Abstract
Phenoxazines have sparked a lot of interest owing to their numerous applications in material science, organic light-emitting diodes, photoredox catalyst, dye-sensitized solar cells and chemotherapy. Among other things, they have antioxidant, antidiabetic, antimalarial, anti-alzheimer, antiviral, anti-inflammatory and antibiotic properties. Actinomycin D, which contains a phenoxazine moiety, functions both as an antibiotic and anticancer agent. Several research groups have worked on various structural modifications over the years in order to develop new phenoxazines with improved properties. Both phenothiazines and phenoxazines have gained prominence in medicine as pharmacological lead structures from their traditional uses as dyes and pigments. Organoelectronics and material sciences have recently found these compounds and their derivatives to be quite useful. Due to this, organic synthesis has been used in an unprecedented amount of exploratory alteration of the parent structures in an effort to create novel derivatives with enhanced biological and material capabilities. As a result, it is critical to conduct more frequent reviews of the work done in this area. Various stages of the synthetic transformation of phenoxazine scaffolds have been depicted in this article. This article aims to provide a state of the art review for the better understanding of the phenoxazine derivatives highlighting the progress and prospects of the same in medicinal and material applications.
Graphical abstract
Keywords: Actinomycin D, Antimalarial, Antiviral, Fluorescent probe, Phenoxazine
Aims and scopes of the review
Starting from its inception, chemists have been particularly interested in the chemistry of phenoxazines owing to its wide range of applications both in pharmacology and industry. For the past few decades, the phenoxazine derivatives have also found widespread applications in the fields of organoelectronics, photophysics, material sciences and biological sciences thereby necessitating the urge of synthetic developments and modifications of the scaffold. The various synthetic transformative stages of phenoxazine scaffolds have been highlighted in this review (discussed in ‘Synthetic methods to prepare several phenoxazine derivatives’). A well-known member of the actinomycin family with strong antitumor properties is actinomycin D. Since 1954, many tumours have been successfully treated with actinomycin D, an anticancer drug that is also a helpful tool in biochemistry and molecular biology. The structural design of actinomycin includes a phenoxazine moiety which suggests that phenoxazine and its derivatives should also have anticancer properties (discussed in ‘Application of phenoxazine as an anticancer drug’). Despite decades of work since the creation of pamaquine in 1924, quinacrine in 1930 and chloroquine in 1934, no single synthetic medication was known to eradicate both the blood and tissue stages of the various types of malaria parasites encountered in human body. The emergence of chloroquine-resistant strains heightened the need for newer, more potent antimalarial drugs. The scientific community hypothesised that medications used to treat neoplasms might also have some toxic effects on plasmodia parasites and, in some cases, vice versa. This was primarily due to the fact that most of the medications used to treat malaria purportedly work by interfering with the parasite's replicative processes and because the host-drug-parasite triangle poses similar challenges in the treatment of cancer. In order to test this hypothesis, clinical trials took into account the antimalarial properties of actinomycin D, one of the most potent antineoplastic agents (discussed in ‘Application of phenoxazine as antimalarial drug’). Worldwide outbreaks and epidemics are periodically brought on by newly and re-emerging viruses which eventually result in global events like the current pandemic of the novel SARS-CoV-2 coronavirus infection COVID-19. Therefore, there is a glaring need for novel antivirals. A brief discussion is worthwhile in this aspect regarding the antiviral activity of phenoxazines and the nucleoside analogues based on phenoxazine scaffold (discussed in ‘Application of phenoxazine as antiviral drug’). When the pancreas does not produce enough insulin to regulate blood sugar levels, type 2 diabetes is primarily caused which raises the risk of heart attack, atherosclerosis and hypertension, all of which are insulin-related diseases. Phenoxazine moiety-containing compounds had been looked into due to the ongoing interest in the development of antidiabetic agents, and these derivatives were found to play a significant role (discussed in ‘Application of phenoxazine as antidiabetic drug’). By using comparatively simple synthetic procedures, several environment sensitive phenoxazine-based fluorophores were designed over the years. These fluorophores were examined for their potential utility as fluorescent labels for amines, amino acids, proteins in general. Even their utility to detect various environments is worth mentioning (discussed in ‘Application of phenoxazine as fluorescent probes’). Organic light-emitting diodes (OLEDs) are optoelectronic devices that use the photoluminescent properties of π-conjugated organic materials which are useful for lighting applications. A wide range of phenoxazine-based OLEDs had been developed by various research groups which have found wide applications in the next-generation flat-panel displays (discussed in ‘Application of phenoxazine as organic light-emitting diodes’). For the past few years, photoredox catalysts (PCs) have garnered wider interest in order to construct several covalent bonds of significant challenges under mild reaction conditions. Most of the PCs have been found to be derived from precious metals. Hence, in recent times, the development of organic analogues as sustainable replacements has received tremendous curiosity and attention. A significant number of research groups have already established phenoxazine-based systems as PCs (discussed in ‘Application of phenoxazine as photo-redox catalyst’).
Concerns about the use of traditional energy sources, such as fossil fuels, have recently grown as a result of environmental and long-term supply issues. Alternative energy research is currently receiving a lot of attention all over the world. The sun's abundance of energy makes solar cells an appealing alternative to the major sources used today. However, the current crystalline silicon-based solar cell production costs are still too high to compete with the market's conventional energy sources. Dye-sensitized solar cells (DSSCs) could be a viable option for effectively lowering production costs. A series of metal-free organic dyes with a phenoxazine chromophore at their core have been synthesised and tested as sensitizers in dye-sensitized solar cells (discussed in ‘Application of phenoxazine in dye-sensitized solar cells’).
Introduction
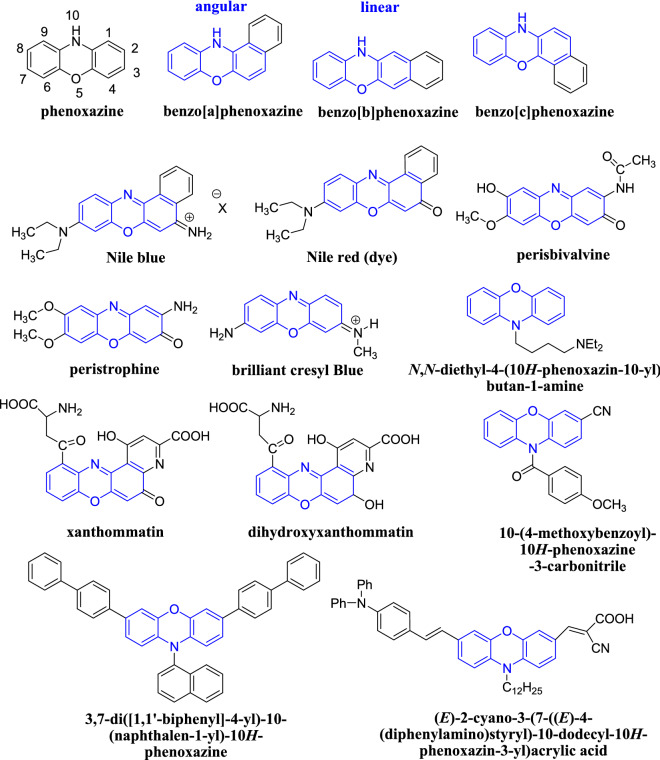
Heterocyclic compounds have been recognised for their immense importance to life as well as their diverse natural resources, making them an important contributor to chemistry and biology over the years [1–7]. Many known drug molecules contain at least one heterocyclic skeleton. A review of the literature on heterocyclic compounds containing both nitrogen and oxygen in its molecular architecture reveals the existence of phenoxazine moiety [4–7]. Phenoxazines are tricyclic heterocycles made up of two benzene rings joined together by an oxazine structure [8–10]. Bernthsen first designed phenoxazine, a unique class of heterocyclic compound, in 1887 by thermal condensation of o-aminophenol and catechol (Scheme 1) [11].
Scheme 1.
Bernthsen synthesis of phenoxazine and representative structures of gallocyanine and Meldola's blue [11]
Two dyestuffs with the phenoxazine ring system, Meldola's blue and gallocyanine, were commercially available even before Bernthsen reported the first synthesis of phenoxazine in 1887 [12]. Phenoxazines were discovered, but their chemistry lay dormant for nearly sixty years. The discovery of biologically active and naturally occurring phenoxazines since 1947 was the prime factor that the interest in this class of compound had been sparked with rekindled interest [12, 13].
Phenoxazine is reputed for its pharmacological and industrial properties. It serves as the primary structural skeleton in a wide range of natural products, including ommochromes (dihydroxanthommatin, xanthommatin) and ommins pigments which primarily control invertebrate skin and eye colours [13, 14]. This compound has primarily been used as a dyeing agent for silk dyeing in the textile industry since its inception and also serves as the foundation for the dyes like Nile blue and Nile red (Scheme 2). These dyes are photostable and highly fluorescent. They can act as photosensitizers as well as histological stains for imaging lysosomes and lipids in vitro. The versatile utility of phenoxazine derivatives piqued the interest of the research community [14]. The literature is replete with various pharmacological activities of phenoxazine derivatives, including antibacterial, antimalarial, antiviral, antifungal, antihistaminic, anticancer, antitumor, anti-inflammatory, antidiabetic, antidepressant, analgesic and schizophrenia activities. Recent research has demonstrated its enormously promising activities in the OLED (organic light-emitting diode), dye-sensitised solar cells and as photoredox catalysts in metal-free polymerizations [12–15].
Scheme 2.
Representative structures of several compounds containing phenoxazine moiety (drawn in blue)
Guttman and Ehrlich reported the clinical cure of two malaria patients in 1891 following the oral administration of methylene blue which contains a phenothiazine moiety; this dye was well known prior to the discovery of penicillin [16, 17]. During the development of antimalarial drugs, phenoxazine, an isostere of phenothiazine, came into the sight again [18]. Ihara et al. investigated the antimalarial activities of oxazines, focussing on brilliant cresyl blue and nile blue compounds [19].
Later in 1940, Waksman and Woodruff isolated the first phenoxazine core containing antibiotic, Actinomycin D, from Streptomyces melanochromogenes which contains the chromophoric phenoxazine dicarboxylic acid [20]. The compound also served the purpose as a chemotherapeutic agent in the therapy of various malignant cancers, namely Wilm's tumour, testicular cancer and choriocarcinoma since 1954. In 1969, in vivo antimalarial activity of Actinomycin D was first observed by Fink and Goldenberg which was later confirmed by Kauser [21]. Achenbach and Blümm discovered phenoxazine derivatives in Pycnoporus sanguineus, a white rot saprobic fungus, in 1991 [22]. Peristrophine, the first phenoxazine alkaloid, was isolated from P. roxburghiana by Qin et al. in [23]. After some time, a group of VAST (Vietnam Academy of Science and Technology) scientists investigated the leaves of the plant Peristrophe, a genus of the Acanthaceae family found primarily in Southern Asia and Africa. This plant was then cultivated for the purpose of colouring foods such as sticky rice and as a traditional medicine. Peristrophe bivalvis, also known as Noja in Indonesia, was used as a source of natural red dye. VAST scientists extracted perisbivalvine (Scheme 2) from this plant in 2009 and studied its NMR spectra [24, 25].
Literature studies have also revealed that phenoxazine acts as a multidrug resistance (MDR) modulator in various cancerous cells [26, 27]. Iwata et al. in [28] and Kato et al. in [29] reported a newly synthesised drug, 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one with antiviral, immunosuppressive and antiproliferative activity [28, 29]. Even the 3,7-bis(dialkylamino) phenoxazinium salts have also been shown to have significant antimalarial activity against Plasmodium falciparum (CQ-resistant K1 strain) and Plasmodium berghei (NK-65 strain) [19]. Phenoxazine derivatives can also be used to treat diabetes. The antidiabetic activity of the derivative, N-(4-hydroxy-phenyl)-1-nitro-10H-phenoxazine 3-sulphonamide, is a renowned one [30]. Although the phenoxazinium derivatives demonstrated potent in vitro activity, some oral efficacy and micromolar level cytotoxicity were observed as well.
The appeal of this skeleton lies in the presence of all four of the planet's most vital biochemical constituents, oxygen, nitrogen, carbon and hydrogen atoms, bound together in a way that makes it simple for the two large π-conjugated systems can easily exhibit their charge transport properties [20, 31]. In dye-sensitized solar cells (DSSCs), it performed better as a semiconductor material because of its potent electron-donating capacity. Several phenoxazine derivatives have recently been used in organic light-emitting diodes (OLEDs), as well as thermally activated delayed fluorescence (TADF) diodes [31–33]. According to one study, phenoxazine can be used as a photoredox catalyst (PC) to produce polymers with controlled molecular weight and low disparities [34]. To study biological samples, several phenoxazine-containing fluorescent probes have been synthesised in the laboratory. Owing to its planar and rigid structure, electron-donating capacity, lipophilicity, high thermal stability, bio adaptability, photophysical and pharmacological activities, phenoxazine has garnered wider interest and has been the important subject area of research [35, 36].
However, a few review articles on phenoxazine have been published over the years [13–15, 20]. However, given its diverse applications in various branches of science in recent years, it has become necessary to review the work done in this area on a more regular basis. The primary focus of this review work is to showcase several routes to synthesise phenoxazine alkaloids and derivatives containing this heterocyclic skeleton, as well as a brief discussion of its photophysical, optoelectronic, pharmacological and biological activities.
Methodology
A thorough analysis of the research articles published in the last 25 years has been conducted on the synthesis of phenoxazine and its derivatives, as well as its biomedical applications, applications in material science, organic light-emitting diodes, photoredox catalyst, dye-sensitized solar cells and chemotherapy. Information has been gathered from several readily available online databases, including Google Scholar, Scopus and PubMed. Apart from synthesis, numerous applications of phenoxazine have drawn significant attention for the past few decades. The main keywords that have been used to search databases include (a) phenoxazine as an anticancer drug, (b) phenoxazine-based antimalarial drug, (c) applications of phenoxazine as fluorophores, (d) phenoxazine as organic light-emitting diodes, (e) use of phenoxazine as photoredox catalyst and (f) application of phenoxazine in dye-sensitized solar cells.
Synthetic methods to prepare several phenoxazine derivatives
Phenoxazine, 1 was first synthesised more than a century ago and over the past few decades, a variety of synthetic routes to the target molecule have been developed. Two key methodologies have been outlined in Scheme 3 for designing the desired moiety via the formation of the middle ring [11].
Scheme 3.
Few reputable synthetic routes to phenoxazine derivatives [11]
In this regard, a retrosynthetic pathway of phenoxazine is worth discussing (Scheme 4). Here the target molecule, 1 can be obtained from an acetyl derivative, 2, obtained from the functionalized diaryl ether, 3 through cyclization of iodo-substituted diaryl ethers under metal-free condition using Bolm's N-arylation methodology [37]. Cross coupling reactions using transition metal as catalyst have also been used to form C–N bonds e.g. a Palladium catalysed double N-arylation reaction using di(2-bromoaryl) ethers [37].
Scheme 4.
Retrosynthetic routes towards phenoxazine [37]
A crucial step in the retrosynthetic route is the formation of diaryl ether, 3. Molecule 3 can be synthesised in two ways: using N-functionalized phenol, 4 with 2-iodophenyl salt, 5 (route A), or using 2-acetamido-substituted salt, 6 with 2-iodophenol, 7 (route B). Since the compound is prone to oxidation, it is difficult to synthesise the iodonium salt, 5, via route A. However, in route B, the chemoselective transfer of the 2-amido aryl group over other aryl groups may necessitate the use of a symmetric iodonium salt (R = NHAc) or an electron-rich dummy group. Route B was initially prioritised in order to investigate the anisyl moiety as a dummy group in the iodonium salt, 6a. The salt, 6a, was prepared from iodoarene, 8 with a high yield involving arylboronic acid [38]. The reaction went well when 2-iodophenol, 7 and salt, 6a were O-arylated in both toluene and THF, with the former producing N-acetylphenoxazine, 2 via tandem arylation followed by cyclization in one pot. Initially, 3 was formed via a T-shaped intermediate, 9 (Scheme 6) which was cyclised to N-acetylphenoxazine, 2 under high yielding O-arylation conditions. Deprotection of 2 was reported to proceed in a very high yield and the synthesis of 2 thus advances under a metal-free conventional phenoxazine, 1 synthesis (Scheme 5) [38].
Scheme 6.
Synthesis of phenoxazine derivative from 2-aminophenol and 3,4-dihaloarene [41]
Scheme 5.
Forward synthetic route to phenoxazine [38]
A variety of synthetic routes can result in the formation of the phenoxazine moiety [39–42]. Eastmond et al. developed a synthetic route involving the transition metal-free pathway (Scheme 6). Here, the molecule of interest was obtained from 2-aminophenol and 3,4-dihaloarenes containing electron withdrawing substituent (3,4-difluorobenzonitrile) [41].
