Abstract
Macrophages are critical sentinel cells armed with multiple regulated necrosis pathways, including pyroptosis, apoptosis followed by secondary necrosis, and necroptosis, and are poised to undergo distinct form(s) of necrosis for tackling dangers of pathogenic infection or toxic exposure. The natural BH3-mimetic gossypol is a toxic phytochemical that can induce apoptosis and/or pyroptotic-like cell death, but what exact forms of regulated necrosis are induced remains largely unknown. Here we demonstrated that gossypol induces pyroptotic-like cell death in both unprimed and lipopolysaccharide-primed mouse bone marrow-derived macrophages (BMDMs), as evidenced by membrane swelling and ballooning accompanied by propidium iodide incorporation and lactic acid dehydrogenase release. Notably, gossypol simultaneously induces the activation of both pyroptotic and apoptotic (followed by secondary necrosis) pathways but only weakly activates the necroptosis pathway. Unexpectedly, gossypol-induced necrosis is independent of nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, as neither inhibitor for the NLRP3 pathway nor NLRP3 deficiency protects the macrophages from the necrosis. Furthermore, necrotic inhibitors or even pan-caspase inhibitor alone does not or only partly inhibit such necrosis. Instead, a combination of inhibitors composed of pan-caspase inhibitor IDN-6556, RIPK3 inhibitor GSK′872 and NADPH oxidase inhibitor GKT137831 not only markedly inhibits the necrosis, with all apoptotic and pyroptotic pathways being blocked, but also attenuates gossypol-induced peritonitis in mice. Lastly, the activation of the NLRP3 pathway and apoptotic caspase-3 appears to be independent of each other. Collectively, gossypol simultaneously induces the activation of multiple subroutines of regulated necrosis in macrophages depending on both apoptotic and inflammatory caspases.
Keywords: BH3-mimetic, gossypol, macrophages, pyroptosis, secondary necrosis, necroptosis
Introduction
Macrophages are critical tissue-resident innate immune cells patrolling tissues to defend pathogenic infections and tissue injury [1]. In response to pathogenic infection or tissue injury, macrophages are poised to undergo various forms of regulated necrosis including pyroptosis, apoptosis followed by secondary necrosis, and necroptosis [ 2– 4]. For example, cytosolic sensors such as nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) in macrophages can be activated by danger signals derived from pathogens or injured tissues to trigger inflammasome assembly, leading to the activation of caspase-1 which cleaves gasdermin D (GSDMD) to generate its N-terminal fragment (GSDMD-NT) that executes pyroptosis [5]. Pyroptosis is a lytic cell death resulting in not only the release of inflammatory components but also the release of intracellular pathogens, thus mediating robust inflammation and allowing the elimination of the pathogens by other phagocytes such as neutrophils [ 6, 7]. However, pathogens can evolve evasion strategies to block some cell death signaling pathways of infected cells [ 8, 9]. To overcome such evasion, macrophages also have evolved strategies to undergo other forms of lytic cell death through caspase-8-mediated cleavage of GSDMD [ 8, 9]. In circumstances when caspase-8 is blocked, one more form of regulated necrosis subroutine named necroptosis can be induced in macrophages and other cells [ 10, 11]. On the other hand, in response to influenza A virus, vesicular stomatitis virus, Listeria monocytogenes, Salmonella enterica serovar Typhimurium [12], Yersinia pseudotuberculosis [13] or murine coronavirus mouse hepatitis virus [14] infection, multiple forms of cell death including pyroptosis, apoptosis and necroptosis, can be induced at the same time in bone marrow-derived macrophages (BMDMs), which has been named PANoptosis [ 15, 16].
Apart from pathogenic infection, recent studies have shown that macrophages can undergo secondary necrosis through caspase-3/ gasdermin E (GSDME) upon chemotherapeutic agent treatment [ 17, 18], which relies on the expression of GSDME protein [18]. Such GSDME-dependent secondary necrosis is likely to represent a possible mechanism of side effects of chemotherapy to macrophages [ 18, 19], but may also contribute to the anticancer effects of chemotherapeutic agents [ 20, 21].
BH3-mimetics are interesting agents that have been shown to mimic the pro-apoptotic BH3-only protein to antagonize the activity of Bcl-2, thus leading to mitochondrial dysfunction and release of cytochrome c and resulting in apoptosis [ 22, 23]. Gossypol, a toxic phytochemical isolated from cotton seeds, has been shown to be a natural BH3-mimetic, acting as pan-Bcl-2 inhibitor that can interact with pro-survival proteins Bcl-2, Bcl-X L, and Mcl-1 to counteract their anti-apoptotic effects, thus inducing mitochondria-mediated apoptosis [ 24, 25]. Due to its toxic effect on mitochondria which causes damage to sperms, the natural BH3-mimetic gossypol has been shown to have an anti-fertility effect in males [26]. Subsequently, owning to the apoptosis induction activity, gossypol and its derivatives (e.g., AT-101) have been tested as anti-cancer agents in a wide range of cancers including prostate cancer [ 23, 27]. Other synthetic BH3-mimetics (e.g., ABT-737) have been developed as therapeutic agents for cancers [22].
Emerging evidence suggests that both extrinsic and intrinsic apoptosis induced by the synthetic BH3-mimetic ABT-737 in combination with protein synthesis inhibitor can induce NLRP3 inflammasome activation and interleukin-1β (IL-1β) secretion in macrophages through Bax/Bak-dependent induction of apoptotic caspase-3/-7 activation downstream of caspase-8 [28]. It has also been found that combination of ABT-737 with Mcl-1 inhibitor (S63845) can drive NLRP3 inflammasome assembly depending on the pannexin-1 activation via the mitochondrial apoptosis [29]. Although those studies suggest potential side effects of BH3-mimetics on immune cells, ABT-737 alone does not induce significant lytic cell death in macrophages [ 28, 29]. Interestingly, previous studies showed that the natural BH3-mimetic gossypol was able to induce apoptosis in tumor cells [ 30, 31]. Our previous studies showed that gossypol induced pyroptotic-like cell death in macrophages [32], indicating that gossypol per semay have potential action on innate immune cells including macrophages. But the mechanisms underlying the induction of lytic cell death by gossypol in macrophages remain largely unknown. Macrophages as aforementioned are armed with various pathways of regulated necrosis, thus serving as good candidate cells for exploring necrosis of toxic substances. The toxic effects of gossypol on macrophages are of great interest to be explored in term of regulated necrosis.
Herein we found that the natural mimetic was able to simultaneously induce both pyroptosis and apoptosis/secondary necrosis but only weak activation of necroptosis in murine macrophages. Interestingly, any inhibitor for one pathway of regulated necrosis was unable to suppress gossypol-induced lytic cell death; however, a combination of inhibitors for multiple cell death pathways (e.g., pan-caspase inhibitor, RIPK3 inhibitor and NAPDH oxidase 1/4 inhibitor) robustly blocked the necrosis induced by gossypol, suggesting that the multiple subroutines of gossypol-induced regulated necrosis are probably independent of each other.
