Abstract
Context
Adrenal insufficiency (AI)-related morbidity persists despite efforts to minimize its effect. Reasons for this are unknown and warrant examination.
Objective
This work aimed to investigate trends in AI hospitalizations and glucocorticoid (GC) replacement therapy use.
Methods
Data on hospitalizations for a principal diagnosis of AI and prescriptions for short-acting GCs between 2000 and 2019 were extracted from national repositories. Age-standardized admission and prescription rates were calculated using census data. Rates were compared over time overall and according to age, sex, and disease subtype.
Results
AI admissions increased by 62.0%, from 36.78/million to 59.59/million (trend P < .0001). Adrenal crisis (AC) admissions also increased, by 90.1% (from 10.73/million to 20.40/million; trend, P < .00001). These increases were more pronounced in the second decade. Prescriptions for short-acting GCs also increased (by 67.2%, from 2198.36/million in 2000/2001 to 3676.00/million in 2017/2018). Females had higher average admission rates and a greater increase in admission rates than males. Increased AI admissions were found in all age groups among females but only in men aged 70+ yrs. Secondary AI (SAI) admission rates increased by 91.7%, whereas admission rates for primary AI (PAI) remained unchanged.
Conclusion
The prevalence of AI and hospitalizations for this disorder (including ACs) have increased since 2000, with a greater increase occurring after 2010. Admission rates for SAI increased but PAI admissions remained stable. Possible causes include immunotherapies for malignancy, increased cranial imaging detecting pituitary tumors and their subsequent treatment, and increased use of low-dose, short-acting GC-replacement therapy.
Keywords: adrenal insufficiency, adrenal crisis, epidemiology, iatrogenic, glucocorticoid, immunotherapy
Adrenal insufficiency (AI) is a chronic disorder with a prevalence of approximately 300 cases/million [1, 2]. While glucocorticoid (GC)-replacement therapy restores health in the majority of patients, all are at risk of an adrenal crisis (AC), which is an acute life-threatening episode of AI with hypotension and other features including severe weakness, syncope, abdominal pain, vomiting, confusion, and biochemical abnormalities (hyponatremia, hyperkalemia, and occasionally hypoglycemia and hypercalcemia) [3, 4]. ACs require urgent hospital care and admission may also be required for less severe episodes (symptomatic AI) to restore health and prevent an AC. Domiciliary preemergent GC stress-dosing (oral and/or intramuscular/subcutaneous) is regarded as a mainstay of prevention but ACs continue to occur at an approximate rate of 6 to 8 ACs/100 patient years and are a cause of increased mortality in AI [4, 5].
Despite efforts to minimize morbidity and mortality from AI, there is evidence to suggest that the burden of illness, measured by AI-related hospitalizations, has not improved and may be increasing [6]. Reasons for this are poorly understood but may reflect an increase in AI prevalence due to changes in the underlying epidemiology of diseases causing AI (including iatrogenic etiologies) or changes to AI management. In Western countries, autoimmune disorders and pituitary adenomas (and their treatment) are common causes of AI, while infections (tuberculosis and HIV) predominate in low- to middle-income populations [7-9]. Malignancies (primary or secondary tumors of the adrenal or pituitary glands) can occur at any age, with adrenal metastases being more common in older patients [10]. Iatrogenic AI can follow exposure to therapeutic doses of GCs, including inhaled GCs, or opioid use and is often undiagnosed [11, 12]. More recently, immunotherapy for treatment of an increasing number of malignancies, can cause hypophysitis, often with selective loss of adrenocorticotropin secretion, or, less commonly, adrenalitis, resulting in AI in some patients [13-15]. In addition, from the 1990s, following reassessment of endogenous cortisol production, there has been gradual acceptance of the use of lower doses of shorter-acting (hydrocortisone and cortisone acetate) GCs, a practice associated, both epidemiologically and in cohort studies, with an increased incidence of ACs in treated AI [6, 16, 17]. Problems with access to specialist care and educational programs to increase competence in self-management may also influence hospitalization rates.
While reports and case series have documented associations between medical disorders (and related treatments) and the development of AI, the effect of these and other factors, including changes to recommended GC-replacement therapy, on the burden of AI in populations has not been evaluated [14, 18, 19]. The aim of the present study, which examined both hospitalization and prescription data from national repositories, was to investigate whether there have been changes to the epidemiology of AI/AC from 2000 to the present.
Materials and Methods
Deidentified data on all episodes of hospitalization in Australia are stored by the Australian Institute of Health and Welfare (AIHW), according to Australian financial year (July 1 to June 30) of admission and principal diagnosis (main reason for hospitalization as identified by the admitting clinician) (coded according to the Australian modification of the International Statistical Classification of Diseases and Related Problems (ICD-10-AM) [20]. These data are made available by the AIHW in its Principal Diagnosis Datacubes (https://www.aihw.gov.au/reports/hospitals/principal-diagnosis-data-cubes/contents/data-cubes). Available data comprise the number of admissions for each principal diagnosis by age (in 5-year groups), sex, and financial year. Only one diagnosis is given for each admission, so no information on the underlying subtype of AI is available for AC admissions.
We extracted data on all admissions between the years 2000/2001 and 2018/2019 inclusive among adult inpatients aged 20 years and older, in which the principal diagnosis was coded as an Addisonian (adrenal) crisis (AC) (E27.2); primary adrenocortical insufficiency (PAI) (E27.1); drug-induced hypoadrenalism (E27.3); other causes and unspecified causes of hypoadrenalism (E27.4); hypopituitarism (E23.0); or postsurgical hypopituitarism (E89.3).
Data on all prescriptions for medications under the Australian Pharmaceutical Benefits scheme (PBS) are stored by the Australian Commonwealth Department of Health and Human Services (DHHS). Deidentified data for the years 2000 to 2018 were extracted from the PBS for the medications by the DHHS, for hydrocortisone (4 and 20 mg) and cortisone acetate (5 and 25 mg) according to year, sex, and 10-year age group of the patient receiving the service.
Information on the Australian population is available from the Australian Bureau of Statistics (https://stat.data.abs.gov.au/Index.aspx), which publishes data on the population in each census year, and estimated populations for intercensal years. We extracted population data on each study year by sex and 5-year age groups. These data were used to give denominator populations for study each year.
Ethics
No ethics clearance was required for data obtained from publicly available data sets. Permission to use pharmaceutical prescription data was obtained by the Commonwealth Department of Health and Human Services and from the University of Notre Dame Human Research Ethics Committee (No. 2021-175S).
Statistical Analysis
All data were extracted from the relevant databases and entered into Excel for processing (Microsoft Corp, 2018. Microsoft Excel). Age-standardized rates were calculated using the age and sex structure of the year 2000 as the base year. Secondary AI (SAI) in this analysis was considered to be any of “drug-induced AI,” hypopituitarism, “other causes and unspecified causes of AI,” or postsurgical hypopituitarism. Drug-induced AI was also considered separately in some analyses. Where appropriate, a z score for the difference in 2 proportions was used to assess the significance of the observed difference between admission and prescription rates between the first and last years of the study. T tests and analysis of variance were used to determine the significance of mean rates between groups, with appropriate adjustments made for differences in variance, where appropriate. Pearson correlation coefficients were calculated for the associations between AI and AC admission rates with GC prescription rates (using data from 2000/2001 to 2017/2018).
