Abstract
The mechanism behind the aberrant expression of S100A6 in osteosarcoma is seldom reported so far. This study sought to explore the regulatory axis targeting S100A6 involved in osteosarcoma progression. Clinical samples collected from osteosarcoma patients were used to detect the expressions of SNHG1, miR-493-5p, and S100A6 by western bolt analysis and reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The effects of S100A6 on proliferation and osteogenic differentiation were investigated by the CCK-8 assay, colony formation assay, Ethynyl deoxyuridine staining, matrix mineralization assay, and alkaline phosphatase assay. The potential of lncRNAs/miRNAs targeting S100A6 was identified by the bioinformatics approach, and the results were verified by the dual luciferase assay and RNA immunoprecipitation assay. Both in vitro and in vivo rescue experiments were performed to investigate the regulatory relationship between the identified lncRNAs and S100A6. The results showed that S100A6 is highly expressed in osteosarcoma. S100A6 overexpression not only increases the proliferation but also reduces the osteogenic differentiation of osteosarcoma cells, while S1006A silence exerts the opposite effects. Then, SNHG1 is identified to directly interact with miR-493-5p to attenuate miR-493-5p binding to the 3′-untranslated region of S100A6. Notably, S100A6 silence partially rescues the effect of SNHG1 overexpression on proliferation and osteogenic differentiation of osteosarcoma cells. Furthermore, the suppressive role of SNHG1 silence in the growth of osteosarcoma xenograft tumors is countered by S100A6 overexpression. Collectively, this study reveals that S100A6 plays an important role in osteosarcoma progression, and SNHG1 promotes S100A6 expression by competitively sponging miR-493-5p.
Keywords: osteosarcoma, S100A6, regulatory mechanism, lncRNA SNHG1, miR-493-5p
Introduction
As the predominant form of bone neoplasms, osteosarcoma is characterized by rapid progression, early metastasis, and poor prognosis. In the past several decades, although great efforts have been made in therapeutic strategies of osteosarcoma, the overall survival of patients has shown mild improvement due to drug resistance and lung metastasis [ 1, 2]. To develop effective targeted therapies for osteosarcoma, extensive basic studies on the molecular mechanisms regulating tumorigenesis and progression of osteosarcoma are still quite necessary.
S100A6 is a calcium-binding protein that has been reported as an oncogene in several cancers, including gastric cancer [3] and hepatocellular carcinoma [4]. Previous studies documented that the expression of S100A6 was increased in osteosarcoma and closely related to osteosarcoma metastasis [ 5, 6]. However, few studies reported the regulatory mechanisms behind the aberrant expression of S100A6 in osteosarcoma.
With the technological advance in whole-genome sequencing, the biological functions of non-coding RNAs have attracted much attention in studies on different diseases, including osteosarcoma [7]. Simply defined as RNAs that do not encode proteins, non-coding RNAs (ncRNAs) have been proven critically important in many biological processes [8]. Multiple studies have indicated that long non-coding RNAs (lncRNAs), a type of ncRNAs with more than 200 nucleotides in length, are often dysregulated in many types of cancers [ 9, 10]. It is widely accepted that lncRNAs play their roles in modulating gene expression at transcriptional [11], post-transcriptional [12], and even translational [13] levels. The most remarkable form of regulation in gene expression is available between lncRNAs and microRNAs (miRNAs) [14]. A previous study reported that lncRNA SNHG20 up-regulated RUNX2 and promoted the malignant phenotypes of osteosarcoma cells by sponging miR-139 [15]. In addition, using in vitro and in vivo studies, Gu et al. [16] demonstrated that lncRNA DICER1-AS1 mediated the miR-30b/ATG5 axis to participate in osteosarcoma development.
Based on the above literature, we speculate that there is also an lncRNA/miRNA pair in the regulation of S100A6 in osteosarcoma. Here, potential lncRNAs and miRNAs that target S100A6 were identified by the bioinformatics analysis, and the regulatory mechanism was validated through in vitro and in vivoexperiments.
Materials and Methods
Clinical sample collection
Ethical approval for this research was obtained from Shengjing Hospital of China Medical University (Shenyang, China). Cancerous and adjacent non-cancerous osteosarcoma samples were collected from 12 osteosarcoma patients undergoing osteosarcoma resection surgery. All clinical samples were stored in liquid nitrogen before analysis. Written informed consent was obtained from all enrolled patients.
Cell culture
Two human osteosarcoma cell lines (MG63 and 134B), a normal human osteoblast cell line (hFOB1.19), and a human embryonic kidney cell line (293T) were obtained from ATCC (Manassas, USA). All cells were cultured in regular growth medium comprised of DMEM and 10% FBS (Gibco, Carlsbad, USA), at 37°C in an incubator containing 5% CO 2.
Western blot analysis
Total protein of tissues and cells was extracted using the RIPA buffer (Abcam, Cambridge, UK) and subsequently quantified using a BCA Kit (Beyotime, Shanghai, China). Western blot analysis was performed as described by Mahmood and Yang [17]. In brief, the proteins were separated by SDS-PAGE before being transferred onto PVDF membrane. Then, the membrane was blocked with skimmed milk for 1.5 h, followed by overnight incubation with primary antibodies and subsequent 2-h incubation with secondary antibodies. Finally, protein bands were visualized using the ECL Substrate Kit (Abcam) and quantified using Image J software. The antibodies used in this study were all purchased from Abcam and used at the following dilutions: anti-S100A6 (#ab181975; 1:10,000), anti-GAPDH (#ab8245; 1:8000), anti-osteocalcin (OCN) (#ab93876; 1:1000), anti-osteopontin (OPN) (#ab166709; 1:500), and Goat Anti-Rabbit IgG H&L (HRP) (#ab6721; 1:3000).
Reverse transcription-quantitative polymerase chain reaction
Total RNA isolation and subsequent reverse transcription were conducted according to standard protocols. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis was performed on the 7500 Real-Time PCR System (Applied Biosystems, Foster City, USA) to detect the expression levels of S100A6, miR-493-5p, SNHG1, OCN, and OPN, while GAPDH and U6 were utilized for normalization. The expression levels were further analyzed based on the 2 –ΔΔCt method [18]. The primer sequences are described in Table 1 .
