Abstract
Although hematopoietic stem cells (HSCs) in the bone marrow are in a state of quiescence, they harbor the self-renewal capacity and the pluripotency to differentiate into mature blood cells when needed, which is key to maintain hematopoietic homeostasis. Importantly, HSCs are characterized by their long lifespan ( e. g., up to 60 months for mice), display characteristics of aging, and are vulnerable to various endogenous and exogenous genotoxic stresses. Generally, DNA damage in HSCs is endogenous, which is typically induced by reactive oxygen species (ROS), aldehydes, and replication stress. Mammalian cells have evolved a complex and efficient DNA repair system to cope with various DNA lesions to maintain genomic stability. The repair machinery for DNA damage in HSCs has its own characteristics. For instance, the Fanconi anemia (FA)/BRCA pathway is particularly important for the hematopoietic system, as it can limit the damage caused by DNA inter-strand crosslinks, oxidative stress, and replication stress to HSCs to prevent FA occurrence. In addition, HSCs prefer to utilize the classical non-homologous end-joining pathway, which is essential for the V(D)J rearrangement in developing lymphocytes and is involved in double-strand break repair to maintain genomic stability in the long-term quiescent state. In contrast, the base excision repair pathway is less involved in the hematopoietic system. In this review, we summarize the impact of various types of DNA damage on HSC function and review our knowledge of the corresponding repair mechanisms and related human genetic diseases.
Keywords: c-NHEJ, DNA inter-strand crosslink, FA/BRCA pathway, hematopoietic stem cell, oxidative damage, replication stress
Introduction
Hematopoiesis refers to the process that generates blood cells of all lineages, where the cellular constituents of blood are continually replenished. Hematopoietic stem cells (HSCs) play a central role in hematopoiesis. The long-term HSCs (LT-HSCs) have self-renewal capacity and can differentiate into short-term HSCs (ST-HSCs), which are also known as multipotent progenitors (MPPs). MPPs lose the ability of self-renewal and further give rise to two types of downstream progenitors, the common myeloid progenitor (CMP) and common lymphoid progenitor (CLP). These progenitor cells then proliferate and differentiate into all types of mature blood cells [ 1, 2] . Hematopoietic homeostasis depends largely on the balance between self-renewal and differentiation of HSCs in the bone marrow (BM). Another distinguishing feature of HSCs is that they can maintain a long-term quiescent state and thus have a long lifespan (ranging from 10 to 60 months in mice) [3], where the cell cycle remains in the G0-phase without dividing [4]. Consequently, HSCs are more likely to accumulate DNA damage during the aging process. Therefore, the DNA repair machinery is important for maintaining the stability of the HSC genome, which is required for the maintenance of HSC function [ 5, 6] .
Hematopoietic cells are mainly threatened by endogenous DNA damage induced by metabolic intermediates, such as reactive oxygen species (ROS) produced by mitochondrial metabolism and aldehydes produced during lipid peroxidation. These metabolites may affect the self-renewal and differentiation of HSCs. Furthermore, they can directly induce multiple DNA lesions, including oxidative damage, DNA inter-strand crosslinks (ICLs), single-strand breaks (SSBs), and double-strand breaks (DSBs), and induce replication stress disrupting the stability of the HSCs genome [ 7– 9] . Previous studies have shown that the expression levels of most DNA damage repair genes in quiescent HSCs are downregulated compared with the downstream progenitors. Once driven into the cell cycle, the expressions of DNA damage repair genes in HSCs are increased significantly, allowing DNA breaks to be repaired stably [10]. Therefore, DNA repair defects can compromise hematopoiesis and cause bone marrow failure (BMF) or hematological malignancies [ 11, 12] .
This review comprehensively summarizes the characteristics of DNA damage and related repair mechanisms in the hematopoietic system and attempts to introduce how they affect the development and function of HSCs.
DNA Damage and the Inducers in Hematopoietic System
Under physiological conditions, DNA damage in HSCs is mainly endogenously generated, typically induced by ROS, aldehydes, and replication stress. Here, we will review the generation mechanism, the main clearance manners, and the primary impacts on HSC development of these endogenous damage inducers.
ROS
ROS mainly refers to hydrogen peroxide (H 2O 2) and superoxide anion radicals (O 2 ·−) which are primarily produced by intracellular mitochondrial metabolism, and are also generated when cells are exposed to ionizing radiation (IR) or exogenous environmental and pharmaceutical carcinogens. ROS can be eliminated by various antioxidant defense enzymes in mammalian cells, where excessive ROS causes various DNA lesions, including abasic sites, base deamination, base oxidations, 8-oxoguanine lesions, and DNA strand breaks that disrupt the functions of DNA, RNA, and cellular proteins. One of the most studied intracellular oxidative DNA lesions is 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxoG) which has been considered as a marker of oxidative stress [ 13, 14] .
Unlike the somatic cells that have high metabolic activity and continuously produce ROS, stem cells generally have a low metabolic rate with less production of ROS [15]. A hypoxic microenvironment and proper level of ROS in BM are essential for the self-renewal and differentiation of HSCs. Compared to the LT-HSCs from mice, ROS is increased in proliferating downstream progenitor populations, which is also true in HSCs when exiting quiescence [ 16, 17] . These results suggest that HSCs in the active cell cycle face higher levels of oxidative stress, which is consistent with the idea that HSC quiescence facilitates genomic stability [18]. Elevated levels of ROS have been considered as a characteristic of aging HSCs, and multiple evidence has shown that increased ROS impairs various functions of HSCs, including lifespan, self-renewal, and differentiation [ 8, 19, 20] .
Aldehydes
Aldehydes, such as acetaldehyde, formaldehyde, 4-hydroxynonenal, malondialdehyde, and acrolein, represent a group of reactive metabolites which are usually resulted from the metabolism of amino acids, carbohydrates, lipids, biogenic amines, vitamins and steroids. For instance, the oxidation of endogenous methanol which is derived from the process of one-carbon metabolism in proteins, will lead to the formation of formaldehyde [21]. Moreover, alcohol drinking is also an important source of aldehydes [9]. At least 19 distinct aldehyde dehydrogenases (ALDHs) have been reported to function as detoxifying enzymes thus far [22]. The most studied members are ADH5 and ALDH2, where the metabolism of formaldehyde requires for both enzymes, while ALDH2 is more important for the metabolizing ethanol-derived acetaldehyde. Excessive aldehydes can induce various DNA lesions, including oxidative stress, DNA-ICLs, and DNA-protein crosslinks (DPCs) [ 23, 24] . As one of the most thoroughly studied types of DNA lesions, DNA-ICLs are formed by the presence of covalent adducts between two strands of DNA, which impede DNA unwinding and block DNA replication and transcription, resulting in cytotoxicity [25].
Clearance of endogenous aldehydes is critical for HSC development. Two research groups recently revealed that simultaneous loss of function of Adh5 and Aldh2 in mice, rather than single gene inactivation, results in elevated blood formaldehyde, and causes increased DNA damage and depletion of LT-HSCs and CLPs accompanied by growth restriction and reduced lifespan [ 26, 27] . Consistently, patients harboring bi-allelic variants in ADH5 in combination with the rs671 defective allele of ALDH2 were identified with BMF, myelodysplasia, and several other congenital abnormalities [ 26– 28] . The results from the patient-based induced pluripotent stem cell (iPSC) model suggest that ADH5 plays a dominant role in formaldehyde metabolism, while ALDH2 plays a secondary role [28].
DNA replication stress
DNA replication stress is defined as the slowing or stalling of replication fork progression, which can be induced by various endogenous and exogenous sources, such as endogenous oxidative DNA lesions and DNA-ICLs, secondary DNA structures and fragile sites, R-loops, and oncogene over-expression. Replication stress is not a DNA lesion with specific structural characteristics, but rather reflects a state when the replication fork is threatened during the DNA replication process, which is more likely to cause genomic instability. In general, replicative stress produces ssDNA that binds to and is protected by replication protein A (RPA), which can recruit multiple downstream components (ATRIP, Rad17, Rad9-Hus1-Rad1 complex, and TOPBP1) to trigger the activation of the ATR/CHK1 pathway to maintain fork stability. In addition, continuous replication stress can cause replication fork stalling and collapse, and eventually leads to DSBs formation [ 29, 30] .
