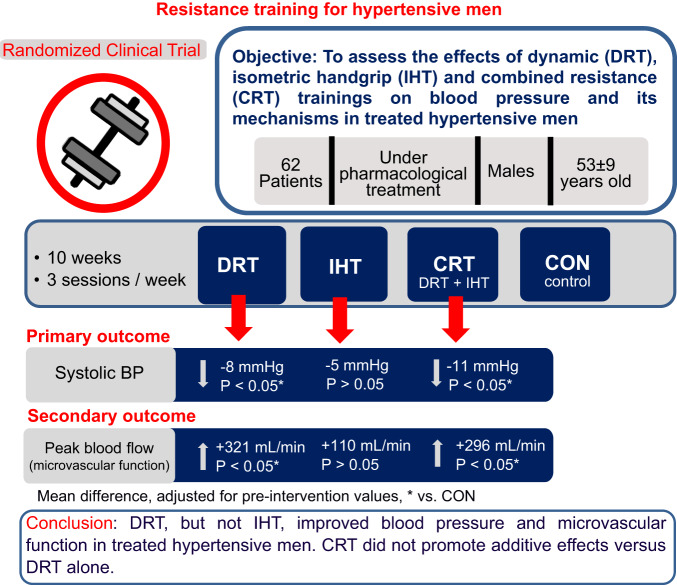
Abstract
Although dynamic resistance training (DRT) and isometric handgrip training (IHT) may decrease blood pressure (BP) in hypertensives, the effects of these types of training have not been directly compared, and a possible additive effect of combining IHT to DRT (combined resistance training—CRT), has not been investigated. Thus, this study compared the effects of DRT, IHT and CRT on BP, systemic hemodynamics, vascular function, and cardiovascular autonomic modulation. Sixty-two middle-aged men with treated hypertension were randomly allocated among four groups: DRT (8 exercises, 50% of 1RM, 3 sets until moderate fatigue), IHT (30% of MVC, 4 sets of 2 min), CRT (DRT + IHT) and control (CON – stretching). In all groups, the interventions were administered 3 times/week for 10 weeks. Pre- and post-interventions, BP, systemic hemodynamics, vascular function and cardiovascular autonomic modulation were assessed. ANOVAs and ANCOVAs adjusted for pre-intervention values were employed for analysis. Systolic BP decreased similarly with DRT and CRT (125 ± 11 vs. 119 ± 12 and 128 ± 12 vs. 119 ± 12 mmHg, respectively; P < 0.05), while peak blood flow during reactive hyperaemia (a marker of microvascular function) increased similarly in these groups (774 ± 377 vs. 1067 ± 461 and 654 ± 321 vs. 954 ± 464 mL/min, respectively, P < 0.05). DRT and CRT did not change systemic hemodynamics, flow-mediated dilation, and cardiovascular autonomic modulation. In addition, none of the variables were changed by IHT. In conclusion, DRT, but not IHT, improved BP and microvascular function in treated hypertensive men. CRT did not have any additional effect in comparison with DRT alone.
Keywords: Hypertension, Strength training, Vascular function, Autonomic modulation, Hemodynamics
Introduction
Hypertension is one of the major modifiable risk factors for cardiovascular disease [1], causing around 8 million deaths per year, mainly due to stroke, myocardial infarction and sudden death [2]. Blood pressure (BP) control among individuals with hypertension remains sub-optimal (i.e. 43.5%) [3], and complementary non-pharmacological interventions, such as exercise training, are recommended to improve BP control [3, 4]. Recently, resistance training has been considered for hypertension treatment with dynamic resistance training (DRT) recommended by both the American and European guidelines [3, 4], while isometric handgrip training (IHT) is advised only by the American guidelines [3].
Meta-analytic data demonstrated that DRT reduces systolic/diastolic blood pressures (SBP/DBP) by −6.11 (95%CI: −10.23 to −1.99)/−2.75 (95%CI: −4.27 to −1.22) mmHg in treated hypertensives [5]. Such effects may be related to vascular adaptations induced by training since studies have reported improved resistance vessel function in healthy [6] and pre-hypertensive [7] individuals after DRT, which still needs to be evidenced in hypertensives. Concerning IHT, a recent meta-analysis [8] indicated that it decreases SBP/DBP by −6.00 (95%CI: −7.75 to −4.26/−2.75 (95%CI: −3.78 to −1.72) mmHg, which might be related to the training effects improving cardiac vagal modulation and vasomotor sympathetic modulation [9].
Current literature has suggested IHT in hypertension management based on its potential of higher adherence given its short duration (11 min per session) and execution with portable device [10]. However, its use as a stand-alone exercise therapy has drawbacks. Differently from DRT that promotes generalised musculoskeletal and metabolic benefits [11], IHT has musculoskeletal effects confined to the small muscle mass exercised and only minor impact on overall health. Given that, IHT is recommended in addition, and not in place of conventional exercise modes, such as DRT [10]. However, by the best of our knowledge, no previous study investigated the possible additive effect of associating IHT to DRT on BP control.
Based on this background, it is possible to hypothesise that the addition of IHT to DRT, in a combined resistance training (CRT), besides improving general health status, may also induce a greater BP decrease in hypertensives as such protocol would combine the DRT vascular effects [6, 7] and the IHT autonomic effects [9].
Therefore, the current clinical trial was designed to assess and compare the effects of DRT alone, IHT alone and CRT on BP, systemic hemodynamics, markers of vascular function, and cardiovascular autonomic modulation in treated hypertensives. The hypotheses were: (i) DRT alone would decrease BP and improve vascular function; (ii) IHT alone would equally decrease BP compared with DRT and would improve cardiovascular autonomic modulation; and (iii) CRT would induce a greater BP-lowering effect than both DRT and IHT, promoting both vascular and autonomic improvements.
Methods
Subjects
This study was registered at the Brazilian Clinical Trials (RBR-4fgknb) at http://www.ensaiosclinicos.gov.br, and all procedures were approved by the Ethics Committee of the School of Physical Education and Sport, University of São Paulo (process 2.870.688). All participants were informed of the benefits and risks of the investigation before providing written consent before enrolment. Experimental procedures were performed at the School of Physical Education and Sport of University of São Paulo. Preliminary medical evaluation was performed at the Hospital das Clínicas of the Medical School of the University of São Paulo.
Middle-aged (30–65 years old) hypertensive men were recruited from advertisements posted at the University of Sao Paulo’s media. The study was conducted with men to avoid the influence of menstrual cycle and menopause status on BP and its mechanisms [12].
The inclusion criteria were: (i) be receiving anti-hypertensive pharmacological treatment with drugs and doses maintained for at least the last 4 months; and (ii) not be physically active (i.e. not accumulating more than 150 min per week of leisure physical activity, not performing exercise training more than 2 times per week, and had not performed resistance training in the previous 6 months). The exclusion criteria were: (i) taking drugs that directly act on cardiac autonomic modulation (i.e. nondihydropyridine calcium channel blockers or beta-adrenergic receptor antagonists); (ii) presence of secondary hypertension; (iii) presence of hypertension-induced target-organ damage; (iv) presence of other cardiovascular disease despite hypertension; (v) presence of symptoms or electrocardiographic alterations during a graded maximal exercise test; (vi) body mass index ≥ 35 kg/m2; (vii) presence of diabetic complications or insulin use; (viii) presence of musculoskeletal problems that impair resistance training execution; and ix) SBP/DBP ≥ 160/105 mmHg that are the maximal BP values recommended for beginning exercise by the Brazilian Hypertension Guidelines [13].