Poisson regression models were constructed using R version 4.0.2 (R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing; https://www.R-project.org/) to determine the significance of secular trends in admissions and prescriptions over the time period of the study. The remaining analyses were conducted using GraphPad Prism (GraphPad Prism version 9.0.0 for Windows, GraphPad Software; www.graphpad.com). Given the large sample sizes and the use of multiple comparisons, a conservative P of less than .001 was used to determine statistical significance.
Results
Admissions for Adrenal Insufficiency
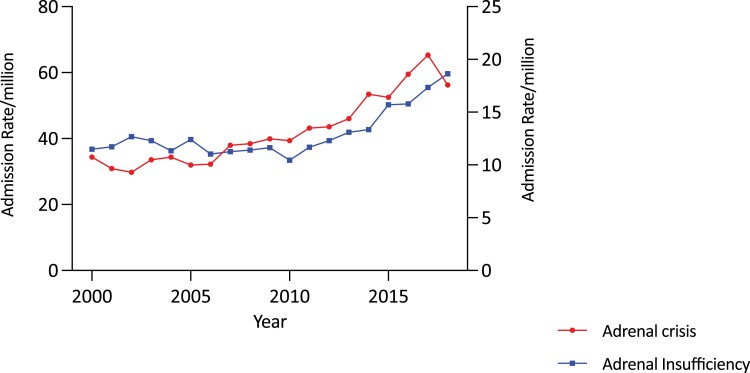
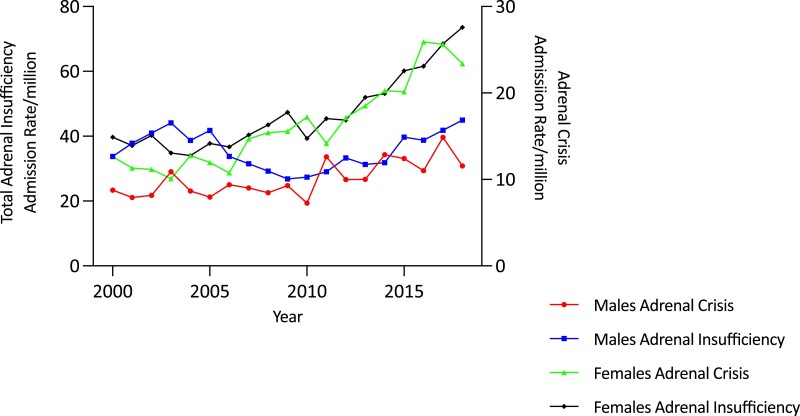
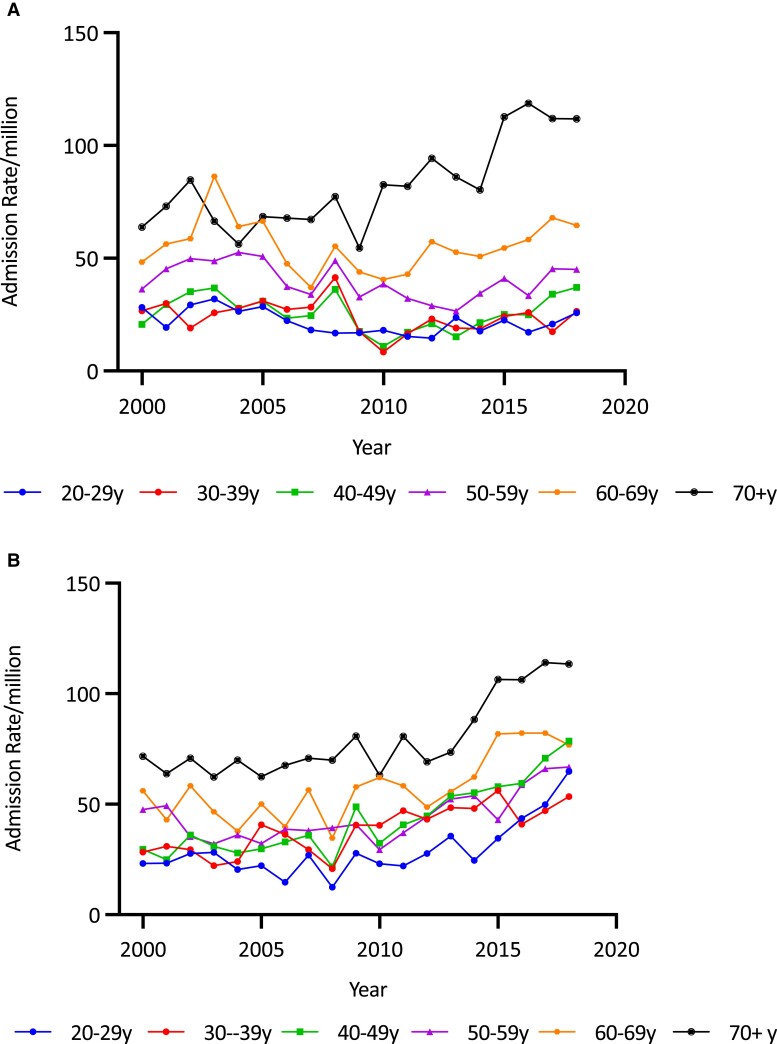
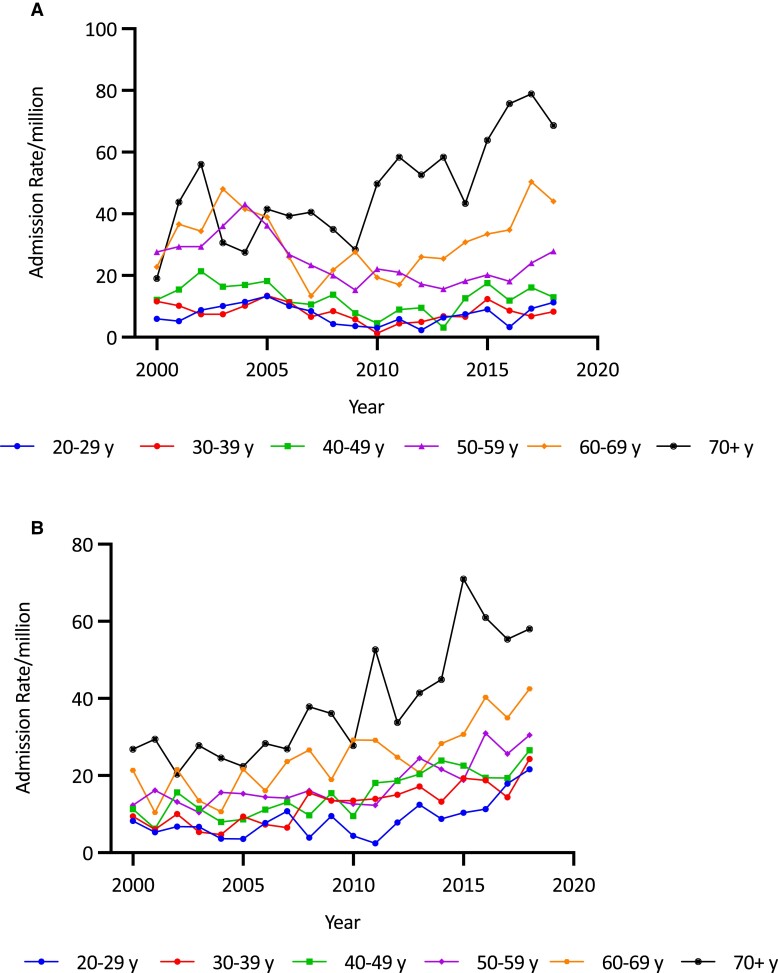
Overall age-adjusted admission rates for all forms of AI increased by 62.0% over the study period, from 36.78/million to 59.59/million (trend P < .0001) (Fig. 1 and Table 1). The increase in admissions was most evident in the second decade (78.1%, from 33.46/million in 2010/2011 to 59.59/million in 2018/2019) and was negligible in the first (1.2%, from 36.78/million in 2000/2001 to 37.23/million in 2009/2010). Over the study period, the secular increases were higher in females (85.3%, from 39.71 to 73.58/million; trend P < .0001) than males (33.2%, from 33.74 to 44.95/million; trend P < .0001) (Fig. 2 and Table 1). Females had a higher average admission rate than males (47.36 [11.78]/million/year vs 35.27 [5.72]/million/year; P < .0001), and average admission rates increased with age, reaching 80.53/million/year in those aged 70 years and older; P < .0001). Age- and sex-specific admission rates showed statistically significant (trend P < .0001) increases in men aged 70 years and older (by 75.2%, from 63.85 to 111.84/million) and in all women, in whom the greatest increases were in those aged 20 to 29 years (179.1%, from 23.22 to 64.81/million) and 40 to 49 years (165.4%, from 29.59 to 78.52/million) (see Table 1 and Fig. 3A and 3B).