Endogenous DNA replication stress has been suggested as a potent driver of functional decline in aging HSCs, which has been observed from the accumulation of γH2AX foci (a widely used molecular marker for DNA damage) in HSCs from old mice; it was not caused by DNA damage, but was strongly associated with stalled and/or collapsed replication forks. In mice, old HSCs display greater levels of DNA damage associated with intracellular replication stress compared to young HSCs, accompanied by a largely decreased expression of MCM helicase components (Mcm2–7), which are pivotal for DNA replication, leading to both a delay in entering phase S and an extended S phase [18]. Consistent with the results of this study [18], the hypomorphic mice model harboring a synonymous A/G transition in exon 9 of Atr showed increased embryonic replication stress, accompanied by the characteristics of Seckel syndrome including accelerated aging, dwarfism, and pancytopenia [31], which have also been described in humans [32] ( Table 1).
Table 1 Summary of the human genetic disorders associated with DNA repair defects in the hematopoietic system
|
Gene |
Function |
Hematological phenotype in mice model |
Disorders (hematologicalphenotype) in human |
|
Key kinases | |||
|
ATM |
Plays a central role in DDR |
BMF with HSCs depletion |
Ataxia-telangiectasia (defective B cell differentiation, decreased circulating T cells) |
|
ATR |
Plays a central role in DDR |
Accelerated aging, dwarfism, pancytopenia accumulation of fat in the BM |
Seckel syndrome (pancytopenia) |
|
Genes in the FA/BRCA pathway | |||
|
FANCA, FANCB, FANCC, FANCE, FANCF, FANCG/XRCC9, FANCL, FANCT/UBE2T |
Constituting FA core complex to promote FANCD2/I ubiquitination |
Do not spontaneously develop a BMF phenotype, unless there is additional exogenous stress |
FA (BMF) |
|
FANCD2, FANCI |
Recruitment of downstream nucleases to excise DNA-ICLs |
||
|
FANCP/SLX4, FANCQ/ERCC4/XPF |
Structure-specific exonucleases |
||
|
FANCD1/BRCA2, FANCJ/BRIP1, FANCN/PALB2, FANCU/XRCC2, FANCW/RFWD3 |
Repair of DSB by promoting HR |
||
|
FANCV/REV7/MAD2L2 |
Bypasss the crosslink remnants; and repair of DSB by promoting NHEJ |
||
|
Genes in DSB repair machinery | |||
|
DNA-PKcs/PRKDC |
Serine/threonine-protein kinase that recruit interacts with LIG4-XRCC4 complex to promote DSB end ligation, and essential for V(D)J recombination |
BMF with HSCs depletion |
Severe combined immunodeficiency (decreased circulating T and B cells) |
|
LIG4 |
ATP-dependent DNA ligase that interacts with XRCC4 to promote DSB end ligation, and essential for V(D)J recombination |
Immunodeficiency, BMF with HSCs depletion |
LIG4 syndrome (pancytopenia) |
|
NBS1 |
A member of MRN complex, promotes DSB end resection |
BMF with HSCs depletion |
Nijmegen breakage syndrome (autoimmune hemolytic anemia,thrombocytopenia post hemolytic anemia, decreased circulating T and B cells) |
|
Genes in MMR pathway | |||
|
MLH1, MSH2, MSH6, PMS2 |
Repair of mismatches |
Hematopoietic malignancies (leukemia and lymphoma |
Mismatch repair cancer syndrome (leukemia) |
|
Others | |||
|
ERCC6L2 |
May play a role in the NER pathway |
None |
BMF syndrome |
|
ADH5 and ALDH2 (Digenic) |
Detoxifying enzymes that metabolize formaldehyde and acetaldehyde, respectively |
Depletion of LT-HSCs and CLPs |
AMED syndrome (BMF) |
BM, bone marrow; BMF, bone marrow failure; CLPs, lymphoid progenitor; DDR, DNA damage response; DNA-ICLs, DNA inter-strand crosslinks; DSB, double-strand break; FA, Fanconi anemia; LT-HSCs, long-term hematopoietic stem cells; MMR, mismatch repair; MRN, MRE11-RAD50-NBS1; NER, nucleotide excision repair; NHEJ, non-homologous end joining; HR, homologous recombination; REF, reference
DNA Repair Mechanisms in the Hematopoietic System
HSCs exhibit a certain ability to utilize the intracellular enzyme metabolism system ( i. e., ALDHs for aldehydes) to eliminate these main endogenous DNA damage inducers. However, cells still need to develop corresponding DNA damage repair mechanisms to cope with the specific damage they induce, especially when the metabolic system is insufficient to eliminate these DNA damage inducers. In the following discussions, we focus on repair mechanisms of the oxidative damage, DNA-ICL, and DSB.
ATM-mediated oxidative damage repair
It is known that the ATM plays a critical role in responding to ROS-induced oxidative damage in HSCs. Atm –/– mice older than 24 months uniformly present with BMF due to defective maintenance of the HSC pool associated with increased ROS [33]. Mechanistically, the increased ROS, which might be resulted from p16 INK4a-Rb pathway activation [33], can limit the lifespan of HSCs in Atm –/– mice through activation of p38-MAPK kinase [34]. In vitro treatment of HSCs from Atm –/– mice with the anti-oxidative agent N-acetyl-L-cysteine (NAC) can significantly decrease intracellular ROS levels and rescue the defective repopulating capacity [33]. Similar results were also obtained after inactivation of the p38-MAPK kinase [34].
In addition, ATM contributes to the fork head box O (FOXO) family of transcription factors-mediated ROS response. The FOXO family comprises four members (FOXO1, FOXO3, FOXO4, and FOXO6), and is another key factor in response of HSCs to oxidative DNA damage. The combined deletion of Foxo1, Foxo3, and Foxo4 results in HSC dysfunction accompanied by increased ROS levels [35]. Specifically, FOXO3 can repress ROS by regulating the expression of ATM. Foxo3 –/– mice display elevated ROS-related oxidative damage and defective cell cycle in HSCs, which can be restored by NAC treatment [ 36, 37] .
Failure to eliminate ROS will inevitably result in the generation of DSBs. It is worth noting that ATM also plays an important role in the repair of ROS-induced endogenous DSBs [38], which may contribute to the maintenance of HSCs. Details will be discussed in the “HR and c-NHEJ to repair DSB” section.
Fanconi anemia (FA) pathway to repair DNA-ICLs
In addition to the endogenous aldehydes, DNA-ICLs are also commonly formed upon the application of exogenous chemotherapy drugs, such as chlorambucil, mitomycin C (MMC), and platinum compounds [39]. The repair of DNA-ICL has a special clinical significance due to its association with the occurrence of FA. FA is a rare genetic disease with an estimated incidence of 1–5/1,000,000 live births and is characterized by progressive BMF, congenital abnormalities, and an increased incidence of cancers. Consequently, cells derived from FA patients show hypersensitivity and increased chromosome abnormalities which are typically characterized by radial aggregation of chromosomes and chromosome breakage, upon treatment with DNA-ICL agents ( e. g., MMC) [ 40, 41] .
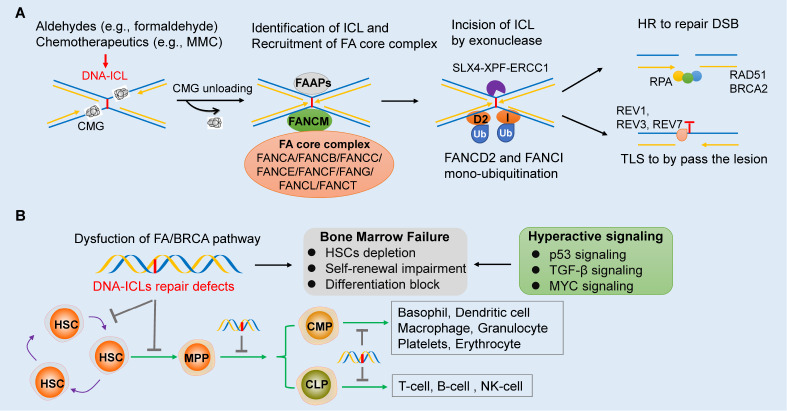
The FA pathway, also known as FA/BRCA pathway, plays a dominant role in the DNA-ICL repair, consisting of 22 key FANC proteins (FANCA-W) and many associated proteins ( e. g., FAAP10, FAAP16, FAAP20, FAAP24, FAAP100, and FAN1). Of the FANC genes, mutations in 18 bona fide genes lead to the occurrence of FA ( Table 1), while the other 4 FANC genes ( FANCM, FANCO/ RAD51C, FANCR/ RAD51, and FANCS/ BRCA1) are associated with FA-like syndrome [41]. The FA/BRCA pathway for DNA-ICL repair is extremely complex, and can be briefly summarized with the following 5 steps ( Figure 1A): (1) The CMG helicase complex (Cdc45/MCM2-7/GINS) is unloaded from the stalled replication fork, allowing the leading strand to approach the ICL; (2) FANCM, a member of the FA core complex (FANCA/FANCB/FANCC/FANCE/FANCF/FANG/FANCL/FANCT), recognizes the ICL and acts as a “bridge” to recruit other members of the core complex to catalyze the mono-ubiquitination of FANCD2 and FANCI; (3) Ubiquitinated FANCD2-I recruits the structure-specific exonuclease (SLX4-XPF-ERCC1) to unhook the ICL with the generation of a DSB; (4) RAD51-dependent HR to resolve the DSB (details can be seen in the next section “HR and c-NHEJ to repair DSB”); and (5) REV1-REV3-REV7 (Polζ)-dependent translesion synthesis (TLS) to bypass the crosslink remnants [ 40, 42, 43] . This process requires the E3 ubiquitin ligase FANCW (also known as RFWD3) to mediate PCNA ubiquitylation, which is an essential step for TLS polymerase recruitment [44].