Table1 Sequences of PCR primers used in this study
|
Gene |
Primer (5′→3′) |
|
|
S100A6 |
Forward |
GGGAGGGTGACAAGCACAC |
|
Reverse |
AGCTTCGAGCCAATGGTGAG |
|
|
SNHG1 |
Forward |
ACGTTGGAACCGAAGAGAGC |
|
Reverse |
GCAGCTGAATTCCCCAGGAT |
|
|
OPN |
Forward |
CTCCATTGACTCGAACGACTC |
|
Reverse |
CAGGTCTGCGAAACTTCTTAGAT |
|
|
OCN |
Forward |
CACTCCTCGCCCTATTGGC |
|
Reverse |
CCCTCCTGCTTGGACACAAAG |
|
|
miR-493-5p |
Forward |
GCAGTTGTACATGGTAGGCT |
|
Reverse |
GGTCCAGTTTTTTTTTTTTTTTAAT |
|
|
GAAAG |
Cell transfection
Cells were cultured in plates to an approximately confluence of 80% and subsequently transfected with different plasmids using Lipofectamine® 2000 reagent (Invitrogen, Carlsbad, USA). Forty-eight hours after transfection, the transfection efficiencies were determined by western blot analysis and RT-qPCR. The plasmids used in this study were as follows: the short hairpin RNA (shRNA) of SNHG1 (sh-SNHG1) and the corresponding negative control (sh-NC), the small interfering RNAs targeting S100A6 (si-S100A6-1 and si-S100A6-2) and SNHG1 (si-SNHG1-1 and si-SNHG1-2), the overexpression plasmids of S100A6 (S100A6) and SNHG1 (SNHG1), and the blank plasmid (vector). The siRNA and shRNA sequences are described in Table 2 .
Table2 Sequences of siRNA
|
siRNA |
Target sequence (5′→3′) |
|
si-S100A6-1 |
GTGGGTAATTGTACAATAAATTT |
|
si-S100A6-2 |
TGGGTAATTGTACAATAAATTTT |
|
si-SNHG1-1 |
CTGAGCAAATAAGGTGTATAAAA |
|
si-SNHG1-2 |
GAGCAAATAAGGTGTATAAAAAA |
CCK-8 assay
The transfected cells were seeded into 96-well plates (Corning, Steuben County, USA) and incubated for 24, 48, and 72 h. At indicated time points, 10 μL of CCK-8 solution (Dojindo, Kumamoto, Japan) was added to each well and subsequently incubated for 2 h. Finally, the absorbance values at 450 nm were examined with a Microplate reader (Bio-Rad, Hercules, USA).
Colony formation assay
The transfected cells were seeded into 6-well plates (Corning) at a density of 2.5×10 2 cells/well and cultured for two weeks. Cell colonies were fixed with 4% paraformaldehyde (PFA) and subsequently stained with 1% crystal violet. The dishes were gently rinsed with distilled water, photographed, and counted under a BX51 microscope (Olympus, Tokyo, Japan).
Ethynyl deoxyuridine assay
In accordance with protocols provided by the manufacturer, the Ethynyl deoxyuridine (EdU) Staining Proliferation Kit (Abcam) was utilized to evaluate the proliferation of transfected cells in different groups. Briefly, transfected cells were incubated with EdU overnight, fixed and stained. Cell nuclei were stained with DAPI (Invitrogen) for 15 min prior to observation, and EdU positive cells were counted under a BX51 microscope (Olympus, Tokyo, Japan).
Matrix mineralization assay
To stimulate osteogenic differentiation, transfected cells were cultivated in an osteogenic induction medium (regular growth medium supplemented with 0.1 μM dexamethasone, 10 mM sodium β-glycerophosphate, and 0.05 mM ascorbic acid-2-phosphate) for two weeks. Afterward, the matrix mineralization of cells was assessed by calcium deposits stained with the Alizarin-Red Staining Solution (Sigma-Aldrich, St Louis, USA).
Alkaline phosphatase assay
A colorimetric Alkaline Phosphatase (ALP) Assay Kit (Abcam) was utilized to determine ALP activity based on the color of p-nitrophenyl phosphate, which turned yellow after being dephosphorylated by ALP. The absorbance was measured at 405 nm on a Microplate reader to assess ALP activity.
Bioinformatics analysis
The starBase ( http://starbase.sysu.edu.cn/) [19] was utilized to predict miRNAs that target S100A6, as well as lncRNAs that may potentially interact with the miRNAs of interest. A GSE28423 dataset provided by Namløs et al. [20] was downloaded from Gene Expression Omnibus (GEO) and used to identify the miRNAs down-regulated in osteosarcoma. The lncRNAs differentially expressed in osteosarcoma were obtained from Lnc2Cancer 3.0 ( http://www.bio-bigdata.net/lnc2cancer) [21].
Dual-luciferase assay
To construct luciferase reporter vectors, the 3′UTRs (wild-type, WT or mutant control, MUT) of SNHG1 and S100A6 cDNA fragments carrying the putative miR-493-5p binding site were amplified and inserted into the pmirGlo dual-luciferase vector (Addgene, Watertown, USA) upstream of the reporter gene. For luciferase assay, 293T cells cultured in a 24-well plate were co-transfected with the constructed vectors (SNHG1 3′UTR WT/MUT; S100A6 3′UTR WT/MUT) and miR-493-5p mimic (#miR10002813-1-5; Guangzhou Ribobio Co., Ltd, Guangzhou, China) or the miRNA control (NC mimic; #miR1N0000001-1-5; Guangzhou Ribobio Co., Ltd). After transfection, the firefly fluorescence was normalized to the renilla fluorescence to calculate the luciferase activity. The sequence information of SNHG1, miR-493-5p, and S100A6 is listed in Supplementary Table S1.
RNA immunoprecipitation
The RNA immunoprecipitation (RIP) assay was conducted following the guidelines by using a Magna RIP Kit (Millipore, Billerica, USA). In brief, cells were lysed in the complete RIP lysis buffer containing protease and RNase inhibitors. Then, the cell extract was immunoprecipitated with magnetic beads pre-coated with the antibody against Ago2 (#ab32381; Abcam) or control anti-IgG antibody (#AB5711; Millipore) for 6 h at 4°C. After discarding the supernatant, the RNA-protein complex was treated with proteinase K (Millipore) at 55°C for half an hour to obtain immunoprecipitated RNA. Finally, the immunoprecipitated RNA was subjected to qRT-PCR to detect the levels of miR-493-5p and S100A6. IgG was used as a negative control.