Inclusion and exclusion criteria were checked through preliminary procedures. In an initial visit, the participants answered an anamnesis, fulfilled a questionnaire, and underwent anthropometric and BP evaluations. The anamnesis involved questions about health history, regular medication use, and physical activity routines. The International Physical Activity Questionnaire was completed [14]. Weight and height were measured (Welmy® W300A, São Paulo, Brazil) and body mass index calculated. Auscultatory BP was measured in triplicate on both arms with the participants in the seated position for at least 5 min. This BP evaluation was repeated in another visit and the six values obtained for each arm were averaged with the highest value between the arms being considered as the BP level of each participant. In another visit, medical evaluations were conducted, including clinical examination and collection of urine and blood samples to exclude secondary hypertension and target-organ lesion. For that, the basic laboratorial evaluation recommended by the Brazilian Hypertension Guidelines [13] were followed and included the analyses of plasma potassium, uric acid, and creatinine; fasting plasma glycose, triglycerides, and total, HDL- and LDL-cholesterol concentrations; conventional urine analyses; and the estimation of glomerular filtration rate. Finally, a graded maximal exercise test was performed on a cycle ergometer (Lode Medical Technology, Corival, Groningen, Netherlands) with electrocardiogram (Welch Allyn, Cardioperfect ST2001 model, Netherlands) evaluated by a physician.
The participants who fulfilled the study criteria underwent two familiarisation sessions to the exercises employed in the study as already done in previous research [15]. In these sessions, they executed 2 sets of 20 repetitions with the lowest workload allowed by each equipment (Edge Line, Movement Fitness, Sao Paulo, Brazil) in 8 dynamic resistance exercises (bench press, leg press, lat pull down, left leg extension, right leg extension, arms curl, left leg curl and right leg curl) followed by the execution of 4 sets of 2 min isometric handgrip exercise at 5% of maximal voluntary contraction (MVC). On another day, they did 1 repetition maximum (1RM) tests in all aforementioned exercises following standardised protocol [16] as already done in previous studies [17, 18]. Afterwards, participants performed a standardised evaluation of handgrip MVC with left and right hands [19].
Procedures
This study was a four-parallel-arm randomised controlled trial designed to evaluate and compare the effects of DRT, IHT and CRT. The pre-specified primary outcome was BP, and the secondary outcomes were muscle strength, systemic hemodynamics, vascular function, and cardiovascular autonomic modulation.
The participants were randomly allocated among four groups: DRT, IHT, CRT and control (CON), with a 1:1:1:1 allocation ratio. Randomisation was performed after the pre-intervention evaluations by an independent researcher (i.e. not involved directly in the recruitment and data collection) using the block method through sealed envelopes (i.e. sorting among the four options in each envelop). In all four groups, the intervention period lasted 10 weeks and the intervention sessions were conducted 3 times per week. Each session was individually supervised by an exercise specialist and conducted at the institution’s gym facility. The outcomes were assessed in experimental sessions conducted pre- and post-interventions, with the post-evaluations being conducted after a minimal interval of 48 h in relation to the last intervention session.
Prior to the experimental sessions, the participants received the following instructions: (i) not to ingest vitaminic supplements in the previous 72 h; (ii) not to perform exercise in the previous 48 h; (iii) not to consume alcoholic beverages in the previous 24 h; (iv) not to smoke in the previous 8 h; (v) to keep their usual daily activities and sleep habits in the previous day; (vi) to use their regular medications as usual; and (vii) to come to the session after fasting for at least 8 h. The experimental sessions started between 07:00 and 07:30 a.m. and the laboratory temperature was maintained between 20 and 22 °C.
During the experimental sessions, assessments started after 10 min of seated rest. Firstly, continuous signals of electrocardiogram, photoplethysmographic BP and respiration were recorded for 10 min for cardiovascular autonomic modulation evaluation. Then, auscultatory BP, cardiac output (CO) and heart rate (HR) were measured in triplicate for systemic hemodynamic evaluation. Afterwards, for vascular evaluation, the participants moved to the supine position, and after a 10 min interval, images and doppler flow signals of the brachial artery were recorded initially for 1 min without any stimulus (baseline) and then for 3 min after 5 min of forearm vascular occlusion (post-occlusion).
Interventions
The DRT group executed the 8 dynamic resistance exercises previously mentioned on specialised equipments (Edge Line, Movement Fitness, Sao Paulo, Brazil). In each exercise, the participants executed 3 sets of repetitions until moderate fatigue (defined by a visual reduction of movement velocity) and kept a 90 s interval between the sets and exercises. The intensity was initially set at 50% of 1RM and was increased by 2–5% and 5–10% for upper- and lower-limb exercises, respectively, when the participants could perform more than 15 repetitions without moderate fatigue in two consecutive sets [20]. This DRT protocol followed the hypertension guidelines [3, 4].
The IHT group executed the isometric handgrip exercise on a specific device (ZonaPlus, Zona Health, Boise, Idaho, USA). In each session, the participants executed 4 sets of 2 min isometric contractions at 30% of MVC, alternating the hands (i.e. 2 sets per hand) and maintaining a 60 s interval between the sets. MVC was measured at the beginning of each training session. After each session, the device provided a score quantifying the performance of the handgrip squeeze, and values ≥80 indicated effective training. This IHT protocol followed the hypertension guidelines [3].
The CRT group executed, in each training session, the same protocol as the DRT group followed by the same protocol performed by the IHT group.
The CON group executed 30 min stretching sessions. In each session, the participants executed 20–25 exercises and in each exercise, they executed 2–3 attempts keeping the highest degree of stretching without pain for 20–30 s. This active control intervention was proposed for this study to assure a similar interaction of the participants with the research team and to multiple BP measurements, since it is known that adaptation to these factors that would happen in the training groups (DRT, IHT and CRT) can decrease BP.
Adherence to each intervention was calculated as the percentage of the 30 offered sessions actually performed by each participant (i.e. sessions performed/30 × 100).
Measurements
Auscultatory BP was measured by a trained evaluator using a calibrated aneroid sphygmomanometer (Mikatos, Missouri, Sao Paulo, Brazil). Measurements were done on the dominant arm employing an adequate cuff size. SBP and DBP were respectively defined as phases I and V of Korotkoff sounds. Mean BP (MBP) was calculated as: MBP = DBP + [1/3 × (SBP – DBP)] [21, 22].
For systemic hemodynamic evaluation, CO was assessed by the indirect Fick method through CO2 rebreathing technique [23] using a gas analyser (Medical Graphics Corporation, CPX/Ultima, Minnesota, USA) and a bag containing hypercapnic gas (8–10% CO2). Firstly, the participants spontaneously breathed the ambient air for the measurement of CO2 production and the estimation of CO2 arterial content from end-tidal CO2 pressure. Then, via a two-way valve, participants started to inhale the hypercapnic gas until CO2 achieved an equilibrium and CO2 venous content could be estimated. Then, CO was calculated as: CO = VCO2/(CO2 venous content – CO2 arterial content). Systemic vascular resistance (SVR) was calculated from: SVR = MBP/CO. Stroke volume (SV) was calculated from: SV = CO/HR.