Figure 1.
Adjusted total adrenal insufficiency (AI) and adrenal crisis (AC) admission rates/million population year in Australia, 2000/2001 to 2018/2019. Admissions for AI and ACs increased significantly in the decade 2010 to 2019.
Table 1.
Adrenal insufficiency admission rates by subtype and sex and age group in years
| Males | Females | Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018/2019, y | AI | AC | SAI | PAI | Drug AI | AI | AC | SAI | PAI | Drug AI | AI | AC | SAI | PAI | Drug AI |
| 20-29 | 27.38 | 11.28 | 11.28 | 4.83 | 0 | 64.81a | 33.24a | 21.60a | 9.97 | 0 | 45.81a | 22.08a | 16.36a | 7.36 | 0 |
| 30-39 | 24.75 | 12.10 | 8.25 | 4.40 | 0 | 53.39a | 19.41a | 24.27a | 9.71 | 2.70 | 39.21a | 15.79a | 16.34a | 7.08 | 1.36 |
| 40-49 | 37.08 | 14.21 | 12.98 | 9.89 | 0 | 78.52a | 28.99a | 26.58a | 22.95 | 1.21 | 58.04a | 21.69a | 19.85a | 16.49 | 0.61a |
| 50-59 | 45.11 | 12.60a | 27.86 | 4.64 | 3.98 | 66.78a | 23.53a | 30.53a | 12.72 | 7.63 | 56.17a | 18.18a | 29.22 | 8.77 | 5.84a |
| 60-69 | 64.50 | 14.95 | 44.05 | 5.51 | 9.44a | 77.51a | 20.12 | 42.48a | 14.91 | 8.94 | 71.18a | 17.60a | 43.24a | 10.33 | 9.18a |
| 70+ | 111.89a | 31.62a | 68.64a | 11.57a | 13.11a | 112.73a | 26.37a | 58.01a | 28.35 | 13.18a | 112.32a | 28.79a | 62.91a | 20.61a | 13.15a |
| Total b | 48.11a | 15.47a | 23.51a | 6.61a | 3.11a | 74.49a | 25.45a | 31.90a | 16.11 | 4.82a | 61.56a | 20.40a | 29.55a | 11.45 | 3.99a |
| 2000/2001, y | |||||||||||||||
| 20-29 | 28.17 | 9.64 | 5.93 | 12.60 | 0 | 23.22 | 7.49 | 8.24 | 7.49 | 0.75 | 25.71 | 8.57 | 7.08 | 10.06 | 0.37 |
| 30-39 | 26.72 | 7.54 | 11.65 | 7.54 | 1.37 | 28.35 | 8.10 | 9.45 | 10.80 | 0.68 | 27.55 | 7.82 | 10.54 | 9.18 | 1.02 |
| 40-49 | 20.70 | 6.42 | 12.14 | 2.14 | 0 | 29.59 | 3.52 | 11.27 | 14.79 | 0 | 25.17 | 4.96 | 11.70 | 8.51 | 0 |
| 50-59 | 36.28 | 5.18 | 27.64 | 3.46 | 1.73 | 47.52 | 19.36 | 12.32 | 15.84 | 3.52 | 41.85 | 12.21 | 20.05 | 9.59 | 2.62 |
| 60-69 | 48.35 | 9.40 | 22.83 | 16.12 | 1.34 | 56.01 | 24.00 | 21.34 | 10.67 | 4.00 | 52.20 | 16.73 | 22.08 | 13.38 | 2.68 |
| 70+ | 63.85 | 19.02 | 19.02 | 25.81 | 0 | 71.63 | 22.88 | 26.86 | 21.89 | 2.98 | 68.34 | 21.25 | 23.55 | 23.55 | 1.72 |
| Total b | 33.74 | 8.76 | 15.33 | 9.64 | 0.73 | 33.74 | 12.63 | 13.75 | 13.33 | 1.68 | 36.78 | 10.73 | 14.53 | 11.52 | 1.22 |
Age-specific admission rate per million.
Abbreviations: AC, adrenal crisis; AI, adrenal insufficiency; All SAI, all secondary adrenal insufficiency (includes “other and drug”); Drug AI, drug-induced adrenal insufficiency; PAI, primary adrenal insufficiency.
At least P less than .001 for trend difference between 2000/2001 and 2018/2019.
Age adjusted.
Figure 2.
Adjusted total adrenal insufficiency (AI) and adrenal crisis (AC) admission rates/million population by sex and year in Australia, 2000/2001 to 2018/2019. The increase in AI and AC admissions is most evident among females.
Figure 3.
A, Adrenal insufficiency (AI) admission rates/million in males by age group and year in Australia, 2000/2001 to 2018/2019. AI admission rates were predominantly seen in males older than 70 years. B, AI admission rates/million in females by age group and year in Australia, 2000/2001 to 2018/2019. In females, AI admission rates were seen in the decade 2010 to 2019, across all age groups, especially in the over 70 age group but also among those aged 20 to 29 years.
