Abstract
Background
Scarce epidemiological studies have characterised allergic rhinitis (AR) and non-allergic rhinitis (NAR) in adults. In a population-based cohort, our aims were to 1) describe rhinitis, AR and NAR, and 2) explore how asthma and conjunctivitis may lead to the identification of novel rhinitis phenotypes.
Methods
In this cross-sectional analysis, current rhinitis was defined as present in the last 12 months using a questionnaire from the French CONSTANCES cohort. Participants with current rhinitis reporting nasal allergies were considered as AR, otherwise as NAR. We described AR and NAR phenotypes, and their phenotypes including co-occurrence with ever-asthma and ever-conjunctivitis.
Results
Among the 20 772 participants included in this analysis (mean±sd age 52.6±12.6 years; 55.2% female), crude prevalences of AR and NAR were 28.0% and 10.9%. AR participants more frequently reported persistent rhinitis (31.6% versus 25.1%) and moderate-to-severe rhinitis (40.1% versus 24.2%) than NAR participants. Among AR or NAR participants, those with ever-asthma reported more moderate-to-severe rhinitis. Participants with AR, ever-asthma and ever-conjunctivitis had an earlier age of rhinitis onset, more severe rhinitis and higher eosinophil counts than participants in other groups. Results were replicated in another cohort.
Conclusions
In this large population-based cohort, 40% reported current rhinitis, with a lower prevalence of moderate-to-severe rhinitis than in clinical practice. For the first time in a general adult population, we showed that AR and NAR alone or in combination with asthma or in combination with asthma and conjunctivitis are different phenotypes. These results provide new insights on how best to manage rhinitis and its multimorbidities.
Short abstract
For the first time in a general adult population, this study shows novel rhinitis phenotypes based on allergic rhinitis and non-allergic rhinitis multimorbidities, and that participants with asthma and conjunctivitis had more severe rhinitis https://bit.ly/3ReCsoN
Introduction
Rhinitis describes nasal symptoms resulting from inflammation and/or dysfunction of the nasal mucosa [1]. It is one of the most common chronic conditions, and is a global health problem causing major burden and disability worldwide [2, 3].
Behind the apparent simplicity of its clinical definition, rhinitis is a complex and heterogeneous disease characterised by several phenotypes. After excluding acute infectious rhinitis, rhinitis can be divided into two major phenotypes: allergic rhinitis (AR) and non-allergic rhinitis (NAR). AR is caused by IgE-mediated reactions to inhaled allergens and NAR is a heterogeneous group of nasal conditions [4]. However, allergic and non-allergic mechanisms are often intertwined. AR and NAR even share some common symptoms (nasal congestion and rhinorrhoea), but they have different clinical features and treatments [5]. AR usually starts in childhood, whereas NAR often appears later. Many patients with AR have a seasonal exacerbation of symptoms, mainly due to aeroallergen exposure, and AR is characterised by the presence of the full spectrum of nasal symptoms, which are frequently associated with eye symptoms [5]. Most NAR patients have symptoms triggered by non-allergenic exposures and ocular symptoms appear to be uncommon [5]. However, most of this knowledge on rhinitis is derived from clinical practice, and there are few epidemiological studies in the general adult population that have assessed the prevalence and characteristics of phenotypes of rhinitis. Furthermore, few of them have assessed NAR [3, 6, 7].
Asthma is a major multimorbidity of both AR and NAR: >80% of patients with asthma have rhinitis and 10–40% of patients with rhinitis have asthma [1]. Surprisingly, although many studies have described the characteristics of asthma in relation to the presence or absence of rhinitis, few population-based epidemiological studies have described rhinitis in relation to asthma multimorbidity [8]. In particular, the association between asthma and severity of rhinitis is unclear [1, 9]. Conjunctivitis is also an important multimorbidity of AR [3] and provides additional information when studying allergic multimorbidities [10]. This suggests that there may be different AR phenotypes depending on the presence or absence of asthma and conjunctivitis. However, to the best of our knowledge, no population-based study in adults has investigated the characteristics of AR in relation to asthma and conjunctivitis.
Our objective was to describe the characteristics of rhinitis, considering AR and NAR separately in adults from the French population-based cohort CONSTANCES, and to explore phenotypic differences between rhinitis alone and rhinitis associated with asthma and/or conjunctivitis. Finally, we externally validated our results in the French case–control epidemiological study on asthma (EGEA).
Methods
Study design
A cross-sectional study was carried out with the data from the 2014 annual follow-up of CONSTANCES. CONSTANCES is a population-based cohort of almost 220 000 adults aged 18–69 years at inclusion, randomly selected from social security affiliates in France (https://www.constances.fr). The participants were enrolled from 2012 to 2020 in 20 administrative districts (supplementary figure S1). At inclusion, participants completed standardised questionnaires and had a complete medical examination [11–13]. Annual follow-ups were done by questionnaire.
All participants who were included until the end of 2013 received the 2014 questionnaire that included two pages of detailed, validated and standardised questions on rhinitis (supplementary material).
All confidentiality, safety and security procedures were approved by the French legal authorities (supplementary material). All participants signed a written informed consent.
Definitions
Rhinitis
We defined current and ever-rhinitis using the standardised questions proposed in the International Study of Asthma and Allergies in Childhood (ISAAC) [14], as recommended by Allergic Rhinitis and its Impact on Asthma (ARIA) [15]. Participants were considered as having ever-rhinitis if they answered yes to “During your lifetime, have you ever had a problem with sneezing, or a runny, or a blocked nose when you did not have a cold or the flu?”, otherwise participants who answered no were classified as never having rhinitis. Among those with ever-rhinitis, participants were classified as having current rhinitis if they answered yes to “Have you had these problems in the last 12 months?”, otherwise participants who answered no were classified as having non-current rhinitis.
To distinguish AR from NAR, we used an adaptation of the European Community Respiratory Health Survey (ECRHS) question to define nasal allergies [16] that was recently shown to be a suitable proxy in epidemiological studies [17]. Participants with rhinitis who answered yes to “Have you ever had nasal allergies in your lifetime, including hay fever?” were classified as AR, otherwise participants who answered no were classified as NAR.
Definitions of rhinitis duration, rhinitis severity and reported symptoms are described in the supplementary material.
Asthma
Participants were considered as having ever-asthma if they answered yes at inclusion to “Have you ever had asthma?” or answered “Asthma” at the 2014 annual follow-up questionnaire to: “Here is a list of health problems. Indicate here the ones you have suffered from in the last 12 months (whether or not there was a work interruption, whether or not there is a treatment)”.
Conjunctivitis
Participants were considered as having ever-conjunctivitis if they answered yes at the 2014 annual follow-up questionnaire to “During your lifetime, have you ever had allergic conjunctivitis?”.
Statistical methods
Analyses were carried out in complete cases, i.e. participants with missing data were excluded and no imputation was performed.
