Abstract
Based on the availability of oxygen, plant growth environment can be normoxic (normal environment), hypoxic (reduced oxygen, <21%), or anoxic (complete depletion of oxygen). Hypoxic/anoxic environment is created when a plant is exposed to stresses such as submergence, flooding, or pathogen attack. Survival of the plants following stress conditions is in part dependent on their ability to overcome the stress induced by anoxia/hypoxia conditions. This shows the need for the development of strategies for understanding the mechanisms involved in plant tolerance to anoxia. Previous studies have employed different methods for establishing an anerobic environment. Here, we describe a simple method for creating anoxic environment using an anaerobic atmosphere generation bag. Anoxic conditions can be maintained in a cylindrical jar, a rectangular box, or a vacuum sealer bag, enabling the screening of a large number of samples. This protocol is particularly useful to screen plant mutants that are tolerant to anoxia. The method is simple, easy, cost-efficient, reproducible, and does not require any sophisticated instruments.
Graphic abstract
Keywords: Anoxia, Arabidopsis, Flooding, Hypoxia, Submergence, Plants
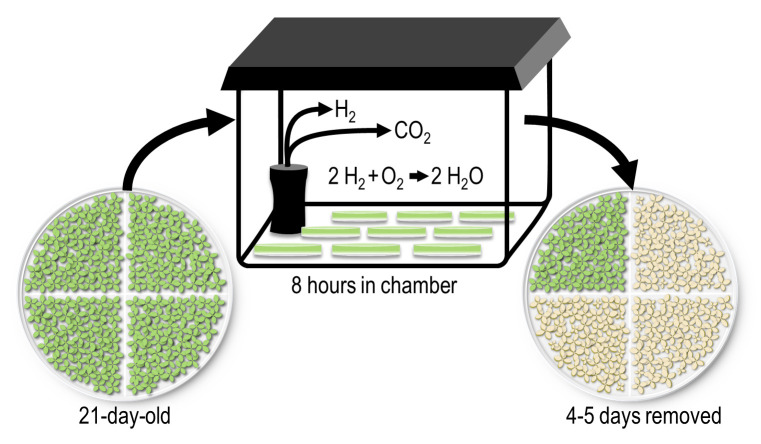
Here, we document a protocol for establishing anoxia stress conditions in Arabidopsis. Plants are grown for 21 days on media and then placed in a GasPakTM anaerobic system to create an oxygen-free environment—a setup widely used for the growth of anaerobic microorganisms. Anaerobic environment can be generated in a cylinder, rectangular jar, or vacuum bag, using an AnaeroGenTM sachet, which rapidly absorbs the oxygen with the simultaneous production of carbon dioxide. After eight hours in the anaerobic system (plus darkness), the plants are placed back in standard growth conditions for multiple days. After four to five days, wildtype plants fail to survive while anoxia-tolerant mutants survive. This system is easy, economical, and highly reproducible. This protocol should be easily modified to various plant species by varying the age of the plants and duration of the stress.
Background
Anaerobic conditions are part of an ensemble of stresses plants incur during submergence. Flooding also imposes temperature changes and altered oxidative conditions (Sasidharan et al., 2018). While submergence assays for plants have been detailed, they are often hard to perform and produce variable results (Bui et al., 2020; Yang et al., 2022; Loreti and Perata, 2020). Meanwhile, multiple methods have been used to create anaerobic conditions (Clark, 2019). Some of these methods include the Hungate technique, which uses a roll-tube approach to culture medium through which anoxic gas is bubbled to remove the remaining oxygen (Hungate, 1969). Other techniques are the VPI (Virginia Polytech Institute) method, a modification to the Hungate technique for large-scale culture using pre-reduced medium (Moore, 1966), and Glove-box chambers, a sealed chamber with attached gloves that are filled with anoxic gases (Clark, 2019). Recently, glass desiccators have been used for creating a hypoxic environment for plants. The method involves flushing oxygen-depleted air (humidified 100% N2 gas) into air-tight closed glass desiccators under dark conditions to generate an anoxic environment (Hartman et al., 2019). Similarly, an enclosed anerobic workstation (Anaerobic System model 1025; Forma Scientific) has been used for generating an anoxic environment for plant experiments (Loreti et al., 2018). Some of the disadvantages of these older methods include accidental gas leaks that expose the chamber to oxygen, high cost of gas tanks, regular maintenance requirements, space limitation, single purpose application, and lower efficiency and complexity (Killgore et al., 1973). Here, we introduce a simple and robust anoxia assay. Compared to previous methods, this protocol is quick, easy to perform, highly reproducible, and economical, requires minimum space, and produces a controlled (0% oxygen) anoxic environment.