Figure 1 .
The FA/BRCA pathway and HSC development
(A) Brief description of the DNA inter-strand crosslink (ICL) repair mechanism by the FA/BRCA pathway. DNA-ICLs induced by various exogenouschemotherapeutics ( e. g., MMC) or endogenous aldehydes ( e. g., formaldehyde) are first recognized by FANCM after the CMG (CDC45/MCM2-7/GINS) complex dissociation from the replication fork. FANCM then acts as a bridge to recruit other core complex members to ICLs to catalyze the mono-ubiquitination of FANCD2 and FANCI. Ubiquitinated FANCD2/FANCI can recruit the SLX4-XPF-ERCC1 exonuclease complex to unhook DNA-ICLs with the generation of DSBs. Lastly, DSBs are further repaired by HR, and the crosslink remnants are bypassed by TLS. (B) In the hematopoietic process, the HSCs must maintain self-renewal to prevent depletion of the stem cell pool in the BM. In addition, the HSCs have the pluripotency to differentiate into MPPs that can further differentiate into CMPs and CLPs and the downstream mature blood cells when needed. Defects in the FA/BRCA pathway will result in DNA-ICLs repair defects and genomic instability of HSCs, leading to bone marrow failure with HSCs depletion, self-renewal impairment, and differentiation block. FA, Fanconi anemia; FAAPs, FA-associated proteins; MMC, mitomycin C.
Dysfunction of the FA/BRCA pathway results in genome instability and causes HSCs developmental defects ( Figure 1B). Reduction in the number, blocked differentiation, and compromised self-renewal of HSCs have been widely observed in the FA-deficient HSCs [ 45– 47] . Recently, Shen et al. [48] uncovered that elevated nuclear formaldehyde generated during hematopoietic differentiation is the endogenous genotoxin causing blood attrition in FA patients. Cellular formaldehyde appears to be necessary for blood progenitor/stem cell differentiation, since the formaldehyde levels of FANCL –/– cells are comparable to those of wild-type cells under V-D3 induction. However, differentiating cells display higher levels of formaldehyde-inflicted DNA-ICLs in the absence of FANCL, and thus encounter a significantly elevated level of genotoxic stress, resulting in defects in cell differentiation in vitro. In addition, systematic studies of human FA cell lines and BM samples, including genome-wide shRNA screening and single-cell RNA sequencing (scRNA-seq) strategies, reported that several signaling pathways are closely related to the occurrence and development of FA. These include the p53, transforming growth factor-β (TGF-β), and MYC signaling pathways ( Figure 1B). In BM and cell samples from FA patients, unrepaired DNA damage induces continuous hyper-activation of p53/p21 CDKN1A , resulting in G0/G1 cell-cycle arrest. Depletion of p53 can rescue hematopoietic stem/progenitor cells (HSPCs) defects in Fancg –/– mice and improve the clonogenicity of CD34 + cells [49]. Zhang et al. [50] reported that the TGF-β pathway is significantly upregulated in FA/BRCA pathway-deficient cells, which has also been observed in myelodysplastic syndrome [51], myelofibrosis [52], and Shwachman-Diamond syndrome, another inherited BMF syndrome (IBMFS) [53]. Hyperactive TGF-β signaling impairs FA cell survival following MMC treatment. Inhibition of TGF-β signaling can rescue the dysfunction of HSCs derived from humans and mice [50]. Rodríguez et al. [54] revealed that MYC is overexpressed in FA samples, which can promote the proliferation of FA HSCs, but with a much higher level of DNA damage, and cause subsequent exhaustion of HSCs in FA BM. Thus, MYC inhibition decreases HSC proliferation; however, it can reduce physiological and genotoxic stress in HSCs from Fancd2 –/– mice.
Although the importance of the FA/BRCA pathway in maintaining the genome stability in human HSPCs has been well established, it has encountered great challenges when using murine models to further explore the molecular mechanisms, mainly because mice with FA/BRCA pathway defects do not naturally develop BMF. One possible explanation is that mice are not under constant physiological stress as human HSCs are [17]. Indeed, treatment of Fanca –/– mice with polyinosinic:polycytidylic acid (pI:pC) impairs the capacity to re-enter the long-term quiescent state and repopulation activity of the HSCs, and eventually causes BMF [17]. Consistent with this idea, combined deletion of the FA gene with Adh5 or Aldh2 in mice can induce DNA damage accumulation in BM cells and cause BMF [ 55, 56] . Therefore, the induction of BMF in FA gene-deficient mice through appropriate exogenous stress stimulation has been widely used to study the pathogenesis of FA.
It should be noted that how the defects in the molecular mechanism of FA/BRCA pathway leads to the occurrence of FA or other types of IBMFSs has not been fully explored. For example, Hodskinson et al. [57] reported a novel acetaldehyde-induced DNA-ICL repair pathway mediated by REV1, which requires fork convergence, but not CMG unloading. Unlike the FA/BRCA pathway, this incision-free pathway does not produce DNA strand breaks or abasic sites, thus avoiding large-scale genome instability. Notably, depletion of REV1 sensitizes cells to MMC and cisplatin, resulting in increased chromosome breakage and radial formation [58]. However, no REV1 variants have been reported in patients with FA or other hematopoietic abnormalities. It will be interesting to investigate whether digenic variants in REV1 and ALDHs contribute to the BMF phenotype in the future. In addition, novel FA/BRCA pathway regulators that function in DNA-ICL repair have been identified. Although the loss of function of some genes, such as NIPA [59] and NLRP12 [60], has been linked to BMF in mice, their molecular mechanisms for repairing DNA-ICLs or their impacts on HSC function and phenotype in humans have not been fully elucidated. Conversely, some genes have shown definitive functions in DNA-ICL repair; however, their roles in hematopoiesis have not been explored, such as SAN1 [61] and NEIL3 [ 62, 63] .
FA/BRCA pathway to repair oxidative damage
The FA/BRCA pathway plays a critical role in the repair of oxidative damage in the hematopoietic system. It has been suggested that the occurrence of FA is not only caused by DNA-ICL repair defects, and oxidative stress is also involved in the pathogenesis of FA. Early evidence is arised from the following: (1) FANCC interacts with NADPH cytochrome-P450 reductase (RED) [64], and (2) FANCG interacts with and suppresses the activity of cytochrome P450 2E1 (CYP2E1) in DNA oxidation [65]. This idea is corroborated by the increasing evidence from both human and mouse FA models. For example, in vitro treatment of FA patients’ cells with H 2O 2 resulted in similar effects to ICL inducers, including decreased colony formation of the BM progenitor cells and G2/M arrest of the lymphoblasts. Mechanistically, FA can prevent the promoters of antioxidant defense genes from being damaged by oxidative stress, thereby maintaining their expression levels [66]. In mice, double knockouts of Fancc and cytosolic Cu/Zn superoxide dismutase ( Sod1) cause a series of blood cell abnormalities, including bone marrow hypocellularity, erythrocytopenia, and leucopenia [67]. Consistently, Fancc –/– hematopoietic progenitors are hypersensitive to H 2O 2, and the anti-oxidative agent NAC can improve the survival of Fancc –/– murine embryo fibroblasts [68].
