Abstract
Objectives
To use national, pre- and post-pandemic electronic health records (EHR) to develop and validate a scenario-based model incorporating baseline mortality risk, infection rate (IR) and relative risk (RR) of death for prediction of excess deaths.
Design
An EHR-based, retrospective cohort study.
Setting
Linked EHR in Clinical Practice Research Datalink (CPRD); and linked EHR and COVID-19 data in England provided in NHS Digital Trusted Research Environment (TRE).
Participants
In the development (CPRD) and validation (TRE) cohorts, we included 3.8 million and 35.1 million individuals aged ≥30 years, respectively.
Main outcome measures
One-year all-cause excess deaths related to COVID-19 from March 2020 to March 2021.
Results
From 1 March 2020 to 1 March 2021, there were 127,020 observed excess deaths. Observed RR was 4.34% (95% CI, 4.31–4.38) and IR was 6.27% (95% CI, 6.26–6.28). In the validation cohort, predicted one-year excess deaths were 100,338 compared with the observed 127,020 deaths with a ratio of predicted to observed excess deaths of 0.79.
Conclusions
We show that a simple, parsimonious model incorporating baseline mortality risk, one-year IR and RR of the pandemic can be used for scenario-based prediction of excess deaths in the early stages of a pandemic. Our analyses show that EHR could inform pandemic planning and surveillance, despite limited use in emergency preparedness to date. Although infection dynamics are important in the prediction of mortality, future models should take greater account of underlying conditions.
Keywords: Clinical, epidemiology, health informatics, infectious diseases, public health
Introduction
Mortality estimates of COVID-19 have been widely reported and followed at local, regional, national and international levels since early in the pandemic, influencing policy and health service planning. Electronic health record (EHR) data informed early identification of risk factors for COVID-19 severity and mortality, leading to UK lockdown and shielding policies.1–3 Moreover, EHR linkage enabled both specialist registry data and pragmatic clinical trials of new treatments at scale.4,5
All-cause and disease-specific mortality prediction in research and clinical practice has included underlying conditions or ‘baseline mortality risk’, often derived and validated using EHR.6–8 Underlying non-communicable diseases (NCDs) are important mortality predictors in infectious diseases,9,10 but baseline mortality risk based on NCDs is largely neglected in pandemic preparedness, which emphasises infection transmissibility and severity, using metrics such as case fatality ratio, infection fatality ratio and reproduction number.11–14 Although COVID-19 is increasingly viewed as a ‘syndemic’15 (with interaction between infectious diseases and NCDs, requiring cross-speciality expertise), efforts to predict excess mortality have focused on dynamic transmission modelling without consideration of baseline risk or use of anonymised, individual-level, population-scale EHR.16,17
On 22 March 2020, before the first UK lockdown, we released a preprint (published on 12 May 2020),1 estimating one-year COVID-19 mortality using a model developed in pre-pandemic population-based linked EHRs from 3.8 million people in the UK (via Clinical Practice Research Datalink [CPRD]). Our EHR-derived model included baseline one-year mortality risk for a range of underlying conditions, incorporating scenario-based assumptions regarding relative risk (RR) of mortality during the pandemic compared to baseline, and population infection rate (IR). Validation of the model is required to establish the actual RR and IR, to update scenario-based assumptions and to assess the accuracy of model predictions.
The NHS Digital Trusted Research Environment (TRE) for England, which became available during 2020, offers the opportunity to validate our approach at whole population level, with longitudinal, individual-level data.18,19 Therefore, using these data, we: (1) ascertained the observed IR of COVID-19 and RR of one-year COVID-19 mortality; and (2) compared the predicted versus observed COVID-19 mortality for conceptual validation of our EHR-derived model.
Methods
Data sources
Conceptual model development
We used a pre-pandemic linked CPRD dataset, including EHR, across primary care, hospital data and death registry with follow-up from 1997 to 2017.1
Model validation
The NHS Digital TRE for England provides secure, remote access to linked, individual-level EHR data,18,19 including primary care, hospital episodes, registered deaths, dispensed medicines, COVID-19 laboratory tests and vaccinations. We used General Practice Extraction Service Data for Pandemic Planning and Research, Hospital Episode Statistics Admitted Patient Care, Second Generation Surveillance System, COVID-19 Hospitalisation in England Surveillance System, Civil Registry Deaths, NHS Business Services Authority dispensed medicines and COVID-19 vaccine datasets, prior to 15 May 2021.19
Cohort specifications
Both model development and validation involved population-based, retrospective cohort analyses with a range of high-risk conditions as exposures and one-year all-cause mortality as outcome. In the validation study, a further exposure was SARS-CoV-2 infection. In the development study, eligible individuals were aged ≥30 years, registered with a GP between 1 January 1997 and 1 January 2017 (Figure S1.A), with ≥1 year of follow-up.
In the validation study, eligible individuals were aged ≥30 years on 1 March 2018. COVID-19-related high-risk conditions were from Public Health England guidance.20 We considered all-cause mortality after COVID-19 as the direct pandemic effect. Deaths in those without COVID-19 include baseline mortality and deaths attributable to indirect pandemic effects. To evaluate direct COVID-19 effects on one-year all-cause mortality, we specified two time periods (Figure S1.B and S1.C). The pre-pandemic period (1 March 2018–1 March 2019) was used for baseline characteristics and outcome (mortality) in the non-exposed (non-COVID-19) group. The pandemic period (1 March 2020–1 March 2021) was used to study COVID-19 cases and deaths in the exposed group (i.e. COVID-19 with or without high-risk conditions). Underlying conditions were assessed on 1 March 2018 in the validation study, minimising the effect of age difference between pre-pandemic and pandemic periods (Figure S2).
