Abstract
Background:
Apremilast, an oral phosphodiesterase 4 inhibitor, is approved in the European Union for the treatment of moderate-to-severe chronic plaque psoriasis in adult patients refractory or contraindicated to or intolerant of other systemic therapies.
Objectives:
The APPRECIATE study assessed apremilast use in real-world practice and its clinical value to physicians and patients. APPRECIATE was a multinational, observational, retrospective, cross-sectional study.
Methods:
Apremilast effectiveness at 6 (±1) months was assessed on the basis of psoriasis severity and health-related quality-of-life scores and treatment satisfaction using physician/patient-reported outcomes, respectively. We report the Austrian cohort of 72 patients.
Results:
At 6 (±1) months, three-quarters of patients remained on apremilast, while physicians and patients reported treatment benefits across all psoriasis symptoms and manifestations. Of patients, the majority were satisfied with their treatment and achieved treatment goals considered most relevant. Patients’ and physicians’ perceptions of treatment effectiveness were aligned, and health-related quality-of-life scores indicated an improvement in the majority of patients. Apremilast tolerability was consistent with the known safety profile.
Conclusions:
Among psoriasis patients receiving apremilast in Austria, improvement in clinical outcomes were observed and satisfaction with apremilast treatment among patients and physicians was high.
Registration:
ClinicalTrials.gov NCT02740218
Keywords: apremilast, health-related quality of life, psoriasis drug therapy, psoriasis severity scores, real-world data
Introduction
Psoriasis is a chronic disease that can occur at any age, with most patients first diagnosed before the age of 35 years.1 In Austria, the life-time prevalence of physician-diagnosed psoriasis is estimated to be 135,650 in adults and 3580 in children; self-reported psoriasis could be twice as high.2 Of note, there are still patients not diagnosed. Psoriasis is considered systemic with an immune mediated inflammatory mechanism.3 Typical symptoms are red, scaly plaques that can occur on all sites of the body surface, but most frequently on the scalp, elbows, knees, and lower back.4,5 The impact on patients’ physical and psychological well-being is high and associated with considerable social stigmatization.2,6
Recently, an individualized, patient-centred approach has been recognized and integrated into the management of psoriatic disease. To objectively assess outcomes of a given treatment, the use of scores is recommended to compare the disease severity at treatment initiation and while on treatment with respect to primary efficacy and its maintenance or loss. The most common psoriasis severity scores are the Body Surface Area (BSA),7,8 the Psoriasis Area and Severity Index (PASI),7,9 the Dermatology Life Quality Index (DLQI),10,11 and the Physicians Global Assessment (PGA).7 The BSA determines the proportion of the body surface area affected and ranges from 0% to 100%; psoriasis is considered mild for BSA < 3%, moderate for BSA 3–10% and severe for BSA > 10%. The PASI divides the body into four sections (head, arms, trunk, and legs). Each of these areas is scored individually and the four scores combined into the final PASI, with a possible range of 0 to 72. A score of <5 is considered mild disease, 5 to 10 is considered moderate disease and a score >10 considered severe. PASI is widely used in clinical trials to measure response to treatment and presented as a percentage response rate, for example, PASI 50 or PASI 75, representing the percentage of patients who have achieved ⩾50% or ⩾75% reduction in their PASI score from baseline. The DLQI is a 10-item patient questionnaire to assess health-related quality of life (HRQoL) with a resulting score ranging from 0 to 30, with 0 to 1 = the psoriasis has no effect on the patient’s HRQoL, 2 to 5 = small effect, 6 to 10 = moderate effect, 10 to 20 = very large effect, and 21 to 30 = extremely large effect. The PGA is a 5-point scoring system (some versions use a 6- or 7-point system) which assesses psoriasis disease severity and ranges from 0 = clear (no signs of psoriasis, but post-inflammatory discoloration may be present) to 4 = very severe (very marked plaque elevation, scaling, and erythema). PGA 0/1 (clear/almost clear) is a frequently used measure of treatment effectiveness in clinical trials.
The Patient Benefit Index (PBI)12 is a two-part tool consisting of the Patient Needs Questionnaire (PNQ) and the Patient Benefit Questionnaire (PBQ). Patients rate the relevance of 25 predefined treatment goals using the PNQ (0 = not important at all to 4 = very important) and can also indicate, if a goal does or does not apply to them. They then rate the extent to which the same set of goals have been met under treatment using the PBQ (0 = treatment did not help at all to 4 = treatment helped a lot). A patient’s PBI total score is calculated as the mean of all PBQ items, weighted by the relative importance of each item in the PNQ with a resulting score of 0 = no benefit to 4 = maximal patient-defined benefit.