In vivo experiments
A total of twenty nude mice (SPF grade, male, 4–6 weeks old, 15–20 g) purchased from Guangdong Medical Laboratory Center (Guangzhou, China) were divided into four groups ( n=5 per group), and housed under constant temperature (22–24°C) and humidity (50%–60%). After acclimatizing to the new environment for seven days, the four groups of mice were subjected to subcutaneous injection with different transfected cells (5×10 6/0.2 mL PBS) as follows: (1) NC group: 143B cells transfected with sh-NC; (2) sh-SNHG1 group: 143B cells transfected with sh-SNHG1; (3) sh-SNHG1+vector group: 143B cells co-transfected with sh-SNHG1 and blank plasmids; and (4) sh-SNHG1+S100A6 group: 143B cells co-transfected with sh-SNHG1 and S100A6-overexpressing plasmids. The volumes of xenografts were recorded every 4 days with digital calipers and calculated as length × width 2/2 (mm 3). Thirty-two days later, the mice were sacrificed, and osteosarcoma xenograft tumors were collected and weighted, followed by storage in liquid nitrogen for the subsequent detection of SNHG1, miR-493-5p, and S1006A. All experiments involving animals were reviewed and approved by the Academy Animal Experimental Ethical Committee.
Statistical analysis
All experiments were performed with at least three replicates. Prism 8.0.1 was used to conduct the statistical analysis of all data. The comparisons among groups and the determination of significant differences ( P<0.05) were conducted by using Student′s t-test or one-way ANOVA followed by Bonferroni’s post hoc test.
Results
S100A6 is up-regulated in osteosarcoma to promote the proliferation of osteosarcoma cells
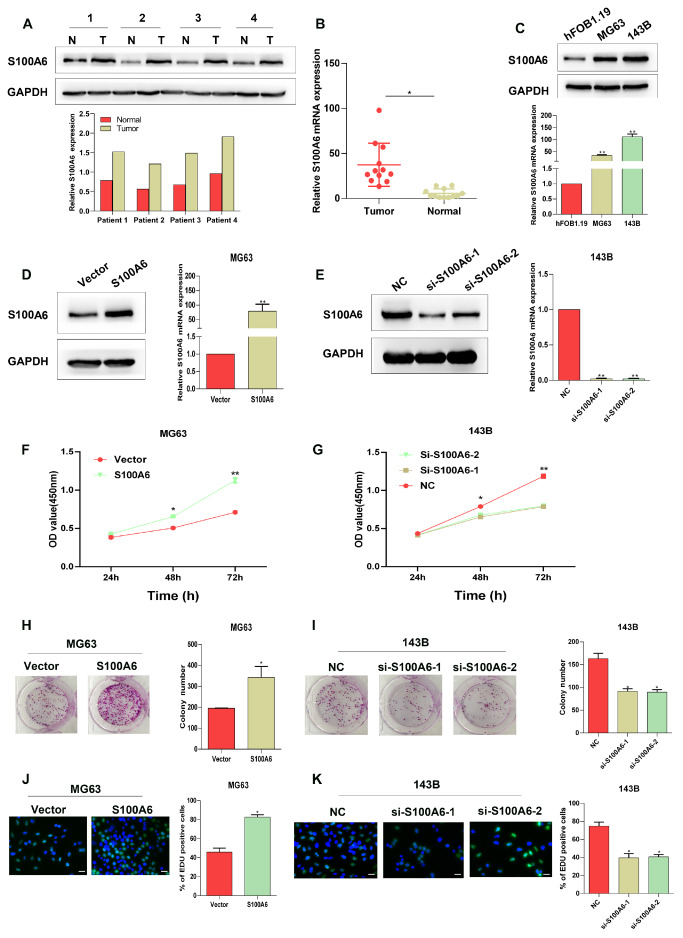
Initially, the expression pattern of S100A6 was investigated in clinical tissue samples. As shown in Figure 1A,B, both the protein and mRNA expression levels of S100A6 were significantly increased in cancerous osteosarcoma tissues as compared with adjacent non-cancerous tissues. Then, the differential expression of S100A6 between normal human osteoblasts (hFOB1.19) and osteosarcoma cells (MG63 and 143B) was also observed. As shown in Figure 1C, compared with that in hFOB1.19 cells, the expression of S100A6 in both MG63 and 143B cells was significantly increased. To explore the functions of S100A6 in osteosarcoma cells, S100A6 was overexpressed and silenced in MG63 and 143B cells, respectively. Western blot analysis and RT-qPCR verified that S100A6-overexpressing and si-S100A6 plasmids were successfully transfected ( Figure 1D,E). CCK-8, colony formation, and EdU assays all showed that the overexpression of S100A6 remarkably enhanced the proliferation of osteosarcoma cells ( Figure 1F,H,J), while silencing of S100A6 exerted an opposite effect ( Figure 1G,I,K). These findings demonstrated that S100A6 is highly expressed and positively related to cell proliferation in osteosarcoma.
Figure1 .
S100A6 is highly expressed in osteosarcoma and promotes the proliferation of osteosarcoma cells(A) The protein and (B) mRNA expression levels of S100A6 in cancerous and adjacent tissues in osteosarcoma patients. (C) The expression pattern of S100A6 in human osteosarcoma cell lines (MG63 and 143B) and osteoblasts (hFOB1.19). (D) The expression of S100A6 in MG63 cells after transfection with blank vectors and S100A6-overexpressing vectors; left: protein level, right: mRNA level. (E) The expression of S100A6 in 143B cells after transfection with siRNAs targeting S100A6 (si-S100A6-1 and si-S100A6-2) or the corresponding negative control (si-NC); left: protein level, right: mRNA level. CCK-8 assay was used to detect the viability of (F) MG63 and (G) 143B cells after different transfection. The colonies formed by (H) MG63 and (I) 143B cells after different transfection. Edu staining of (J) MG63 and (K) 143B cells after different transfection. Scale bar=100 μm. *P<0.05 and **P<0.01.