Exposures and outcomes of interest
Exposures were presence (versus absence) of high-risk conditions for COVID-1920 including cardiovascular disease (CVD), chronic kidney disease (CKD), diabetes, chronic obstructive pulmonary disease (COPD), body mass index (BMI) over 40 kg/m2, chronic liver disease, age >70 years and history of oral steroid therapy. For all conditions, except steroid therapy, the minimum period between earliest diagnosis date and baseline date (1 March 2018) was one year. For steroid therapy, event date was based on first dispensing date between 1 March 2018 and 1 March 2019, since prescription/dispensed medication data were only available since April 2018. Outcome was one-year all-cause mortality.
To define underlying conditions, we used extended CALIBER phenotyping algorithms.21 Phenotypes with earliest diagnosis dates between 1 March 2017 and 1 March 2018 were excluded, to allow ≥1 year history of conditions prior to cohort entry. The CVD phenotype was a composite, including heart failure, stroke (non-specified, ischaemic, haemorrhagic, transient ischaemic attack, subarachnoid haemorrhagic), arrhythmias, acute myocardial infarction, cardiomyopathy, atrial fibrillation, deep vein thrombosis, isolated calf vein thrombosis and pulmonary embolism. The dispensed oral corticosteroid phenotype was determined based on the CALIBER phenotype mapped to British National Formulary codes.22 To define COVID-19 cases, we used positive swab testing results and Public Health England labs and NHS hospitals, community swab testing results, primary care and hospital episode data, vaccination and death registration.23
Model development and validation
Our prediction model in the development study was a conceptual model based on baseline mortality, RR of death in those exposed to COVID-19 versus those not exposed to COVID-19 (pre-pandemic) and IR of COVID-19
In the development study, we calculated scenario-based COVID-19 excess deaths using baseline mortality by high-risk underlying conditions and plausible RR/IR (0.001%, 1%, 10% and 80% for total, partial, moderate and no suppression, respectively).2 For each IR scenario, we applied RRs (1.2, 1.5 and 3), and scaled up to mid-2018 population of England aged ≥30 using estimates of the Office for National Statistics.24
Full validation was beyond scope and not possible in the rapidly changing timelines of the pandemic. Validation in our study involved use of observed IR and RR values (TRE for England; Figure S1.B) in the conceptual model to predict COVID-19 deaths in the development and validation cohorts. This constituted ‘model verification’ (‘determining that the model's inputs and outputs are consistent with actual data and accepted theories’) and ‘conceptual model validation’ (‘determining that the theories and assumptions underlying the conceptual model are correct and the model representation of the problem entity and the model's structure, logic and mathematical and causal relationships are reasonable for the intended purpose of the model’).25 In order to capture direct COVID-19 mortality effects, we selected unexposed and exposed groups in pre-pandemic and pandemic periods, respectively. We estimated baseline one-year mortality in the pre-pandemic period (Figure S1.B) by Kaplan–Meier survival analysis. We calculated baseline and COVID-19 mortality risk (using RR) in pre-pandemic and pandemic periods, respectively, by high-risk conditions. To calculate IR in each sub-sample, we divided the COVID-19 population by those at risk at the start of the period. The final IR was the average of IRs of two sub-samples (refer to Supplementary materials).
Results
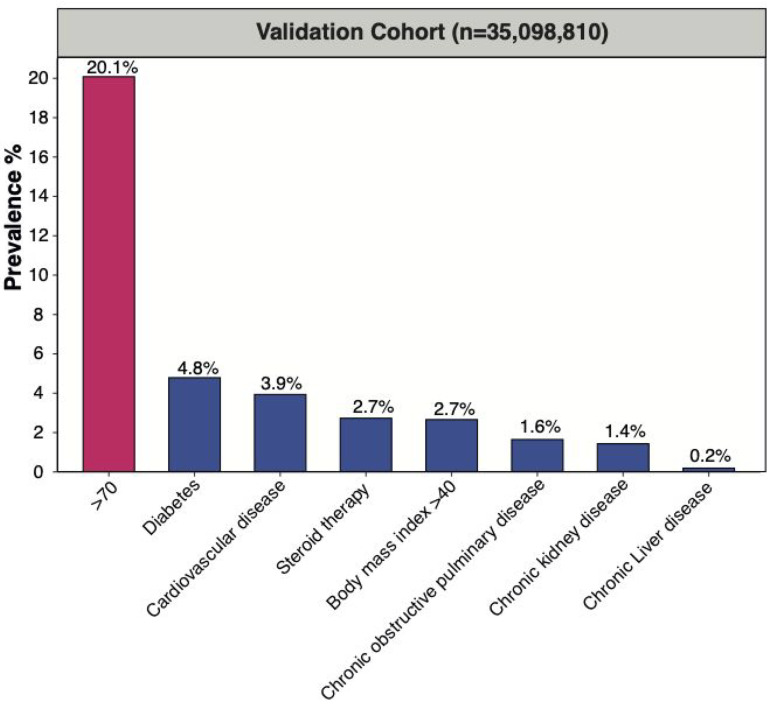
In the validation cohort, we included 35,098,810 individuals aged ≥30 years at baseline (Figure S2). Of all individuals aged ≥30 years on 1 March 2018, 18,361, 665 (52.3%) were female; mean age was 55.0[SD 16.2] in both sexes; 28,049,984 (79.9%) were aged ≤70 (mean age 48.7 [SD 11.6] years in females and 49.1 [SD 11.5] years in males) and 7,048,826(20.1%) were >70 (mean 79.7 [SD 6.8] years in females and 78.5 [SD 6.1] years in males). Prevalence for CVD, diabetes, CKD, COPD, BMI > 40 kg/m2, chronic liver disease and steroid therapy was 5.56% and 2.76%, 4.59% and 3.75%, 2.03% and 2.84%, 1.83% and 1.81%, 1.41% and 2.07%, 0.15% and 0.10%, and 3.52% and 5.07% in males and females, respectively. Prevalence of 0, 1, 2 and ≥3 underlying conditions was 35.57% and 39.95%, 8.15% and 8.48%, 8.82% and 2.79%, and 1.13% and 1.09% in males and females, respectively. Prevalence of all underlying conditions was higher in individuals >70 years and males (Figure 1, Table 1).