The Chi-squared test for categorical variables and the t-test (when comparing two groups) or ANOVA comparison of variances (when comparing more than two groups) for continuous variables were used. We also conducted sensitivity analyses using alternative definitions to distinguish AR from NAR: one definition based on the triggers of nasal symptoms and one definition based on the classification tree obtained by Burte et al. [18] with an unsupervised approach to identify AR and NAR (supplementary material).
As asthma is a major multimorbidity of AR and NAR, we described AR or NAR according to whether or not participants had reported ever-asthma. Subsequently, we described phenotypes of AR including ever-conjunctivitis: participants who reported only AR, those who reported AR plus ever-conjunctivitis only, those who reported AR plus ever-asthma only and those who reported AR plus ever-conjunctivitis plus ever-asthma. This step could only be done for AR as there were not enough participants with NAR reporting both ever-asthma and ever-conjunctivitis.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Replication study
To validate our findings, we replicated some analyses in the case–control EGEA study. We used a rhinitis definition based on very similar questions. EGEA participipants also had biomarkers and tests of allergic sensitisation (total IgE and skin prick tests (SPTs) to 12 allergens) (supplementary material).
Results
Demographic characteristics of the participants
The 2014 annual follow-up questionnaire was sent to 26 737 participants included by 2013 and was completed by 21 507 (80%) of them. Participants with missing data regarding the definitions of rhinitis (n=497) or nasal allergies (n=238) were excluded from the analyses (supplementary figure S2). Non-included participants were on average more often male, younger, smokers, with a lower level of education and a higher body mass index, and reported more ever-asthma than included participants (supplementary table S1).
There were 20 772 participants included in the main analyses (mean±sd age 52.6±12.6 years; 55.2% female). Among them, 13.5% were current smokers, 12.7% reported ever-asthma and 29.2% reported ever-conjunctivitis (table 1).
TABLE 1.
Characteristics of participants according to their rhinitis status
| All (n=20 772) | Never-rhinitis (n=9674) | Non-current rhinitis (n=3029) | Current rhinitis (n=8069) | p-value | |
| Sex | <0.0001 | ||||
| Male | 9297 (44.8) | 4358 (45.0) | 1459 (48.2) | 3480 (43.1) | |
| Female | 11 475 (55.2) | 5316 (55.0) | 1570 (51.8) | 4589 (56.9) | |
| Age, years | <0.0001 | ||||
| <30 | 1057 (5.1) | 470 (4.9) | 123 (4.1) | 464 (5.8) | |
| [30–45[ | 4924 (44.1) | 2181 (22.5) | 645 (21.3) | 2098 (26.0) | |
| [45–60[ | 7648 (36.8) | 3659 (37.8) | 1184 (39.1) | 2805 (34.8) | |
| ≥60 | 7143 (34.4) | 3364 (34.8) | 1077 (35.6) | 2702 (33.5) | |
| Tobacco status | 0.063 | ||||
| Never-smoker | 9164 (46.4) | 4353 (47.3) | 1323 (46.0) | 3488 (45.5) | |
| Ex-smoker | 7904 (40.0) | 3588 (39.0) | 1157 (40.2) | 3159 (41.2) | |
| Current smoker | 2675 (13.5) | 1259 (13.7) | 398 (13.8) | 1018 (13.3) | |
| Educational level | <0.0001 | ||||
| Less than high school | 1938 (9.4) | 927 (9.7) | 316 (10.6) | 695 (8.7) | |
| High school | 6755 (32.9) | 3321 (34.7) | 1036 (34.8) | 2398 (30.1) | |
| University | 11 825 (57.6) | 5316 (55.6) | 1625 (54.6) | 4884 (61.2) | |
| Body mass index, kg·m−2 | 0.019 | ||||
| <18.5 | 460 (2.3) | 222 (2.3) | 66 (2.2) | 172 (2.2) | |
| [18.5–25[ | 11 510 (56.5) | 5327 (56.2) | 1610 (54.1) | 4573 (57.9) | |
| [25–30[ | 6283 (30.9) | 2970 (31.3) | 966 (32.5) | 2347 (29.7) | |
| ≥30 | 2104 (10.3) | 968 (10.2) | 332 (11.2) | 804 (10.2) | |
| Asthma | <0.0001 | ||||
| Never-asthma | 17 793 (87.3) | 8948 (94.2) | 2485 (83.8) | 6360 (80.4) | |
| Ever-asthma | 2579 (12.7) | 549 (5.8) | 481 (16.2) | 1549 (19.6) | |
| Conjunctivitis | <0.0001 | ||||
| Never-conjunctivitis | 13 354 (70.8) | 7582 (85.3) | 1803 (65.9) | 3969 (54.8) | |
| Ever-conjunctivitis | 5509 (29.2) | 1308 (14.7) | 931 (34.1) | 3270 (45.2) | |
| Eczema | <0.0001 | ||||
| Never-eczema | 13 631 (71.9) | 7166 (79.4) | 1883 (68.7) | 4582 (63.7) | |
| Ever-eczema | 5337 (28.1) | 1863 (20.6) | 858 (31.3) | 2616 (36.3) | |
| Blood eosinophil count, cells·mm−3 | 185.5±126.2 | 172.0±112.2 | 189.7±125.5 | 199.8±139.7 | <0.0001 |
| Age of onset of rhinitis, years | 26.3±16.1 | NA | 25.2±15.1 | 26.7±16.5 | 0.0002 |
Data are presented as n (%) or mean±sd, unless otherwise stated. NA: not applicable.
AR and NAR
The crude prevalences of ever-rhinitis, ever-AR and ever-NAR were 53.4% (95% CI 52.8–54.1%), 36.5% (95% CI 35.9–37.2%) and 16.9% (95% CI 16.4–17.4%), respectively. The crude prevalences of current rhinitis, current AR and current NAR were 38.9% (95% CI 38.2–39.5%), 28.0% (95% CI 27.3–28.6%) and 10.9% (95% CI 10.5–11.3%), respectively.
The characteristics of the participants according to their rhinitis status (never, non-current or current) are presented in table 1. Participants with current rhinitis were more often female, younger, with a higher education level, had higher eosinophil counts, and had more ever-asthma, ever-conjunctivitis and ever-eczema than participants with never-rhinitis or with non-current rhinitis.
Participants with current AR reported significantly more often ever-asthma, ever-conjunctivitis and ever-eczema than those with NAR (table 2). The age of rhinitis onset was on average 10 years earlier for AR compared with NAR. AR participants reported more nasal symptoms than NAR participants; the highest differences between the two groups were observed for associated eye symptoms (68.8% versus 35.3%) and nasal itching (67.0% versus 39.7%). AR participants reported more persistent symptoms (31.6% versus 25.1%) and more moderate-to-severe rhinitis (40.1% versus 24.2%) than NAR participants. Triggers of rhinitis symptoms differed between AR and NAR: the largest differences were observed for pollens (52.3% versus 10.2%), dust mites/house dust (33.4% versus 10.4%) and unknown triggers (27.1% versus 48.6%). Treatments by oral antihistamines and intranasal corticosteroids were reported by 32.0% of AR participants and 6.0% of NAR participants.