Materials and Methods
Reagents and antibodies
(±)Gossypol (with ~95% purity) (the structure is shown in Figure 1A), lipopolysaccharide (LPS) ( Escherichia coli O111:B4), Hoechst 33342, propidium iodide (PI) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St Louis, USA). Ac-DEVD-CHO, ferrostatin-1, cyclosporin A, and disulfiram were bought from MedChemExpress (Princeton, USA). IDN-6556, GKT137831 (Setanaxib), GSK′872, Necrostatin-1, VX-765, and MCC950 were obtained from Selleck (Houston, USA). CytoTox 96 Non-Radioactive Cytotoxicity Assay kit was bought from Promega (Madison, USA). Dulbecco’s modified Eagle’s medium (DMEM) with high glucose, Opti-MEM, fetal bovine serum (FBS), streptomycin and penicillin were purchased from ThermoFisher/Gibco (Carlsbad, USA). Gossypol was freshly dissolved in DMSO at 50 mM. The antibody against NLRP3 was bought from Adipogen AG (Liestal, Switzerland). Specific antibodies against IL-1β, cleaved caspase-9, cleaved caspase-8, PARP, cleaved caspase-3, caspase-3, β-actin, p-MLKL, MLKL, AlexaFluor488-conjugated anti-ASC, horseradish peroxidase (HRP)-conjugated horse-anti-mouse IgG, and HRP-conjugated goat-anti-rabbit IgG were all obtained from Cell Signaling Technology (Danvers, USA). Rabbit monoclonal antibodies against pro-caspase1+p10+p12, caspase-8, GSDMD, and DFNA5/GSDME were purchased from Abcam (Cambridge, UK). CF568-conjugated goat-anti-rabbit IgG was obtained from Biotium (Hayward, USA). PE-labeled anti-Ly-6G (Ly-6G-PE) and FITC-labeled anti-CD11b (CD11b-FITC) were obtained from BioLegend (San Diego, USA).
Figure1 .
BH3-mimetic gossypol induces pyroptotic-like necrosis in LPS-primed macrophagesBMDMs were primed with 500 ng/mL LPS for 4 h, and then treated with indicated concentrations of gossypol (GOS) and indicated time periods. (A) Structure of gossypol. (B) Lytic cell death was measured by lactic acid dehydrogenase (LDH) release in culture supernatants (n = 3). (C,D) The cells were treated with gossypol for 3 h (C) or with graded doses of gossypol for indicated periods (D). Representative cell death images showing bright-field images combined with propidium iodide (PI, red fluorescence indicating dead cells) and Hoechst 33342 (blue, staining all nuclei). The white arrow in each image indicates a typical cell with ballooning morphology. Scale bar, 20 μm. (E,F) Quantification of PI-positive cells in five randomly chosen fields and ratios of PI-positive over all cells (revealed by Hoechst 33342) are used to show the percent of lytic cell death (n = 5). Data are shown as the mean±SD. **P < 0.01, ***P < 0.001.
Animals
C57BL/6J mice (6–8 weeks of age) were purchased from the Experimental Animal Center of Southern Medical University (Guangzhou, China). C57BL/6J NLRP3 +/− mice were purchased from GemPharmatech (Nanjing, China). Knockout mice were backcrossed to the C57BL/6J background for more than eight generations and NLRP3 −/− mice were identified as described previously [33]. All animals were maintained under a 12/12 h light/dark cycle with ad libitum access to food and water and were acclimatized for one week before experiments. Mouse experiments were approved by the Committee on the Ethics of Animal Experiments of Jinan University.
Bone marrow-derived macrophages
BMDMs were prepared as described previously [34]. Briefly, bone marrow cells from hind femora and tibias of C57BL/6J mice were cultured in DMEM supplemented with 10% FBS, 20% L-929-conditioned medium, 100 U/mL penicillin, and 100 μg/mL streptomycin. BMDMs were seeded into 96, 24 and 6-well plates at a density of 5×10 4 cells/well, 2.5×10 5 cells/well and 1.6×10 6 cells/well, respectively, in complete DMEM (DMEM containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin), and incubated overnight at 37°C before experiments.
Lytic cell death
Lytic cell death was measured by PI incorporation essentially as described previously [ 35, 36]. Briefly, cells were stained with 2 μg/mL PI and 5 μg/mL Hoechst 33342 in PBS for 10 min, followed by observation with a Zeiss Axio Observer D1 microscope (Carl Zeiss, Gottingen, Germany). Lytic cell death was also assayed by lactic acid dehydrogenase (LDH) release using the CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega) according to instructions of the manufacturer.
Western blot analysis
Immunoblotting was performed as described previously to detect proteins in both cell lysates and supernatants, respectively [ 34, 35]. For the analyses of secreted proteins, soluble proteins in culture supernatants (equal volume from each sample) were precipitated as previously described [34].
Immunofluorescence microscopy
BMDMs were seeded in glass-bottom dishes and immunofluorescence microscopy was performed as previously reported [34]. In brief, cells were fixed with paraformaldehyde and permeabilized with methanol. After being blocked with 5% goat serum in PBS, the cells were incubated with the following primary antibodies overnight at 4°C: anti-caspase-8 antibody (1:300), anti-cleaved caspase-3 antibody (1:300) or anti-p-MLKL antibody (1:300), respectively. The dishes were then incubated with CF568-conjugated goat-anti-rabbit IgG for 1 h at room temperature, followed by incubation with AlexaFluor488-conjugated anti-ASC antibody (1:100) overnight at 4°C. The nuclei were revealed by Hoechst 33342 staining (5 μg/mL in PBS). Fluorescent images of cells were captured under the Zeiss Axio Observer D1 microscope. Images were analyzed by using ZEN software (Carl Zeiss).
Mitochondrial membrane potential measurement
The mitochondrial membrane potential was measured using a mitochondrial membrane potential assay kit with JC-1 (C2006; Beyotime, Shanghai, China) according to instructions of the manufacturer.
Mouse peritonitis
Mice were intraperitoneally (i.p.) pre-treated with indicated inhibitors for 1 h prior to injection (i.p.) with gossypol or vehicle (2% Tween-80 in PBS) for 8 h. The animals were sacrificed via cervical dislocation and peritoneal lavage cells were collected and washed with PBS-F (PBS containing 0.1% NaN 3 and 1% FBS). Neutrophils in the peritoneal lavage cells were stained with anti-mouse CD11b-FITC and anti-mouse Ly-6G-PE. Data were analyzed by flow cytometry on the Attune NxT Flow Cytometer (Thermo Fisher Scientific (Carlsbad, USA).
Statistical analysis
Data were presented as the mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, USA). One-way analysis of variance (ANOVA) followed by Bonferroni post hoc test and unpaired Student’s t-test was used to analyze the statistical significance among multiple groups and between two groups, respectively. P<0.05 was considered of statistically significant difference.