Figure 1.
Prevalence of high-risk conditions for COVID-19 mortality in validation cohort (n = 35,098,810) cohort aged ≥30 years.
Table 1.
Underlying conditions in the validation cohort (NHS Digital TRE, n = 35,098,810, aged 30 years or older).
| Count (% of total population) |
|||||||
|---|---|---|---|---|---|---|---|
| Underlying condition | Male Age ≤ 70 yearsN = 13,587,089 | MaleAge > 70 yearsN = 3,150,056 | MaleAll agesN = 16,737,145 | FemaleAge ≤ 70 yearsN = 14,462,895 | FemaleAge > 70 yearsN = 3,898,770 | FemaleAll agesN = 18,361,665 | |
| CVD | 873,001 (2.49) | 1,080,487 (3.08) | 1,953,488 (5.56) | 509,450 (1.45) | 968,909 (2.76) | 1,478,359 (4.21) | |
| Diabetes | 965,436 (2.75) | 647,269 (1.84) | 1,612,705 (4.59) | 716,309 (2.04) | 600,494 (1.71) | 1,316,803 (3.75) | |
| CKD | 227,924 (0.65) | 483,972 (1.38) | 711,896 (2.03) | 274,852 (0.78) | 720,582 (2.05) | 995,434 (2.84) | |
| COPD | 291,294 (0.83) | 351,684 (1.00) | 642,978 (1.83) | 287,287 (0.82) | 349,463 (0.99) | 636,750 (1.81) | |
| BMI >40 kg/m2 | 373,213 (1.06) | 120,512 (0.34) | 493,725 (1.41) | 561,351 (1.60) | 165,612 (0.47) | 726,963 (2.07) | |
| Chronic liver disease | 42,789 (0.12) | 10,966 (0.03) | 53,755 (0.15) | 25,807 (0.07) | 9875 (0.03) | 35,682 (0.10) | |
| Steroid therapy | 762,449 (2.17) | 472,571 (1.35) | 1,235,020 (3.52) | 1,183,308 (3.37) | 596,578 (1.70) | 1,779,886 (5.07) | |
| 0 | 11,167,965 (31.82) | 1,317,372 (3.75) | 12,485,337 (35.57) | 12,137,332 (35.58) | 1,885,800 (5.37) | 14,023,132 (39.95) | |
| 1 | 1,835,747 (5.23) | 1,025,674 (2.92) | 2,861,421 (8.15) | 1,800,831 (5.13) | 1,174,823 (3.35) | 2,975,654 (8.48) | |
| 2 | 451,492 (1.29) | 541,211 (1.54) | 992,703 (8.82) | 406,504 (1.16) | 573,786 (1.63) | 980,290 (2.79) | |
| ≥3 | 131,885 (0.38) | 265,799 (0.76) | 397,684 (1.13) | 118,228 (0.34) | 264,361 (0.75) | 382,589 (1.09) | |
Figure 2.
Baseline one-year mortality in England (age ≥30 years) according to underlying conditions in the validation cohort (n = 35,098,810).
One-year mortality
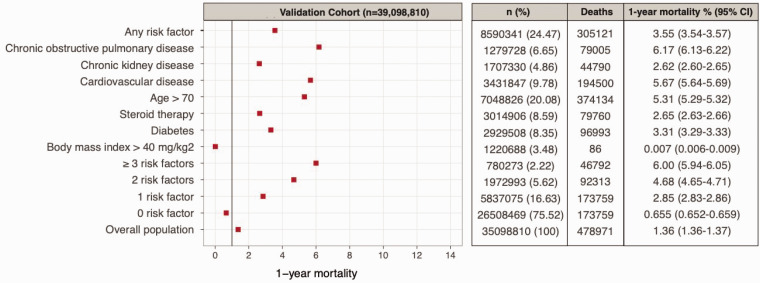
Among individuals with at least one high-risk condition, estimated pre-pandemic one-year mortality risk was observed to be 3.55% (3.54–3.57). One-year mortality risk in individuals aged >70 years was 9.24% (9.17–9.31), 3.37% (3.34–3.40), 8.36% (8.32–8.40) and 6.38% (6.34–6.42) for COPD, CKD, CVD and diabetes, respectively. In individuals aged >70 years, one-year mortality risks in men were 9.45% (9.35–9.55), 3.91% (3.85–3.96), 7.92% (7.98–9.20), 6.48% (6.42–6.54) for COPD, CKD, CVD and diabetes, respectively; and in women, 9.02% (8.92–9.11), 3.00% (2.96–3.04), 8.84% (8.78–9.11) and 6.27% (6.21–6.33), respectively.
Validation and replication of the conceptual model
In March 2020, we predicted 73,498 one-year COVID-19-related deaths for the population of England, by scaling from the development cohort (3,862,012 aged ≥30 years) to the mid-2018 population of England and assuming a scenario of IR = 10% and RR = 3.2 In the validation study, from March 2020 until March 2021, we ascertained 127,020 COVID-19-related all-cause deaths. We estimated pre-pandemic one-year mortality risk by age group, sex and number of high-risk conditions in the absence of COVID-19.