S100A6 plays a suppression role in osteogenic differentiation in osteosarcoma cells
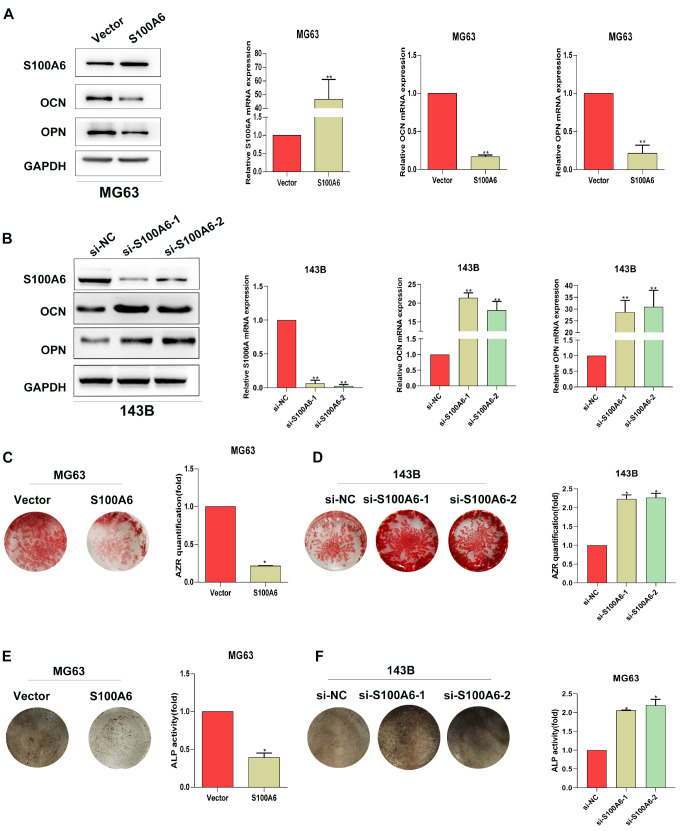
The effect of S100A6 on the osteogenic differentiation of osteosarcoma cells was investigated by detecting OCN and OPN expression, matrix mineralization, as well as ALP activity in MG63 and 143B cells. As depicted in Figure 2A, the expression levels of OCN and OPN were both noticeably reduced in MG63 cells after overexpressing S100A6. Simultaneously, silencing of S100A6 significantly elevated the expression levels of OCN and OPN in comparison with those in the NC group ( Figure 2B), further supporting that S100A6 could down-regulate the expressions of OCN and OPN. The results of matrix mineralization assay showed that the overexpression of S100A6 remarkably reduced the calcium deposit formation in osteosarcoma cells induced by the osteogenic induction medium, while silencing of S100A6 significantly increased the level of calcium deposits ( Figure 2C,D). The results of the ALP assay are depicted in Figure 2E,F, which indicated that the overexpression of S100A6 significantly inhibited the ALP activity, whereas the knocking down of S100A6 induced the opposite effect. All these data indicated that S100A6 could probably modulate the osteogenic differentiation of osteosarcoma cells.
Figure2 .
S100A6 negatively regulates the osteogenic differentiation of osteosarcoma cellsOsteosarcoma cells were cultured in the osteogenic induction medium for 14 days after transfection with different plasmids. The expressions of S100A6, OPN, and OCN in (A) MG63 and (B) 143B cells; left: protein level, right: mRNA level. The matrix mineralization of (C) MG63 and (D) 143B cells was detected by Alizarin Red Staining. ALP staining and ALP activity of (E) MG63 and (F) 143B cells; left: ALP staining, right: ALP activity. *P<0.05 and **P<0.01.
miR-493-5p directly targets S100A6
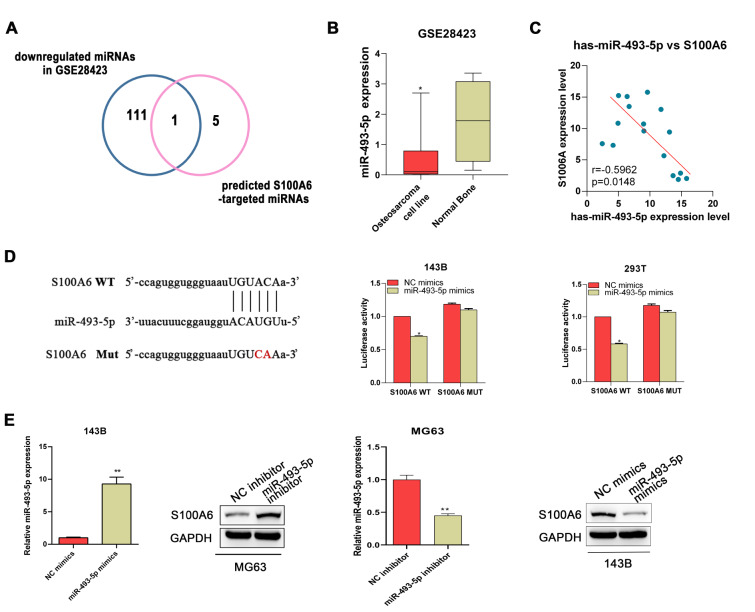
To understand the potential regulatory mechanism of S100A6 in osteosarcoma, a bioinformatics analysis approach was applied to predict miRNAs that potentially targeted S100A6. First, a total of 112 down-regulated miRNAs were identified in osteosarcoma based on the GSE28423 dataset. In addition, six miRNAs that may biologically target S100A6 were predicted by a bioinformatics online platform (Starbase). After pooling the results of these analyses, we found that miR-493-5p may be a regulator of S100A6 in osteosarcoma ( Figure 3A). The expression profile of miR-493-5p from the GSE28423 dataset was described in Figure 3B. Then, a significantly inverse correlation between the expressions of S100A6 and miR-493-5p was observed in the osteosarcoma samples of this study ( Figure 3C). As shown in Figure 3D, the dual-luciferase assay verified that S100A6 is a direct target of miR-493-5p. Moreover, western blot and RT-qPCR analyses indicated that miR-493-5p could negatively regulate the expression level of S100A6 at both mRNA and protein levels ( Figure 3E).
Figure3 .
S100A6 is a target of miR-493-5p(A) S100A6-targeting miRNAs obtained by intersecting the miRNAs predicted by Starbase and the down-regulated miRNAs identified based on GSE28423. (B) The expression profile of miR-493-5p based on the GSE28423 dataset. (C) The correlation between S100A6 and miR-493-5p in osteosarcoma tissues. (D) Relationship between S100A6 and miR-493-5p expression was confirmed by the dual-luciferase assay. (E) The protein and mRNA expression levels of S100A6 in osteosarcoma cells after transfection with miR-493-5p mimics or inhibitors. *P<0.05 and **P<0.01.