Results
Natural BH3-mimetic gossypol induces pyroptotic-like cell death in macrophages
To study whether the natural BH3 mimetic gossypol ( Figure 1A)induces the activation of multiple forms of necrosis in macrophages, we initially examined lytic cell death in wild-type BMDMs primed with LPS by assaying LDH release and PI uptake. LPS priming was adopted to induce the expression of pro-IL-1β and upregulation of NLRP3 protein for exploring the role of NLRP3 inflammasome. The results showed that gossypol treatment induced LDH release in a time- and dose-dependent manner ( Figure 1B), indicating the induction of lytic cell death in the macrophages. Consistent with LDH release, PI uptake also revealed a similar time- and dose-dependent lytic cell death ( Figure 1C–F). Notably, gossypol-induced lytic cell death had a characteristic of pyroptosis, i.e. cell swelling and ballooning accompanied by PI incorporation ( Figure 1C,D). In addition, gossypol was also able to induce lytic cell death in unprimed macrophages ( Supplementary Figure S1A,B). These results indicated that gossypol induces pyroptotic-like cell death in both unprimed and LPS-primed macrophages.
BH3-mimetic gossypol simultaneously induces activation of multiple regulated necrosis pathways in macrophages
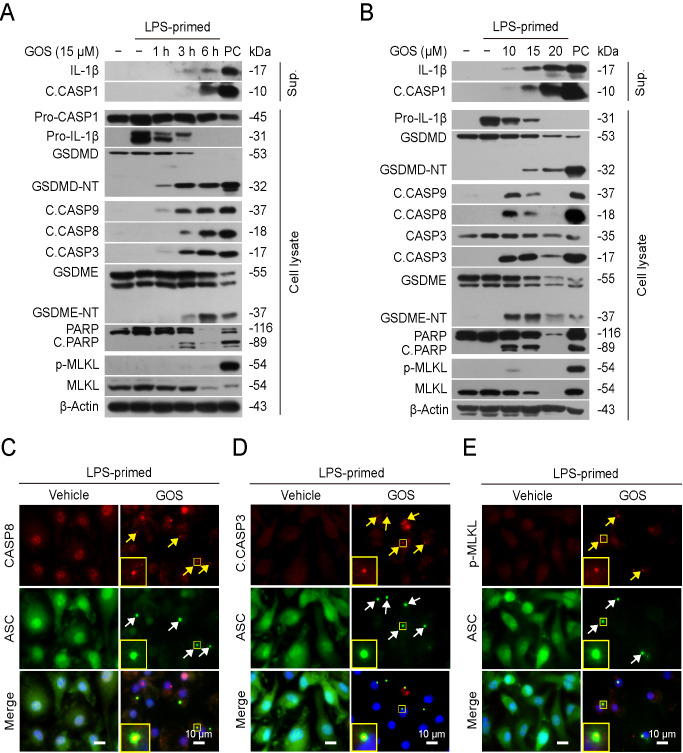
Recent studies revealed that both apoptotic caspases (such as caspase-8/-9/-3) and inflammatory caspases (such as caspase-1) can mediate necrosis by cleaving respective executioner substrates GSDMD [ 37– 41] and GSDME [ 17, 18], while p-MLKL may also translocate to the plasma membrane to induce necrosis [ 42– 45]. Consistent with previous studies [ 30– 32], western blot analysis in this study showed that in LPS-primed macrophages, gossypol time- and dose-dependently induced the release of cleaved caspase-1p10 and mature IL-1β (17 kDa) in culture supernatants accompanied by the generation of GSDMD-NT in cell lysates ( Figure 2A,B), the hallmarks of inflammasome activation. Consistent with this, ASC specks were observed in macrophages upon gossypol treatment ( Figure 2C and Supplementary Figure S2A), indicating induction of inflammasome assembly. Interestingly, concomitant with inflammasome assembly, both cleaved caspase-8 and cleaved caspase-9 were detected in gossypol-treated cells in a time-dependent manner ( Figure 2A), indicating the activation of both extrinsic and intrinsic apoptotic signaling pathways. The downstream caspase-3 was also activated, as revealed by cleaved caspase-3 and its substrate PARP fragment (89 kDa) ( Figure 2A). The caspase-3 activation and the GSDME-NT (37 kDa) generation might be indicative of the induction of secondary necrosis [ 17, 18]. Notably, p-MLKL (marker for necroptosis) was only weakly detected in cells treated with a low dose of gossypol (10 μM) ( Figure 2B), at which gossypol induced robust production of cleaved caspase-8/-9/-3 concomitant with cleavage of caspase-3 substrates PARP and GSDME. However, gossypol-induced cleavage of caspase-8/-9/-3, PARP and GSDME was not dose-dependent but appeared to be reciprocal to the changes of caspase-1p10 and GSDMD-NT, suggesting that there was a transition between pyroptosis and other forms of cell death. In addition, fluorescence microscopy showed that approximately 40% of caspase-8, cleaved caspase-3 and p-MLKL aggregates were co-localized with ASC specks ( Figure 2C–E and Supplementary Figure S2B–D), implicating interconnections among them. Similar to LPS-primed macrophages, gossypol induced cleavage of GSDME and PARP as well as phosphorylation of MLKL in unprimed cells ( Supplementary Figure S1C), indicating that both apoptosis and necroptosis pathways had been activated. However, GSDMD cleavage was not observed in unprimed cells, which was likely due to the low expression of NLRP3 and pro-IL-1β ( Supplementary Figure S1D) and thus the insufficient assembly of NLRP3 inflammasome in unprimed cells as compared with LPS-primed ones. Together, these results indicated that gossypol dose-dependently induces the activation of multiple subroutines of regulated necrosis, including pyroptosis, apoptosis/secondary necrosis and possibly low level necroptosis in macrophages.
Figure2 .
Gossypol induces robust activation of both apoptotic and pyroptotic pathways but only weak activation of necroptotic signalingBMDMs were treated as in Figure1. (A,B) Western blot analysis showed the expression levels of indicated proteins in cell lysates and culture supernatants. β-Actin was adopted as a loading control for cell lysates. Positive control (PC) for detection of IL-1β and caspase-1p10 (C.CASP1) in supernatants or GSDMD in lysates was culture supernatants and cell lysates from BMDMs primed with LPS (500 ng/mL) for 4 h, followed by stimulation with ATP (5 mM) for 1 h. PC for detection of C.CASP3, C.CASP8, C.CASP9, GSDME and PARP was cell lysates from BMDMs primed with LPS (500 ng/mL) for 4 h, followed by treatment with doxorubicin (10 μM) for 16 h. PC for detection of MLKL and p-MLKL was cell lysates from BMDMs treated with LPS (500 ng/mL) and Z-VAD-FMK (50 μM) for 6 h. (C−E) Immunofluorescence microscopy analyses of cells stained with indicated antibodies. The nuclei were revealed by Hoechst 33342 staining. The insets show a magnified area of one cell with an ASC speck co-localized with caspase-8, cleaved caspase-3 or p-MLKL aggregates. White arrowheads indicate ASC specks which are co-localized with caspase-8, cleaved caspase-3 and p-MLKL aggregates, respectively, indicated by yellow arrowheads. Scale bar, 10 μm. Sup, supernatant.
