Abstract
Secretory carcinoma (SC) is a salivary gland tumor with a generally low grade microscopic appearance, a characteristic immunophenotype, and a recurrent translocation leading to ETV6::NTRK3 fusion gene. Rare cases are reported in children. The maxillary sinus is an unusual localization. SC have an overall favorable prognosis, but cases with high grade morphology have been described in adult population and are related to a more aggressive clinical course. We present a pediatric case of secretory carcinoma involving the maxillary sinus with high grade morphology, with a review of the literature of secretory carcinomas with high grade component.
Keywords: pediatric secretory carcinoma high grade
Introduction
Secretory carcinoma (SC) is a low grade salivary gland carcinoma characterized by morphological resemblance to mammary secretory carcinoma and by the presence of the ETV6::NTRK3 gene fusion.1 SC mainly affects adults (average age: 45 years),2 with rare cases reported in children.3-6 SC usually occurs in major and minor salivary glands, with rare cases reported in the sinonasal mucosa.7,8 It’s composed of medium-sized cells with eosinophilic granular or vacuolated cytoplasm and small, uniform nuclei, arranged in a microcystic/solid, tubular, follicular, and papillary-cystic pattern. A distinctive PAS-positive luminal secretion is seen.1,9 Immunohistochemistry shows positivity for S100, Mammaglobin, CK7, Vimentin, GCDFP-15, EMA, and negativity for myoepithelial cell markers (p63, Calponin, CK14, SMA, and CK5/6) and for DOG1.9-11 The neoplasm harbors a recurrent translocation t(12;15)(p13;q25) leading to a ETV6::NTRK3 fusion gene9,12 that encodes an activated membrane receptor kinase protein promoting cell proliferation and survival through the activation of the Ras-MAP kinase and PI3K-Akt pathways.13 SC have an overall favorable prognosis. High grade morphology has been described in rare adult cases and it’s related to a more aggressive clinical course.14 We report an exceptional case of sinonasal secretory carcinoma with a high grade morphology occurring in a 12 years old girl.
Case Report
A 12 years old female presented with a large disfiguring mass of the right cheekbone of recent onset.
CT scans demonstrated a voluminous mass (62 mm × 48 mm) obliterating the maxillary sinus, with osteolytic appearance and uneven post-contrast enhancement.
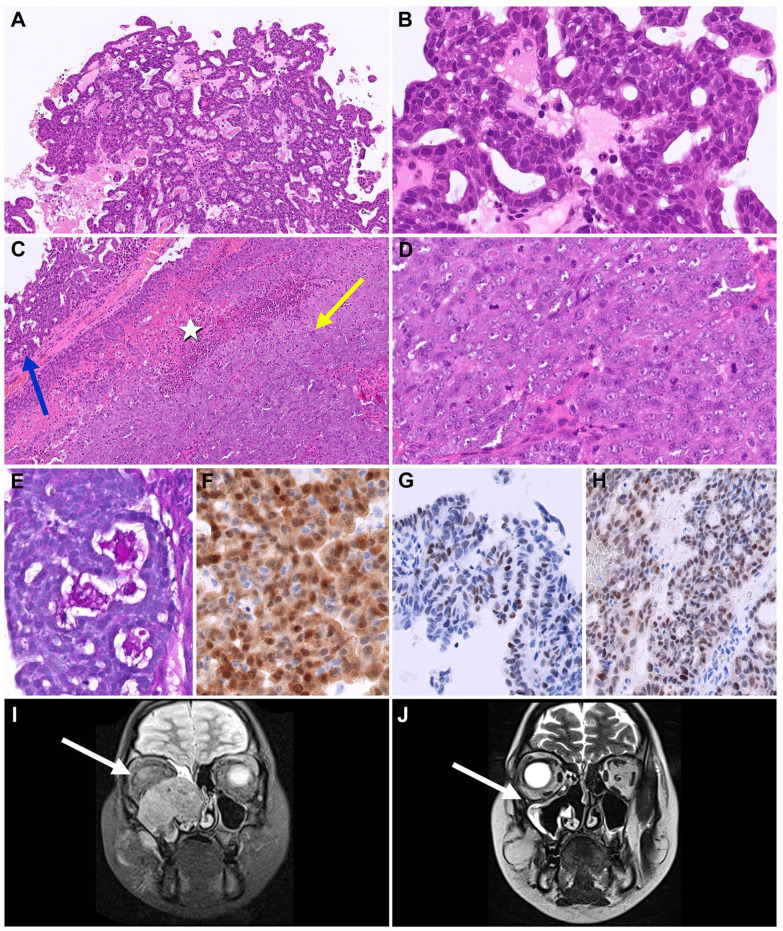
Microscopically, the biopsy showed areas of papillary and tubular/follicular growth by cells with vacuolated eosinophilic cytoplasm, high nuclear/cytoplasmic ratio, round nuclei with dense chromatin, slight nuclear pleomorphism, and small visible nucleoli, with PAS and PAS-D positive luminal secretion. Areas of solid growth by cells with large round nuclei, nuclear pleomorphism, vesicular chromatin, and prominent single or multiple nucleoli were readily apparent (see Figure 1A-E). Mitotic index was high in both areas (16 mitoses/10 HPF). There were foci of necrosis and perineural invasion. There was no invasion of blood vessels.