Materials and Reagents
Aluminum foil
Anaerobic atmosphere generation bags, OxoidTM AnaeroGenTM 2.5 L sachet (Thermo Scientific; catalog number: OXAN0025A)
AnaeroPackTM 2.5 L rectangular jar (Thermo Scientific, catalog number: R685025)
FalconTM 15 mL conical tubes (CorningTM, catalog number: 14-959-53A)
Protect laboratory bottles (DURAN, PSC-47-11619)
Anaerobic system/cylindrical jar (BD GasPakTM 150 Systems, catalog number: 11-816)
Microcentrifuge tubes, 1.5 mL (PierceTM, catalog number: 69715)
Petri plates with clear lid (FisherbrandTM, catalog number: S33580A)
Surgical tape (Micropore, available through Amazon, USA)
Vacuum sealer bags (Wevac, available through Amazon, USA)
Anaerobic indicator (Thermo Scientific, catalog number: BR0055B)
Vaseline® (available through Amazon, USA)
-
Half-strength MS media (see Recipes; modified from Cheng et al., 2003)
Murashige and Skoog basal salt mixture (MS) media (Sigma-Aldrich, catalog number: M5524)
Sucrose (Sigma-Aldrich, catalog number: S1888)
Agar (Sigma-Aldrich, catalog number: A1296)
2-(N-Morpholino) ethanesulfonic acid (MES) (Sigma-Aldrich, catalog number: M3671)
Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: 221473)
-
20% (V/V) bleach (see Recipes)
Commercially available bleach (Clorox concentrated household bleach; available through Amazon, USA)
-
80% (V/V) acetone (see Recipes)
Acetone (Sigma-Aldrich, catalog number: 179124)
Plant materials
Recently, we have established that loss of a tonoplast-localized H+/Ca transporter, CAX1, imparts tolerance to anoxia. A mutation in another H+/Ca transporter, CAX3, is sensitive to anoxia in a manner indistinguishable from wildtype Colombia-0 (Col-0) (Yang et al., 2022). In the present study, we have used the same three mutants (two cax1 alleles and one cax3 mutant) along with Col-0 to standardize the anoxia assay conditions; the details are given below.
Arabidopsis wild type (Col-0)
-
Arabidopsis mutants:
cax1-1 - CS25435 (Cheng et al., 2003)
cax1-2 - SALK_021486 (Catala et al., 2003)
cax3-1 - CS25429 (Cheng et al., 2003)
Equipment
Varian Cary 50 Bio UV-visible spectrophotometer
Laminar air flow hood (SteriGard Hood, The Baker Company, Main, US)
Pipettes (GILSON, PIPETMAN CLASSIC P1000, catalog number: F123602 and GILSON, PIPETMAN L P20L, 2–20 µL, catalog number: FA10003M)
Nikon D80 Digital SLR camera (Nikon Corp., Tokyo, Japan)
Plant growth chamber (Geneva Scientific, CU-36L6 tissue culture chamber)
Heat poly bag sealer (Thomas Scientific, catalog number: 1147J64)
Procedure
Prepare half-strength MS media plates and 20% (V/V) bleach (see Recipes).
-
Seed sterilization
Transfer 100–200 seeds from each genotype to separate microcentrifuge tubes.
Add 1 mL of 20% (V/V) bleach to the tubes.
Mix the seeds thoroughly by brief vortexing.
Incubate the seeds in bleach solution for 15 min with occasional vortexing.
Give a short spin so that the seeds settle to the bottom of the tube. Do the remaining steps in a sterile environment.
Decant the bleach solution carefully with a 1 mL pipette. It is normal to have some seeds (poorly developed/empty seeds) to float on the supernatant bleach solution.