Adrenal Crisis Admissions
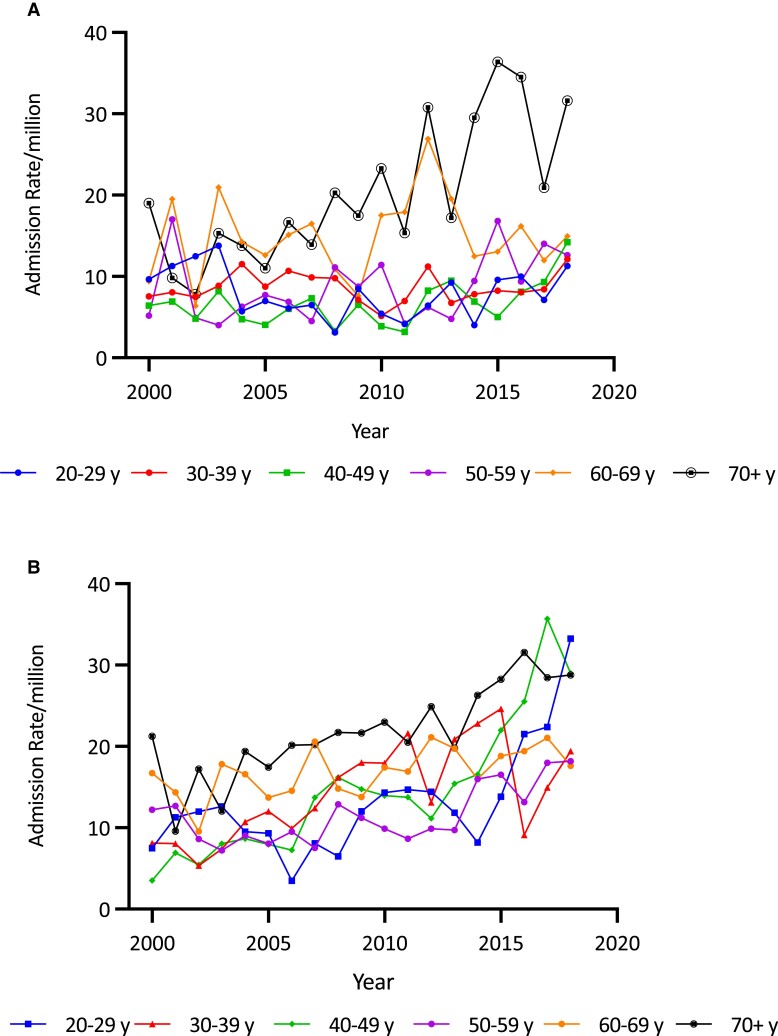
Overall, there was a 90.1% increase in the age-adjusted AC admission rate over the study period (from 10.73/million to 20.40/million; trend P < .00001) (see Table 1 and Fig. 1). The increase was greater in the second decade (63.6%, from 12.47/million in 2010/2011 to 20.40/million in 2018/2019) than in the first (19.0%, from 10.73/million in 2000/2001 to 12.77/million in 2009/2010) (Fig. 1). AC admission rate increases were greater in females (102.9%, from 12.63 to 25.62/million; trend P < .0001) than males (69.6%, from 8.76 to 14.86/million; trend P < .0001) (see Fig. 2 and Table 1). Females had a higher average AC admission rate (16.25 [4.95]/million/year) than males (10.05 [2.05]/million/year) (P < .0001). Average AC admission rates varied by age, being higher in the older than the younger groups (P < .0001). Within the age- and sex-specific groups, AC rates increased in females in all but those aged 60 to 69 years. The most marked increases were in those aged 20 to 29 years (by 343.7%, from 7.49 to 33.24/million; trend P < .0001) and 40 to 49 years (by 723.1%, from 3.52 to 28.99/million; trend P < .001). (Fig. 4A and Table 1). By comparison, increases were found in men older than 50 years (Fig. 4B and Table 1).
Figure 4.
A, Adrenal crisis (AC) admission rates per million population in males by age group and year in Australia, 2000/2001 to 2018/2019. ACs among males occurred predominantly in the 60 to 69 and over 70 age group. B, AC admission rates per million population in females by age group and year in Australia, 2000/2001 to 2018/2019. AC rates increased in most age groups among females with a notable increase in recent years among women older than 70 years, similar to that seen in men and also in the younger age group 20 to 29 years.
Admission Rates for Primary Adrenal Insufficiency
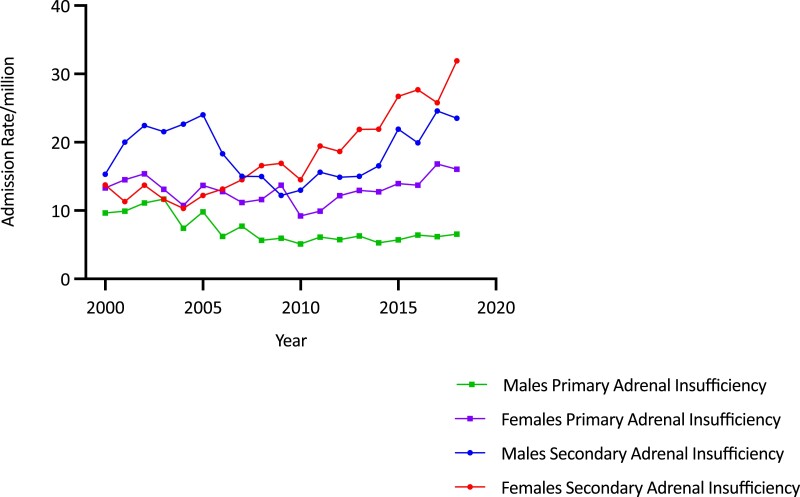
Overall, admission rates for PAI averaged 10.21 (1.58)/million/year over the study period, with no significant secular trend (Fig. 5). Rates were stable among females but decreased in males (by 32.2%, from 9.64 to 6.54/million; trend P < .0001) (see Fig. 5). Average age-adjusted PAI admission rates were higher in females than in males (13.03 [1.97]/million/year vs 7.28 [2.07]/million/year; P < 0.0001) and highest in the oldest age group (16.56 [4.52]/million/year; P < .0001 for overall difference between the age groups). Age- and sex-specific analysis found no significant trends among females but there were significant decreases in men aged 60 to 69 years (−65.8%, from 16.12 to 5.51/million; trend P < .001) and 70 years and older (−55.2%, from 25.81 to 11.57/million; trend P < .001).
Figure 5.
Age-adjusted admission rates for primary (AI) and secondary adrenal insufficiency (SAI) per million by sex and year in Australia, 2000/2001 to 2018/2019. The increase in AI admission rates is most marked among females with SAI.