Figure 1.
(A) Low-grade component with papillary/tubular architecture. (B) Higher magnification of the low-grade component, with cells with dense chromatin and inconspicuous nucleoli; (C) High grade component with solid pattern. Note the transition from the low-grade (blue arrow) to the high-grade (yellow arrow) component and the microscopic focus of necrosis (white star); (D) Higher magnification of the high-grade component, with cells with vesicular chromatin, evident nucleoli, and higher mitotic index; (E) PAS-D positive luminal secretion; (F) S100; (G) GATA3; (H) pan-TRK; (I) MRI, neoplasm before treatment; (J) MRI, after 3 courses of chemotherapy.
Immunohistochemistry showed diffuse positivity for CK (AE1/AE3), Vimentin, CK7, EMA, S100 (nuclear and cytoplasmic) and SOX10, diffuse but weak positivity for GATA3 and focal and weak positivity for MUC4. DOG1 showed focal positivity of the apical membrane surface. Pan-TRK immunostain was diffusely positive. GCDFP-15, SMA, and p63 were negative. Ki67 index was approximately 60% (see Figure 1(F)-(H)).
Next Generation Sequencing has been performed using the Archer FusionPlex OPBG custom panel from areas of papillary and tubular/follicular architecture (RNA extraction with kit ReliaPrep FFPE total RNA—Promega, RNA amplification and sequencing with Illumina—Miseq, data analysis with Archer® Analysis 6.0 software) and it showed the presence of the ETV6 (exon 5)::NTRK3 (exon 15) fusion. The same fusion was also identified from areas of solid growth through real time PCR (RNA extraction with kit ReliaPrep FFPE total RNA—Promega, amplification with EasyPGX® qPCR instrument 96 and analysis with EasyPGX® ready NTRK Fusion, Diatech Pharmacogenetics—IVD Kit).
A diagnosis of secretory carcinoma with high grade morphology was given.
The patient was treated with 4 courses of 5-Fluorouracil and Cisplatin, with a mass reduction of about 75% on MRI evaluation (see Figure 1(I) and (J)). After the fourth course of chemotherapy the patient started an oral TRK-inhibitor drug (Larotrectinib). The patient subsequently underwent surgery for a suspected neoplastic residue in the ethmoid area, without lymph node dissection.
Histological examination of the surgical specimen revealed complete pathological response.
Adjuvant protontherapy on surgical bed (61.4 Gy) and on an area including ipsilateral laterocervical lymph nodes (54.05 Gy) was given.
Larotrectinib therapy was continued for 1 year. At last follow up, 16 months after diagnosis, the patient is alive and in complete remission.