Add 1 mL of sterile water to the seeds and mix it thoroughly by inverting the tubes 3–4 times.
Remove the water and add fresh sterile water to the seeds.
Repeat steps B7–8 five more times to remove any trace of bleach.
Add 1 mL of sterile water to the seeds. At this stage, you can store the seeds or immediately proceed for seed plating. The seeds can be stored at 4°C for a week without losing its germination frequency.
-
Seed plating
Bring the half-strength MS media plates and sterilized seeds to room temperature. Seed plating is done in a laminar flow hood. Using a 20 µL pipette, draw up the sterilized seeds such that they are individually lined up in the pipette tip (use cut tips).
Carefully release the seeds onto the agar plate in the desired location (if two seeds are clumped together, add a small drop of water, separate the seeds, draw up one seed, and place it in another location). You can plate up to 50–60 seeds in a single plate. Make sure that the seeds are placed equidistantly.
Leave the plate open in the flow hood surface for 2–3 min to dry out the water from the seeds.
-
Cover the plates with surgical tape and keep them in a plant growth chamber maintained at 12 h light and 22°C, and 12 h night and 20°C for three weeks.
Note: Depending on the plant species, the optimal plant size/growth stage for the anoxia assay may vary. We recommend modifying the protocol based on the plant species by performing the anoxia assay at different stages of plant growth to acquire optimal plant size. For Arabidopsis, the assay was performed at several stages of plant growth and 3-week-old plants showed the optimal phenotype. Preliminary data with tobacco (Nicotiana tabacum) and tomato (Solanum lycopersicum) suggest that anoxia conditions for young seedlings will be in the range of conditions used for 3-week-old Arabidopsis; however, the initial tests with rice (Oryza sativa) and medicago (Medicago truncatula) lines suggest they are much more anoxia-tolerant and may require days to show sensitivity.
-
Anoxia experiment
-
Using a cylindrical jar (refer to Video 1):
Remove the surgical tape from the Petri dishes.
Stack the plates (nine plates/stack) into the anaerobic system/cylindrical jar (BD GasPakTM).
Apply a light coat of Vaseline on the rims of the jar and ensure proper sealing of the box.
Open a single sachet of AnaeroGenTM 2.5 L GasPakTM and place it in the jar on the side.
Place 2–3 tissue papers between the GasPakTM and the plates to dissipate heat generated from the GasPakTM.
-
Tighten the jar, cover with aluminum foil, and incubate in the dark at room temperature for required period of time.
Note: Depending on the plant species, optimal anoxia treatment time may vary. We recommend modifying the protocol based on the plant species by performing the anoxia assay for multiple periods to acquire optimal duration. For Arabidopsis, the assay was performed for 4, 6, 8, and 10 h. At 8 h, tolerant and sensitive lines showed the most distinctive phenotype.
Optional: Place an anaerobic indicator in the container to ensure the creation of anoxic environment.
After the anoxia treatment, re-tape and incubate the plates in the plant growth chamber maintained at 12 h light and 22°C, and 12 h night and 20°C for 4–5 days. Photograph the plates once the phenotype is clear (after 4–5 days).
-
Using a rectangular 2.5 L AnaeroPackTM system (refer to Video 2):
Remove the surgical tape from the Petri dishes.
Arrange the Petri plates containing plants in two stacks (four plates/stack) in the box.
Apply a light coat of Vaseline on the rims of the box and ensure proper sealing of the box.
Open a single sachet of AnaeroGenTM 2.5 L GasPakTM and place it inside the small compartment within the rectangular box; place 2–3 tissue papers between the GasPakTM and the plates to dissipate heat generated from the GasPakTM.
Cover the jar with aluminum foil and incubate in the dark at room temperature for required period of time.
After the anoxia treatment, return the plants to standard growth conditions. Photograph the plates after 4–5 days.
Using a vacuum sealer bag (refer to Video 3):
Vacuum sealer bag is used for performing anoxia for a small number of plates (1–2).- Remove the surgical tape from the Petri dishes.
- Cover both sides of the Petri dish with aluminum foil.
- Place the Petri dish on top of a layer of folded tissue paper inside the Wevac vacuum sealer bag.
- Open a single sachet of AnaeroGenTM 2.5 L GasPakTM and place it beneath the tissue paper inside the bag.