Crivello et al. synthesised the simpler form of linear benzophenoxazine by refluxing 2,3 dihydroxynaphthalene with 2-aminophenol for 5 h and then diluting it with 95% ethanol to obtain 12H-benzo[b]phenoxazine (Scheme 7) [43].
Scheme 7.
Synthesis of 12H-benzo[b]phenoxazine from 2,3dihydroxynaphthalene 2-aminophenol [43]
Mickevičienė et al. reported another route to benzo[b]phenoxazine synthesis by refluxing several derivatives of 2-aminophenol 10 (a-c) with crotonic acid, yielding 3-[(2-hydroxyphenyl) amino]butanoic acids 11 (a–c). The reaction of the corresponding acids and hydrazine hydrate then produced a series of hydrazides, 12 (a-c) which on reaction with several aldehydes produced hydrazones, 13 (d–g) [44]. On reaction of compounds, 12 (a–c) with 2,5-hexanedione, 14 afforded N-(2,5-dimethyl-1H-pyrrol-1-yl)-3-[(2-hydroxyphenyl)amino]butanamides, 15. Compounds, 11 (a–c) on reaction with 2,3-dichloronaphthalene-1,4-dione, 19 yielded compounds 3-(6,11-dioxo-6H-benzo[b]phenoxazin-12(11H)-yl)butanoic acids, 20(a–c). These products on further treatment with ethyl iodide and sodium carbonate in acetone medium gave ethyl 3-(6,11-dioxo-6H-benzo[b]phenoxazin-12(11H)-yl)butanoates, 21 (a–c). The reaction of ethyl-3-oxobutanoate, 16 with the ortho-aminophenol derivatives yielded a series of ethyl 3-(2-hydroxyphenylamino)but-2-enoates, 17 (a-c) which when treated with 19 yielded 18 (a–c) (Scheme 8). Again, N′-substituted-3-(6,11-dioxo-6,11-dihydro-12H-benzo[b]phenoxazin-12yl)butane hydrazides, 22 (d–g) were obtained from intermediates, 13 (d–g) and butanamides, 23 were achieved from intermediates, 15 (Scheme 8).
Scheme 8.
Synthetic route to the formation of various intermediates from substituted ortho- aminophenol and preparation of corresponding benzo[b]phenoxazine derivatives [44]
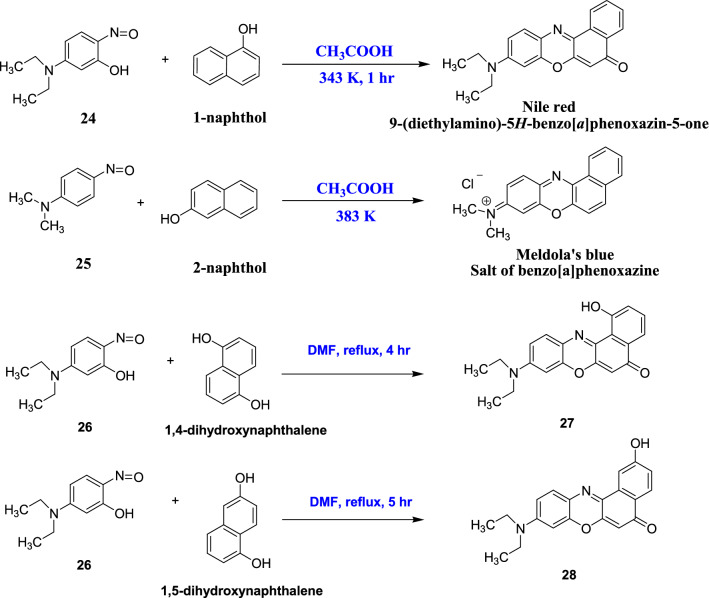
The nonlinear benzo[a]phenoxazine was initially discovered by Goldstein and Semelitch [45]. Later, Jose and Burgess reported the synthetic pathway for other nonlinear phenoxazine, Nile Red and Meldola's blue which was obtained by the condensation of nitroso compounds, 24 and 25 with 1-naphthol and 2-naphthol at very high temperatures in acetic acid medium (Scheme 9) [46]. A few Nile red derivatives (1-hydroxy derivative, 27 and 2-hydroxy derivative, 28) were also synthesised by refluxing 5-diethylamino-2-nitrophenol, 26 with 1,4-dihydroxynaphthalene and 1,5-dihydroxynaphthalene in DMF medium (Scheme 9), respectively [46].
Scheme 9.
Synthetic strategy to obtain of benzo[a]phenoxazine from nitroso compound and 1-hydroxy (27) and 2-hydroxy (28) derivatives of Nile Red [46]
The synthesis of fluoro derivatives of Nile red from tetrafluoronaphthalene and diethylaminephenol was reported in the scientific literature (Scheme 10) [46]. It is worth mentioning that ‘FLAsH dyes’ based on the phenoxazine core were synthesised using 1-hydroxynaphthoquinone and 2-amino-5-nitrophenol as starting components (Scheme 11) [46].
Scheme 10.
Synthesis of fluoro derivative of Nile Red [46]
Scheme 11.
Synthesis of FLAsH dyes based on Nile red [46]
Jose et al. reported the synthesis of sulfonated benzophenoxazines, 30 and 32 from 1,3 and 3-,6-disubstituted sulfonic acid along with 29 and 31, respectively (Scheme 12) [47].
Scheme 12.
Synthetic route to form sulfonated benzophenoxazines [47]
In 2015, Ezeokonkwo et al. identified a pathway for the synthesis of 6-chloro-5H-benzo[a]phenoxazin-5-one, 33 an antimalarial drug [48]. At a higher temperature, they used a coupling reaction of 2,3-dichloro-1,4-naphthaquinone with 2-aminophenol in presence of sodium acetate and benzene medium that yielded a yellow coloured solid after recrystallisation from ethanol–water (2:1) (Scheme 13). In the presence of Pd(0)/Xphos, compound 33 produced 6-(hex-1-yn-1-yl)-5H-phenoxazin-5-one, 34 and 6-(phenylethynyl)-5H-benzo[a]phenoxazin-5-one, 35. Again, compound 33 on treatment with aryl boronic acid and styryl boronic acid resulted in the formation of 6-phenyl-5H-benzo[a]phenoxazin-5-one, 36 and (E)-6-styryl-5H-benzo[a]phenoxazin-5-one 37, respectively, via Suzuki–Miyaura Mechanism (Scheme 13) [49].
Scheme 13.
Synthetic route to obtain various phenoxazine derivatives using 6-chlor-5H-benzo[a]phenoxazin-5-one [49]
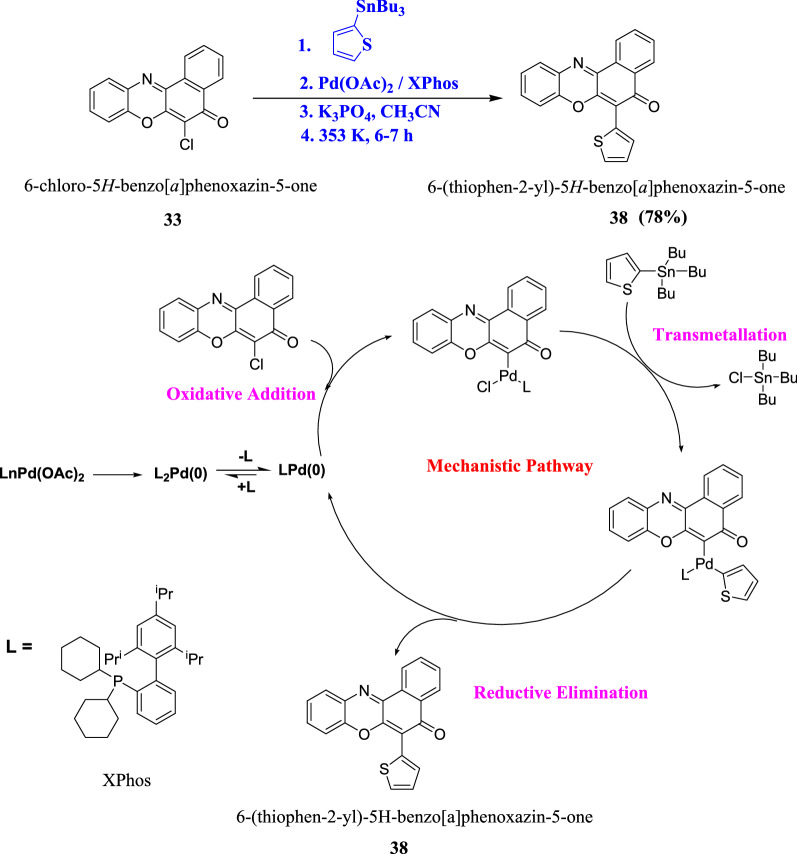
Stille cross coupling involving compound 33 and tributylthiophenylstannane yielded 6-(thiophen-2-yl)-5H-benzo[a]phenoxazin-5-one, 38 (Scheme 14) [50].
Scheme 14.
Mechanistic representation of synthesis of 6-(thiophen-2-yl)-5H-benzo[a]phenoxazin-5-one from 6-chloro-5H-benzo[a]phenoxazin-5-one via Stille coupling [50]
In this regard, it is important to note that the synthesis of various derivatives of 6-chloro-5H-naphtho[2,1-b]pyrido[2,3-e][1,4]oxazin-5-one, 39 was reported by Onoabedje et al. following the same pathway as discussed for 6-chloro-5H-benzo[a]phenoxazin-5-one, 33 (Scheme 15) [50].
Scheme 15.
Synthesis of various derivatives of 6-chloro-5H-naphtho[2,1-b]pyrido[2,3-e][1,4]oxazin-5-one [50]
Ayogu et al. in 2007 reported the synthesis of a 6-anilino derivative of angular phenoxazine which required the condensation of 2,4-dichloro-1,4-naphthaquinone with 2-aminophenol in basic medium to produce 6-chlorobenzo[a]phenoxazine-5-one, 45 (Scheme 16) [51]. Under the conditions of Buchwald-Hartwig cross coupling reaction with several amines, namely 2-aminophenol, 4-nitroaniline, 2-amino-4-methylpyridine, 2-amino-5-nitropyridine, 2-aminopyrazine and aniline in DMF/toluene at 110 °C, 45 resulted in corresponding amino derivatives, 46(a-f) (Scheme 16) [51].
Scheme 16.
Synthetic route to obtain various 6-anilino derivative of angular phenoxazine [51]
In 2014, Mizukawa et al. reported a one-pot synthesis of benzo[a]phenoxazine SSJ-183, a very promising antimalarial drug [52]. Even in blood, it also showed a strong inhibition of the growth of Plasmodium falciparum [53]. The drug was commercially synthesised by reducing Nile blue A with Zn/Fe in an acidic medium, followed by an in situ reaction with 4-chloropyridine at 900C and subsequent oxidation in air (Scheme 17 a). Because of its nucleophilic nature, Nile Blue A readily participated in the substitution reaction with 4-chloropyridine to produce SSJ-183. Using the same method, bis-N,N-deethylated metabolite, 49 was synthesised from commercially available Darrow red via a one-pot reduction and oxidation reaction [52]. Again, an N-deethylated metabolite, 48, was produced by reducing Darrow red with Zn and pyridine hydrochloride, yielding an intermediate, N-((5E)-5-(pyridin-4-ylimino)-5H-benzo[a]phenoxazin-9-yl)acetamide, 47. In dioxane medium, the intermediate, 47, was treated with sodium bis(2-methoxyethoxy)aluminium hydride, yielding a compound, 48 (Scheme 17 b). Similarly, the synthetic route towards bis-N,N-deethylated metabolite, 49, has also been showcased in Scheme 17 b [53].
Scheme 17.
a One-pot synthesis of the antimalarial drug SSJ-183 [52]. b Synthetic route to N-deethylated metabolite and bis-N,N-deethylated metabolite [53]
Several methods for obtaining aza-fused phenoxazine molecules were designed by Oni et al. in 2015 using the Mizoroki–Heck arylation method. The reaction of 7-chloro-5,8-quinolinequinone, 50 with 2-aminophenol in the presence of sodium acetate produced 1-azabenzo[a]phenoxazin-5-one, 51. On treatment with 4,5-diamino-6-hydroxypyrimidine, 52, compound 50 yielded 53, 11-amino-1,8,10-triazabenzo[a]phenoxazin-5-one (Scheme 18 a) [54]. Imai et al. synthesised 6-substituted 11-aza-5H-pyrido[2,3-a]phenoxazine-5-one, 56 and 6-substituted 11-aza-5H-pyrido[3,2-a]phenoxazine-5-one, 57 using a condensation reaction between 6,7-dibromo-5,8-dioxoquinoline, 54 and 2-amino-3-hydroxypyridine, 55 in ethanol/benzene medium in the presence of potassium acetate at room temperature (Scheme 18 b) [55].
Scheme 18.
a Synthesis of 1-azabenzo[a]phenoxazin-5-one and 11-amino-1,8,10-triazabenzo[a]phenoxazin-5-one [54]. b Synthetic route to 6-substituted-11-aza-5H-pyrido phenoxazine compound [55]
Nan’ya et al. reported the synthesis of 11-aza-5H-benzo[a]phenoxazin-5-one derivatives, 59 by the condensation of 5-substituted-2,3-dihalogeno-1,4–naphthoquinone, 58 with 2-amino-3-hydroxypyridine, 55 in the presence of potassium acetate and in benzene/ethanol medium (Scheme 19a) [56]. Ishii reported the acid-catalysed synthesis of pyrido[3,2-a]phenoxazine, 61 which was obtained by the condensation of 2-aminophenol and 4,6-dihydroxyquinoline-5,8-dione, 60 (Scheme 19b) [57]. Okafor et al. reported the synthesis of another phenoxazine-containing compound, 6-chlorobenzo[a]-11-azaphenoxazine-5-one, 63 by refluxing 2,3-dichloro-1,4-naphthoquinone, 62 with 2-amino-3-pyridinol, 55 in chloroform and anhydrous sodium carbonate medium (Scheme 19c) [58].
Scheme 19.
a Synthesis of 11-aza-5H-benzo[a]phenoxazin-5-one derivatives via condensation reaction [56]. b Synthetic route to pyrido[3,2-a]phenoxazine [57]. c Synthetic pathway to synthesise 6-chlorobenzo[a]-11-azaphenoxazine-5-one [58]
David et al. reported a series of new compounds based on the angular triazaphenoxazinone moiety via a copper-catalysed arylation pathway [59]. Here, 11-amino-1,8,10-triazabenzo[a]phenoxazin-5-one, 68 was obtained by the reaction between 11-amino-1, 8, 10-triazabenzo[a]phenoxazin-5-one, 64 and substituted potassium phenyltriolborates, 67 in the presence of some N-oxide, copper acetate and 4 Å molecular sieves (ms). The role of molecular sieve was to absorb the water molecules formed in the reaction mixture. The yield of the product varied depending on the different substituents present on the substituted potassium phenyltriolborates. It is to be noted that the reaction of 7-chloro-5,8-quinolinequinone, 65 and 4,5-diamino-6-hydroxypyrimidine, 66 yielded the compound, 64 (Scheme 20) [59].
Scheme 20.
Synthetic route obtain new compounds of angular triazaphenoxazine by copper-catalysed arylation reaction [59]
In comparison to conventional heating conditions, oxazine-based heterocycles are now constructed using ultrasound -mediated condensation which is a greener approach for the synthesis of benzo[a]phenoxazinium chlorides, 71. Numerous organic reactions are carried out using ultrasonic irradiation which results in higher yield of products, shorter reaction times and increased product selectivity even under milder conditions. Raju et al. reported the ultrasound-mediated condensation synthesis of benzo[a]phenoxazinium chloride, 71 using N-alkylnaphthalen-1-amine, 69 and 5-(ethylamino)-4-methyl-2-nitrosophenol hydrochloride, 70 (Scheme 21) [60].
Scheme 21.
Ultrasound-assisted and thermal synthesis of benzo[a]phenoxazinium chlorides, 71 [60]
Bruyneel et al. reported laccase-mediated synthetic routes to substituted phenoxazine. Laccase is a copper-containing enzyme that is responsible for one electron oxidation in a wide range of phenolic compounds. The reaction between sulfonated aminophenol, 72 and enzyme-mediated dimerization resulted in the formation of phenoxazines, 77. The presence of sulfonyl substituent was responsible for the water solubility of the compound (Scheme 22) [61].
Scheme 22.