Discussion
The diagnosis was especially challenging due to unusual clinical and morphological features. Secretory carcinomas (SCs) may occur in children, but they are rare in comparison with their adult counterpart.3 Sinonasal location is not typical for SC, with only few cases reported.7 Moreover, the presence of a high grade component has been reported in adults but, to the best of our knowledge, it has never been described in children. In our case, the typical immunophenotype and the identification of the recurrent ETV6::NTRK3 gene fusion allowed us to exclude other salivary gland malignancies. ETV6::NTRK3 gene fusion is present in more than 95% of SC and it has not been demonstrated in other salivary gland tumors.13,15 Recently, ETV6::NTRK3 gene rearrangement has been described in sinonasal low-grade non-intestinal adenocarcinoma (non-ITAC) in adults. Non-ITACs are all of low-grade appearance, they are positive for CK7, DOG1, GCDFP-15, and SOX10 and they show just scattered tumor cells positive for S-100.16 We could exclude this diagnosis due to the high grade morphology and to the different immunophenotype of our lesion.
A high grade component is rare in SC, and mostly reported as single cases or small series. To date (including the current case), high grade component has been observed in 23 cases, but it has never been reported in the pediatric age group8,11,14,17-26 (see Table 1). High grade morphology is associated with a more aggressive course. A 8 out of 19 adult patients died of disease progression. Moreover, local recurrence, lymph nodes metastasis and distant metastasis have been observed respectively in 11, 11, and 9 cases.
Table 1.
Cases of Published High Grade Secretory Carcinoma.
| A/S | Localization | D (cm) | LR | LN Met | Dist Met | Treatment | Follow up | |
|---|---|---|---|---|---|---|---|---|
| Present case | 12/F | Maxillary sinus | 6.2 | – | na | – | CT, TRK-I, S, RT | DF (16 m) |
| Xu et al8 | 61/M | Maxillary sinus | 4.1 | – | – | – | S, RT | DF (8 m) |
| Majewska et al11 | 73/M | Parotid | 3 | + | + | – | CT, RT | DOD (79 m) |
| Majewska et al11 | 60/M | Parotid | 4 | + | + | Lung, Bones (16 m) | S, RT | DOD (20 m) |
| Luo et al17 | 41/F | Palate | 2 | – | + | – | S, RT | DF (10 m) |
| Skalova et al18 | 55/M | Parotid | 4 | + (6 y) | – | – | RT | Died (cause not known) |
| Skalova et al18 | 61/M | Parotid | 4 | – | + | + | RT | DOD (20 m) |
| Skalova et al18 | 73/M | Parotid | Multiple | + | + | – | RT, CT | DOD (4 y) |
| Jung et al19 | na | na | na | na | na | na | na | na |
| Cipriani et al20 | 44/M | Oral cavity | 3.5 | – | – | Pleural cavity (2 m) | S, CT, Crizotinib | DOD (4 m) |
| Asai et al14 | 44/M | Oral cavity | 4 | – | – | Lung (12 m) | S | DF (19 m after metastasis resection) |
| Baghai et al21 | 55/M | Parotid | 7 | – | na | Bones (15 m) | S, RT, CT | DOD (24 m) |
| Baghai et al21 | 58/M | Parotid | 4 | – | + | – | S | na |
| Sun et al22 (3 cases) | 63.7/M (mean age) | Parotid (all) | 3.7 (average) | + (All) | + (All) | Lung (1 case) | S (all cases), RT in one case | No one DOD |
| Taverna et al23 (3 cases) | na | na | na | na | na | na | na | na |
| Numano et al24 | 65/F | Parotid | 8.5 | + | + | Lung (3 m) | CT (for concurrent SCLC) | DOD (8 m) |
| Suzuki et al25 | 74/M | Cervical | na | + (18 m) | – | Lung (18 m) | TRK-I | DF (27 m) |
| Suzuki et al25 | 61/M | Parotid | na | + | – | Lung (18 y) | S | DOD (18 y) |
| Skálová et al26 | 61/M | Submandibular gland | 1.9 | + (4 y) | + (4 y) | – | S, RT | na |
Abbreviations: A, age; S, sex; F, female; M, male; na, not available; D, dimension; LR, local recurrence; m, months; y, years; −, negative; +, positive; LN met, lymph nodes metastasis; Dist Met, distant metastasis; CT, chemotherapy; RT, radiotherapy; S, surgery; TRK-I, TRK inhibitors; SCLC, small cell lung carcinoma; DF, disease free; DOD, died of disease.