- Immediately seal the bag with a heat poly bag sealer and incubate at room temperature for required period of time.
- After the anoxia treatment, return the plates to standard growth conditions and photograph after 4–5 days.
-
-
Chlorophyll estimation (modified from Liang et al., 2017)
Grind 50 mg of tissue from both pre- and post-anoxia samples in liquid nitrogen.
Add 1 mL of 80% (V/V) acetone to the ground tissue and mix thoroughly by vortexing.
Incubate at room temperature for 30 min.
Centrifuge at 17,900 × g for 1 min.
Transfer the supernatant to a 15 mL centrifuge tube.
Repeat steps E2–5 three more times or until the pellet becomes colorless.
Add 80% (V/V) acetone to a final volume of 6 mL.
Read the absorbance of the pooled supernatant at wavelengths of 645 and 663 nm with a spectrophotometer.
Calculate the total chlorophyll in the sample using Arnon’s equation [total chlorophyll (µg/mL) = 20.2 (A645) + 8.02 (A663)].
Repeat the experiment at least two more times and represent the data as average ± SE (standard error).
Video 1. Anoxia treatment using “The Jar”.
Video 2. Anoxia treatment using “The Box”.
Video 3. Anoxia treatment using “The Bag”.
Data analysis
Results from anoxia experiments were confirmed three times with 13–15 seeds tested each time.
Chlorophyll estimation was performed for three biological replicates and represented as average ± SE (standard error).
Notes
While we do not see any advantage or disadvantage, we observed minor variations in the result of anoxia depending upon the chamber used, plant age, anoxia persistence, and diurnal conditions as given below.
Comparison of three anoxia chambers suggests that the rectangular box and vacuum sealer bag produce a more consistent response than the cylindrical jar. The cylindrical jar sometimes produced more variability, as plants in closer proximity to the gas pack occasionally displayed enhanced sensitivity than those placed further from the gas pack in the same jar. Thus, here we used the rectangular box/vacuum sealer bags for subsequent experiments. The smaller volume containers may produce more rapid anoxic conditions; however, these differences were not significant enough to produce phenotypic changes in these assay conditions with Arabidopsis.
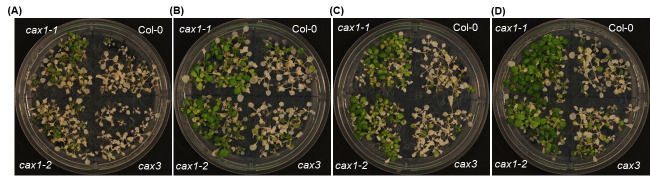
Plant age: Using the mutants and control, we assessed the impact of plant age on anoxia tolerance (Boyes et al., 2001). Plants at the 9-rosette leaf stage (21 days old) and 6–7-rosette leaf stage (18 days old) were slightly more tolerant than plants at the 5-rosette leaf stage (14 days old; Figure 1). For all the remaining assays, plants at the 7-rosette leaf stage (18 days old) were used.
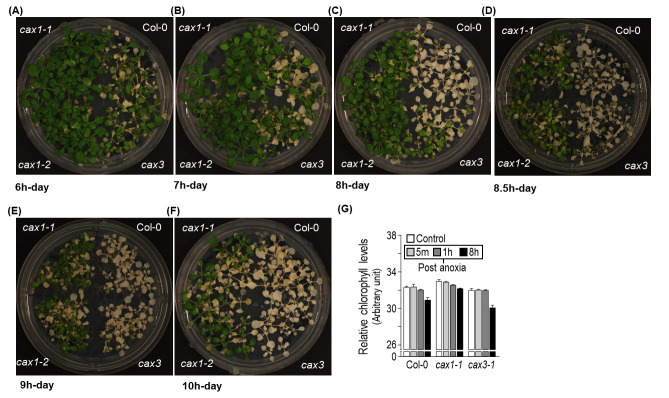
Duration of treatment: The effect of anoxia persistence was then assayed. A difference in the phenotype was observed as soon as at 6 h of treatment in the rectangular 2.5 L AnaeroPackTM system (Figure 2A). The phenotype became very clear with prolonged incubation time. At 8–9 h of anoxia treatment, there was a clear difference between the phenotype of sensitive and tolerant genotypes. The two sensitive genotypes (Col-0 and cax3) did not survive after anoxia (Figure 2B–E). However, when the incubation was extended for 10 h, the survival rate for the tolerant genotypes (cax1-1 and cax1-2) reduced significantly (Figure 2F). The survival rate following anoxia was indirectly measured by estimating the residual chlorophyll content five days after the anoxia treatment. As shown in figure 2G, cax1 exhibited less chlorophyll loss compared to Col-0 and cax3.