We calculated cross-validated one-year (March 2020–2021) RR and IR of COVID-19 as 4.34%(95% CI, 4.31–4.38) and 6.27% (95% CI, 6.26–6.28), respectively. Tables S1 and S2 show the cross-validated IR and RR, respectively, across two random subsamples of the cohort shown in Figure S1. Table S3 shows the sensitivity analysis for underfitting and further cross-validation. We found that the effect of vaccination on overall RR or IR between December 2020 and March 2021 was negligible compared to effects of under-reported COVID-19 cases pre-vaccination (Table S4). We applied our prediction model using observed RR (4.34) and IR (6.27) and baseline mortality risk data in the validation cohort (Tables S5 and S6).
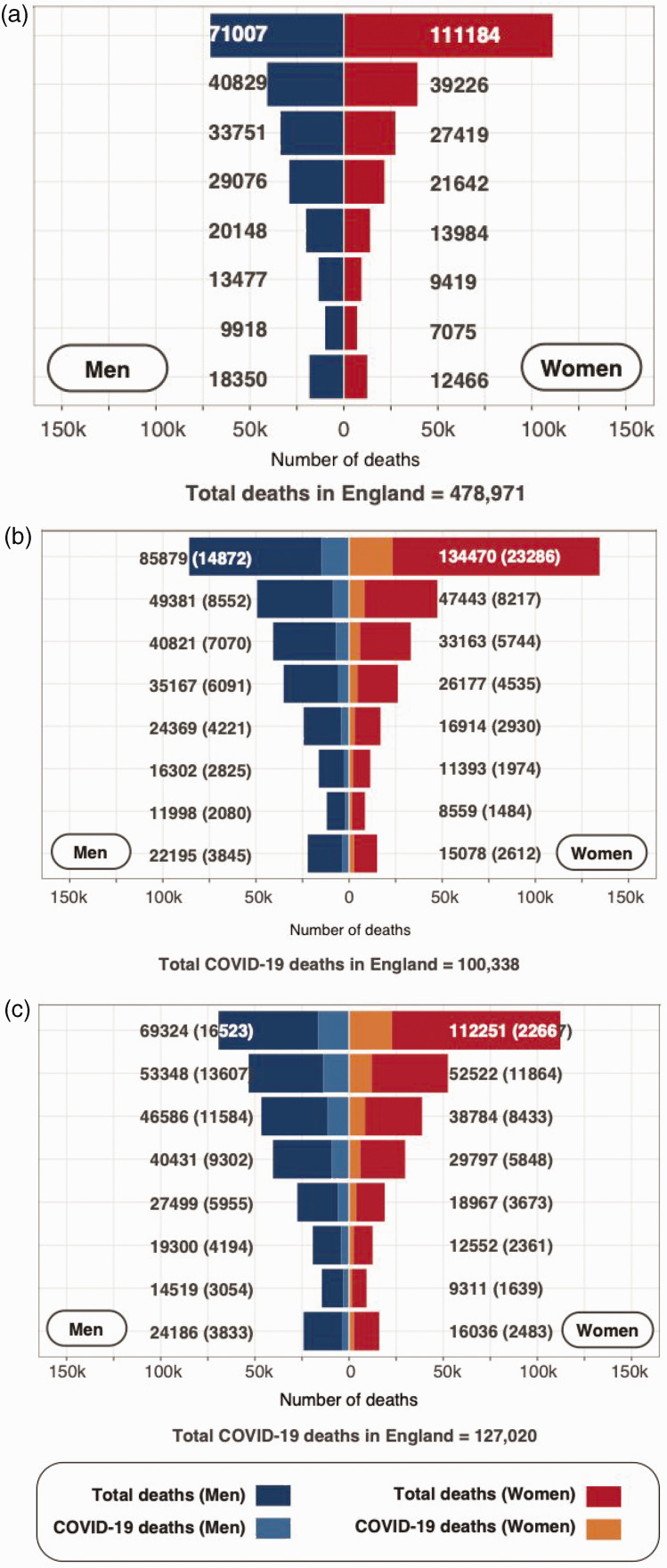
Figures 3 and S4 show the predicted one-year COVID-19-related all-cause deaths, based on baseline mortality risk (March 2018–2019 for validation cohort), RR = 4.34, and IR = 6.27% compared to observed excess deaths (March 2020–2021). The observed and model-predicted COVID-19 deaths were 127,020 and 100,338 (79.0% of observed), respectively (Tables 2, Figure 3).
Figure 3.
Baseline deaths, model-predicted COVID-19-related all-cause deaths and observed deaths among those with COVID-19 in England (age ≥30 years) over one year, stratified by age and sex in the validation cohort (n = 35,098,810). (a) Baseline one-year mortality. (b) Total (and model-predicted COVID-19) one-year mortality based on RR = 4.34%, IR = 6.27%. (c) Total (and observed COVID-19) one-year mortality.
Table 2.