SNHG1 directly interacts with miR-493-5p to regulate S100A6
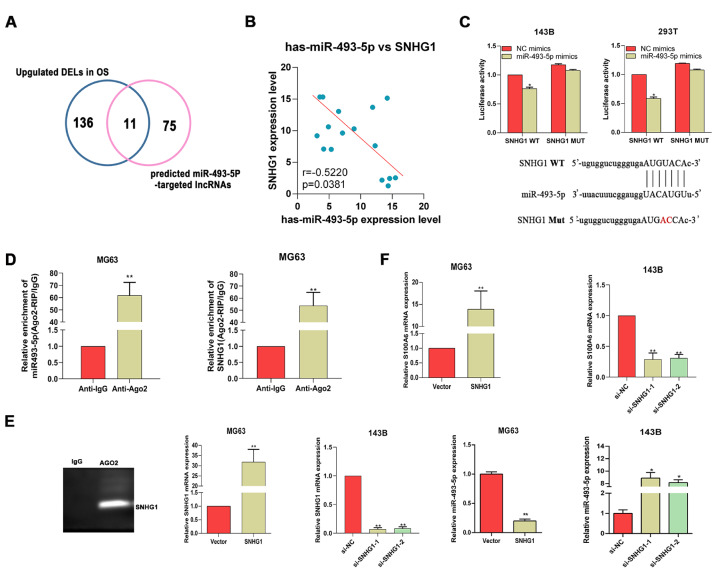
Given that lncRNAs may act as competing endogenous RNAs (ceRNAs) of miRNAs to regulate the target genes of miRNAs, lncRNAs that potentially target miR-493-5p in osteosarcoma were further identified using bioinformatics tools. As shown in Figure 4A, a total of 11 lncRNAs were obtained, which included SNHG3, KCNQ1OT1, GAS6-AS2, LUCAT1, ILF3-AS1, NEAT1, MIR17HG, SNHG1, MALAT1, FTX, and LINC00324. SNHG1 was selected as a target for further analysis. The Pearson’s correlation analysis of the clinical tissue samples showed that SNHG1 was inversely correlated with miR-493-5p in osteosarcoma ( Figure 4B). Then, the dual-luciferase assay confirmed the direct interaction between miR-493-5p and SNHG1 ( Figure 4C). Moreover, the results of the RIP assay in MG63 cells showed that both miR-493-5p and SNHG1 were obviously enriched in immunoprecipitation complexes, further confirming that SNHG1 could spatially interact with miR-493-5p via Ago2 protein ( Figure 4D).
Figure4 .
SNHG1 acts as a competing ceRNA of miR-493-5p to regulate the expression of S100A6(A) miR-493-5p-regulating lncRNAs predicted by Starbase and lnc2cancer. (B) The correlation between SNHG1 and miR-493-5p in osteosarcoma tissues. (C) Relationship between SNHG1 and miR-493-5p expression was confirmed by the dual-luciferase assay. (D) Correlation of SNHG1 and miR-493-5p with Ago2 was predicted by the RIP assay. The expressions of SNHG1, miR-493-5p (E), and S100A6 (F) in MG63 cells after transfection with blank vectors or SNHG1-overexpressing vectors, and in 143B cells after transfection of si- SNHG1 (si-SNHG1-1 and si-SNHG1-2) or si-NC. *P<0.05 and **P<0.01.
Based on the above results, we speculated that SNHG1 may be involved in the regulation of S100A6 in osteosarcoma via miR-493-5p. In order to prove this speculation, the expression of S100A6 in osteosarcoma cells was investigated after knocking down or overexpressing SNHG1. The transfection efficiency of the SNHG1 overexpression vector and si-SNHG1 was verified by RT-qPCR ( Figure 4E ). It was found that the expression of miR-493-5p could be down-regulated by overexpressing SNHG1, and up-regulated by knocking down SNHG1 ( Figure 4E). As expected, the expression of S100A6 in osteosarcoma cells was significantly increased by the overexpression of SNHG1, and obviously suppressed by the silencing of SNHG1 ( Figure 4F). This result suggested that SNHG1 is able to regulate the expression of S100A6 in osteosarcoma cells via miR-493-5p.
SNHG1 aggravates the malignant phenotypes of osteosarcoma cells through S100A6
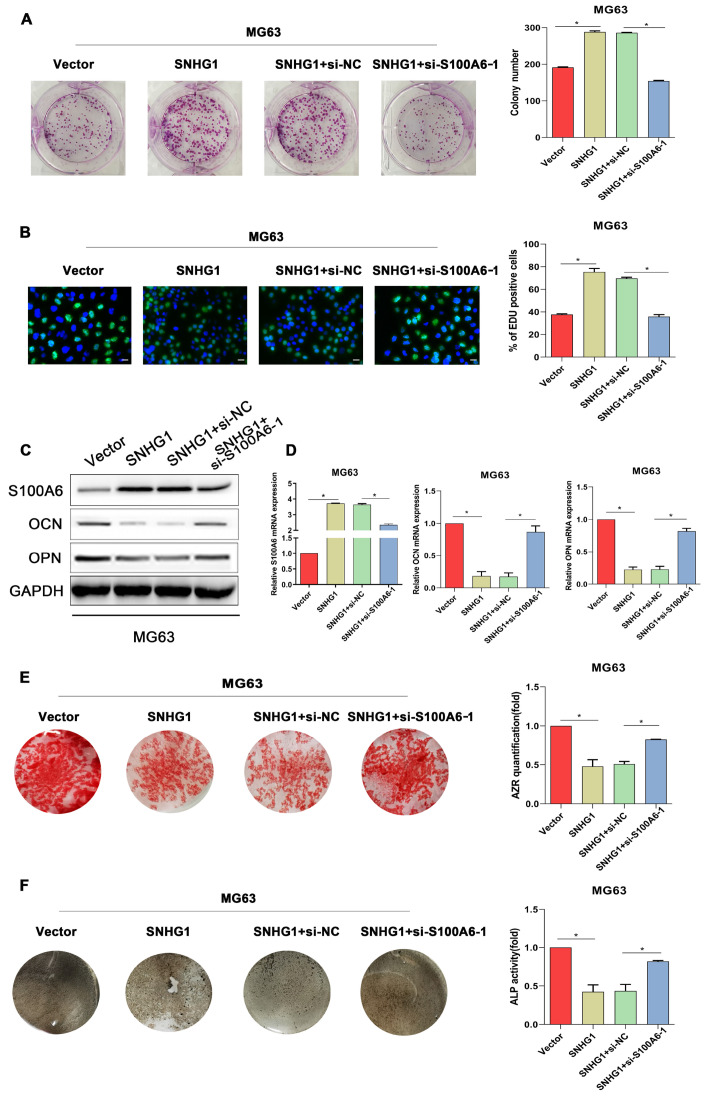
Since the previous results revealed a regulatory role of SNHG1 in S100A6 expression in osteosarcoma cells, whether S100A6 mediates SNHG1-induced changes of cell biological functions was investigated. As depicted in Figure 5A,B, overexpression of SNHG1 significantly enhanced the proliferation of MG63 cells, but this effect could be blocked by silencing S100A6. In terms of osteogenic differentiation ability, SNHG1 overexpression led to reduced expression levels of osteogenic markers, and decreased calcium deposits and ALP activity ( Figure 5C–F), suggesting that SNHG1 overexpression could impaire the capacity of osteogenic differentiation in AGS cells. Simultaneously, the reduced osteogenic differentiation induced by SNHG1 overexpression was counteracted by S100A6 silencing ( Figure 5C–F). These results suggested that SNHG1 regulates the expression of S100A6 to promote osteosarcoma progression.