Apremilast, an oral nonbiologic (small molecule) PDE4 inhibitor, is approved in the European Union for the treatment of moderate-to-severe chronic plaque psoriasis in adult patients who failed to respond to or who have a contraindication to, or are intolerant of other systemic therapy including cyclosporine, methotrexate or psoralen and ultraviolet-A light (PUVA). Other indications include psoriatic arthritis and Behçet’s disease.13 In Austria, apremilast is reimbursed in the psoriasis indication in accordance with the European Medicines Agency label.14 The 2021 S3 guidelines15,16 recommend the initiation of a conventional systemic therapy in patients with moderate to severe psoriasis, defined as (BSA > 10% or PASI > 10) and DLQI > 10. Among systemic therapies, the use of biologics or nonbiologics, including apremilast, is recommended second-line in patients not responding to, contraindicated to or intolerant of conventional therapy. First-line use of such agents is recommended, where conventional therapies are not expected to induce sufficient response. These so-called ‘upgrade criteria’ are high disease severity [PASI ⩾ 20]), rapid disease progression, or in case of involvement of nails, genital area, or scalp, or severely impaired HRQoL, for example, DLQI ⩾ 15.16,17 In addition, the perception of ‘sufficient response’ is subject to individual patient treatment goals. In patients with mild disease as determined using disease severity scores, strongly impaired HRQoL may still be indicative of moderate or severe disease, especially when special areas are affected.18,19
The Apremilast Clinical Treatment Experience in Psoriasis (APPRECIATE) study was a multinational, observational, retrospective, cross-sectional study in psoriasis patients treated with apremilast in real-world clinical practice. The study objectives were to describe the characteristics of patients with psoriasis treated with apremilast in clinical practice, evaluate real-world outcomes and patient and physician perspectives on treatment outcomes. Overall results from a European cohort of six countries (Austria, Ireland, Germany, Sweden, Switzerland, and the United Kingdom) have been reported previously.20,21 We report data from the Austrian cohort.
Methods
The study involved multiple European countries. We report data from patients treated at 13 sites across Austria, and their physicians. The methods of this multinational, observational, retrospective, cross-sectional study have been described by Augustin et al.20 and Klein et al.21 and are summarized in brief below.
Eligibility criteria
The study included patients ⩾18 years with physician-diagnosed chronic plaque psoriasis treated with apremilast according to routine clinical practice, who could be contacted 6 (±1) months after apremilast initiation.20,21 All consecutive patients at each site were considered to minimize bias in patient selection. Patients who provided written informed consent were enrolled regardless of whether they were continuing apremilast treatment. Patients who were participating in another clinical trial were excluded from the study.
Data collection
At 6 (±1) months after initiation of apremilast treatment (study visit), data were retrospectively collected from medical records, and patients and physicians were asked to complete study-specific questionnaires.
Outcome measures
Supplemental Figure S1.1 (part 1 of the supplemental materials) provides an overview of the study outcomes. Patient demographics and characteristics at apremilast treatment initiation, comorbidities, prior psoriasis treatments, time since psoriasis diagnosis, clinical manifestations of psoriasis, presence of psoriatic arthritis, other significant disease history, and reasons for apremilast initiation were retrospectively collected from medical records.
The apremilast outcome assessment at 6 (±1) months included apremilast status (ongoing or discontinued; if discontinued, reasons for discontinuation and subsequent treatment), disease severity [mean (SD) change in scores for PASI, PGA, BSA; proportion of patients achieving PASI 75, PASI 50, PGA 0/1], impact on patient health-related quality of life [mean (SD) change in DLQI score], and adverse events during apremilast therapy (including, where possible, an assessment of severity and relationship to apremilast).
Apremilast satisfaction at 6 (±1) months was assessed using study-specific patient and physician questionnaires, which assessed treatment effect on their psoriasis symptoms. Each patient was also requested to complete the PBI, consisting of the PNQ and the PBQ questionnaires.
Questionnaires were administered to patients in a prospective manner to minimize recall bias. An overview of all physician and patient-specific questionnaires used can be found in the supplemental material (part 2).
Statistical methods
All analyses were descriptive in nature. Categorical variables were summarized as number, percentage, and 95% confidence intervals (CIs). Continuous variables were summarized as mean, standard deviation (SD), 95% CI, median, interquartile range (IQR), and range. Data were analysed with no imputation for missing data. In an exploratory analysis, results were summarized by treatment setting, that is, hospital- versus office-based setting; no formal comparisons were conducted. Results by treatment setting are shown in the supplementary materials.
Since the intent of this study was exploratory, the sample size was based on what was considered a meaningful cohort relative to the prescribing use within the relevant country rather than statistical grounds.
Results
Patient disposition
In total, 72 patients were enrolled in Austria between May 2016 and July 2018; 38 were treated in the hospital-based setting and 34 were treated in the office-based setting. All patients were included in the analysis reported herein.
Apremilast treatment initiation
Patient demographics and disease characteristics
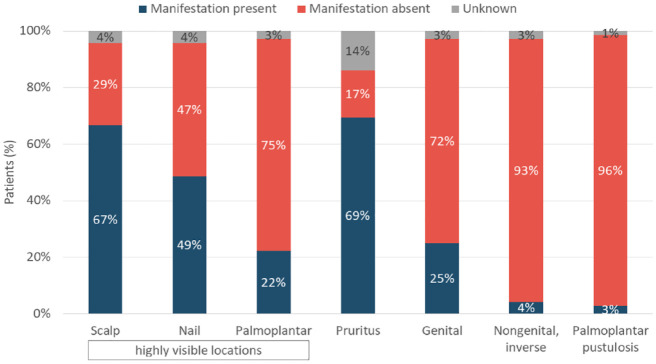
Patients were predominantly male (63%) and had a mean age of 54.2 (SD 15.3) years (Table 1, Supplemental Table S1.1). Most patients (97%, 70/72) were Caucasian, two patients (3%) were Asian. Mean body mass index at baseline was 29.0 (SD 6.0) kg/m2. Most patients had moderate to severe psoriasis (Table 1). The most frequently reported psoriasis manifestations were pruritus (69%), scalp psoriasis (67%), and nail psoriasis (49%) (Figure 1; Supplemental Figure S1.2). At apremilast initiation, comorbidities were recorded in 47% (34/72) of patients; the most common (>5%) being psoriatic arthritis (35%, 25/72), hypertension (17%, 12/72), metabolic syndrome (15%, 11/72), obesity (7%, 5/72), and malignant neoplasm (6%, 4/72).