TABLE 2.
Characteristics of participants with current rhinitis, current allergic rhinitis (AR) or current non-allergic rhinitis (NAR)
| All current rhinitis (n=8069) | AR (n=5806) | NAR (n=2263) | p-value | |
| Sex | 0.072 | |||
| Male | 3480 (43.1) | 2468 (42.5) | 1012 (44.7) | |
| Female | 4589 (56.9) | 3338 (57.5) | 1251 (55.3) | |
| Age, years | 51.9±12.9 | 51.7±12.8 | 52.4±13.3 | 0.036 |
| Tobacco status | 0.0044 | |||
| Never-smoker | 3488 (45.5) | 2534 (46.0) | 954 (44.3) | |
| Ex-smoker | 3159 (41.2) | 2289 (41.5) | 870 (40.4) | |
| Current smoker | 1018 (13.3) | 688 (12.5) | 330 (15.3) | |
| Educational level | 0.77 | |||
| Less than high school | 695 (8.7) | 495 (8.6) | 200 (8.9) | |
| High school | 2398 (30.1) | 1717 (29.9) | 681 (30.4) | |
| University | 4884 (61.2) | 3528 (61.5) | 1356 (60.6) | |
| Body mass index, kg·m−2 | 0.34 | |||
| <18.5 | 172 (2.2) | 124 (2.2) | 48 (2.2) | |
| [18.5–25[ | 4573 (57.9) | 3264 (57.5) | 1309 (59.0) | |
| [25–30[ | 2347 (29.7) | 1690 (29.8) | 657 (29.6) | |
| ≥30 | 804 (10.2) | 599 (10.6) | 205 (9.2) | |
| Asthma | <0.0001 | |||
| Never-asthma | 6360 (80.4) | 4304 (75.7) | 2056 (92.5) | |
| Ever-asthma | 1549 (19.6) | 1383 (24.3) | 166 (7.5) | |
| Conjunctivitis | <0.0001 | |||
| Never-conjunctivitis | 3969 (54.8) | 2317 (44.7) | 1652 (80.4) | |
| Ever-conjunctivitis | 3270 (45.2) | 2868 (55.3) | 402 (19.6) | |
| Eczema | <0.0001 | |||
| Never-eczema | 4582 (63.7) | 3021 (59.2) | 1561 (74.5) | |
| Ever-eczema | 2616 (36.3) | 2081 (40.8) | 535 (25.5) | |
| Blood eosinophil count, cells·mm−3 | 199.8±139.7 | 208.6±146.4 | 177.3±117.9 | <0.0001 |
| Age of onset of rhinitis, years | 26.7±16.5 | 24.3±15.3 | 34.6±17.5 | <0.0001 |
| Reported triggers of rhinitis symptoms# | ||||
| Dust mites or house dust | 2172 (26.9) | 1937 (33.4) | 235 (10.4) | <0.0001 |
| Animals | 732 (9.1) | 677 (11.7) | 55 (2.4) | <0.0001 |
| Air pollution | 1847 (22.9) | 1560 (26.9) | 287 (12.7) | <0.0001 |
| Change in weather | 2278 (28.2) | 1677 (28.9) | 601 (26.6) | 0.037 |
| Tobacco | 452 (5.6) | 359 (6.2) | 93 (4.1) | 0.0003 |
| Pollens | 3266 (40.5) | 3035 (52.3) | 231 (10.2) | <0.0001 |
| Cold air | 2050 (25.4) | 1423 (24.5) | 627 (27.7) | 0.0030 |
| Other | 1017 (12.6) | 735 (12.7) | 282 (12.5) | 0.81 |
| Unknown | 2673 (33.1) | 1573 (27.1) | 1100 (48.6) | <0.0001 |
| Reported symptoms# | ||||
| Rhinorrhoea | 5266 (70.7) | 4051 (75.1) | 1215 (59.0) | <0.0001 |
| Nasal congestion/obstruction | 5257 (72.4) | 4000 (76.3) | 1257 (62.1) | <0.0001 |
| Nasal itching | 4212 (59.5) | 3445 (67.0) | 767 (39.7) | <0.0001 |
| Sneezing | 5128 (69.4) | 4073 (75.9) | 1055 (52.2) | <0.0001 |
| Associated eye symptoms | 4391 (59.8) | 3693 (68.8) | 698 (35.3) | <0.0001 |
| Reported symptoms, n | 3.2±1.4 | 3.6±1.3 | 2.3±1.3 | <0.0001 |
| Rhinitis severity | <0.0001 | |||
| Mild | 4253 (64.3) | 2862 (59.9) | 1391 (75.8) | |
| Moderate-to-severe | 2358 (35.7) | 1913 (40.1) | 445 (24.2) | |
| Rhinitis duration | <0.0001 | |||
| Intermittent | 5422 (70.2) | 3798 (68.4) | 1624 (74.9) | |
| Persistent | 2302 (29.8) | 1758 (31.6) | 544 (25.1) | |
| Rhinitis treatment | <0.0001 | |||
| Neither OA nor INCS | 3507 (45.1) | 1911 (34.1) | 1596 (73.5) | |
| OA only | 1322 (17.0) | 1204 (21.5) | 118 (5.4) | |
| INCS only | 1025 (13.2) | 700 (12.5) | 325 (15.0) | |
| OA and INCS | 1925 (24.7) | 1794 (32.0) | 131 (6.0) |
Data are presented as n (%) or mean±sd, unless otherwise stated. OA: oral antihistamine; INCS: intranasal corticosteroid. #: several possible answers.
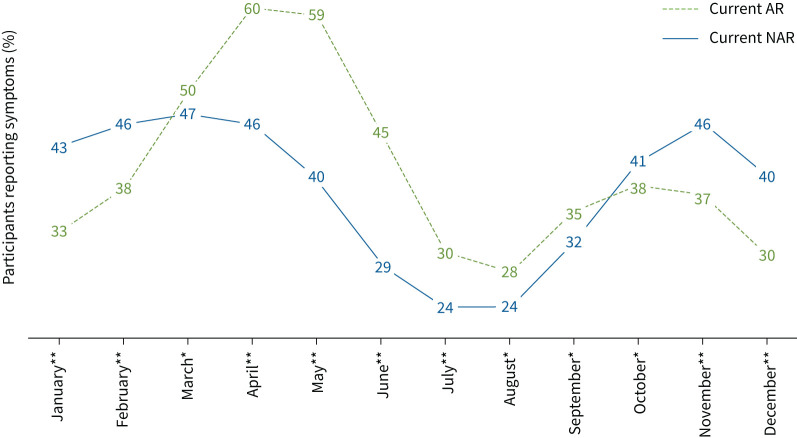
The seasonality of symptoms differed between AR and NAR: AR participants reported symptoms mainly during the spring, whereas NAR participants reported symptoms mainly during the winter (figure 1).