FA/BRCA pathway to protect replication forks
Increasing evidence shows that the FA/BRCA pathway is involved in replication fork protection and the restart of stalled replication forks, suggesting that replication stress may contribute to the occurrence of FA. BRCA1, BRCA2, and RAD51 have shown a strong ability to protect stalled replication forks from the nucleolytic degradation independent of DSB repair. Additionally, FANCA, FANCD2, and FANCM exhibit the ability to protect the replication forks [ 69– 72] . In response to replication fork stalling, RPA is polyubiquitinated by FANCW and facilitates the restart of stalled replication forks [73]. In addition, the FA/BRCA pathway directs break-induced replication (BIR) machinery to promote stalled replication forks involved in fork cleavage by SLX4 and FAN1 endonucleases [ 74, 75] . Interestingly, although extensive evidence confirms that the FA/BRCA pathway is directly involved in the protection of replication forks under replication stress, most FA-deficient cells seem to be resistant to replication stress agents, such as hydroxyurea (HU) [76]. Xu et al. [75] demonstrated that the FA-deficient cells are hypersensitive to replication stress only when persistently treated with low doses of HU or aphidicolin for two to three weeks. Consistently, daily injection of HU into Fancl-deficient adult mice for six weeks resulted in BMF. Several negative regulators of replication fork protection, including the DNA/RNA helicase SLFN11 [77] and LNK/SH2B3 [78], have recently been identified that may contribute to the attrition of HSCs in FA. The high expression of SLFN11 in HSCs promotes fork degradation in PD20 (FANCD2 –/–) cells. Depletion of SLFN11 in PD20 cells can prevent replication fork degradation and rescue the FA phenotype of cells [77]. Similarly, depletion of Lnk restores HSC function in Fancd2 −/− mice [78]. These new findings once again indicate that replication stress is an important cause of HSCs dysfunction in FA patients and has strong therapeutic significance.
HR and c-NHEJ to repair DSB
Endogenous DSBs in the hematopoietic system mainly arise from the collapsed replication forks and the incision of the aldehyde-induced DNA-ICLs by the FA/BRCA pathway. In addition, HSCs are under stress from exogenous DSBs, especially when patients with malignant tumors are treated with IR, radiomimetic compounds ( i. e., bleomycin), topoisomerase inhibitors ( i. e., camptothecin and etoposide), and ribonucleotide reductase inhibitors ( i. e., HU) [ 7, 79] . As the most cytotoxic type of DNA lesion, the repair of DSBs in vertebrate cells is mainly accomplished by the HR and classical non-homologous end-joining (c-NHEJ) pathways, and there are stricter cell cycle restrictions: c-NHEJ mainly occurs in the G0/G1 phase, while HR mainly functions in the S/G2- phase [80]. c-NHEJ is error-prone because the DSB end is blocked by Ku70/80 and DNA-PKcs, and can only be repaired by blunt end ligation by LIG4, independent of sequence homology. Ligation is catalyzed by LIG4, which is recruited to the DNA ends by XRCC4 ( Figure 2). In contrast, HR is usually error-free with the use of a sister or homologous chromatid as a template. DSBs generated from DNA-ICLs incision by the FA/BRCA pathway are mainly repaired by HR; therefore, cells from FA patients exhibit high c-NHEJ activity, leading to chromosomal abnormalities.
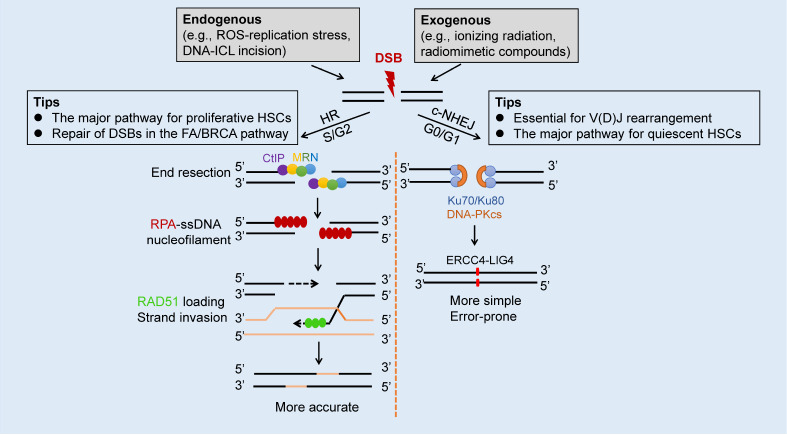
Figure 2 .
Brief description of the DSB repair mechanism in hematopoietic system
In HSCs, DSBs are mainly caused by excessive endogenous replication stress ( e. g., ROS-induced) and DNA-ICL incision or application of exogenous chemotherapeutics ( e. g., ionizing radiation and radiomimetic, compounds). Two major pathways, termed HR and c-NHEJ facilitate DSB repair. HR mainly occurs in the S/G2-phase, where the DSB ends first undergo nucleolytic resection performed by the CtIP-MRN (MRE11-RAD50-NBS1) complex to generate single-strand DNA (ssDNA). The subsequent steps include replication protein A (RPA)-ssDNA nucleofilament formation, RAD51 loading to replace RPA, and strand invasion. The proliferative HSCs mainly use HR to complete DSB repair, which is also true for the repair of the DSBs generated in the FA/BRCA pathway. c-NHEJ repairs DSBs in the G0/G1-phase, where the DNA ends are blocked by Ku70/Ku80/DNA-PKcs and then undergo blunt end ligation by XRCC4-LIG4. It is well known that c-NHEJ is essential for V(D)J rearrangement during lymphocyte development. In addition, c-NHEJ plays the dominant role in DSBs repair for quiescent HSCs, which are in the G0-phase of the cell cycle. HSC, hematopoietic stem cell; ssDNA, single-strand DNA.
Mounting evidence has shown that the c-NHEJ pathway is essential for hematopoiesis, especially for V(D)J rearrangement in developing lymphocytes [81]. Accordingly, HSCs from DNA- PKcs –/– and Ku80 –/– mice did not develop into mature B and T cells [ 82, 83] . In addition, the mice with mutations at the phosphorylation sites of DNA- PKcs (DNA-PKcs 3A/3A) died early due to congenital BMF [84]. Ku80 –/– and the hypomorphic Lig4 Y288C mutant mice display HSC defects under stress conditions, which are particularly evident in aging HSCs [ 85, 86] . Consistently, bi-allelic germline variants of DNA-PKcs and LIG4 in humans have been reported to cause severe combined immunodeficiency (decreased circulating T and B cells) [87] and LIG4 syndrome (pancytopenia) [88], respectively ( Table 1). These results are consistent with the fact that HSCs tend to utilize c-NHEJ rather than HR to maintain genomic stability. HSPCs from mice have a higher baseline level of NHEJ and show resistance to low doses of IR compared to CMPs. Irradiated HSPCs showed cell cycle arrest, whereas CMPs showed cell death. Further studies suggested that quiescent HSPCs use error-prone NHEJ to complete IR-induced DSBs for survival, and the remaining surviving CMPs use HR to repair DSBs. Interestingly, HR activity was significantly increased after IR treatment in proliferative HSPCs, indicating that the quiescent state of HSPCs greatly limits their ability to use high-fidelity HR [89].
The key event that determines the path of DSB repair is the end resection of DNA. DSBs without end resection can only be repaired by c-NHEJ, and once the DSB undergoes end resection, it is mainly repaired by HR [ 80, 90] . The initial stage of DSB end resection is mediated by the nuclease CtIP and MRN complex (MRE11-RAD50-NBS1). In the second stage of extensive resection, more helicases and exonucleases, including DNA2, BLM, WRN, and EXO1, are required to cut the DSB into single-stranded DNA (ssDNA) [ 91, 92] . RPA is immediately recruited to ssDNA and subsequently replaced by RAD51 ( Figure 2). The formation of ssDNA-RAD51 nucleofilaments is essential for mediating the formation of the D-loop of DNA strands [93]. ssDNA-RPA not only protects the DNA ends from being degraded after resection, but also recruits ATR and triggers the ATR signaling pathway (CHK1 phosphorylation) to ensure that HR repair proceeds smoothly in the S/G2 phase [ 80, 94] .
The MRN complex recruits and activates ATM, whose activation plays a central role in DSB repair signaling by phosphorylatingseveral downstream effectors, such as BRCA1 and ATR. In addition to the crucial role in DSB repair, MRN complex may also help HSCs face replication stress by directly promoting the restart of stalled replication forks or by suppressing R-loop formations at transcription-replication conflicts [ 95– 97] . In line with this hypothesis, hematopoietic abnormalities have been observed in mice models deficient of Atm [ 33, 34] , Mre11 [98], Rad50 [99], and Nbs1 [ 100, 101] , respectively. In addition, ATM and NBS1 have been associated with hematopoietic phenotypes in humans, and bi-allelic variants of each gene can lead to defects in T and B cells [ 102, 103] ( Table 1).