Table 1.
Patient demographics and disease characteristics.
| All patients (N = 72) | |
|---|---|
| Sex, n (%) | |
| Male | 45 (63) |
| Female | 27 (38) |
| Age (years) | |
| Mean (SD) | 54.2 (15.3) |
| Age category, n (%) | |
| <35 years | 9 (13) |
| 35–65 years | 42 (58) |
| >65 years | 21 (29) |
| Psoriasis disease severity and HRQoL scores at treatment initiation, mean (SD) | |
| PASI (points), n = 46a | 10.3 (5.9) |
| PGA (points), n = 28a | 2.3 (0.6) |
| BSA (%), n = 9a | 9.1 (6.7) |
| DLQI (points), n = 8a | 9.6 (7.4) |
BSA, Body Surface Area; DLQI, Dermatology Life Quality Score; HRQoL, health-related quality of life; PASI, Psoriasis Area Severity Index; PGA, Physician Global Assessment; SD, standard deviation.
Number of patients with scores recorded.
Figure 1.
Specific manifestation of psoriasis.
Percentages are calculated from the overall cohort (N = 72).
Prior medication and reasons for apremilast initiation
Most patients had received one (42%) or two (22%) previous therapies, mostly conventional systemic therapy (49%; Supplemental Figure S1.3). The mean time between psoriasis diagnosis and apremilast initiation was 18.8 years (SD = 16.6). Most patients initiated apremilast due to ineffectiveness of previous therapy (83%, Table 2; Supplemental Table S1.2).
Table 2.
Apremilast use.
| All patients (N = 72) | |
|---|---|
| Time between psoriasis diagnosis and apremilast initiation, years | |
| Mean (SD) | 18.8 (16.6) |
| Reason for apremilast initiation, n (%) | |
| Previous therapy ineffective | 60 (83) |
| Intolerant to previous therapy | 5 (7) |
| Contraindications to conventional therapies | 4 (6) |
| Patient choice | 2 (3) |
| Other | 1 (1) |
| Apremilast treatment status at 6 (±1) months, n (%) | |
| Ongoing | 53 (74) |
| Discontinued | 19 (26) |
| Reason for apremilast discontinuation, n (%) | |
| Safety/tolerability | 10 (14) |
| Lack of efficacy | 7 (10) |
| Other | 1 (1) |
| Unknown | 1 (1) |
SD, standard deviation.
Apremilast outcome assessment
Apremilast treatment status at 6 (±1) months
Almost three-quarters of patients (74%) were still receiving apremilast after 6 (±1) months, 26% had discontinued. Reasons for apremilast discontinuation were safety or tolerability (14%), lack of efficacy (10%), and other (1%) or unknown (1%; Table 2; Supplemental Table S1.2). The mean duration of apremilast treatment at the study visit was 186 (SD = 25) days in patients with ongoing treatment and 104 (SD = 52) days in patients who had discontinued treatment.
Disease severity and impact on patient well-being
PASI, DLQI, PGA, and BSA scores at treatment initiation and at 6 (±1) months are summarized in Supplemental Table S1.3. PASI was the most commonly recorded score; approximately two-thirds (64%) of patients had a PASI recorded at apremilast initiation, falling to 52% at 6 (±1) months. At apremilast initiation, 39% of patients had a PGA recorded, falling to 31% at 6 (±1) months. Less than 20% of patients had DLQI or BSA scores recorded. When recorded, all scores improved between apremilast initiation and 6 (±1) months. Mean PASI fell from 10.3 (SD = 5.9) to 2.7 (SD = 4.3) at 6 (±1) months. In patients with a PASI score recorded at both timepoints, 85% (28/33) achieved PASI 50% and 70% (23/33) achieved PASI 75. In patients with ongoing treatment at 6 (±1) months and recorded PGA, PGA 0/1 was achieved by 91% (20/22). Mean body mass index at 6 (±1) months was 28.1 (SD 5.9) kg/m2.
Safety
Of the 72 patients in the Austrian cohort, 46% (33) patients experienced a total of 54 adverse events, the majority of events which (41/54 [76%]) were mild. The most frequently reported adverse events (occurring in >5% of patients) were diarrhoea (21%), nausea (15%), and weight loss (6%; Supplemental Table S1.4). Withdrawals due to adverse events were reported in 10 (14%) patients. One patient experienced a serious adverse event of Guillain-Barré syndrome which resolved. The relationship of this event to apremilast was based on the timing of apremilast initiation and onset of symptoms; however, prior respiratory infection and other medications may have been confounding factors.
Apremilast satisfaction assessment
Physician assessment of apremilast effectiveness
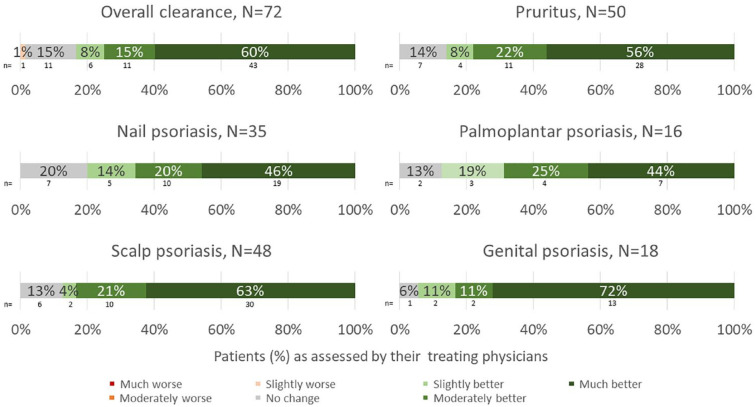
Physician-perceived apremilast effectiveness on psoriasis manifestations at 6 (±1) months is summarized in Figure 2 (and Supplemental Figure S1.4). Three-quarters of physicians reported clinical improvement (defined as ‘moderately’ or ‘much’ better) in overall skin clearance; 78% reported clinical improvements in pruritis, 66%, 69%, 84%, and 83% reported clinical improvements in nail, palmoplantar, scalp, and genital psoriasis, respectively.