BH3-mimetic gossypol-induced regulated necrosis is not efficiently inhibited by blocking any single cell death pathway in macrophages
Having observed that gossypol-induced regulated necrosis is morphologically similar to pyroptosis, we next sought to investigate whether gossypol-triggered NLRP3 inflammasome activation is a cause for other cell death pathways. MCC950, a specific inhibitor of NLRP3 inflammasome activation [46], was used to pre-treat BMDMs to block NLRP3 activation, followed by gossypol treatment. Both LDH release and PI uptake analysis showed that gossypol-induced necrosis was unaffected by MCC950 ( Figure 3A,B). Consistent with this, cell ballooning, a hallmark of pyroptosis, was unaffected by MCC950 treatment ( Figure 3C). To further corroborate this, we examined lytic cell death in BMDMs derived from NLRP3 −/− mice. The data showed that gossypol dose-dependently induced necrosis (as revealed by PI uptake) in NLRP3 −/− BMDMs and that the percentages of PI-positive cells were similar between NLRP3 −/− and wild-type BMDMs ( Figure 3D). Gossypol also induced cell swelling and ballooning in NLRP3 −/− BMDMs similar to wild-type cells ( Figure 3E). As caspase-1 activation and GSDMD-NT generation are downstream events of NLRP3 assembly [ 39– 41], we further used caspase-1 inhibitor VX-765 and GSDMD-NT inhibitor disulfiram [47] to examine whether gossypol-induced necrosis could be suppressed by these inhibitors. PI uptake revealed that neither VX-765 nor disulfiram had any inhibitory effects on the necrosis in macrophages upon gossypol treatment ( Supplementary Figure S3A,B).
Figure3 .
Gossypol-induced necrosis in macrophages is independent of NLRP3 inflammasome activationBMDMs were first primed with 500 ng/mL LPS for 4 h, and pre-treated with or without MCC950 (NLRP3 inhibitor) for 1 h, followed by treatment with 15 μM gossypol (GOS) for 3 h in the presence or absence of each inhibitor. (A,B) Percentages of lytic cell death was measured by lactic acid dehydrogenase (LDH) release (A) in culture supernatants (n=3), or by propidium iodide (PI) staining (B) (n=5). (C) Images show morphological characteristics of cell death by bright-field merging with PI (red, staining lytic cells) and Hoechst 33342 (blue, staining all nuclei). (D,E) BMDMs from wild-type (WT) and NLRP3−/− mice were primed for 4 h with LPS (500 ng/mL) and stimulated with GOS for a further 3 h. Percentages of lytic cell death were measured by PI staining (D) and bright-field images merged with PI (red) and Hoechst 33342 (blue) fluorescence (E) were captured by fluorescence microscopy. Data are shown as the mean±SD (n=5). Scale bar, 20 μm. ns, not significant.
Apart from inhibitors for the NLRP3 pathway, we also examined whether inhibitors for other regulated necrosis pathways could block gossypol-induced necrosis. First, necrostatin-1, an inhibitor of RIPK1 (upstream signaling for the necroptosis pathway) had no effect on gossypol-induced necrosis ( Supplementary Figure S3C). GSK′872, an inhibitor of RIPK3 (a critical signaling component for necroptosis), slightly but not significantly inhibited the necrosis ( Supplementary Figure S3D). In addition, neither the ferroptosis inhibitor ferrostatin-1 nor the mitochondrial permeability transition (MPT)-driven necrosis inhibitor cyclosporin A showed any effects on the necrosis ( Supplementary Figure S3E,F). Furthermore, caspase-3 inhibitor Ac-DEVD-CHO had no effect on the necrosis either, whereas the pan-caspase inhibitor IDN-6556 (Emricasan) did not inhibit but instead significantly increased necrosis in the presence of gossypol ( Supplementary Figure S3G,H). Interestingly, IDN-6556 alone induced high level necrosis in LPS-primed macrophages probably through induction of necroptosis, which is consistent with previous findings that caspase-8 inhibition induces necroptosis [ 48– 50]. In addition, the only effective inhibitor we found at present that could partially inhibit gossypol-induced necrosis ( Supplementary Figure 3I) was GKT137831, an inhibitor for NADPH oxidase 1/4 (NOX1/4) that mediates the production of cellular superoxide anions [ 51, 52].
Together, these results indicated that the blockade of one subroutine of regulated necrosis alone could not efficiently block gossypol-induced necrosis, consistent with the aforementioned findings that multiple subroutines of regulated necrosis in macrophages are simultaneously induced by gossypol.
A combination of inhibitors for multiple signaling pathways markedly suppresses the regulated necrosis induced by gossypol
Considering the above-mentioned results that single inhibitor for each regulated cell death pathway is unable to markedly inhibit gossypol-induced multiple subroutines of regulated necrosis, we presumed that a combination of different necrosis pathway inhibitors might be able to block the necrosis. Thus, we pre-treated BMDMs with different combinations of inhibitors followed by gossypol treatment, and necrosis was assayed by PI uptake. The results showed that addition of MCC950 to GKT137831 did not further enhance the inhibitory effect of GKT137831 ( Figure 4A). However, as shown in Figure 4B, pan-caspase inhibitor (IDN-6556) in combination with RIPK3 inhibitor (GSK′872) reduced gossypol-induced necrosis by ~50% even though IDN-6556 alone did increase necrosis. Furthermore, addition of GKT137831 to IDN-6556+GSK′872 further decreased the level of gossypol-induced necrosis. Again, further addition of MCC950 to this combination (GKT137831+IDN-6556+GSK′872) did not significantly decrease necrosis level when compared with GKT137831+IDN-6556+GSK′872 ( Figure 4C). Similar results were obtained in NLRP3 −/− BMDMs ( Figure 4D). Taken together, these results indicated that combined administration of multiple inhibitors (such as GKT137831, IDN-6556 and GSK′872) is able to robustly inhibit gossypol-induced necrosis, but NLRP3 inhibitor MCC950 has no additive effect on the necrosis.
Figure4 .
Gossypol-induced necrosis can be markedly inhibited by a combination of inhibitors for multiple pathwaysBMDMs were first primed with 500 ng/mL LPS for 4 h, and pre-treated with or without inhibitors for 1 h, followed by treatment with 15 μM gossypol (GOS) for 3 h. (A) BMDMs were pre-treated with GKT137831 or GKT137831 plus MCC950 for 1 h. (B) BMDMs were pre-treated with combination of inhibitors, 20 μM IDN-6556 and 5 μM GSK′872, for 1 h. PI staining in the wild-type (C) and NLRP3−/− (D) BMDMs were pre-treated with combination of inhibitors (2 μM MCC950, 10 μM GKT137831, 20 μM IDN-6556 and 5 μM GSK′872). Lytic cell death was observed by fluorescence microscopy. PI-positive cells in five randomly chosen fields each containing ~100 cells were quantified. Data are shown as the mean±SD (n=5). ***P < 0.001. ns, not significant.
