Manganese (Mn) is an essential trace element for the normal development of human body. It plays an important role in the regulation of cellular energy level, blood sugar balance, function of immunity, and bone growth, and works as an essential part of many indispensable enzyme functions. Meanwhile, overexposure to divalent manganese (Mn 2+) could cause a series of physiological and pathological dysfunctions, and Mn has been recognized as an environmental toxicant and an occupational hazard [1]. With the increasing exposure risk in environmental settings, such as contaminated food and polluted water and soil, Mn 2+-induced toxicities have become an important public health issue.
Neurotoxicity induced by Mn 2+ exposure has been extensively studied, and neuroinflammation has been recognized as the key player that significantly increases the risk of neurodegenerative disorders. Besides neurotoxicity, Mn 2+-induced immunotoxicity has also been observed. It has been shown that Mn 2+ exposure could enhance the activity of natural killer cells through type I interferons (IFNs) and elevate the humoral immune response in mice [2]. However, the underlying mechanism in Mn 2+-induced immunotoxicity is yet to be explored. As disruption of the immune system may impact the body defense against infectious diseases, oxidative stress, and tumorigenesis, etc. [3], Mn 2+-induced immunotoxicity can lead to a broad range of disorders and should be further investigated.
The innate immunity functions at the frontier of body responses to invading microbes or environmental stimuli. Macrophages are a chief player in the innate immunity that control inflammatory responses and secret important inflammatory cytokines [ 4, 5]. In macrophages, initiation of inflammatory responses involves the activation of inflammasomes. Inflammasomes are important for defending pathogens, but their inappropriate activation is harmful for potentially causing immune-related disorders. To understand the immunotoxicity of Mn 2+, study on inflammasomes of macrophages is a pivotal direction [ 6, 7].
In the present study, mouse peritoneal macrophages (PMs) were used as an in vitro model to investigate the immunotoxicity of Mn 2+. Female C57BL/6 mice (6–8 weeks old; Zhejiang Experimental Animal Center, Hangzhou, China) were individually housed in cages with a light/dark cycle of 12/12 h and free access to sterile food and water under the specific pathogen-free (SPF) condition of the Laboratory Animal Center of China Pharmaceutical University (Nanjing, China). All procedures have been approved by the Animal Care Commission of China Pharmaceutical University.
For collection and culture of resident PMs, PMs were harvested in cold PBS from the peritoneal cavities of the mice after intraperitoneally inoculated with 2.5 mL of 5% thioglycollate medium (Solarbio Science & Technology, Beijing, China) for 4 days. Then the PMs were suspended in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin (Solarbio Science & Technology), and incubated in individual flat-bottom culture plates at 37°C in an incubator with 5% CO 2. For western blot analysis, the cell culture supernatant was centrifuged at 1,500 g for 5 min to remove the cell debris. Subsequently, 1 mL of the supernatant was processed to obtain the protein sample using chloroform and methanol. The protein pellet was dissolved in 1% SDS. For the cell lysates, proteins were extracted using the RIPA Lysis Buffer containing 1 mM PMSF (Beyotime, Shanghai, China). The protein samples were subject to 15% SDS-PAGE and transferred to PVDF membranes. Membranes were blocked with 5% skimmed milk and incubated with indicated primary antibodies, followed by incubation with HRP-conjugated secondary antibodies (Proteintech, Wuhan, China). The signals were developed using the ECL Plus western blotting detection reagent (Merck Millipore, Bedford, USA). For RNA extraction and quantitative real-time PCR (qPCR), total RNA was isolated from PMs using the Total RNA Extraction Reagent (Vazyme, Nanjing, China). qPCR reactions were performed on a StepOne Real-Time PCR System (Thermo Fisher Scientific, Waltham, USA) using the qPCR SYBR Green Master Mix (Vazyme). β-Actin was used as the housekeeping gene control. The primer sequences are shown in Table 1. Data were presented as the mean±SEM of at least three independent experiments using Graphpad Prism 5.0. ANOVA Bonferroni multiple comparisons test, Tukey post-hoc test, and/or unpaired Student’s t-test were used with SPSS version 11.0. P<0.05 is considered as statistically significant.