Figure5 .
Silencing of S100A6 reverses the effect of SNHG1 overexpression on cell proliferation and osteogenic differentiation in osteosarcoma cells MG63 cells were respectively transfected with blank vectors, SNHG1-overexpressing vectors, and the combination of SNHG1-overexpressing vectors with si-NC or si-S100A6-1.
(A) The colonies formed by MG63 cells after different transfection. (B) EdU staining of MG63 cells after different transfection. (C,D) S100A6, OPN and OCN expressions, (E) matrix mineralization, (F) ALP staining and ALP activity of transfected MG63 cells after being cultured in the osteogenic induction medium for 14 days. *P<0.05.
SNHG1 silencing suppresses osteosarcoma development by regulating S100A6 via miR-493-5p
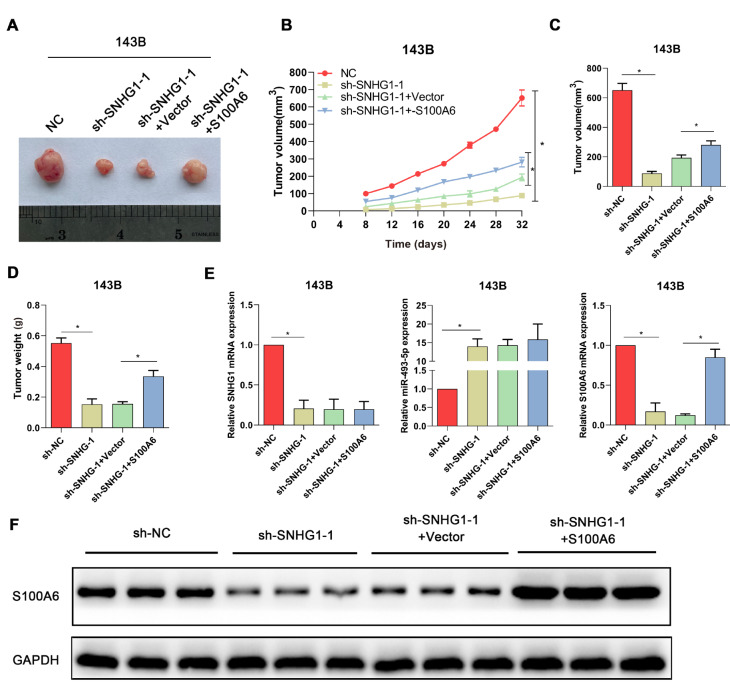
Finally, in vivo experiments were carried out to further confirm the role of SNHG1/S100A6 in osteosarcoma. The xenograft tumors in nude mice of different groups are depicted in Figure 6A, which indicated that the xenograft tumors of the sh-SNHG1 group and the sh-SNHG1+vector group were obviously smaller than those of the NC group and the sh-SNHG1+S100A6 group. Similar results could be found in Figure 6B–D, which revealed that the silencing of SNHG1 inhibited the growth of osteosarcoma, while S100A6 overexpression could rescue the change induced by SNHG1 silencing. These data corroborated previous findings, indicating that SNHG1 promotes the progression of osteosarcoma by up-regulating S100A6. Furthermore, the expression levels of SNHG1, miR-493-5p, and S1006A were detected in xenograft tumors of mice from each group. As shown in Figure 6E, RT-qPCR indicated that SNHG1 knockdown could significantly enhance the expression of miR-493-5p and reduce the expression of S1006A. Notably, it was found that S100A6 overexpression had no effect on the changes induced by SNHG1 silencing in the expression of SNHG1 and miR-493-5p. Furthermore, the expression of S100A6 protein in the xenograft tumors was detected. The result of western blot analysis was similar to that of RT-qPCR, indicating that SNHG1 knockdown significantly reduced the expression of S1006A ( Figure 6F). Collectively, these data supported the speculation that the up-regulation of S100A6 in osteosarcoma is regulated by SNHG1 via miR-493-5p.
Figure6 .
S100A6 overexpression counteracts the suppressive role of SNHG1 silencing in tumor growth in nude mice(A) Representative images of xenograft tumors from each group. (B) The growth curve of tumor volume. (C) The volume and (D) weight of xenograft tumors recorded on the last day. (E) RT-qPCR was used to detect the expressions of SNHG1, miR-493-5p, and S1006A in xenograft tumors of mice from each group. (F) Western blot analysis was used to detect the expression of S1006A protein in xenograft tumors of mice from each group. *P<0.05.
Discussion
Given that the prognosis of osteosarcoma is not optimal, research on its pathogenesis is still of great significance to the development of novel strategies in osteosarcoma therapy. S100A6 has been documented to be associated with aggressive biological behavior in many human malignancies, including osteosarcoma [22]. Li et al. [23] demonstrated based on an in vivostudy that S100A6 serves as an oncogene to promote osteosarcoma growth. Nevertheless, the molecular mechanism underlying the up-regulation of S100A6 in osteosarcoma remains ill-defined. Herein, we uncovered that SNHG1 is involved in regulating S100A6 expression in osteosarcoma by directly binding to miR-493-5p, an miRNA directly targeting S100A6, thereby exerting tumorigenic effects.
Consistent with a previous report [5], the results of this study exhibited that the expression of S100A6 was obviously up-regulated in cancerous osteosarcoma tissues compared with that in adjacent non-cancerous tissues. Functional studies revealed that S100A6 overexpression not only markedly reinforced the proliferation of osteosarcoma cells, but also impeded their osteogenic differentiation, suggesting a role of S100A6 in promoting osteosarcoma development. This finding was confirmed by the studies on S100A6 silencing. Since defective osteogenic differentiation is one of the important features in osteosarcoma tumorigenesis and contributes to the high malignant potential of osteosarcoma [24], osteosarcomas can maintain their proliferative phenotypes and overall aggressiveness by preventing terminal differentiation.