Figure 2.
Physician perceived effectiveness of apremilast at 6 (±1) months.
Treatment needs and satisfaction
The treatment goals patients most frequently reported were applicable to their needs were healing of affected skin [71/72 (99%) ‘get better skin quickly’, 68/72 (94%) ‘be healed of all skin defects’], confidence in therapy [71/72 (99%)], less time needed for daily treatment [67/72 (93%)], be less dependent on doctor and clinic visits [67/72 (93%)], find a clear diagnosis and therapy [67/72 (93%)], and not fearing the disease would become worse [66/72 (92%)] (Supplemental Figure S1.5A). At 6 (±1) months, among patients reporting these treatment goals were applicable to them, three-quarters strongly agreed (responding ‘quite’ or ‘very’) that apremilast therapy helped reduce the time needed for daily treatment [46/62 (74%)], alleviate their fear that psoriasis would worsen [48/64 (75%)], and find a clear diagnosis and treatment [51/89 [(79%)]. Approximately two-thirds strongly agreed that apremilast helped them achieve healing of affected skin [46/69 (67%) ‘get better skin quickly’, 45/68 (66%) ‘be healed of all skin defects’] and be less dependent on doctor and clinic visits [40/64 (63%)]. Approximately 70% strongly agreed apremilast helped them have confidence in treatment (n = 47/67), (Supplemental Figure S1.5B).
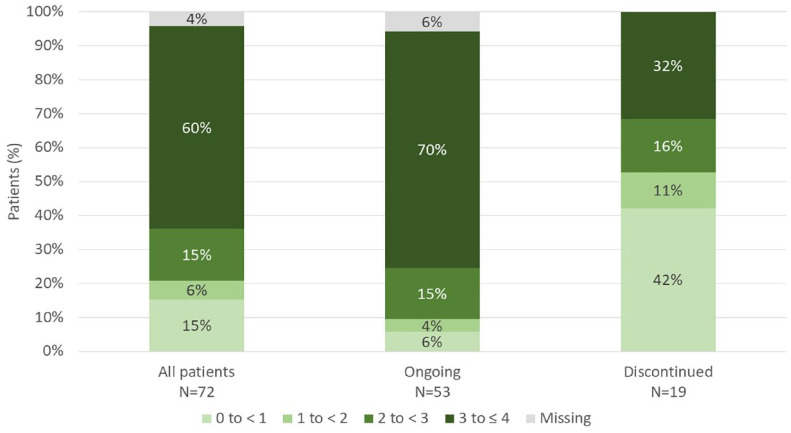
As measured by the global PBI score, 60% of all patients reported a high benefit (score of 3 to ⩽4) after apremilast treatment for 6 (±1) months; 70% of patients continuing apremilast treatment at 6 (±1) months and 32% of patients who had discontinued apremilast at 6 (±1) months reported a high benefit, respectively (Figure 3). The mean global PBI score was 2.8 (SD = 1.31; 95% CI = 2.5–3.1) for all patients and was higher for patients continuing apremilast treatment at 6 (±1) months [3.2 (SD = 0.98; 95% CI = 2.9–3.5)] compared with patients who had discontinued apremilast [1.6 (SD = 1.37; 95% CI = 1.0–2.3)].
Figure 3.
Distribution of global Patient Benefit Index (PBI) scores.
Percentages are calculated from the overall cohort (N = 72).
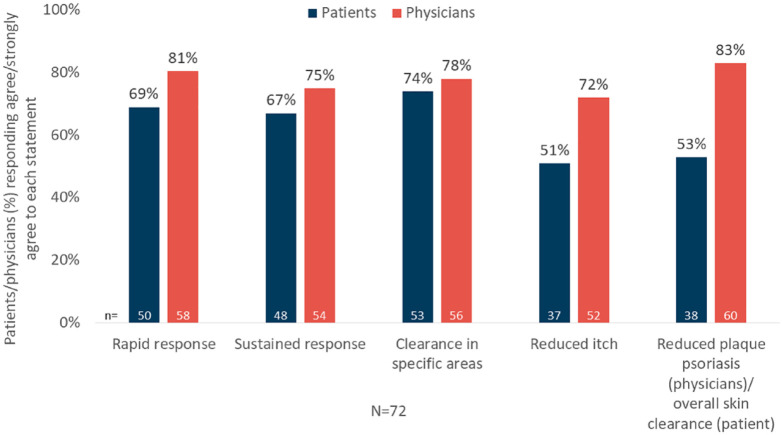
Overall, patients’ and physicians’ satisfaction with apremilast treatment, expressed as ‘agree’ or ‘strongly agree’ on a given statement, was high (Figure 4; Supplemental Figure S1.6).
Figure 4.
Patient and physician satisfaction with apremilast treatment.