Table1 Sequences of primers used in qPCR
|
Gene |
GenBank acce-ssion No. |
Primer sequence (5′→3′) |
|
IL-6 |
Forward: TGCCTTCTTGGGACTGATGC |
|
|
Reverse: GCAAGTGCATCATCGTTGTTC | ||
|
IL-12 |
Forward: GGAAGAGTCCCCCAAAAGCT |
|
|
Reverse: CAGCAAAGGTGTCATGATGAACTT | ||
|
IL-18 |
Forward: ACCAAGTTCTCTTCGTTGAC |
|
|
Reverse: CTTCACAGAGAGGGTCACAG | ||
|
IL-1β |
Forward: AGGAGAACCAAGCAACGACA |
|
|
Reverse: CTCTGCTTGTGAGGTGCTGA | ||
|
TNF-α |
Forward: GACGTGGAACTGGCAGAAGA |
|
|
Reverse: GGCTACAGGCTTGTCACTCG | ||
|
NOD1 |
Forward: GATTGGAGACGAAGGGGCAA |
|
|
Reverse: CGTCTGGTTCACTCTCAGCA | ||
|
NOD2 |
Forward: GCCAGTACGAGTGTGAGGAG |
|
|
Reverse: GCGAGACTGAGTCAACACCA | ||
|
NLRP1 |
Forward: ATAAACAAGCCACCCCCAGT |
|
|
Reverse: TGTGCCCAATGTCGATCTCA | ||
|
NLRP3 |
Forward: AGCCAGAGTGGAATGACACG |
|
|
Reverse: CGTGTAGCGACTGTTGAGGT | ||
|
NLRC4 |
Forward: GCTCAGTCCTCAGAACCTGC |
|
|
Reverse: ACCCAAGCTGTCAATCAGACC | ||
|
ACTIN |
Forward: GCCATGTACGTAGCCATCCA |
|
|
Reverse: ACGCACGATTTCCCTCTCAG | ||
|
Caspase-1 |
Forward: AACCACTCGTACACGTCTTGC |
|
|
Reverse: ATCCTCCAGCAGCAACTTCA |
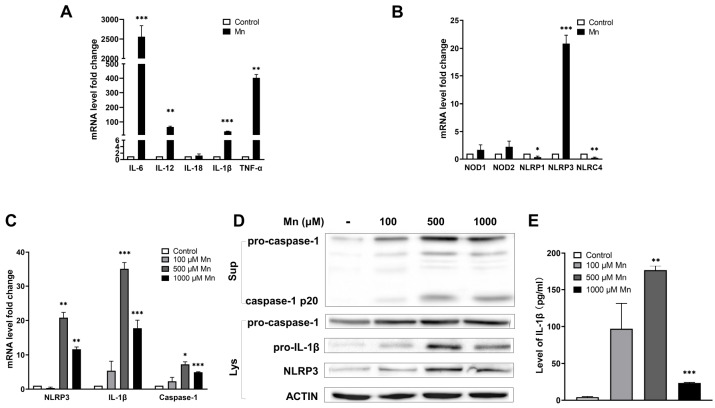
We found that in response to Mn 2+ exposure, the expression levels of IL-6, IL-12, IL-1β and TNF-α were significantly upregulated, while the expression of IL-18 remained unchanged ( Figure 1A). For the inflammasome-related genes, NLRP1 and NLRC4 were slightly down-regulated, and NLRP3 was greatly up-regulated ( Figure 1B). These results suggested that inflammasome response is involved in the Mn 2+ exposure-induced effects of PMs and NLRP3 inflammasome may play an important role in the Mn 2+-induced inflammation of PMs.
Figure1 .
Activation of NLRP3 inflammasome in Mn 2+-induced PMs For induction, PMs were treated with 500 μM of Mn2+or three different concentrations of Mn2+ for 12 h. (A) The relative transcription levels of IL-6, IL-12, IL-1β and TNF-α were measured by qPCR. (B) The relative transcription levels of NOD1, NOD2, NLRP1, NLRP3 and NLRC4 were measured by qPCR. (C) The relative transcription levels of NLRP3, IL-1β and caspase-1 were measured by qPCR. (D) Expressions of pro-caspase-1, cleaved caspase-1 (p20), pro-IL-1β and NLRP3 were analyzed by western blot analysis. Sup, supernatant. Lys, cell lysate. (E) Secretion of IL-1β to the supernatant was measured by ELISA. The data represented results from three independent experiments and were presented as the mean±SEM. *P<0.05, **P<0.01, ***P<0.001 compared with the negative controls that were not Mn2+-induced.
Mn 2+, at concentrations above 500 μM, greatly up-regulated three key components of NLRP3 inflammasome, including secretion of IL-1β, the activation of caspase-1 and the expression of NLRP3 in PMs ( Figure 1C). The protein levels of these key components were also increased, accompanied by the appearance of cleaved caspase-1 p20, with Mn 2+ induction as determined by western blot analysis ( Figure 1D). The release of active IL-1β to the supernatant was found to be induced by Mn 2+ exposure as revealed by ELISA ( Figure 1E). These results indicated that activation of NLRP3 inflammasome was part of the Mn 2+-induced inflammation in PMs.