Observed COVID-19 one-year mortality in England (NHS Digital TRE; n = 35,098,810 aged ≥30 years; 1 March 2020 to 1 March 2021).
| Age ≤70 years |
Age >70 years |
All ages |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N total (%) | Total deaths | N COVID-19 (%) | COVID-19 deaths | N total (%) | Total deaths | N COVID-19 (%) | COVID-19 deaths | N total (%) | Total deaths | N COVID-19 (%) | COVID-19 deaths | |
| ≥1 Underlying condition excluding age >70 years | 4,634,608 (13.58) | 70,202 | 314,587 (0.92) | 16,203 | 3,340,209 (9.79) | 317,114 | 209,190 (0.61) | 74,276 | 7,974,817 (23.36) | 70,479 | 523,777 (1.53) | 90,479 |
| Age > 70 years | – | – | – | – | 6,299,844 (18.46) | 443,043 | 317,798 (0.93) | 99,828 | – | – | – | – |
| Diabetes | 1,645,037 (4.82) | 29,688 | 123,984 (0.36) | 8338 | 1,087,148 (3.18) | 106,124 | 75,870 (0.22) | 27,474 | 2,732,158 (8.00) | 135,902 | 199,854 (0.58) | 35,812 |
| CVD | 1,335,614 (3.91) | 30,301 | 80,174 (0.23) | 6966 | 1,710,348 (5.01) | 200,644 | 121,772 (0.36) | 46,744 | 3,045,962 (8.92) | 230,945 | 201,946 (0.59) | 53,710 |
| BMI > 40 kg/m2 | 932,120 (2.73) | 8454 | 73,399 (0.21) | 2333 | 280,331 (0.82) | 19,410 | 15,690 (0.04) | 4911 | 1,212,451 (3.55) | 27,864 | 89,089 (0.26) | 7244 |
| Steroid therapy | 1,889,695 (5.54) | 44,671 | 149,685 (0.44) | 7655 | 923,584 (2.70) | 111,144 | 67,321 (0.20) | 24,354 | 2,813,279 (8.24) | 155,815 | 217,006 (0.64) | 32,009 |
| COPD | 549,304 (1.61) | 18,905 | 29,797 (0.09) | 3733 | 574,369 (1.68) | 70,701 | 43,183 (0.13) | 16,872 | 112,3673 (3.29) | 89,606 | 72,980 (0.21) | 20,605 |
| CKD | 492,763 (1.44) | 11,102 | 33,377 (0.10) | 3255 | 1,100,918 (3.25) | 121,830 | 75,622 (0.22) | 29,332 | 1,593,680 (4.67) | 132,932 | 108,999 (0.32) | 32,587 |
| Chronic liver disease | 60,270(0.18) | 3584 | 3769 (0.18) | 556 | 15,556 (0.04) | 2291 | 1213 (0.003) | 483 | 75,826(0.22) | 5875 | 4982(0.01) | 1039 |
| 3+ Underlying conditions | 233,799 (0.68) | 12,645 | 18,267 (0.05) | 1470 | 442,569 (1.30) | 67,507 | 40,625 (0.12) | 17,304 | 676,368 (1.98) | 80,152 | 58,892 (0.17) | 20,774 |
| 2 Underlying conditions | 827,803 (2.472) | 20,516 | 55,977 (0.16) | 4885 | 956,907 (2.80) | 104,452 | 66,645 (0.19) | 24,693 | 1,784,710 (5.23) | 12,4968 | 122,622 (0.36) | 29,578 |
| 1 underlying condition | 3,573,006 (10.47) | 37,041 | 240,343 (0.70) | 7848 | 1,940,733 (5.68) | 145,155 | 101,920 (0.30) | 32,279 | 5,513,739 (16.15) | 182,196 | 342,263 (1.00) | 40,127 |
| No underlying condition | 23,197,624 (47.96) | 72,168 | 1,615,026 (4.73) | 10,989 | 2,959,635 (8.67) | 125,929 | 108,608 (0.32) | 23,869 | 26,157,259 (76.63) | 198,097 | 1,723,634 (5.05) | 36,541 |
| Overall population | 27,832,232 (81.54) | 142,370 | 1,929,613 (5.65) | 27,192 | 6,299,844 (18.46) | 443,043 | 317,798 (0.93) | 99,828 | 34,132,076 (100) | 585,413 | 2,247,411 (6.58) | 127,020 |
Discussion
In anonymised, individual-level, population-scale, national EHR data between March 2020 and March 2021, we conducted the first study to predict and validate one-year mortality among those with COVID-19 using baseline (pre-pandemic) mortality risk. We provide the first detailed, scenario-based mortality risk assessment before and during the pandemic, based on absolute risk estimates in national population data. We show that a simple, parsimonious model incorporating baseline risk of mortality, IR and RR of the pandemic can be used to predict one-year COVID-19 mortality.
Strengths and weaknesses
Our analysis uses anonymised, national, individual-level EHR data with unprecedented scale and whole population inclusivity and validated EHR phenotypes. It highlights the importance of EHR data, baseline mortality and scenario-based assumptions in risk assessment at early stages of a pandemic where dynamics of the new infectious disease are not yet known.
Our analysis used only the most frequent high-risk conditions. Our simple model made assumptions regarding static RR and IR over the course of the pandemic and did not incorporate infectivity or population dynamics of the original or later strains of SARS-CoV-2, the impact of COVID-19-related policies or vaccination rates. Generalisability of our findings to other countries and contexts requires further validation. Our study only investigated COVID-19, and applicability to other infectious diseases or pandemics is unknown. There are differences between development and validation cohorts in terms of data coding systems (e.g. lack of standardised one-to-one mapping between coding terminologies), and limited availability of fields in CPRD (e.g. ethnicity) and in the TRE for England (e.g. medication use before 2018 and multiple index of deprivation), which restricted analyses. Overall, national mortality estimates in people with COVID-19 were similar in development and validation cohorts, with differences in mortality risk at baseline in stratified analyses. For example, mortality risk was similar for younger people in both cohorts, but mortality risk was relatively higher in the development cohort for individuals aged >70 years due to the earlier cohort entry date in the CPRD study population.1 Also, the number of estimated deaths was lower in the development cohort in all age categories, perhaps because the one-year mortality in CPRD data were calculated after study entry date, when these individuals were younger (mean age 43.5 [SD 11.7] years), compared to the validation cohort in March 2018–2019 (mean age 55.0 [SD 16.2] years). Another explanation is that the actual IR over one year is higher than our observed rate (and probably greater than the 10% we used in prediction), due to incomplete availability of COVID-19 testing, especially during the early months of the pandemic.