Laccase-mediated synthetic pathway to obtain substituted phenoxazine derivatives [61]
Phenoxazines can be found in a variety of natural and synthetic chromes that have been used for centuries. Almost 80 years ago, in 1939, Becker first described ommatidia found in an insect [62]. Following Becker's detailed chemical study, scientists continued to focus on ommochromes. Bolognese et al. identified ommochromes which are decarboxylated forms of xanthommatin and H2-xanthommatin, found in the in vitro oxidation of ommochrome precursors in 1988 [63]. Vogliardi et al. identified the same in 2004 [64]. In 2017, two developmental biologists Sekimura and Nijhout used butterfly ommochromes to understand the mechanism and diversity of colour patterning [65]. The first few steps of ommochrome biosynthesis cover the formation of kynurenines via tryptophan oxidation (Figon and Casa [66]). Ommochromes were produced in some specialized ommochromasomes. Two hypotheses were proposed for the formation of ommatins from 3-hydroxykynurenine. One of them involved the initiation of xanthommatin via oxidative dimerization of 3-hydroxykynurenine followed by intramolecular cyclization. This was considered as the biosynthesis pathway for ommatins. Iwasahi and Ishii reported in 1997 that uncyclized xanthommatin was apparently recognised in the in vitro oxidation of 3-hydroxykynurenine. The oxidative dimerization of 3-hydroxykynurenine yielded xanthommatin and its decarboxylated forms [67, 68]. A schematic representation has been given in Scheme 23.
Scheme 23.
Kynurenine pathway to obtain xanthommatin and its analogues [67, 68]
Peristrophine, a natural alkaloid, was synthesised in the laboratory from vanillin, a xylochemical that produced the homodimerization product peristrophine, 78 followed by aerial oxidation and several steps (Scheme 24) [69–71].
Scheme 24.
Applications of phenoxazine as an anticancer drug
Phenoxazines played a significant role in medicinal chemistry as well as a number of industrial applications. Among its diverse applications in medicinal chemistry, its effectiveness as an antiproliferative agent is a crucial one. Since 1954, many tumours have been successfully treated with actinomycin D, an anticancer drug containing phenoxazine moiety that is also a helpful tool in biochemistry and molecular biology [20, 21]. Actinomycin D accomplishes this by attaching to DNA at the transcription initiation complex thereby preventing the elongation of the RNA chain by RNA polymerase. Cancer is a disease that involves uncontrolled cell division and cell growth [72]. Notably, Actinomycin D finds applications in the treatment of Wilms' tumour, Ewing's sarcoma, gestational trophoblastic disease and rhabdomyosarcoma, among others. Actinomycin D, a naturally occurring chromopeptide, consisting of a chromophore based on heterocyclic skeleton and two cyclic pentapeptide-based lactone rings, has been demonstrated in cell biology to have the ability to impede transcription. The compound's colour and intercalative properties are due to the heterocyclic fragment, a phenoxazine derivative with a quinonimine part and this made the researchers believe that various derivatives of phenoxazine should also possess anticancer properties [73]. The capacity of actinomycin D to produce superoxide radicals was first noted in 1978 and its most likely mechanism has been outlined in Scheme 25 [74].
Scheme 25.
Representative structure of Actinomycin D and its probable mechanism [74]
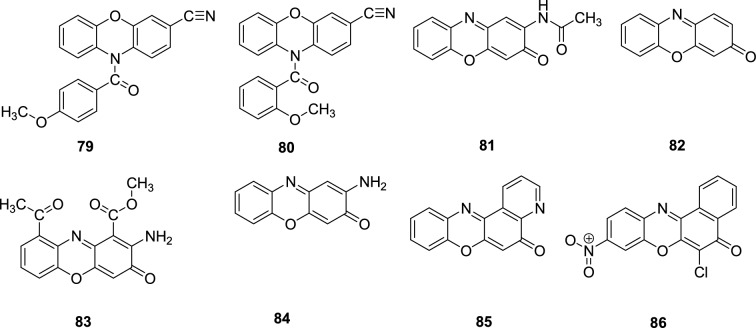
According to the report published by Kobayashi et al. in [75], several phenoxazine compounds have been synthesised since 1970 [75]. Surprisingly, the majority of them were synthesised without much direction. Most of the compounds were later tested for anticancer activity. To monitor the interaction with tubulin polymerization, Prinz et al. carefully examined the synthesis, structure activity relationship and anticancer activity studies of a number of N-benzoylatedphenoxazines [76]. It was discovered that the phenoxazine derivatives 10-(4-methoxybenzoyl)-10H-phenoxazine-3-carbonitrile, 79 and its isovanillic analogue, 80 were found to be the potential inhibitors of multiple cancer cell lines. The antiproliferative properties of a number of polycyclic phenoxazine compounds including 2-acetylamino-3H-phenoxazin-3-one, 81, 3H-phenoxazin-3-one, 82, 2-amino-1,9-diacetyl-3H-phenoxazine, 83, 2- amino-3H-phenoxazin-3-one, 84 and 5H-pyrido[3,2-a]phenoxazin-5-one, 85 were synthesized, and their antiproliferative properties were studied in detail (Scheme 26) [76].
Scheme 26.
Representative structures of a few phenoxazine derivatives having antiproliferative properties [76]
With the best antiproliferative inhibition property, these substances showed antileukemic activity at sub-micromolar concentrations. The benzo[a]phenoxazine derivatives showed more activity than that of a simple phenoxazine skeleton [76, 77]. A study was carried out considering eleven benzo[a]phenoxazine and four phenoxazines to modulate multidrug resistance (MDR) in a P-glycoprotein overexpressing T lymphoma cell line in mice. It was done by the distinguishable accumulation of rhodamine 123 by sensitive multidrug-resistant cells which was based on flow cytometric functional test [78]. P-glycoprotein inhibition was observed to a certain extent for seven benzo[a]phenoxazine derivatives accounting for the increased accumulation of rhodamine 123 by resistant cells. However, it is worthwhile to mention that none of the simple phenoxazine frameworks were able to suppress P-glycoprotein; rather benzo[a]phenoxazine was found to be an effective MDR modulator. Experimental results revealed that dimethyl benzo[a]phenoxazine derivatives were found to be more effective against carcinoma than leukaemia cell lines. Martin et al. described the behaviour of the anticancer drug 6-chloro-9-nitro-5-oxo-5H-benzo[a] phenoxazine (CNOB), 86 and found that CNOB/ChR6 therapy was effective at killing cancer cell lines in vitro [79]. Almost 40% complete remission was observed for 10 mg/Kg dose of CNOB for the treatment of a tumour in mice. In this aspect, it is worth mentioning the role of ChR6. This prodrug activates the bacterial enzymes nitroreductase, thereby reduces CNOB into the anticancer compound 9-amino-6-chloro-5H-benzo[a]phenoxazin-5-one (MCHB). MCHB binds with DNA and at a non-lethal dose and it leads to the cell accumulation at the S-phase. However, under lethal concentration, it influences cell surface Annexin V, caspase-3 and caspase-9 activities [79].
Phenoxazine as antimalarial drug
Since Guttmann and Ehrlich reported the clinical cure of two patients after oral administration of a synthetic dye, methylene blue which contains a phenothiazine moiety, in 1891, numerous synthetic dyes were investigated for their antimalarial properties [16, 80]. The only adverse effect they reported was ‘periodic irritation of the bladder’ which was somewhat alleviated by taking nutmeg powder. Methylene blue was chosen as an antimalarial agent due to its ability to specifically stain plasmodia which was demonstrated by Celli and Guarnieri, as well as Ehrlich and Leppmann's successful clinical use of this dye to treat neuralgia. Phenoxazine, an isostere of phenothiazine, came into focus over the past few decades with renewed enthusiasm for the development of new antimalarial medications [17, 81].
Synthetic oxazine dyes with antimalarial properties included Brilliant Cresyl Blue, Nile Blue and Gallocyanine [82]. Recently, Takasu et al. investigated the antimalarial properties of oxazines with a focus on Nile Blue and Brilliant Cresyl Blue [19]. The antimalarial efficacy of the 3,7-bis(dialkylamino)phenoxazinium salts against Plasmodium berghei (NK-65 strain) and Plasmodium falciparum was reported to be improved (CQ-resistant K1 strain). When compared to methylene blue and Brilliant Cresyl Blue, Compound 87 offers up to 100% parasitemia clearance with a good survival effect and a minute toxicity at a dose of 25 mg/Kg for 4 days [83]. In continuation of this antiprotozoal activity of phenoxazines, a series of phenoxazinium compounds were obtained that showed in vitro activity against Trypanosoma brucei rhodesiense, T. cruzi, Plasmodium falciparum and Leishmania donovani [53, 84] though remarkably the compound showed increased activity in case of Plasmodium falciparum and T. cruzi. According to earlier studies, 3,7-bis(diethylamino)phenoxazinium chloride, 88 showed a selectivity index of 2400 (IC50 = 0.002 M) compared to chloroquine (IC50 = 0.148 M). According to the structure–activity relationship (SAR) of these drugs, derivatives with shorter alkyl chains (–CH3, –C2H5) exhibited good selectivity, higher activity and less toxic properties. But as the alkyl chains increased with carbon atoms, the toxicity and selectivity increased. The drug's active site must be flat and may include a tricyclic moiety. Even though the compounds 87 and 88 in Scheme 27's representation of phenoxazine derivatives showed oral effectiveness and in vitro activity, some sort of cytotoxicity was still seen at very low (micromolar) concentrations [85].
Scheme 27.
Phenoxazine-containing antimalarial drug [85]
In order to estimate antimalarial activity, several benzo[a]phenoxazine derivatives were created, with the hope of obtaining the compounds in high yield and with low toxicity [84]. When benzo[a]phenoxazine with 4-aminopyridine incorporation was used as a medication to treat malaria, an IC50 of 7.6nM was recorded against P. falciparum with a selectivity index higher than 7300. The oral administration of the compound to Plasmodium Berghei infected mice at a dose of 100 mg/Kg resulted in the ability to treat malaria, and this was safely supported by severe toxicity testing in mice at single doses of 2000 mg/kg po, chromosome aberration testing, in vivo as well as in vitro micronucleus testing. Ge et al. described a study on SSJ-183, a benzo[a]phenoxazine compound, along with its N-de-ethylated and bis-N,N-deethylated metabolites (Scheme 18). The activity of such benzo[a]phenoxazine class of SSJ-183 compounds against Plasmodium falciparum was reported. The presence of a heteroaromatic ring at the position 6 of such compound was found to be the causative for its rapid action similar to that of artesunate antimalarial medications [84, 85].
Application of phenoxazine as antiviral drug
The number of new virus strains is growing every day and includes COVID-19 as well as the flu, polio, herpes, dengue, ebola and shingles. To combat the problems, proper research must be done on new antiviral agents to treat all of these viral infections [86]. In related research, Iwata et al. discovered the antiviral effects of 2- amino-4,4α-dihydro-4α-7-dimethyl-3H-phenoxazine-3-one (Phx-1), 89 and 3- amino-1,4α-dihydro-4α-8-dimethyl-2H-phenoxazine-2-one (Phx-2), 90 on several viruses including vesicular stomatitis virus (VSV), porcine parvovirus, simian virus-40 (SV-40), herpes simplex virus-1 (HSV-1) and poliovirus [28, 87]. Additional research suggested that Phx-1 and Phx-2 might help to prevent the spread of infections brought on by the poliovirus and porcine parvovirus in future by opening a new pathway for the creation of antiviral medications. When the host cells were previously treated with these phenoxazines, Iwata et al. found that Phx-1 and Phx-2 significantly inhibited the replication of porcine parvovirus and poliovirus. These could be explained by the fact that phenoxazines modulated the host cells' intracellular homeostasis, enhancing the host cells' protective activity against porcine parvovirus and poliovirus. In 2008, Hayashi et al. looked into the antiviral activities of Phx-1, Phx-2 and Phx-3 (2-aminophenoxazine-3-one, 91) against herpes viruses (Scheme 28). Interestingly, all of them prohibited the replication of herpes simplex virus (HSV) [88]. The phenoxazine scaffold was proposed to be frequently used to stabilise nucleic acid duplexes. Even the antiviral activity of a nucleoside analogue could be enhanced by adjusting the size and shape of the aromatic heterocyclic base in addition to the sugar residue. In this regard, it is important to note that Kozlovskaya et al. in [89] reported the synthesis of a wide variety of phenoxazine-based nucleoside compounds and their antiviral activities against a number of viruses that are structurally distinct from one another [89]. According to additional research, 3-(2’-Deoxy-β-D-ribofuranosyl)-1,3-diaza-2- oxophenoxazine, 92 acted as a promising inhibitor of VZV (Varicella-Zoster Virus) DNA replication with superior activity against wild type than thymidine kinase deficient strains (EC50 0.06 and 10 µM, respectively) [90, 91]. Other research involved the synthesis of a new class of 1,3-diaza-2-oxophenoxazine nucleosides with fatty C8–10 alkoxy substituents in the aromatic moiety [90, 91].
Scheme 28.
Representatives of a few antiviral drugs containing phenoxazine moiety [89–91]
Antiviral activities of the newly derived compounds were analysed against both DNA and on a wide array of RNA viruses, including TBEV, Powassan virus (POWV), chikungunya virus (CHIKV, alphavirus), Omsk haemorrhagic fever virus (OHFV) (flaviviruses) and respiratory syncytial virus (RSV, pneumovirus). While this research of Kozlovskaya et al. was going on, the COVID-19 pandemic broke out. A group of Russian scientists isolated SARS-Cov-2 and checked the efficacy of these compounds against such virus [92]. In micromolar concentrations, one of the compounds, 93, demonstrated multiple activities that suppressed both RNA (flavi- and alpha-) and DNA (herpes-) viruses. Rest of the available phenoxazine derivatives were tested against SARS-CoV-2, and compounds with low micromolar activities were identified [91, 92].
Application of phenoxazine as antidiabetic drug
Diabetes mellitus is a polygenic disorder that has affected a large number of people in the recent decades. The main cause of type 2 diabetes is when the pancreas does not produce enough insulin to control blood sugar levels which increases the risk of heart attack, atherosclerosis and hypertension, all of which are insulin-related disorders [93]. In this context, efforts were made to develop a pan-agonist of peroxide proliferator-activated receptors (PPAR) with a heterocyclic scaffold and a side chain containing a sulfonamide moiety with geometry complementary to that of PPAR. Indeglitazar, a compound containing a sulfonamide moiety, was discovered. It was an antidiabetic agent capable of showing PPAR pan activity [94].
Owing to the continued interest in the development of antidiabetic agents, phenoxazine moiety-containing compounds were investigated and found to act as a dual PPAR agonist with convincing anti-hyperglycemic properties. Again, to continue the thread taking Indeglitazar as a lead drug and also incorporating phenoxazine moiety, several sulfonamides were also designed. Ullmann et al. reported the synthesis of 1-nitro-10H-phenoxazine-3-sulfonic acid in 1909 starting from chlorobenzene. However, the proper synthetic route to convert a sulfonic acid unit into the corresponding sulfonamide moiety appeared to be a difficult one to the scientists at the time [95–97]. 1-nitro-10H-phenoxazine-3-sulfonic acid was converted to the corresponding sulfonyl chloride using phosphorus oxychloride in the absence of any solvent. In later times, Pal et al. synthesised and evaluated a large variety of N-(alkyl/aryl/heteroaryl)-1-nitro-10H-phenoxazine-3-sulfonamides, 94 (a–o) from 1-nitro-10H-phenoxazine-3-sulfonyl chloride via an economically synthetic route (Scheme 29) [30, 96].
Scheme 29.
Synthesis of N-(alkyl/aryl/heteroaryl)-1-nitro-10H-phenoxazine-3-sulfonamides [30, 96]
All of the compounds were tested for hypoglycemic, hyperglycemic and oral antidiabetic activity. Among all the derivatives, N-(4-hydroxyphenyl)-1-nitro-10H-phenoxazine-3-sulfonamide exhibited notable antidiabetic activities comparable to glibenclamideone. The compound showed an increase in serum insulin levels, indicating its potential as a novel insulin secretagogue. Rajagopalan et al. reported a phenoxazine analogue of phenyl propanoic acid, Ragaglitazar (DRF 2725) or (2S)-2-ethoxy-3-[4-[2-(10 hphenoxazin-10-yl)ethoxy]phenyl] propanoic acid, 95. It showed dual (PPARα and PPARγ) agonist properties in an in vitro transactivation assay and it was envisioned to restore insulin sensitivity along with correcting diabetic dyslipidemia [93]. The synthetic scheme has been shown below (Scheme 30). Phenoxazine on reaction with p-bromomethoxybenzaldehyde yielded a benzaldehyde derivative that after consecutive steps yielded the corresponding propanoic acid in racemic form. On resolution with (S) (+)-2-phenyl glycinol followed by hydrolysis using 1(M) sulfuric acid, it yielded DRF 2725 in (-) form.