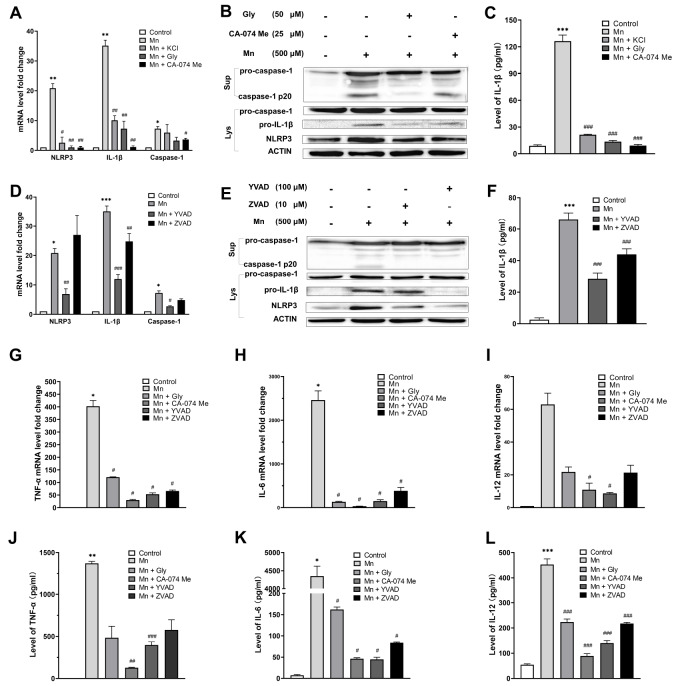
We further confirmed the involvement of NLRP3 inflammasome in the Mn 2+-induced inflammation in PMs by using NLRP3 inhibitors. The elevated transcription levels of the three key components of NLRP3 inflammasome with Mn 2+ exposure were suppressed by NLRP3 inhibitors, i.e., Glibenclamide (Gly; inhibits K + efflux) and CA-074 methyl ester (CA-074 Me; inhibits cathepsin B) ( Figure 2A). The protein levels of NLRP3, IL-1β and cleaved caspase-1 p20 that were augmented with Mn induction were also decreased in the presence of the inhibitors ( Figure 2B). The secretion of IL-1β to the cell supernatant with Mn 2+ induction was also suppressed ( Figure 2C). These results indicated that NLRP3 inhibitors could restrain the expression of NLRP3, the activation of caspase-1, and the secretion of IL-1β, suggesting that NLRP3 plays an important role in the inflammation of Mn 2+-induced PMs.
Figure2 .
Inflammation in Mn 2+-induced PMs blocked by NLRP3, extracellular K +, and caspase inhibitors In samples with the inhibitors, PMs were treated with 50 μM of Gly, 25 μM of CA-074 Me, 100 μM of YVAD or 10 μM of ZVAD for 60 min, followed by induction with 500 μM of Mn2+ for 12 h. For treatment with extracellular K+, PMs were treated with 50 mM KCl for 60 min, followed by induction with 500 μM of Mn2+ for 12 h. (A,D) The relative transcription levels of NLRP3, IL-1β and caspase-1 were measured by qPCR. (B,E) Expression of pro-caspase-1, cleaved caspase-1 (p20), pro-IL-1β and NLRP3 were analyzed by western blot analysis. Sup, supernatant. Lys, cell lysate. (C,F) Secretion of IL-1β to the supernatant was measured by ELISA. (G–I) The relative transcription levels of TNF-α, IL-6 and IL-12 were measured by qPCR. (J–L) Secretion of inflammatory cytokines TNF-α, IL-6 and IL-12 to the supernatant was measured by ELISA. Data are presented as the mean±SEM from three independent experiments. *P<0.05, **P<0.01, ***P<0.001, compared with the negative controls that were not Mn2+-induced. #P<0.05, ##P<0.01, ###P<0.001, compared with the Mn2+-induced samples without inhibitor treatment.
Evidence have shown that K + efflux is a common signaling event of NLRP3 inflammasome activation, though there are exceptions [8]. To test if extracellular K + could suppress the inflammation, PMs were pre-treated with KCl before the Mn 2+ induction. Indeed, extracellular K + suppressed the inflammatory signals ( Figure 2A,C), suggesting the involvement of K + efflux in the Mn 2+-induced NLRP3 inflammasome activation.