Discussion
The APPRECIATE study assessed the use of apremilast in routine clinical practice across several countries and its clinical value to physicians and patients.20 Apremilast effectiveness and tolerability in the Austrian cohort was consistent with that observed in the overall study population, and in clinical trials and prior real-world studies, with improvement in skin involvement and special locations and symptoms of psoriasis. Patients in this cohort showed a high burden of disease, though, with the majority meeting their treatment goals and being satisfied with the apremilast treatment. Physicians’ and patients’ evaluations were largely aligned.
Our results are an improvement on data previously reported for clinical practice in Austria. For example, in an Austrian national survey of 1,184 psoriasis patients primarily treated in tertiary care centres conducted in 2014/2015, the majority of individual treatment goals were not achieved to a sufficient degree.22 Within the 4 weeks preceding the survey, 88% of patients had received topical therapy, 33% had received systemic therapies, and 22% had received UV phototherapy. In general, this discrepancy between patients’ treatment goals and their achievement was smaller in the Austrian APPRECIATE cohort. Among patients considering the disappearance of all skin lesions very much or quite important (i.e. 94% in the Austrian APPRECIATE cohort and 93% in the Austrian national survey), two-thirds of patients in the Austrian APPRECIATE cohort achieved that goal to a large extent (quite or very much), compared with fewer than half (47%) in the Austrian national survey.
In the Austrian cohort of APPRECIATE, physicians reported overall skin clearance to have improved in 83% of patients. This is similar to the overall APPRECIATE study population, in which physicians reported overall skin clearance improved in 76% of patients.20 Observed effectiveness of apremilast was not limited to plaque skin psoriasis and extended to special areas such as scalp, palmoplantar, nail, and genital psoriasis. This is in line with reports on apremilast effectiveness in difficult-to-treat areas in real-life from other countries.23–27
Amid the physicians’ subjective perception, treatment effectiveness was also confirmed using objective clinical assessment scores such as PASI, PGA, DLQI, and BSA. Our data highlight that these scores were infrequently used in clinical practice and suggest they are more applicable for clinical trials. The PASI score was used in 64% of patients at treatment initiation and 51% at 6 (±1) months, the PGA score was used in 39% and 31%, respectively. DLQI and BSA were used in <20% of patients. Mean PASI and DLQI scores at treatment initiation were lower in Austria compared with the overall population [10.3 (SD = 5.9) versus 13.1 (8.7) for PASI, and 9.6 (7.4) versus 12.8 (7.3) for DLQI], suggesting apremilast is used in more moderate disease in Austria compared with other participating countries. Given the infrequent use of these clinical assessment scores, the results should be interpreted with caution. An analysis of 367 patients from the Austrian Psoriasis Registry who were treated with apremilast also observed a reduction in PASI from a mean of 6.5 (SD = 6.4) at baseline to 3.2 (5.0) after 6 months of apremilast treatment.28 The Austrian Psoriasis Registry analysis also found that PASI was infrequently assessed, with 44% and 28% of patients having a PASI score documented at treatment start and follow-up, respectively.28 DLQI was also assessed in the above-mentioned Austrian nationwide survey.22 Across all treatments, 29% of patients had a DLQI of 2 to 5, indicating mild impact of disease, and 19% a DLQI of 6 to 10, indicating moderate impact of disease on the patients’ health-related quality of life. In the Austrian cohort of APPRECIATE, mean DLQI fell from 9.6 (SD = 7.4) at apremilast initiation to 1.6 (1.5) at 6 (±1) months, indicating an improvement from moderate to mild impairment of health-related quality of life.
Inconsistent use of these clinical assessment scores in clinical practice was noted across other countries participating in the APPRECIATE study.20 However, assessment scores such as DLQI may underestimate the disease-specific burden as individual items of the questionnaire may be of lower importance in patients with an overall higher disease burden.29,30 Thus, physicians may feel that their use inadequately captures patients’ needs in routine practice. Efforts have been undertaken to avoid miscategorization of patients’ disease severity. Salgado-Boquete et al.31 propose a combined use of PASI, BSA and static physician global assessment (sPGA) for classification of mild, moderate, and severe psoriasis, capturing the dermatologists’ and the patients’ perspectives. Strober et al.32 reverted to a simpler classification based on treatment needs – topical or systemic – where candidates for systemic therapy are those with a BSA >10%, involvement of special areas, or failure of topical therapy.
Apremilast use was maintained over time, with 74% of patients in the APPRECIATE Austria cohort continuing apremilast at 6 (±1) months, similar to the overall study population (72%) and findings from the Austrian Psoriasis Registry (74.1%). The Austrian Psoriasis Registry analysis found that apremilast discontinuation was higher in patients younger than 40 years. These had a significantly higher rate of inverse and scalp involvement compared with older patients. The authors suggest that younger patients place more importance on quick symptom resolution to feel comfortable in partnerships and in public life, a goal possibly hampered by the higher incidence of difficult-to-treat psoriasis manifestations in that age group.28 This study did not estimate drug survival in the long-term. In the published literature of apremilast drug survival in the real-world setting, the 6-months drug survival rate was 69.5% in one study,33 the 1-year drug survival rate ranged from 34.7% to 57.3%.28,33,34 Median drug survival varied between studies and ranged from 3.1 to 15.7 months.25,28,33–36 It was found that drug survival correlated with primary efficacy, loss of efficacy, safety, age, the burden of comorbidities, and previous exposure to biologics.25,28,36
The adverse events reported in the Austrian cohort of APPRECIATE were consistent with the overall study population and the known safety profile of apremilast. The most frequently reported events (>5% of patients) were diarrhoea, nausea and headache overall, and diarrhoea, nausea, and weight loss in the Austrian cohort. Withdrawals due to adverse events (14%) or lack of efficacy (10%) were comparable with the overall population (12% and 14%, respectively).20 Weight loss was observed in a substantially lower proportion of patients in Austria (6%) compared with the overall APPRECIATE population (23%). The summary of product characteristics states that in the pivotal trials, a total of 14.3% of patients receiving apremilast had observed weight loss between 5% and 10% while 5.7% had observed weight loss greater than 10%.13 A potential explanation for this finding may be that weight data are not collected routinely in clinical practice in Austria and weight change may be presumed to originate from lifestyle changes rather than association with apremilast therapy and therefore not identified or reported as an unexpected/adverse event.