Vascular function evaluation was assessed through a linear array probe attached to a high-resolution ultrasound machine (General Eletric Medical Systems, LOGIQ 7, California, USA) following guidelines [24, 25]. Assessments were performed at the brachial artery of the dominant arm, ~5 cm proximal to the antecubital fossa, and using an insonation angle of 60°. Firstly, vascular images and doppler flow signal were continuously recorded for 1 min as baseline. From these records, arterial diameter was automatically detected, and blood flow velocity was quantified (Quipu, Cardiovascular Suite, Pisa, Italy). Blood flow (BF) was calculated as: BF = arterial cross-sectional area × blood flow velocity. Vascular conductance (VC) was calculated as: VC = BF/MBP. For vascular function assessment, a vascular occlusion period was initiated immediately after the baseline assessment using a cuff positioned at the forearm that was inflated to 250 mmHg for 5 min. When the cuff pressure was released, recordings of vascular images and doppler signals were taken for 3 min. Microvascular function (i.e. resistance vessels function) was assessed by the peak BF (i.e. highest absolute value) achieved during the reactive hyperaemia following cuff deflation [25]. Arterial endothelial function was assessed by flow-mediated dilation (FMD) [24] calculated by arterial diameter change from the baseline to the post-occlusion period as: FMD (%) = [(peak arterial diameter – baseline arterial diameter)/baseline diameter] × 100. The stimulus underlying FMD was evaluated by peak shear rate calculated at the post-occlusion period as: peak shear rate = 4 × peak BF velocity/arterial diameter.
Cardiovascular autonomic modulation evaluation followed the respective Task Force guidelines [26]. Briefly, HR was continuously measured through three-lead electrocardiogram (EMG System of Brazil, EMG 030110/00B), Sao Paulo, Brazil), beat-by-beat BP was monitored using finger photoplethysmography (Finapress Measurement System, Finometer, Arnhem, Netherland) and respiratory movements were measured via elastic thoracic belt (Pneumotrace 2, UFI, Morro Bay, USA). These signals were continuously acquired and recorded through a data acquisition system (Dataq Instruments, DI-720, Akron, Ohio, USA) with a sampling rate of 500 Hz/channel. Temporal sequences of R-R intervals, SBP and respiration were generated and analysed at the frequency domain through the autoregressive model using the Heart Scope II Software (A.M.P.S. LLC, Version 1.3.0.3, New York, USA). Cardiac sympathovagal balance was defined by the ratio between the low- and high-frequency bands of R-R interval variability (LF/HFR-R). Sympathetic vasomotor modulation was defined by the low-frequency band of SBP variability (LFSBP). Baroreflex sensitivity (BRS) was evaluated by the transfer function method [27].
Statistical analysis
The minimal sample size estimated for this study was 60 participants (i.e. 15 per study arm). This number was calculated for the primary outcome (SBP), considering an effect size (d) of −0.41 [28], a statistical power of 0.90, an alpha value of 0.05 and a correlation among repeated measures of 0.68 [29].
Data normality was checked by Shapiro–Wilks test and outliers identified through box plots. Non-normal data were transformed by natural logarithm to meet assumptions of the subsequent inferential analysis. The efficacy of interventions on the study’s outcomes was analysed by two-way mixed ANOVAs considering group as a between factor (DRT vs. IHT vs. CRT vs. CON) and time (pre- vs. post-intervention) as a within factor. When significant main effects or interactions were observed, pairwise comparisons were done by Newman-Keuls post-hoc tests. In addititon, changes (∆ = post-intervention – pre-intervention) adjusted for pre-intervention values were compared between the groups by ANCOVAs, and Bonferroni post-hoc tests were applied for pairwise comparisons when a significant effect was observed.
Data are presented as mean ± standard deviation, and significance level was set at P value < 0.05 for all analyses.
Results
Data recruitment took place from September 2018 to November 2021. Due to coronavirus 2019 disease, the study´s procedures were interrupted or restrained from March 2020 to September 2020 and from March 2021 to June 2021.
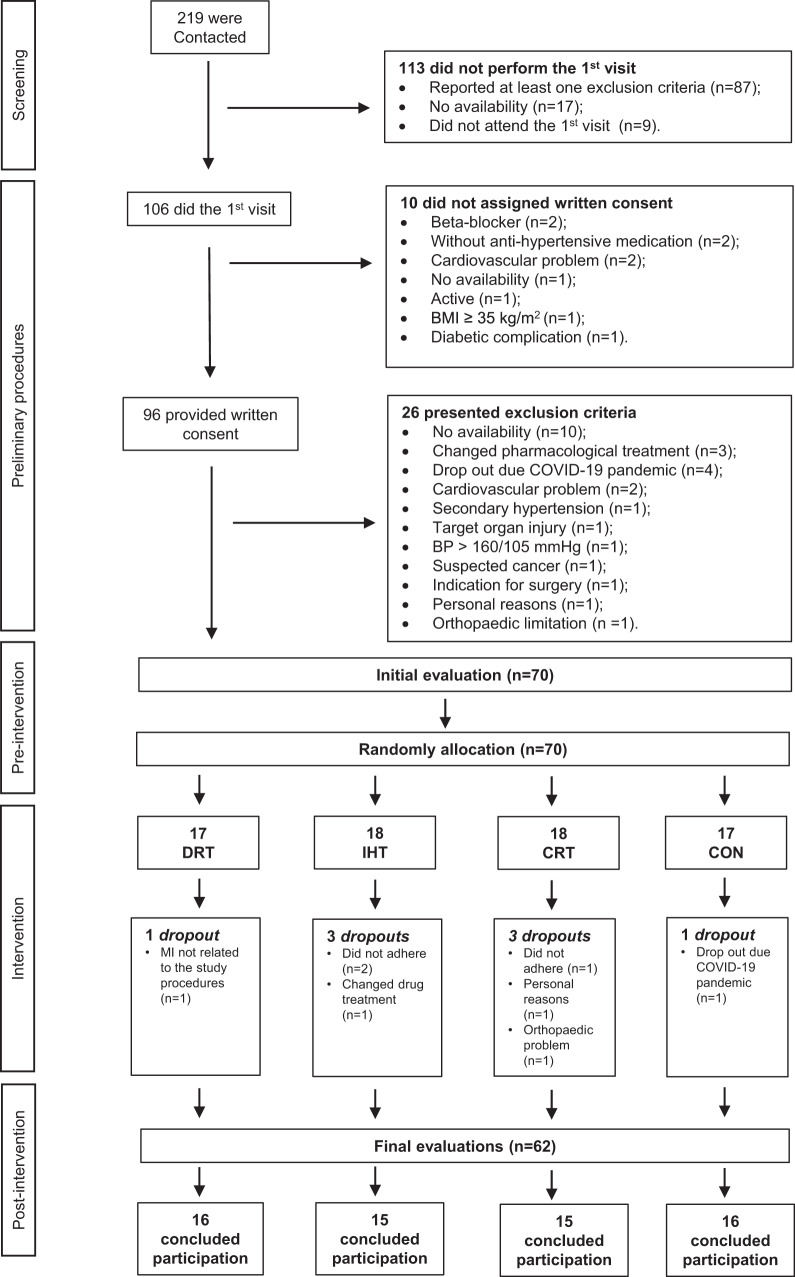
The clinical trial flowchart is shown in Fig. 1. Two hundred and nineteen participants were contacted, 106 performed the initial visit, 96 provided written consent and 70 were randomly allocated into the study’s groups. The clinical trial ended after the assignment of 70 participants considering the minimal sample size required (i.e. 60 participants) and a dropout rate of 15.0% [30]. Indeed, there were 8 (11.4%) dropouts during the intervention period and 62 participants concluded the entire experimental protocol. Due to technical issues, data for the autonomic modulation evaluation was missed for two participants (DRT: n = 1 and CON: n = 1). As the study was designed to evaluate and compare the efficacy of DRT, IHT and CRT, only data from the subjects who finished the experimental protocol were analysed. Groups characteristics were similar at the beginning of the study as shown in Table 1.
