C, Lamba S, Lorenzato A, Arena S, Montone M, Mussolin B, Bian Y, et al. : EGFR Blockade Reverts Resistance to KRAS(G12C) Inhibition in Colorectal Cancer. Cancer Discov 2020, 10:1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan MB, Fece de la Cruz F, Phat S, Myers DT, Wong E, Shahzade HA, Hong CB, Corcoran RB: Vertical Pathway Inhibition Overcomes Adaptive Feedback Resistance to KRAS(G12C) Inhibition. Clin Cancer Res 2020, 26:1633–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan MB, Coker O, Sorokin A, Fella K, Barnes H, Wong E, Kanikarla P, Gao F, Zhang Y, Zhou L, et al. : KRAS(G12C)-independent feedback activation of wild-type RAS constrains KRAS(G12C) inhibitor efficacy. Cell Rep 2022, 39:110993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuboki YYR, Fakih MG, Strickler JH, Masuishi T, Kim EJ, Bestvina CM, Langer CJ, Krauss JC, Puri S, Cardona P, Chan E, Tran Q, Hong DS: 315O - Sotorasib in combination with panitumumab in refractory KRAS G12C-mutated colorectal cancer: Safety and efficacy for phase Ib full expansion cohort. Annals of Oncology 2022, 33:S136–S196. [Google Scholar]
- 25.Ramalingam S, Fakih M, Strickler J, Govindan R, Li BT, Goldberg S, Gandara D, Burns T, Barve M, Shu C, et al. : Abstract P05–01: A phase 1b study evaluating the safety and efficacy of sotorasib, a KRASG12C inhibitor, in combination with trametinib, a MEK inhibitor, in KRAS p.G12C-Mutated Solid Tumors. Molecular Cancer Therapeutics 2021, 20:P05–01–P05–01. [Google Scholar]
- 26.Garraway LA, Janne PA: Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov 2012, 2:214–226. [DOI] [PubMed] [Google Scholar]
- 27.Nusrat M, Roszik J, Holla V, Cai T, Hong M, Coker O, Johnson B, Ahnert JR, Janku F, Kopetz S, et al. : Therapeutic vulnerabilities among KRAS G12C mutant (mut) advanced cancers based on co-alteration (co-alt) patterns. Journal of Clinical Oncology 2020, 38:3625–3625. [Google Scholar]
- 28.Lou K, Steri V, Ge AY, Hwang YC, Yogodzinski CH, Shkedi AR, Choi ALM, Mitchell DC, Swaney DL, Hann B, et al. : KRAS(G12C) inhibition produces a driver-limited state revealing collateral dependencies. Sci Signal 2019, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li BT, Velcheti V, Price TJ, Hong DS, Fakih M, Kim D-W, Falchook GS, Delord J-P, Dy GK, Ramalingam SS, et al. : Largest evaluation of acquired resistance to sotorasib in KRAS p.G12C-mutated non–small cell lung cancer (NSCLC) and colorectal cancer (CRC): Plasma biomarker analysis of CodeBreaK100. Journal of Clinical Oncology 2022, 40:102–102. [Google Scholar]
- 30.Prenen H, Fakih M, Falchook G, Strickler J, Hindoyan A, Anderson A, Ang A, Kurata T, Price T: SO-39 Evaluation of acquired resistance to sotorasib in KRAS p.G12C-mutated colorectal cancer: Exploratory plasma biomarker analysis of CodeBreaK 100. Annals of Oncology 2022, 33:S373. [Google Scholar]
- 31.Awad MM, Liu S, Rybkin II, Arbour KC, Dilly J, Zhu VW, Johnson ML, Heist RS, Patil T, Riely GJ, et al. : Acquired Resistance to KRAS(G12C) Inhibition in Cancer. N Engl J Med 2021, 384:2382–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka N, Lin JJ, Li C, Ryan MB, Zhang J, Kiedrowski LA, Michel AG, Syed MU, Fella KA, Sakhi M, et al. : Clinical Acquired Resistance to KRAS(G12C) Inhibition through a Novel KRAS Switch-II Pocket Mutation and Polyclonal Alterations Converging on RAS-MAPK Reactivation. Cancer Discov 2021, 11:1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Allen S, Blake JF, Bowcut V, Briere DM, Calinisan A, Dahlke JR, Fell JB, Fischer JP, Gunn RJ, et al. : Identification of MRTX1133, a Noncovalent, Potent, and Selective KRASG12D Inhibitor. Journal of Medicinal Chemistry 2022, 65:3123–3133. [DOI] [PubMed] [Google Scholar]