Admission Rates for Secondary Adrenal Insufficiency
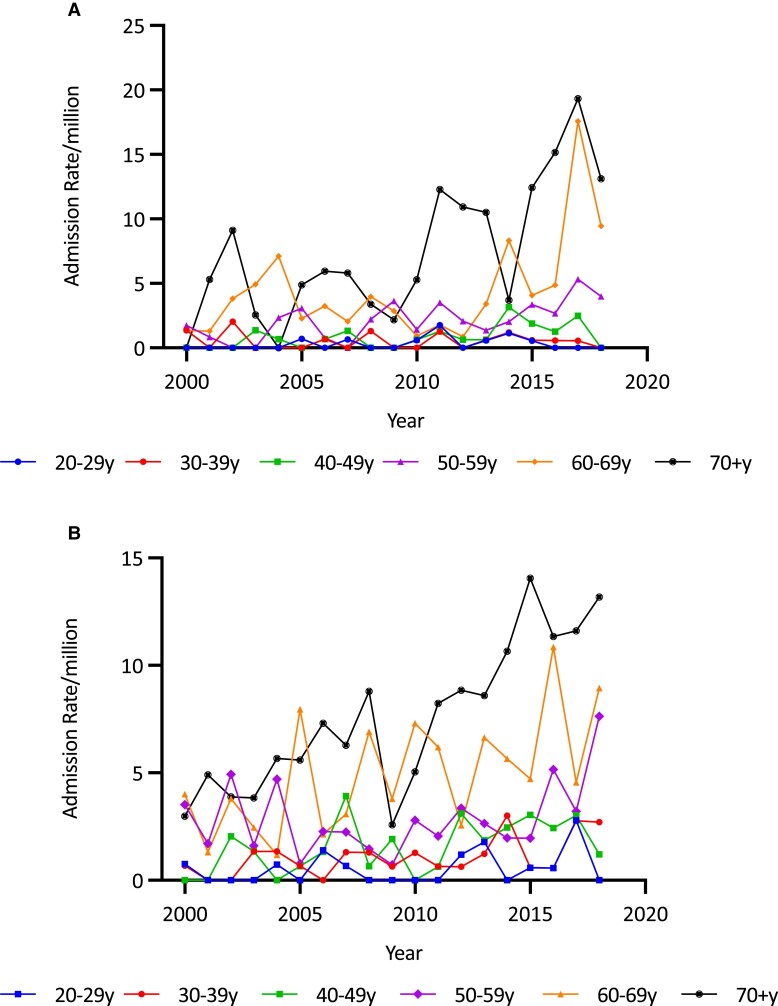
Overall age-adjusted admission rates for SAI increased by 91.7% (from 14.53 to 27.85/million; trend P < .0001) (see Fig. 5). Average age-adjusted SAI admission rates were comparable between the sexes (18.50 [4.03]/million/year for males and 18.03 [6.33]/million/year for females) but the secular increase among females (132.0%, from 13.75/million to 31.90/million; trend P < .00001) was more than double that in males (53.3%, from 15.33 to 23.51/million; trend P < .00001) (see Fig. 5). Average SAI admission rates were higher in older patients, with those aged 70 years and older having the highest average rate of 44.56/million (P < .0001 for overall age differences). Age- and sex-specific analysis showed that rates increased significantly among women in all age groups (all P < .00001), while rates increased only in men older than 70 years, in whom the admission rate increased by 260.9% (from 19.02/million to 68.64/million) (Fig. 6A and 6B and Table 1).
Figure 6.
A, Secondary adrenal insufficiency (SAI) admission rates per million in males by age group and year in Australia, 2000/2001 to 2018/2019. SAI admission rates have increased predominantly in men aged 60 to 69 and 70 to 79 years. B, SAI admission rates per million in females by age group and year in Australia, 2000/2001 to 2018/2019. SAI admission rates increased across most age ranges with a notable increase in the older age groups 60 to 69 years and 70 to 79 years, as well as those aged 20 to 29 years.
Drug-induced Adrenal Insufficiency Admission Rates
Admissions for the principal diagnosis of drug-induced AI were uncommon but increased by 227.0% over the study period (from 1.22/million to 3.99/million; trend P < .0001). Rates in males increased by 326.0% (from 0.73/million to 3.11/million) and for females by 186.9% (from 1.68/million to 4.82/million) (see Table 1). Average admission rates were highest in those 70 years and older (7.95 [5.54-10.36]/million) (P < .0001 for overall age difference). Within the age- and sex-specific categories, statistically significant rate increases were found in males 60 and older and in women older than 70 years (Fig. 7A and 7B and Table 1).
Figure 7.
A, Drug-induced adrenal insufficiency (AI) admission rates per million in males by age group and year in Australia, 2000/2001 to 2018/2019. A recent increase was noted in older males. B, Drug-induced AI admission rates/million in females by age group and year in Australia, 2000/2001 to 2018/2019. A recent increase was notable in older age groups.
Glucocorticoid Prescriptions
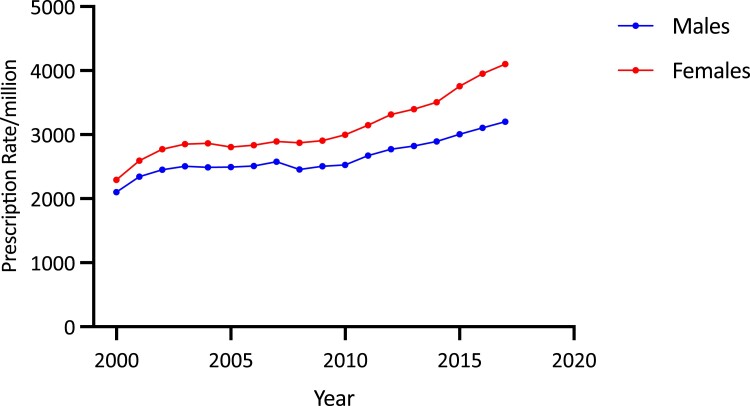
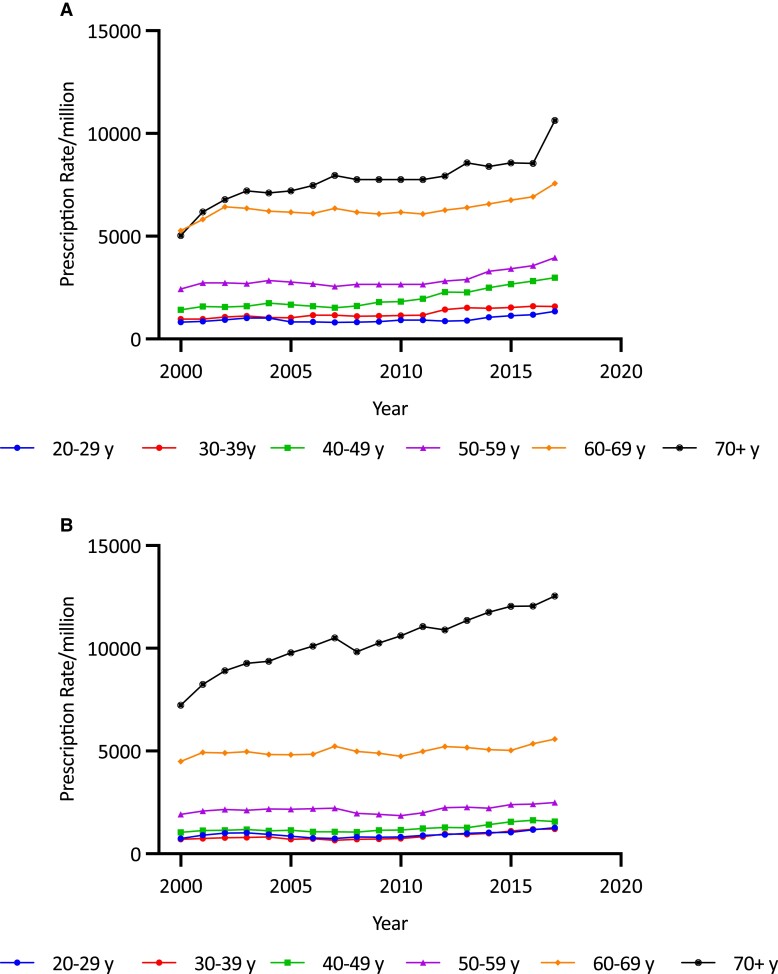
Overall age-adjusted GC prescription rates increased by 67.2%, from 2198.36/million in 2000/2001 to 3676.00/million in 2017/2018 (trend P < .0001). The increase over the second decade (35.4%, from 2715.76/million to 3676.00/million) was higher than that in the first (21.6%, from 2198.36/million to 2673.45/million). Females had a higher average GC prescription rate than males (3103.7 [478.1]/million/year vs 2635.9 [281.4]/million/year; P < .001) and a larger secular increase in GC prescription rates (78.9%, from 2292.01 to 4100.51/million vs 52.5%, from 2100.88 to 3202.25/million in males) (both P < .0001) (Fig. 8). Average GC prescription rates were higher in the older than younger age groups, being highest in those older than 70 years (8694.97/million) and lowest among 20- to 29-year-olds (940.02/million) (overall difference P < .001). Age-sex-specific analysis showed that prescription rates increased significantly in each age and sex category (all P < .00001) (Fig. 9A and 9B). The most pronounced increases were in men 70 and older (by 73.5% from 7230.27/million to 12 543.34/million) (see Fig. 9B), in women 70 years and older (by 111.1% from 5036.31/million to 10 629.05/million), and women aged 40 to 49 years (by 110.65% from 1414.50/million to 2879.59/million) (see Fig. 9A).
Figure 8.
Adjusted total glucocorticoid prescription rates per million population by sex and year in Australia, 2000/2001 to 2017/2018.
Figure 9.
A, Age-specific total glucocorticoid (GC) prescription rates per million in females by year, 2000/2001 to 2017/2018. Increases were seen broadly but particularly in older age groups, especially those older than 70 years. B, Age-specific total GC prescription rates per million in males by year, 2000/2001 to 2017/2018. Most of the increase was seen in men older than 70 years.