Fig. 1.
Flow diagram of the current trial. N number of participants, BMI body mass index, COVID-19 coronavirus disease 2019, HIV human immunodeficiency virus, BP blood pressure, EXP experimental session, MI myocardial infarction, DRT dynamic resistance training, IHT isometric handgrip training, CRT combined resistance training, CON control
Table 1.
Sample characteristics obtained at preliminary procedures
| DRT | IHT | CRT | CON | P | |
|---|---|---|---|---|---|
| N | 16 | 15 | 15 | 16 | |
| Age (years old) | 54 ± 7 | 55 ± 7 | 50 ± 11 | 52 ± 10 | 0.457 |
| COVID-19 without hospitalisation – n (%) | 2 (13) | 1 (7) | 2 (13) | 1 (6) | 0.862 |
| Physical activity levels (minutes/week) | 41 ± 43 | 57 ± 55 | 35 ± 41 | 57 ± 50 | 0.476 |
| Anthropometric | |||||
| Height (m) | 1.75 ± 0.06 | 1.74 ± 0.08 | 1.77 ± 0.09 | 1.76 ± 0.06 | 0.617 |
| Weight (kg) | 91 ± 12 | 86 ± 15 | 91 ± 18 | 88 ± 11 | 0.642 |
| BMI (kg/m2) | 29.8 ± 3.5 | 28.1 ± 3.5 | 28.8 ± 4.0 | 28.4 ± 3.5 | 0.591 |
| Blood pressure | |||||
| SBP (mmHg) | 130 ± 12 | 131 ± 13 | 134 ± 12 | 127 ± 10 | 0.505 |
| DBP (mmHg) | 88 ± 9 | 88 ± 7 | 88 ± 8 | 85 ± 7 | 0.621 |
| Pharmacological treatment | |||||
| Anti-hypertensive treatment duration (months) | 118 ± 91 | 105 ± 87 | 95 ± 78 | 114 ± 80 | 0.883 |
| Anti-hypertensive monotherapy – n (%) | 9 (56) | 8 (53) | 6 (40) | 9 (56) | 0.810 |
| Anti-hypertensive polytherapy – n (%) | 7 (44) | 7 (47) | 9 (60) | 7 (44) | 0.810 |
| ARB – n (%) | 12 (75) | 11 (73) | 11 (73) | 10 (63) | 0.894 |
| ACEi – n (%) | 2 (13) | 1 (7) | 4 (27) | 4 (25) | 0.440 |
| CCB – n (%) | 5 (31) | 5 (33) | 7 (47) | 5 (31) | 0.812 |
| DIU – n (%) | 6 (38) | 6 (40) | 5 (33) | 4 (25) | 0.854 |
| Statins – n (%) | 1 (6) | 3 (20) | 3 (20) | 1 (6) | 0.510 |
Data: mean ± standard deviation or number (percentage). Physical activity levels were evaluated by the International Physical Activity Questionnaire. Analysis = One-way ANOVA for continuous data and Fisher’s exact test for categorial data
DRT dynamic resistance training, IHT isometric handgrip training, CRT combined resistance training, C control, COVID-19 coronavirus disease 2019, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, ARB angiotensin receptor blocker, ACEi angiotensin-converting enzyme inhibitor, CCB calcium channel blocker, DIU diuretic
Adherences to the intervention sessions were high and similar among the groups (DRT: 89 ± 7%; IHT: 90 ± 9%; CRT: 90 ± 7%; CON: 88 ± 9%, p = 0.917). During the interventions, participants from CRT executed dynamic and isometric exercises with similar intensities and volumes as DRT and IHT, respectively (data not shown).
None of the interventions changed isometric handgrip MVC of the left nor the right arm (left: +1 ± 6, +3 ± 5, +2 ± 9, and −1 ± 4 kg; and right: +1 ± 6, +3 ± 4, +2 ± 5, and −1 ± 6 kg for DRT, IHT, CRT and CON, respectively, all p > 0.05). On the other hand, DRT and CRT significantly increased 1RM strength (all pgroup × time < 0.05) in all exercises (bench press: +11 ± 11 and +11 ± 10 kg; leg press: +33 ± 24 and +32 ± 26 kg; lat pull down: +12 ± 9 and +11 ± 7 kg; left leg extension: +10 ± 11 and +11 ± 10 kg; right leg extension: +10 ± 12 and +11 ± 10 kg; arms curl: +12 ± 8 and +7 ± 12 kg; left leg curl: +8 ± 5 and +7 ± 4 kg; and right leg curl: +8 ± 4 and +6 ± 5 kg for DRT and CRT, respectively), while no change was observed for the IHT and the CON groups (bench press: −1 ± 4 and +3 ± 7 kg; leg press: −4 ± 16 and +5 ± 17 kg; lat pull down: 0 ± 4 and +1 ± 10 kg; left leg extension: −3 ± 9 and +2 ± 8 kg; right leg extension: −4 ± 10 and +1 ± 7 kg; arms curl: −2 ± 3 and −1 ± 5 kg; left leg curl: 0 ± 4 and 0 ± 3 kg; and right leg curl: 0 ± 4 and 1 ± 4 kg for IHT and CON, respectively).
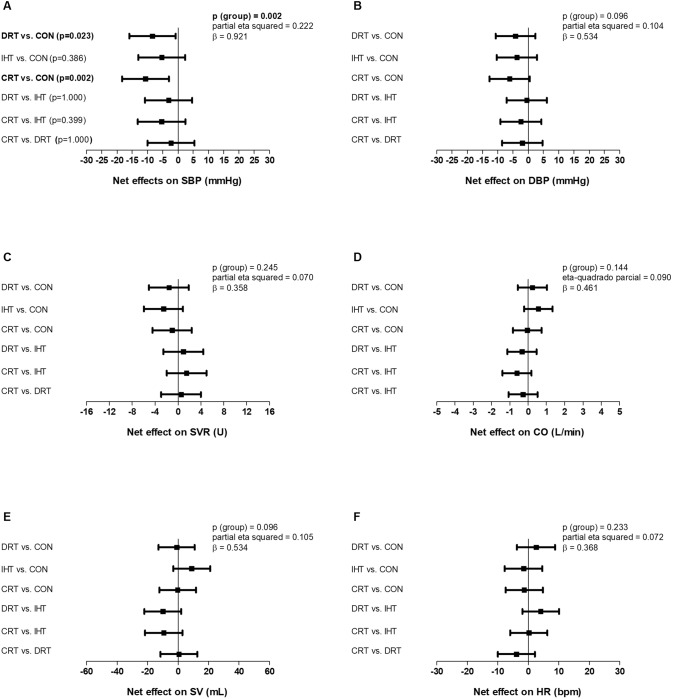
SBP decreased significantly from pre- to post-intervention after the DRT and the CRT and did not change after the IHT and the CON (pgroup × time = 0.003, Table 2). In addition, SBP changes adjusted to pre-intervention values observed with DRT and CRT were significantly different from CON (p = 0.002, Fig. 2). DBP did not change significantly in any group (p > 0.05, Table 2) and changes in DBP adjusted for pre-intervention values were similar among the groups (p = 0.096, Fig. 2).