NOX1/4 inhibitor combined with pan-caspase and RIPK3 inhibitors blocks all cell death pathways for pyroptosis and apoptosis/secondary necrosis in macrophages with or without NLRP3 expression
As the aforementioned data ( Figure 4C,D) showed that GKT137831 (NOX1/4 inhibitor) in combination with pan-caspase inhibitor IDN-6556 plus RIPK3 inhibitor GSK′872) displayed the most pronounced suppression of gossypol-induced necrosis, we further explored whether gossypol-induced cell death pathways for different subroutines of regulated necrosis are blocked by the inhibitors or their combination and whether NLRP3 plays a role in this process. As shown in Figure 5A, the activation of caspase-8/-9/-3 and cleavage of GSDME and PARP were observed in both wild-type and NLRP3 −/− BMDMs, suggesting that gossypol-induced activation of these pathways are independent of NLRP3 expression. Interestingly, the levels of both cleaved caspase-3 and GSDME-NT in NLRP3 −/− cells were higher than those in wild-type ones upon gossypol treatment ( Figure 5A–C). However, the activation of caspase-1 (as indicated by cleaved caspase-1p10 and IL-1β in culture supernatants) and generation of GSDMD-NT were dependent on NLRP3 expression ( Figure 5A), confirming that gossypol-induced inflammasome activation relies on NLRP3 expression. Although GKT137831 combined with IDN-6556 and GSK′872 inhibited gossypol-induced necrosis more substantially than IDN-6556 plus GSK′872, both of these combinations were able to fully block the activation of the cell death pathways for pyroptosis and apoptosis/secondary necrosis either in wild-type cells or in NLRP3 −/− cells ( Figure 5A), indicating that their action is independent of NLRP3 inflammasome. Together, these data indicated the combined inhibitor regimens were able to completely suppress the signaling pathways for pyroptosis and apoptosis/secondary necrosis independently of NLRP3 inflammasome activation, and suggested that GKT137831, acting as an inhibitor of NOX1/4, might contribute to the suppression of gossypol-induced regulated necrosis via an as-yet-unidentified pathway.
Figure5 .
Gossypol-induced activation of regulated necrosis pathways can be markedly suppressed by a combination of multiple pathway inhibitors with or without NLRP3 proteinBMDMs were first primed with 500 ng/mL LPS for 4 h, and pre-treated with or without inhibitors for 1 h, followed by treatment with 15 μM gossypol (GOS) for 3 h. (A) Western blot analysis of indicated proteins in the culture supernatants (Sup.) or cells lysates of the wild-type and NLRP3−/− BMDMs. (B,C) Relative gray values of GSDME-NT and C.CASP3 blots were quantified to respective actin blot (n=3). β-Actin was used as a loading control. ***P<0.001. ns, not significant.
Caspase-3 activation and NLRP3/ASC pathway are independent of each other in gossypol-treated macrophages
Having found that ASC speck is co-localized with caspase-8, cleaved caspase-3 and p-MLKL ( Figure 2C–E), we next asked whether there is any association between these signaling pathways. Western blot analysis showed that although IDN-6556 in combination with GSK′872 completely inhibited the activation of all caspases including caspase-1 ( Figure 5A), this combined regimen could not prevent the assembly of ASC speck ( Figure 6A,D), suggesting that gossypol-induced inflammasome assembly is independent of the activation of all caspases. Given that caspase-8- or caspase-9-dependent apoptosis activates channel-forming glycoprotein pannexin-1 to release ATP to drive NLRP3 inflammasome activation [29], we tested whether blocking ATP release or its action has any effect on NLRP3/ASC assembly. As shown in Figure 6B,F, blocking ATP receptor P2X7R with competitive antagonist A-804598 or blocking pannexin-1 activity by trovafloxacin (a well-established panexin-1 inhibitor [53]) did not display any effects on ASC speck formation in macrophages treated with gossypol, indicating that NLRP3/ASC inflammasome assembly is independent of released ATP via the pannexin-1 channel, consistent with the results from IDN-6556 plus GSK′872 ( Figure 6A,D).
Figure6 .
The activation of caspase-3 and ASC speck assembly are independent of but is associated with each otherThe wild-type and NLRP3−/− BMDMs planted in glass-bottom dishes were primed with 500 ng/mL LPS for 4 h, and pre-treated with or without inhibitor (2 μM MCC950, 1 μM A-804598, and 30 μM trovafloxacin for 1 h, followed by stimulation with 15 μM GOS for 3 h in the presence or absence of inhibitors. After fixation and permeabilization, the cells were stained with AlexaFluor488-conjugated anti-ASC antibody only (A) or anti-cleaved caspase-3 (C.CASP3) antibody (B,C), anti-p-MLKL antibody (E), and were stained with CF568-conjugated goat-anti-rabbit IgG, followed by AlexaFluor488-conjugated anti-ASC antibody. The nuclei were revealed by Hoechst 33342 staining. Fluorescence images were captured by fluorescence microscopy. The insets show a magnified area of one cell with an ASC speck co-localized with C.CASP3/p-MLKL punctum. White arrows indicate ASC specks co-localized with C.CASP3/p-MLKL dots which are indicated by yellow arrows. Scale bar, 10 μm. (D,F) The ratios of cells containing ASC specks were calculated by the number of cells with ASC specks relative to the total number of cells from five random fields. Data are shown as the mean±SD (n=5). ***P<0.001. ns, not significant.
On the other hand, we also asked whether NLRP3/ASC assembly plays any roles in the activation of apoptotic caspases. We first used co-staining of cleaved caspase-3 and ASC in gossypol-treated macrophages in the presence of MCC950 to block NLRP3/ASC assembly. The results showed that MCC950-mediated blockade of ASC speck formation had little effect on caspase-3 activation, as revealed by cleaved caspase-3 staining ( Figure 6C). Further, in BMDMs with NLRP3 being genetically deleted, gossypol-induced ASC speck formation was fully blocked but induction of cleaved caspase-3 was unaffected ( Figure 6C). Together, these results indicated that the activation of apoptotic caspases is independent of NLRP3/ASC assembly and vice versa.
Meanwhile, neither MCC950-meidtaed blockade of NLRP3 nor NLRP3 knockout influenced gossypol-induced phosphorylation of MLKL in cells treated with gossypol ( Figure 6E), suggesting that MLKL phosphorylation is independent of NLRP3/ASC, which still needs further investigations.
Considering that gossypol acts as a natural BH3-mimetic to induce mitochondria-mediated apoptosis [ 24, 25], we asked whether gossypol could induce mitochondrial dysfunction in macrophages. As expected, gossypol dose-dependently induced a decrease in mitochondrial membrane potential, as revealed by JC-1 staining ( Supplementary Figure S4A,B). However, neither IDN-6556+GSK′872 nor GKT137831+IDN-6556+GSK′872 had any influence on gossypol-induced mitochondrial dysfunction ( Supplementary Figure S4C,D), suggesting that gossypol’s effect on mitochondria might be upstream or independent of those signaling pathways targeted by these inhibitors.
Gossypol-induced peritonitis in mice is partly inhibited by the combined inhibitors
As lytic cell death is an inflammatory form of cell death, we lastly assayed whether gossypol could induce inflammatory responses in a mouse model of peritonitis. The results showed that gossypol administration caused a significant recruitment of neutrophils into the peritoneal cavity of both WT and NLRP3-deficient mice when compared with the vehicle control ( Figure 7A,B). In addition, we found that the combination of GKT137831+IDN-6556+GSK′872 could partly but significantly attenuated the recruitment of neutrophils into the peritoneal cavity ( Figure 7C,D). These results suggested that gossypol-induced inflammation is at least partly mediated by regulated necrosis.
Figure7 .