Recently, Shao et al. [104] revealed that DNA-PKcs plays an rRNA-dependent role in hematopoiesis, which is independent of its classical function in c-NHEJ. Defects of phosphorylation at the T2609 cluster of DNA-PKcs impair 18S rRNA processing, cause global translation defects in hematopoietic cells, and ultimately lead to BMF in mice. Similarly, γH2AX, a sensitive molecular marker of DSBs, which is phosphorylated by ATM, ATR, and DNA-PKcs kinases on serine 139, was also found to have functions beyond the DNA damage response [105]. γH2AX can transcriptionally inhibit rDNA genes, leading to impairment of ribosome biogenesis in quiescent old HSCs [18]. These indicate that the regulation of ribosome biogenesis by classical DNA damage/DNA repair genes may present a new study topic for HSC aging and BMF.
BER, MMR, and NER in hematopoiesis
The base excision repair (BER), DNA mismatch repair (MMR), and nucleotide excision repair (NER) pathways are the three excision repair pathways that primarily resolve single-stranded DNA damage. Little is known about BER and hematopoiesis, hence we focus on MMR and NER in this section. The MMR pathway is used for the repair of mismatches which mainly arise from replication errors when confronted with base pair anomalies, such as O 6-mG and 8-oxoG [106]. MMR defects are associated with microsatellite instability (MSI), a marker of genomic instability, which is frequently observed in colorectal cancer [107]. In humans, the MMR pathway consists of seven members, including MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2 [ 108, 109] . HSCs from older individuals (>45 years old) display greater methylation in the MLH1 promoter compared to HSCs from younger individuals (<45 years old), leading to lower MLH1 expression and increased MSI [110], suggesting that MLH1-mediated MMR plays an important role in maintaining genomic instability of HSCs during aging. Defects in the MMR pathway will drive HSC malignancy and lead to hematopoietic malignancies [111], whereas BMF is rare in patients with MMR deficiency. Indeed, bi-allelic germline variants in MLH1 have been reported in the early onset of leukemia and lymphoma [112]. Moreover, Mlh1 +/-mice show an increased incidence of lymphomagenesis upon simulated space radiation exposure [113]. Similar evidence can be observed in both patients and (or) mouse models with defects in MSH2, MSH6, and PMS2 [ 112, 114, 115] ( Table 1).
NER is particularly important for the repair of ultraviolet light-induced lesions, such as thymine dimers and 6,4-photoproducts. Thus, NER defects are strongly associated with the risk of developing skin cancer. Germline variants of classic genes ( e. g., ERCC2/ XPD, ERCC3/ XPB, ERCC4/ XPF, ERCC5/ XPG, ERCC6/ CSB, and ERCC8) in the NER pathway frequently cause Xeroderma pigmentosum, trichothiodystrophy, or Cockayne syndrome [116]. Hematopoietic abnormalities are rarely associated with NER, except for ERCC4/ XPF, which has been linked to FA [117]. Nevertheless, evidence does show that NER is involved in hematopoietic processes. For instance, although the establishment, maintenance, or expansion of LT-HSCs during aging is normal, the numbers of CMPs and CLPs in XPD-deficient mice are significantly reduced [86]. Moreover, ERCC6L2 is reportedly associated with NER, as depletion of ERCC6L2 causes mild sensitivity to MMC and Irofulven, but not to topoisomerase inhibitors. Bi-allelic ERCC6L2 variants have been reported in multiple BMF patients [ 118, 119] .
Conclusions and Perspectives
This review attempts to classify the DNA damage that blood cells may encounter and specifically describe the repair mechanisms involved in each type of DNA damage. These DNA lesions, as well as the repair pathways, are not isolated from each other, which is reflected not only in the fact that a single DNA damage may require multiple repair mechanisms, but also in the fact that a single repair mechanism plays a role in multiple types of DNA damage. For example, DNA-ICL and ROS can cause replication stress, and if the replication stress cannot be resolved in time, the replication fork may collapse and generate DSBs. The classic FA/BRCA pathway requires the cooperation of HR to repair DNA-ICLs because DSBs are inevitably produced in this process.
Due to the difficulty in obtaining human HSCs, most studies have been conducted in mice. Given that FA-knockout mice do not spontaneously develop a BMF phenotype, additional exogenous stress, such as pI:pC, aldehydes, and HU treatment should be considered to test the hematopoietic phenotype when studying the pathogenesis of a novel candidate FA gene. In addition, several DNA repair genes have been shown to be important for HSC function, but only a few have been validated in humans ( Table 1). For example, no FA patients harbor germline variants in FA-associated genes, which are essential for ICL repair. Moreover, HSC dysfunction has been demonstrated in Ku80 –/– mice [86], but related phenotypes ( e. g., BMF or blood cell reduction) have not been reported in humans. Exploring the underlying mechanisms of these phenomena is essential for understanding the pathogenesis of related diseases and may also provide opportunities for the development of potential treatments.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the Program of Shanghai Academic/Technology Research Leader (No. 19XD1422600 to J.W.), Shuguang Program supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (No. 18SG14 to J.W.), and the Shanghai Pujiang Program (No. 20PJ1409900 to N.L.).
References
- 1.Laurenti E, Göttgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. . 2018;553:418–426. doi: 10.1038/nature25022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. . 2019;20:303–320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sieburg HB, Rezner BD, Muller-Sieburg CE. Predicting clonal self-renewal and extinction of hematopoietic stem cells. Proc Natl Acad Sci U S A. . 2011;108:4370–4375. doi: 10.1073/pnas.1011414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Velthoven CTJ, Rando TA. Stem cell quiescence: dynamism, restraint, and cellular idling. Cell Stem Cell. . 2019;24:213–225. doi: 10.1016/j.stem.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li T, Zhou ZW, Ju Z, Wang ZQ. DNA damage response in hematopoietic stem cell ageing. Genomics Proteomics BioInf. . 2016;14:147–154. doi: 10.1016/j.gpb.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biechonski S, Yassin M, Milyavsky M. DNA-damage response in hematopoietic stem cells: an evolutionary trade-off between blood regeneration and leukemia suppression. Carcinogenesis. . 2017;38:367–377. doi: 10.1093/carcin/bgx002. [DOI] [PubMed] [Google Scholar]
- 7.Tubbs A, Nussenzweig A. Endogenous DNA damage as a source of genomic instability in cancer. Cell. . 2017;168:644–656. doi: 10.1016/j.cell.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y, Fang Y, Cai J, Li X, Xu F, Yuan N, Zhang S, et al. ROS functions as an upstream trigger for autophagy to drive hematopoietic stem cell differentiation. Hematology. . 2016;21:613–618. doi: 10.1080/10245332.2016.1165446. [DOI] [PubMed] [Google Scholar]
- 9.Garaycoechea JI, Crossan GP, Langevin F, Mulderrig L, Louzada S, Yang F, Guilbaud G, et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature. . 2018;553:171–177. doi: 10.1038/nature25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. . 2014;15:37–50. doi: 10.1016/j.stem.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delia D, Mizutani S. The DNA damage response pathway in normal hematopoiesis and malignancies. Int J Hematol. . 2017;106:328–334. doi: 10.1007/s12185-017-2300-7. [DOI] [PubMed] [Google Scholar]
- 12.Gueiderikh A, Maczkowiak-Chartois F, Rosselli F. A new frontier in Fanconi anemia: from DNA repair to ribosome biogenesis. Blood Rev. . 2021;52:100904. doi: 10.1016/j.blre.2021.100904. [DOI] [PubMed] [Google Scholar]
- 13.Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. . 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 14.Moehrle BM, Geiger H. Aging of hematopoietic stem cells: DNA damage and mutations? Exp Hematol. . 2016;44:895–901. doi: 10.1016/j.exphem.2016.06.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsogtbaatar E, Landin C, Minter-Dykhouse K, Folmes CDL. Energy metabolism regulates stem cell pluripotency. Front Cell Dev Biol. . 2020;8:87. doi: 10.3389/fcell.2020.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. . 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter D, Lier A, Geiselhart A, Thalheimer FB, Huntscha S, Sobotta MC, Moehrle B, et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. . 2015;520:549–552. doi: 10.1038/nature14131. [DOI] [PubMed] [Google Scholar]