-
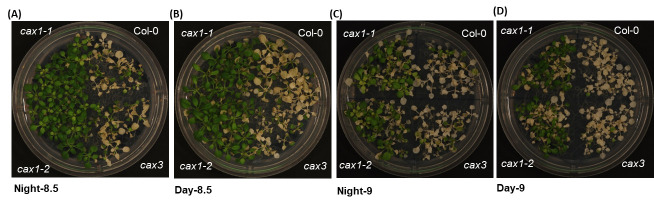
Diurnal variations: We then sought to better understand how the photoperiod influences anoxia tolerance. While the anoxia treatment was always performed in the dark, plants treated during the daytime were slightly more sensitive than those treated at night (Figure 3). Hence, anoxia treatment at night without disturbing the diurnal rhythm was consistently used in our experiments.
By limiting variations in the above-mentioned parameters, differences in the response of a particular genotype to anoxia can be minimized. Although there was a slight difference in the extent of chlorophyll loss within a genotype (Figure 4), the assay clearly distinguished the tolerant from the susceptible genotypes.
We have used the assay for Arabidopsis thaliana in this study, but this approach should be applicable to other plant species as well.
Figure 1. Effect of plant age on anoxia response.
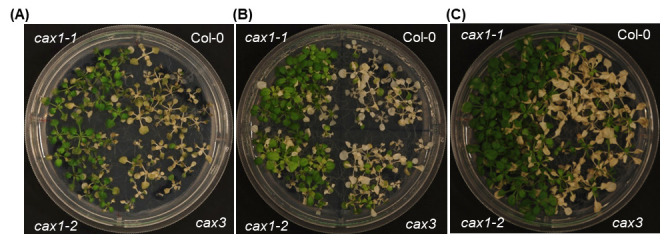
Plants belonging to 5-rosette (A), 7-rosette (B), and 9-rosette (C) leaf stages were exposed to an anaerobic environment for 9 h during the night. The experiment was repeated three times, with 13–15 plants analyzed for each mutant in each replicate. Col-0 (wild type), cax1-1 and cax1-2 (two alleles of cax1 with T-DNA insertions at different locations), and cax3 (cax3 mutant). For further information including statistical comparisons and transcriptomic and proteomics data analyses refer to Yang et al. (2022).
Figure 2. Effect of the duration of treatment to anoxia response.
Plants belonging to 7-rosette leaf stage (18 days old) were exposed to anaerobic environment for 6 (A), 7 (B), 8 (C), 8.5 (D), 9 (E), and 10 h (F) during the day. Differential responses of the genotype to anoxia represented in terms of their chlorophyll loss (G). cax1 exhibited less chlorophyll loss than Col-0 and cax3. For further information including chlorophyll measurements, statistical comparisons, and transcriptomic and proteomics data analyses refer to Yang et al. (2022).
Figure 3. Effect of diurnal phase of treatment on response to anoxia.
Plants belonging to 7-rosette leaf stage (18 days old) were exposed to an anaerobic environment for 8.5 h during the night (A), 8.5 h during the day (B), 9 h during the night (C), and 9 h during the day (D).
Figure 4. Variations in the anoxia response with similar external conditions.
Plants belonging to 7-rosette leaf stage (18 days old) were exposed to anaerobic condition during the night.
Recipes
-
Half-strength MS media
(Preparation of 1 L media; enough for preparing approximately 20 plates)
Dissolve the following components in 800 mL of double distilled water:
2.15 g of MS media
5 g of sucrose
500 mg of MES
Adjust the pH to 5.6–5.8 by adding KOH while stirring. Make up the volume to 1 L.
Add 8% (W/V) agar into two 500 mL bottles.