Gossypol-induced peritonitis can be attenuated by a combination of multiple signaling pathway inhibitors(A,B) Wild-type (WT) and NLRP3−/− mice were treated with 500 μM gossypol (100 μL/mouse) by intraperitoneal injection. After 8 h, neutrophils (CD11b+Ly-6G+) in the peritoneal cavity were analyzed by flow cytometry. (C,D) WT mice pre-treated with intraperitoneal injection of combined inhibitors (10 μM GKT137831+10 μM IDN-6556+10 μM GSK′872) (100 μL/mouse, 1 h) or PBS with 0.1% DMSO, followed by treatment with gossypol (500 μM, 100 μL/mouse) or treatment with PBS with 0.1% DMSO for 8 h. (A,C) Representative dot-plots of flow cytometry. The number within each dot-plot is the percentage of neutrophils in peritoneal exudate cells. (B,D) Quantification of neutrophils within the gate in (A,C). Data are shown as the mean±SD (n=3). *P < 0.05, **P < 0.01, ***P < 0.001. ns, not significant. GKT, GKT137831; GOS, gossypol.
Discussion
Regulated necrosis has been defined as a genetically controlled lytic cell death that eventually culminated in disruption of plasma membrane with cell swelling and ballooning as morphological hallmarks [ 2, 3]. Multiple forms of regulated necrosis have been recently identified, such as necroptosis, ferroptosis, MPT-driven necrosis, pyroptosis, and secondary necrosis [2]. Although apoptosis has been the focus of research of regulated cell death, recent studies have revealed that regulated necrosis is not only relevant to various diseases but also implicated in xenobiotics-elicited toxicity, thus representing as new therapeutic target and having implications in toxicity in the context of anticancer therapies [ 4, 54]. In this study, we found that the natural BH3-mimetic gossypol, as a potential anticancer agent, is able to robustly induce, in macrophages, multiple subroutines of regulated necrosis at least including pyroptosis, apoptosis/secondary necrosis, but only weak necroptosis. The GSDMD-NT-mediated pyroptosis was a result of NLRP3 inflammasome activation as this process was completely blocked by specific inhibitor MCC950 or by genetic depletion of NLRP3. Intriguingly, the NLRP3 inflammasome assembly occurred simultaneously with the activation of apoptotic caspases even in the same cells. However, the activation of apoptotic caspases appeared to be independent of the NLRP3 pathway and vice versa. The necrosis induced by gossypol was only efficiently inhibited by a combination of multiple cell death pathway inhibitors, but not any one of them. Our results suggested the natural BH3-mimetic can induce various subroutines of regulated necrosis including pyroptosis, secondary necrosis and probably necroptosis in LPS-primed macrophages, thus providing a further layer of mechanistic explanation for previous findings of pyroptotic-like cell death [32].
Our finding that gossypol-induced multiple subroutines of regulated necrosis is to some extent consistent with recent studies showing that pyroptosis, apoptosis and necroptosis can be triggered in macrophages by various pathogens [ 12– 14]. Such multiple cell death has been named PANoptosis [ 15, 16] which is mediated by the PANoptosome that has been regarded as the platform for triggering PANoptosis [12]. Interestingly, such complex necrosis of macrophages induced by pathogens, such as including influenza A virus and Yersinia pseudotuberculosis, was not or only weakly blocked by deletion of individual component of PANoptosome but instead was blocked by combined deletion of the PANoptosome components (e.g., caspase-1/-11, RIPK3 and caspase-8) [12], which seems in line with our combined pan-caspase and RIPK3 inhibitors to inhibit gossypol-induced regulated necrosis. However, our results are different from those previous studies in that we only observed weak activation of the necroptotic signaling, as evidenced by low level of p-MLKL, thus whether gossypol-induced necrosis can be regarded as PANoptosis needs to be further clarified, even though ASC speck is co-localized with caspase-8, p-MLKL or cleaved caspase-3. Collectively, apart from pathogenic microorganisms, the natural BH3-mimetic gossypol can also induce multiple forms of regulated necrosis in macrophages, suggesting that BH3-mimetics may exert its toxicity by inducing distinct forms of cell death in innate immune cells including macrophages.
Several recent studies have shown that different forms of cell death can be switched from one to another if one signaling pathway is blocked by pathogens. Yersinia effector protein YopJ inhibits TAK1 or IKK kinase by activating caspase-8 via RIPK1 to cleave GSDMD, thereby inducing both apoptotic and pyroptotic-like cell death in macrophages (BMDMs) [8]. A similar study showed that macrophages stimulated with LPS in the context of TAK1 inhibition resulted in necrosis accompanied by the activation of pan-caspases, while Yersiniainfection induced similar pyroptotic-like cell death likely mediated by RIPK3/caspase-8-dependent cleavage of both GSDMD and GSDME [9]. More recently, it has been demonstrated that caspase-8 acts as a switch for apoptosis, necroptosis and pyroptosis [ 10, 55]. Activated caspase-8, previously known as an initiator for apoptosis, can inhibit the phosphorylation of MLKL (p-MLKL) mediated by RIPK3, resulting in the blockade of necroptosis, while cells with caspase-8 being blocked by pan-caspase inhibitor or by genetic deletion leads to necroptosis [ 48– 50]. Mice deficient with caspase-8 is embryonic lethal but can be rescued by further deletion of Ripk3 or Mlkl [50]. Interestingly, expression of enzymatically inactive caspase-8 induced both necroptosis and pyroptosis thus also causing embryonic lethality, suggesting a pro-death scaffolding function of the inactive caspase-8, whereas blocking necroptosis by further deletion of Mlkl in cells with inactive caspase-8 triggered the activation of inflammasome (formation of ASC speck and activation of caspase-1) [ 10, 55]. Consistent with these studies, our study showed that the blockade of gossypol-induced activation of pan-caspases (including caspase-8) by pan-caspase inhibitor IDN-6556 resulted in increased necrosis, indicative of triggering of necroptosis. When IDN-6556-triggered necroptosis was inhibited by co-treatment with RIPK3 inhibitor GSK′872, both necroptosis and p-MLKL were suppressed; IDN-6556 plus GSK′872-mediated inhibitory effect on gossypol-induced necrosis was further enhanced by addition of inhibitor for NOX1/4, which mediates the production of cellular superoxide anions [ 51, 52]. In addition, we also found that depletion of NLRP3 increased the activation of caspase-3 and cleavage of GSDME ( Figure 5A–C), whereas gossypol dose-dependently increased caspase-1 activation but decreased caspase-3/GSDME cleavage ( Figure 2B), suggestive of a transition between pyroptosis and other forms of cell death, though the mechanism of such transition is unclear at present. Taken together, the aforementioned studies and ours highlight a notion that combined inhibition of multiple signaling pathways is needed to efficiently suppress regulated necrosis that may undergo switch between subroutines such as necroptosis, pyroptosis, and apoptosis/secondary necrosis.