Overall GC prescription rates correlated with AC and AI admission rates in females and with AC admission rates in males (Table 2). Within age- and sex-specific groups, females showed the strongest correlation between AI/AC admissions and GC prescription rates, whereas there were no statistically significant correlations for males in any of the age groups (see Table 2).
Table 2.
Pearson correlation coefficients (95% CIs) between glucocorticoid prescription rates/million and adrenal crisis admission rates/million and adrenal insufficiency admission rates/million by sex and age group
| Adrenal crisis, y | Men | Women |
|---|---|---|
| 20-29 | 0.28 (−0.21 to 0.66) | 0.74 (0.42 to 0.90)a |
| 30-39 | −0.05 (−0.50 to 0.43) | 0.49 (0.03 to 0.80) |
| 40-49 | 0.40 (−0.09 to 0.73) | 0.86 (0.70 to 0.90)a |
| 50-59 | 0.22 (−0.28 to 0.62) | 0.60 (0.20 to 0.80) |
| 60-69 | 0.28 (−0.21 to 0.66) | 0.40 (−0.08 to 0.70) |
| 70+ | 0.64 (0.25 to 0.85) | 0.57 (−0.10 to 0.80) |
| Total | 0.78 (0.49 to 0.90)a | 0.91 (0.77 to 0.97)a |
| Adrenal insufficiency, y | ||
| 20-29 | 0.06 (−0.42 to 0.51) | 0.77 (0.48 to 0.91)a |
| 30-39 | −0.28 (−0.66 to 0.22) | 0.72 (0.40 to 0.90)a |
| 40-49 | −0.01 (−0.47 to 0.46) | 0.92 (0.80 to 1.00)a |
| 50-59 | −0.03 (−0.49 to 0.44) | 0.74 (0.40 to 0.90)a |
| 60-69 | 0.18 (−0.31 to 0.66) | 0.66 (0.30 to 0.90) |
| 70+ | 0.70 (0.35 to 0.88) | 0.70 (0.30 to 0.90) |
| Total | 0.18 (−0.31 to 0.60) | 0.91 (0.77 to 0.97)a |
For the years 2000/2001 to 2017/2018.
P value less than .001.
Discussion
The results of this comprehensive, population-based study show that, from the year 2000, age-adjusted admission rates for AI and ACs increased by 62.0% and 90.1%, respectively, with these increases being more marked in the second decade. Over the same time, prescription rates for short-acting GCs rose by 67.2%. Together these results indicate that, since the beginning of this century, there has been an increase in AI prevalence, an apparently proportionate increase in AI hospitalizations but a disproportionate increase in the severity of AI presentations to the hospital.
The secular changes varied between patient groups, suggesting that the reasons for this epidemiological transition are multifactorial and inconsistent according to age and sex. Possible relevant contributory factors include an increase in iatrogenic AI from use of immunotherapies for malignant disease, more common in older people; greater use of cranial imaging leading to increased detection of pituitary adenomas with subsequent development of treatment-related AI; augmented use of opioids and inhaled corticosteroids, both of which may cause AI; and increased use over time of the recommended lower doses of short-acting GCs as replacement therapy, potentially predisposing some patients to episodes of symptomatic AI and/or AC events [6, 12, 14, 17, 18, 21]. Differential access to specialist endocrinology services, and to self-management education and support programs, may also be relevant in a large and diverse health care setting encompassing rural and remote communities, especially for newly diagnosed patients and those with considerable comorbid diseases including malignancies. Psychosocial factors that impede effective self-management may also play a role, and these are likely to differ between the sexes and across the lifecycle.
There were substantial increases in GC prescriptions and AI hospitalizations among older people, especially during the second decade, reflecting a previously undetected rapid rise in AI prevalence in this age group. Factors potentially responsible for this phenomenon include increasing rates of cancer immunotherapy-related AI and increased detection and treatment (surgery/radiotherapy) of pituitary tumors and other factors such as opioid and therapeutic GC exposures [13, 14, 18]. The considerable increases in admissions for the principal diagnoses of drug-induced AI and total SAI in this age group support this hypothesis. An estimated 10% of patients treated with a CTLA-4–based therapy develop hypophysitis/hypopituitarism leading to permanent AI, with this risk increasing when an anti-PD-1/PD-L1 agent is added [13]. Common malignancies treated with immunotherapy, melanoma, and renal cancer are more prevalent in older people, especially men, with Australia having one of the highest rates of melanoma globally [22, 23]. Concomitant GC or opiate use may also cause AI, and GC use may mask symptoms of AI until the GC is withdrawn [24].