Scheme 30.
Synthetic route to obtain Ragaglitazar [93]
When compared to rosiglitazone, Ragaglitazar demonstrated good oral bioavailability and pharmacokinetic characteristics, establishing the drug as a better antidiabetic agent that lowered plasma glucose and triglyceride levels [98, 99]. Kristensen et al. developed new synthetic methods for labelling Ragaglitazar with carbon-14 and tritium which were later published [99].
Application of phenoxazine as fluorescent probes
Since the discovery of small molecule organic fluorophores in the late nineteenth century, significant attention has been paid to the design and characterisation of such molecules, particularly from the perspective of biochemical and biophysical studies [100, 101]. These are highly valued due to their ability to probe a wide range of micro-environments and unearth important facts in the fields of drug discovery, disease diagnosis, material science, advanced cell biology research, tissue diagnostics, enzyme substrates, molecular biology and detecting environmental contaminants with high sensitivity, selectivity, temporal and spatial resolution by employing light of a specific wavelength. Though fluoresceins, quinines, rhodamines, pyrenes, oxazines, squaraines, coumarins, cyanine dyes, bodipy dyes, ellipticines, carbazoles and other fluorophores are well known, efforts to develop new ones are still ongoing due to their importance of visualising a biophysical or biochemical process [100–112]. In this context, we can discuss Nile red and Nile blue, two organic dyes from the benzo[a]phenoxazine family with high fluorescence and photostability [113–123]. These dyes have been used as histological stains in vitro to image lysosomes and lipids. [123]. They have a high quantum yield and solvent-dependent optical properties, making them an appealing scaffold for the development of pH probes and local polarity indicators [121–123].
The investigation of the effects of solvent on the fluorescence spectra of a variety of organic molecules establishes an important subject area for photophysical research. This is essential for the growth and expansion of solution chemistry [124–129]. Several examples can be cited where different types of solvent molecules have had a significant impact on the behaviour of solute molecules in solution, both in vitro and in vivo. This causes the probe molecule to stabilise, typically through dipolar relaxation. Specific interactions, such as hydrogen bonding, are also frequently responsible for such stabilisations [130–135].
Nile Blue's fluorescence lifetime in ethanol medium was measured ~ 1.42 ns which is comparatively shorter than Nile Red's corresponding value i.e. ~ 3.65 ns. The lifetime of Nile Blue is relatively constant at dilute concentrations (10–3- 10–8 mol dm−3) but increases with concentration and shows different effects in different solvents [114]. This is most likely due to the interdependence between spectroscopic properties and the degree of aggregation in solution. To support this claim, it was discovered that the viscosity of the medium has no effect on the lifetimes; rather, small changes in temperature cause a deviation in the lifetimes [135, 136].
Nile Red's broad applicability stems from its excellent solvatochromism. Both the steady-state and time-resolved emission properties are found to be strongly medium dependent. Fluorescence yield typically increases as the solvent polarity decreases along with a hypsochromic shift [114]. It was also discovered that the fluorescence lifetime of Nile Red is not sensitive to dielectric solvent–solute interactions but decreases significantly as the hydrogen bond donating ability in alcohols increases. The polarity-sensitive fluorescence of Nile red has been attributed to a Twisted Intramolecular Charge Transfer (TICT) state [137–140]. Because of the presence of the flexible diethylamino end group attached to the rigid structure of the molecule, TICT would be possible in this molecule. In most of the TICT molecules, we generally observe dual fluorescence [138, 139]. However, in case of Nile red, no such dual fluorescence is observed though study regarding the existence of the same in methanol–water mixture has been reported. Several observations in various homogeneous and binary solvent mixtures lead to the conclusion that Nile Red can act as a solvatochromic and fluorescence anisotropy probe [114].
Nile blue, on the other hand, a renowned DNA binding probe, along with others in the same family of benzo[a]phenoxazinium dyes, has been found to be selectively localised in animal tumours. The planar hydrophobic phenoxazine moiety of Nile Blue is expected to easily intercalate between the DNA bases, with the positive charge neutralised by the phosphate groups of the DNA. When compared to other conventional intercalators such as Ethidium bromide, the use of Nile blue has the advantage of having low toxicity and comparable sensitivity for DNA quantification. The photophysics of Nile blue in pure solvents is well documented in the literature [141–143], and there are also some quantum mechanical calculations [144]. Both steady-state and ultrafast time-resolved spectroscopic studies led to the conclusion that Nile blue exhibits either an intermolecular electron transfer process around 100 fs in electron-donating solvents or an intermolecular proton transfer process in the range of a few ps in hydrogen bond accepting solvents [142–144].
An overview of the photophysical properties of some cationic phenoxazine derivatives (Scheme 31) is worth noting here. Table 1 shows the maximum absorption and emission wavelengths, as well as the fluorescence quantum yield, ΦF, for both ethanol and water at pH 7.4.
Scheme 31.
Representative chemical structure of a few studied compounds [114]
Table 1.
Photophysical properties of cationic phenoxazine derivatives in ethanol and water (pH ~ 7.4)
| Compound | Ethanolic medium | Aqueous medium | ||||
|---|---|---|---|---|---|---|
| Maximum absorption wavelength (nm) | Maximum emission wavelength (nm) | Fluorescence quantum yield ΦF | Maximum absorption wavelength (nm) | Maximum emission wavelength (nm) | Fluorescence quantum yield ΦF | |
| A | 500 nm | 612 nm | 0.051 | 640 nm | 682 nm | 0.10 |
| B | 625 nm | 669 nm | 0.110 | 635 nm | 682 nm | 0.094 |
| C | 633 nm | 620 nm | 0.053 | 640 nm | 678 nm | 0.080 |
| D | 515 nm | 643 nm | 0.0017 | – | – | – |
| E | 625 nm | 661 nm | 0.19 | – | – | – |
| F | 638 nm | 618 nm | 0.049 | 650 nm | 685 nm | 0.065 |
| G | 635 nm | 666 nm | 0.225 | 645 nm | 684 nm | 0.080 |
| H | 520 nm | 622 nm | 0.0022 | – | – | – |
| I | 625 nm | 644 nm | 0.44 | 625 nm | 652 nm | 0.28 |
| J | 630 nm | 643 nm | 0.50 | 625 nm | 654 nm | 0.32 |
| K | 615 nm | 643 nm | 0.49 | 620 nm | 650 nm | 0.19 |
The position of the absorption maximum for compound A shifts dramatically from ~ 500 nm (in ethanol) to ~ 640 nm (in water). The common solvatochromic effect on π–π∗ electronic transitions could explain this fact. The observed shift value of 140 nm is too large. It is indeed true that most of the compounds except A, D, G and L exhibit maximum absorbance near ~ 600 nm in ethanol medium. Again, charged dyes are known to aggregate in water due to the high dielectric constant of water which reduces electrostatic repulsion. The same class of compounds, oxazine dyes, have been shown to form H-aggregates [145]. This type of dimer has an absorption band similar to the monomer's blue absorption band and a very low fluorescence quantum yield. Variations in dimerization equilibrium constants with the solvent can then explain the observed differences in absorption maximum. To validate the proposition, the absorption spectra of compound A at various concentrations in ethanol and water were measured. Approximately, 40 nm separation between the monomer and dimer absorption maxima indicated the formation of H-aggregates in water. However, the substituents present in benzo[a]phenoxazine control how much dimerization occurs. The predomination of the blue (500 nm) absorption band is observed in solvents with high hydrogen bond accepting capacity (acetone, DMF, dioxane and ethyl acetate) indicating that the solvents interact with such benzo[a]phenoxazinium dyes either by accepting a hydrogen bond or by completely removing the amine proton leading to the corresponding base. An interesting observation experienced by Douhal et al. in [141]. They demonstrated that for Nile Blue, the hydrogen bond interaction results in a ∼5 nm hypsochromic shift. The complete removal of the proton from the amine resulted in the formation of a new band shifted ∼100 nm to the blue. Thus, the band near 500 nm can be assigned to the basic form of the studied compounds [141].
We have seen the importance of organised assemblies on biological and photophysical processes over the last three decades [131, 133, 146–149]. Because of their applications in assorted biophysical and biochemical processes, there has been a developing interest in the study of various photoprocesses occurring in electronically excited molecules in various organised microheterogeneous environments such as micelles, reverse micelles, cyclodextrins and so on [146–149]. The importance of such studies lies in the comprehension of various confining environments and their ability to influence various photoprocesses. When reactants are accommodated in molecular assemblies such as micelles, reverse micelles and so on, they achieve a higher degree of organisation than when they are in homogeneous solution. These organised assemblies can mimic bio-system reactions and have the potential for energy storage. The interaction of Nile Blue with micelles and genomic DNA has recently been reported using its photophysical behaviour [150]. Previous studies of Nile Red in biophysically interesting systems included sodium dodecylbenzenesulfonate micelles [151], Triton X-100 reverse micelles [152], DNA on gold electrodes with the potential for DNA sensor development [153] and DNA intercalation with the potential for electrophoresis gel detection [154]. The key findings were that Nile Red exhibits acid–base equilibrium with a neutral and basic form coexisting. It frequently undergoes aggregations in negative surfaces and it interacts with DNA via both intercalative and electrostatic binding [153–156].
In order to identify hypoxic cells in solid tumours, Wilson et al. synthesised a new class of bioreductive and long-wavelength fluorescent markers. Phenoxazine was utilised as the fluorescent matrix in a related study of such fluorescent probes [157]. Again, BPO-N3, 96 which had a high quantum yield and the capacity to detect H2S, was synthesised by Lv et al. using resorcinol (Scheme 32) [158].
Scheme 32.
Synthetic route to obtain BPO-N3 [158]
The findings showed extremely high selectivity and sensitivity to exogenous and endogenous H2S in living cells with a detection limit of 30 nM [158]. With the addition of H2S, the spectral characteristics of the probe were examined (maximum absorption is observed at 600 nm). Additionally, the effects of pH and temperature were also looked into.
Long-wavelength fluorescent amino acid bioconjugates were reported by Frade et al. using a series of side chain carboxylated 5,9-diaminobenzo[a]phenoxazinium salts [159]. Alves et al. synthesised fluorescent benzo[a]phenoxazinium chlorides in a related study that can recognise the micellization process [160]. Although hypochlorous acid (HOCl) is crucial for biological processes, uncontrolled oxidation can harm tissues and lead to cell inflammation. For the purpose of detecting hypochlorous acid, another novel near-infrared (NIR) fluorescent probe, BR-1, 97, was developed by connecting the N,N-dimethyl-thiocarbonyl (DMTC) group to the phenoxazine framework in a one-pot reaction (Scheme 33).
Scheme 33.
Synthesis of BR-1 to detect HOCl [161]
BR-1 is powerful enough to detect endogenous HOCl in the inflammation area of arthritis mouse models [161]. Even the molecule is capable of displaying anti-interference against Hg2+/Hg2+ + H2O2 when DMTC is connected to the phenoxazine skeleton.
In 2017, Chadar et al. synthesized a heterocyclic aromatic fluorescent benzo[a]phenoxazine namely, 6-methyl-9-nitro-5Hbenzo[a]phenoxazin-5-one, 100a and 6-methyl-10-nitro-5H-benzo[a]phenoxazin-5-one, 100b from Vitamin K3, 98 and nitro aminophenol, 99 (Scheme 34) [162].
Scheme 34.
Synthesis of 6-methyl-9-nitro-5Hbenzo[a]phenoxazin-5-one and 6-methyl-10-nitro-5H-benzo[a]phenoxazin-5-one from Vitamin K3 and nitro aminophenol [162]
Phenoxazine as organic light-emitting diodes
Since their initial introduction in 1987 at Eastman Kodak, organic light-emitting diodes (OLEDs) have advanced significantly. Due to their promising qualities, OLEDs are now highly prized in the display and lighting industries. OLED device technology has advanced steadily over the past 30 years as one of the research areas that both motivates and inspires advancement in academia and industry. These are optoelectronic devices whose working abilities are based on the electroluminescent properties of π-conjugated organic materials [163]. Working ability and durability of OLEDs have been improved by the incorporation of emissive materials having very high luminescence quantum yield which is also capable of accepting both electrons and holes [164, 165]. Several thermally stable N, N’-dialkyl and -diaryl substituted 1,4-benzoxazino[2,3-b]phenoxazines were synthesized by Okamoto et al. [163]. These compounds had a low oxidation potential ranging from + 0.30 to + 0.41 V vs SCE (Standard Calomel Electrode). The N,N′-dimethyl derivative produced a stable radical cation salt or low conductive charge transfer complex via electrochemical oxidation with tetracyanoquinomethane (TCNQ). N,N'-diaryl compounds are used in organic light-emitting diodes as hole injection materials (HIMs) because of their high thermal stability which allows to test the device's performance by measuring its luminous efficiency and decay time at a high current density (50 mA cm−1). The results showed that naphthyl containing N,N′-bis-aryl compounds containing gave the best results when compared to a reference device of similar composition without HIM.
According to recent studies, adding a second 2D layer increases the device's stability by up to 5 times whereas also significantly increasing emitting efficiency of the same. Researchers' interest in metal-free thermally activated delayed fluorescence (TADF)-based fluorophores has recently increased because these entirely organic materials for organic light-emitting diodes (OLEDs) can incorporate both singlet (S1) and triplet (T1) excited states for luminescence through an effective reverse intersystem crossing (RISC) process, leading to a theoretical maximum internal quantum efficiency (IQE) of 100 percent.
Wang et al. designed the synthesis of highly effective orange TADF diodes to advance research in this area [164]. The emitter, 7,10- bis(phenoxazine)-2,3-dicyanopyrazinophenanthrene (PXZ-DCPP) 104 was synthesized by using phenoxazine (PXZ), 103 as the electron donor unit and 2,3-dicyanopyrazinophenanthrene (DCPP), 102 as the electron acceptor unit (Scheme 35) [164].
Scheme 35.
Synthesis of PXZ-DCPP [164]
The lowest singlet and triplet states have a very small energy gap, resulting in a relatively long fluorescence lifetime. In related studies, Zhu et al. developed novel phenoxazine-based compounds like 107 by incorporating phenyl quinoline moiety via Friedlander condensation (Scheme 36). Later, they reported that those compounds had improved emitting characteristics [165].
Scheme 36.
Synthesis of phenoxazine via incorporation of phenylquinoline [165]
Pure compounds were produced using column chromatography and recrystallised from benzene. By using 1H NMR, mass spectrometry, Fourier transform infrared spectroscopy and X-ray single crystal analysis, their molecular structures were identified [166–169]. Tavgeniene et al. synthesised a thermally stable phenoxazine framework with a number of new compounds for use as potential host materials for green phosphorescent OLEDs [167, 168]. When the host 3-[bis(9-ethylcarbazol-3-yl)methyl]-10-hexylphenoxazine, 110 is used, the device performs better with an external quantum efficiency of more than 5.9%, a maximum current efficiency of 18.3 cd/A, a maximum brightness of 5366 cd/m2 and a low turn-on voltage of 3.1 V (Scheme 37).
Scheme 37.
OLED formation via incorporating carbazole skeleton [167]
The carbozolyl moiety based on phenoxazine has the capacity to form stable amorphous films which lessens the impact of concentration quenching [168, 170, 171]. Triplet emitters are used as emitting guests in phosphorescent devices to decrease quenching in accordance with the relatively long excited state lifetimes of triplet emitters in a host material.
For next-generation full-colour displays with great colour purity, multi-resonance thermally activated delayed fluorescence (MR-TADF) emitters with narrowband emissions based on a boron/nitrogen (B/N) framework are essential [172–174]. Based on a skeleton of phenoxazine units and amplification at the para-position of the boron atom by various electron-donating groups (phenoxazine/tert-butylcarbazole), Hu et al. devised a straightforward molecular design technique for MR-TADF materials. TPXZBN and DPXZCZBN, two novel phenoxazine-fused MR-TADF materials, displayed green emissions with sharp peaks at 502 nm and 500 nm, respectively, along with a narrow full-width at half maximum (FWHM) bandwidths of 33 nm and 32 nm (Scheme 38) [175]. Small singlet–triplet state energy gaps (ΔEsts) of 0.16 eV and 0.13 eV are also present in TPXZBN and DPXZCZBN, respectively, as well as high photoluminescence quantum yields (PLQYs) of 91% and 90% in toluene solutions and 99% and 94% in doped films. Scheme 38 has shown the representative structures of TPXZBN and DPXZCZBN and the corresponding designing of the molecules. Synthetic strategy of TPXZBN and DPXZCZBN has been depicted in Scheme 39.
Scheme 38.
Representative structures of TPXZBN and DPXZCZBN and molecular designing [175]
Scheme 39.