It has been revealed that synthetic BH3-mimetic ABT-737 in combination with TAK1 inhibitor induces caspase-8 or caspase-9-dependent lytic cell death and NLRP3 inflammasome activation, the latter of which requires the channel-forming pannexin-1 [29]. Pannexin-1 is activated by caspase-3/-7-dependent cleavage at its C-terminus during apoptosis [ 56, 57] and the removal of this inhibitory C-terminal domain leads to the opening of pannexin-1 channel to release ATP and potassium efflux [ 56, 58], resulting in the activation of NLRP3 inflammasome. However, in our study, we found that NLRP3/ASC inflammasome assembly was independent of pannexin-1 channel-mediated release of ATP, as blocking pannexin-1 channel by trovafloxacin or inhibiting ATP receptor P2X7R by its competitive antagonist A-804598 had no effect on ASC speck formation, suggesting that pannaxin-1 might not be involved in gossypol-induced NLRP3 inflammasome activation. Besides pannexin-1, MLKL activation through phosphorylation by RIPK3 triggers potassium efflux and NLRP3 inflammasome assembly [ 59, 60]. Yet in our study, p-MLKL was only weakly increased and more importantly GSK′872 plus IDN-6556 did not block the formation of ASC speck, indicating that p-MLKL plays no role in this process. On the other hand, our evidence indicated that the NLRP3 pathway plays a minimal role in mediating gossypol-induced activation of caspase-8/-9/-3 in that pharmacological inhibition or genetic deletion of NLRP3 did not inhibit caspase-8/-9/-3 activation. Altogether, our results suggest that the BH3-mimetic gossypol may induce both the activation of apoptotic caspases and the assembly of NLRP3 inflammasome, thus leading to inflammatory caspase-1 activation, through mechanisms that appear independent of each other.
Although we have provided evidence for the activation of both apoptotic caspase-8/-9/-3 and inflammatory caspase-1, it remains unclear how they are activated by gossypol. As a pan-Bcl-2 inhibitor, the natural BH3-mimetic gossypol can counteract the anti-apoptotic effect of Bcl-2, Bcl-X L and Mcl-1, thus leading to the activation of pro-apoptotic Bax and Bak, which mediates the release of cytochrome c thus eventually resulting in caspase-3/-7 activation and apoptosis [23]. In our study, we detected robust activation of caspase-8/-9/-3, suggesting that the mitochondrial pathway plays a role in this process. However, reactive oxygen species (ROS) might also be involved in the action of gossypol-induced activation of NLRP3 inflammasome, as NOX1/4 blockade could decrease caspase-1 activation. In line with this, it has been documented that gossypol can cause mitochondrial dysfunction by inhibiting cell respiration and stimulating the generation of ROS [61]. Consistently, we found that gossypol could induce a marked decrease in the mitochondrial membrane potential in murine macrophages. However, recent studies also showed that gossypol induces ROS-independent apoptosis and mitochondrial dysfunction in human A375 melanoma cells [30]. Future research is therefore needed to clarify the precise mechanism(s).
In summary, we found in this study that the natural BH3-mimetic gossypol, as a toxic phytochemical, is able to induce multiple subroutines of regulated necrosis in macrophages, accompanied by the release of inflammatory factors including IL-1β. Different from apoptosis, such regulated necrosis represents inflammatory cell death that can trigger inflammatory responses in vivo, which is supported by our preliminary data in a mouse model of peritonitis. Our findings may have some implications in the study of gossypol as an anticancer agent. As emerging evidence indicates that excessive level of IL-1β has been associated with poor prognosis in various cancers [ 62, 63], gossypol-induced release of IL-1β from LPS-primed macrophages, as well as other inflammatory cellular components from both primed and unprimed macrophages, during regulated necrosis may have potential influences on the efficiency of anticancer therapy. Future investigations are therefore warranted to ensure proper application of BH3-mimetic in clinic considering its toxic effects in inducing multiple subroutines of regulated necrosis in innate immune cells including macrophages.
Supporting information
Supplementary Data
Supplementary data is available at Acta Biochimica et Biphysica Sinica online.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (No. 81773965 to X.H. and No. 81873064 to D.O.).
References
- 1.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. . 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. . 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 3.Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol. . 2014;35:24–32. doi: 10.1016/j.semcdb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Conrad M, Angeli JPF, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. . 2016;15:348–366. doi: 10.1038/nrd.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. . 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. . 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Kovacs SB, Miao EA. Gasdermins: effectors of pyroptosis. Trends Cell Biol. . 2017;27:673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. . 2018;362:1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection . Proc Natl Acad Sci U S A. . 2018;115:E10888–E10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritsch M, Günther SD, Schwarzer R, Albert MC, Schorn F, Werthenbach JP, Schiffmann LM, et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. . 2019;575:683–687. doi: 10.1038/s41586-019-1770-6. [DOI] [PubMed] [Google Scholar]
- 11.Newton K, Wickliffe KE, Dugger DL, Maltzman A, Roose-Girma M, Dohse M, Kömüves L, et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature. . 2019;574:428–431. doi: 10.1038/s41586-019-1548-x. [DOI] [PubMed] [Google Scholar]
- 12.Christgen S, Zheng M, Kesavardhana S, Karki R, Malireddi RKS, Banoth B, Place DE, et al. Identification of the PANoptosome: a molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis) Front Cell Infect Microbiol. . 2020;10:237. doi: 10.3389/fcimb.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malireddi RKS, Kesavardhana S, Karki R, Kancharana B, Burton AR, Kanneganti TD. RIPK1 distinctly regulates yersinia-induced inflammatory cell death, PANoptosis. ImmunoHorizons. . 2020;4:789–796. doi: 10.4049/immunohorizons.2000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng M, Williams EP, Malireddi RKS, Karki R, Banoth B, Burton A, Webby R, et al. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J Biol Chem. . 2020;295:14040–14052. doi: 10.1074/jbc.RA120.015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malireddi RKS, Kesavardhana S, Kanneganti TD. ZBP1 and TAK1: master regulators of NLRP3 inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis) Front Cell Infect Microbiol. . 2019;9:406. doi: 10.3389/fcimb.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Place DE, Lee SJ, Kanneganti TD. PANoptosis in microbial infection. Curr Opin MicroBiol. . 2021;59:42–49. doi: 10.1016/j.mib.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. . 2017;8:14128. doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. . 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 19.Mai FY, He P, Ye JZ, Xu LH, Ouyang DY, Li CG, Zeng QZ, et al. Caspase‐3‐mediated GSDME activation contributes to cisplatin‐ and doxorubicin‐induced secondary necrosis in mouse macrophages. Cell Prolif. . 2019;52:e12663. doi: 10.1111/cpr.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H, Zhang S, Wu J, Chen M, Cai MC, Fu Y, Li W, et al. Molecular targeted therapies elicit concurrent apoptotic and GSDME-dependent pyroptotic tumor cell death. Clin Cancer Res. . 2018;24:6066–6077. doi: 10.1158/1078-0432.CCR-18-1478. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Li S, Qi J, Chen Z, Wu Y, Guo J, Wang K, et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. . 2019;10:193. doi: 10.1038/s41419-019-1441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billard C. BH3 mimetics: status of the field and new developments. Mol Cancer Ther. . 2013;12:1691–1700. doi: 10.1158/1535-7163.MCT-13-0058. [DOI] [PubMed] [Google Scholar]