- 18.Flach J, Bakker ST, Mohrin M, Conroy PC, Pietras EM, Reynaud D, Alvarez S, et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. . 2014;512:198–202. doi: 10.1038/nature13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Q, Yao W, Chen Y, Yan B, Liu C, Yuan M, Zhou Y, et al. GRK6 regulates ROS response and maintains hematopoietic stem cell self-renewal. Cell Death Dis. . 2016;7:e2478. doi: 10.1038/cddis.2016.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porto ML, Rodrigues BP, Menezes TN, Ceschim SL, Casarini DE, Gava AL, Pereira TMC, et al. Reactive oxygen species contribute to dysfunction of bone marrow hematopoietic stem cells in aged C57BL/6 J mice. J Biomed Sci. . 2015;22:97. doi: 10.1186/s12929-015-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadalutti CA, Prasad R, Wilson SH. Perspectives on formaldehyde dysregulation: Mitochondrial DNA damage and repair in mammalian cells. DNA Repair. . 2021;105:103134. doi: 10.1016/j.dnarep.2021.103134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Wang C, Xu H, Gao Y. Aldehyde dehydrogenase, liver disease and cancer. Int J Biol Sci. . 2020;16:921–934. doi: 10.7150/ijbs.42300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Housh K, Jha JS, Haldar T, Amin SBM, Islam T, Wallace A, Gomina A, et al. Formation and repair of unavoidable, endogenous interstrand cross-links in cellular DNA. DNA Repair. . 2021;98:103029. doi: 10.1016/j.dnarep.2020.103029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei X, Peng Y, Bryan C, Yang K. Mechanisms of DNA−protein cross-link formation and repair. Biochim Biophys Acta (BBA) - Proteins Proteomics. . 2021;1869:140669. doi: 10.1016/j.bbapap.2021.140669. [DOI] [PubMed] [Google Scholar]
- 25.Nalepa G, Clapp DW. Fanconi anaemia and cancer: an intricate relationship. Nat Rev Cancer. . 2018;18:168–185. doi: 10.1038/nrc.2017.116. [DOI] [PubMed] [Google Scholar]
- 26.Dingler FA, Wang M, Mu A, Millington CL, Oberbeck N, Watcham S, Pontel LB, et al. Two aldehyde clearance systems are essential to prevent lethal formaldehyde accumulation in mice and humans. Mol Cell. . 2020;80:996–1012.e9. doi: 10.1016/j.molcel.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oka Y, Hamada M, Nakazawa Y, Muramatsu H, Okuno Y, Higasa K, Shimada M, et al. Digenic mutations in ALDH2 and ADH5 impair formaldehyde clearance and cause a multisystem disorder, AMeD syndrome . Sci Adv. . 2020;6:eabd7197. doi: 10.1126/sciadv.abd7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mu A, Hira A, Niwa A, Osawa M, Yoshida K, Mori M, Okamoto Y, et al. Analysis of disease model iPSCs derived from patients with a novel Fanconi anemia–like IBMFS ADH5/ALDH2 deficiency . Blood. . 2021;137:2021–2032. doi: 10.1182/blood.2020009111. [DOI] [PubMed] [Google Scholar]
- 29.Gaillard H, García-Muse T, Aguilera A. Replication stress and cancer. Nat Rev Cancer. . 2015;15:276–289. doi: 10.1038/nrc3916. [DOI] [PubMed] [Google Scholar]
- 30.Kotsantis P, Petermann E, Boulton SJ. Mechanisms of oncogene-induced replication stress: jigsaw falling into place. Cancer Discov. . 2018;8:537–555. doi: 10.1158/2159-8290.CD-17-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murga M, Bunting S, Montaña MF, Soria R, Mulero F, Cañamero M, Lee Y, et al. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat Genet. . 2009;41:891–898. doi: 10.1038/ng.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler MG, Hall BD, Maclean RN, Lozzio CB, Opitz JM, Reynolds JF. Do some patients with Seckel syndrome have hematological problems and/or chromosome breakage? Am J Med Genet. . 1987;27:645–649. doi: 10.1002/ajmg.1320270318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. . 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 34.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. . 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 35.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. . 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, Vercherat C, Sarkar A, et al. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem. . 2008;283:25692–25705. doi: 10.1074/jbc.M800517200. [DOI] [PubMed] [Google Scholar]
- 37.Bigarella CL, Li J, Rimmelé P, Liang R, Sobol RW, Ghaffari S. FOXO3 transcription factor is essential for protecting hematopoietic stem and progenitor cells from oxidative DNA damage. J Biol Chem. . 2017;292:3005–3015. doi: 10.1074/jbc.M116.769455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodbine L, Brunton H, Goodarzi AA, Shibata A, Jeggo PA. Endogenously induced DNA double strand breaks arise in heterochromatic DNA regions and require ataxia telangiectasia mutated and Artemis for their repair. Nucleic Acids Res. . 2011;39:6986–6997. doi: 10.1093/nar/gkr331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semlow DR, Walter JC. Mechanisms of vertebrate DNA interstrand cross-link repair. Annu Rev Biochem. . 2021;90:107–135. doi: 10.1146/annurev-biochem-080320-112510. [DOI] [PubMed] [Google Scholar]
- 40.Niraj J, Färkkilä A, D′Andrea AD. The Fanconi anemia pathway in cancer. Annu Rev Cancer Biol. . 2019;3:457–478. doi: 10.1146/annurev-cancerbio-030617-050422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li N, Ding L, Li B, Wang J, D′Andrea AD, Chen J. Functional analysis of Fanconi anemia mutations in China. Exp Hematol. . 2018;66:32–41.e8. doi: 10.1016/j.exphem.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Ceccaldi R, Sarangi P, D′Andrea AD. The Fanconi anaemia pathway: new players and new functions. Nat Rev Mol Cell Biol. . 2016;17:337–349. doi: 10.1038/nrm.2016.48. [DOI] [PubMed] [Google Scholar]
- 43.Shakeel S, Rajendra E, Alcón P, O′Reilly F, Chorev DS, Maslen S, Degliesposti G, et al. Structure of the Fanconi anaemia monoubiquitin ligase complex. Nature. . 2019;575:234–237. doi: 10.1038/s41586-019-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallina I, Hendriks IA, Hoffmann S, Larsen NB, Johansen J, Colding-Christensen CS, Schubert L, et al. The ubiquitin ligase RFWD3 is required for translesion DNA synthesis. Mol Cell. . 2021;81:442–458.e9. doi: 10.1016/j.molcel.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parmar K, Kim J, Sykes SM, Shimamura A, Stuckert P, Zhu K, Hamilton A, et al. Hematopoietic stem cell defects in mice with deficiency of Fancd2 or Usp1. Stem Cells. . 2010;28:1186–1195. doi: 10.1002/stem.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang QS, Marquez-Loza L, Eaton L, Duncan AW, Goldman DC, Anur P, Watanabe-Smith K, et al. Fancd2 −/− mice have hematopoietic defects that can be partially corrected by resveratrol . Blood. . 2010;116:5140–5148. doi: 10.1182/blood-2010-04-278226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mgbemena VE, Signer RAJ, Wijayatunge R, Laxson T, Morrison SJ, Ross TS. Distinct brca1 mutations differentially reduce hematopoietic stem cell function. Cell Rep. . 2017;18:947–960. doi: 10.1016/j.celrep.2016.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen X, Wang R, Kim MJ, Hu Q, Hsu CC, Yao J, Klages-Mundt N, et al. A surge of DNA damage links transcriptional reprogramming and hematopoietic deficit in Fanconi anemia. Mol Cell. . 2020;80:1013–1024.e6. doi: 10.1016/j.molcel.2020.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ceccaldi R, Parmar K, Mouly E, Delord M, Kim JM, Regairaz M, Pla M, et al. Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells. Cell Stem Cell. . 2012;11:36–49. doi: 10.1016/j.stem.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Kozono DE, O′Connor KW, Vidal-Cardenas S, Rousseau A, Hamilton A, Moreau L, et al. TGF-β inhibition rescues hematopoietic stem cell defects and bone marrow failure in fanconi anemia. Cell Stem Cell. . 2016;18:668–681. doi: 10.1016/j.stem.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bewersdorf JP, Zeidan AM. Transforming growth factor (TGF)-β pathway as a therapeutic target in lower risk myelodysplastic syndromes. Leukemia. . 2019;33:1303–1312. doi: 10.1038/s41375-019-0448-2. [DOI] [PubMed] [Google Scholar]