In the past several years, many studies have reported that the dysregulation of some lncRNAs is closely related to osteosarcoma development and progression. ANRIL has been proven to be a lncRNA related to the prognosis of osteosarcoma, and ANRIL up-regulation can promote proliferation, invasion, as well as phosphorylated PI3K and AKT level of osteosarcoma cells [25]. Furthermore, several lncRNAs have been demonstrated to be correlated with chemoresistance in osteosarcoma [ 26, 27]. For example, a previous study revealed that FOXC2-AS1 can promote doxorubicin resistance in osteosarcoma cells, which is closely associated with poor prognosis in osteosarcoma patients [27]. In the context of osteosarcoma, several lncRNA/miRNA pairs have been investigated. Recently, He et al. [28] revealed that lncRNA LMCD1-AS1 plays an important role in osteosarcoma progression by sponging miR-106b-5p, and its overexpression is closely related to poor clinical outcomes of osteosarcoma patients. Therefore, it was speculated that there may be a lncRNA/miRNA axis involved in regulating the abnormal expression of S100A6 in osteosarcoma. By integrated bioinformatics analysis, SNHG1/miR-493-5p was identified for further analyses. This study proved that miR-493-5p directly down-regulated the expression of S100A6 in osteosarcoma cells. In addition, it was found that SNHG1 exhibited a negative correlation with miR-493-5p in osteosarcoma tissues, and could competitively bind to miR-493-5p in osteosarcoma cells. It has been widely reported that lncRNAs serve as ceRNAs of miRNAs to reduce the interaction of miRNAs with their target genes, thereby enhancing the expression of the target genes. Inspired by this view, we speculated that SNHG1 may work as an important regulator in the expression of S100A6 in osteosarcoma progression.
Mounting evidence implicated SNHG1 as a well-characterized oncogenic lncRNA that regulates tumor initiation and progression in multiple cancers [29], including esophageal cancer [30], neuroblastoma [31], and osteosarcoma [32]. Wang et al. [33] found that SNHG1 was highly expressed in osteosarcoma tissues and cell lines, and revealed a role of SNHG1 in promoting the cell growth and metastasis of osteosarcoma in vitro and in vivo. Another study conducted by Jiang et al. [32] also demonstrated the oncogenic role of SNHG1 in the progression of osteosarcoma. In line with the aforementioned literature, the data of this study showed that overexpression of SNHG1 significantly potentiated the proliferation of osteosarcoma cells. It was found that the overexpression of SNHG1 could effectively suppress the differentiation of osteosarcoma cells into osteoblasts. Notably, these changes induced by SNHG1 overexpression were rescued by co-transfection with S100A6 siRNA. Moreover, in vivo experiments showed that the suppressive effect of SNHG1 silencing on tumor growth was partly blocked by overexpressing S100A6, suggesting that the effect of SNHG1 on osteosarcoma was partly exerted by regulating the expression of S100A6. Previous studies have demonstrated that SNHG1 participates in the tumorigenesis and development of osteosarcoma via miR-326/NOB1 [33] and miR-577/WNT2B/Wnt/β-catenin axis [32]. In this study, our data uncovered an miR-493-5p/S100A6 axis that contributes to the oncogenic role of SNHG1 in osteosarcoma. RT-qPCR analysis on the expressions of SNHG1, miR-493-5p, and S1006A further implied that the expression of S100A6 is regulated by SNHG1 via suppressing miR-493-5p in osteosarcoma.
Taken together, this study identified the involvement of SNHG1, an oncogenic lncRNA, in the abnormal expression of S100A6 in osteosarcoma by regulating miR-493-5p, thereby promoting the progression of osteosarcoma. Nevertheless, this study is preliminary in nature, and more details on the functions of S100A6/SNHG1/miR-493-5p axis in osteosarcoma are still required to further explore its role in the future. Given that different subtypes of osteosarcoma, such as conventional osteosarcoma (COS) and telangiectatic osteosarcoma (TOS), have completely different prognosis, the functions of SNHG1 in the progression of COS and TOS may also be different. Therefore, in future studies, elucidating the role of SNHG1 in different subtypes of osteosarcoma is also required.
The present study revealed that S100A6, a highly expressed gene in osteosarcoma, promotes the progression of osteosarcoma in vitro and in vivo, and its expression is regulated by the SNHG1/miR-493-5p axis. The findings of this study provide new insights into the pathogenesis of osteosarcoma, and lay the foundation for innovative RNA-based anticancer strategies targeting S100A6.
Supporting information
Supplementary Data
Supplementary Data is available at Acta Biochimica et Biphysica Sinica online.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grant from the Shengjing Hospital of China Medical University.