Table 2.
Blood pressure and systemic hemodynamics parameters measured pre- and post-interventions in the four experimental groups: dynamic resistance training (DRT); isometric handgrip training (IHT); combined resistance training (CRT) and control (CON)
| DRT | IHT | CRT | CON | ||
|---|---|---|---|---|---|
| SBP (mmHg) | P group = 0.511 | ||||
| PRE | 125 ± 11 | 128 ± 13 | 128 ± 12 | 127 ± 14 | P time = 0.000 |
| POST | 119 ± 12a | 125 ± 14 | 119 ± 12a | 129 ± 16 | P group × time = 0.003 |
| DBP (mmHg) | P group = 0.764 | ||||
| PRE | 85 ± 10 | 87 ± 8 | 87 ± 6 | 86 ± 9 | P time = 0.642 |
| POST | 84 ± 10 | 86 ± 10 | 84 ± 8 | 89 ± 10 | P group × time = 0.091 |
| CO (L/min) | P group = 0.107 | ||||
| PRE | 5.6 ± 1.0 | 5.0 ± 0.9 | 5.1 ± 1.0 | 4.8 ± 0.8 | P time = 0.158 |
| POST | 5.2 ± 1.0 | 5.3 ± 1.1 | 4.7 ± 0.9 | 4.6 ± 0.6 | P group × time = 0.201 |
| SVR (U) | P group = 0.133 | ||||
| PRE | 18 ± 4 | 21 ± 4 | 21 ± 4 | 21 ± 4 | P time = 0.449 |
| POST | 19 ± 5 | 20 ± 5 | 21 ± 4 | 23 ± 3 | P group × time = 0.306 |
| SV (mL) | P group = 0.995 | ||||
| PRE | 82 ± 17 | 77 ± 15 | 81 ± 24 | 83 ± 17 | P time = 0.101 |
| POST | 76 ± 17 | 83 ± 16 | 76 ± 16 | 77 ± 15 | P group × time = 0.066 |
| HR (bpm) | P group = 0.060 | ||||
| PRE | 69 ± 11 | 66 ± 11 | 65 ± 13 | 60 ± 7 | P time = 0.908 |
| POST | 70 ± 7 | 65 ± 9 | 64 ± 12 | 61 ± 6 | P group × time = 0.379 |
Data: mean ± standard deviation. Analysis: Two-way mixed ANOVA
SBP systolic blood pressure, DBP diastolic blood pressure, CO cardiac output, SVR systemic vascular resistance, SV stroke volume, HR heart rate
aSignificantly different from pre-intervention (P < 0.05)
Bold values mean significant result
Fig. 2.
Between-groups comparisons of changes (post-intervention – pre-intervention) adjusted for pre-intervention values for the following variables: systolic blood pressure (SBP – A), diastolic blood pressure (DBP – B), systemic vascular resistance (SVR – C), cardiac output (CO – D), stroke volume (SV – E) and heart rate (HR – F). DRT dynamic resistance training, IHT isometric handgrip training, CRT combined resistance training, CON control. Analysis: One-way ANCOVA adjusted for pre-intervention values. Bold values mean significant result
SVR, CO, SV and HR did not change significantly in any group (all p > 0.05, Table 2) and changes in these variables adjusted for pre-intervention values were similar among the groups (all p > 0.05, Fig. 2).
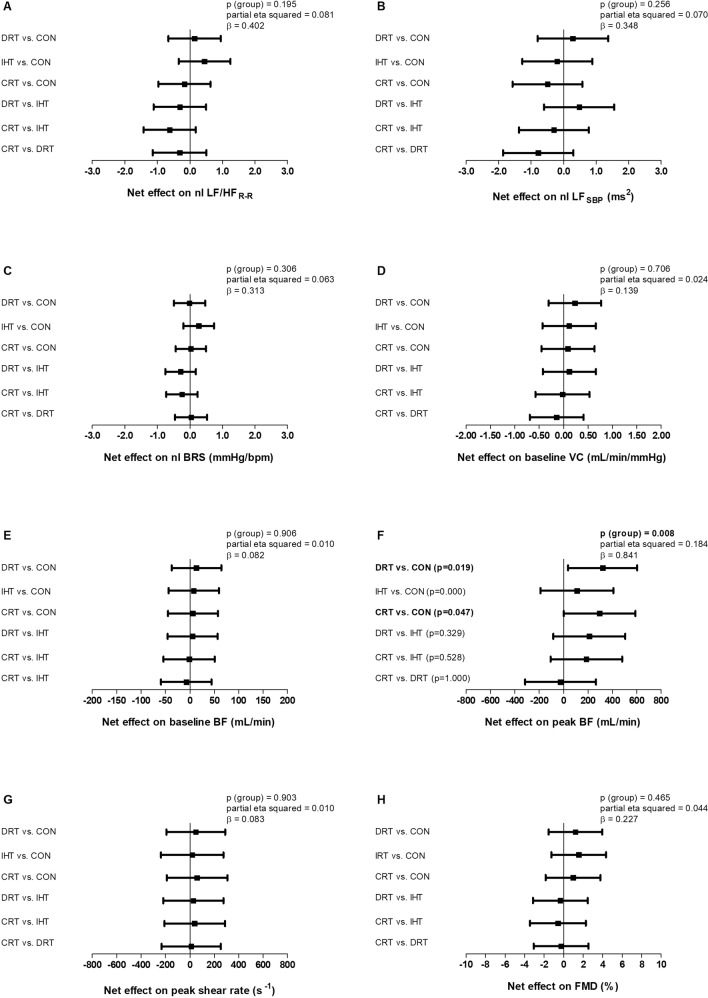
Baseline BF and VC as well as FMD did not change significantly in any group (all p > 0.05, Table 3) and changes in these variables adjusted for pre-intervention values were similar among the groups (all p > 0.05, Fig. 3). There was significant main effect of time for peak shear rate (ptime = 0.011), demonstrating that peak shear rate increased significantly and similarly from pre- to post-intervention in all groups, including CON. Accordingly, changes in peak shear rate adjusted for pre-intervention values were similar between the groups (p = 0.903). On the other hand, peak BF increased significantly from pre- to post-intervention after DRT and CRT and did not change after IHT and CON (pgroup × time = 0.007). In addition, peak BF changes adjusted to pre-intervention values observed with DRT and CRT were significantly different from CON (p = 0.008).
Table 3.