- 52.Teodorescu P, Pasca S, Jurj A, Gafencu G, Joelsson JP, Selicean S, Moldovan C, et al. Transforming growth factor β‐mediated micromechanics modulates disease progression in primary myelofibrosis. J Cell Mol Med. . 2020;24:11100–11110. doi: 10.1111/jcmm.15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joyce CE, Saadatpour A, Ruiz-Gutierrez M, Bolukbasi OV, Jiang L, Thomas DD, Young S, et al. TGF-β signaling underlies hematopoietic dysfunction and bone marrow failure in Shwachman-Diamond syndrome. J Clin Investigation. . 2019;129:3821–3826. doi: 10.1172/JCI125375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodríguez A, Zhang K, Färkkilä A, Filiatrault J, Yang C, Velázquez M, Furutani E, et al. MYC promotes bone marrow stem cell dysfunction in Fanconi anemia. Cell Stem Cell. . 2021;28:33–47.e8. doi: 10.1016/j.stem.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langevin F, Crossan GP, Rosado IV, Arends MJ, Patel KJ. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature. . 2011;475:53–58. doi: 10.1038/nature10192. [DOI] [PubMed] [Google Scholar]
- 56.Rosado IV, Langevin F, Crossan GP, Takata M, Patel KJ. Formaldehyde catabolism is essential in cells deficient for the Fanconi anemia DNA-repair pathway. Nat Struct Mol Biol. . 2011;18:1432–1434. doi: 10.1038/nsmb.2173. [DOI] [PubMed] [Google Scholar]
- 57.Hodskinson MR, Bolner A, Sato K, Kamimae-Lanning AN, Rooijers K, Witte M, Mahesh M, et al. Alcohol-derived DNA crosslinks are repaired by two distinct mechanisms. Nature. . 2020;579:603–608. doi: 10.1038/s41586-020-2059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mirchandani KD, McCaffrey RM, D′Andrea AD. The Fanconi anemia core complex is required for efficient point mutagenesis and Rev1 foci assembly. DNA Repair. . 2008;7:902–911. doi: 10.1016/j.dnarep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kreutmair S, Erlacher M, Andrieux G, Istvanffy R, Mueller-Rudorf A, Zwick M, Rückert T, et al. Loss of the Fanconi anemia–associated protein NIPA causes bone marrow failure. J Clin Investigation. . 2020;130:2827–2844. doi: 10.1172/JCI126215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin Q, Wu L, Ma Z, Chowdhury,1 FA, Mazumder HH, Du W. Persistent DNA damage–induced NLRP12 improves hematopoietic stem cell function. JCI Insight. . 2020;5:e133365. doi: 10.1172/jci.insight.133365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andrews AM, McCartney HJ, Errington TM, D′Andrea AD, Macara IG. A senataxin-associated exonuclease SAN1 is required for resistance to DNA interstrand cross-links. Nat Commun. . 2018;9:2592. doi: 10.1038/s41467-018-05008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Semlow DR, Zhang J, Budzowska M, Drohat AC, Walter JC. Replication-dependent unhooking of DNA interstrand cross-links by the NEIL3 glycosylase. Cell. . 2016;167:498–511.e14. doi: 10.1016/j.cell.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li N, Wang J, Wallace SS, Chen J, Zhou J, D′Andrea AD. Cooperation of the NEIL3 and Fanconi anemia/BRCA pathways in interstrand crosslink repair. Nucleic Acids Res. . 2020;48:3014–3028. doi: 10.1093/nar/gkaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kruyt FAE, Hoshino T, Liu JM, Joseph P, Jaiswal AK, Youssoufian H. Abnormal microsomal detoxification implicated in Fanconi anemia group C by interaction of the FAC protein with NADPH cytochrome P450 reductase. Blood. . 1998;92:3050–3056. doi: 10.1182/blood.V92.9.3050. [DOI] [PubMed] [Google Scholar]
- 65.Futaki M, Igarashi T, Watanabe S, Kajigaya S, Tatsuguchi A, Wang J, Liu JM. The FANCG Fanconi anemia protein interacts with CYP2E1: possible role in protection against oxidative DNA damage. Carcinogenesis. . 2002;23:67–72. doi: 10.1093/carcin/23.1.67. [DOI] [PubMed] [Google Scholar]
- 66.Du W, Rani R, Sipple J, Schick J, Myers KC, Mehta P, Andreassen PR, et al. The FA pathway counteracts oxidative stress through selective protection of antioxidant defense gene promoters. Blood. . 2012;119:4142–4151. doi: 10.1182/blood-2011-09-381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hadjur S, Ung K, Wadsworth L, Dimmick J, Rajcan-Separovic E, Scott RW, Buchwald M, et al. Defective hematopoiesis and hepatic steatosis in mice with combined deficiencies of the genes encoding Fancc and Cu/Zn superoxide dismutase. Blood. . 2001;98:1003–1011. doi: 10.1182/blood.V98.4.1003. [DOI] [PubMed] [Google Scholar]
- 68.Saadatzadeh MR, Bijangi-Vishehsaraei K, Hong P, Bergmann H, Haneline LS. Oxidant hypersensitivity of Fanconi anemia type C-deficient cells is dependent on a redox-regulated apoptotic pathway. J Biol Chem. . 2004;279:16805–16812. doi: 10.1074/jbc.M313721200. [DOI] [PubMed] [Google Scholar]
- 69.Kais Z, Rondinelli B, Holmes A, O′Leary C, Kozono D, D′Andrea AD, Ceccaldi R. FANCD2 maintains fork stability in BRCA1/2-deficient tumors and promotes alternative end-joining DNA repair. Cell Rep. . 2016;15:2488–2499. doi: 10.1016/j.celrep.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mijic S, Zellweger R, Chappidi N, Berti M, Jacobs K, Mutreja K, Ursich S, et al. Replication fork reversal triggers fork degradation in BRCA2-defective cells. Nat Commun. . 2017;8:859. doi: 10.1038/s41467-017-01164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu W, Krishnamoorthy A, Zhao R, Cortez D. Two replication fork remodeling pathways generate nuclease substrates for distinct fork protection factors. Sci Adv. . 2020;6:eabc3598. doi: 10.1126/sciadv.abc3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panday A, Willis NA, Elango R, Menghi F, Duffey EE, Liu ET, Scully R. FANCM regulates repair pathway choice at stalled replication forks. Mol Cell. . 2021;81:2428–2444.e6. doi: 10.1016/j.molcel.2021.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elia AEH, Wang DC, Willis NA, Boardman AP, Hajdu I, Adeyemi RO, Lowry E, et al. RFWD3-dependent ubiquitination of RPA regulates repair at stalled replication forks. Mol Cell. . 2015;60:280–293. doi: 10.1016/j.molcel.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Y, Ning S, Wei Z, Xu R, Xu X, Xing M, Guo R, et al. 53BP1 and BRCA1 control pathway choice for stalled replication restart. eLife. . 2017;6:e30523. doi: 10.7554/eLife.30523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu X, Xu Y, Guo R, Xu R, Fu C, Xing M, Sasanuma H, et al. Fanconi anemia proteins participate in a break-induced-replication-like pathway to counter replication stress. Nat Struct Mol Biol. . 2021;28:487–500. doi: 10.1038/s41594-021-00602-9. [DOI] [PubMed] [Google Scholar]
- 76.Chen X, Bosques L, Sung P, Kupfer GM. A novel role for non-ubiquitinated FANCD2 in response to hydroxyurea-induced DNA damage. Oncogene. . 2016;35:22–34. doi: 10.1038/onc.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okamoto Y, Abe M, Mu A, Tempaku Y, Rogers CB, Mochizuki AL, Katsuki Y, et al. SLFN11 promotes stalled fork degradation that underlies the phenotype in Fanconi anemia cells . Blood. . 2021;137:336–348. doi: 10.1182/blood.2019003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balcerek J, Jiang J, Li Y, Jiang Q, Holdreith N, Singh B, Chandra V, et al. Lnk/Sh2b3 deficiency restores hematopoietic stem cell function and genome integrity in Fancd2 deficient Fanconi anemia. Nat Commun. . 2018;9:3915. doi: 10.1038/s41467-018-06380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mehta A, Haber JE. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harbor Perspectives Biol. . 2014;6:a016428. doi: 10.1101/cshperspect.a016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ceccaldi R, Rondinelli B, D′Andrea AD. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. . 2016;26:52–64. doi: 10.1016/j.tcb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chi X, Li Y, Qiu X. V(D)J recombination, somatic hypermutation and class switch recombination of immunoglobulins: mechanism and regulation. Immunology. . 2020;160:233–247. doi: 10.1111/imm.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kirchgessner CU, Patil CK, Evans JW, Cuomo CA, Fried LM, Carter T, Oettinger MA, et al. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. . 1995;267:1178–1183. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- 83.Nussenzweig A, Chen C, da Costa Soares V, Sanchez M, Sokol K, Nussenzweig MC, Li GC. Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature. . 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 84.Zhang S, Yajima H, Huynh HD, Zheng J, Callen E, Chen HT, Wong N, et al. Congenital bone marrow failure in DNA-PKcs mutant mice associated with deficiencies in DNA repair. J Cell Biol. . 2011;193:295–305. doi: 10.1083/jcb.201009074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. . 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 86.