In exploratory analyses, we summarized the data for patients treated in office- and hospital-based settings. These data are presented in the supplementary materials; patient profiles, patient and physician satisfaction, outcome measures, and adverse events were comparable between settings.
The APPRECIATE study has some limitations, which are described in more detail by Augustin et al.20 In brief, documentation of routine clinical practice was incomplete, and the study lacked a control group. The retrospective study design is prone to some degree of recall bias, especially with regards to the patients’ questionnaires. Patient selection bias cannot be excluded and may have led to more frequent inclusion of patients with good treatment response who may also have been more inclined to participate in such a study. In addition, the subjective judgement of treatment satisfaction may be subject to cultural differences. The sample size, especially in the subgroups of the hospital- and office-based settings, was small, rendering interpretation difficult.
Conclusions
In this study of real-world clinical practice in Austria, the effectiveness and safety profile of apremilast treatment was consistent with the overall study population, and prior clinical trials and real-world studies. Patients had a high burden of disease and reported substantial improvement in skin involvement including special locations and specific symptoms of psoriasis under apremilast treatment. Three-quarters of patients remained on apremilast at 6 (±1) months, and physicians and patients reported a treatment benefit across all psoriasis manifestations and treatment needs, with physicians’ and patients’ evaluations largely aligned. Safety and tolerability of apremilast were in line with the known safety profile.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223231152785 for Characteristics and outcomes of patients with psoriasis treated with apremilast in the real-world in Austria – results the APPRECIATE study by Constanze Jonak, Isolde Göttfried, Sylvia Perl-Convalexius, Barbara Gruber, Martina Schütz-Bergmayr, Igor Vujic, Wolfgang Weger, Nikolaus Schicher, Lydia Semlin, Margit Hemetsberger, Myriam Cordey and Paul Sator in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-2-taj-10.1177_20406223231152785 for Characteristics and outcomes of patients with psoriasis treated with apremilast in the real-world in Austria – results the APPRECIATE study by Constanze Jonak, Isolde Göttfried, Sylvia Perl-Convalexius, Barbara Gruber, Martina Schütz-Bergmayr, Igor Vujic, Wolfgang Weger, Nikolaus Schicher, Lydia Semlin, Margit Hemetsberger, Myriam Cordey and Paul Sator in Therapeutic Advances in Chronic Disease
Acknowledgments
Margit Hemetsberger, hemetsberger medical services, Vienna, received a fee from Amgen GmbH for medical writing and editing of this manuscript. Claire Desborough of Amgen provided editorial assistance. The authors thank Lucia Maria Leitner of Amgen for her valuable contributions in managing the publication process.
Footnotes
ORCID iD: Margit Hemetsberger  https://orcid.org/0000-0003-1877-6838
https://orcid.org/0000-0003-1877-6838
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Constanze Jonak, Department of Dermatology, Medical University of Vienna, Vienna, Austria.
Isolde Göttfried, Dermatology Practice, Vienna, Austria.
Sylvia Perl-Convalexius, Dermatology Practice, Vienna, Austria.
Barbara Gruber, Department of Dermatology and Venerology, Klinikum Wels-Grieskirchen, Wels, Austria.
Martina Schütz-Bergmayr, Department of Dermatology, Kepler University Hospital, Linz, Austria.
Igor Vujic, Faculty of Medicine and Dentistry, Danube Private University, Krems an der Donau, Austria; Department of Dermatology, Klinik Landstraße, Vienna, Austria.
Wolfgang Weger, Department of Dermatology, Medical University Graz, Graz, Austria.
Nikolaus Schicher, Dermatology Practice, Klagenfurt, Austria.
Lydia Semlin, Amgen GmbH, Vienna, Austria.
Margit Hemetsberger, hemetsberger medical services, Vienna, Austria.
Myriam Cordey, Amgen Inc, Thousand Oaks, CA, USA.
Paul Sator, Department of Dermatology, Municipal Hospital Hietzing, Wolkersbergenstraße 1, A-1130 Vienna, Austria.
Declarations
Ethics approval and consent to participate: The study was approved by the national central ethics committees of each participating country as detailed in the primary publication;20 the ethics committee of the Vienna Medical University approved the study in Austria (ethics committee approval number: 1482/2017). All patients provided written informed consent prior to any data collection. APPRECIATE was registered in ClinicalTrials.gov (NCT02740218).
Consent for publication: Not applicable.
Author contributions: Constanze Jonak: Investigation; Writing – review & editing.
Isolde Göttfried: Investigation; Writing – review & editing.
Sylvia Perl-Convalexius: Investigation; Writing – review & editing.
Barbara Gruber: Investigation; Writing – review & editing.