The rise in AI in older people is concerning, given that diagnosis and management of AI may be particularly challenging in this age group because of their higher prevalence of substantial comorbidities and other problems such as cognitive deficits and social isolation, all of which make AI self-management more difficult [25]. An increase in AI prevalence in this age group may therefore translate into relatively higher levels of hospitalization compared to that in younger people. This phenomenon may not be evident when only the principal diagnosis is considered, however, as AI-related hospitalizations are known to be comparatively underestimated in older people, for whom the main reason for a hospital stay in AI patients is more likely to be another illness, such as an infection [25, 26]. AC rates may also be underestimated in this age group, if the AC definition used does not consider relative hypotension among hypertensive individuals as an indicator of hemodynamic compromise [27].
Hospitalizations for AI/AC and short-acting GC prescriptions were higher in females, as were the corresponding rate increases. This was unexpected, as AI prevalence is generally comparable between the sexes in Western countries, although there are some differences in the age distribution of incident cases (from earlier detection of pituitary tumors and some increased risk of autoimmune adrenalitis in females) [8, 18, 19]. The considerable rise in AC admissions in younger women (up to 50 years) may reflect a range of factors including greater use of multiday dosing and of short-acting GCs, perhaps at lower doses per body surface area to reduce the likelihood of weight gain, rather than an increase in AI prevalence. This may be compounded by other factors that are more common in young adults, as presented in an earlier analysis of emerging adults (up to age 24 years), such as difficulties with transition to systems of adult care; emergence of a range of psychosocial issues such as drug and alcohol use; eating disorders (more common in females); and financial and other stresses, all of which may make management of AI more complex and difficult [28]. Younger women have been shown to have greater problems than men with the more common endocrine disorder requiring high levels of self-management, type 1 diabetes mellitus, with a female preponderance of diabetic ketoacidosis episodes [29, 30]. Conversely, hypervigilance and anxiety about AC events may result in a lower threshold to seek medical care and may also contribute to higher levels of hospitalization.
The higher prevalence of SAI relative to PAI in this population is consistent with the established epidemiology of AI in Western countries [1, 2]. However, the relative increase in SAI admissions has not been previously reported. While the reasons for this phenomenon are uncertain, and interpretation is made more difficult because the cause of nonprimary AI is not clearly defined in this data set, it may be due to hypophysitis/hypopituitarism from immunotherapy, as mentioned earlier, and treatment of pituitary adenomas, which are common in older people and have increased in frequency, a change that has been attributed to higher levels of identification due to increased imaging for unrelated symptoms and a shift in the diagnostic cutoffs for insulin-like growth factor-1 for the diagnosis of acromegaly [18]. Other common medications such as therapeutic GCs and opioids are also associated with the development of SAI, although the extent to which these result in the need for AI-related hospitalization appears to be low, and their effect may be differentially distributed by age and sex [12, 31].
Irrespective of the underlying cause, all patients with AI are at risk of an AC during physiological stress (annual risk of ∼6%-8%). All patients require ongoing education about stress dosing (oral or subcutaneous/intramuscular) during periods of illness, should have access to hydrocortisone for injection, carry an emergency card, and wear medical jewelry indicating their AI diagnosis [3, 4]. Although educational initiatives are regarded as a mainstay of prevention, ACs continue to cause morbidity and occasional mortality [32]. Some patients may present with an AC, usually after experiencing symptoms of AI that are undiagnosed, and there appears to be a subgroup of patients who seem to be particularly vulnerable to AC events [5]. Patients at risk of iatrogenic AI (such as those receiving immunotherapy for a malignancy) should be assessed according to recommended protocols to preempt presentation in acute AI [24]. The results of this study suggest that demands on services by patients with AI are increasing and that, as a consequence, patients with AI may be treated by a wider range of health workers, possibly leading them to miss the necessary support and education services to manage their AI effectively to maximize their quality of life and avoid ACs.
Data used in this study were from large population-based national repositories comprising nearly 20 years of admission and prescription data and encompassed the experience of urban, regional, and remote populations and health services. These data, therefore, provide a great source of information on the evolving epidemiology of AI. However, limitations of these data include the availability of the principal diagnosis for each admission only, so that the underlying diagnosis of patients presenting in an AC was not available. Prescriptions for prednisolone/prednisone, dexamethasone, and fludrocortisone were not included, as these formulations are not exclusively prescribed for AI. In addition, these data were unmatched for individuals, so that repeated admissions from a small number of patients, which may be influential with regard to rates, could not be assessed. The diagnosis of AI and its subtypes, including an AC diagnosis, was made by the clinician supervising that admission and therefore reflected the definitions for AC/symptomatic AI used by individual practitioners. The possibilities of misclassification of AC and AI as principal diagnosis are, therefore, inherent in these data. While the definitions of an AC have evolved over time, the nonuniformity of AC/AI rate changes between age and sex groups suggest that a widespread change in diagnostic criteria for an AC did not occur.
In summary, the results of the present study, which spans the years 2000/2001 to 2018/2019, provide important information on the changing epidemiological landscape of AI in Australia, with these results likely to apply to a number of populations worldwide. During this period, AI and AC admission rates increased substantially, accompanied by a rise in SAI relative to PAI diagnoses among hospitalized patients, likely due to a number of factors including increased pituitary interventions arising from more widespread use of planar imaging of the head for screening; use of immune checkpoint inhibitors as an antineoplastic, especially in older people; exposure to other agents, such as opioids; and a gradual increase in the use of short-acting GCs. An increase in presentation rates in young and middle-aged women may be due to greater use of short-acting GCs relative to males and older age groups, or other factors inapparent in this data set that require further investigation. Overall, these results highlight the value of periodic examination of routinely collected data sets and the need for ongoing surveillance of AI admissions. They also demonstrate the increasing influence of iatrogenesis on modern AI epidemiology, the extent of which may not be recognized without examination of data repositories.
Abbreviations
- AC
adrenal crisis
- AI
adrenal insufficiency
- DHHS
Australian Commonwealth Department of Health and Human Services
- GC
glucocorticoid
- PAI
primary adrenal insufficiency
- PBS
Australian Pharmaceutical Benefits scheme
- SAI
secondary adrenal insufficiency
Contributor Information
R Louise Rushworth, Email: rosemary.louise.rushworth@gmail.com, School of Medicine Sydney, The University of Notre Dame, Australia, Darlinghurst, New South Wales 2010, Australia.
David J Torpy, Endocrine and Metabolic Unit, Royal Adelaide Hospital, University of Adelaide, Adelaide, South Australia 5000, Australia.
Financial Support
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosures
The authors have nothing to disclose.
Data Availability
Data used to determine admission rates (numbers of hospital admissions by diagnosis) are freely available at https://www.aihw.gov.au/reports/hospitals/principal-diagnosis-data-cubes/contents/data-cubes. Data on prescription rates are not publicly available.