Comparison with other studies
We searched for systematic reviews published after March 2020 in PubMed using combinations of equivalent Mesh terms: ‘COVID’, ‘prediction’, ‘mortality’, ‘model’, ‘underlying condition’, ‘relative risk’ and ‘infection rate’. A systematic review of 107 multivariate prediction models for COVID-19 mortality showed that variables were selected from signs, symptoms and risk factors from COVID-19 patients during the pandemic.26 All models had unclear or high risk of bias, including non-representative data sources, unreliable COVID-19 case definition, excluding patients who had not experienced outcomes of interest, and model overfitting. We found no studies of excess mortality prediction based on pre-pandemic mortality in people with high-risk underlying conditions and RR and IR associated with COVID-19. In our study, all patients, regardless of outcome of interest, were included in analyses. Moreover, we conducted model cross-validation to minimise overfitting (Table S3).
We used EHR data of the whole population in England to validate our model for predicting one-year excess mortality in people exposed to COVID-19. The data used in our study are derived from anonymised, individual-level and linked EHRs of the whole population in England, making our model highly representative. We have used validated phenotype definitions for high-risk underlying conditions and COVID-19 cases. Our study highlights the significance of pre-pandemic longitudinal EHR data to predict the direct effects of the pandemic for preparedness and early response.
Our model is a simple, conceptual model for formulating worst-case to best-case scenarios at the start of the pandemic. We developed the model in CPRD data with assumed parameters and replicated the model in NHS Digital TRE using observed RR and IR values. Hence, our model is more suitable for risk assessment for pandemic preparedness and early response rather than high-precision estimation of the mortality.
Meaning of the study: possible mechanisms and implications
Pre-pandemic mortality risks
Baseline mortality risk can be used to predict COVID-19-related mortality over one year at the national level, and underlying conditions and age are major determining factors of the risk. We show that national data EHRs, such as the NHS Digital TRE, and sampled less complete data, such as CPRD, can be used to estimate and monitor baseline risk at scale. Such data are available across diseases, risk factors and countries via the Global Burden of Disease Study and other efforts, and have already been used to project high-risk populations for COVID-19.27 There is public demand for such information, which can be provided in an interpretable, usable format employing open phenotypes, coding and standards.18–21,28
Infection rate over one year
Surveillance of SARS-CoV-2 IRs has been crucial across countries throughout the pandemic by different methods, including incident or prevalent cases, over weeks or months, by antigen or antibody tests, or by static or dynamic rates. Our model used population IR over one year, which we estimated using comprehensive testing, primary care, hospital data and death data in the NHS Digital TRE in a mostly pre-vaccination era. Our estimates of IR represent nearly the whole English population, consistent with pre-vaccination antibody rates in the UK29 and a recent study using the same data.23 However, under-estimation is still possible and, moreover, likely, due to initially limited testing capacity and asymptomatic infection. Future research and models should incorporate higher vaccination rates, novel variants, potential impact of reinfection and dynamic IRs over time.
Relative risk associated with the pandemic
Excess mortality associated with COVID-19 has been a focus in health policy since the early stages of the pandemic. Comparisons with flu persist until now, including ‘winter excess deaths’, which have been estimated as 20% higher than the baseline mortality rate.1 In our model, we used RR estimates of 1.5, 2 and 3, and in the national data, we observed 4.34 in the overall population. Assuming an under-estimation of IR, we may have over-estimated RR, but our estimates are in line with a recent time-series analysis of excess mortality in the first pandemic wave in the UK. That study showed that certain underlying conditions were associated with higher RR of excess pandemic mortality, compared with the pre-pandemic period.30
Implications for public health and policy makers
There are three public health and policy implications. First, EHRs were designed and used for reimbursement, clinical care and quality improvement, with limited use in emergency preparedness. Our analyses show that EHRs could and should be part of pandemic planning and surveillance. Second, pre-pandemic mortality risk can be estimated at individual, subgroup and national levels, and is important in pandemic mortality prediction as well as preparedness including shielding and vaccination prioritisation. Third, our data support the syndemic lens, which views COVID-19 not just as an infectious disease, but one with social, environmental and NCD determinants and effects, signalling the need for multidisciplinary public health and policy approaches in pandemics.
Research implications
First, there are more than 80 diseases, risk factors and underlying conditions designated as moderate and high risk for COVID-19 by the UK government.20 We will validate COVID-19 mortality estimates for the comprehensive list, providing condition-specific IR and RR estimates, stratified by ethnicity, deprivation and vaccination, with future application for models in COVID-19 and other pandemics. Second, the policy need for region- and country-specific data is well recognised, and our UK-based analyses may not be generalisable to other countries and datasets. Third, we only considered direct pandemic impact on mortality, not indirect and long-term (Long COVID) impact, which need to be studied and incorporated into future pandemic impact models. Fourth, baseline mortality risk estimation (using models such as ours) could be combined with existing methods of dynamic transmission modelling to predict and mitigate future pandemics.
Conclusions
The impact of the COVID-19 pandemic on excess mortality can be predicted using national EHRs and is related to baseline mortality risk, population IRs and pandemic-associated RR. In public health, policy and research, there are implications for expertise, data and resources in future pandemic preparedness.