In our case, despite the large size, the tumor was highly responsive to chemotherapy, with a tumoral regression of 75% on MRI scan, in keeping with the high responsivity to chemotherapy of some pediatric tumors showing ETV6-driven fusion (i.e., infantile fibrosarcoma). The detection of the ETV6 gene fusion was of great importance for the possibility to administer a target therapy. The addition of Larotrectinib contributed to a more extensive regression, with a documented complete pathological response on the surgical specimen.
Acknowledgments
We thank Mr Gabriele Bacile for iconography preparation.
Footnotes
List of Abbreviations: SC Secretory Carcinoma
PAS Periodic Acid-Schiff
PAS-D Periodic Acid-Schiff with Diastase
HPF High power field
FFPE Formalin fixed paraffin embedded
MRI Magnetic Resonance Imaging
ACC Acinic cell carcinoma
Non-ITAC Non-intestinal type adenocarcinoma
FISH Fluorescence in situ
PCR Protein chain reaction
NGS Next generation sequencing
CK Cytokeratin
GCDFP-15 Gross Cystic Disease Fluid Protein 15
EMA Epithelial membrane antigen
SMA Smooth Muscle Actin
DOG1 Discovered on GIST 1
TRK Tropomyosin receptor kinase
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Antonello Cardoni  https://orcid.org/0000-0003-3468-0473
https://orcid.org/0000-0003-3468-0473
References
- 1. El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO Classification of Head and Neck Tumours. 4th ed. IARC; 2017. [Google Scholar]
- 2. Alves LDB, de Melo AC, Farinha TA, et al. A systematic review of secretory carcinoma of the salivary gland: where are we? Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;132:e143-e152. [DOI] [PubMed] [Google Scholar]
- 3. Ngouajio AL, Drejet SM, Phillips DR, Summerlin DJ, Dahl JP. A systematic review including an additional pediatric case report: pediatric cases of mammary analogue secretory carcinoma. Int J Pediatr Otorhinolaryngol. 2017;100:187-193. [DOI] [PubMed] [Google Scholar]
- 4. Quattlebaum SC, Roby B, Dishop MK, Said MS, Chan K. A pediatric case of mammary analogue secretory carcinoma within the parotid. Am J Otolaryngol. 2015;36:741-743. [DOI] [PubMed] [Google Scholar]
- 5. Hwang MJ, Wu PR, Chen CM, Chen CY, Chen CJ. A rare malignancy of the parotid gland in a 13-year-old Taiwanese boy: case report of a mammary analogue secretory carcinoma of the salivary gland with molecular study. Med Mol Morphol. 2014;47:57-61. [DOI] [PubMed] [Google Scholar]
- 6. Rastatter JC, Jatana KR, Jennings LJ, Melin-Aldana H. Mammary analogue secretory carcinoma of the parotid gland in a pediatric patient. Otolaryngol Head Neck Surg. 2012;146:514-515. [DOI] [PubMed] [Google Scholar]
- 7. Willis K, Bullock M, Rigby MH. A case report of surgical resection of secretory carcinoma in the maxillary and ethmoid sinus. Int J Surg Case Rep. 2021;81:105750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu B, Aryeequaye R, Wang L, Katabi N. Sinonasal secretory carcinoma of salivary gland with high grade transformation: a case report of this under-recognized diagnostic entity with prognostic and therapeutic implications. Head and Neck Pathol. 2018;12:274-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599-608. [DOI] [PubMed] [Google Scholar]
- 10. Luk PP, Selinger CI, Eviston TJ, et al. Mammary analogue secretory carcinoma: an evaluation of its clinicopathological and genetic characteristics. Pathology. 2015;47:659-666. [DOI] [PubMed] [Google Scholar]
- 11. Majewska H, Skálová A, Stodulski D, et al. Mammary analogue secretory carcinoma of salivary glands: a new entity associated with ETV6 gene rearrangement. Virchows Arch. 2015;466:245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Connor A, Perez-Ordoñez B, Shago M, Skálová A, Weinreb I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol. 2012;36:27-34. [DOI] [PubMed] [Google Scholar]