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. . 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 87.Mathieu AL, Verronese E, Rice GI, Fouyssac F, Bertrand Y, Picard C, Chansel M, et al. PRKDC mutations associated with immunodeficiency, granuloma, and autoimmune regulator–dependent autoimmunity. J Allergy Clin Immunol. . 2015;135:1578–1588.e5. doi: 10.1016/j.jaci.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun B, Chen Q, Wang Y, Liu D, Hou J, Wang W, Ying W, et al. LIG4 syndrome: clinical and molecular characterization in a Chinese cohort. Orphanet J Rare Dis. . 2020;15:131. doi: 10.1186/s13023-020-01411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohrin M, Bourke E, Alexander D, Warr MR, Barry-Holson K, Le Beau MM, Morrison CG, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. . 2010;7:174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Symington LS. Mechanism and regulation of DNA end resection in eukaryotes. Crit Rev Biochem Mol Biol. . 2016;51:195–212. doi: 10.3109/10409238.2016.1172552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ceppi I, Howard SM, Kasaciunaite K, Pinto C, Anand R, Seidel R, Cejka P. CtIP promotes the motor activity of DNA2 to accelerate long-range DNA end resection. Proc Natl Acad Sci U S A. . 2020;117:8859–8869. doi: 10.1073/pnas.2001165117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deshpande RA, Myler LR, Soniat MM, Makharashvili N, Lee L, Lees-Miller SP, Finkelstein IJ, et al. DNA-dependent protein kinase promotes DNA end processing by MRN and CtIP. Sci Adv. . 2020;6:eaay0922. doi: 10.1126/sciadv.aay0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Špírek M, Taylor MRG, Belan O, Boulton SJ, Krejci L. Nucleotide proofreading functions by nematode RAD51 paralogs facilitate optimal RAD51 filament function. Nat Commun. . 2021;12:5545. doi: 10.1038/s41467-021-25830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peterson SE, Li Y, Wu-Baer F, Chait BT, Baer R, Yan H, Gottesman ME, et al. Activation of DSB processing requires phosphorylation of CtIP by ATR. Mol Cell. . 2013;49:657–667. doi: 10.1016/j.molcel.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lemaçon D, Jackson J, Quinet A, Brickner JR, Li S, Yazinski S, You Z, et al. MRE11 and EXO1 nucleases degrade reversed forks and elicit MUS81-dependent fork rescue in BRCA2-deficient cells. Nat Commun. . 2017;8:860. doi: 10.1038/s41467-017-01180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chang EYC, Tsai S, Aristizabal MJ, Wells JP, Coulombe Y, Busatto FF, Chan YA, et al. MRE11-RAD50-NBS1 promotes Fanconi Anemia R-loop suppression at transcription–replication conflicts. Nat Commun. . 2019;10:4265. doi: 10.1038/s41467-019-12271-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matmati S, Lambert S, Géli V, Coulon S. Telomerase repairs collapsed replication forks at telomeres. Cell Rep. . 2020;30:3312–3322.e3. doi: 10.1016/j.celrep.2020.02.065. [DOI] [PubMed] [Google Scholar]
- 98.Morales M, Liu Y, Laiakis EC, Morgan WF, Nimer SD, Petrini JHJ. DNA damage signaling in hematopoietic cells: a role for Mre11 complex repair of topoisomerase lesions. Cancer Res. . 2008;68:2186–2193. doi: 10.1158/0008-5472.CAN-07-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bender CF, Sikes ML, Sullivan R, Huye LE, Le Beau MM, Roth DB, Mirzoeva OK, et al. Cancer predisposition and hematopoietic failure in Rad50 S/S mice . Genes Dev. . 2002;16:2237–2251. doi: 10.1101/gad.1007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Y, Sun J, Ju Z, Wang ZQ, Li T. Nbs1‐mediated DNA damage repair pathway regulates haematopoietic stem cell development and embryonic haematopoiesis. Cell Prolif. . 2021;54:e12972. doi: 10.1111/cpr.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim JH, Grosbart M, Anand R, Wyman C, Cejka P, Petrini JHJ. The Mre11-Nbs1 interface is essential for viability and tumor suppression. Cell Rep. . 2017;18:496–507. doi: 10.1016/j.celrep.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taylor AMR, Rothblum-Oviatt C, Ellis NA, Hickson ID, Meyer S, Crawford TO, Smogorzewska A, et al. Chromosome instability syndromes. Nat Rev Dis Primers. . 2019;5:64. doi: 10.1038/s41572-019-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weitering TJ, Takada S, Weemaes CMR, van Schouwenburg PA, van der Burg M. ATM: translating the DNA damage response to adaptive immunity. Trends Immunol. . 2021;42:350–365. doi: 10.1016/j.it.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 104.Shao Z, Flynn RA, Crowe JL, Zhu Y, Liang J, Jiang W, Aryan F, et al. DNA-PKcs has KU-dependent function in rRNA processing and haematopoiesis. Nature. . 2020;579:291–296. doi: 10.1038/s41586-020-2041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mah LJ, El-Osta A, Karagiannis TC. γH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. . 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 106.Iyer RR, Pluciennik A, Burdett V, Modrich PL. DNA mismatch repair: functions and mechanisms. Chem Rev. . 2006;106:302–323. doi: 10.1021/cr0404794. [DOI] [PubMed] [Google Scholar]
- 107.Randrian V, Evrard C, Tougeron D. Microsatellite instability in colorectal cancers: carcinogenesis, neo-antigens, immuno-resistance and emerging therapies. Cancers. . 2021;13:3063. doi: 10.3390/cancers13123063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Z, Pearlman AH, Hsieh P. DNA mismatch repair and the DNA damage response. DNA Repair. . 2016;38:94–101. doi: 10.1016/j.dnarep.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bateman AC. DNA mismatch repair proteins: scientific update and practical guide. J Clin Pathol. . 2021;74:264–268. doi: 10.1136/jclinpath-2020-207281. [DOI] [PubMed] [Google Scholar]
- 110.Kenyon J, Fu P, Lingas K, Thomas E, Saurastri A, Santos Guasch G, Wald D, et al. Humans accumulate microsatellite instability with acquired loss of MLH1 protein in hematopoietic stem and progenitor cells as a function of age. Blood. . 2012;120:3229–3236. doi: 10.1182/blood-2011-12-401950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vaish M. Mismatch repair deficiencies transforming stem cells into cancer stem cells and therapeutic implications. Mol Cancer. . 2007;6:26. doi: 10.1186/1476-4598-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang X, Song Y, Chen W, Ding N, Liu W, Xie Y, Wang Y, et al. Germline variants of DNA repair genes in early onset mantle cell lymphoma. Oncogene. . 2021;40:551–563. doi: 10.1038/s41388-020-01542-2. [DOI] [PubMed] [Google Scholar]
- 113.Patel R, Zhang L, Desai A, Hoenerhoff MJ, Kennedy LH, Radivoyevitch T, Ban Y, et al. Mlh1 deficiency increases the risk of hematopoietic malignancy after simulated space radiation exposure. Leukemia. . 2019;33:1135–1147. doi: 10.1038/s41375-018-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ripperger T, Schlegelberger B. Acute lymphoblastic leukemia and lymphoma in the context of constitutional mismatch repair deficiency syndrome. Eur J Med Genet. . 2016;59:133–142. doi: 10.1016/j.ejmg.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 115.Bhattacharya P, Patel TN. Microsatellite instability and promoter hypermethylation of DNA repair genes in hematologic malignancies: a forthcoming direction toward diagnostics. Hematology. . 2018;23:77–82. doi: 10.1080/10245332.2017.1354428. [DOI] [PubMed] [Google Scholar]
- 116.Krasikova Y, Rechkunova N, Lavrik O. Nucleotide excision repair: from molecular defects to neurological abnormalities. Int J Mol Sci. . 2021;22:6220. doi: 10.3390/ijms22126220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bogliolo M, Schuster B, Stoepker C, Derkunt B, Su Y, Raams A, Trujillo JP, et al. Mutations in ERCC4, encoding the DNA-repair endonuclease XPF, cause Fanconi anemia. Am J Hum Genet. . 2013;92:800–806. doi: 10.1016/j.ajhg.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tummala H, Kirwan M, Walne AJ, Hossain U, Jackson N, Pondarre C, Plagnol V, et al. ERCC6L2 mutations link a distinct bone-marrow-failure syndrome to DNA repair and mitochondrial function. Am J Hum Genet. . 2014;94:246–256. doi: 10.1016/j.ajhg.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tummala H, Dokal AD, Walne A, Ellison A, Cardoso S, Amirthasigamanipillai S, Kirwan M, et al. Genome instability is a consequence of transcription deficiency in patients with bone marrow failure harboring biallelic ERCC6L2 variants . Proc Natl Acad Sci U S A. . 2018;115:7777–7782. doi: 10.1073/pnas.1803275115. [DOI] [PMC free article] [PubMed] [Google Scholar]