FIGURE 1.
Percentage of current allergic rhinitis (AR) and current non-allergic rhinitis (NAR) participants reporting symptoms by month. *: p<0.05; **: p<0.0001.
Using alternative definitions of rhinitis, the crude prevalence of current AR varied from 20.3% to 28.4% and that of current NAR varied from 10.0% to 18.6% (supplementary table S2). The κ concordance coefficient between the main definition and the alternative definitions was the highest for the alternative definition based on the classification tree adaptation (κ=0.96) and the lowest for the alternative definition based on the symptom triggers (κ=0.37). Similar differences between AR and NAR were found with the main and alternative definitions in terms of multimorbidities, reported symptoms, triggers, seasonality and treatments (supplementary table S3 and supplementary figures S3 and S4).
Rhinitis phenotypes including ever-asthma and ever-conjunctivitis status
Among AR participants, 1383 reported ever-asthma (24.3%). Among NAR participants, 166 reported ever-asthma (7.5%) (table 3). Among both AR and NAR participants, those who reported ever-asthma had higher eosinophil counts, an earlier age of rhinitis onset and more moderate-to-severe rhinitis than those without asthma. Participants with AR and ever-asthma also reported more ever-conjunctivitis and ever-eczema than those with AR only. These differences were not significant for participants with NAR, even if the tendency was the same.
TABLE 3.
Characteristics of participants with current allergic rhinitis (AR) or current non-allergic rhinitis (NAR) including ever-asthma status
| AR | NAR | |||||
| Never-asthma (n=4304) | Ever-asthma (n=1383) | p-value | Never-asthma (n=2056) | Ever-asthma (n=166) | p-value | |
| Sex | 0.090 | 0.25 | ||||
| Male | 1851 (43.0) | 559 (40.4) | 925 (45.0) | 67 (40.4) | ||
| Female | 2453 (57.0) | 824 (59.6) | 1131 (55.0) | 99 (59.6) | ||
| Age, years | 52.5±12.6 | 48.9±12.7 | <0.0001 | 52.7±13.3 | 48.6±13.8 | 0.0001 |
| Tobacco status | 0.65 | 0.18 | ||||
| Never-smoker | 1883 (46.1) | 613 (46.4) | 879 (44.8) | 59 (37.8) | ||
| Ex-smoker | 1702 (41.7) | 536 (40.6) | 786 (40.1) | 67 (42.9) | ||
| Current smoker | 498 (12.2) | 172 (13.0) | 297 (15.1) | 30 (19.2) | ||
| Educational level | 0.17 | 0.70 | ||||
| Less than high school | 380 (8.9) | 104 (7.6) | 177 (8.7) | 17 (10.5) | ||
| High school | 1285 (30.1) | 396 (28.9) | 619 (30.4) | 50 (30.9) | ||
| University | 2604 (61.0) | 868 (63.5) | 1242 (60.9) | 95 (58.6) | ||
| Body mass index, kg·m−2 | 0.13 | 0.0098 | ||||
| <18.5 | 95 (2.3) | 26 (1.9) | 42 (2.1) | 5 (3.1) | ||
| [18.5–25[ | 2446 (58.1) | 760 (56.0) | 1200 (59.6) | 83 (50.9) | ||
| [25–30[ | 1244 (29.6) | 407 (30.0) | 599 (29.7) | 49 (30.1) | ||
| ≥30 | 423 (10.1) | 165 (12.2) | 174 (8.6) | 26 (16.0) | ||
| Conjunctivitis | <0.0001 | 0.19 | ||||
| Never-conjunctivitis | 1855 (48.6) | 418 (33.0) | 1511 (80.8) | 113 (76.4) | ||
| Ever-conjunctivitis | 1958 (51.4) | 848 (67.0) | 360 (19.2) | 35 (23.6) | ||
| Eczema | <0.0001 | 0.36 | ||||
| Never-eczema | 2329 (62.0) | 639 (51.3) | 1426 (74.8) | 110 (71.4) | ||
| Ever-eczema | 1426 (38.0) | 607 (48.7) | 481 (25.2) | 44 (28.6) | ||
| Blood eosinophil count, cells·mm−3 | 194.6±133.7 | 252.1±175.1 | <0.0001 | 173.8±114.1 | 224.2±150.9 | <0.0001 |
| Age of onset of rhinitis, years | 26.4±15.4 | 18.3±13.4 | <0.0001 | 35.2±17.6 | 28.8±16.2 | 0.0004 |
| Reported triggers of rhinitis symptoms# | ||||||
| Dust mites or house dust | 1167 (27.1) | 729 (52.7) | <0.0001 | 194 (9.4) | 38 (22.9) | <0.0001 |
| Animals | 338 (7.9) | 331 (23.9) | <0.0001 | 39 (1.9) | 14 (8.4) | <0.0001 |
| Air pollution | 1083 (25.2) | 443 (32.0) | <0.0001 | 257 (12.5) | 27 (16.3) | 0.16 |
| Change in weather | 1189 (27.6) | 442 (32.0) | 0.0019 | 549 (26.7) | 46 (27.7) | 0.78 |
| Tobacco | 210 (4.9) | 142 (10.3) | <0.0001 | 83 (4.0) | 9 (5.4) | 0.39 |
| Pollens | 2119 (49.2) | 861 (62.3) | <0.0001 | 199 (9.7) | 26 (15.7) | 0.014 |
| Cold air | 1026 (23.8) | 359 (26.0) | 0.11 | 565 (27.5) | 48 (28.9) | 0.69 |
| Other | 534 (12.4) | 186 (13.4) | 0.31 | 254 (12.4) | 20 (12.0) | 0.91 |
| Unknown | 1301 (30.2) | 240 (17.4) | <0.0001 | 1019 (49.6) | 63 (38.0) | 0.0040 |
| Reported symptoms# | ||||||
| Rhinorrhoea | 2951 (74.1) | 1022 (78.3) | 0.0022 | 1096 (58.5) | 90 (62.1) | 0.39 |
| Nasal congestion/obstruction | 2861 (74.3) | 1056 (82.2) | <0.0001 | 1131 (61.3) | 103 (70.5) | 0.027 |
| Nasal itching | 2456 (65.0) | 918 (72.7) | <0.0001 | 683 (38.9) | 69 (49.3) | 0.015 |
| Sneezing | 2945 (74.4) | 1039 (79.6) | 0.0002 | 951 (51.8) | 80 (54.4) | 0.54 |
| Associated eye symptoms | 2621 (66.1) | 990 (76.3) | <0.0001 | 615 (34.1) | 71 (49.3) | 0.0002 |
| Reported symptoms, n | 3.4±1.3 | 3.8±1.2 | <0.0001 | 2.3±1.3 | 2.7±1.4 | 0.0004 |
| Rhinitis severity | <0.0001 | 0.0042 | ||||
| Mild | 2198 (62.8) | 609 (51.5) | 1285 (76.6) | 83 (65.4) | ||
| Moderate-to-severe | 1301 (37.2) | 574 (48.5) | 392 (23.4) | 44 (34.6) | ||
| Rhinitis duration | 0.0025 | 0.74 | ||||
| Intermittent | 2853 (69.5) | 870 (65.1) | 1481 (75.1) | 116 (73.9) | ||
| Persistent | 1252 (30.5) | 467 (34.9) | 492 (24.9) | 41 (26.1) | ||
| Rhinitis treatment | <0.0001 | <0.0001 | ||||
| Neither OA nor INCS | 1618 (39.0) | 259 (19.3) | 1475 (74.6) | 94 (60.3) | ||
| OA only | 869 (20.9) | 307 (22.8) | 102 (5.2) | 15 (9.6) | ||
| INCS only | 552 (13.3) | 132 (9.8) | 293 (14.8) | 23 (14.7) | ||
| OA and INCS | 1113 (26.8) | 646 (48.1) | 106 (5.4) | 24 (15.4) | ||
Data are presented as n (%) or mean±sd, unless otherwise stated. OA: oral antihistamine; INCS: intranasal corticosteroid. #: several possible answers.