Synthetic procedure towards TPXZBN and DPXZCZBN [175]
Kulszewicz-Bajer et al. in the year 2022 developed and synthesised six acridone (quinacridone) derivatives with carbazole or phenoxazine substituents to investigate the influence of the donor (D) and acceptor (A) connecting pattern (D–A, D–A–D, or D–π–A–π–D) on their photophysical and electrochemical capabilities (Scheme 40) [176]. These novel electroactive compounds have reversible electrochemical oxidation with high luminescence. The electrochemically determined ionisation potentials (IPs) of these compounds range from 5.09 to 5.45 eV, with carbazole derivatives having higher IPs than phenoxazine derivatives. With the exception of the quinacridone derivative, which has an EA of − 3.03 eV, the measured electron affinities (EAs) range from − 2.53 to − 2.64 eV. Their vacuum-deposited films radiate throughout a broad spectrum, from sky-blue to red. The carbazole-containing compounds (a, b, e) displayed immediate fluorescence and aggregation-induced quenching. When taken in toluene solution, their photoluminescent quantum yields reached up to 69%. Phenoxazine-containing compounds (c, d, f) showed aggregation-induced emission enhancement and thermally activated delayed fluorescence.
Scheme 40.
Representative structures of six acridone (quinacridone) derivatives with carbazole or phenoxazine substituents [176]
Phenoxazines as photoredox catalyst
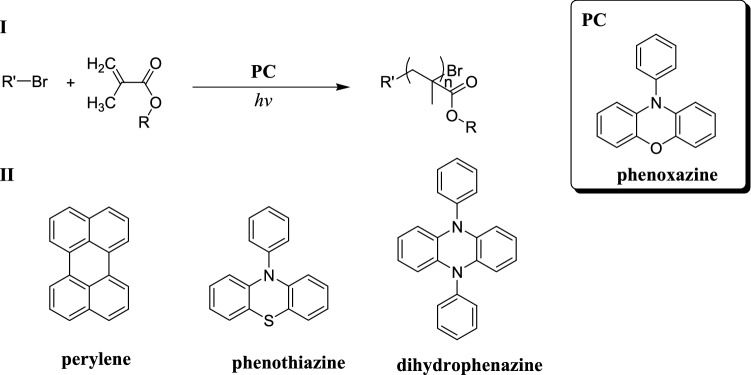
The development of polymerization techniques reveals that atom transfer radical polymerization (ATRP) is the most used controlled radical polymerization (CRP) for the synthesis of polymers with controlled molecular weight (MW), dispersity (Đ), architecture and composition. In the past, performing controlled polymerizations correctly was difficult enough due to the extremely demanding reaction conditions that limited their utility for industrial use [34, 177–179]. Organocatalysed atom transfer radical polymerization (O-ATRP), a recently discovered controlled polymerization method, uses organic photoredox catalysts (PC) to synthesise polymers under mild, metal-free conditions. O-ATRP catalyst radical cations were synthesised and characterised by Corbin et al. using spectroscopic, electrochemical and X-ray diffraction techniques [177]. The reactivity of these compounds in solution, in deactivation model reactions and in O-ATRP, was investigated by the same research group to better understand their role and potential side reactions in O-ATRP. They even discovered that under the suitable conditions, these compounds can be reactive from both the ground and photoexcited states. However, the mechanism of this excited state reactivity is not properly known and further research into radical cation photophysics is required to fully comprehend this intriguing phenomenon (Scheme 41).
Scheme 41.

Proposed mechanism of O-ATRP and the species involved [177]
The ability of one PC•+ to deactivate alkyl radicals was demonstrated using a deactivation model reaction by identifying the expected deactivation product. This model reaction was then used to investigate the effect of various factors on deactivation kinetics, such as halide identity or PC•+ structure. These experiments ultimately yielded a few major conclusions like (a) In O-ATRP, the most likely deactivator is PC•+Br−; (b) the deactivation mechanism is a concerted one involving a bimolecular reaction of PC•+Br− with the propagating radical; (c) since Cl− is more challenging to oxidize, deactivation with Br− is comparatively faster. In another significant study, made by Pearson et al. in [34], they reported the synthesis of N-Aryl phenoxazines [34]. Through the use of structure–property relationships, N-Aryl phenoxazines have been proven to be effective PCs for O-ATRP and other photoredox-catalysed small molecule and polymer syntheses (Scheme 42).
Scheme 42.
I O-ATRP mediated by organic PCs using alkyl bromide initiators and aryl phenoxazines. II Organic PCs investigated previously [34]
It can absorb light in the visible range (400–700 nm) and produce polymers with controlled molecular weights with a very low disparity ranging from 1.13 to 1.31, outperforming other organic PCs for O-ATRP that have been previously reported.
Again, six phenoxazine compounds [112 (a–f)] were examined by Kientz et al. in 2022 as visible light photosensitizers (Scheme 43) [180]. Such photosensitizers were used for photochemical conversion of CO2 to CO in organic medium with an iron porphyrin catalyst. To functionalize the phenoxazine core, electron-donating or -withdrawing groups were utilised. Photophysical properties were thereby modified. Both the singlet and triplet excited state potentials of sensitizers spanned a wide range of several hundred mV. They discovered that there is no relationship between CO production and the excited state potential of the phenoxazine, which governs the catalyst's ability to transfer electrons. On the other hand, a direct relationship was established between the phenoxazine's oxidation potentials and the formation of CO. This finding implied that the procedure was hampered by the regeneration of photosensitizers and emphasised the fact that electron transfers that are unrelated to catalyst activation could be important in homogenous photocatalytic systems.
Scheme 43.
Representative structures of the phenoxazine-based sensitizers [180]
Application of phenoxazines in dye-sensitized solar cells
Due to their potential lighting applications, such as solid-state lighting [94], full-colour flexible flat-panel displays, as well as alternatives to liquid–crystal displays, electroluminescent devices, such as fluorescent and phosphorescent organic light-emitting diodes (FOLEDs & PHOLEDs) have attracted enormous academic and commercial interest in recent years [181, 182]. Perylenes [183], carbazoles [184], triarylamine [185], indoline [186] and several other chromophores [33, 186–190] act as the donating groups in the various metal-free organic dyes that have recently been developed. These dyes have some potential use in dye-sensitised solar cells (DSSC) because they are simple to make, environmentally friendly and have higher molar extinction coefficients. Hetero-anthracenes, specifically phenoxazine and phenothiazine, have also been discovered to exhibit better utility in DSSC due to the extremely strong electron-donating capacity and simple structure elucidation [33]. However, designing of organic dyes that are more effective still presents a difficult task. According to some recent research, intramolecular charge transfer (ICT) and energy transfer (EnT) processes in dyes have a beneficial impact on enhancing the performance of DSSC. According to research by Haque and colleagues, the antenna group of hole transport materials (HTM) can actually reduce the rate of recombination between photoinjected electrons and oxidised HTM by spatially separating the holes from the electrons in the semiconductor, increasing the effectiveness of DSSC.
Based on these studies, Tian et al. designed and produced a novel organic dye (TH305) in 2009 by modifying a basic phenoxazine dye (TH301), with a triphenylamine energy antenna for use in a dye-sensitized solar cell with a notable overall conversion efficiency of 7.7% [33]. In a related development, Tan et al. synthesised phenoxazines 113 (a-d) (Scheme 44) in 2013 with applications to dye-sensitised solar cells and conversion efficiencies of 6.6 percent, 7.8 percent, 7.1 percent and 6.4 percent under simulated AM 1.5G conditions [189].
Scheme 44.
Structures of the compounds TH305, TH301 and 113 (a–d) [189]
Karlsson et al. [32] created a series of metal-free core phenoxazine chromophores (MP03, MP05, MP08, MP12 and MP13) for dye-sensitized solar cells (Schemes 45, 46, 47).
Scheme 45.
Synthetic pathway to the dyes, MP03 and MP13 [32]
Scheme 46.
Synthetic route to obtain dye, MP08 [32]
Scheme 47.
Synthetic route to obtain dyes, MP05 and MP12 [32]
Under standard AM1.5G illumination at a light intensity of 100 mW cm−2, overall conversion efficiencies of 6.03–7.40% were achieved when examining sensitizers for DSSC applications. Dyes with a furan-conjugated linker had a shorter lifetime than dyes with the acceptor group attached directly to the phenoxazine [32]. Addition of an extra donor unit containing insulating alkoxyl chains in the 7-position of the phenoxazine increased the lifetime further leading to an open circuit voltage of 800 mV if used in conjunction with the additives in the electrolyte to raise the conduction band [32]. Nowadays, phenoxazine compounds are designed and synthesised for dye-sensitised solar cells where they serve as a link between the electron-rich thiophene derivatives that serve as antennas and cyanoacrylic acid which serves as the acceptor [190].
Again, Ooyama et al. reported the synthesis of a few donor–acceptor π-conjugated benzofuro[2,3-c]oxazolo[4,5-a]carbazole-based fluorescent dyes, 127, 128, 129 and 130 (Scheme 48) [191]. In all those structures, a carboxyl group was present at the different positions of the chromophoric unit. The fluorescent dyes' absorption and fluorescence spectra, as well as their cyclic voltammograms, agree very well, indicating that the position of the carboxyl group has no significant effect on their photophysical and electrochemical possessions. However, when these dyes are used in dye-sensitized solar cells, their photovoltaic performances differ significantly.
Scheme 48.
Representative structures of benzofuro[2,3-c]oxazolo[4,5-a]carbazole-based fluorescent dyes, 127, 128, 129 and 130 [191]
Conclusions and future directions
Phenoxazine and benzo[a]phenoxazine-based molecules are renowned for their anti-alzheimer, muscle-relaxing, antibiotic, anti-inflammatory, anti-tubercular, antioxidant and antimicrobial properties. They also possess antimalarial, anticancer, antidiabetic and antiviral possessions. The skeleton also finds its importance in industrial applications and material science. It is worth noting that the majority of the synthetic process is made up of simple condensation reactions followed by cyclization. When a catalyst is present, however, some reactions exhibit coupling mechanisms. Since 2014, a greener approach to its synthesis has been used and it has been discovered that the reaction yield is significantly higher than that of other conventional methods while also being more time-efficient. The likelihood of discovering straightforward, highly efficient chemotherapeutic agents that can be commercialised has increased as a result of recent systematic SAR studies on the phenoxazine motif. Notably, there are phenoxazine-based antimalarial drug candidates currently on the market with parameters for in vivo, in vitro and bioavailability that are comparable to chloroquine. Actinomycin D used to be outperformed by synthetic antitumor phenoxazine derivatives but they now have potency comparable to that of doxorubicin, a well-known antineoplastic drug used to treat a number of cancers. Further research into the mechanism of action of phenoxazine is necessary, especially in the light of its antimalarial activity against Plasmodium falciparum, as this information would serve as a solid foundation for improved drug development. Although the incorporation of emissive materials with phenoxazine as the electron donor unit and electron acceptor units such as 2,3-dicyanopyrazinophenanthrene (DCPP) and phenylquinoline has improved the performance and durability of organic light-emitting diodes, more research would produce emitters and sensitised solar cells with higher efficiencies and quantum yields.
More focussed SARs investigations of promising lead compounds would be beneficial for the use of phenoxazine compounds in the search for phenoxazine-based chemotherapies and industrial materials, opening doors to addressing some of the global sustainability goals. The synthetic field is certain to discover more inventive and sympathetic novel strategies using phenoxazines in multicomponent reactions to efficiently build new heterocyclic scaffolds that will lead to significant advances in synthetic organic chemistry or a shift towards biosynthesis due to less ambiguity of procedural requirements, even in conventional methods.
Acknowledgements
The authors would like to acknowledge Prof. Ashutosh Ghosh, the honourable Vice-Chancellor of the Rani Rashmoni Green University, West Bengal, India for his continuous support and encouragement to this work. Amrit Krishna Mitra, the corresponding author of this manuscript would like to acknowledge the financial assistance provided by the Department of Science & Technology and Biotechnology, Government of West Bengal, India (Memo No. 100(Sanc.)/STBT-11012(25)/5/2019-ST SEC dated 28/04/2022). The authors are extremely thankful to Ms. Sayantani Mitra, Senior Teacher, Department of English, Sushila Birla Girls’ School, Kolkata, India, for her valuable suggestions regarding enhancing the quality of the English language of this manuscript. Both the authors appreciate the interest and encouragement of Mr. Subhendu Adhikari and Mr. Subhadip Sinharoy of Rani Rashmoni Green University, West Bengal, India. The authors also declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gomtsyan A. Heterocycles in drugs and drug discovery. Chem Heterocycl Compd. 2012;48(1):7–10. doi: 10.1007/s10593-012-0960-z. [DOI] [Google Scholar]
- 2.Zhang TY (2017) The evolving landscape of heterocycles in drugs and drug candidates. In: Advances in heterocyclic chemistry. Academic Press, vol 121, pp 1–12 10.1016/bs.aihch.2016.05.001
- 3.Broughton HB, Watson IA. Selection of heterocycles for drug design. J Mol Graph Model. 2004;23(1):51–58. doi: 10.1016/j.jmgm.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AP, Robinson RP, Fobian YM, Blakemore DC, Jones LH, Fadeyi O. Modern advances in heterocyclic chemistry in drug discovery. Org Biomol Chem. 2016;14(28):6611–6637. doi: 10.1039/C6OB00936K. [DOI] [PubMed] [Google Scholar]
- 5.Li JJ, editor. Heterocyclic chemistry in drug discovery. New York: Wiley; 2013. [Google Scholar]