Distribute the media equally into each bottle containing agar (500 mL).
Sterilize the media by autoclaving at 121°C for 20 min.
Once the media has cooled, pour each bottle into 10 Petri plates in the laminar flow hood.
Allow the media to solidify by leaving it on the laminar flow hood for 30 min.
The media can be stored at 4°C for 2–3 weeks. Bring the media to room temperature and inspect the plate for any contamination prior to use.
-
20% bleach (V/V)
Mix 10 mL of bleach with 40 mL of autoclaved double distilled water in a 50 mL centrifuge tube.
Mix it thoroughly by inverting the tube.
Bleach can be stored at room temperature for 2–3 weeks.
80% acetone (V/V)
Mix 40 mL of acetone with 10 mL of double distilled water.
Mix it thoroughly by inverting the tube or vortexing.
The solution can be stored at room temperature in a flame-resistant area.
Acknowledgments
This work was supported by grants (to K.D.H) from the National Science Foundation (1557890), USDA (3092-51000-061-00D), and National Institute of Health (R03 AI149201-02). This protocol is derived from Yang et al. (2022).
Competing interests
The authors declare that there is no conflict of interest.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
Q&A
Post your question about this protocol in Q&A and get help from the authors of the protocol and some of its users.
References
- 1.Boyes D. C., Zayed A. M., Ascenzi R., McCaskill A. J., Hoffman N. E., Davis K. R. and Gorlach J.(2001). Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13(7): 1499-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bui L. T., Shukla V., Giorgi F. M., Trivellini A., Perata P., Licausi F. and Giuntoli B.(2020). Differential submergence tolerance between juvenile and adult Arabidopsis plants involves the ANAC017 transcription factor. Plant J 104(4): 979-994. [DOI] [PubMed] [Google Scholar]
- 3.Catala R., Santos E., Alonso J. M., Ecker J. R., Martinez-Zapater J. M. and Salinas J.(2003). Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis. Plant Cell 15(12): 2940-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng N. H., Pittman J. K., Barkla B. J., Shigaki T. and Hirschi K. D.(2003). The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 15(2): 347-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark H.(2019). Culturing anaerobes. Nature Portfolio.
- 6.Hartman S., Liu Z., van Veen H., Vicente J., Reinen E., Martopawiro S., Zhang H., van Dongen N., Bosman F., Bassel G. W., et al.(2019). Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat Commun 10(1): 4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hungate R. E.(1969). Chapter IV: A Roll Tube Method for Cultivation of Strict Anaerobes. In: Methods in Microbiology. Norris, J. R. and Ribbons, D. W.(Eds.). Academic Press. 3: 117-132. [Google Scholar]
- 8.Killgore G. E., Starr S. E., Del Bene V. E., Whaley D. N. and Dowell V. R., Jr (1973). Comparison of three anaerobic systems for the isolation of anaerobic bacteria from clinical specimens. Am J Clin Pathol 59(4): 552-559. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y., Urano D., Liao K. L., Hedrick T. L., Gao Y. and Jones A. M.(2017). A nondestructive method to estimate the chlorophyll content of Arabidopsis seedlings. Plant Methods 13: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loreti E. and Perata P.(2020). The Many Facets of Hypoxia in Plants. Plants(Basel) 9(6): 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loreti E., Valeri M. C., Novi G. and Perata P.(2018). Gene Regulation and Survival under Hypoxia Requires Starch Availability and Metabolism. Plant Physiol 176(2): 1286-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore W. E. C.(1966). Techniques for routine culture of fastidious anaerobes. Intern J Syst Bacteriol 16: 173-190. [Google Scholar]
- 13.Sasidharan R., Hartman S., Liu Z., Martopawiro S., Sajeev N., van Veen H., Yeung E. and Voesenek L.(2018). Signal Dynamics and Interactions during Flooding Stress. Plant Physiol 176(2): 1106-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J., Mathew I. E., Rhein H., Barker R., Guo Q., Brunello L., Loreti E., Barkla B. J., Gilroy S., Perata P. and Hirschi K. D.(2022). The vacuolar H+/Ca transporter CAX1 participates in submergence and anoxia stress responses. Plant Physiol 190(4): 2617-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]