- 13. Toper MH, Sarioglu S. Molecular pathology of salivary gland neoplasms: diagnostic, prognostic, and predictive perspective. Adv Anat Pathol. 2021;28:81-93. [DOI] [PubMed] [Google Scholar]
- 14. Asai S, Sumiyoshi S, Yamada Y, et al. High-grade salivary gland carcinoma with the ETV6-NTRK3 gene fusion: A case report and literature review of secretory carcinoma with high-grade transformation. Pathol Int. 2021;71:427-434. [DOI] [PubMed] [Google Scholar]
- 15. Skálová A, Stenman G, Simpson RHW, et al. The role of molecular testing in the differential diagnosis of salivary gland carcinomas. Am J Surg Pathol. 2018;42:e11-e27. [DOI] [PubMed] [Google Scholar]
- 16. Andreasen S, Skálová A, Agaimy A, et al. ETV6 gene rearrangements characterize a morphologically distinct subset of sinonasal low-grade non-intestinal-type adenocarcinoma: a novel translocation-associated carcinoma restricted to the sinonasal tract. Am J Surg Pathol. 2017;41:1552-1560. [DOI] [PubMed] [Google Scholar]
- 17. Luo W, Lindley SW, Lindley PH, Krempl GA, Seethala RR, Fung KM. Mammary analog secretory carcinoma of salivary gland with high-grade histology arising in hard palate, report of a case and review of literature. Int J Clin Exp Pathol. 2014;7:9008-9022. [PMC free article] [PubMed] [Google Scholar]
- 18. Skálová A, Vanecek T, Majewska H, et al. Mammary analogue secretory carcinoma of salivary glands with high-grade transformation: report of 3 cases with the ETV6-NTRK3 gene fusion and analysis of TP53, β-catenin, EGFR, and CCND1 genes. Am J Surg Pathol. 2014;38:23-33. [DOI] [PubMed] [Google Scholar]
- 19. Jung MJ, Song JS, Kim SY, et al. Finding and characterizing mammary analogue secretory carcinoma of the salivary gland. Pathol. 2013;47:36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cipriani NA, Blair EA, Finkle J, et al. Salivary gland secretory carcinoma with high-grade transformation, CDKN2A/B loss, distant metastasis, and lack of sustained response to crizotinib. Int J Surg Pathol. 2017;25:613-618. [DOI] [PubMed] [Google Scholar]
- 21. Baghai F, Yazdani F, Etebarian A, Garajei A, Skalova A. Clinicopathologic and molecular characterization of mammary analogue secretory carcinoma of salivary gland origin. Pathol Res Pract. 2017;213:1112-1118. [DOI] [PubMed] [Google Scholar]
- 22. Sun J, Wang L, Tian Z, Hu Y, Xia R, Li J. Higher Ki67 index, nodal involvement, and invasive growth were high risk factors for worse prognosis in conventional mammary analogue secretory carcinoma. J Oral Maxillofac Surg. 2019;77:1187-1202. [DOI] [PubMed] [Google Scholar]
- 23. Taverna C, Baněčková M, Lorenzon M, et al. MUC4 is a valuable marker for distinguishing secretory carcinoma of the salivary glands from its mimics. Histopathology. 2021;79:315-324. [DOI] [PubMed] [Google Scholar]
- 24. Numano Y, Ogawa T, Ishikawa T, et al. Parotid secretory carcinoma with high-grade transformation. Auris Nasus Larynx. 2020;47:1043-1048. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki K, Harada H, Takeda M, et al. Clinicopathological investigation of secretory carcinoma cases including a successful treatment outcome using entrectinib for high-grade transformation: a case report. BMC Med Genomics. 2022;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Skálová A, Banečkova M, Thompson LDR, et al. Expanding the molecular spectrum of secretory carcinoma of salivary glands with a novel VIM-RET fusion. Am J Surg Pathol. 2020;44:1295-1307. [DOI] [PubMed] [Google Scholar]