- 6.Heravi MM, Zadsirjan V. Prescribed drugs containing nitrogen heterocycles: an overview. RSC Adv. 2020;10(72):44247–44311. doi: 10.1039/D0RA09198G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amini-Rentsch L, Vanoli E, Richard-Bildstein S, Marti R, Vilé G. A novel and efficient continuous-flow route to prepare trifluoromethylated N-fused heterocycles for drug discovery and pharmaceutical manufacturing. Ind Eng Chem Res. 2019;58(24):10164–10171. doi: 10.1021/acs.iecr.9b01906. [DOI] [Google Scholar]
- 8.Ionescu M, Mantsch H (1967) Phenoxazines. In: Advances in heterocyclic chemistry. Academic Press Doi: 10.1016/S0065-2725(08)60604-2
- 9.Hiscock LK, Maly KE, Dawe LN. Crystal packing of a series of 1, 2, 3, 4-substituted phenoxazine and dibenzodioxin heterocycles. Cryst Growth Des. 2019;19(12):7298–7307. doi: 10.1021/acs.cgd.9b01184. [DOI] [Google Scholar]
- 10.Katsamakas S, Zografos L, Zografos AV. Advances of phenoxazines: Synthesis, reactivity and their medicinal applications. Curr Med Chem. 2016;23(26):2972–2999. doi: 10.2174/0929867323666160510123023. [DOI] [PubMed] [Google Scholar]
- 11.Bernthsen A. Ueber ein neues Chromogen, das Phenazoxin. Ber Dtsch Chem Ges. 1887;20(1):942–944. doi: 10.1002/cber.188702001214. [DOI] [Google Scholar]
- 12.Hughes N (1964) Phenazine, oxazine, thiazine and sulphur dyes. In: Rodd's chemistry of carbon compounds. Elsevier, pp. 403–43610.1016/B978-044453345-6.50793-8
- 13.Onoabedje EA, Ayogu JI, Odoh AS. Recent development in applications of synthetic phenoxazines and their related congeners: a mini-review. ChemistrySelect. 2020;5(28):8540–8556. doi: 10.1002/slct.202001932. [DOI] [Google Scholar]
- 14.Motohashi N, Mitscher LA, Meyer R. Potential antitumor phenoxazines. Med Res Rev. 1991;11(3):239–294. doi: 10.1002/med.2610110302. [DOI] [PubMed] [Google Scholar]
- 15.Shukla S, Dwivedi J, Yaduvanshi N, Jain S. Medicinal and biological significance of phenoxazine derivatives. Mini Rev Med Chem. 2021;21(12):1541–1555. doi: 10.2174/1389557520666201214102151. [DOI] [PubMed] [Google Scholar]
- 16.Vennerstrom JL, Makler MT, Angerhofer CK, Williams JA. Antimalarial dyes revisited: xanthenes, azines, oxazines and thiazines. Antimicrob Agents Chemother. 1995;39(12):2671–2677. doi: 10.1128/AAC.39.12.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takasu K. π-Delocalized lipophilic cations as new candidates for antimalarial, antitrypanosomal and antileishmanial agents: synthesis, evaluation of antiprotozoal potency and insight into their action mechanisms. Chem Pharm Bull. 2016;64(7):656–667. doi: 10.1248/cpb.c16-00234. [DOI] [PubMed] [Google Scholar]
- 18.Ashley EA, Phyo AP. Drugs in development for malaria. Drugs. 2018;78(9):861–879. doi: 10.1007/s40265-018-0911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takasu K, Shimogama T, Satoh C, Kaiser M, Brun R, Ihara M. Synthesis and antimalarial property of orally active phenoxazinium salts. J Med Chem. 2007;50(10):2281–2284. doi: 10.1021/jm070201e. [DOI] [PubMed] [Google Scholar]
- 20.Hollstein U. Actinomycin: chemistry and mechanism of action. Chem Rev. 1974;74(6):625–652. doi: 10.1021/cr60292a002. [DOI] [Google Scholar]
- 21.Fink E, Goldenberg DM. Antimalarial activity of actinomycin D and cyclophosphamide. Proc Soc Exp Biol Med. 1969;132(1):165–167. doi: 10.3181/00379727-132-34172. [DOI] [PubMed] [Google Scholar]
- 22.Achenbach H, Blümm E. Investigation of the Pigments of pycnoporus sanguineus-pycnosanguin and new phenoxazin-3-ones. Arch Pharm. 1991;324(1):3–6. doi: 10.1002/ardp.19913240103. [DOI] [Google Scholar]
- 23.Qin JP, Xu XJ, Wu LZ, Ling ZJ, Pu QL. Structure elucidation of two compounds from Peristrophe roxburghiana. Acta Pharmaceutica Sinica. 1999;34:599–603. [Google Scholar]
- 24.Thuy TT, et al. Natural phenoxazine alkaloids from Peristrophe bivalvis (L.) Merr. Biochem Systemat Ecol. 2012;44:205. doi: 10.1016/j.bse.2012.05.009. [DOI] [Google Scholar]
- 25.Evitasari RT, Rahayuningsih E, Mindaryani A (2019) Dyeing of cotton fabric with natural dye from peristrophe bivalvis extract. In: AIP Conference Proceedings. AIP Publishing LLC, 2085(1), pp 020055) Doi: 10.1063/1.5095033
- 26.Thimmaiah KN, Horton JK, Seshadri R, Israel M, Houghton JA, Harwood FC, Houghton PJ. Synthesis and chemical characterization of N-substituted phenoxazines directed toward reversing vinca alkaloid resistance in multidrug-resistant cancer cells. J Med Chem. 1992;35(18):3358–3364. doi: 10.1021/jm00096a009. [DOI] [PubMed] [Google Scholar]
- 27.Mayur YC, Jagadeesh S, Thimmaiah KN. Targeting calmodulin in reversing multi drug resistance in cancer cells. Mini Rev Med Chem. 2006;6(12):1383–1389. doi: 10.2174/138955706778993021. [DOI] [PubMed] [Google Scholar]
- 28.Iwata A, Yamaguchi T, Sato K, Yoshitake N, Tomoda A. Suppression of proliferation of poliovirus and porcine parvovirus by novel phenoxazines, 2-amino-4, 4α-dihydro-4α-7-dimethyl-3H-phenoxazine-3-one and 3-amino-1, 4α-dihydro-4α-8-dimethyl-2H-phenoxazine-2-one. Biol Pharm Bull. 2005;28(5):905–907. doi: 10.1248/bpb.28.905. [DOI] [PubMed] [Google Scholar]
- 29.Kato S, et al. Anticancer effects of phenoxazine derivatives combined with tumor necrosis factor-related apoptosis-inducing ligand on pancreatic cancer cell lines, KLM-1 and MIA-PaCa-2. Oncol Rep. 2006;15(4):843–848. doi: 10.3892/or.15.4.843. [DOI] [PubMed] [Google Scholar]
- 30.Reddy SV, et al. Construction of phenoxazine rings containing nitro and sulfonic acid groups leading to phenoxazine-3-sulfonamide derivatives: their evaluation as novel and potential insulin secretagogues. MedChemComm. 2014;5(5):587–592. doi: 10.1039/C3MD00377A. [DOI] [Google Scholar]
- 31.Yang XB, Yang BX, Ge JF, Xu YJ, Xu QF, Liang J, Lu JM. Benzo [a] phenoxazinium-based red-emitting chemosensor for zinc ions in biological media. Org Lett. 2011;13(10):2710–2713. doi: 10.1021/ol2008022. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson KM, Jiang X, Eriksson SK, Gabrielsson E, Rensmo H, Hagfeldt A, Sun L. Phenoxazine dyes for dye-sensitized solar cells: relationship between molecular structure and electron lifetime. Chemistry A. 2011;17(23):6415–6424. doi: 10.1002/chem.201003730. [DOI] [PubMed] [Google Scholar]
- 33.Tian H, et al. Tuning of phenoxazine chromophores for efficient organic dye-sensitized solar cells. Chem Commun. 2009;41:6288–6290. doi: 10.1039/B912746A. [DOI] [PubMed] [Google Scholar]
- 34.Pearson RM, Lim CH, McCarthy BG, Musgrave CB, Miyake GM. Organocatalyzed atom transfer radical polymerization using N-aryl phenoxazines as photoredox catalysts. J Am Chem Soc. 2016;138(35):11399–11407. doi: 10.1021/jacs.6b08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frade VH, Sousa MJ, Moura JC, Gonçalves MST. Synthesis, characterisation and antimicrobial activity of new benzo [a] phenoxazine based fluorophores. Tetrahedron Lett. 2007;48(47):8347–8352. doi: 10.1016/j.tetlet.2007.09.108. [DOI] [Google Scholar]
- 36.Bag SS, Ghorai S, Jana S, Mukherjee C. Solvatochromic fluorescent cyanophenoxazine: design, synthesis, photophysical properties and fluorescence light-up sensing of ct-DNA. RSC Adv. 2013;3(16):5374–5377. doi: 10.1039/C3RA23463K. [DOI] [PubMed] [Google Scholar]
- 37.Thome I, Bolm C. Transition-metal-free intramolecular N-arylations. Org Lett. 2012;14(7):1892–1895. doi: 10.1021/ol3005134. [DOI] [PubMed] [Google Scholar]
- 38.Bielawski M, Aili D, Olofsson B. Regiospecific one-pot synthesis of diaryliodonium tetrafluoroborates from arylboronic acids and aryl iodides. J Org Chem. 2008;73(12):4602–4607. doi: 10.1021/jo8004974. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt DM, Bonvicino GE. The halogen-activated Smiles rearrangement 2. J Org Chem. 1984;49(9):1664–1666. doi: 10.1021/jo00183a039. [DOI] [Google Scholar]
- 40.Kervefors G, Becker A, Dey C, Olofsson B. Metal-free formal synthesis of phenoxazine. Beilstein J Org Chem. 2018;14(1):1491–1497. doi: 10.3762/bjoc.14.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eastmond GC, Gilchrist TL, Paprotny J, Steiner A. Cyano-activated fluoro displacement reactions in the synthesis of cyanophenoxazines and related compounds. New J Chem. 2001;25(3):385–390. doi: 10.1039/B008503K. [DOI] [Google Scholar]
- 42.Bonvicino GE, Yogodzinski LH, RHardy RA. Synthesis of 2-chloro-10-(3-dimethylaminopropyl) phenoxazine:(a) the o-phenoxyaniline route; (b) a Modification of the turpin reaction. J Org Chem. 1961;26(8):2797–2803. doi: 10.1021/jo01066a042. [DOI] [Google Scholar]
- 43.Crivello JV. Benzophenothiazine and benzophenoxazine photosensitizers for triarylsulfonium salt cationic photoinitiators. J Polym Sci Part A. 2008;46(11):3820–3829. doi: 10.1002/pola.22731. [DOI] [Google Scholar]
- 44.Mickevičienė K, Baranauskaitė R, Kantminienė K, Stasevych M, Komarovska-Porokhnyavets O, Novikov V. Synthesis and antimicrobial activity of N-substituted-β-amino acid derivatives containing 2-hydroxyphenyl, benzo [b] phenoxazine and quinoxaline moieties. Molecules. 2015;20(2):3170–3189. doi: 10.3390/molecules20023170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okafor CO. Synthesis, properties and uses of angular phenoxazines. Dyes Pigm. 1986;7(2):103–131. doi: 10.1016/0143-7208(86)85003-3. [DOI] [Google Scholar]
- 46.Jose J, Burgess K. Syntheses and properties of water-soluble Nile Red derivatives. J Org Chem. 2006;71(20):7835–7839. doi: 10.1021/jo061369v. [DOI] [PubMed] [Google Scholar]
- 47.Jose J, Burgess K. Benzophenoxazine-based fluorescent dyes for labeling biomolecules. Tetrahedron. 2006;62(48):11021–11037. doi: 10.1016/j.tet.2006.08.056. [DOI] [Google Scholar]
- 48.Ezeokonkwo MA, Ugwuona FO, Ugwu IC. Synthesis and antibacterial studies of some alkynylated benzo [a] phenoxazin-5-one and 1, 4-naphthoquinone derivatives. Asian J Chem. 2015 doi: 10.14233/ajchem.2015.19012. [DOI] [Google Scholar]
- 49.Shruti DJ, KishoreSain DS. Recent advancement in the synthesis of phenoxazine derivatives and their analogues. Synth Commun. 2018;48(12):1377–1402. doi: 10.1080/00397911.2018.1448090. [DOI] [Google Scholar]
- 50.Onoabedje EA, Okoro UC, Knight DW. Rapid access to new angular phenothiazine and phenoxazine dyes. J Heterocycl Chem. 2017;54(1):206–214. doi: 10.1002/jhet.2569. [DOI] [Google Scholar]
- 51.Ayogu JI, Enoo O, Okoro UC. Synthesis of 6-anilino derivatives of benzo [a] Phenoxazin-5-one and related aza analogues via palladium-catalyzed buchwald–hartwig amination reaction. Int J Sci Res. 2007;16:2319–7064. doi: 10.21275/ART20172906. [DOI] [Google Scholar]
- 52.Mizukawa Y, et al. Novel synthetic route for antimalarial benzo [a] phenoxazine derivative SSJ-183 and two active metabolites. Bioorg Med Chem. 2014;22(14):3749–3752. doi: 10.1016/j.bmc.2014.04.066. [DOI] [PubMed] [Google Scholar]
- 53.Ge JF, et al. Discovery of novel benzo [a] phenoxazine SSJ-183 as a drug candidate for malaria. ACS Med Chem Lett. 2010;1(7):360–364. doi: 10.1021/ml100120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oni TT, Okoro UC, Ugwu DI. Synthesis of 1-azabenzo [a] phenoxazin-5-one and 11-amino-1, 8, 10-triazabenzo [a] phenoxazin-5-one and their functionalized aryl derivatives via mizoroki-heck arylation methodology. Orient J Chem. 2015;31(1):371–378. doi: 10.13005/ojc/310144. [DOI] [Google Scholar]
- 55.Imai S, Shimazu A, Furihata K, Furihata K, Hayakawa Y, Seto H. Isolation and structure of a new phenoxazine antibiotic, exfoliazone, produced by Streptomyces exfoliatus. J Antibiot. 1990;43(12):1606–1607. doi: 10.7164/antibiotics.43.1606. [DOI] [PubMed] [Google Scholar]
- 56.Nan'ya S, Maekawa E, Kang WB, Ueno Y. Synthesis of 11-aza-5H-benzophenoxazin-5-one and 11-Aza-5H-pyridophenoxazin-5-one derivatives. J Heterocycl Chem. 1986;23(6):1697–1700. doi: 10.1002/jhet.5570230619. [DOI] [Google Scholar]
- 57.Ishii S (1961) “Chizu Drug Manufacturing Co. Japanese Patent. Chem”. Abstract (1961), 55
- 58.Okafor CO, Okerulu IO, Okeke SI. Vat dyes from three new heterocyclic ring systems. Dyes Pigm. 1987;8(1):11–24. doi: 10.1002/chin.198731302. [DOI] [Google Scholar]
- 59.David JA, Chris OU. Copper-catalyzed arylation reaction in the synthesis of new derivatives of angular triazaphenoxazinone. Chem Mater Res. 2014;6(9):2224–3224. [Google Scholar]
- 60.Raju BR, Sampaio DM, Silva MM, Coutinho PJ, Goncalves MST. Ultrasound promoted synthesis of Nile Blue derivatives. Ultrason Sonochem. 2014;21(1):360–366. doi: 10.1016/j.ultsonch.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Bruyneel F, Payen O, Rescigno A, Tinant B, Marchand-Brynaert J. Laccase-mediated synthesis of novel substituted phenoxazine chromophores featuring tuneable water solubility. Chem A. 2009;15(33):8283–8295. doi: 10.1002/chem.200900681. [DOI] [PubMed] [Google Scholar]
- 62.Becker E. Uber die Natur des Augenpigments von Ephestia kuhniella und seinen Vergleich mit den Augenpigmenten anderer Insekten. Biologisches Zentralblatt. 1939;59:597–627. [Google Scholar]
- 63.Bolognese A, Liberatore R. Photochemistry of ommochrome pigments. J Heterocycl Chem. 1988;25(4):1243–1246. doi: 10.1002/jhet.5570250438. [DOI] [Google Scholar]
- 64.Vogliardi S, Bertazzo A, Comai S, Costa CV, Allegri G, Seraglia R, Traldi P. An investigation on the role of 3-hydroxykynurenine in pigment formation by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom. 2004;18(13):1413–1420. doi: 10.1002/rcm.1497. [DOI] [PubMed] [Google Scholar]
- 65.Sekimura T, Nijhout HF. Diversity and evolution of butterfly wing patterns: an integrative approach. New York: Springer Nature; 2017. [Google Scholar]
- 66.Figon F, Casas J. Ommochromes in invertebrates: biochemistry and cell biology. Biol Rev. 2019;94(1):156–183. doi: 10.1111/brv.12441. [DOI] [PubMed] [Google Scholar]
- 67.Figon F, Munsch T, Croix C, Viaud-Massuard MC, Lanoue A, Casas J. Uncyclized xanthommatin is a key ommochrome intermediate in invertebrate coloration. Insect Biochem Mol Biol. 2020;124:103403. doi: 10.1016/j.ibmb.2020.103403. [DOI] [PubMed] [Google Scholar]
- 68.Li J, Beerntsen BT, James AA. Oxidation of 3-hydroxykynurenine to produce xanthommatin for eye pigmentation: a major branch pathway of tryptophan catabolism during pupal development in the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1999;29(4):329–338. doi: 10.1016/S0965-1748(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 69.Orphanos DG, Taurins A. Preparation of 3, 4-methylenedioxy-and 3, 4-dimethoxy-6-aminophenol. Can J Chem. 1966;44(15):1875–1879. doi: 10.1139/v66-283. [DOI] [Google Scholar]