Supplemental Material
Supplemental material, sj-pdf-1-jrs-10.1177_01410768221131897 for Using national electronic health records for pandemic preparedness: validation of a parsimonious model for predicting excess deaths among those with COVID-19–a data-driven retrospective cohort study by Mehrdad A Mizani, Ashkan Dashtban, Laura Pasea, Alvina G Lai, Johan Thygesen, Chris Tomlinson, Alex Handy, Jil B Mamza, Tamsin Morris, Sara Khalid, Francesco Zaccardi, Mary Joan Macleod, Fatemeh Torabi, Dexter Canoy, Ashley Akbari, Colin Berry, Thomas Bolton, John Nolan, Kamlesh Khunti, Spiros Denaxas, Harry Hemingway, Cathie Sudlow, Amitava Banerjee on behalf of the CVD-COVID-UK Consortium in Journal of the Royal Society of Medicine
Declarations
Competing Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JBM and TM are employees of AstraZeneca. KK is chair of the ethnicity subgroup of the Independent Scientific Advisory Group for Emergencies (SAGE) and director of the University of Leicester Centre for Black Minority Ethnic Health. KK and AB are trustees of the South Asian Health Foundation (SAHF). CS is Director of the BHF Data Science Centre. All other authors report no competing interests.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The British Heart Foundation Data Science Centre (grant no. SP/19/3/34678, awarded to Health Data Research (HDR) UK) funded co-development (with NHS Digital) of the TRE, provision of linked datasets, data access, user software licences, computational usage, and data management and wrangling support, with additional contributions from the HDR UK data and connectivity component of the UK Government Chief Scientific Adviser’s National Core Studies programme to coordinate national COVID-19 priority research. Consortium partner organisations funded the time of contributing data analysts, biostatisticians, epidemiologists and clinicians. AB, MAM, MHD and LP were supported by research funding from AstraZeneca. AB has received funding from the National Institute for Health Research (NIHR), British Medical Association and UK Research and Innovation. AB, SD and HH are part of the BigData@Heart Consortium, funded by the Innovative Medicines Initiative-2 Joint Undertaking under grant agreement No 116074. KK is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC-EM) and NIHR Lifestyle BRC.
Ethics approval
Approval for the study in CPRD was granted by the Independent Scientific Advisory Committee (20_074R) of the Medicines and Healthcare products Regulatory Agency in the UK in accordance with the Declaration of Helsinki. The North East-Newcastle and North Tyneside 2 research ethics committee provided ethical approval for the CVD-COVID-UK research programme (REC No 20/NE/0161).
Information governance
The data used in this study are available in NHS Digital’s TRE for England, but as restrictions apply, they are not publicly available (https://digital.nhs.uk/coronavirus/coronavirus-data-services-updates/trusted-research-environment-service-for-england). The CVD-COVID-UK/COVID-IMPACT programme led by the BHF Data Science Centre (https://www.hdruk.ac.uk/helping-with-health-data/bhf-data-science-centre/) received approval to access data in NHS Digital’s TRE for England from the Independent Group Advising on the Release of Data (https://digital.nhs.uk/about-nhs-digital/corporate-information-and-documents/independent-group-advising-on-the-release-of-data) via an application made in the Data Access Request Service Online system (ref. DARS-NIC-381078-Y9C5K) (https://digital.nhs.uk/services/data-access-request-service-dars/dars-products-and-services). The CVD-COVID-UK/COVID-IMPACT Approvals & Oversight Board (https://www.hdruk.ac.uk/projects/cvd-covid-uk-project/) subsequently granted approval to this project to access the data within NHS Digital’s TRE for England. The de-identified data used in this study were made available to accredited researchers only.
The open-source code and utilised phenotype code-lists used this study are available in a repository in the British Heart Foundation Data Science Centre’s GitHub organisation (https://github.com/BHFDSC/CCU003_03).
Guarantor
AB.
Contributorship
Research question, approach and study oversight: AB. Leading data engineering, coding and analysis: MAM. Data analysis, quality assurance and phenotyping: AD, JT, CT, AH, TB, JN. Study design and review: LP, SD, HH, CS, MJM, DC, CB, KK. Data visualisation: AGL. Coordinating approval for and access to data within NHS Digital’s TRE for England for CVD-COVID-UK/COVID-IMPACT: CS. Drafting initial and final versions of manuscript: AB and MAM. Critical review of early and final versions of manuscript: All authors.
Acknowledgements
This work is carried out with the support of the BHF Data Science Centre led by HDR UK (BHF Grant no. SP/19/3/34678) and makes use of de-identified data held in NHS Digital’s TRE for England, made available via the BHF Data Science Centre’s CVD-COVID-UK/COVID-IMPACT consortium. This work uses data provided by patients and collected by the NHS as part of their care and support. We would also like to acknowledge all data providers who make health relevant data available for research.
Provenance
Not commissioned; peer reviewed by Julie Morris, and pre-submission peer review comments.
ORCID iDs
Chris Tomlinson https://orcid.org/0000-0002-0903-5395
Ashley Akbari https://orcid.org/0000-0003-0814-0801
Amitava Banerjee https://orcid.org/0000-0001-8741-3411
Supplemental material
Supplemental material for this article is available online.