For replication analyses in EGEA, 842 participants with current rhinitis were included (n=174 AR plus never-asthma, n=380 AR plus ever-asthma, n=176 NAR plus never-asthma and n=83 NAR plus ever-asthma). Similar results were observed (supplementary table S4). Furthermore, those who reported ever-asthma had higher serum IgE levels and allergen sensitisation assessed by SPTs. Participants with ever-asthma had on average a higher mean number of positive SPTs than those with never-asthma for both AR (2.6 versus 1.9) and NAR (1.6 versus 0.4). When using the SPT definition instead of the questionnaire definition the results were almost identical (supplementary table S5).
In CONSTANCES, the characteristics of AR participants with and without ever-asthma and ever-conjunctivitis (n=1855 AR only, n=1958 AR plus ever-conjunctivitis, n=418 AR plus ever-asthma and n=848 AR plus ever-conjunctivitis plus ever-asthma) are shown in table 4. Statistically significant differences were observed for almost all the characteristics. In particular, participants with AR plus ever-conjunctivitis plus ever-asthma had an earlier age of rhinitis onset, more moderate-to-severe rhinitis, reported more comedication (intranasal corticosteroids and antihistamines) and had higher blood eosinophil counts than participants from all other groups.
TABLE 4.
Characteristics of participants with current allergic rhinitis (AR) including ever-asthma (A) and ever-conjunctivitis (C) status
| AR alone (n=1855) | AR+C (n=1958) | AR+A (n=418) | AR+C+A (n=848) | p-value | |
| Sex | <0.0001 | ||||
| Male | 941 (50.7) | 671 (34.3) | 196 (46.9) | 308 (36.3) | |
| Female | 914 (49.3) | 1287 (65.7) | 222 (53.1) | 540 (63.7) | |
| Age, years | 51.9±12.9 | 51.6±12.5 | 48.7±12.7 | 48.1±12.6 | <0.0001 |
| Tobacco status | 0.0030 | ||||
| Never-smoker | 775 (43.9) | 920 (49.4) | 167 (42.0) | 397 (48.7) | |
| Ex-smoker | 756 (42.8) | 740 (39.7) | 169 (42.5) | 321 (39.4) | |
| Current smoker | 236 (13.4) | 202 (10.8) | 62 (15.6) | 97 (11.9) | |
| Educational level | 0.033 | ||||
| Less than high school | 148 (8.0) | 154 (7.9) | 34 (8.2) | 54 (6.4) | |
| High school | 573 (31.1) | 519 (26.7) | 123 (29.8) | 229 (27.3) | |
| University | 1120 (60.8) | 1270 (65.4) | 256 (62.0) | 556 (66.3) | |
| Body mass index, kg·m−2 | 0.086 | ||||
| <18.5 | 32 (1.8) | 50 (2.6) | 6 (1.5) | 18 (2.2) | |
| [18.5–25[ | 1041 (57.6) | 1151 (59.9) | 249 (60.6) | 455 (54.4) | |
| [25–30[ | 548 (30.3) | 530 (27.6) | 109 (26.5) | 265 (31.7) | |
| ≥30 | 187 (10.3) | 189 (9.8) | 47 (11.4) | 98 (11.7) | |
| Eczema | <0.0001 | ||||
| Never-eczema | 1398 (75.6) | 915 (52.6) | 301 (72.7) | 334 (43.2) | |
| Ever-eczema | 450 (24.4) | 823 (47.4) | 113 (27.3) | 440 (56.8) | |
| Blood eosinophil count, cells·mm−3 | 196.8±134.0 | 191.6±134.2 | 260.9±183.3 | 247.4±169.1 | <0.0001 |
| Age of onset of rhinitis, years | 27.3±15.7 | 24.7±14.4 | 21.4±14.6 | 16.4±12.1 | <0.0001 |
| Reported triggers of rhinitis symptoms# | |||||
| Dust mites or house dust | 441 (23.8) | 620 (31.7) | 210 (50.2) | 470 (55.4) | <0.0001 |
| Animals | 118 (6.4) | 200 (10.2) | 91 (21.8) | 224 (26.4) | <0.0001 |
| Air pollution | 412 (22.2) | 571 (29.2) | 114 (27.3) | 292 (34.4) | <0.0001 |
| Change in weather | 474 (25.6) | 568 (29.0) | 135 (32.3) | 271 (32.0) | 0.0011 |
| Tobacco | 78 (4.2) | 107 (5.5) | 42 (10.0) | 88 (10.4) | <0.0001 |
| Pollens | 789 (42.5) | 1149 (58.7) | 217 (51.9) | 591 (69.7) | <0.0001 |
| Cold air | 445 (24.0) | 459 (23.4) | 111 (26.6) | 220 (25.9) | 0.35 |
| Other | 225 (12.1) | 247 (12.6) | 49 (11.7) | 126 (14.9) | 0.22 |
| Unknown | 617 (33.3) | 492 (25.1) | 87 (20.8) | 114 (13.4) | <0.0001 |
| Reported symptoms# | |||||
| Rhinorrhoea | 1230 (70.3) | 1394 (76.5) | 291 (74.2) | 651 (80.0) | <0.0001 |
| Nasal congestion/obstruction | 1198 (71.0) | 1364 (76.9) | 308 (79.8) | 656 (82.1) | <0.0001 |
| Nasal itching | 982 (58.7) | 1235 (71.0) | 251 (65.2) | 614 (77.2) | <0.0001 |
| Sneezing | 1231 (71.1) | 1395 (77.0) | 297 (74.6) | 669 (82.2) | <0.0001 |
| Associated eye symptoms | 912 (53.1) | 1488 (80.3) | 245 (62.0) | 689 (84.7) | <0.0001 |
| Reported symptoms, n | 3.2±1.3 | 3.7±1.2 | 3.5±1.3 | 4.0±1.1 | <0.0001 |
| Rhinitis severity | <0.0001 | ||||
| Mild | 1047 (66.1) | 947 (58.8) | 196 (54.4) | 377 (50.1) | |
| Moderate-to-severe | 537 (33.9) | 664 (41.2) | 164 (45.6) | 375 (49.9) | |
| Rhinitis duration | 0.0016 | ||||
| Intermittent | 1268 (70.9) | 1275 (68.4) | 284 (70.1) | 521 (63.4) | |
| Persistent | 520 (29.1) | 590 (31.6) | 121 (29.9) | 301 (36.6) | |
| Rhinitis treatment | <0.0001 | ||||
| Neither OA nor INCS | 819 (45.5) | 603 (31.9) | 114 (28.2) | 120 (14.5) | |
| OA only | 329 (18.3) | 472 (24.9) | 91 (22.5) | 207 (25.0) | |
| INCS only | 272 (15.1) | 200 (10.6) | 44 (10.9) | 60 (7.2) | |
| OA and INCS | 380 (21.1) | 617 (32.6) | 155 (38.4) | 442 (53.3) |
Data are presented as n (%) or mean±sd, unless otherwise stated. OA: oral antihistamine; INCS: intranasal corticosteroid. #: several possible answers.