References
- 1.Scott MC, Temiz NA, Sarver AE, LaRue RS, Rathe SK, Varshney J, Wolf NK, et al. Comparative transcriptome analysis quantifies immune cell transcript levels, metastatic progression, and survival in osteosarcoma. Cancer Res. . 2018;78:326–337. doi: 10.1158/0008-5472.CAN-17-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009, 152: 3–13 . [DOI] [PubMed]
- 3.Wang XH, Du H, Li L, Shao DF, Zhong XY, Hu Y, Liu YQ, et al. Increased expression of S100A6 promotes cell proliferation in gastric cancer cells. Oncol Lett. . 2017;13:222–230. doi: 10.3892/ol.2016.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Tang M, Ling B, Liu S, Zheng Y, Nie C, Yuan Z, et al. Increased expression of S100A6 promotes cell proliferation and migration in human hepatocellular carcinoma. J Mol Med. . 2014;92:291–303. doi: 10.1007/s00109-013-1104-3. [DOI] [PubMed] [Google Scholar]
- 5.Luu HH, Zhou L, Haydon RC, Deyrup AT, Montag AG, Huo D, Heck R, et al. Increased expression of S100A6 is associated with decreased metastasis and inhibition of cell migration and anchorage independent growth in human osteosarcoma. Cancer Lett. . 2005;229:135–148. doi: 10.1016/j.canlet.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Luo X, Sharff KA, Chen J, He TC, Luu HH. S100A6 expression and function in human osteosarcoma. Clin Orthop Relat Res. . 2008;466:2060–2070. doi: 10.1007/s11999-008-0361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang JY, Yang Y, Ma Y, Wang F, Xue A, Zhu J, Yang H, et al. Potential regulatory role of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed Pharmacother. . 2020;121:109627. doi: 10.1016/j.biopha.2019.109627. [DOI] [PubMed] [Google Scholar]
- 8.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. . 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 9.Guo LP, Zhang ZJ, Li RT, Li HY, Cui YQ. Influences of LncRNA SNHG20 on proliferation and apoptosis of glioma cells through regulating the PTEN/PI3K/AKT signaling pathway. Eur Rev Med Pharmacol Sci. 2019, 23: 253-261 . [DOI] [PubMed]
- 10.Hu Q, Ye Y, Chan LC, Li Y, Liang K, Lin A, Egranov SD, et al. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat Immunol. . 2019;20:835–851. doi: 10.1038/s41590-019-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azofeifa JG, Allen MA, Hendrix JR, Read T, Rubin JD, Dowell RD. Enhancer RNA profiling predicts transcription factor activity. Genome Res. . 2018;28:334–344. doi: 10.1101/gr.225755.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jadaliha M, Gholamalamdari O, Tang W, Zhang Y, Petracovici A, Hao Q, Tariq A, et al. A natural antisense lncRNA controls breast cancer progression by promoting tumor suppressor gene mRNA stability. PLoS Genet. . 2018;14:e1007802. doi: 10.1371/journal.pgen.1007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rashid F, Shah A, Shan G. Long non-coding RNAs in the cytoplasm. Genomics Proteomics BioInf. . 2016;14:73–80. doi: 10.1016/j.gpb.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. . 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Luo P, Guo W, Shi Y, Xu D, Zheng H, Jia L. LncRNA SNHG20 knockdown suppresses the osteosarcoma tumorigenesis through the mitochondrial apoptosis pathway by miR-139/RUNX2 axis. Biochem Biophys Res Commun. . 2018;503:1927–1933. doi: 10.1016/j.bbrc.2018.07.137. [DOI] [PubMed] [Google Scholar]
- 16.Gu Z, Hou Z, Zheng L, Wang X, Wu L, Zhang C. LncRNA DICER1-AS1 promotes the proliferation, invasion and autophagy of osteosarcoma cells via miR-30b/ATG5. Biomed Pharmacother. . 2018;104:110–118. doi: 10.1016/j.biopha.2018.04.193. [DOI] [PubMed] [Google Scholar]
- 17.Mahmood T, Yang PC. Western blot: technique, theory, and trouble shooting. N Am J Med Sci. . 2012;4:429. doi: 10.4103/1947-2714.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ C T method . Methods. . 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein–RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. . 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Namløs HM, Meza-Zepeda LA, Barøy T, Østensen IH, Kresse SH, Kuijjer ML, Serra M, et al. Modulation of the osteosarcoma expression phenotype by microRNAs. PLoS ONE. . 2012;7:e48086. doi: 10.1371/journal.pone.0048086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ning S, Zhang J, Wang P, Zhi H, Wang J, Liu Y, Gao Y, et al. Lnc2Cancer: a manually curated database of experimentally supported lncRNAs associated with various human cancers. Nucleic Acids Res. . 2016;44:D980–D985. doi: 10.1093/nar/gkv1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donato R, Sorci G, Giambanco I. S100A6 protein: functional roles. Cell Mol Life Sci. . 2017;74:2749–2760. doi: 10.1007/s00018-017-2526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Wagner ER, Yan Z, Wang Z, Luther G, Jiang W, Ye J, et al. The calcium-binding protein S100A6 accelerates human osteosarcoma growth by promoting cell proliferation and inhibiting osteogenic differentiation. Cell Physiol Biochem. . 2015;37:2375–2392. doi: 10.1159/000438591. [DOI] [PubMed] [Google Scholar]
- 24.Wagner ER, Luther G, Zhu G, Luo Q, Shi Q, Kim SH, Gao JL, et al. Defective osteogenic differentiation in the development of osteosarcoma. Sarcoma. . 2011;2011:1–12. doi: 10.1155/2011/325238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu G, Liu G, Yuan D, Dai J, Cui Y, Tang X. Long non-coding RNA ANRIL is associated with a poor prognosis of osteosarcoma and promotes tumorigenesis via PI3K/Akt pathway. J Bone Oncol. . 2018;11:51–55. doi: 10.1016/j.jbo.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han Z, Shi L. Long non-coding RNA LUCAT1 modulates methotrexate resistance in osteosarcoma via miR-200c/ABCB1 axis. Biochem Biophys Res Commun. . 2018;495:947–953. doi: 10.1016/j.bbrc.2017.11.121. [DOI] [PubMed] [Google Scholar]
- 27.Zhang CL, Zhu KP, Ma XL. Antisense lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2. Cancer Lett. . 2017;396:66–75. doi: 10.1016/j.canlet.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 28.He JW, Li DJ, Zhou JH, Zhu YL, Yu BQ. SP1-mediated upregulation of lncRNA LMCD1-AS1 functions a ceRNA for miR-106b-5p to facilitate osteosarcoma progression. Biochem Biophys Res Commun. . 2020;526:670–677. doi: 10.1016/j.bbrc.2020.03.151. [DOI] [PubMed] [Google Scholar]
- 29.Thin KZ, Tu JC, Raveendran S. Long non-coding SNHG1 in cancer. Clinica Chim Acta. . 2019;494:38–47. doi: 10.1016/j.cca.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Jin X, Wang Z, Zhang X, Liu S, Liu G. Downregulation of SNHG1 suppresses cell proliferation and invasion by regulating Notch signaling pathway in esophageal squamous cell cancer. Cancer Biomark. . 2017;21:89–96. doi: 10.3233/CBM-170286. [DOI] [PubMed] [Google Scholar]
- 31.Sahu D, Hsu CL, Lin CC, Yang TW, Hsu WM, Ho SY, Juan HF, et al. Co-expression analysis identifies long noncoding RNA SNHG1 as a novel predictor for event-free survival in neuroblastoma . Oncotarget. . 2016;7:58022–58037. doi: 10.18632/oncotarget.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Z, Jiang C, Fang J. Up-regulated lnc-SNHG1 contributes to osteosarcoma progression through sequestration of miR-577 and activation of WNT2B/Wnt/β-catenin pathway. Biochem Biophys Res Commun. . 2018;495:238–245. doi: 10.1016/j.bbrc.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Cao L, Wu J, Wang Q. Long non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326 and promotes tumorigenesis in osteosarcoma. Int J Oncol. . 2017;52:77. doi: 10.3892/ijo.2017.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.