Martina Schütz-Bergmayr: Investigation; Writing – review & editing.
Igor Vujic: Investigation; Writing – review & editing.
Wolfgang Weger: Investigation; Writing – review & editing.
Nikolaus Schicher: Investigation; Writing – review & editing.
Lydia Semlin: Conceptualization; Data curation; Writing – original draft; Writing – review & editing.
Margit Hemetsberger: Formal analysis; Visualization; Writing – original draft; Writing – review & editing.
Myriam Cordey: Conceptualization; Methodology; Writing – review & editing.
Paul Sator: Investigation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Celgene; Amgen Inc. acquired the worldwide rights to Otezla® (apremilast) on November 21, 2019. Kantar Health GmbH, Munich, Germany acted as the clinical research organization, sponsored by Celgene.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P.S.: AbbVie, Actelion, Amgen, Almirall, Celgene Corporation, Janssen, LEO Pharma, Lilly, Maruho, Merck Sharp & Dohme, Novartis, Pfizer and UCB – research funding, consulting and speaker fees, travel support. I.G.: AbbVie, Celgene Corporation, Eli Lilly and Novartis – consulting fees, travel support. S.P.C.: ALK, Celgene Corporation, Eli Lilly, HAL Allergy and Pelpharma – consulting and speaker fees and travel support. B.G.: AbbVie, Bristol-Myers Squibb, Eli Lilly, LEO Pharma and Janssen-Cilag – consulting and speaker fees and travel support. M.S.B.: AbbVie, Celgene Corporation, Eli Lilly, Janssen, Novartis and Pfizer – consulting and speaker fees and travel support. C.J.: Department of Dermatology, Medical University of Vienna, Austria – employment; AbbVie, Almirall, Amgen, Böhringer Ingelheim, Janssen, LEO, Lilly, Kyowa Kirin, Novartis, Pfizer, Recordati Rare Diseases, Sandoz, Takeda, UCB – consulting and speaker fees; Lilly, Novartis, LEO, 4SC – study investor. I.V.: Bristol-Myers Squibb, Celgene Corporation, LEO Pharma and Novartis – consulting and speaker fees. W.W.: AbbVie, Amgen, Celgene Corporation, Eli Lilly, Janssen, LEO Pharma, Merck Sharp & Dohme, Novartis, Pfizer, Sandoz and UCB – research funding, consulting and speaker fees, travel support. L.S.: Amgen – employment relationship, shares; Celgene – Stocks. M.H.: Amgen – consulting fees, shares; M.C.: Amgen – employment; Celgene – former employment at the time of study conduct. N.S.: no conflict of interest.
Availability of data and materials: Qualified researchers may request data from Amgen clinical studies. Complete data are available at the following: https://wwwext.amgen.com/about/how-we-operate/policies-practices-and-disclosures/ethical-research/clinical-data-transparency-practices/clinical-trial-data-sharing-request.
References
- 1. Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis 2005; 64: ii18–ii23; discussion ii24–ii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ 2020; 369: m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370: 263–271. [DOI] [PubMed] [Google Scholar]
- 4. Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Am Acad Dermatol 2014; 70: 871–881. [DOI] [PubMed] [Google Scholar]
- 5. Lambert J, Dowlatshahi EA, de la Brassinne M, et al. A descriptive study of psoriasis characteristics, severity and impact among 3269 patients: results of a Belgian cross sectional study (BELPSO). Eur J Dermatol 2012; 22: 231–237. [DOI] [PubMed] [Google Scholar]
- 6. Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc 2004; 9: 136–139. [DOI] [PubMed] [Google Scholar]
- 7. Spuls PI, Lecluse LLA, Poulsen M-LNF, et al. How Good are clinical severity and outcome measures for psoriasis? Quantitative evaluation in a systematic review. J Invest Dermatol 2010; 130: 933–943. [DOI] [PubMed] [Google Scholar]
- 8. Ramsay B, Lawrence CM. Measurement of involved surface area in patients with psoriasis. Br J Dermatol 1991; 124: 565–570. [DOI] [PubMed] [Google Scholar]
- 9. Ashcroft DM, Wan Po AL, Williams HC, et al. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol 1999; 141: 185–191. [DOI] [PubMed] [Google Scholar]
- 10. Basra MK, Fenech R, Gatt RM, et al. The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008; 159: 997–1035. [DOI] [PubMed] [Google Scholar]
- 11. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 12. Feuerhahn J, Blome C, Radtke M, et al. Validation of the patient benefit index for the assessment of patient-relevant benefit in the treatment of psoriasis. Arch Dermatol Res 2012; 304(6): 433–441. [DOI] [PubMed] [Google Scholar]
- 13. European Medicines Agency. Otezla summary of medicinal product characteristics, 2021, https://www.ema.europa.eu/en/documents/product-information/otezla-epar-product-information_en.pdf (accessed 28 January 2022).
- 14. Österreichische Sozialversicherung. Erstattungskodex, 2022, https://www.sozialversicherung.at/cdscontent/?contentid=10007.844498&portal=svportal (accessed 28 January 2022).