References
- 1. Arlt W, Allolio B.. Adrenal insufficiency. Lancet. 2003;361(9372):1881–1893. [DOI] [PubMed] [Google Scholar]
- 2. Regal M, Páramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol (Oxf). 2001;55(6):735–740. [DOI] [PubMed] [Google Scholar]
- 3. Allolio B. Extensive expertise in endocrinology. Adrenal crisis. Eur J Endocrinol. 2015;172(3):R115–R124. [DOI] [PubMed] [Google Scholar]
- 4. Rushworth RL, Torpy DJ, Falhammar H. Adrenal crisis. N Engl J Med. 2019;381(9):852–861. [DOI] [PubMed] [Google Scholar]
- 5. Hahner S, Spinnler C, Fassnacht M, et al. High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab. 2015;100(2):407–416. [DOI] [PubMed] [Google Scholar]
- 6. Rushworth RL, Torpy DJ. Adrenal insufficiency in Australia: is it possible that the use of lower dose, short-acting glucocorticoids has increased the risk of adrenal crises? Horm Metab Res. 2015;47(6):427–432. [DOI] [PubMed] [Google Scholar]
- 7. Oshino S, Saitoh Y, Kinoshita M, Mukai K, Otsuki M, Kishima H. Characteristics of nonfunctioning pituitary adenomas that cause secondary adrenal insufficiency. World Neurosurg. 2021 Sep;153:e275–e281. [DOI] [PubMed] [Google Scholar]
- 8. Meyer G, Neumann K, Badenhoop K, Linder R. Increasing prevalence of Addison's disease in German females: health insurance data 2008-2012. Eur J Endocrinol. 2014;170(3):367–373. [DOI] [PubMed] [Google Scholar]
- 9. Mukhopadhyay P, Pandit K, Ghosh S. Adrenal disorders in the tropics. 2021 May 22. In: Feingold KR, Anawalt B, Boyce A, et al., eds. Endotext [Internet]. MDText.com Inc; 2000. [Google Scholar]
- 10. Lubomski A, Falhammar H, Torpy DJ, Rushworth RL. The epidemiology of primary and secondary adrenal malignancies and associated adrenal insufficiency in hospitalised patients: an analysis of hospital admission data, NSW, Australia. BMC Endocr Disord. 2021;21(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Einarsdottir MJ, Trimpou P, Olsson DS, Johannsson G, Ragnarsson O. The prevalence of oral glucocorticoid use in doses associated with risk of tertiary adrenal insufficiency. Endocrine Abstracts. 2019;63:P1011. [Google Scholar]
- 12. Li T, Cunningham JL, Gilliam WP, Loukianova L, Donegan DM, Bancos I. Prevalence of opioid-induced adrenal insufficiency in patients taking chronic opioids. J Clin Endocrinol Metab. 2020;105(10):e3766–e3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wright JJ, Powers AC, Johnson DB. Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. 2021;17(7):389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raschi E, Fusaroli M, Massari F, et al. The changing face of drug-induced adrenal insufficiency in the Food and Drug Administration adverse event reporting system. J Clin Endocrinol Metab. 2022;107(8):e3107–e3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi Y, Shen M, Zheng X, Yang T. Immune checkpoint inhibitor-induced adrenalitis and primary adrenal insufficiency: systematic review and optimal management. Endocr Pract. 2020;27(2):165–169. [DOI] [PubMed] [Google Scholar]
- 16. Rushworth RL, Torpy DJ. Modern hydrocortisone replacement regimens in adrenal insufficiency patients and the risk of adrenal crisis. Horm Metab Res. 2015;47(9):637–642. [DOI] [PubMed] [Google Scholar]
- 17. El-Maouche D, Hargreaves CJ, Sinaii N, Mallappa A, Veeraraghavan P, Merke DP. Longitudinal assessment of illnesses, stress dosing, and illness sequelae in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2018;103(6):2336–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crowther S, Rushworth RL, Rankin W, Falhammar H, Phillips LK, Torpy DJ. Trends in surgery, hospital admissions and imaging for pituitary adenomas in Australia. Endocrine. 2018;59(2):373–382. [DOI] [PubMed] [Google Scholar]
- 19. Meyer G, Badenhoop K, Linder R. Addison's disease with polyglandular autoimmunity carries a more than 2.5-fold risk for adrenal crises: German health insurance data 2010-2013. Clin Endocrinol (Oxf). 2016;85(3):347–353. [DOI] [PubMed] [Google Scholar]
- 20. National Centre for Classification in Health . International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD-10-AM). 5th ed.Australian Classification of Health Interventions (ACHI), Australian Coding Standards (ACS), National Centre for Classification in Health; 2006. [Google Scholar]
- 21. Lapi F, Kezouh A, Suissa S, Ernst P. The use of inhaled corticosteroids and the risk of adrenal insufficiency. Eur Respir J. 2013;42(1):79–86. [DOI] [PubMed] [Google Scholar]
- 22. Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of renal cell carcinoma. World J Oncol. 2020;11(3):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raimondi S, Suppa M, Gandini S. Melanoma epidemiology and sun exposure. Acta Derm Venereol. 2020;100(11):adv00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayes AG, Rushworth RL, Torpy DJ. Risk assessment, diagnosis, and treatment of cancer treatment-related adrenal insufficiency. Expert Rev Endocrinol Metab. 2022;17(1):21–33. [DOI] [PubMed] [Google Scholar]
- 25. Rushworth RL, Torpy DJ, Falhammar H. Adrenal crises in older patients. Lancet Diabetes Endocrinol. 2020;8(7):628–639. [DOI] [PubMed] [Google Scholar]
- 26. Rushworth RL, Torpy DJ. A descriptive study of adrenal crises in adults with adrenal insufficiency: increased risk with age and in those with bacterial infections. BMC Endocr Disord. 2014;14(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rushworth RL, Goubar T, Ostman C, McGrath S, Torpy DJ. Interaction between hypotension and age on adrenal crisis diagnosis. Endocrinol Diabetes Metab. 2020;4(2):e00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chrisp GL, Quartararo M, Torpy DJ, Falhammar H, Rushworth RL. Trends in hospital admissions for adrenal insufficiency in adolescents and young adults in the 21st century. Front Endocrinol (Lausanne). 2022 Sep 20;13:986342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Australian Institute of Health and Welfare . Diabetic Ketoacidosis (DKA) Among Children and Young People with Type 1 Diabetes. AIHW; 2016. [Google Scholar]
- 30. Australian Institute of Health and Welfare . Australia's Health 2020. AIHW; 2020. [Google Scholar]
- 31. Rushworth RL, Chrisp GL, Torpy DJ. Glucocorticoid-induced adrenal insufficiency: a study of the incidence in hospital patients and a review of peri-operative management. Endocr Pract. 2018;24(5):437–445. [DOI] [PubMed] [Google Scholar]
- 32. Hahner S, Loeffler M, Bleicken B, et al. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol. 2010;162(3):597–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used to determine admission rates (numbers of hospital admissions by diagnosis) are freely available at https://www.aihw.gov.au/reports/hospitals/principal-diagnosis-data-cubes/contents/data-cubes. Data on prescription rates are not publicly available.