- 70.Gavara L, Boisse T, Hénichart JP, Daïch A, Rigo B, Gautret P. Toward new camptothecins: part 6: synthesis of crucial ketones and their use in Friedländer reaction. Tetrahedron. 2010;66(38):7544–7561. doi: 10.1016/j.tet.2010.07.048. [DOI] [Google Scholar]
- 71.Kühlborn J, Konhäuser M, Groß J, Wich PR, Opatz T. Xylochemical synthesis of cytotoxic 2-aminophenoxazinone-type natural products through oxidative cross coupling. ACS Sustain Chem Eng. 2019;7(4):4414–4419. doi: 10.1021/acssuschemeng.8b06353. [DOI] [Google Scholar]
- 72.Gao M, Yu F, Lv C, Choo J, Chen L. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem Soc Rev. 2017;46(8):2237–2271. doi: 10.1039/C6CS00908E. [DOI] [PubMed] [Google Scholar]
- 73.Shimamoto T, Tomoda A, Ishida R, Ohyashiki K. Antitumor effects of a novel phenoxazine derivative on human leukemia cell lines in vitro and in vivo. Clin Cancer Res. 2001;7(3):704–708. [PubMed] [Google Scholar]
- 74.Avendaño C, Menéndez JC. Anticancer drugs acting via radical species, photosensitizers and photodynamic therapy of cancer. Med Chem Anticancer Drugs. 2008 doi: 10.1016/B978-0-444-52824-7.00004-4. [DOI] [Google Scholar]
- 75.Kobayashi M, Sakagami H, Kawase M, Motohashi N (2008) Changes in polyamine levels during cell death induced by heterocycles. In: Bioactive heterocycles VI. Springer, Berlin pp 161–171. Doi:10.1007/7081_2007_102
- 76.Prinz H, et al. N-benzoylated phenoxazines and phenothiazines: synthesis, antiproliferative activity and inhibition of tubulin polymerization. J Med Chem. 2011;54(12):4247–4263. doi: 10.1021/jm200436t. [DOI] [PubMed] [Google Scholar]
- 77.Bolognese A, et al. Antitumor agents: 2: synthesis, structure− activity relationships and biological evaluation of substituted 5 H-pyridophenoxazin-5-ones with potent antiproliferative activity. J Med Chem. 2002;45(24):5217–5223. doi: 10.1021/jm020918w. [DOI] [PubMed] [Google Scholar]
- 78.Wesolowska O, et al. Benzo [a] phenoxazines: a new group of potent P-glycoprotein inhibitors. In Vivo. 2006;20(1):109–113. [PubMed] [Google Scholar]
- 79.Thorne SH, Barak Y, Liang W, Bachmann MH, Rao J, Contag CH, Matin A. CNOB/ChrR6, a new prodrug enzyme cancer chemotherapy. Mol Cancer Ther. 2009;8(2):333–341. doi: 10.1158/1535-7163.MCT-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tougan T, et al. In vitro and in vivo characterization of anti-malarial acylphenoxazine derivatives prepared from basic blue 3. Malaria J. 2019;18(1):1–12. doi: 10.1186/s12936-019-2873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kananda CM (2018) Synthesis and photophysical properties of phenoxazine analogs designed as potential antimalarials. Doi:10.57709/12604841
- 82.De Beer F, Petzer JP, Petzer A. Monoamine oxidase inhibition by selected dye compounds. Chem Biol Drug Des. 2020;95(3):355–367. doi: 10.1111/cbdd.13654. [DOI] [PubMed] [Google Scholar]
- 83.Ge JF, Arai C, Kaiser M, Wittlin S, Brun R, Ihara M. Synthesis and in vitro antiprotozoal activities of water-soluble, inexpensive 3, 7-bis (dialkylamino) phenoxazin-5-ium derivatives. J Med Chem. 2008;51(12):3654–3658. doi: 10.1021/jm8003619. [DOI] [PubMed] [Google Scholar]
- 84.Lu J, Arai C, Md AB, Ihara M. Plasmodium berghei proteome changes in response to SSJ-183 treatment. Bioorg Med Chem. 2011;19(13):4144–4147. doi: 10.1016/j.bmc.2011.04.051. [DOI] [PubMed] [Google Scholar]
- 85.Schleiferböck S, et al. In vitro and in vivo characterization of the antimalarial lead compound SSJ-183 in Plasmodium models. Drug Des Dev Ther. 2013;7:1377. doi: 10.2147/DDDT.S51298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitra AK. Familiar fixes for a modern malady: a discussion on the possible cures of COVID-19. AIMS Mol Sci. 2020;7(3):269–280. doi: 10.3934/molsci.2020012. [DOI] [Google Scholar]
- 87.Akazawa M, Koshibu-Koizumi J, Iwamoto T, Takasaki M, Nakamura M, Tomoda A. Effects of novel phenoxazine compounds, 2-amino-4, 4α-dihydro-4α, 7-dimethyl-3h-phenoxazine-3-one and 3-amino-1, 4α-dihydro-4α, 8-dimethyl-2h-phenoxazine-2-one on proliferation of phytohemagglutinin-or anti-human IgM-activated human peripheral blood mononuclear cells. Tohoku J Exp Med. 2002;196(3):185–192. doi: 10.1620/tjem.196.185. [DOI] [PubMed] [Google Scholar]
- 88.Hayashi K, Hayashi T, Tomoda A. Phenoxazine derivatives inactivate human cytomegalovirus, herpes simplex virus-1 and herpes simplex virus-2 in vitro. J Pharmacol Sci. 2008;106(3):369–375. doi: 10.1254/jphs.FP0071679. [DOI] [PubMed] [Google Scholar]
- 89.Kozlovskaya LI, et al. Antiviral activity spectrum of phenoxazine nucleoside derivatives. Antivir Res. 2019;163:117–124. doi: 10.1016/j.antiviral.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Deville-Bonne D, El Amri C, Meyer P, Chen Y, Agrofoglio LA, Janin J. Human and viral nucleoside/nucleotide kinases involved in antiviral drug activation: structural and catalytic properties. Antivir Res. 2010;86(1):101–120. doi: 10.1016/j.antiviral.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 91.Kozlovskaya LI, et al. Phenoxazine nucleoside derivatives with a multiple activity against RNA and DNA viruses. Eur J Med Chem. 2021;220:113467. doi: 10.1016/j.ejmech.2021.113467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kozlovskaya L, et al. Isolation and phylogenetic analysis of SARS-CoV-2 variants collected in Russia during the COVID-19 outbreak. Int J Infect Dis. 2020;99:40–46. doi: 10.1016/j.ijid.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lohray BB, et al. (−) 3-[4-[2-(Phenoxazin-10-yl) ethoxy] phenyl]-2-ethoxypropanoic acid [(−) DRF 2725]: a dual PPAR agonist with potent antihyperglycemic and lipid modulating activity. J Med Chem. 2001;44(16):2675–2678. doi: 10.1021/jm010143b. [DOI] [PubMed] [Google Scholar]
- 94.Artis DR, et al. Scaffold-based discovery of indeglitazar, a PPAR pan-active anti-diabetic agent. Proc Natl Acad Sci. 2009;106(1):262–267. doi: 10.1073/pnas.0811325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ullmann F, Engi G, Wosnessensky N, Kuhn E, Herre E. Studien über aromatische Verbindungen mit labilem Halogen. Justus Liebigs Ann Chem. 1909;366(1–2):79–118. doi: 10.1002/jlac.19093660104. [DOI] [Google Scholar]
- 96.Blotny G. A new, mild preparation of sulfonyl chlorides. Tetrahedron Lett. 2003;44(7):1499–1501. doi: 10.1016/S0040-4039(02)02853-8. [DOI] [Google Scholar]
- 97.De Luca L, Giacomelli G. An easy microwave-assisted synthesis of sulfonamides directly from sulfonic acids. J Org Chem. 2008;73(10):3967–3969. doi: 10.1021/jo800424g. [DOI] [PubMed] [Google Scholar]
- 98.Lebovitz HE, Dole JF, Patwardhan R, Rappaport EB, Freed MI, Rosiglitazone Clinical Trials Study Group Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab. 2001;86(1):280–288. doi: 10.1210/jcem.86.1.7157. [DOI] [PubMed] [Google Scholar]
- 99.Kristensen JB, Johansen SK, Valsborg JS, Martiny L, Foged C. [14C] and [3H]-labelling of Ragaglitazar: a dual acting PPARα and PPARγ agonist with hypolipidemic and anti-diabetic activity. J Labell Compds Radiopharm. 2003;46(5):475–488. doi: 10.1002/jlcr.690. [DOI] [Google Scholar]
- 100.Lakowicz JR, editor. Principles of fluorescence spectroscopy. Boston: Springer; 2006. [Google Scholar]
- 101.Mitra AK. I am the light beneath your eyes. Resonance. 2019;24(6):623–632. doi: 10.1007/s12045-019-0821-5. [DOI] [Google Scholar]
- 102.Sam B, George L, Varghese A. Fluorescein based fluorescence sensors for the selective sensing of various analytes. J Fluoresc. 2021;31(5):1251–1276. doi: 10.1007/s10895-021-02770-9. [DOI] [PubMed] [Google Scholar]
- 103.Jun JV, Chenoweth DM, Petersson EJ. Rational design of small molecule fluorescent probes for biological applications. Org Biomol Chem. 2020;18(30):5747–5763. doi: 10.1039/D0OB01131B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang L, Du W, Hu Z, Uvdal K, Li L, Huang W. Hybrid rhodamine fluorophores in the visible/NIR region for biological imaging. Angew Chem Int Ed. 2019;58(40):14026–14043. doi: 10.1002/anie.201901061. [DOI] [PubMed] [Google Scholar]
- 105.Wang XQ, Ling QH, Wang W, Xu L. Pyrene-based metallocycles and metallocages: more than fluorophores. Mater Chem Front. 2020;4(11):3190–3200. doi: 10.1039/D0QM00484G. [DOI] [Google Scholar]
- 106.Gulati GK, Gulati LK, Kumar S. Recent progress in multi-stimulable photochromic oxazines with their wide-ranging applications. Dyes Pigments. 2021;192:109445. doi: 10.1016/j.dyepig.2021.109445. [DOI] [Google Scholar]
- 107.Ta DD, Dzyuba SV. Squaraine-based optical sensors: designer toolbox for exploring ionic and molecular recognitions. Chemosensors. 2021;9(11):302. doi: 10.3390/chemosensors9110302. [DOI] [Google Scholar]
- 108.Cao D, Liu Z, Verwilst P, Koo S, Jangjili P, Kim JS, Lin W. Coumarin-based small-molecule fluorescent chemosensors. Chem Rev. 2019;119(18):10403–10519. doi: 10.1021/acs.chemrev.9b00145. [DOI] [PubMed] [Google Scholar]
- 109.Pandey RK, James N, Chen Y, Dobhal MP. Cyanine dye-based compounds for tumor imaging with and without photodynamic therapy. Heterocyclic Polymethine Dyes. 2008 doi: 10.1007/7081_2008_113. [DOI] [Google Scholar]
- 110.Boens N, Leen V, Dehaen W. Fluorescent indicators based on BODIPY. Chem Soc Rev. 2012;41(3):1130–1172. doi: 10.1039/C1CS15132K. [DOI] [PubMed] [Google Scholar]
- 111.Mitra AK. Synthesis, biological activity and photophysical studies of ellipticine and its derivatives: state of the art. Chem Heterocyclic Compds. 2022 doi: 10.1007/s10593-022-03070-1. [DOI] [Google Scholar]
- 112.Mitra AK. Sesquicentennial birth anniversary of carbazole, a multifaceted wonder molecule: a revisit to its synthesis, photophysical and biological studies. J Iran Chem Soc. 2021 doi: 10.1007/s13738-021-02444-0. [DOI] [Google Scholar]
- 113.Martinez V, Henary M. Nile red and nile blue: applications and syntheses of structural analogues. Chem A. 2016;22(39):13764–13782. doi: 10.1002/chem.201601570. [DOI] [PubMed] [Google Scholar]
- 114.Coutinho PJ (2009). Photophysics and biophysical applications of benzo [a] phenoxazine type fluorophores. In: Reviews in fluorescence 2007. Springer, pp. 335–362, Doi:10.1007/978-0-387-88722-7_14
- 115.Greenspan P, Fowler SD. Spectrofluorometric studies of the lipid probe, nile red. J Lipid Res. 1985;26(7):781–789. doi: 10.1016/S0022-2275(20)34307-8. [DOI] [PubMed] [Google Scholar]
- 116.Ira and, Krishnamoorthy G (2001) Probing the link between proton transport and water content in lipid membranes. J Phys Chem B 105(7), 1484–1488. Doi:10.1021/jp003009w
- 117.Sackett DL, Wolff J. Nile red as a polarity-sensitive fluorescent probe of hydrophobic protein surfaces. Anal Biochem. 1987;167(2):228–234. doi: 10.1016/0003-2697(87)90157-6. [DOI] [PubMed] [Google Scholar]
- 118.Davis DM, Birch DJ. Extrinsic fluorescence probe study of human serum albumin using nile red. J Fluoresc. 1996;6(1):23–32. doi: 10.1007/BF00726723. [DOI] [PubMed] [Google Scholar]
- 119.Maiti NC, Krishna MMG, Britto PJ, Periasamy N. Fluorescence dynamics of dye probes in micelles. J Phys Chem B. 1997;101(51):11051–11060. doi: 10.1021/jp9723123. [DOI] [Google Scholar]
- 120.Datta A, Mandal D, Pal SK, Bhattacharyya K. Intramolecular charge transfer processes in confined systems: Nile red in reverse micelles. J Phys Chem B. 1997;101(49):10221–10225. doi: 10.1021/jp971576m. [DOI] [Google Scholar]
- 121.Jose J, Ueno Y, Burgess K. Water-soluble Nile blue derivatives: syntheses and photophysical properties. Chemistry A. 2009;15(2):418–423. doi: 10.1002/chem.200801104. [DOI] [PubMed] [Google Scholar]
- 122.Fowler SD, Greenspan P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: comparison with oil red O. J Histochem Cytochem. 1985;33(8):833–836. doi: 10.1177/33.8.4020099. [DOI] [PubMed] [Google Scholar]
- 123.Bonilla EDUARDO, Prelle ALESSANDRO. Application of nile blue and nile red, two fluorescent probes, for detection of lipid droplets in human skeletal muscle. J Histochem Cytochem. 1987;35(5):619–621. doi: 10.1177/35.5.3559182. [DOI] [PubMed] [Google Scholar]
- 124.Reichardt C. Solvent and solvent effects in organic chemistry. Weinheim: Wiley; 2004. [Google Scholar]
- 125.Baur ME, Nicol M. Solvent stark effect and spectral shifts. J Chem Phys. 1966;44(9):3337–3343. doi: 10.1063/1.1727234. [DOI] [Google Scholar]
- 126.Kobayashi T, Nagakura S. Photoelectron spectra of substituted benzenes. Bull Chem Soc Jpn. 1974;47(10):2563–2572. doi: 10.1246/bcsj.47.2563. [DOI] [Google Scholar]
- 127.Airinei A, Homocianu MIHAELA, Dorohoi DO. Changes induced by solvent polarity in electronic absorption spectra of some azo disperse dyes. J Mol Liq. 2010;157(1):13–17. doi: 10.1016/j.molliq.2010.07.011. [DOI] [Google Scholar]
- 128.Mitra AK. The journey of 1-Keto-1, 2, 3, 4-tetrahydrocarbazole based fluorophores: from inception to implementation. J Fluoresc. 2022 doi: 10.1007/s10895-022-03004-2. [DOI] [PubMed] [Google Scholar]
- 129.Mitra AK, Ghosh S, Chakraborty S, Basu S, Saha C. Synthesis and spectroscopic exploration of carboxylic acid derivatives of 6-hydroxy-1-keto-1, 2, 3, 4-tetrahydrocarbazole: hydrogen bond sensitive fluorescent probes. J Lumin. 2013;143:693–703. doi: 10.1016/j.jlumin.2013.06.021. [DOI] [Google Scholar]
- 130.Ghosh S, Mitra AK, Saha C, Basu S. Tuning the solution phase photophysics of two de novo designed hydrogen bond sensitive 9-methyl-2, 3, 4, 9-tetrahydro-1H-carbazol-1-one derivatives. J Fluoresc. 2013;23(6):1179–1195. doi: 10.1007/s10895-013-1249-z. [DOI] [PubMed] [Google Scholar]
- 131.Mitra AK, Ghosh S, Sau A, Saha C, Basu S. Solution phase photophysics of 5, 7-dimethoxy-2, 3, 4, 9-tetrahydro-1H-carbazol-1-one: analysing the lineaments of a new fluorosensor to probe different micro-environments. J Lumin. 2015;167:233–248. doi: 10.1016/j.jlumin.2015.06.048. [DOI] [Google Scholar]
- 132.Mitra AK, Ghosh S, Chakraborty S, Sarangi MK, Saha C, Basu S. Photophysical properties of an environment sensitive fluorophore 1-keto-6, 7-dimethoxy-1, 2, 3, 4-tetrahydrocarbazole and its excited state interaction with N,N-dimethylaniline: a spectroscopic investigation. J Photochem Photobiol, A. 2012;240:66–74. doi: 10.1016/j.jphotochem.2012.05.006. [DOI] [Google Scholar]
- 133.Ghosh S, Mitra AK, Pal U, Basu S, Saha C. Evidence of two structurally related solvatochromic probes complexed with β-cyclodextrin by using spectroscopic methods. J Mol Struct. 2017;1130:810–817. doi: 10.1016/j.molstruc.2016.10.097. [DOI] [Google Scholar]