Vascular function and cardiovascular autonomic modulation parameters measured pre- and post- interventions in the four experimental groups: dynamic resistance training (DRT); isometric handgrip training (IHT); combined resistance training (CRT) and control (CON)
| DRT | IHT | CRT | CON | ||
|---|---|---|---|---|---|
| VASCULAR FUNCTION | |||||
| Baseline VC (mL.min−1.mmHg−1) | P group = 0.489 | ||||
| PRE | 1.16 ± 0.70 | 1.09 ± 0.64 | 0.93 ± 0.46 | 0.98 ± 0.60 | P time = 0.137 |
| POST | 1.34 ± 0.63 | 1.19 ± 0.73 | 1.10 ± 0.50 | 1.03 ± 0.55 | P group × time = 0.940 |
| Baseline BF (mL/min) | P group = 0.614 | ||||
| PRE | 110 ± 59 | 107 ± 55 | 90 ± 40 | 96 ± 57 | P time = 0.205 |
| POST | 121 ± 50 | 114 ± 70 | 105 ± 51 | 102 ± 53 | P group × time = 0.968 |
| Peak BF (mL/min) | P group = 0.161 | ||||
| PRE | 774 ± 377 | 581 ± 298 | 654 ± 321 | 828 ± 358 | P time = 0.000 |
| POST | 1067 ± 461a | 714 ± 336 | 954 ± 464a | 786 ± 223 | P group × time = 0.007 |
| Peak shear rate (s−1) | P group = 0.161 | ||||
| PRE | 723 ± 289 | 564 ± 206 | 656 ± 253 | 849 ± 412 | P time = 0.011 |
| POST | 819 ± 309a | 688 ± 266a | 788 ± 353a | 851 ± 314a | P group × time = 0.510 |
| FMD (%) | P group = 0.711 | ||||
| PRE | 6.0 ± 3.3 | 6.6 ± 4.2 | 5.6 ± 2.6 | 6.2 ± 4.0 | P time = 0.588 |
| POST | 6.6 ± 2.9 | 7.1 ± 4.2 | 6.2 ± 2.2 | 5.5 ± 2.7 | P group × time = 0.642 |
| CARDIOVASCULAR AUTONOMIC MODULATION | |||||
| nl LF/HFR-R | P group = 0.110 | ||||
| PRE | 0.76 ± 0.86 | 0.33 ± 0.83 | 0.06 ± 1.03 | 0.38 ± 0.82 | P time = 0.065 |
| POST | 0.45 ± 1.05 | 0.48 ± 0.51 | −0.30 ± 1.02 | 0.05 ± 1.12 | P group × time = 0.320 |
| nl LFSBP (ms2) | P group = 0.310 | ||||
| PRE | 1.97 ± 1.08 | 1.70 ± 1.27 | 1.63 ± 1.05 | 1.52 ± 1.01 | P time = 0.692 |
| POST | 2.10 ± 1.00 | 1.55 ± 1.03 | 1.22 ± 1.38 | 1.69 ± 1.00 | P group × time = 0.596 |
| nl BRS (mmHg/bpm) | P group = 0.124 | ||||
| PRE | 1.41 ± 0.55 | 1.54 ± 0.51 | 1.92 ± 0.47 | 1.76 ± 0.58 | P time = 0.046 |
| POST | 1.56 ± 0.48a | 1.90 ± 0.45a | 1.91 ± 0.56a | 1.79 ± 0.76a | P group × time = 0.161 |
Data: mean ± standard deviation. Analysis: Two-way mixed ANOVA
DRT dynamic resistance training, IHT isometric handgrip training, CRT combined resistance training, CON control, BF blood flow, VC vascular conductance, FMD flow-mediated dilation, nl natural logarithm, LF/HFR-R ratio between low- and high-frequency bands of R-R interval variability, LFSBP low-frequency band of systolic blood pressure variability, BRS baroreflex sensitivity
aSignificantly different from pre-intervention (P < 0.05)
Bold values mean significant result
Fig. 3.
Between-groups comparisons of changes (post-intervention – pre-intervention) adjusted for pre-intervention values for the following variables: ratio between low- and high-frequency bands of R-R interval variability (LF/HFR-R –A), low-frequency band of systolic blood pressure variability (LFSBP – B), baroreflex sensitivity (BRS – C), baseline vascular conductance (VC – D), baseline blood flow (BF – E), peak blood flow (F), peak shear rate (G) and flow-mediated dilation (FMD - H). DRT dynamic resistance training, IHT isometric handgrip training, CRT combined resistance training, CON control, nl natural logarithm. Analysis: One-way ANCOVA adjusted for pre-intervention values. Bold values mean significant result
Regarding autonomic modulation responses, there were no significant main effects nor interactions (group vs. time) for LF/HFR-R, nor LFSBP (all p > 0.05, Table 3). Accordingly, changes between the groups adjusted for pre-intervention values were similar for these variables (all p > 0.05, Fig. 3). There was a significant main effect of time for BRS (ptime = 0.046), showing that BRS increased significantly and similarly from pre- to post-intervention in all groups, including CON. Accordingly, changes in BRS adjusted for pre-intervention values were similar among the groups (p = 0.306).
Discussion
The current study has two main findings. First, DRT, but not IHT, decreased BP and improved microvascular function in treated hypertensive men. Second, the addition of IHT to DRT, in the CRT, did not promote any additive effect in comparison to DRT alone on either BP, systemic hemodynamics, vascular function or autonomic modulation.
DRT produced a net reduction (i.e. DRT vs. CON, Fig. 2) of −8.4 [95%CI: −15.9 to −0.8] mmHg in SBP, which is in accordance with the study hypothesis and within the range of reduction reported in a previous meta-analysis for SBP in treated hypertensives after DRT (−6.1; 95%CI: −10.2 to −2.0 mmHg) [5]. Moreover, the BP-reduction observed is comparable to the net effect reported for aerobic training (−8.3; 95%CI: −10.7 to −6.0 mmHg) [31], and for the main anti-hypertensive drug classes used in monotherapy (−8.8; 95%IC: −9.6 to −8.0 mmHg) [32]. This BP-lowering effect induced by DRT might have clinical relevance given that a 5 mmHg decrease in SBP has been shown to reduce the risk of major cardiovascular events by about 9% [33]. Indeed, 75% (n = 12) of the participants in the DRT group presented this clinically meaningful reduction in SBP (Supplementary Fig. 1).
The BP-lowering effect induced by DRT was accompanied by an increase in peak BF during hyperaemia, which reflects an improvement in microvascular function [25]. As BP is mainly regulated by resistance vessels, such improvement in microvascular function may be responsible, at least in part, for the reduction in SBP induced by DRT. By our knowledge, this is the first study to demonstrate that DRT improves microvascular function in treated hypertensives. This adaptation was probably triggered by mechanism deflagrated during each exercise execution. Along this line, skeletal muscle activity produces vasodilatory factors (e.g. adenosine, CO2, lactate/H+, and K+) [34], but during the concentric phase of dynamic resistance exercise, blood flow is restricted [35]. However, during the rest periods between the exercise repetitions and sets, blood flow increases, producing shear stress and vasodilation, which reveals ischemia/reperfusion cycles [36] that may chronically improve microvascular function [37]. In addition, such microvascular function improvement after DRT might have clinical relevance once microvascular dysfunction is typical in hypertension [7], and an attenuated reactive hyperaemia is associated with higher risk of major cardiovascular events [38]. On the other hand, DRT did not improve arterial endothelial function evaluated by FMD. Likewise, a previous study [6] with healthy individuals with preserved endothelial function also found unchanged FMD and increased peak BF after DRT. Thus, the absence of FMD changes after DRT in the current study might be related, at least in part, to the apparently preserved baseline FMD presented by the participants; which might be due to the fact that almost all the sample was taking angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors that already improve FMD [39].