The results observed in EGEA were almost identical (supplementary table S6 and supplementary figure S5). In addition, we observed that participants with AR plus ever-conjunctivitis plus ever-asthma had the highest mean number of positive SPTs. When using the SPT definition the results were also almost identical (supplementary table S7).
Discussion
In a large population-based study, four out of 10 adults reported current rhinitis and 30% of them reported moderate-to-severe rhinitis. We found the well-known characteristics of AR and NAR with questionnaire-based definitions. We showed that AR and NAR alone or with asthma or with asthma and conjunctivitis are different phenotypes.
Strengths and limitations
CONSTANCES is the largest French population-based epidemiological study in adults. CONSTANCES is, however, not fully representative of the French adult population as 1) participants were randomly selected from the beneficiaries of the National Health Insurance Fund (“Caisse Nationale d'Assurance Maladie” (CNAM)) that covered ∼85% of the French population and 2) some geographical areas of France were not included. This may lead to a misestimation of the actual rhinitis prevalence. Furthermore, the 2014 population is even more selected, mainly regarding age, with few participants <30 years old. This may have slightly impacted the characterisation and identification of our phenotypes. Recall bias is possible, more likely for ever-asthma than for current rhinitis as the questions on current rhinitis characteristics covered the last 12 months, limiting memory issues.
We acknowledge the lack of SPTs and/or serum specific IgE measurement is a limitation. However, we have defined AR and NAR using validated and standardised questions [16] that were shown to be a good alternative to differentiate AR from NAR in epidemiological studies [17] even if classification errors cannot be excluded. We observed that a few participants classified as NAR reported allergic triggers and we could not exclude that they could have AR without knowing it. By using alternative definitions of AR and NAR, the prevalence and characteristics of AR and NAR were very similar regardless of the definitions used, showing the robustness of our results. Considering asthma status, we observed that, even among NAR, participants with ever-asthma reported more allergic triggers of rhinitis symptoms compared with those with never-asthma. It is possible that among those with ever-asthma, some participants with AR were misclassified as NAR. Even if misclassification exists, it could not explain by itself the contrasting results observed between the four groups. The present study did not distinguish local AR and mixed rhinitis from other forms of AR. We also acknowledge that the rhinitis definition based on symptoms did not distinguish rhinitis from chronic rhinosinusitis that shares some symptoms including nasal congestion.
We defined ever-asthma based on a validated and standardised question used in ECRHS [16], and recently published in CONSTANCES [19, 20].
Interpretation of results
The prevalence of current rhinitis in our population was 40%. This result is difficult to compare with the literature as the definition of rhinitis varies greatly between studies and ranges from <10% to >50% [1]. According to ARIA 2008 [1], the prevalence of AR was estimated to be ∼25% of the general European population, which is consistent with our findings. Three studies conducted in Belgium, Sweden and Italy found very similar prevalences of NAR, between 9.6% and 12.0% [21–23]. Recently, a study in the Netherlands found a prevalence of NAR of 26% [7]. The actual prevalence of rhinitis may be underestimated because most studies had only considered AR. Studies considering both AR and NAR are needed to obtain an overall prevalence of rhinitis and to confirm our results.
Most of the current knowledge about AR and NAR is derived from clinical knowledge. Few population-based studies have described the characteristics of rhinitis. We confirmed the main differences observed in clinical practice [24]: AR participants reported on average more allergic multimorbidities, an earlier age of onset and more allergic triggers than NAR participants.
We observed that the seasonal patterns of AR and NAR are different. Indeed, NAR showed a peak of declarations during the cold months in France and fewer declarations during the summer months. For AR there was a major peak during the spring season, which is when the main pollens are released. It is interesting to note that there was also a second, smaller peak for AR in autumn, which corresponds to the season when ragweed pollens (notably Ambrosia artemisiifolia) are released in France. About half of AR participants reported antihistamine treatments. The majority of NAR participants did not report antihistamine or corticosteroid treatments, consistent with the lack of effective treatments for NAR [25]. The seasonality, triggers and treatments we observed for AR and NAR are consistent with the literature and clinical settings, suggesting that the questionnaire-based definition used is a good proxy to identify AR and NAR.
We observed that the age of AR onset was on average lower than that of NAR; it is often reported that AR starts in childhood, whereas in our study the average age of AR onset was 24 years. We cannot exclude the possibility that a memory bias exists for age of onset, as some participants with childhood rhinitis may not remember it. Regarding the symptoms, we also found well-known differences: AR participants reported more sneezing, nasal itching and eye symptoms than NAR participants. Itching and eye symptoms are commonly described as rare in NAR [24]. In our study, >30% of participants with NAR reported these symptoms. Similar results were found in many studies that have considered definitions of AR and NAR based on medical diagnoses or biological tests [26, 27]. There are still many gaps in our knowledge about NAR, but it is possible that some of its forms present symptoms usually considered as allergic. Another explanation could be that AR and NAR are not necessarily mutually exclusive, as up to 50% of rhinitis patients may have mixed rhinitis combining AR and NAR [28].