Then we determined whether caspases play an essential role in the Mn 2+-induced inflammation by using Z-VAD-FMK (ZVAD; an inhibitor of pan-caspase) and Ac-YVAD-CHO (YVAD; an inhibitor of caspase-1 and caspase-4). The inhibitor YVAD had a more significant effect on the transcriptions of the three key components of NLRP3 inflammasome induced by Mn 2+ exposure than the inhibitor ZVAD ( Figure 2D). Western blot analysis and ELISA demonstrated the suppression of caspase-1 activation and IL-1β secretion by both caspase inhibitors, and YVAD had a more significant effect ( Figure 2E,F). These results suggest that caspases, particularly caspase-1, are required for the Mn 2+-induced inflammation in PMs.
Three additional inflammatory cytokines, including TNF-α ( Figure 2G,J), IL-6 ( Figure 2H,K) and IL-12 ( Figure 2I,L), showed elevated expression and secretion with Mn 2+ exposure in PMs, as confirmed by qPCR and ELISA. Moreover, their expression and secretion were significantly down-regulated by the NLRP3 and caspase inhibitors. Thus, the NLRP3 inflammasome signaling pathway plays a key role in the Mn 2+-induced inflammation in PMs by mediating the secretion of important inflammatory factors, including TNF-α, IL-6, IL-12, and IL-1β.
In summary, this study for the first time reported the activation of NLRP3 inflammasome in macrophages as a critical event of the Mn 2+-induced immunotoxicity. The NLRP3 inflammasome in PMs was activated by Mn 2+ exposure and mediated the activation of caspase-1 and the release of IL-1β. PMs rarely express pro-IL-1β without the presence of innate immunity inducers [9]. The expression of pro-IL-1β and secretion of its active form were significantly upregulated in PMs with Mn 2+ induction, and the relationship with activation of NLRP3 inflammasome was clearly demonstrated. The K + efflux was an upstream signal of the NLRP3 inflammasome activation. These observations are similar to the phenomenon in microglia of Mn 2+ exposure, in which the NF-κB pathway plays a critical role in the formation of NLRP3 inflammasome, and consequently causes the release of mature IL-1β and other inflammatory factors [10]. This suggests the involvement of NLRP3 inflammasome in the overall effect of Mn 2+-related toxicities. The NOD-like receptor protein 3-caspase 1 (NLRP3-CASP1) pathway could be a common therapeutical target in both immune and nervous systems for inflammatory damages caused by Mn 2+ exposure. Potential effective drug targets in this pathway should be screened in the future.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (No. 31470275), the Key Natural Science Research Project of the Jiangsu Higher Education Institutions of China (No. 20KJA416002), the Natural Science Foundation of Jiangsu Higher Education Institutions of China (No. 20KJB350008), the Science and Technology Project of Lianyungang High-tech Zone of China (No. HZ201901), the Open-end Funds of Jiangsu Key Laboratory of Marine Pharmaceutical Compound Screening (No. HY202004), the “Blue Project” of Jiangsu Higher Education Institutions of China, and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
References
- 1.Tinkov AA, Paoliello MMB, Mazilina AN, Skalny AV, Martins AC, Voskresenskaya ON, Aaseth J, et al. Molecular targets of manganese-induced neurotoxicity: a five-year update. Int J Mol Sci. . 2021;22:4646. doi: 10.3390/ijms22094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Zhang R, Wei X, Lv M, Jiang Z. Metalloimmunology: the metal ion-controlled immunity. Adv Immunol 2020, 145: 187–241 . [DOI] [PubMed]
- 3.Kühl AA, Erben U, Kredel LI, Siegmund B. Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol. . 2015;6:1–7. doi: 10.3389/fimmu.2015.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barksby HE, Lea SR, Preshaw PM, Taylor JJ. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. . 2007;149:217–225. doi: 10.1111/j.1365-2249.2007.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. . 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 6.Moloudizargari M, Moradkhani F, Asghari N, Fallah M, Asghari MH, Moghadamnia AA, Abdollahi M. NLRP inflammasome as a key role player in the pathogenesis of environmental toxicants. Life Sci. . 2019;231:116585. doi: 10.1016/j.lfs.2019.116585. [DOI] [PubMed] [Google Scholar]
- 7.Zheng D, Liwinski T, Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. . 2020;6:36. doi: 10.1038/s41421-020-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Chen M, He X, Ouyang D. A mini-review on ion fluxes that regulate NLRP3 inflammasome activation. Acta Biochim Biophys Sin. . 2021;53:131–139. doi: 10.1093/abbs/gmaa155. [DOI] [PubMed] [Google Scholar]
- 9.Schroder K, Tschopp J. The Inflammasomes. Cell. . 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar S, Rokad D, Malovic E, Luo J, Harischandra DS, Jin H, Anantharam V, et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci Signal. . 2019;12:eaat9900. doi: 10.1126/scisignal.aat9900. [DOI] [PMC free article] [PubMed] [Google Scholar]