Contrary to the study’s hypothesis, IHT did not reduce SBP nor DBP. Indeed, although meta-analytic data indicates that IHT reduces BP in general population [8], a recent evidence-based Consensus Document [40] concluded that such hypotensive effect is greater in normotensive than hypertensive individuals; suggesting that the target population may explain, at least in part, this current result. Therefore, as the BP-lowering is clinically important in hypertension, more research is required to actually elucidate whether IHT can decrease BP in this specific population, i.e. treated hypertensives.
The present results also do not support an effect of IHT on cardiovascular autonomic modulation. Although, prior data [9] reported improvements in cardiovascular autonomic markers after IHT in hypertensives, a meta-analysis [41] published during this study execution concluded that IHT does not modify cardiac autonomic modulation in hypertensives. Therefore, the current results support that IHT does not improve cardiovascular autonomic control in treated hypertensives.
CRT produced a net reduction (i.e. CRT vs. CON, Fig. 2) of −10.7 [95%CI: −18.3 to −3.0] mmHg in SBP, with 60% (n = 9) of the participants of this group presenting a clinically meaningful (>5 mmHg) reduction in SBP (Supplementary Fig. 1). In addition, CRT increased peak BF during reactive hyperaemia. These responses, however, were similar to DRT, demonstrating that CRT effects were driven by DRT and IHT had no additive effect. Interestingly, a previous meta-analysis [31] also reported no additive effect of the combination between DRT and aerobic exercise training in BP reduction. Therefore, obtaining an additive BP-lowering effect through the addition of different exercise modes seems to be challenging.
The current study has important clinical implications. The findings support DRT as a valuable additional non-pharmacological intervention for hypertension management since it reduced BP and improved microvascular function even in hypertensive patients already taking pharmacologic treatment. On the other hand, the results raise caution regarding the replace of conventional exercise modes by IHT for hypertension management given the observed lack of efficacy. Lastly, the present results do not also support the association of IHT to DRT given the absence of additive effects in comparison to DRT alone.
It is important to mention the limitations of the current study. Participants were non-active middle-aged men without cardiovascular disease. Thus, caution is needed when extrapolating the current results to individuals with other characteristics, such as elderly, women and patients with cardiovascular disease. Few participants (n = 6, 9% of final sample) had been infected by SARS-CoV-2 before the study enrolment, but none of them had to be hospitalised, and their prevalence was similar among the study’s groups. As in many clinical trials, although adequately powered for the primary outcome (SBP: β = 0.921), analysis for secondary outcomes can be underpowered. Finally, the results regarding the comparisons among the training protocols (DRT, IHT and CRT) are restricted to the specific protocols employed in the present study. It is possible to speculate that the divergent responses between DRT and IHT might be explained, at least in part, by the different amount of muscle mass involved in each protocol, since DRT enrolled a whole-body training and the vascular adaptations induced by training are greater in regions directly mobilised during the exercise sessions [37, 42]. Nevertheless, the protocols employed in the present study were designed based on the recommendations of the hypertension guidelines [3, 4, 13] but the employment of other protocols might reveal different results.
In conclusion, DRT, but not IHT, reduced BP and improved microvascular function in treated hypertensive men. The addition of IHT to DRT, in a CRT protocol, did not produce additive effects when compared to DRT alone.
Supplementary information
Acknowledgements
The authors want to acknowledge the volunteers of the current study. We declare that authors or any organization with which we are associated do not have any competing interests.
Funding
This study was supported by the National Council for Scientific and Technological Development (CNPQ, process 304436/2018-6), the São Paulo Research Foundation (FAPESP, process 2018/12390-1, 2018/19151-2, 2018/23653-3 and 2019/02649-0) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES, process 0001).
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41440-023-01202-4.
References
- 1.Brouwers S, Sudano I, Kokubo Y, Sulaica EM. Arterial hypertension. Lancet. 2021;398:249–61. doi: 10.1016/S0140-6736(21)00221-X. [DOI] [PubMed] [Google Scholar]
- 2.Lawes CM, Hoorn SV, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–8. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 3.Whelton PK, Carey RM, Aronow WS, Ovbiagele B, Casey DE, Smith SC, et al. 2017 ACC / AHA / AAPA / ABC / ACPM / AGS / APhA / ASH / ASPC / NMA / PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults A Report of the American College of Cardiology / American Heart Association T. Hypertension. 2018;71:1269–324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 4.Williams B, Mancia G, Spiering W, Rosei E, Azizi M, Burnier M. 2018 ESC/ESH Guidelines for the management of arterial hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 5.Oliver-Martínez PA, Ramos-Campo DJ, Martínez-Aranda LM, Martínez-Rodríguez A, Rubio-Arias J. Chronic effects and optimal dosage of strength training on SBP and DBP: a systematic review with meta-analysis. J Hypertens. 2020;38:1909–18. doi: 10.1097/HJH.0000000000002459. [DOI] [PubMed] [Google Scholar]
- 6.Rakobowchuk M, Mcgowan CL, de Groot PC, Hartman JW, Phillips SM, MacDonald MJ. Endothelial function of young healthy males following whole body resistance training. J Appl Physiol. 2005;98:2185–90. doi: 10.1152/japplphysiol.01290.2004. [DOI] [PubMed] [Google Scholar]
- 7.Beck DT, Martin JS, Casey DP, Braith RW. Exercise training improves endothelial function in resistance arteries of young prehypertensives. J Hum Hypertens. 2014;28:303–9. doi: 10.1038/jhh.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Valenciano A, Ruiz-Pérez I, Ayala F, Sánchez-Meca J, Vera-Garcia F. Updated systematic review and meta-analysis on the role of isometric resistance training for resting blood pressure management in adults ˜. J Hypertens. 2019;37:1320–33. doi: 10.1097/HJH.0000000000002022. [DOI] [PubMed] [Google Scholar]
- 9.Taylor AC, McCartney N, Kamath MV, Wiley RL. Isometric training lowers resting blood pressure and modulates autonomic control. Med Sci Sports Exerc. 2003;35:251–6. doi: 10.1249/01.MSS.0000048725.15026.B5. [DOI] [PubMed] [Google Scholar]
- 10.Millar P, Paashuis A, McCartney N. Isometric Handgrip Effects on Hypertension. Curr Hypertens Rev. 2009;5:54–60. doi: 10.2174/157340209787314351. [DOI] [Google Scholar]
- 11.Westcott WL. Resistance Training is Medicine: Effects of strength training on health. Am Coll Sport Med. 2012;11:209–16. doi: 10.1249/JSR.0b013e31825dabb8. [DOI] [PubMed] [Google Scholar]
- 12.Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: An age-old debate. Hypertension. 2008;51:952–9. doi: 10.1161/HYPERTENSIONAHA.107.105742. [DOI] [PubMed] [Google Scholar]
- 13.Barroso W, Rodrigues C, Bortolotto L, Gomes M, Brandão A, Feitosa A. Brazilian Guidelines of Hypertension - 2020. Arq Bras Cardiol. 2021;116:516–658. doi: 10.36660/abc.20201238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-Country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 15.Queiroz ACC, Sousa JCS, Silva ND, Tobaldini E, Ortega KC, De Oliveira EM, et al. Captopril does not Potentiate Post-Exercise Hypotension: A Randomized Crossover Study. Int J Sports Med. 2017;38:270–7. doi: 10.1055/s-0042-123044. [DOI] [PubMed] [Google Scholar]
- 16.Maud PJ, Foster C. Physiological Assessment of Human Fitness. 1st ed. Champaign, IL: Human Kinetics 10.1097/00005768-199607000-00027.