There are few epidemiological studies in adults that estimate the proportion of persistent and severe rhinitis in the general population, especially for NAR. In the present study, the prevalence of persistent rhinitis represents 31% of AR and 25% of NAR. Moderate-to-severe rhinitis was found in 40% for AR and 24% for NAR. These figures are lower than those found in clinical practice as most patients who consult a physician have severe rhinitis [29]. This shows that studies in the general population are needed to reflect the actual situation of rhinitis.
For the first time in adults from a population-based study, we described phenotypes of AR and NAR accounting for ever-asthma status. We observed contrasting characteristics across the groups. Our results showed that compared with AR participants without ever-asthma, those with ever-asthma reported 1) earlier onset of the disease, 2) more frequent moderate-to-severe rhinitis, 3) more conjunctivitis and eczema multimorbidities, 4) a higher level of blood eosinophils, and 5) more comedication. This latter finding suggests a more severe AR [30]. We validated these results by finding similar characteristics for all groups in the EGEA study. Moreover, allergen sensitisation is available in EGEA and there was an increased number of sensitisations in AR with asthma [10]. It therefore appears that rhinitis alone and rhinitis and asthma represent two different diseases as found in real-life [31, 32] and genetic studies: rhinitis alone being associated with Toll-like receptors, and rhinitis and asthma multimorbidity with interleukin (IL)-5 (associated with eosinophilia) and IL-33 [33]. However, biological pathways that are involved in rhinitis alone versus rhinitis with asthma are only partly understood and further studies are needed to understand the underlying biological mechanisms involved in the aetiology of these phenotypes.
We further described the AR characteristics including ever-asthma and ever-conjunctivitis status. There was an extreme rhinitis phenotype in terms of rhinitis severity (symptoms and treatment) and eosinophil counts, with the three multimorbidities associated. It is possible that the natural history of rhinitis alone or in multimorbidity differs. Based on our results, another potential explanation is that the intensity of allergic sensitisation increases the risk of having more than one condition or vice versa. It is of paramount importance that these newly described phenotypes can be observed in this large population-based study.
Implications of all the available evidence
The high rhinitis prevalence is an issue for health policy, especially as more than a third of the participants reported having symptoms that interfered with their daily activities or sleep in the past 12 months. In this context, it is important to study the risk factors that could explain the increase in the prevalence of rhinitis. These factors are not all clearly identified at present, in particular environmental factors. Further studies on rhinitis aetiology are needed.
We showed that AR and NAR alone, or with asthma, or with asthma and conjunctivitis had different characteristics and thus may have different aetiologies. This raises the question of how best to manage rhinitis and its multimorbidities in terms of natural history, impact on ageing processes and management. Furthermore, studying the evolution of these rhinitis phenotypes over time is an essential research perspective, especially in adults for whom data are scarce. How does rhinitis evolve with advanced age? How does rhinitis severity evolve over time? Does rhinitis lead to multimorbidities or vice versa? Do other morbidities appear over time? There is an unmet need to answer these questions.
Conclusions
In a large population-based cohort among adults we found a high prevalence of self-reported rhinitis, with, as expected but never shown before, a lower prevalence of moderate-to-severe rhinitis than in clinical practice. We showed that AR and NAR alone, or with asthma, or with asthma and conjunctivitis are different phenotypes. These results were replicated in the EGEA study. These rhinitis phenotypes will have major implications in terms of clinical practice.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00943-2022.Supplement (1,008.9KB, pdf)
Shareable PDF
Acknowledgements
This work is part of a thesis prepared in the framework of the Doctoral Network in Public Health coordinated by the EHESP. This work was supported by the French Environment and Energy Management Agency (ADEME) and the Université Paris-Saclay. The authors acknowledge the CONSTANCES Respiratory Group: M.C. Delmas, O. Dumas, V. Giraud, Y. Itwasubo, B. Leynaert, N. Le Moual, R. Nadif, T. Perez, N. Roche and R. Varraso. The authors thank the team of the “Population-based Cohorts Unit” (Cohortes en Population) that designed and manages the CONSTANCES Cohort Study. They also thank the French National Health Insurance Fund (“Caisse Nationale d'Assurance Maladie” (CNAM)) and its Health Screening Centres (“Centres d'Examens de Santé”), which collected a large part of the data, as well as the French National Old-Age Insurance Fund (“Caisse Nationale d'Assurance Vieillesse” (CNAV)) for its contribution to the constitution of the cohort, and ClinSearch, Asqualab and Eurocell, which conducted the data quality control.
Footnotes
Conflict of interest: M. Savouré reports that this work is a part of a thesis supported by the French Environment and Energy Management Agency (ADEME) and the Université Paris-Saclay. J. Bousquet reports lecture honoraria from Cipla, Menarini, Mylan, Novartis, Purina, Sanofi-Aventis, Teva and Uriach, outside the submitted work, and is a shareholder of KYomed Innov and MASK-air-SAS. All other authors have nothing to disclose.