- 34.Koltun ES, Rice MA, Gustafson WC, Wilds D, Jiang J, Lee BJ, Wang Z, Chang S, Flagella M, Mu Y, et al. : Abstract 3597: Direct targeting of KRASG12X mutant cancers with RMC-6236, a first-in-class, RAS-selective, orally bioavailable, tri-complex RASMULTI(ON) inhibitor. Cancer Research 2022, 82:3597–3597. [Google Scholar]
- 35.Hofmann MH, Gmachl M, Ramharter J, Savarese F, Gerlach D, Marszalek JR, Sanderson MP, Kessler D, Trapani F, Arnhof H, et al. : BI-3406, a Potent and Selective SOS1-KRAS Interaction Inhibitor, Is Effective in KRAS-Driven Cancers through Combined MEK Inhibition. Cancer Discov 2021, 11:142–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofmann MH, Lu H, Duenzinger U, Gerlach D, Trapani F, Machado AA, Daniele JR, Waizenegger I, Gmachl M, Rudolph D, et al. : Abstract CT210: Trial in Process: Phase 1 studies of BI 1701963, a SOS1::KRAS Inhibitor, in combination with MEK inhibitors, irreversible KRASG12C inhibitors or irinotecan. Cancer Research 2021, 81:CT210–CT210. [Google Scholar]
- 37.Lal N, White BS, Goussous G, Pickles O, Mason MJ, Beggs AD, Taniere P, Willcox BE, Guinney J, Middleton GW: KRAS Mutation and Consensus Molecular Subtypes 2 and 3 Are Independently Associated with Reduced Immune Infiltration and Reactivity in Colorectal Cancer. Clin Cancer Res 2018, 24:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lal N, Beggs AD, Willcox BE, Middleton GW: An immunogenomic stratification of colorectal cancer: Implications for development of targeted immunotherapy. Oncoimmunology 2015, 4:e976052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P, Li J, Wang G, Lan Z, Li J, et al. : KRAS-IRF2 Axis Drives Immune Suppression and Immune Therapy Resistance in Colorectal Cancer. Cancer Cell 2019, 35:559–572.e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, Gould SE, Maecker H, Irving BA, Kim JM, et al. : MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016, 44:609–621. [DOI] [PubMed] [Google Scholar]
- 41.Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, Gaida K, Holt T, Knutson CG, Koppada N, et al. : The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575:217–223. [DOI] [PubMed] [Google Scholar]
- 42.Briere DM, Calinisan A, Aranda R, Sudhakar N, Hargis L, Gatto S, Fernandez-Banet J, Pavlicek A, Engstrom LD, Hallin J, et al. : Abstract LB-C09: The KRAS G12C inhibitor MRTX849 reconditions the tumor immune microenvironment and leads to durable complete responses in combination with anti-PD-1 therapy in a syngeneic mouse model. Molecular Cancer Therapeutics 2019, 18:LB-C09–LB-C09. [Google Scholar]
- 43.Li BT, Falchook GS, Durm GA, Burns TF, Skoulidis F, Ramalingam SS, Spira A, Bestvina CM, Goldberg SB, Veluswamy R, et al. : OA03.06 CodeBreaK 100/101: First Report of Safety/Efficacy of Sotorasib in Combination with Pembrolizumab or Atezolizumab in Advanced KRAS p.G12C NSCLC. Journal of Thoracic Oncology 2022, 17:S10–S11. [Google Scholar]
- 44.Eng C, Kim TW, Bendell J, Argiles G, Tebbutt NC, Di Bartolomeo M, Falcone A, Fakih M, Kozloff M, Segal NH, et al. : Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2019, 20:849–861. [DOI] [PubMed] [Google Scholar]
- 45.Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, et al. : T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med 2016, 375:2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maoz A, Rennert G, Gruber SB: T-Cell Transfer Therapy Targeting Mutant KRAS. N Engl J Med 2017, 376:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang QJ, Yu Z, Griffith K, Hanada K, Restifo NP, Yang JC: Identification of T-cell Receptors Targeting KRAS-Mutated Human Tumors. Cancer Immunol Res 2016, 4:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]