- 17.Queiroz ACC, Sousa JCS, Cavalli AAP, Silva ND, Jr, Costa LAR, Tobaldini E, et al. Post-resistance exercise hemodynamic and autonomic responses: Comparison between normotensive and hypertensive men. Scand J Med Sci Sport. 2015;25:486–94. doi: 10.1111/sms.12280. [DOI] [PubMed] [Google Scholar]
- 18.Rezk CC, Marrache RCB, Tinucci T, Mion D, Forjaz CLM. Post-resistance exercise hypotension, hemodynamics, and heart rate variability: Influence of exercise intensity. Eur J Appl Physiol. 2006;98:105–12. doi: 10.1007/s00421-006-0257-y. [DOI] [PubMed] [Google Scholar]
- 19.Carlson DJ, Inder J, Palanisamy SKA, McFarlane JR, Dieberg G, Smart NA. The efficacy of isometric resistance training utilizing handgrip exercise for blood pressure management: a randomized trial. Med (Baltim) 2016;95:e5791. doi: 10.1097/MD.0000000000005791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratamess NA, Alvar BA, Evetoch TK, Housh TJ, Kibler WB, Kraemer WJ, et al. Progression Models in Resistance Training for Healthy Adults. Med Sci Sport Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 21.Malachias M, Souza W, Plavnik F, Rodrigues C. Sociedade Brasileira de Cardiologia. 7a Diretriz Brasileira de Hipertensão Arterial. Arq Bras Cardiol. 2016;107:1–83. [Google Scholar]
- 22.Sharman JE, Lagerche A. Exercise blood pressure: Clinical relevance and correct measurement. J Hum Hypertens. 2015;29:351–8. doi: 10.1038/jhh.2014.84. [DOI] [PubMed] [Google Scholar]
- 23.Collier CR. Determination of mixed venous CO2 tensions by rebreathing. J Appl Physiol. 1956;9:25–9. doi: 10.1152/jappl.1956.9.1.25. [DOI] [PubMed] [Google Scholar]
- 24.Thijssen DHJ, Bruno RM, van Mil ACCM, Holder SM, Faita F, Greyling A, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40:1–14. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 25.Limberg JK, Casey DP, Trinity JD, Nicholson WT, Wray DW, Tschakovsky ME, et al. Assessment of resistance vessel function in human skeletal muscle: guidelines for experimental design, Doppler ultrasound, and pharmacology. Am J Physiol Hear Circ Physiol. 2020;318:H301–H325. doi: 10.1152/ajpheart.00649.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Society of Cardiology; North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–81. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 27.Robbe HW, Mulder LJ, Rüddel H, Langewitz WA, Veldman JB, Mulder G. Assessment of Baroreceptor Reflex Sensitivity by Means of Spectral Analysis. Hypertension. 1987;10:538–43. doi: 10.1161/01.HYP.10.5.538. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald HV, Johnson BT, Huedo‐Medina TB, Livingston J, Forsyth KC, Kraemer WJ, et al. Dynamic Resistance Training as Stand‐Alone Antihypertensive Lifestyle Therapy: A Meta-Analysis. J Am Heart Assoc. 2016;5:e003231. doi: 10.1161/JAHA.116.003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bottini B, Carr A, Prisant L, Rhoades R. Variability and similarity of manual office and automated blood pressures. J Clin Pharm. 1992;32:614–9. doi: 10.1002/j.1552-4604.1992.tb05770.x. [DOI] [PubMed] [Google Scholar]
- 30.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–21. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- 31.Cornelissen V, Smart N. Exercise Training for Blood Pressure: A Systematic Review and Meta-analysis. J Am Heart Assoc. 2013;2:e004473. doi: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naci H, Salcher-Konrad M, Dias S, Blum MR, Sahoo SA, Nunan D, et al. How does exercise treatment compare with antihypertensive medications? A network meta-analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br J Sports Med. 2018;0:859–69. doi: 10.1136/bjsports-2018-099921. [DOI] [PubMed] [Google Scholar]
- 33.Adler A, Agodoa L, Algra A, Asselbergs FW, Beckett NS, Berge E, et al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397:1625–36. doi: 10.1016/S0140-6736(21)00590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarelius I, Pohl U. Control of muscle blood flow during exercise: local factors and integrative mechanisms. Acta Physiol. 2010;199:349–65. doi: 10.1111/j.1748-1716.2010.02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asmussen E. Similarities and dissimilarities between static and dynamic exercise. Circ Res. 1981;48:I3–10. doi: 10.1249/01.MSS.0000115224.88514.3A. [DOI] [PubMed] [Google Scholar]
- 36.Mohrman DE, Heller LJ. Cardiovascular Physiology. 8th ed. New York (NY): McGraw-Hill Education; 2013.
- 37.Green DJ, Hopman MTE, Padilla J, Laughlin MH, Thijssen DHJ. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol Rev. 2017;97:495–528. doi: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, et al. Microvascular function predicts cardiovascular events in primary prevention: Long-term results from the firefighters and their endothelium (FATE) study. Circulation. 2011;123:163–9. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 39.Shahin Y, Khan JA, Samuel N, Chetter I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: A meta-analysis of randomised controlled trials. Atherosclerosis. 2011;216:7–16. doi: 10.1016/j.atherosclerosis.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 40.Hanssen H, Boardman H, Deiseroth A, Moholdt T, Simonenko M, Kränkel N, et al. Personalized exercise prescription in the prevention and treatment of arterial hypertension: a Consensus Document from the European Association of Preventive Cardiology (EAPC) and the ESC Council on Hypertension. Eur J Prev Cardiol. 2022;29:205–15. doi: 10.1093/eurjpc/zwaa141. [DOI] [PubMed] [Google Scholar]
- 41.Almeida JPA, de S, Bessa M, Lopes LTP, Gonçalves A, Roever L, et al. Isometric handgrip exercise training reduces resting systolic blood pressure but does not interfere with diastolic blood pressure and heart rate variability in hypertensive subjects: a systematic review and meta-analysis of randomized clinical trials. Hypertens Res. 2021;44:1205–12. doi: 10.1038/s41440-021-00681-7. [DOI] [PubMed] [Google Scholar]
- 42.McGowan CL, Visocchi A, Faulkner M, Verduyn R, Rakobowchuk M, Levy AS, et al. Isometric handgrip training improves local flow-mediated dilation in medicated hypertensives. Eur J Appl Physiol. 2007;99:227–34. doi: 10.1007/s00421-006-0337-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.