- 23.Opydo-Chanek M, Gonzalo O, Marzo I. Multifaceted anticancer activity of BH3 mimetics: Current evidence and future prospects. Biochem Pharmacol. . 2017;136:12–23. doi: 10.1016/j.bcp.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Liu H, Guo R, Ling Y, Wu X, Li B, Roller PP, et al. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem Pharmacol. . 2003;66:93–103. doi: 10.1016/S0006-2952(03)00248-X. [DOI] [PubMed] [Google Scholar]
- 25.Oliver CL, Bauer JA, Wolter KG, Ubell ML, Narayan A, O′Connell KM, Fisher SG, et al. In vitro effects of the BH3 mimetic, (−)-gossypol, on head and neck squamous cell carcinoma cells . Clin Cancer Res. . 2004;10:7757–7763. doi: 10.1158/1078-0432.CCR-04-0551. [DOI] [PubMed] [Google Scholar]
- 26.Wu D. An overview of the clinical pharmacology and therapeutic potential of gossypol as a male contraceptive agent and in gynaecological disease. Drugs. . 1989;38:333–341. doi: 10.2165/00003495-198938030-00001. [DOI] [PubMed] [Google Scholar]
- 27.Zeng Y, Ma J, Xu L, Wu D. Natural product gossypol and its derivatives in precision cancer medicine. Curr Med Chem. . 2019;26:1849–1873. doi: 10.2174/0929867324666170523123655. [DOI] [PubMed] [Google Scholar]
- 28.Vince JE, De Nardo D, Gao W, Vince AJ, Hall C, McArthur K, Simpson D, et al. The mitochondrial apoptotic effectors BAX/BAK activate caspase-3 and -7 to trigger NLRP3 inflammasome and caspase-8 driven IL-1β activation. Cell Rep. . 2018;25:2339–2353.e4. doi: 10.1016/j.celrep.2018.10.103. [DOI] [PubMed] [Google Scholar]
- 29.Chen KW, Demarco B, Heilig R, Shkarina K, Boettcher A, Farady CJ, Pelczar P, et al. Extrinsic and intrinsic apoptosis activate pannexin‐1 to drive NLRP 3 inflammasome assembly . EMBO J. . 2019;38:e101638. doi: 10.15252/embj.2019101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haasler L, Kondadi AK, Tsigaras T, von Montfort C, Graf P, Stahl W, Brenneisen P. The BH3 mimetic (±) gossypol induces ROS-independent apoptosis and mitochondrial dysfunction in human A375 melanoma cells in vitro . Arch Toxicol. . 2021;95:1349–1365. doi: 10.1007/s00204-021-02987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Sun S, Wang Y, Mo Y, Xiong F, Zhang S, Zeng Z, et al. Gossypol induces apoptosis of multiple myeloma cells through the JUN-JNK pathway. Am J Cancer Res 2020, 10: 870-883 . [PMC free article] [PubMed]
- 32.Lin QR, Li CG, Zha QB, Xu LH, Pan H, Zhao GX, Ouyang DY, et al. Gossypol induces pyroptosis in mouse macrophages via a non-canonical inflammasome pathway. Toxicol Appl Pharmacol. . 2016;292:56–64. doi: 10.1016/j.taap.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 33.Shu JX, Zhong CS, Shi ZJ, Zeng B, Xu LH, Ye JZ, Wang YF, et al. Berberine augments hypertrophy of colonic patches in mice with intraperitoneal bacterial infection. Int Immunopharmacol. . 2021;90:107242. doi: 10.1016/j.intimp.2020.107242. [DOI] [PubMed] [Google Scholar]
- 34.Ye J, Zeng B, Zhong M, Li H, Xu L, Shu J, Wang Y, et al. Scutellarin inhibits caspase-11 activation and pyroptosis in macrophages via regulating PKA signaling. Acta Pharm Sin B. . 2021;11:112–126. doi: 10.1016/j.apsb.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng CY, Li CG, Shu JX, Xu LH, Ouyang DY, Mai FY, Zeng QZ, et al. ATP induces caspase-3/gasdermin E-mediated pyroptosis in NLRP3 pathway-blocked murine macrophages. Apoptosis. . 2019;24:703–717. doi: 10.1007/s10495-019-01551-x. [DOI] [PubMed] [Google Scholar]
- 36.Py BF, Jin M, Desai BN, Penumaka A, Zhu H, Kober M, Dietrich A, et al. Caspase-11 Controls Interleukin-1β Release through Degradation of TRPC1. Cell Rep. . 2014;6:1122–1128. doi: 10.1016/j.celrep.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. . 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 38.He W, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. . 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. . 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. . 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 41.Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, et al. Gsdmd membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. . 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. . 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. . 2014;24:105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. . 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, Pierotti C, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci U S A. . 2014;111:15072–15077. doi: 10.1073/pnas.1408987111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coll RC, Robertson AAB, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. . 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, Ruan J, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. . 2020;21:736–745. doi: 10.1038/s41590-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. . 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, et al. Catalytic activity of the caspase-8–flipl complex inhibits RIPK3-dependent necrosis. Nature. . 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvarez-Diaz S, Dillon CP, Lalaoui N, Tanzer MC, Rodriguez DA, Lin A, Lebois M, et al. The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity. . 2016;45:513–526. doi: 10.1016/j.immuni.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moon JS, Nakahira K, Chung KP, DeNicola GM, Koo MJ, Pabón MA, Rooney KT, et al. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat Med. . 2016;22:1002–1012. doi: 10.1038/nm.4153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. . 2012;56:2316–2327. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poon IKH, Chiu YH, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, Ravichandran KS. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature. . 2014;507:329–334. doi: 10.1038/nature13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aki T, Funakoshi T, Uemura K. Regulated necrosis and its implications in toxicology. Toxicology. . 2015;333:118–126. doi: 10.1016/j.tox.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Newton K, Wickliffe KE, Maltzman A, Dugger DL, Reja R, Zhang Y, Roose-Girma M, et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature. . 2019;575:679–682. doi: 10.1038/s41586-019-1752-8. [DOI] [PubMed] [Google Scholar]
- 56.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. . 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandilos JK, Chiu YH, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, Bayliss DA. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J Biol Chem. . 2012;287:11303–11311. doi: 10.1074/jbc.M111.323378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. . 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conos SA, Chen KW, De Nardo D, Hara H, Whitehead L, Núñez G, Masters SL, et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci U S A. . 2017;114:E961–E969. doi: 10.1073/pnas.1613305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gutierrez KD, Davis MA, Daniels BP, Olsen TM, Ralli-Jain P, Tait SWG, Gale Jr M, et al. MLKL activation triggers NLRP3-mediated processing and release of IL-1β independently of gasdermin-D. J Immunol. . 2017;198:2156–2164. doi: 10.4049/jimmunol.1601757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keshmiri-Neghab H, Goliaei B. Therapeutic potential of gossypol: an overview. Pharmaceutical Biol. . 2014;52:124–128. doi: 10.3109/13880209.2013.832776. [DOI] [PubMed] [Google Scholar]
- 62.Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med. . 2006;4:48. doi: 10.1186/1479-5876-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β–dependent adaptive immunity against tumors. Nat Med. . 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.