- 15. Nast A, Altenburg A, Augustin M, et al. Deutsche S3-Leitlinie zur Therapie der Psoriasis vulgaris, adaptiert von EuroGuiDerm – Teil 2: Therapiemonitoring, besondere klinische Situationen und Vorliegen von Komorbidität. J Dtsch Dermatol Ges 2021; 19: 1092–1117. [DOI] [PubMed] [Google Scholar]
- 16. Nast A, Altenburg A, Augustin M, et al. German S3-Guideline on the treatment of psoriasis vulgaris, adapted from EuroGuiDerm – Part 1: treatment goals and treatment recommendations. J Dtsch Dermatol Ges 2021; 19: 934–150. [DOI] [PubMed] [Google Scholar]
- 17. Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res 2011; 303: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kavanaugh A, Helliwell P, Ritchlin CT. Psoriatic arthritis and burden of disease: patient perspectives from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Rheumatol Ther 2016; 3: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reich K, Gisondi P, Stein Gold L, et al. Differences in patient and dermatologist perspectives on psoriasis treatment: results From the UPLIFT survey. In: 30th European academy of dermatology and venereology (EADV) congress, Virtual, 2021. [Google Scholar]
- 20. Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: results from the APPRECIATE study. J Eur Acad Dermatol Venereol 2021; 35: 123–134. [DOI] [PubMed] [Google Scholar]
- 21. Klein TM, Blome C, Kleyn CE, et al. Real-world experience of patient-relevant benefits and treatment satisfaction with apremilast in patients with psoriasis: an analysis of the APPRECIATE study. Dermatol Ther 2022; 12: 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolf P, Weger W, Legat F, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges 2018; 16: 981–990. [DOI] [PubMed] [Google Scholar]
- 23. Filippi F, Patrizi A, Iezzi L, et al. Use of Apremilast® in the psoriasis treatment: a real-life multicenter Italian experience. Ital J Dermatol Venerol 2022; 157: 313–317. [DOI] [PubMed] [Google Scholar]
- 24. Pavia G, Gargiulo L, Cortese A, et al. Apremilast for the treatment of palmo-plantar non-pustular psoriasis: a real-life single-center retrospective study. Dermatol Ther 2022; 35: e15253. [DOI] [PubMed] [Google Scholar]
- 25. Kapniari E, Dalamaga M, Papadavid E. Comorbidities burden and previous exposure to biological agents may predict drug survival of apremilast for psoriasis in a real-world setting. Dermatol Ther 2020; 33: e14168. [DOI] [PubMed] [Google Scholar]
- 26. de Vlam K, Toukap AN, Kaiser MJ, et al. Real-world efficacy and safety of apremilast in Belgian patients with psoriatic arthritis: results from the prospective observational APOLO study. Adv Ther 2022; 39: 1055–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghislain PD, Lambert J, Hoai XL, et al. Real-life effectiveness of apremilast for the treatment of psoriasis in Belgium: results from the observational OTELO study. Adv Ther 2022; 39: 1068–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graier T, Weger W, Sator PG, et al. Effectiveness and clinical predictors of drug survival in psoriasis patients receiving apremilast: a registry analysis. JAAD Int 2021; 2: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Augustin M, Langenbruch A, Gutknecht M, et al. Definition of psoriasis severity in routine clinical care: current guidelines fail to capture the complexity of long-term psoriasis management. Br J Dermatol 2018; 179: 1385–1391. [DOI] [PubMed] [Google Scholar]
- 30. Langenbruch A, Radtke MA, Gutknecht M, et al. Does the Dermatology Life Quality Index (DLQI) underestimate the disease-specific burden of psoriasis patients? J Eur Acad Dermatol Venereol 2019; 33: 123–127. [DOI] [PubMed] [Google Scholar]
- 31. Salgado-Boquete L, Carrascosa JM, Llamas-Velasco M, et al. A new classification of the severity of psoriasis: what’s moderate psoriasis? Life 2021; 11: 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strober B, Ryan C, van de Kerkhof P, et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol 2020; 82: 117–122. [DOI] [PubMed] [Google Scholar]
- 33. Distel J, Cazzaniga S, Seyed Jafari SM, et al. Long-term effectiveness and drug survival of apremilast in treating psoriasis: a real-world experience. Dermatology 2022; 238: 267–275. [DOI] [PubMed] [Google Scholar]
- 34. Kishimoto M, Komine M, Kamiya K, et al. Drug survival of apremilast in a real-world setting. J Dermatol 2019; 46: 615–617. [DOI] [PubMed] [Google Scholar]
- 35. Lee EB, Amin M, Wu JJ. Drug survival of apremilast in patients treated for psoriasis in a real-world setting. J Am Acad Dermatol 2018; 79: 760–761. [DOI] [PubMed] [Google Scholar]
- 36. Vujic I, Herman R, Sanlorenzo M, et al. Apremilast in psoriasis – a prospective real-world study. J Eur Acad Dermatol Venereol 2018; 32: 254–259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223231152785 for Characteristics and outcomes of patients with psoriasis treated with apremilast in the real-world in Austria – results the APPRECIATE study by Constanze Jonak, Isolde Göttfried, Sylvia Perl-Convalexius, Barbara Gruber, Martina Schütz-Bergmayr, Igor Vujic, Wolfgang Weger, Nikolaus Schicher, Lydia Semlin, Margit Hemetsberger, Myriam Cordey and Paul Sator in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-2-taj-10.1177_20406223231152785 for Characteristics and outcomes of patients with psoriasis treated with apremilast in the real-world in Austria – results the APPRECIATE study by Constanze Jonak, Isolde Göttfried, Sylvia Perl-Convalexius, Barbara Gruber, Martina Schütz-Bergmayr, Igor Vujic, Wolfgang Weger, Nikolaus Schicher, Lydia Semlin, Margit Hemetsberger, Myriam Cordey and Paul Sator in Therapeutic Advances in Chronic Disease