References
- 1.Banerjee A, Pasea L, Harris S, Gonzalez-Izquierdo A, Torralbo A, Shallcross L, et al. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet 2020; 395: 1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clift AK, Coupland CAC, Keogh RH, Diaz-Ordaz K, Williamson E, Harrison EM, et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ 2020; 371: m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020; 369: m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al.; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008; 336: 1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogelsang RP, Bojesen RD, Hoelmich ER, Orhan A, Buzquurz F, Cai L, et al. Prediction of 90-day mortality after surgery for colorectal cancer using standardized nationwide quality-assurance data. BJS Open 2021; 5: zrab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajnakina O, Agbedjro D, McCammon R, Faul J, Murray RM, Stahl et al. Development and validation of prediction model to estimate 10-year risk of all-cause mortality using modern statistical learning methods: a large population-based cohort study and external validation. BMC Med Res Methodol 2021; 21: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolge SC, Kariburyo F, Yuce H, Fleischhackl R. Predictors and outcomes of hospitalization for influenza: real-world evidence from the United States medicare population. Infect Dis Ther 2021; 10: 213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma HM, Tang WH, Woo J. Predictors of in-hospital mortality of older patients admitted for community-acquired pneumonia. Age Ageing 2011; 40: 736–741. [DOI] [PubMed] [Google Scholar]
- 11.Huppert A, Katriel G. Mathematical modelling and prediction in infectious disease epidemiology. Clin Microbiol Infect 2013; 19: 999–1005. [DOI] [PubMed] [Google Scholar]
- 12.Biggerstaff M, Cowling BJ, Cucunubá ZM, Dinh L, Ferguson NM, Gao H, et al. WHO COVID-19 modelling parameters group. Early insights from statistical and mathematical modeling of key epidemiologic parameters of COVID-19. Emerg Infect Dis 2020; 26: e1–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laydon DJ, Mishra S, Hinsley WR, Samartsidis P, Flaxman S, Gandy A, et al. Modelling the impact of the tier system on SARS-CoV-2 transmission in the UK between the first and second national lockdowns. BMJ Open 2021; 11: e050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 2020; 20: 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horton R. Offline: COVID-19 is not a pandemic. Lancet 2020; 396: 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee A, Chen S, Pasea L, Lai AG, Katsoulis M, Denaxas S, et al. Excess deaths in people with cardiovascular diseases during the COVID-19 pandemic. Eur J Prev Cardiol 2021; 28: 1599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai AG, Pasea L, Banerjee A, Hall G, Denaxas S, Chang WH, et al. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. BMJ Open 2020; 10: e043828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood A, Denholm R, Hollings S, Cooper J, Ip S, Walker V, et al. Linked electronic health records for research on a nationwide cohort of more than 54 million people in England: data resource. BMJ 2021; 373: n826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CVD-COVID-UK/COVID-IMPACT TRE Dataset Provisioning Dashboard, British Heart Foundation Data Science Centre, Health Data Research UK. See www.hdruk.ac.uk/wp-content/uploads/2022/02/220210-CVD-COVID-UK-COVID-IMPACT-TRE-Dataset-Provisioning-Dashboard.pdf (last checked 11 February 2022).
- 20.Who is at high risk from coronavirus (COVID-19). See www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/who-is-at-high-risk-from-coronavirus/ (last checked 1 February 2022).
- 21.Denaxas S, Gonzalez-Izquierdo A, Direk K, Fitzpatrick NK, Fatemifar G, Banerjee A, et al. UK phenomics platform for developing and validating electronic health record phenotypes: CALIBER. J Am Med Inform Assoc 2019; 26: 1545–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.OpenPrescribing, https://openprescribing.net/bnf/0603/ (last checked 1 February 2022).
- 23.Thygesen JH, Tomlinson C, Hollings S, Mizani MA, Handy A, Akbari A, et al. COVID-19 trajectories among 57 million adults in England: a cohort study using electronic health records. Lancet Digit Health 2022; 4: e542–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estimates of the population for the UK, England and Wales, Scotland and Northern Ireland, Office for National Statistics. See www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (last checked 1 February 2022).
- 25.Kopec JA, Finès P, Manuel DG, Buckeridge DL, Flanagan WM, Oderkirk J, et al. Validation of population-based disease simulation models: a review of concepts and methods. BMC Public Health 2010; 10: 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ 2020; 369: m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health 2020; 8: e1003–e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee A, Pasea L, Manohar S, Lai AG, Hemingway E, Sofer I, et al. ‘What is the risk to me from COVID-19?': Public involvement in providing mortality risk information for people with ‘high-risk' conditions for COVID-19 (OurRisk.CoV). Clin Med (Lond) 2021; 21: e620–e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coronavirus (COVID-19) Infection survey: characteristics of people testing positive for COVID-19 in England and antibody data for the UK: December 2020. See www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsinthecommunityinengland/december2020 (last checked 2 February 2022).
- 30.Strongman H, Carreira H, De Stavola BL, Bhaskaran K, Leon DA. Factors associated with excess all-cause mortality in the first wave of the COVID-19 pandemic in the UK: a time series analysis using the Clinical Practice Research Datalink. PLoS Med 2022; 19: e1003870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jrs-10.1177_01410768221131897 for Using national electronic health records for pandemic preparedness: validation of a parsimonious model for predicting excess deaths among those with COVID-19–a data-driven retrospective cohort study by Mehrdad A Mizani, Ashkan Dashtban, Laura Pasea, Alvina G Lai, Johan Thygesen, Chris Tomlinson, Alex Handy, Jil B Mamza, Tamsin Morris, Sara Khalid, Francesco Zaccardi, Mary Joan Macleod, Fatemeh Torabi, Dexter Canoy, Ashley Akbari, Colin Berry, Thomas Bolton, John Nolan, Kamlesh Khunti, Spiros Denaxas, Harry Hemingway, Cathie Sudlow, Amitava Banerjee on behalf of the CVD-COVID-UK Consortium in Journal of the Royal Society of Medicine