Support statement: This work is a part of Marine Savouré's thesis supported by the French Environment and Energy Management Agency (ADEME) and the Université Paris-Saclay. The CONSTANCES Cohort Study was supported and funded by the French National Health Insurance Fund (“Caisse Nationale d'Assurance Maladie” (CNAM)). The CONSTANCES Cohort Study is an “Infrastructure Nationale en Biologie et Santé” and benefits from a grant from the French National Agency for Research (ANR-11-INBS-0002). CONSTANCES is also partly funded by Merck Sharp & Dohme, AstraZeneca, Lundbeck and L'Oréal. None of the funding sources had any role in the design of the study, the collection, analysis and interpretation of data, or in the writing of the report and decision to publish. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 (in collaboration with the World Health Organization, GA2LEN and AllerGen). Allergy 2008; 63: Suppl. 86, 8–160. doi: 10.1111/j.1398-9995.2007.01620.x [DOI] [PubMed] [Google Scholar]
- 2.Vandenplas O, Vinnikov D, Blanc PD, et al. Impact of rhinitis on work productivity: a systematic review. J Allergy Clin Immunol Pract 2018; 6: 1274–1286. doi: 10.1016/j.jaip.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Primers 2020; 6: 95. doi: 10.1038/s41572-020-00227-0 [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulos NG, Guibas GV. Rhinitis subtypes, endotypes, and definitions. Immunol Allergy Clin North Am 2016; 36: 215–233. doi: 10.1016/j.iac.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greiwe J, Bernstein JA. Nonallergic rhinitis: diagnosis. Immunol Allergy Clin North Am 2016; 36: 289–303. doi: 10.1016/j.iac.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 6.Hellings PW, Klimek L, Cingi C, et al. Non-allergic rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy 2017; 72: 1657–1665. doi: 10.1111/all.13200 [DOI] [PubMed] [Google Scholar]
- 7.Avdeeva KS, Fokkens WJ, Segboer CL, et al. The prevalence of non-allergic rhinitis phenotypes in the general population: a cross-sectional study. Allergy 2022; 77: 2163–2174. doi: 10.1111/all.15223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bousquet J, Schünemann HJ, Samolinski B, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol 2012; 130: 1049–1062. doi: 10.1016/j.jaci.2012.07.053 [DOI] [PubMed] [Google Scholar]
- 9.Li AR, Zhang K, Reddy PD, et al. Systematic review of measures of disease severity in rhinitis. Int Forum Allergy Rhinol 2021; 11: 1367–1377. doi: 10.1002/alr.22794 [DOI] [PubMed] [Google Scholar]
- 10.Siroux V, Boudier A, Nadif R, et al. Association between asthma, rhinitis, and conjunctivitis multimorbidities with molecular IgE sensitization in adults. Allergy 2019; 74: 824–827. doi: 10.1111/all.13676 [DOI] [PubMed] [Google Scholar]
- 11.Zins M, Goldberg M, CONSTANCES Team . The French CONSTANCES population-based cohort: design, inclusion and follow-up. Eur J Epidemiol 2015; 30: 1317–1328. doi: 10.1007/s10654-015-0096-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg M, Carton M, Descatha A, et al. CONSTANCES: a general prospective population-based cohort for occupational and environmental epidemiology: cohort profile. Occup Environ Med 2017; 74: 66–71. doi: 10.1136/oemed-2016-103678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henny J, Nadif R, Got SL, et al. The CONSTANCES Cohort Biobank: an open tool for research in epidemiology and prevention of diseases. Front Public Health 2020; 8: 605133. doi: 10.3389/fpubh.2020.605133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995; 8: 483–491. doi: 10.1183/09031936.95.08030483 [DOI] [PubMed] [Google Scholar]
- 15.Bousquet J, Van Cauwenberge P, Khaltaev N, et al. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol 2001; 108: 5 Suppl., S147–S334. doi: 10.1067/mai.2001.118891 [DOI] [PubMed] [Google Scholar]
- 16.European Community Respiratory Health Survey . Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS). Eur Respir J 1996; 9: 687–695. doi: 10.1183/09031936.96.09040687 [DOI] [PubMed] [Google Scholar]
- 17.Savouré M, Bousquet J, Burte E, et al. Questionnaire as an alternative of skin prick tests to differentiate allergic from non-allergic rhinitis in epidemiological studies. Allergy 2021; 76: 2291–2294. doi: 10.1111/all.14812 [DOI] [PubMed] [Google Scholar]
- 18.Burte E, Bousquet J, Varraso R, et al. Characterization of rhinitis according to the asthma status in adults using an unsupervised approach in the EGEA study. PLoS One 2015; 10: e0136191. doi: 10.1371/journal.pone.0136191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsiavia T, Henny J, Goldberg M, et al. Blood inflammatory phenotypes were associated with distinct clinical expressions of asthma in adults from a large population-based cohort. EBioMedicine 2022; 76: 103875. doi: 10.1016/j.ebiom.2022.103875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delmas M-C, Bénézet L, Ribet C, et al. Prévalence de l'asthme chez l'adulte en France, données de la cohorte Constances. [Prevalence of asthma among adults in France, data from the Constances cohort study.] Rev Mal Respir 2021; 38: 797–806. doi: 10.1016/j.rmr.2021.05.007 [DOI] [PubMed] [Google Scholar]
- 21.Cazzoletti L, Ferrari M, Olivieri M, et al. The gender, age and risk factor distribution differs in self-reported allergic and non-allergic rhinitis: a cross-sectional population-based study. Allergy Asthma Clin Immunol 2015; 11: 36. doi: 10.1186/s13223-015-0101-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Engvall K, Smedje G, et al. Rhinitis, asthma and respiratory infections among adults in relation to the home environment in multi-family buildings in Sweden. PLoS One 2014; 9: e105125. doi: 10.1371/journal.pone.0105125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachert C, van Cauwenberge P, Olbrecht J, et al. Prevalence, classification and perception of allergic and nonallergic rhinitis in Belgium. Allergy 2006; 61: 693–698. doi: 10.1111/j.1398-9995.2006.01054.x [DOI] [PubMed] [Google Scholar]
- 24.Quillen DM, Feller DB. Diagnosing rhinitis: allergic vs. nonallergic. Am Fam Physician 2006; 73: 1583–1590. [PubMed] [Google Scholar]
- 25.Tran NPB, Vickery JD, Blaiss MS. Management of rhinitis: allergic and non-allergic. Allergy Asthma Immunol Res 2011; 3: 148–156. doi: 10.4168/aair.2011.3.3.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campo P, Rondón C, Gould HJ, et al. Local IgE in non-allergic rhinitis. Clin Exp Allergy 2015; 45: 872–881. doi: 10.1111/cea.12476 [DOI] [PubMed] [Google Scholar]
- 27.Mølgaard E, Thomsen SF, Lund T, et al. Differences between allergic and nonallergic rhinitis in a large sample of adolescents and adults. Allergy 2007; 62: 1033–1037. doi: 10.1111/j.1398-9995.2007.01355.x [DOI] [PubMed] [Google Scholar]
- 28.Greiwe JC, Bernstein JA. Allergic and mixed rhinitis: diagnosis and natural evolution. J Clin Med 2019; 8: 2019. doi: 10.3390/jcm8112019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bousquet J, Annesi-Maesano I, Carat F, et al. Characteristics of intermittent and persistent allergic rhinitis: DREAMS study group. Clin Exp Allergy 2005; 35: 728–732. doi: 10.1111/j.1365-2222.2005.02274.x [DOI] [PubMed] [Google Scholar]
- 30.Bédard A, Basagaña X, Anto JM, et al. Mobile technology offers novel insights into the control and treatment of allergic rhinitis: the MASK study. J Allergy Clin Immunol 2019; 144: 135–143. doi: 10.1016/j.jaci.2019.01.053 [DOI] [PubMed] [Google Scholar]
- 31.Bousquet J, Devillier P, Anto JM, et al. Daily allergic multimorbidity in rhinitis using mobile technology: a novel concept of the MASK study. Allergy 2018; 73: 1622–1631. doi: 10.1111/all.13448 [DOI] [PubMed] [Google Scholar]
- 32.Shamji MH, Sharif H, Layhadi JA, et al. Diverse immune mechanisms of allergen immunotherapy for allergic rhinitis with and without asthma. J Allergy Clin Immunol 2022; 149: 791–801. doi: 10.1016/j.jaci.2022.01.016 [DOI] [PubMed] [Google Scholar]
- 33.Lemonnier N, Melén E, Jiang Y, et al. A novel whole blood gene expression signature for asthma, dermatitis, and rhinitis multimorbidity in children and adolescents. Allergy 2020; 75: 3248–3260. doi: 10.1111/all.14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00943-2022.Supplement (1,008.9KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00943-2022.Shareable (486KB, pdf)