- 134.Ghosh S, Mitra AK, Basu S, Chakraborty S, Saha C. 5, 6, 7, 9-Tetrahydro-[1, 3] dioxolo [4, 5-h] carbazol-8-one: a solvatochromic PET-acceptor fluorescent probe. J lumin. 2014;153:296–303. doi: 10.1016/j.jlumin.2014.03.046. [DOI] [Google Scholar]
- 135.Cser A, Nagy K, Biczók L. Fluorescence lifetime of Nile Red as a probe for the hydrogen bonding strength with its microenvironment. Chem Phys Lett. 2002;360(5–6):473–478. doi: 10.1016/S0009-2614(02)00784-4. [DOI] [Google Scholar]
- 136.Coutinho PJ, Castanheira EM, Céu Rei M, Real Oliveira MEC. Nile red and DCM fluorescence anisotropy studies in C12E7/DPPC mixed systems. J Phys Chem B. 2002;106(49):12841–12846. doi: 10.1021/jp026479u. [DOI] [Google Scholar]
- 137.Dutt GB, Doraiswamy S, Periasamy N. Molecular reorientation dynamics of polar dye probes in tertiary-butyl alcohol–water mixtures. J Chem Phys. 1991;94(8):5360–5368. doi: 10.1063/1.460521. [DOI] [Google Scholar]
- 138.Ghoneim N. Photophysics of Nile red in solution: steady state spectroscopy. Spectrochim Acta Part A. 2000;56(5):1003–1010. doi: 10.1016/S1386-1425(99)00199-7. [DOI] [PubMed] [Google Scholar]
- 139.Dutta AK, Kamada K, Ohta K. Spectroscopic studies of nile red in organic solvents and polymers. J Photochem Photobiol A. 1996;93(1):57–64. doi: 10.1016/1010-6030(95)04140-0. [DOI] [Google Scholar]
- 140.Sarkar N, Das K, Nath DN, Bhattacharyya K. Twisted charge transfer processes of nile red in homogeneous solutions and in faujasite zeolite. Langmuir. 1994;10(1):326–329. doi: 10.1021/la00013a048. [DOI] [Google Scholar]
- 141.Douhal A. Photophysics of Nile blue A in proton-accepting and electron-donating solvents. J Phys Chem. 1994;98(50):13131–13137. doi: 10.1021/j100101a007. [DOI] [Google Scholar]
- 142.Kobayashi T, Takagi Y, Kandori H, Kemnitz K, Yoshihara K. Femtosecond intermolecular electron transfer in diffusionless, weakly polar systems: Nile Blue in aniline and NN-dimethylaniline. Chem Phys Lett. 1991;180(5):416–422. doi: 10.1016/0009-2614(91)85142-J. [DOI] [Google Scholar]
- 143.Grofcsik A, Kubinyi M, Jones WJ. Intermolecular photoinduced proton transfer in Nile Blue and oxazine 720. Chem Phys Lett. 1996;250(3–4):261–265. doi: 10.1016/0009-2614(96)00003-6. [DOI] [Google Scholar]
- 144.Grofcsik A, Kubinyi M, Ruzsinszky A, Veszprémi T, Jones WJ. Quantum chemical studies on excited state intermolecular proton transfer of oxazine dyes. J Mol Struct. 2000;555(1–3):15–19. doi: 10.1016/S0022-2860(00)00583-4. [DOI] [Google Scholar]
- 145.Mitra AK, Ghosh S, Sarangi MK, Sau A, Saha C, Basu S. Influence of microheterogeneity on the solution phase photophysics of a newly synthesised, environment sensitive fluorophore 2-((7, 8-dimethyl-1-oxo-2, 3, 4, 9-tetrahydro-1H-carbazol-6-yl) oxy) acetic acid and its tagged derivative. J Photochem Photobiol A. 2015;296:66–79. doi: 10.1016/j.jphotochem.2014.09.009. [DOI] [Google Scholar]
- 146.Mitra AK, Ghosh S, Sarangi MK, Chakraborty S, Saha C, Basu S. Photophysics of a solvent sensitive keto-tetrahydrocarbazole based fluorophore and its interaction with triethylamine: a spectroscopic inquest under surfactant and β-CD confinement. J Mol Struct. 2014;1074:617–628. doi: 10.1016/j.molstruc.2014.06.038. [DOI] [Google Scholar]
- 147.Mitra AK, Sau A, Pal U, Saha C, Basu S. Constrained photophysics of 5, 7-dimethoxy-2, 3, 4, 9-tetrahydro-1H-carbazol-1-one in the bioenvironment of serum albumins: a spectroscopic endeavour supported by molecular docking analysis. J Fluoresc. 2017;27(4):1547–1558. doi: 10.1007/s10895-017-2094-2. [DOI] [PubMed] [Google Scholar]
- 148.Mitra AK, Sau A, Bera SC, Chakraborty S, Saha C, Basu S. Monitoring the competence of a new keto-tetrahydrocarbazole based fluorosensor under homogeneous, micro-heterogeneous and serum albumin environments. J Fluoresc. 2015;25(6):1931–1949. doi: 10.1007/s10895-015-1685-z. [DOI] [PubMed] [Google Scholar]
- 149.Sarangi MK, Mitra AK, Sengupta C, Ghosh S, Chakraborty S, Saha C, Basu S. Hydrogen bond sensitive probe 5-methoxy-1-keto-1, 2, 3, 4-tetrahydro carbazole in the microheterogeneity of binary mixtures and reverse micelles. J Phys Chem C. 2013;117(5):2166–2174. doi: 10.1021/jp310923q. [DOI] [Google Scholar]
- 150.Mitra RK, Sinha SS, Pal SK. Interactions of Nile Blue with micelles, reverse micelles and a genomic DNA. J Fluoresc. 2008;18(2):423–432. doi: 10.1007/s10895-007-0282-1. [DOI] [PubMed] [Google Scholar]
- 151.Gao HW, Ye QS, Liu WG. Langmuir aggregation of Nile blue and safranine T on sodium dodecylbenzenesulfonate surface and its application to quantitative determination of anionic detergent. Anal Sci. 2002;18(4):455–459. doi: 10.2116/analsci.18.455. [DOI] [PubMed] [Google Scholar]
- 152.Das K, Jain B, Patel HS. Nile Blue in Triton-X 100/benzene–hexane reverse micelles: a fluorescence spectroscopic study. Spectrochim Acta Part A. 2004;60(8–9):2059–2064. doi: 10.1016/j.saa.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 153.Chen QY, Li DH, Yang HH, Zhu QZ, Xu JG, Zhao Y. Interaction of a novel red-region fluorescent probe, Nile Blue, with DNA and its application to nucleic acids assay. Analyst. 1999;124(6):901–906. doi: 10.1039/A901174I. [DOI] [PubMed] [Google Scholar]
- 154.Yang YI, Hong HY, Lee IS, Bai DG, Yoo GS, Choi JK. Detection of DNA using a visible dye, Nile blue, in electrophoresed gels. Anal Biochem. 2000;280(2):322–324. doi: 10.1006/abio.2000.4537. [DOI] [PubMed] [Google Scholar]
- 155.Nasr C, Hotchandani S. Excited-state behavior of Nile Blue H-aggregates bound to SiO2 and SnO2 colloids. Chem Mater. 2000;12(6):1529–1535. doi: 10.1021/cm0000752. [DOI] [Google Scholar]
- 156.Parafiniuk K, Sznitko L, Zelechowska M, Mysliwiec J. Near-infrared distributed feedback laser emission based on cascade Förster resonance energy transfer to Nile Blue aggregates. Org Electron. 2016;33:121–127. doi: 10.1016/j.orgel.2016.03.007. [DOI] [Google Scholar]
- 157.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 158.Lv L, Luo W, Diao Q. A novel ratiometric fluorescent probe for selective detection and imaging of H2S. Spectrochim Acta Part A. 2021;246:118959. doi: 10.1016/j.saa.2020.118959. [DOI] [PubMed] [Google Scholar]
- 159.Frade VH, Coutinho PJ, Moura JC, Gonçalves MST. Functionalised benzo [a] phenoxazine dyes as long-wavelength fluorescent probes for amino acids. Tetrahedron. 2007;63(7):1654–1663. doi: 10.1016/j.tet.2006.12.005. [DOI] [Google Scholar]
- 160.Alves CM, Naik S, Coutinho PJ, Gonçalves MST. New long alkyl side-chain benzo [a] phenoxazines as micellisation probes. Tetrahedron Lett. 2009;50(31):4470–4474. doi: 10.1016/j.tetlet.2009.05.055. [DOI] [Google Scholar]
- 161.Yang J, Zheng W, Shen Y, Xu Y, Lv G, Li C. A novel near-infrared fluorescent probe based on phenoxazine for the specific detection of HOCl. J Lumin. 2020;226:117460. doi: 10.1016/j.jlumin.2020.117460. [DOI] [Google Scholar]
- 162.Chadar D, Chakravarty D, Lande DN, Gejji SP, Sahoo S, Salunke-Gawali S. Crystal structure and spectral properties of vitamin K3 based nitrobenzo [a] phenoxazines. J Mol Struct. 2017;1149:84–91. doi: 10.1016/j.molstruc.2017.07.091. [DOI] [Google Scholar]
- 163.Okamoto T, Kozaki M, Doe M, Uchida M, Wang G, Okada K. 1, 4-Benzoxazino [2, 3-b] phenoxazine and its sulfur analogues: Synthesis, properties and application to organic light-emitting diodes. Chem Mater. 2005;17(22):5504–5511. doi: 10.1021/cm050723n. [DOI] [Google Scholar]
- 164.Wang B, Qiao X, Yang Z, Wang Y, Liu S, Ma D, Wang Q. Realizing efficient red thermally activated delayed fluorescence organic light-emitting diodes using phenoxazine/phenothiazine-phenanthrene hybrids. Org Electron. 2018;59:32–38. doi: 10.1016/j.orgel.2018.04.045. [DOI] [Google Scholar]
- 165.Zhu Y, Kulkarni AP, Jenekhe SA. Phenoxazine-based emissive donor− acceptor materials for efficient organic light-emitting diodes. Chem Mater. 2005;17(21):5225–5227. doi: 10.1021/cm050743p. [DOI] [Google Scholar]
- 166.Zhu Y, Alam MM, Jenekhe SA. Regioregular head-to-tail poly (4-alkylquinoline) s: synthesis, characterization, self-organization, photophysics and electroluminescence of new n-type conjugated polymers. Macromolecules. 2003;36(24):8958–8968. doi: 10.1021/ma0348021. [DOI] [Google Scholar]
- 167.Tavgeniene D, Blazevicius D, Eidimtas M, Krucaite G, Zhang B, Sutkuviene S, Grigalevicius S. Phenoxazines having various electron acceptor or donor fragments as new host materials for green phosphorescent OLEDs. Dyes Pigments. 2020;172:107839. doi: 10.1016/j.dyepig.2019.107839. [DOI] [Google Scholar]
- 168.Jou JH, et al. A wet-and dry-process feasible carbazole type host for highly efficient phosphorescent OLEDs. J Mater Chem C. 2015;3(47):12297–12307. doi: 10.1039/C5TC02889B. [DOI] [Google Scholar]
- 169.Yeh SJ, et al. New dopant and host materials for Blue-Light-Emitting phosphorescent organic electroluminescent devices. Adv Mater. 2005;17(3):285–289. doi: 10.1002/adma.200401373. [DOI] [Google Scholar]
- 170.So F, Kido J, Burrows P. Organic light-emitting devices for solid-state lighting. MRS Bull. 2008;33(7):663–669. doi: 10.1557/mrs2008.137. [DOI] [Google Scholar]
- 171.Park Y, Kim B, Lee C, Lee J, Lee JH, Park J. High efficiency new hole injection materials for organic light emitting diodes based on dimeric phenothiazine and phenoxazine moiety derivatives. J Nanosci Nanotechnol. 2012;12(5):4356–4360. doi: 10.1166/jnn.2012.5886. [DOI] [PubMed] [Google Scholar]
- 172.Tang CW, VanSlyke SA. Organic electroluminescent diodes. Appl Phys Lett. 1987;51(12):913–915. doi: 10.1063/1.98799. [DOI] [Google Scholar]
- 173.Zhang Q, Li B, Huang S, Nomura H, Tanaka H, Adachi C. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat Photonics. 2014;8(4):326–332. doi: 10.1038/nphoton.2014.12. [DOI] [Google Scholar]
- 174.Hirata S, et al. Highly efficient blue electroluminescence based on thermally activated delayed fluorescence. Nat Mater. 2015;14(3):330–336. doi: 10.1038/nmat4154. [DOI] [PubMed] [Google Scholar]
- 175.Hu JJ, Luo XF, Zhang YP, Mao MX, Ni HX, Liang X, Zheng YX. Green multi-resonance thermally activated delayed fluorescence emitters containing phenoxazine units with highly efficient electroluminescence. J Mater Chem C. 2022;10(2):768–773. doi: 10.1039/D1TC04595D. [DOI] [Google Scholar]
- 176.Kulszewicz-Bajer I. Acridone and quinacridone derivatives with carbazole or phenoxazine substituents: synthesis, electrochemistry, photophysics and application as TADF electroluminophores. J Mater Chem C. 2022;10(34):12377–12391. doi: 10.1039/D2TC02270B. [DOI] [Google Scholar]
- 177.Corbin DA, McCarthy BG, van de Lindt Z, Miyake GM. Radical cations of phenoxazine and dihydrophenazine photoredox catalysts and their role as deactivators in organocatalyzed atom transfer radical polymerization. Macromolecules. 2021;54(10):4726–4738. doi: 10.1021/acs.macromol.1c00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Matyjaszewski K, Tsarevsky NV. Macromolecular engineering by atom transfer radical polymerization. J Am Chem Soc. 2014;136(18):6513–6533. doi: 10.1021/ja408069v. [DOI] [PubMed] [Google Scholar]
- 179.Boyer C, et al. Copper-mediated living radical polymerization (atom transfer radical polymerization and copper (0) mediated polymerization): from fundamentals to bioapplications. Chem Rev. 2016;116(4):1803–1949. doi: 10.1021/acs.chemrev.5b00396. [DOI] [PubMed] [Google Scholar]
- 180.Kientz M, Lowe G, McCarthy BG, Miyake GM, Bonin J, Robert M. Phenoxazine-sensitized CO2-to-CO reduction with an iron porphyrin catalyst: a redox properties-catalytic performance study. ChemPhotoChem. 2022 doi: 10.1002/cptc.202200009. [DOI] [Google Scholar]
- 181.Bodedla GB, Thomas KJ, Kumar S, Jou JH, Li CJ. Phenothiazine-based bipolar green-emitters containing benzimidazole units: synthesis, photophysical and electroluminescence properties. RSC Adv. 2015;5(106):87416–87428. doi: 10.1039/C5RA18372C. [DOI] [Google Scholar]
- 182.Wolak MA, Delcamp J, Landis CA, Lane PA, Anthony J, Kafafi Z. High-performance organic light-emitting diodes based on dioxolane-substituted pentacene derivatives. Adv Func Mater. 2006;16(15):1943–1949. doi: 10.1002/adfm.200500809. [DOI] [Google Scholar]
- 183.Ferrere S, Gregg BA. New perylenes for dye sensitization of TiO2. New J Chem. 2002;26(9):1155–1160. doi: 10.1039/B203260K. [DOI] [Google Scholar]
- 184.Li J, Liu D, Li Y, Lee CS, Kwong HL, Lee S. A high Tg carbazole-based hole-transporting material for organic light-emitting devices. Chem Mater. 2005;17(5):1208–1212. doi: 10.1021/cm034731k. [DOI] [Google Scholar]
- 185.Ning Z, Zhang Q, Wu W, Pei H, Liu BO, Tian HE. Starburst triarylamine based dyes for efficient dye-sensitized solar cells. J Org Chem. 2008;73(10):3791–3797. doi: 10.1021/jo800159t. [DOI] [PubMed] [Google Scholar]
- 186.Zhu W, et al. Organic D-A-π-A solar cell sensitizers with improved stability and spectral response. Adv Func Mater. 2011;21(4):756–763. doi: 10.1002/adfm.201001801. [DOI] [Google Scholar]
- 187.Ning Z, Zhang Q, Pei H, Luan J, Lu C, Cui Y, Tian H. Photovoltage improvement for dye-sensitized solar cells via cone-shaped structural design. J Phys Chem C. 2009;113(23):10307–10313. doi: 10.1021/jp902408z. [DOI] [Google Scholar]
- 188.Tian H, et al. Effect of different electron donating groups on the performance of dye-sensitized solar cells. Dyes Pigm. 2010;84(1):62–68. doi: 10.1016/j.dyepig.2009.06.014. [DOI] [Google Scholar]
- 189.Tan H, et al. Phenoxazine-based organic dyes with different chromophores for dye-sensitized solar cells. Org Electron. 2013;14(11):2795–2801. doi: 10.1016/j.orgel.2013.07.008. [DOI] [Google Scholar]
- 190.Reddy MA, et al. Highly conjugated electron rich thiophene antennas on phenothiazine and phenoxazine-based sensitizers for dye sensitized solar cells. Synth Met. 2014;195:208–216. doi: 10.1016/j.synthmet.2014.06.009. [DOI] [Google Scholar]
- 191.Ooyama Y, Shimada Y, Kagawa Y, Imae I, Harima Y. Photovoltaic performance of dye-sensitized solar cells based on donor–acceptor π-conjugated benzofuro [2, 3-c] oxazolo [4, 5-a] carbazole-type fluorescent dyes with a carboxyl group at different positions of the chromophore skeleton. Org Biomol Chem. 2007;5(13):2046–2054. doi: 10.1039/B705694J. [DOI] [PubMed] [Google Scholar]